Abstract
Brain activation studies generally utilize blood oxygenation level dependent (BOLD) contrast, most commonly measured using the gradient‐echo echo‐planar imaging (EPI) technique. BOLD contrast arises from regional changes in cerebral blood flow (CBF), cerebral blood volume (CBV), and the local metabolic rate of oxygen consumption. An alternative to BOLD is the detection of activation through direct measurement of these parameters. A noninvasive approach to measure activation‐related CBV changes is the vascular space occupancy (VASO) method, which exploits blood as an endogenous contrast agent by selectively nulling the magnetization of the water spins in the blood. Using a recently developed multislice variant of VASO that enables single‐shot whole‐brain coverage by virtue of a three‐dimensional GRASE readout, we here present the first application of VASO to an fMRI study with a whole‐brain cognitive task. Within acceptable measurement times (∼12 minutes), brain activation during a Stroop color‐word matching task could be detected reliably both on the group (N = 12) and single subject level, as evident from a qualitative comparison with separately acquired BOLD data and literature reports. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: VASO fMRI, 3D GRASE, CBV weighted, BOLD, cognitive activation study
INTRODUCTION
The vascular space occupancy (VASO) method [Lu et al.,2003] was proposed as an fMRI method that allows the detection of activation‐related cerebral blood volume (CBV) changes without the need for a blood pool contrast agent. A volume‐selective inversion pulse is applied, and the intravascular signal effectively nulled by acquiring the image at the zero‐crossing of the blood water magnetization. As a consequence, vasodilation and CBV increase will be observed as a signal decrease in activated voxels. VASO was received with considerable interest, and over the last years has led to an appreciable number of studies devoted to mechanistic investigations into the cerebral hemodynamics [Donahue et al.,2009a,b,c; Lin et al.,2009]. Given the converging interpretation that blood volume changes occur primarily in the capillaries and/or arterioles [Hillman et al.,2007; Kim et al.,2007; Lu et al.,2003], VASO in principle offers a promising opportunity to detect activation with a spatial specificity much higher than that of blood oxygenation level dependent (BOLD) fMRI, which at typical field strengths is by its nature sensitive to signal changes originating downstream of the activation, that is signal changes in the venules and veins. The original VASO method was proposed as a single slice technique based on a gradient‐echo echo planar imaging (GE‐EPI) readout, which has precluded its application to fMRI studies beyond simple visual and primary motor stimulation. However, the method has meanwhile seen improvements along two directions: Its sensitivity could be increased by the use of a spin‐echo (SE‐) EPI [Donahue et al.,2006] or HASTE [Poser and Norris,2007] readout, or adding a magnetization transfer preparation [Hua et al.,2009a], as well as an adaptation for high‐resolution scans on small animals [Jin and Kim,2008]. Second, the severely limited volume coverage due to single slice acquisition has been alleviated by the introduction of multislice VASO variants based on the multiple acquisitions using global inversion cycling (MAGIC) scheme [Lu et al.,2004b; Scouten and Constable,2007], which could be extended to whole‐brain coverage and used in combined experiments with visual, motor, and auditory stimulation [Scouten and Constable,2007]. An alternative whole‐brain VASO technique is the recently proposed VASO with a three‐dimensional gradient‐ and spin‐echo (3D GRASE) readout [Poser and Norris,2009b]. The benefits of 3D GRASE have been firmly established for arterial spin labeling [Fernandez‐Seara et al.,2007,2008; Gunther et al.,2005; MacIntosh et al.,2008], but it also lends itself to the use in VASO fMRI for similar reasons: As the overall signal decay is determined by T2 instead of T2* contrast, it is not only free of signal voids but also minimizes the detrimental BOLD signal contributions [Lu and van Zijl,2005], while also allowing enough time for an entire imaging volume to be acquired in a single shot, at typical spatial resolution for fMRI. The 3D sampling of k‐space further means that optimum use can be made of parallel imaging methods, which can be applied along both the k y‐ and k z‐phase encoding directions to drastically reduce echo train length and power deposition. In contrast to MAGIC‐like multislice VASO where the train of slice selective excitations that are applied around, but importantly not at the blood nulling point results in slice‐dependent CBV weighting, single‐shot 3D GRASE requires only a single inversion and a single slab‐selective excitation for the entire volume to be acquired, thereby guaranteeing position independent CBV weighting. In the initial experiments with visual und motor stimulation we demonstrated robust detection of activation in the visual and primary motor cortices as well as supplementary motor areas, with a sensitivity comparable to that of single‐slice GE‐EPI VASO [Poser and Norris,2009b]. Also other authors have recently started using a 3D GRASE readout for VASO and VASO‐FLAIR fMRI experiments [Donahue et al.,2009a].
We here use this single‐shot 3D GRASE VASO sequence to investigate its suitability for whole‐brain fMRI with a more complex cognitive task, as was indicated by preliminary results [Poser and Norris,2009a], on the single and multisubject level. The functional experiments in this study comprise a color‐word interference Stroop task which is well known to elicit positive and negative BOLD responses across the entire brain [Zysset et al.,2001], and should therefore be ideal for probing the method's ability to image activation in the different brain regions. Conventional GE‐EPI BOLD data were acquired for reference.
MATERIALS AND METHODS
All experiments were performed on a 3T Trio TIM systems (Siemens, Erlangen, Germany) equipped with the product 32‐channel head coil. Twelve volunteers (eight men, mean age 26.8 ± 5.2 years, all right handed) were scanned in accordance with the local ethics regulations. VASO acquisitions were performed using the single‐shot 3D GRASE sequence with the following parameters: matrix size 64 × 64 × 20 + 4 (20%) slice oversampling with axial slab orientation, voxel size 3.5 × 3.5 × 5 mm3, TR/TE/TI = 2,500/13.9/780 ms, spectral‐selective pre‐saturation for fat suppression, and vendor‐provided GRAPPA parallel imaging with factor 4 and 2 acceleration applied along k y and k z, respectively (coil calibration data were acquired over the central 34 lines and 22 partitions of k‐space at the beginning of the scan and used throughout). Slab‐selective 180° pulses were used to generate a total of 12 echoes (k x–k y planes) per scan, resulting in a total echo train length of 170 ms; readout bandwidth was 2,170 Hz/px. Importantly, the inversion pulse (like all other RF pulses) was transmitted using the body coil which ensures blood nulling in an as large as possible volume and thereby reduces inflow effects, and hence CBF weighting of the VASO signal [Donahue et al.,2006]. TI was calculated based on a blood T1 of 1,627 ms [Lu et al.,2004a] and taking into account that T1 relaxation effectively starts only after the last refocusing pulse. Further details of the sequence, which is shown in the top panel of Figure 1, can be found in Poser and Norris [2009b]. As a reference experiment, BOLD fMRI data were collected using a multiecho 2D EPI sequence with TE = 25, 35, 45 ms and TR = 1,500 ms, but otherwise identical geometric and acquisition parameters; sensitivity weighted echo combination (“CNR weighting”) was performed according to Poser et al. [2006]. T1‐weighted anatomical data at 1 mm resolution were acquired using a standard MP‐RAGE protocol (TR/TI = 2,300/1,100 ms, α = 8°, FoV = 256 mm, 192 = slices, BW = 130 Hz/px, factor‐2 GRAPPA acceleration with 24 auto‐calibation lines; total acquisition time 5 minutes 21 seconds).
Figure 1.
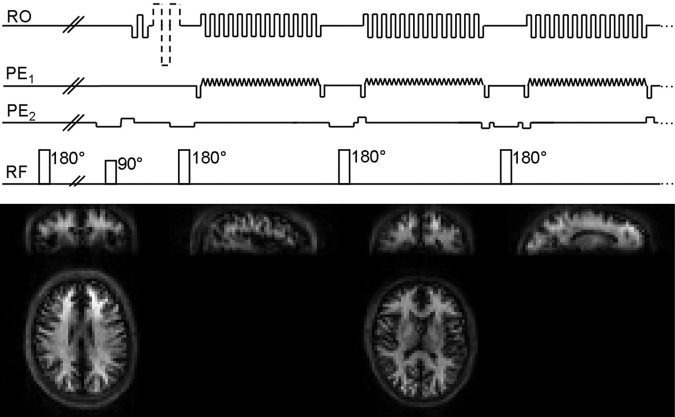
Schematic diagram showing the 3D GRASE VASO sequence (top). Following nonselective blood inversion and slab‐selective excitation, 3D k‐space is covered in a centre‐out fashion along k z to minimize TE; a complete k x–k y plane is acquired per RF interval using an EPI‐like readout. More details of the sequence can be found in a study by Poser and Norris [2009b]. Bottom, sample VASO images from two subjects, showing axial, coronal, and sagittal views at two different positions to demonstrate the acceptable degree of blurring along the secondary phase encoding (head‐feet) direction.
A cognitive Stroop color‐word interference task was used for this activation study [Zysset et al.,2001]. The stimulus consisted of three different task conditions (“neutral,” “congruent,” “incongruent”) which were intermixed in order to maintain the subjects' attention but were not considered independently during the analysis as the actual role of the different brain regions in stimulus processing was not subject of this investigation. Starting with 30 seconds of baseline, the different conditions were presented pseudo‐randomly in blocks of 30 seconds, separated by 15 seconds of baseline (30 seconds after every third block). The total experimental duration for VASO was 12′28″ per run including coil calibration data acquisition, yielding 292 time points; the BOLD experiment lasted 6′26″, yielding 249 data points. Further details on the Stroop task can be found in Zysset et al. [2001]. Stimuli were shown using Presentation software (Neurobehavioral Systems, Inc.), and subjects responded by button presses with their right index and middle fingers which were recorded and used to verify that they were performing the task.
The functional VASO and BOLD data were analyzed in SPM8 (available at: http://www.fil.ion.ucl.ac.uk/spm/). Preprocessing included image realignment for motion correction, coregistration with the anatomical data, and subsequent transformation tod standard space by T1 to T1‐template normalization, resampling to isotropic 2 mm voxels and 3D image smoothing with an 8‐mm Gaussian kernel. No temporal smoothing in addition to the “SPM default” high‐pass filtering was applied. The coregistration results were checked by visual inspection, and the realignment parameters used to verify that the data had not been corrupted by excessive subject motion (>0.5 pixels) during acquisition. The realignment parameters were, to zeroth order, included as regressors of no interests in the GLM for subsequent statistical analysis.
The 12 VASO datasets were analyzed by calculating the t‐contrasts for “activation > baseline” and “activation < baseline,” and combined in a fixed effect analysis (FFX) over all 12 subjects (P < 0.00001, cluster threshold of 100 pixels). A second‐level (random effects, RFX) analysis as now most commonly employed in fMRI was also performed in order to see whether the VASO method would hold up to this type of analysis (P < 0.005, cluster threshold of 100 pixels). The BOLD data were analyzed analogously but for display were thresholded at higher t‐value due to their higher sensitivity. Single‐subject activation maps were also generated in order to qualitatively gain an impression of the ability of VASO to detect the complex cognitive brain activation in individual subjects. Note that for ease of comparison with conventional activation maps, all VASO activations in this study are shown with reversed contrast to account for the fact that signal changes upon increased blood volume during brain activation are negative, and hence opposite to BOLD signal changes.
RESULTS
The bottom panel of Figure 1 show sample VASO data from two subjects in axial, sagittal and coronal view, illustrating the anatomical detail inherent to VASO data, and the acceptable degree of blurring along the secondary phase‐encoding direction (k z, head‐feet direction). The images are entirely free of residual parallel imaging artifacts, despite the relatively high acceleration factor (factor 4 by 2) used for the GRASE scans. In contrast, the quality of the coregistration of functional and anatomical data was found to be remarkably good which is largely attributable to the high in‐plane acceleration, and the resulting very low degree of image distortion along the A‐P direction. No data sets showed excessive motion (>0.5 pixels) and hence the VASO and BOLD data of all 12 subjects were included in the further analysis.
The statistical fixed effects analysis over the 12 subjects yielded an activation pattern that is in very good agreement with other reports for this type of Stroop experiments [Norris et al.,2002; Poser and Norris,2009c; Zysset et al.,2001] and the activation results from the GE BOLD data that were acquired for reference. These typical Stroop activations include positive BOLD/CBV/CBF changes in the lateral occipitotemporal gyri, the intraparietal sulci, the posterior and anterior inferior frontal sulci, as well as the occipital cortices and motor areas as a consequence of the visual stimulus and button‐press response, respectively. Negative BOLD/CBV/CBF changes are typically observed in the cingulate cortices, the lateral parietal sulci, the frontal cortices, and the right lateral superior parietal lobe. Figure 2 shows the activation maps of the fixed effects analysis for the VASO and BOLD data, illustrating clearly the ability of VASO to detect the same activations throughout the entire brain. The maximum t‐scores and corresponding coordinates for each observed cluster of activation are listed in the left half of Table I; also shown are the results for the GE‐EPI BOLD data for reference, but we point out that the purpose here is not a comparison of the sensitivity of the methods. At this significance threshold and with the experimental durations used in this study, there is one region that is found active in the VASO but not BOLD data (right inferior frontal pole), but also one region that is detected in BOLD but not VASO (right lateral occipitoparietal). It appears from the activation maps that the frontal and superior frontal deactivations are relatively lower in the BOLD data. Because these regions are in GE‐EPI typically strongly affected by through‐plane dephasing due to inhomogeneity gradients, and relatively thick slices were used here, this reduced sensitivity may be the consequence of EPI signal voids; in GRASE as a spin‐echo technique such effects would be refocused by the refocusing pulses. However, when lowering the detection threshold the frontal activations are also visible in the GE‐EPI as would be expected.
Figure 2.
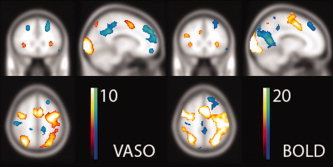
The fixed effects analysis of the VASO data (N = 12, P < 0.0001, t > 5, left panel) yields the typical Stroop activation pattern that agrees very well with the BOLD activation maps that were obtained from the GE‐EPI data on the same twelve subjects (P < 0.0001, t > 10, right panel), and the observations for this type of Stroop experiment elsewhere in the literature. The activation maps are shown overlaid on the SPM T1 template brain, at coordinate [−12, 26, 52]. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table I.
Summary of the detected clusters of activation in the VASO and BOLD data
| FFX model (N = 12) | RFX model (N = 12) | |||||||
|---|---|---|---|---|---|---|---|---|
| (P < 0.00001) | P < 0.005 | |||||||
| VASO | BOLD | VASO | BOLD | |||||
| Brain region | Max t | Position | Max t | Position | Max t | Position | Max t | Position |
| L intraparietal sulcus | 9.51 | [−28, −70, 48] | 39.16 | [−28, −66, 52] | 5.33 | [−22, −72, 52] | 8.63 | [−32, −60, 52] |
| R intraparietal sulcus | 10.84 | [32, −66, 54] | 27.73 | [32, −58, 52] | 7.78 | [24, −60, 48] | 11.31 | [32, −60, 46] |
| L posterior inferior frontal sulcus | 13.53 | [−32, −14, 58] | 34.24 | [−36, −10, 62] | 8.08 | [−34, −10, 46] | 9.68 | [−44, 0, 36] |
| R posterior inferior frontal sulcus | 9.85 | [38, −6, 52] | 25.82 | [36, −8, 52] | 3.38 | [32, −8,45] | 3.63 | [44, 0, 42] |
| L anterior inferior frontal sulcus | 6.53 | [−32, 20, 6] | 18.73 | [−30, 26, 4] | — | — | 4.54 | [−28, 22, 6] |
| R anterior inferior frontal sulcus | 5.91 | [34, 24, 2] | 15.38 | [34, 25, 3] | — | — | 4.61 | [40, 12, 6] |
| L occipital cortex | 19.29 | [−24, −98, 2] | 61.18 | [−22, −98, 6] | 11.38 | [−18, −96, −8] | 12.51 | [−16, −92, −14] |
| R occipital cortex | 21.22 | [30, −94, 4] | 48.93 | [22, −84, −10] | 11.31 | [16, −92, −10] | 7.85 | [14, −90, −10] |
| L lateral occipitotemporal gyrus | 17.34 | [−34, −92, −5] | 41.48 | [−42, −80, −10] | 6.77 | [−48, −74, −14] | 12.59 | [−32, −52, −18] |
| R lateral occipitotemporal gyrus | 10.17 | [24, −78, −12] | 45.76 | [36, −86, −2] | 3.79 | [28, −62, −20] | 5.46 | [38, −46, −20] |
| SMA | 12.73 | [−5, 5, 50] | −38.01 | [−4, 4, 54] | 5.30 | [−4, 4, 48] | 10.49 | [−4, 6, 50] |
| L ventral frontal cortex | −8.33 | [−12, 54, 32] | −10.13 | [−12, 42, 40] | −5.70 | [−12, 8, 28] | −5.48 | [−14, 36, 54] |
| R ventral frontal cortex | −7.23 | [14, 48, 38] | −8.65 | [22, 36, 40] | −13.05 | [18, 42, 40] | — | — |
| L inferior frontal pole | −7.41 | [−10, 56, −14] | −9.14 | [18, 64, 6] | −4.20 | [−10, 58, −10] | −3.33 | [−18, 64, 4] |
| R inferior frontal pole | −8.41 | [10, 50, −12] | — | — | −6.71 | [1, −54, −10] | — | — |
| L lateral occipitoparietal | −8.98 | [−50, −60, 28] | −27.13 | [−42, −84, 32] | −7.81 | [−46, −74, 38] | −8.71 | [−44, −78, 26] |
| R lateral occipitoparietal | — | — | −17.66 | [48, −78, 32] | — | — | −6.71 | [44, −80, 34] |
| Posterior cingulate cortex | −8.01 | [−10, −48, 34] | −17.25 | [−20, −62, 14] | −7.84 | [−8, −56, 34] | −8.48 | [−6, −62, 16] |
| R lateral superior parietal | −6.64 | [48, −24, 54] | −10.41 | [46, −24, 58] | −6.66 | [44, −22, 52] | −6.34 | [52, −22, 60] |
Maximum t‐score and corresponding coordinate, for the fixed and random effects analyses.
The activation maps obtained from the second‐level analysis are shown in Figure 3, and the results summarized on the right hand side of Table I. For both VASO and BOLD, there are clusters that do not survive the random effects analysis at this threshold of P < 0.005 (Table I), but again the inferior frontal regions appear to suffer relatively reduced sensitivity in the EPI data. On the whole, it can be noted that BOLD proves clearly much more sensitive than VASO in the first‐level analysis as would be expected; at the second level, however, the relative difference is markedly reduced, and for many regions the observed maximum t‐scores are even comparable.
Figure 3.
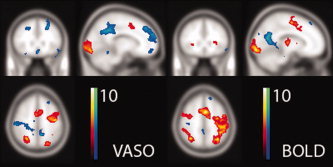
Activation maps from the random effects analysis (N = 12, P < 0.005) of the VASO and GE‐EPI BOLD. At this threshold there are clusters in both methods that are seen in the FFX but not the RFX analysis. In particular, the sensitivity of EPI appears to be compromised in the inferior frontal regions, likely due to its proneness to inhomogeneity artifacts; however, the activations in the central brain are now no longer detected in GRASE. Again, the activation maps are shown at the same position [−12, 26, 52]. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For an impression of the quality of activation maps that can be obtained from single‐subject data, Figure 4 shows VASO and BOLD activation maps for six individual subjects. Although the detection threshold was here adjusted arbitrarily to account for the strong difference in activation strength between subjects and methods, qualitative inspection reveals the striking similarity between the activation patterns as detected with VASO and BOLD.
Figure 4.
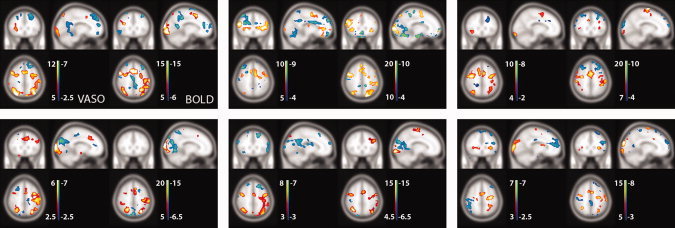
Single‐subject VASO and BOLD activation maps shown for six of the subjects reveal the similarity of the activation pattern between the two methods even at the single subject level (VASO and BOLD shown on the left and right of each panel, respectively). Due to the large variation in activation strength between the different individuals, the thresholds for activation detection were here chosen arbitrarily as indicated by the color bars. Again, the three planes intersect at position [−12, 26, 52]. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION AND CONCLUSION
In this work, the novel 3D GRASE VASO method was used for a cognitive fMRI study based on CBV contrast. Due to their various limitations, the application of VASO methods had thus far been restricted to mechanistic studies, for example of cerebral hemodynamics, and hence very simple stimuli have typically been applied. The aim of this study was thus the application of VASO to measure activation with whole‐brain coverage during a cognitively more challenging task, and to determine whether the same activations that are found with GE‐EPI BOLD can be detected throughout the brain. As such, the purpose of this work was not a comparison of the functional sensitivity of VASO with the much more sensitive BOLD, nor the investigation into the functional role of activated brain regions. Although the sensitivity of VASO is obviously lower, the present results convincingly demonstrate that CBV‐weighted VASO fMRI can detect the much more complex brain activation during a cognitive task and not just activation in the primary cortices. The experimental duration of only little more than 12 minutes for the VASO acquisitions must here be regarded as very moderate for this type of experiment. We had a priori no estimate of how sensitive the technique would be in practice and as such the choice of experimental duration and activation criteria for subsequent analysis to reveal the known activated brain regions may be seen as somewhat arbitrary. More experience with VASO applied to cognitive challenges will likely be needed for more reliable a priori power estimates as is now possible with BOLD fMRI.
As with all 3D sequences and a long readout train, there are two potential technical limitations to the single‐shot 3D VASO sequence proposed here. The first is spatial blurring due to T2 decay along the slow sampling direction which may be caused when long echo trains are used; this is especially the case with centric phase encoding (as used here to achieve the short TE) because this effectively doubles the width of the point spread function. The blurring will remain within one pixel if the echo train length ETL is shorter than (π × T2)/2, where the factor of two accounts for the centric rather than linear readout. With the readout of 170 ms and a T2 of about 100 ms this criterion is nearly met, and the blurring remains within acceptable limits as evident fro Figure 1. This, however, underlines the importance of parallel imaging which is absolutely vital to achieve the necessary shortening of the echo train, here by a factor of 8, especially if a large volume coverage is desired. In the present experiment we were able to sample 24 slices of which four edge slices needed discarding due to wrap‐around. This constitutes the second potential disadvantage of the 3D technique and is caused by imperfections in the slab profile even with good excitation and refocusing pulses.
The coregistration of the VASO and anatomical data worked surprisingly well and reliably, as verified by careful visual inspection for each subject. This can be attributed to the SPM coregistration routine which uses a mutual‐information based algorithm and can therefore be used to coregister images from different modalities and with very different image contrast. We had also acquired one brief EPI scan immediately before each VASO run to perform the coregistration based on that, but this did not turn out to be necessary.
One important issue pertaining to the analysis of VASO data is the application of spatial smoothing which cannot be circumvented in a group analysis as done here: the dual purpose of typically strong smoothing is to increase the image SNR, but also to reduce differences between individual subjects that remain after spatial normalization. However, much more so than T2*‐weighted BOLD data, VASO images are characterized by very low pixel intensity in the gray matter regions where the activation takes place, as a consequence of the blood inversion which considerably attenuates the gray matter signal (Fig. 1). The smoothing process thus strongly reduces the VASO signal changes while to some degree increasing SNR [Zimine et al.,2005]. For the purpose of this group study we chose the typical value of 8 mm for the smoothing kernel, but in a given data set there is likely an optimum kernel size <8 mm that would yield the greatest contrast to (temporal) noise and hence sensitivity in a single subject analysis. Future studies might focus on optimized VASO data (pre‐) processing, for instance using nonuniform or structure‐specific smoothing kernels as for instance suggested by Tabelow et al. [2009].
One conceptionally very attractive feature of VASO data that is clearly reduced by the application of smoothing is the inherently higher spatial specificity. In contrast to the GE BOLD signal which, as it is primarily driven by flow induced changes in deoxyhemoglobin concentration, largely originates from the vasculature downstream of the activation, VASO is thought to be better localized to the neuronal activation if indeed the CBV changes take place in the capillaries or arterioles as indicated by several recent studies [e.g. Hillman et al.,2007; Kim et al.,2007; Lu et al.,2003]. If the spatial resolution is further increased, one might hence argue that the potential of VASO is best exploited on the single subject level. If blocked task paradigms are used, as in this study, one may then also consider the use of temporal rather than spatial smoothing to increase sensitivity without sacrificing spatial resolution. Investigating the spatial differences between BOLD and VASO as already done by high‐resolution animal studies might constitute a subject for futures studies. To investigate any spatial differences between VASO and BOLD activation that might be expected because the underlying contrast mechanisms are fundamentally different, however, one should use a higher spatial resolution and concentrate on a within‐subject analysis since any differences are likely lost due to the spatial smoothing and intersubject variability.
The repetition time of our VASO experiments was 2.5 seconds. This meets the typical demands for activation studies, and would even be compatible with event‐related fMRI. For VASO, a TR of 2.5 seconds should already be regarded as short, and it is worth noting that the restriction is not caused by the (GRASE) signal readout, but rather the implicit requirement of VASO for sufficient relaxation of the longitudinal magnetization before the next inversion can be applied. It has been shown that for a TR below 3 seconds there is a blood flow contribution to the VASO signal due to inflow effects, even if a large volume of blood is effectively inverted by the use of the body RF coil [Donahue et al.,2006], indicating that TR should ideally be long if pure CBV contrast is desired; the TR of 2.5 seconds was here chosen as an acceptable compromise that is more compatible with the demands of typical activations studies. As for BOLD fMRI, there is an unwanted contribution from the CSF to the VASO signal especially at long TR [Donahue et al.,2006]. Although likely not confounding with the choice of TR in this study, it would generally be preferable to remove the CSF contribution by using a double‐inversion (VASO‐FLAIR); but this would further lengthen the preparation experiment and the additional gain would have to be weighed against the expense in TR.
As an alternative to VASO for measuring blood volume changes Shen et al. have suggested an fMRI technique that is based on the nulling of the extravascular gray matter [Shen et al.,2009]. In that study, the (positive) CBV signal changes were detected with nearly factor 2 higher sensitivity than VASO, suggesting that CBV weighted fMRI can be further improved by adapting this strategy. A drawback of this technique is that it is residually sensitive to BOLD changes, and one would have to investigate whether the SNR gain outweighs this potentially confounding contribution; this may depend on whether a pure CBV signal is required by the experimental question to be addressed. The method was presented as a single‐slice EPI method, but gray matter nulled fMRI can very easily be combined with the 3D GRASE readout used here and thereby extended to whole‐brain coverage. Another interesting possibility is the recently proposed “inflow VASO” (iVASO) technique [Hua et al.,2009b] which nulls the fresh blood flowing into the imaging volume and is therefore predominantly sensitized to the arterial CBV changes; the consequences of using iVASO with a thick 3D imaging slab and therefore by implication a mix of effective transit times, however, would need some investigation.
In summary, we have investigated the ability of the recently presented whole‐brain 3D GRASE VASO technique to measure brain activation during a cognitive Stroop task. In contrast to numerous previous studies which have used different VASO variants with simple visual, motor, or auditory stimulation, this is to our knowledge the first application of VASO in a “real” cognitive fMRI context. Brain activation was detected reliably at the single‐subject and group level using both first and second‐level statistical analyses, as could be verified by BOLD data that were acquired for reference. Despite their overall lower functional sensitivity, one advantage of CBV‐weighted fMRI techniques may include their conceivably higher spatial specificity that would result from the better colocalization of CBV changes and neuronal activity.
Acknowledgements
The authors thank Siemens Healthcare in Erlangen, Germany, for kindly making available the 32‐channel head coil, and Markus Barth and Emily van Mierlo for their assistance with the data acquisition.
REFERENCES
- Donahue MJ,Blicher JU,Ostergaard L,Feinberg DA,MacIntosh BJ,Miller KL,Gunther M,Jezzard P ( 2009a): Cerebral blood flow, blood volume, and oxygen metabolism dynamics in human visual and motor cortex as measured by whole‐brain multi‐modal magnetic resonance imaging. J Cereb Blood Flow Metab 29: 1856–1866. [DOI] [PubMed] [Google Scholar]
- Donahue MJ,Lu H,Jones CK,Edden RA,Pekar JJ,van Zijl PC ( 2006): Theoretical and experimental investigation of the VASO contrast mechanism. Magn Reson Med 56: 1261–1273. [DOI] [PubMed] [Google Scholar]
- Donahue MJ,Stevens RD,de Boorder M,Pekar JJ,Hendrikse J,van Zijl PC ( 2009b): Hemodynamic changes after visual stimulation and breath holding provide evidence for an uncoupling of cerebral blood flow and volume from oxygen metabolism. J Cereb Blood Flow Metab 29: 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue MJ,van Laar PJ,van Zijl PC,Stevens RD,Hendrikse J ( 2009c): Vascular space occupancy (VASO) cerebral blood volume‐weighted MRI identifies hemodynamic impairment in patients with carotid artery disease. J Magn Reson Imaging 29: 718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Seara MA,Edlow BL,Hoang A,Wang J,Feinberg DA,Detre JA ( 2008): Minimizing acquisition time of arterial spin labeling at 3T. Magn Reson Med 59: 1467–1471. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Seara MA,Wang J,Wang Z,Korczykowski M,Guenther M,Feinberg DA,Detre JA ( 2007): Imaging mesial temporal lobe activation during scene encoding: Comparison of fMRI using BOLD and arterial spin labeling. Hum Brain Mapp 28: 1391–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther M,Oshio K,Feinberg DA ( 2005): Single‐shot 3D imaging techniques improve arterial spin labeling perfusion measurements. Magn Reson Med 54: 491–498. [DOI] [PubMed] [Google Scholar]
- Hillman EM,Devor A,Bouchard MB,Dunn AK,Krauss GW,Skoch J,Bacskai BJ,Dale AM,Boas DA ( 2007): Depth‐resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation. Neuroimage 35: 89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J,Donahue MJ,Zhao JM,Grgac K,Huang AJ,Zhou J,van Zijl PC ( 2009a): Magnetization transfer enhanced vascular‐space‐occupancy (MT‐VASO) functional MRI. Magn Reson Med 61: 944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J,Qin Q,Donahue M,Zhou J,Pekar J,van Zijl P ( 2009b): In: Proceeding of the 17th Scientific Meeting, International Society for Magnetic Resonance in Medicine (ISMRM). Functional MRI Using Arteriolar Cerebral Blood Volume Changes. Honolulu. pp 12.
- Jin T,Kim SG ( 2008): Improved cortical‐layer specificity of vascular space occupancy fMRI with slab inversion relative to spin‐echo BOLD at 9.4 T. Neuroimage 40: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T,Hendrich KS,Masamoto K,Kim SG ( 2007): Arterial versus total blood volume changes during neural activity‐induced cerebral blood flow change: Implication for BOLD fMRI. J Cereb Blood Flow Metab 27: 1235–1247. [DOI] [PubMed] [Google Scholar]
- Lin AL,Fox PT,Yang Y,Lu H,Tan LH,Gao JH ( 2009): Time‐dependent correlation of cerebral blood flow with oxygen metabolism in activated human visual cortex as measured by fMRI. Neuroimage 44: 16–22. [DOI] [PubMed] [Google Scholar]
- Lu H,Clingman C,Golay X,van Zijl PC ( 2004a): Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med 52: 679–682. [DOI] [PubMed] [Google Scholar]
- Lu H,Golay X,Pekar JJ,van Zijl PCM ( 2003): Functional magnetic resonance imaging based on changes in vascular space occupancy. Magn Reson Med 50: 263–274. [DOI] [PubMed] [Google Scholar]
- Lu H,van Zijl PC ( 2005): Experimental measurement of extravascular parenchymal BOLD effects and tissue oxygen extraction fractions using multi‐echo VASO fMRI at 1.5 and 3.0 T. Magn Reson Med 53: 808–816. [DOI] [PubMed] [Google Scholar]
- Lu H,van Zijl PC,Hendrikse J,Golay X ( 2004b): Multiple acquisitions with global inversion cycling (MAGIC): A multislice technique for vascular‐space‐occupancy dependent fMRI. Magn Reson Med 51: 9–15. [DOI] [PubMed] [Google Scholar]
- MacIntosh BJ,Pattinson KT,Gallichan D,Ahmad I,Miller KL,Feinberg DA,Wise RG,Jezzard P ( 2008): Measuring the effects of remifentanil on cerebral blood flow and arterial arrival time using 3D GRASE MRI with pulsed arterial spin labelling. J Cereb Blood Flow Metab 28: 1514–1522. [DOI] [PubMed] [Google Scholar]
- Norris DG,Zysset S,Mildner T,Wiggins CJ ( 2002): An investigation of the value of spin‐echo‐based fMRI using a Stroop color‐word matching task and EPI at 3 T. Neuroimage 15: 719–726. [DOI] [PubMed] [Google Scholar]
- Poser BA,Norris DG ( 2007): Measurement of activation‐related changes in cerebral blood volume: VASO with single‐shot HASTE acquisition. Magn Reson Mater Phy 20: 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser BA,Norris DG ( 2009a): In: Proceeding of the 17th Scientific Meeting, International Society for Magnetic Resonance in Medicine (ISMRM). 3D Single‐Shot VASO FMRI Using a Maxwell‐Gradient Compensated GRASE Sequence. Honolulu. pp 15. [DOI] [PubMed]
- Poser BA,Norris DG ( 2009b): 3D single‐shot VASO using a Maxwell gradient compensated GRASE sequence. Magn Reson Med 62: 255–262. [DOI] [PubMed] [Google Scholar]
- Poser BA,Norris DG ( 2009c): Investigating the benefits of multi‐echo EPI for fMRI at 7 T. Neuroimage 45: 1162–1172. [DOI] [PubMed] [Google Scholar]
- Poser BA,Versluis MJ,Hoogduin JM,Norris DG ( 2006): BOLD contrast sensitivity enhancement and artifact reduction with multiecho EPI: Parallel‐acquired inhomogeneity‐desensitized fMRI. Magn Reson Med 55: 1227–1235. [DOI] [PubMed] [Google Scholar]
- Scouten A,Constable RT ( 2007): Applications and limitations of whole‐brain MAGIC VASO functional imaging. Magn Reson Med 58: 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y,Kauppinen RA,Vidyasagar R,Golay X ( 2009): A functional magnetic resonance imaging technique based on nulling extravascular gray matter signal. J Cereb Blood Flow Metab 29: 144–156. [DOI] [PubMed] [Google Scholar]
- Tabelow K,Voss HU,Polzehl J ( 2009): Structural adaptive smoothing methods for high‐resolution fMRI. In: Proceeding of the 13th Scientific Meeting, International Society for Magnetic Resonance in Medicine (ISMRM). San Francisco. pp 386.
- Zimine I,Petersen ET,Ho Y‐CL,Golay X ( 2005): Partial Volume Effects in VASO‐fMRI. Miami. [Google Scholar]
- Zysset S,Muller K,Lohmann G,von Cramon DY ( 2001): Color‐word matching stroop task: Separating interference and response conflict. Neuroimage 13: 29–36. [DOI] [PubMed] [Google Scholar]


