Abstract
This study set out to determine whether there is white matter involvement in essential tremor (ET), the most common movement disorder. We collected diffusion MRI and analysed differences in fractional anisotropy (FA) and mean diffusivity (MD) between ET patients and control subjects as markers of white matter integrity. We used both classical ROI‐based statistics and whole‐brain analysis techniques, including voxel‐wise analysis with SPM5 and tract‐based spatial statistics (TBSS). Using region of interest (ROI) analysis, we found increased MD bilaterally in the inferior cerebellar peduncles (ICP) and reduced FA in the right‐sided ICP of ET patients. Whole‐brain analyses with TBSS detected increased MD distributed in both motor and nonmotor white matter fibers of ET patients predominantly in the left parietal white matter, while there were no significant FA differences in these areas between ET patients and controls. Voxel‐wise analysis with SPM detected significant increase of MD congruent with the highest probability of difference as detected by TBSS. VBM analysis of T1 images did not detect significant differences in either gray or white matter density between our study groups. In summary, we found evidence for changes in white matter MRI properties in ET. The circumscript pathology of the ICP corroborates the pathogenetic concept of the cerebellum and its projections as key structures for tremor generation in ET. Moreover, increased diffusivity in white matter structures of both hemispheres suggests widespread alterations of fiber integrity in motor and nonmotor networks in ET patients. The underlying cause of the DTI changes observed remains to be elucidated. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: essential tremor, diffusion tensor imaging, diffusion, cerebellum, motor networks, pathology
INTRODUCTION
Essential tremor (ET) is the most common movement disorder [Louis et al., 1998] and one of the most frequent neurological disorders in general [Louis, 2005]. Typical symptoms are a postural and kinetic tremor of medium to high frequency [Elble, 1996], often exacerbated by emotional stress or physical exertion [Deuschl et al., 1998; Findley, 1996].
Symptom presentation can be mild, such that patients never seek medical attention [Louis, 2005]. This fact contributes to the notable discrepancies observed in prevalence estimates of ET in community versus service‐based studies, which range from 400–4,000 per 100,000 [Elble, 2006; Louis et al., 1998]. When patients do seek medical attention, a limited number of drugs have shown modest benefit, yet their mechanisms of action in ET remain to be elucidated [Louis, 2005].
Despite its high prevalence, there are only few studies on the neuropathology of ET available, but the most frequently reported changes are related to the cerebellar Purkinje cells [Louis et al., 2006, 2007). Involvement of white matter is still controversial [Martinelli et al., 2007; Shin et al., 2008]. Magnetic resonance diffusion‐weighted imaging [Le Bihan and Breton, 1985] provides a unique window on tissue microstructure [Johansen‐Berg and Behrens, 2006]. It allows for quantitative assessment of white matter structure, and measures derived from the diffusion tensor model have been shown to correlate with the severity and with progression rates of degenerative [Ciccarelli et al., 2006] and inflammatory [Benedetti et al., 2009; Bodini et al., 2009] diseases of the brain. The most widely used measurements derived from the tensor model are the fractional anisotropy (FA) and the mean diffusivity (MD), both of which provide complementary data on tissue microstructure. Reduction in local fiber density, or, more generally, disturbed fiber integrity is expected to result in a decrease of FA as a measure of diffusion directionality and an increase of MD as a measure of overall water diffusion.
Two studies applied diffusion‐weighted imaging in ET patients [Martinelli et al., 2007; Shin et al., 2008]. One study [Martinelli et al., 2007] analysed MD within prespecified ROIs, and did not report significant changes in ET subjects. Another study using diffusion tensor imaging (DTI) found widespread FA reduction in the entire brain [Shin et al., 2008] using voxel‐wise statistics, but failed to achieve cluster‐corrected statistical significance. We investigated white matter involvement in ET using an optimized diffusion imaging and image analysis protocol in age‐matched groups of ET patients and controls. We performed a region‐of‐interest (ROI) based analysis of FA and MD in the cerebellar peduncles, since they carry all information processed by the cerebellum in a highly collinear fiber system. In addition, we also performed whole‐brain analyses of the data, exploring further involvement of white matter in ET. Finally, we performed a voxel‐based morphometry (VBM) analysis of gray and white matter density assessed by T1‐weighted imaging to exclude macroscopic differences between the study groups.
SUBJECTS AND METHODS
Study Subjects
The local ethics committee approved the study, and all subjects provided informed consent before inclusion. We studied 14 patients with ET (age ± standard deviation: 61.2 years ±12.0) and 20 age‐matched control subjects (60.2 ± 8.1). The diagnosis of ET had been established according to criteria of the Tremor Investigation group [Deuschl et al., 1998], with 10 patients meeting criteria of definite ET, and four meeting criteria of probable ET. Tremor rating was performed using the Fahn Tremor Rating Scale [Fahn et al., 1998], and videotaped assessment was cross‐validated by an experienced movement disorders specialist (R.H.). All patients and control subjects were right‐handed, receiving a score of 100 on the Edinburgh Handedness Inventory (EHI) [Oldfield, 1971]. Demographics of the patients are reported in Table I. We report tremor lateralization, self‐reported family history, and whether symptoms responded positively to alcohol ingestion. Some subjects did not drink any alcohol and were therefore unable to answer the last question.
Table I.
Patient demographics
| Patient no. | Gender | Age | Duration of ET | Fahn tremor score | Family history | Affected side | Handedness (EHI) | Response to alcohol | Head tremor | Medication |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 73 | 30 | 29 | Pos | R>L | 100 | + | — | PRI, MET |
| 2 | F | 75 | 7 | 14 | Neg | R=L | 100 | ? | — | PRO |
| 3 | M | 43 | 5 | 9 | Pos | R>L | 100 | ? | — | None |
| 4 | M | 65 | 15 | 28 | Pos | R=L | 100 | ? | — | PRO |
| 5 | F | 47 | 8 | 15 | Pos | L>R | 100 | + | — | PRO |
| 6 | F | 72 | 4 | 12 | Neg | R>L | 100 | ‐ | — | None |
| 7 | F | 64 | 7 | 11 | Pos | L>R | 100 | ? | — | None |
| 8 | M | 65 | 3 | 16 | Pos | R>L | 100 | + | — | PRO |
| 9 | M | 68 | 7 | 13 | Pos | R>L | 100 | ? | — | Alfazosin |
| 10 | M | 38 | 7 | 18 | Neg | R=L | 100 | + | — | PRO |
| 11 | M | 47 | 1.5 | 6 | Neg | L>R | 100 | ? | — | PRO |
| 12 | M | 63 | 40 | 20 | Neg | R>L | 100 | ? | — | Diltiazem |
| 13 | M | 69 | 20 | 31 | Pos | R=L | 100 | + | — | Gabapentin |
| 14 | F | 67 | 30 | 16 | Pos | R=L | 100 | + | — | MET |
All patients had bilateral tremor. Some patients were unable to assess response to alcohol due to abstinence.
PRO, propranolol; PRI, primidone; MET, metoprolol.
Diffusion‐Weighted MR Imaging
All subjects underwent diffusion MRI in a Siemens Trio 3T (Siemens, Erlangen, Germany) scanner, using an 8‐channel array head coil for signal reception and the built‐in body coil for radiofrequency transmission. Diffusion was measured in 60 isotropically distributed directions using spin‐echo echo‐planar imaging [Turner et al., 1991] and sensitivity‐encoded read‐out acceleration (SENSE) [Jaermann et al., 2006; Pruessmann et al., 1999] (SE‐EPI, TE 95ms, TR 9.3s, 70 axial slices, isotropic voxel size 2 mm, SENSE factor 2) with a b‐value of 1,000 s mm−2 and 10 nondiffusion weighted reference images. To increase the signal to noise ratio (SNR), scanning was repeated three times for averaging, resulting in a total scanning time of ∼45 min.
Anatomical MR Imaging
Subjects underwent T1‐weighted imaging using a Fast Low Angle Shot sequence [Frahm et al., 1986] (FLASH, TE 2.4 ms, TR 7.6 ms, flip angle 18°, 160 sagittal slices, isotropic voxel size 1 mm). Scanning was repeated twice for off‐line averaging to increase SNR. Before averaging, datasets were realigned using FLIRT [Jenkinson et al., 2002].
In addition, subjects received Fluid Attenuated Inversion Recovery T2‐weighted imaging to exclude gross pathology (3D‐FLAIR, TE 353 ms, TR 6,000 ms, TI 2,200 ms, 72 sagittal slices, voxel size 1 × 1 × 2.07 mm3).
Data Analysis—VBM
T1‐weighted images were subjected to VBM using SPM 5 (The Wellcome Department of Cognitive Neurology, London, http://www.fil.ion.ucl.ac.uk/spm). All analyses were performed in ICBM‐152 space, transforming individual images using the T1 template supplied with SPM. After correcting for intensity nonuniformity, estimates of gray and white matter density were generated. Density estimates were modulated with the Jacobian of the transformation matrix to address local compression or expansion due to spatial normalization. Then, a multiple regression model was created using age as a confound regressor.
Data Analysis—Diffusion Data
Diffusion data were corrected for eddy currents and head movement by affine registration to a nondiffusion‐weighted reference volume [Jenkinson et al., 2002]. The three acquisitions were averaged to increase SNR, and a mask was created to limit analysis to brain tissue only. Diffusion tensors were fitted using a conventional least‐squares approach with tools from the FMRIB software library [Smith et al., 2004].
ROI‐Based Analysis
For each subject and each side of the brain, masks were drawn in the superior (SCP), medial (MCP) and inferior cerebellar peduncle (ICP). To visualize these structures, color‐coded representations of tensor orientation were displayed [Douek et al., 1991], using FA images to modulate image luminosity. This way, both FA (luminosity‐encoded) and principal diffusion direction (color‐coded) are visible simultaneously, facilitating manual separation of the peduncles. For SCP, the ROI centre was located on sagittal images and ten voxels were selected on up to three sagittal image planes around the SCP's centre, taking care to stay clear of the SCP's merging with the brain stem. For MCP, two coronal sections in the middle of the rostro‐caudal extent of the peduncle were selected. On each of these two coronal sections, nine voxels were selected in the centre of the MCP, resulting in a total of 18 voxels marked. For ICP, four voxels each were selected on two adjacent transversal planes in the middle of the rostro‐caudal extent of this peduncle, resulting in a ROI with eight voxels in total. Thus, we created six ROIs in original space for each of the study subjects. Subsequently, samples of FA and MD were taken from these ROIs. Figure 1 shows ROI placement in a representative study subject. We tested for (a) group differences and (b) correlations of FA and MD in these ROIs with Fahn tremor score and disease duration using SPSS 17.0 (SPSS Inc., Chicago). To account for residual age effects in our study cohorts, a multiple regression model was used with age as confound regressor. F‐tests and post hoc two‐sided two‐sample t tests were applied to assess between‐group mean differences (P < 0.05).
Figure 1.
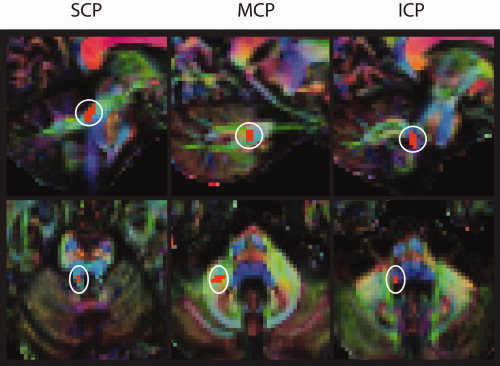
ROIs overlaid onto an example subject for right superior (SCP), middle (MCP), and inferior (ICP) cerebellar peduncle. Color‐coded representations of principal diffusion direction, luminosity modulated by FA. Red indicates left‐to‐right, green rostral‐to‐occipital, and blue cranial‐to‐caudal direction of diffusion. Sagittal view in upper, transaxial view in lower row.
Common Space
To account for differences of individual anatomy, FA images were registered to a high‐definition template in ICBM‐152 space using nonlinear warping [Andersson et al., 2008] (FNIRT). The parameters obtained from this procedure were also used to transform individual images of mean diffusivity (MD) to the same standard space, since MD images do not provide sufficient tissue contrast to estimate registration parameters. Movie loops of standard space data across all subjects were evaluated by eye to exclude any gross misregistrations.
Tract‐Based Spatial Statistics
Tract‐based spatial statistics (TBSS) is a technique for analyzing group effects in diffusion‐based imaging [Smith et al., 2006]. TBSS aims to isolate dominant pathways of the brain in a skeletonized representation of white matter, and projects the nearest maximum FA values onto this skeleton. This way, residual variability after nonlinear registration is reduced. We tested for FA and MD differences including age as a confound variable, using spatial normalization parameters and skeleton projection vectors derived from FA analysis for both parameters. Skeletonization used an FA threshold of 0.2, as suggested by the authors of TBSS (cf. http://www.fmrib.ox.ac.uk/fsl/tbss).
In addition, we performed an exploratory analysis testing for correlations between either Fahn score or disease duration, and FA or MD in ET patients. Also, we tested for significant difference of either FA or MD between those patients who reported a positive family history, and those who did not.
For statistical inference, including correction for multiple comparisons across space, we used permutation testing [Nichols and Holmes, 2002] on our data implemented in RANDOMISE, a part of the FSL software package. We followed previously described methods [Douaud et al., 2009], calculating 5,000 permutations of our data and testing for significant group differences (or correlations, respectively) at a level of P < 0.05. Correction for multiple comparisons and cluster formation preceded with threshold‐free cluster enhancement (TFCE) [Smith and Nichols, 2009], a technique that does not need spatial smoothing and initial thresholding of the intermediate statistical images, making it well‐suited to the essentially 2D skeletonized data.
For correlation analysis, we performed linear regression on the thus identified regions of significant correlation with MD and FA, respectively, in a multiple regression design using SPSS to calculate R 2 values. The same correlation analysis was applied to peduncular parameters.
SPM Analysis
To compare our own to previously published data, we also performed classical, voxel‐wise statistical inference. After registration to ICBM‐152 space, FA and MD images were subjected to voxel‐wise [Wright et al., 1995] using SPM5 (http://www.fil.ion.ucl.ac.uk/spm). Images were smoothed with an 8 mm Gaussian kernel to enable GLM analysis and satisfy smoothness prerequisites of random field theory [Friston, 1995; Kiebel et al., 1999]. The data were fed into a multiple regression model with a binary regressor modeling patient/control group membership, and including age as a confound regressor. We tested for between‐group differences with family‐wise error correction (FWE) at the significance level of P < 0.05. No minimum cluster size was prescribed.
RESULTS
On visual inspection, significant pathology was absent on FLAIR imaging, but some focal white matter hyperintensities were present in most study subjects, as expected in elderly individuals. VBM analysis of T1 images did not detect significant differences in either gray or white matter density between our study groups.
In the ROI analysis, we found a significant group difference for FA and MD with F statistics. Post hoc t testing detected decreased FA in the right ICP of ET patients versus controls (P = 0.046), whereas there was no significant difference on the left side. Moreover, we found significantly increased MD in both the left (P = 0.035) and the right (P = 0.034) ICP of the ET group. In contrast, significant mean differences of either FA or MD were lacking in the SCP and MCP of both sides. The study results of the ROI analysis are summarized in Table II.
Table II.
Fractional anisotropy (FA) and mean diffusivity (MD), reported as mean with standard deviation in parentheses, in left and right superior (SCP), middle (MCP) and inferior (ICP) cerebellar peduncle
| Controls | ET patients | P values | ||||
|---|---|---|---|---|---|---|
| FA | MD | FA | MD | FA | MD | |
| Left SCP | 0.67 (0.089) | 0.99 (0.155) | 0.70 (0.054) | 0.91 (0.092) | 0.259 | 0.075 |
| Right SCP | 0.64 (0.094) | 1.0 (0.162) | 0.68 (0.072) | 0.94 (0.157) | 0.168 | 0.306 |
| Left MCP | 0.72 (0.064) | 0.63 (0.020) | 0.71 (0.068) | 0.63 (0.043) | 0.474 | 0.785 |
| Right MCP | 0.68 (0.042) | 0.67 (0.053) | 0.66 (0.065) | 0.67 (0.037) | 0.299 | 0.798 |
| Left ICP | 0.59 (0.073) | 0.73 (0.074) | 0.56 (0.082) | 0.79 (0.074) | 0.334 | 0.035* |
| Right ICP | 0.60 (0.049) | 0.74 (0.063) | 0.56 (0.053) | 0.78 (0.059) | 0.046* | 0.034* |
MD reported in mm2 sec−1 10−3, and associated p values of two‐sample t‐tests between groups.
significant at P < 0.05
SPM analysis showed a cluster of significant MD increase adjacent to the left parieto‐occipital sulcus in ET patients versus controls (peak voxel at X = −31, Y = −61, Y = 29, P = 0.005, Fig. 2), but no FA differences in either positive or negative direction.
Figure 2.
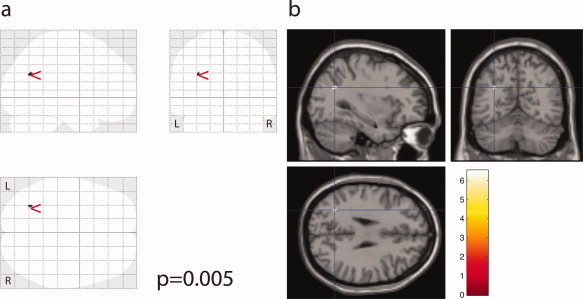
Increased MD in ET patients compared to control is detected in white matter adjacent to the left parieto‐occipital sulcus (P = 0.005). Visualisation as SPM “glass brain” maximum intensity projection (a), and overlaid onto a canonical single subject brain (b).
In the ET group, TBSS analysis uncovered increased MD bilaterally in the frontal and parietal white matter and in the left occipital and temporal lobe, excluding the callosal body (see Fig. 3). The peak significant voxel in ICBM‐152 space was found at [X = −22, Y = −52, Z = 19], P = 0.008, and MD within the cluster was 0.78 ± 0.038 mm2 sec−1 10−3 for controls, and 0.80 ± 0.048 mm2 sec−1 10−3 in ET patients (mean ± standard deviation). Frontal and parietal changes were more pronounced in the left hemisphere and included the left internal capsule in the region of the pyramidal tract. TBSS analysis of FA values did not detect any significant group differences between patients and controls, neither did voxel‐wise analysis.
Figure 3.
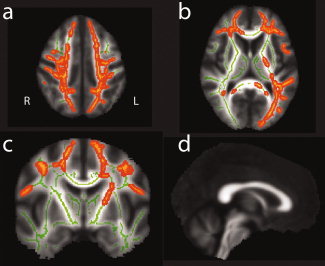
TBSS detects increase of MD in ET patients in bilateral frontal and parietal white matter (a, dorsal, b, ventral), excluding the callosal body, and in left‐hemispheric occipital and temporal white matter. Dorsal (a) and ventral (b) axial views, coronal slice through the pyramidal tract (c), and sagittal view of callosum (d). The skeleton generated by TBSS is rendered in green, and the yellow‐orange overlay depicts areas of significant MD increase in ET patients.
In the ET group, we detected a significant positive correlation between Fahn tremor scores and MD values in a region covering frontal and parietal white matter (peak voxel coordinates in ICBM‐152 space [X = 27, Y = 19, Z = 22], peak voxel value P < 0.01, whole cluster R 2 = 0.80, cf. Supporting Information Fig. 1). A smaller region in frontal white matter exhibited a negative correlation between Fahn score and FA ([34, 17, 22], P = 0.02, R 2 = 0.81). Peduncluar ROIs exhibited no significant correlation with Fahn scores.
We did not find significant correlations between disease duration and FA or MD. There was no significant difference in FA or MD values between subjects with and without a positive family history for tremor.
DISCUSSION
This study investigated white matter pathology in ET patients using noninvasive MR diffusion imaging as a tool sensitive to local tissue microstructure [Le Bihan, 2003]. We found evidence for localized pathology of cerebellar circuits and more general alterations of white matter in the brain hemispheres. These results confirm that integrity of neuronal fibers is impaired in ET, which might play a pathogenetic role for tremor generation in the central nervous system.
We demonstrated decreased FA and increased MD in the ICP of ET patients, suggesting cerebellar pathology in these individuals. Since information processed by the cerebellum is anatomically confined to propagate via the cerebellar peduncles, pathology of the cerebellum can be expected to be reflected in them. Thus, our DTI findings suggest selective involvement of the lower cerebellar input tracts. The ICP carries, among others, the olivocerebellar [Grant and Oscarsson, 1966] and spinocerebellar [Grant et al., 1966] tracts, which form the majority of this peduncle. Both terminate on Purkinje cells within the cerebellar cortex, where olivocerebellar fibers directly interface with Purkinje cells, while spinocerebellar afferents are connected with the latter via granular interneurons in the mossy fiber system [Baehr et al., 2005]. From a methodological point of view, the cerebellar peduncles are an attractive target for diffusion imaging: Similar to the callosal body, which is a focus of diffusion‐based research [Johansen‐Berg et al., 2007; Wahl et al., 2007], fibers running within the cerebellar peduncles are highly collinear, and there are no interdigitating crossing fiber bundles hampering analysis.
Our MRI data compare favorably with previous neuropathological studies on ET. Current evidence in neuropathological studies has found two major themes [Louis et al., 2007]: First, there are the more frequent cases of ET with predominantly cerebellar pathology, involving pathology of the Purkinje cells. These account for approximately three quarters of cases reported in the neuropathological literature. Second, there are cases with Lewy‐body type pathology within the brainstem [Louis et al., 2005], notably the locus coeruleus, with relatively preserved cerebellar integrity. In the cerebellar ET subtype, a 31–38% reduction of Purkinje cell density versus controls was reported [Louis et al., 2007; Louis and Vonsattel, 2008]. Additionally, axonal swellings (“torpedoes”) of surviving Purkinje cells suggested injury to these neurons. Functional imaging studies with [15]O‐water positron emission tomography (PET) have found overactivity in the cerebellum of ET patients [Jenkins et al., 1993; Wills et al., 1994], but these cannot distinguish between a primary cerebellar pathology or secondary activation due to a proprioceptive feed‐back mechanism [Deuschl et al., 2001]. With respect to our patient collective, there is no way to distinguish between the cerebellar and brainstem subtype of the disease in vivo. However, given the distribution of cases found on neuropathology, it is reasonable to assume that the majority of subjects belong to the former group.
In contrast to MD, we found an isolated unilateral FA decrease in the right ICP of ET patients. Given a bilateral tremor in all patients under investigation, this finding warrants further discussion. One possible explanation relates to the fact that the right ICP is addressing proprioceptive input of the dominant hand in all study subjects, which is likely to have received a large amount of motor training: Trained movements of the dominant hand range from simple tasks such as picking up an object or striking a match, to much more complex tasks, such as writing. We hypothesize that this amount of motor training enhances the tract's overall integrity or myelination [Demerens et al., 1996], and could thus provide a target for analysis that has high inter‐subject congruence in our purely right‐handed study collective. Moreover, it is likely that the nondominant hand has received a widely variable degree of training across the study group, depending on factors like profession or the ability to play a musical instrument, and this might lead to a greater variability of measurement in left ICP.
Five ET patients had tremor lateralization to the right, and three to the left side of the body. Therefore, tremor laterality may be another contributing factor to the asymmetry of peduncular measurements observed.
On voxel‐wise analysis of MD, we detected a significant cluster of increased diffusivity at the bottom of the left parieto‐occipital sulcus in ET patients. Subsequent TBSS analysis revealed a widespread distribution of increased MD in the brain comprising motor and nonmotor regions, but sparing the callosal body. In agreement with voxel‐wise SPM results, the most pronounced MD increase was found in the left parietal white matter. Interestingly, the left pyramidal tract was included in the MD increase, as opposed to the contralateral hemisphere. This finding points to a more pronounced affection of motor circuits controlling the dominant hand, similar to our peduncular findings. Unlike a recent study by Shin and colleagues, who reported decreased FA in ET patients at a level of P < 0.005 uncorrected [Shin et al., 2008], our TBSS and conventional SPM analysis failed to detect significant FA difference between the study groups. One possible explanation for this discrepancy is that the MRI data acquisition differed substantially between the studies. Other factors that might explain the absence of FA differences between groups in our study are the different sample properties, e.g., the age distribution of and exclusion of head tremor in our subjects, and a relatively conservative analysis approach.
It is an ongoing discussion whether ET is a neurodegenerative disorder [Raethjen and Deuschl, 2009]. Since our data are cross‐sectional in nature, they do not allow us to determine whether the white matter changes observed are progressive over time.
Diffusion data do not provide insight into the exact histopathological changes within the brain tissue investigated. It has been demonstrated that changes in the packing density of axons, in their collinearity and, to a lesser degree, in myelination influence measures such as FA and MD [Le Bihan, 2003]. Therefore, a loss of Purkinje cells, as suggested by neuropathological studies, could lead to degeneration of their afferents, which could in turn lead to reduced numbers of axons and, thus, packing density within the ICP. On the other hand, diffuse MD alterations in the hemispherical white matter suggest a systemic disturbance of myelin sheaths in ET. MD is a predictor of myelin content [Schmierer et al., 2008], and it has been argued that decrease in FA could indicate axonal loss, as opposed to pure disturbance of myelination [Beaulieu, 2009]. Thus, it is possible that changes in brain regions with isolated MD elevation are subtle and do not involve structural loss of axons. Recently, a variant of the LINGO1 gene, which is involved in the myelination of the central nervous system, has been linked to an increased risk to develop ET [Stefansson et al., 2009]. LINGO1 is an adhesion and cell‐cell interaction molecule involved in the coordination of myelin membrane formation [Laursen and Ffrench‐Constant, 2007], whose inactivation protects against neurodegeneration [Mi et al., 2008] and enhances neuronal survival in the cerebellum. Moreover, tremor has been shown in some animal models with myelination defects in the central nervous system [Oliver and Davies, 2005]. Therefore, the association of LINGO1 gene variants with the occurrence of ET suggests a role for myelin dysfunction in the pathophysiology of ET, which also would be in agreement with the hypothesis of a distributed network of tremor generators rather than only a localized cerebellar pathology [Deuschl et al., 2001; Raethjen and Deuschl, 2009]. The strong and contrasting correlations detected between Fahn score and white matter parameters MD and FA in localized but disparate white matter regions might fit with the pathophysiological concept of diffuse myelin disintegration in ET, perhaps as part of a network hypothesis.
With all due caution, our DTI finding of widespread increased diffusivity in cerebral white matter beyond the motor circuits fits well with the pathophysiological concept of diffuse myelin disintegration in ET.
Although our ROI‐based results provide evidence for the involvement of the cerebellar peduncles in ET, whole‐brain analysis techniques were unable to pick this up. This might be explained by the fact that the cerebellar peduncles are small relative to available voxel size, and spatial registration techniques driven by FA only are unlikely to correctly align the centers of these structures. This is especially true for the ICP, which runs directly adjacent to the MCP and can be seen on color‐coded maps of principal diffusion direction, but is hard to distinguish from the MCP on FA maps (see Fig. 4). Therefore, manually placed ROIs, having the advantage of using additional information on principal diffusion direction, are likely to provide a more reliable way to assess changes in the ICP. When ROI placement is guided by a strong hypothesis, this classical method can provide more robust statistical inference than voxel‐based techniques: Evidence from multiple voxels can be gathered without partial voluming, as introduced by spatial smoothing. TBSS projection of maximum FA in ICP may be confounded by directly adjacent MCP, as this structure generally exhibits higher FA than ICP (cf. Table II). This problem is not present in ROI analysis, since the principal diffusion direction of MCP and ICP is rather different and hence, the two can be separated.
Figure 4.
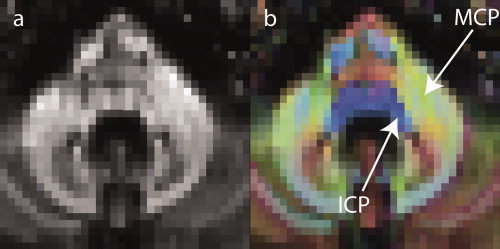
While ICP is hard to separate from MCP on FA only images (a), the two peduncles are easily distinguished on colour‐coded representations of principal diffusion direction (b). Red shades denote fiber estimates in a left‐to‐right (or vice versa) direction, green shades those in a rostro‐occipital, and blue shades estimates in a cranio‐caudal direction.
Last, a likely contributing factor to this discrepancy is the relatively small sample size, with a total of 34 subjects included in the study.
In conclusion, this study provides in vivo evidence for dysintegrity of cerebellar projection fibers and widespread white matter tracts in the cerebral hemispheres of ET patients. The data corroborate the pathophysiological concept that the cerebellum and its connections play a key role for tremor generation in the disease. Moreover, DTI demonstrated diffuse white matter alterations in the brain, which could be compatible with the network hypothesis of tremor pathophysiology in ET patients.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1
Acknowledgements
The authors gratefully acknowledge help with data analysis and compute resources generously provided by Prof. Stephen Smith, FMRIB Centre, Oxford.
REFERENCES
- Andersson JLR, Smith S, Jenkinson M ( 2008): FNIRT—FMRIB's Non‐linear Image Registration Tool. Presented at Fourteenth Annual Meeting of the Organization for Human Brain Mapping—HBM, Melbourne.
- Baehr M, Frotscher M, Duus P ( 2005): Duus' Topical Diagnosis in Neurology: Anatomy, Physiology, Signs, Symptoms, Vol. 14 Stuttgart: Thieme; 517 p. [Google Scholar]
- Beaulieu C ( 2009): The biological basis of diffusion anisotropy In: Johansen‐Berg H, Behrens TEJ, editors. Diffusion MRI. Oxford: Academic Press; pp 105–126. [Google Scholar]
- Benedetti B, Rocca MA, Rovaris M, Caputo D, Zaffaroni M, Capra R, Bertolotto A, Martinelli V, Comi G, Filippi M ( 2010): A diffusion tensor MRI study of cervical cord damage in benign and secondary progressive MS patients. J Neurol Neurosurg Psychiatry 81: 26–30. [DOI] [PubMed] [Google Scholar]
- Bodini B, Khaleeli Z, Cercignani M, Miller DH, Thompson AJ, Ciccarelli O ( 2009): Exploring the relationship between white matter and gray matter damage in early primary progressive multiple sclerosis: An in vivo study with TBSS and VBM. Hum Brain Mapp 30: 2852–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli O, Behrens TE, Altmann DR, Orrell RW, Howard RS, Johansen‐Berg H, Miller DH, Matthews PM, Thompson AJ ( 2006): Probabilistic diffusion tractography: a potential tool to assess the rate of disease progression in amyotrophic lateral sclerosis. Brain 129: 1859–1871. [DOI] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C ( 1996): Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci USA 93: 9887–9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G, Bain P, Brin M ( 1998): Consensus statement of the movement disorder society on tremor. Ad hoc scientific committee. Mov Disord 13 ( Suppl 3): 2–23. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Raethjen J, Lindemann M, Krack P ( 2001): The pathophysiology of tremor. Muscle Nerve 24: 716–735. [DOI] [PubMed] [Google Scholar]
- Douaud G, Mackay C, Andersson J, James S, Quested D, Ray MK, Connell J, Roberts N, Crow TJ, Matthews PM, Smith S, James A ( 2009): Schizophrenia delays and alters maturation of the brain in adolescence. Brain 132 ( Part 9): 2437–2448. [DOI] [PubMed] [Google Scholar]
- Douek P, Turner R, Pekar J, Patronas N, Le Bihan D ( 1991): MR color mapping of myelin fiber orientation. J Comput Assist Tomogr 15: 923–929. [DOI] [PubMed] [Google Scholar]
- Elble RJ ( 1996): Central mechanisms of tremor. J Clin Neurophysiol 13: 133–144. [DOI] [PubMed] [Google Scholar]
- Elble RJ ( 2006): Report from a U.S. conference on essential tremor. Mov Disord 21: 2052–2061. [DOI] [PubMed] [Google Scholar]
- Fahn S, Tolosa E, Marin C ( 1998): Clinical rating scale for tremor In: Jankovic J, Tolosa E, editors. Parkinson's Disease and Movement Disorders, 3rd ed Munich: Urban and Schwarzer; pp 225‐234. [Google Scholar]
- Findley LJ ( 1996): Classification of tremors. J Clin Neurophysiol 13: 122–132. [DOI] [PubMed] [Google Scholar]
- Frahm J, Haase A, Matthaei D ( 1986): Rapid NMR imaging of dynamic processes using the FLASH technique. Magn Reson Med 3: 321–327. [DOI] [PubMed] [Google Scholar]
- Friston KJ ( 1995): Commentary and opinion. II. Statistical parametric mapping: Ontology and current issues. J Cereb Blood Flow Metab 15: 361–370. [DOI] [PubMed] [Google Scholar]
- Grant G, Oscarsson O ( 1966): Mass discharges evoked in the olivocerebellar tract on stimulation of muscle and skin nerves. Exp Brain Res 1: 329–337. [DOI] [PubMed] [Google Scholar]
- Grant G, Oscarsson O, Rosen I ( 1966): Functional organization of the spinoreticulocerebellar path with identification of its spinal component. Exp Brain Res 1: 306–319. [DOI] [PubMed] [Google Scholar]
- Jaermann T, Pruessmann KP, Valavanis A, Kollias S, Boesiger P ( 2006): Influence of SENSE on image properties in high‐resolution single‐shot echo‐planar DTI. Magn Reson Med 55: 335–342. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Bain PG, Colebatch JG, Thompson PD, Findley LJ, Frackowiak RS, Marsden CD, Brooks DJ ( 1993): A positron emission tomography study of essential tremor: evidence for overactivity of cerebellar connections. Ann Neurol 34: 82–90. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S ( 2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H, Behrens TE ( 2006): Just pretty pictures? What diffusion tractography can add in clinical neuroscience. Curr Opin Neurol 19: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen‐Berg H, Della‐Maggiore V, Behrens TE, Smith SM, Paus T ( 2007): Integrity of white matter in the corpus callosum correlates with bimanual co‐ordination skills. Neuroimage ( 36 Suppl 2): T16–T21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebel SJ, Poline JB, Friston KJ, Holmes AP, Worsley KJ ( 1999): Robust smoothness estimation in statistical parametric maps using standardized residuals from the general linear model. Neuroimage 10: 756–766. [DOI] [PubMed] [Google Scholar]
- Laursen LS, Ffrench‐Constant C ( 2007): Adhesion molecules in the regulation of CNS myelination. Neuron Glia Biol 3: 367–375. [DOI] [PubMed] [Google Scholar]
- Le Bihan D ( 2003): Looking into the functional architecture of the brain with diffusion MRI. Nat Rev 4: 469–480. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Breton E ( 1985): Invivo magnetic‐resonance imaging of diffusion. Comptes Rendus Acad Sci Ser 2 301: 1109–1112. [Google Scholar]
- Louis ED ( 2005): Essential tremor. Lancet Neurol 4: 100–110. [DOI] [PubMed] [Google Scholar]
- Louis ED, Vonsattel JP ( 2008): The emerging neuropathology of essential tremor. Mov Disord 23: 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Ottman R, Hauser WA ( 1998): How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Mov Disord 13: 5–10. [DOI] [PubMed] [Google Scholar]
- Louis ED, Honig LS, Vonsattel JP, Maraganore DM, Borden S, Moskowitz CB ( 2005): Essential tremor associated with focal nonnigral Lewy bodies: A clinicopathologic study. Arch Neurol 62: 1004–1007. [DOI] [PubMed] [Google Scholar]
- Louis ED, Vonsattel JP, Honig LS, Ross GW, Lyons KE, Pahwa R ( 2006): Neuropathologic findings in essential tremor. Neurology 66: 1756–1759. [DOI] [PubMed] [Google Scholar]
- Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, Rajput A, Pahwa R, Lyons KE, Ross GW, Borden S, Moskowitz CB, Lawton A, Hernandez N ( 2007): Neuropathological changes in essential tremor: 33 Cases compared with 21 controls. Brain 130 ( Part 12): 3297‐3307. [DOI] [PubMed] [Google Scholar]
- Martinelli P, Rizzo G, Manners D, Tonon C, Pizza F, Testa C, Scaglione C, Barbiroli B, Lodi R ( 2007): Diffusion‐weighted imaging study of patients with essential tremor. Mov Disord 22: 1182–1185. [DOI] [PubMed] [Google Scholar]
- Mi S, Sandrock A, Miller RH ( 2008): LINGO‐1 and its role in CNS repair. Int J Biochem Cell Biol 40: 1971–1978. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP ( 2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Oliver PL, Davies KE ( 2005): Analysis of human neurological disorders using mutagenesis in the mouse. Clin Sci (Lond) 108: 385–397. [DOI] [PubMed] [Google Scholar]
- Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P ( 1999): SENSE: Sensitivity encoding for fast MRI. Magn Reson Med 42: 952–962. [PubMed] [Google Scholar]
- Raethjen J, Deuschl G ( 2009): Tremor. Curr Opin Neurol 22: 400–405. [DOI] [PubMed] [Google Scholar]
- Schmierer K, Wheeler‐Kingshott CA, Tozer DJ, Boulby PA, Parkes HG, Yousry TA, Scaravilli F, Barker GJ, Tofts PS, Miller DH ( 2008): Quantitative magnetic resonance of postmortem multiple sclerosis brain before and after fixation. Magn Reson Med 59: 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DH, Han BS, Kim HS, Lee PH ( 2008): Diffusion tensor imaging in patients with essential tremor. AJNR Am J Neuroradiol 29: 151–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE ( 2009): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44: 83–98. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM ( 2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 ( Suppl 1): S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ ( 2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Steinberg S, Petursson H, Gustafsson O, Gudjonsdottir IH, Jonsdottir GA, Palsson ST, Jonsson T, Saemundsdottir J, Bjornsdottir G, Bottcher Y, Thorlacius T, Haubenberger D, Zimprich A, Auff E, Hotzy C, Testa CM, Miyatake LA, Rosen AR, Kristleifsson K, Rye D, Asmus F, Schols L, Dichgans M, Jakobsson F, Benedikz J, Thorsteinsdottir U, Gulcher J, Kong A, Stefansson K ( 2009): Variant in the sequence of the LINGO1 gene confers risk of essential tremor. Nat Genet 41: 277–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R, Le Bihan D, Chesnick AS ( 1991): Echo‐planar imaging of diffusion and perfusion. Magn Reson Med 19: 247–253. [DOI] [PubMed] [Google Scholar]
- Wahl M, Lauterbach‐Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U ( 2007): Human motor corpus callosum: Topography, somatotopy, and link between microstructure and function. J Neurosci 27: 12132–12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills AJ, Jenkins IH, Thompson PD, Findley LJ, Brooks DJ ( 1994): Red nuclear and cerebellar but no olivary activation associated with essential tremor: A positron emission tomographic study. Ann Neurol 36: 636–642. [DOI] [PubMed] [Google Scholar]
- Wright IC, McGuire PK, Poline JB, Travere JM, Murray RM, Frith CD, Frackowiak RS, Friston KJ ( 1995): A voxel‐based method for the statistical analysis of gray and white matter density applied to schizophrenia. Neuroimage 2: 244–252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1


