Abstract
The d‐amino acid oxidase activator gene (G72) has been found associated with several psychiatric disorders such as schizophrenia, major depression, and bipolar disorder. Impaired performance in verbal fluency tasks is an often replicated finding in the mentioned disorders. In functional neuroimaging studies, this dysfunction has been linked to signal changes in prefrontal and lateral temporal areas and could possibly constitute an endophenotype. Therefore, it is of interest whether genes associated with the disorders, such as G72, modulate verbal fluency performance and its neural correlates. Ninety‐six healthy individuals performed a semantic verbal fluency task while brain activation was measured with functional MRI. All subjects were genotyped for two single nucleotide polymorphisms (SNP) in the G72 gene, M23 (rs3918342) and M24 (rs1421292), that have previously shown association with the above‐mentioned disorders. The effect of genotype on brain activation was assessed with fMRI during a semantic verbal fluency task. Although there were no differences in performance, brain activation in the right middle temporal gyrus (BA 39) and the right precuneus (BA 7) was positively correlated with the number of M24 risk alleles in the G72 gene. G72 genotype does modulate brain activation during language production on a semantic level in key language areas. These findings are in line with structural and functional imaging studies in schizophrenia, which showed alterations in the right middle temporal gyrus. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: G72, fMRI, verbal fluency, middle temporal gyrus, BA 39
INTRODUCTION
Schizophrenia and bipolar disorder have a high heritability of about 80% [McGuffin et al., 2003; Sullivan et al., 2003]. Aetiology, symptom clusters, and course are heterogeneous [Kirov et al., 2005] and are known to be under genetic control.
As a locus of susceptibility, G72 was first identified in a Canadian and a Russian sample by Chumakov [2002] to be associated with schizophrenia, a finding which has been replicated in independent samples [Addington et al., 2004; Chumakov et al., 2002; Li and He, 2007]. It has also been found to be associated with several other psychiatric disorders such bipolar disorder [Schulze et al., 2005], major depression [Rietschel et al., 2008], and panic disorder [Schumacher et al., 2005] (see Abou Jamra et al. [2006], Detera‐Wadleigh and McMahon [2006], and Li and He [2007] for review and meta‐analyses). There are several possible reasons for the associations with more than one disorder: categorical classification of disorders is somewhat artificial and might neglect similarities on a symptomatic level. Another reason may lie in a common vulnerability that could later lead to different disorders. It has been shown that neuroticism is predictive for major depression [e.g. Kendler et al., 2006] and psychosis [Berenbaum and Fujita, 1994; Dinzeo and Docherty, 2007]. There is evidence that G72 has a significant influence on neuroticism [Rietschel et al., 2008], which accordingly may explain the association with several disorders.
Of all studied markers of G72, M23 (rs3918342) and M24 (rs1421292) showed the highest single marker associations with schizophrenia [Detera‐Wadleigh and McMahon, 2006], although, for M23, reported risk alleles vary between populations [Chumakov et al., 2002; Korostishevsky et al., 2004; Rietschel et al., 2008; Schulze et al., 2005].
On a functional level, increased transcripts of G72 were found in dorsolateral prefrontal cortices of patients with schizophrenia compared to healthy controls in postmortem brains [Korostishevsky et al., 2004]. In addition, G72 has been found to interact with the gene d‐amino acid oxidase (DAAO) and has an influence on NMDA receptors via regulation of d‐serine levels [e.g. Chumakov et al., 2002; Moghaddam, 2003]. To date, the exact pathway of G72 influence on reduction of d‐serine levels remains uncertain. It was hypothesized that pLG72, which represents the longest open reading frame (153 amino acids), acts as an activator of the human FAD‐containing flavoprotein d‐amino acid oxidase (hDAAO). Under pathological conditions, either an overexpression of pLG72 leads to an increase in hDAAO activity that decreases the local concentration of d‐serine, or low expression of pLG72 could result in hyperactivation of hDAAO and a decrease of d‐serine concentration. The decrease in d‐serine concentration results in a lower amount of activated NMDA receptors and, thus, in a hypofunction of the glutamatergic neurotransmission [Chumakov et al., 2002; Sacchi et al., 2008]. In a mouse model, it was found that G72 transgenic mice exhibited a reduced prepulse inhibition compared to wild‐type mice that could be ameliorated with haloperidol [Otte et al., 2009]. These mice also showed impaired motor learning and performance as well as higher sensitivity to the NMDA receptor antagonist PCP [Otte et al., 2009].
Cognition is known to be generally impaired in schizophrenia [Glahn et al., 2007; Heinrichs and Zakzanis, 1998] and to a lesser extent in bipolar disorder [Quraishi and Frangou, 2002; Tiihonen et al., 2005] and major depression [Zakzanis et al., 1998]. Among the different cognitive domains, verbal memory, word fluency, and attention display the highest effect sizes when compared with healthy control subjects. In schizophrenia, between 61 and 78% of patients perform below the median of aggregated patient–control samples in these domains [Heinrichs and Zakzanis, 1998]. The impairments in bipolar disorder and major depression are less severe [Quraishi and Frangou, 2002; Reichenberg et al., 2002; Zakzanis et al., 1998]. It could be shown that these impairments can be found in risk groups and relatives of patients and also show a significant level of heritability, although heritability can be lower for cognitive domains than for the disorder itself [Antila et al., 2007; Greenwood et al., 2007]. Thus, these domains are under potential genetic influence, although in which way is not known yet.
The cognitive domain investigated in this study covers word production using a traditional semantic verbal fluency paradigm. Impairment in semantic, but not lexical fluency, has been most consistently found in behavioral studies of patients suffering from schizophrenia. Despite extensive research in healthy populations (for a review on word production, see Indefrey and Levelt [2004]), the neural correlates of verbal fluency have not been studied in bipolar disorder and have only been sparsely studied in patients with schizophrenia, mostly corresponding to performance during lexical fluency tasks (for an overview of lexical verbal fluency, see Spence et al. [2000]; for semantic fluency, see Kircher et al. [2008] and Ragland et al. [2007]). For both fluency versions, BOLD responses in healthy subjects have been reported predominantly in prefrontal and temporal regions of the perisylvian language network, yet the data is heterogeneous. Compared to healthy control subjects, response enhancement in schizophrenia patients of the left superior temporal cortex has been reported during lexical verbal fluency besides comparable left prefrontal activation patterns (for an overview, see Spence et al. [2000]). Other neuroimaging studies failed to show such temporal response patterns but stressed the relevance of impaired executive functions associated with the lateral and medial (i.e. anterior cingulate cortex) prefrontal cortices in schizophrenia. Again, the results ranged from hypo‐ to hyperactivations in patients compared to control subjects during lexical fluency [Boksman et al., 2005; Curtis et al., 1998; Dye et al., 1999; Fletcher et al., 1996; Frith et al., 1995; Fu et al., 2005; Spence et al., 2000; Yurgelun‐Todd et al., 1996]. Suggestions have been made to describe the BOLD‐response patterns in a more systematic way, implying an interaction between prefrontal and temporal structures during expressive language tasks [Fletcher et al., 1996; Ford et al., 2002; Frith et al., 1995; Yurgelun‐Todd et al., 1996].
So far, to our knowledge, there are only two studies investigating the effect of G72 on neural correlates of cognition [Goldberg et al., 2006; Hall et al., 2008]. Goldberg et al. [2006] found an effect of M24 in the hippocampus and the parahippocampus during memory encoding and retrieval in a sample of 14 healthy subjects, Hall et al. [2008] found an influence of M23 in the left hippocampus and the right inferior frontal gyrus in the Hayling (sentence completion) task in 61 subjects at high risk of psychosis.
In this study, the performance on a semantic verbal fluency task and its neural correlates were linked to genetic G72 status in healthy subjects. We genotyped subjects for two single nucleotide polymorphisms (SNP) in the G72 gene, M23 (rs3918342 [C/T]) and M24 (rs1421292 [T/A]), that have previously shown association with schizophrenia, bipolar disorder, major depression, and high levels of neuroticism. Differences in brain activation between genotypic groups were expected in key regions underlying verbal fluency performance in healthy and schizophrenia subjects, such as left lateral, medial prefrontal, and temporal cortices.
MATERIALS AND METHODS
Subjects
Subjects were recruited at RWTH Aachen University; the majority of them were students. Ninety‐six subjects (64 men and 32 women) were enrolled into the study. The inclusion criteria were aged 18–55 years and no psychiatric disorder according to ICD‐10 past or present. The subjects had a mean age of 23.3 years (SD = 3.04), were all right handed (as tested with the Edinburgh Laterality Scale [Oldfield, 1971]), and had 15.7 (2.6) years of education. Their fathers were educated for 15.3 (4.6) and their mothers for 13.9 (4.1) years on average. Our present sample is a subsample of the subjects described in Rietschel et al. [2008], which were tested for association between G72 and neuroticism. All subjects were unrelated and had no relatives with a history of psychosis. The German MWT‐B multiple choice vocabulary test [Lehrl et al., 1995] was used as an assessment for verbal intelligence estimation. Scores were converted into IQ estimates. All subjects were of Western‐ or Middle European descent. After a complete description of the procedure, subjects provided written informed consent to participate in the study. The protocol was approved by the local ethics committee according to the declaration of Helsinki. After participants provided consent, the cognitive tests and fMRI were assessed and blood was taken. After analyzing the fMRI data, five subjects had to be excluded from further analyses for not following instructions. Characteristics of this sample are given in Table I.
Table I.
Sociodemographic data of the behavioral sample, standard deviations in parentheses (n = 91)
| Variable | F | P | |||
|---|---|---|---|---|---|
| M23 (rs3918342) | C/C | T/C | T/T | ||
| Sex ratio (men/women) | 18/8 | 27/13 | 18/7 | χ = 0.15 | n.s. |
| Age | 23.3 (3.0) | 23.5 (3.0) | 22.8 (2.8) | 0.37 | n.s. |
| Education | 15.6 (2.2) | 15.8 (2.8) | 15.6 (2.6) | 0.12 | n.s. |
| Estimated verbal IQ | 112.3 (11.0) | 112.5 (13.0) | 112.1 (13.1) | 0.01 | n.s. |
| M24 (rs1421292) | T/T | A/T | A/A | ||
| Sex ratio (men/women) | 22/6 | 23/15 | 17/7 | χ = 2.5 | n.s. |
| Age | 23.5 (2.9) | 23.3 (3.1) | 22.8 (2.9) | 0.38 | n.s. |
| Education | 15.6 (2.2) | 15.8 (2.9) | 15.6 (2.7) | 0.07 | n.s. |
| Estimated verbal IQ | 113.4 (11.0) | 111.6 (13.1) | 111.4 (12.8) | 0.23 | n.s. |
n.s., nonsignificant (all P > 0.1).
Genetic Analysis
Subjects were genoytped as part of a sample described in Rietschel et al. [2008] for two G72 SNPs (M23 = rs3918342[C/T], positioned at 104983750 and M24 = rs1421292[A/T], positioned at 104,996,236; intermarker distance 12.5 kilobases) using the MassARRAY® system (Sequenom, San Diego, CA). For quality comparison purposes, we genotyped a subset of the sample in duplicate to estimate the replicate error rate. Two of 96 DNA samples per plate were randomly chosen for this purpose. For the SNPs genotyped, all genotypes between duplicates were consistent (0% replicate error rate).
By a standard 1 df chi‐square test, there were no significant deviations from Hardy–Weinberg equilibrium for the genotype distributions of the studied sample.
fMRI Task
Task and stimuli
The task has been used previously in another study of ours successfully [Kircher et al., 2008]. Stimuli were presented with Presentation software package (Neurobehavioral Systems, San Francisco, CA). This semantic verbal fluency task used a block design with two alternating conditions: Subjects had to read aloud single German nouns presented to them (high‐level baseline condition) or, in response to a German noun, they had to name one member of the category this noun represented (e.g., say “dog” when the word “animal” was presented; semantic verbal fluency condition). There were four blocks for each condition. At the beginning of each block, an instruction slide was shown for 2,000 ms (semantic verbal fluency: “generate a category member”; baseline: “read the word”). Then, a fixation cross appeared in the center of the screen for 500 ms, which was followed by the stimulus word for 3,000 ms. Subjects were required to respond within this time frame. Each block consisted of 10 stimuli with a duration of 34 s each. In sum, subjects had to generate 40 words. Appearance of the #‐symbol for 6,000 ms indicated the end of each block. Words were presented in white color on a black background.
Data Acquisition
Imaging was performed on a 3‐T Tim Trio MR scanner (Siemens Medical Systems) in the Institute of Neuroscience and Biophysics—Medicine, Research Centre Jülich. Functional images were collected with echo planar imaging sensitive to BOLD contrast (T2*, 64 × 64 matrix, FoV 200 mm × 200 mm, 36 slices, 3‐mm thickness, TR = 2.25 s, TE = 30 ms, flipangle = 90°). Slices covered the whole brain and were positioned transaxially parallel to the anterior–posterior commissural line (AC‐PC). 157 functional images were collected, and the initial three images excluded from further analysis to remove the influence of T1‐stabilization effects.
Data Analyses
Because there were enough data points in all three groups, statistical analyses of genotype effects in the behavioral as well as fMRI data were performed in accordance with a codominant model (regression analysis), thus checking if the number of risk alleles (M23:C and M24:T) per SNP influences brain activation. As the data by Hall et al. [2008] implied linear effects with the number of risk alleles in G72, regression analyses in this study were performed in this linear way only. By doing so, multiple comparisons were minimized.
fMRI Data Analysis
FMRI data analyses were calculated using SPM5. After realignment, unwarping and stereotaxic normalization (2 mm × 2 mm × 2 mm), a 6‐mm full‐width‐at‐half‐maximum (FWHM) Gaussian smoothing kernel was applied to increase the signal‐to‐noise ratio and compensate for intersubject anatomical variation. The volume of interest was restricted to gray matter voxels by use of an inclusive mask created from the segmentation of the standard brain template (SPM2).
Semantic verbal fluency‐related brain activation was analyzed for each subject contrasting the semantic verbal fluency condition with high‐level baseline. In the first step, a one sample t‐test was calculated for the whole sample (n = 91) to investigate general semantic verbal fluency activation. Because we used an overt semantic fluency task, which could lead to head movement, realignment parameters were entered as a covariate on the first‐level analysis. All subjects had tolerable head movement smaller than one voxel size, similar to previous reports [Kircher et al., 2008]. As additional covariates, numbers of errors in the task, neuroticism and the factors “cognitive perceptual deficits” and “disorganisation” of the schizotypal personality questionnaire (SPQ‐B, [Axelrod et al., 2001]). These covariates were entered, because the number of errors could reflect task difficulty and G72 has been shown to have an effect on personality [Rietschel et al., 2008], which might also lead to differences in task‐related differences in the fMRI data.
To investigate genotype effects, resulting contrasts were entered in a multiple regression using genotype (either M23 or M24) as a covariate. The multiple regressions were calculated both ways to detect increasing brain activation depending on increasing number of T‐alleles or C‐alleles in M23 or A‐alleles and T‐alleles in M24. The results were corrected on a voxel‐wise threshold of P < 0.001. Hereby, a Monte Carlo simulation of the brain volume of the current study was conducted to establish an appropriate voxel contiguity threshold [Slotnick et al., 2003]. The basic principle of the Monte Carlo approach involves the simulation of brain activity whereby each individual voxel of the functional image matrix becomes activated with a set type I error probability (i.e., 0.001). The resulting activation map is smoothed with a FWHM Gaussian kernel (i.e., 6 mm), and the size of each contiguous voxel cluster is determined. After multiple, consecutive simulations (i.e., 10,000), the extent threshold k is chosen such that the relative frequency of clusters of size k or greater in the sample is smaller than the desired corrected P value of P < 0.05. Assuming an individual voxel type I error of P < 0.001 in our study, a cluster extent of 26 contiguous resampled voxels was indicated as necessary to correct for multiple voxel comparisons at P < 0.05. Brain activations were plotted on the anatomical SPM template.
RESULTS
Genetic Analysis
Resulting distribution of M23 (rs3918342) was 26 subjects with C/C genotype (18 male), 40 subjects with T/C genotype (27 male), and 25 subjects with T/T genotype (18 male). Resulting distribution of M24 (rs1421292) was 28 subjects with T/T genotype (22 male), 38 subjects with T/A genotype (23 male), and 24 subjects with T/T genotype (17 male). The resulting groups and variables are displayed in Table I.
Behavioral Data
Recorded speech of all subjects was analyzed. Five subjects had to be excluded from further analyses for not following instructions. They did not name category members but freely associated about the word stimulus. Remaining data were analyzed and checked for errors in the semantic verbal fluency task. Mean error rate of the M23 groups was 2.65 (SD = 1.9) for T/T status, 3.56 (3.1) for T/C status, and 3.43 (2.6) for C/C status. For M24, errors were 2.63 (SD = 2.0) for A/A status, 3.45 (3.0) for A/T status, and 3.58 (2.0) for T/T status (absolute values). There was no significant influence of genotype on errors (F 2,89 = 0.77, n.s. for both M23 and M24).
fMRI Data
The fMRI group analysis (n = 91) of the contrast “semantic verbal fluency” > “reading aloud” revealed activations in the left inferior frontal gyrus (BA 47), left middle temporal gyrus (BA19), and the cingulate gyrus (BA 24) (FWE corrected at P < 0.05). Activated regions are listed in Table II.
Table II.
Brain activation for the whole fMRI sample (n = 91), P = 0.05 corr., and cluster extent 20 voxels
| BA | x | y | z | k E | Max. SPM (T) | ||
|---|---|---|---|---|---|---|---|
| “Semantic verbal fluency” > “reading aloud” | |||||||
| Middle temporal gyrus | L | 19 | −30 | −56 | 14 | 63 | 7.20 |
| Parahippocampal gyrus | L | 37 | −36 | −39 | −1 | 55 | 6.85 |
| Hypothalamus | R | – | 4 | −6 | −5 | 21 | 6.58 |
| Inferior frontal gyrus | L | 47 | −28 | 21 | −4 | 23 | 5.54 |
| Cingulate gyrus | L | 24 | −8 | 21 | 41 | 32 | 5.34 |
| “Reading aloud”>”semantic verbal fluency” | |||||||
| Putamen | L | – | −14 | 9 | −11 | 428 | 15.98 |
| Anterior cingulated | R | 25 | 6 | 13 | −7 | 341 | 15.76 |
| Transverse temporal gyrus | L | 42 | −57 | −13 | 12 | 1293 | 13.39 |
| Transverse temporal gyrus | R | 42 | 59 | −13 | 8 | 300 | 12.39 |
| nferior temporal gyrus | L | 37 | −45 | −66 | 0 | 38 | 11.89 |
| Middle temporal gyrus | R | 39 | 46 | −74 | 28 | 62 | 11.26 |
Coordinates are listed in Talairach and Tournoux atlas space [Talairach and Tournoux, 1988]. BA is the Brodmann area nearest to the coordinate and should be considered approximate.
Regression analyses revealed a linear effect of minor alleles in M24 in the right middle temporal gyrus (MTG, Brodmann Area 39) and the right precuneus (BA 7) for the contrast “semantic verbal fluency” > “reading aloud” (see Fig. 1). The peak voxel in the MTG was located at x = 40, y = −57, and z = 23 (Talairach and Tournoux atlas space [Talairach and Tournoux, 1988], t = 4.42, P = 0.001, and R 2 = .135). The peak voxel in the precuneus was located at x = 18, y = −47, z = 40, t = 3.77, P = 0.001, and R 2 = 0.125). The corresponding cluster in the MTG consisted of 75 voxels, the cluster in the precuneus consisted of 62 voxels (see Fig 1). Peak voxel activation for M24 in the MTG was as follows: homozygous minor allele (T/T) carriers had a mean activation of 0.02 (SD = 0.26), heterozygous (T/A) carriers had −0.21 (.39), and homozygous A allele carriers had −0.34 (.39). This result indicates that homozygous minor allele (T/T) carriers showed small activations compared to deactivations on the other groups in this area.
Figure 1.
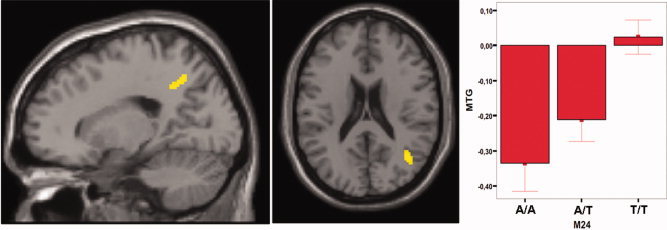
Regression analysis of BOLD response (M24 A/A < A/T < T/T) for “semantic verbal fluency” > “reading aloud.” During semantic verbal fluency versus reading BOLD response increased with the number of T‐alleles in a linear fashion (P < 0.001, corrected by Monte‐Carlo simulations; cluster extend = 26 voxels) in the right precuneus (left image) and the right middle temporal gyrus (middle image). Right side shows parameter estimates for the MTG, error bars represent standard mean error. Cluster size = 75 voxels (middle temporal gyrus) and 62 voxels (precuneus).
The regression analyses for M23 led essentially to an identical results (same location of the cluster), but activation for M23 failed to reach significance due to cluster size (only 21 voxels).
Because the BA 39 falls within the predefined regions of interest (see Introduction section), a post hoc small volume correction as implemented in SPM5 was calculated with a sphere of 5 mm around the maximum activation difference at x = 40, y = −57, and z = 23. The results remained highly significant after FWE and FDR corrections (P FWE‐CORR < 0.001 and P FDR‐CORR < 0.001).
To test for the opposite linear effects (increase in activation), regression analyses were calculated using the number of A‐ or T alleles of M24 and M23 as covariate. No increasing brain activation with respect to an increasing number of A‐ or T‐alleles in M24 and M23, respectively, was observed. These according results are supported by the high‐linkage disequilibrium between M23 and M24 (D′ = 0.996, r 2 = 0.904 (Rietschel et al., 2008]). In this sample, the correlation between M23 and M24 was r = 0.942, P < 0.001.
DISCUSSION
In this study, the effect of G72 on the neural correlates of semantic verbal fluency was investigated in a sample of healthy subjects. Signal change of the right middle temporal gyrus (MTG, BA 39) was correlated significantly with G72 genotype.
The results of the fMRI regression analysis showed an increase in BOLD‐response during verbal fluency compared to reading aloud with the number of minor alleles (T) in M24. A linear increase in activation was found in the MTG, which has been found to show hyperactivations in patients with schizophrenia compared to controls the same task in previous studies [Kircher et al., 2001; Ragland et al., 2007]. Despite differences on the neural level, verbal fluency performance was comparable for all genotype groups during fMRI measurement. As only one instance for each category presented had to be produced by the subjects, it could be argued that the task was not difficult enough to elicit differences in performance between groups. The results support a genetic influence onto brain activity during verbal fluency in a component of the semantic language network, which is not explainable by behavioral performance.
Previous investigations have shown that both, patients with schizophrenia and those at genetic risk of schizophrenia, exhibit a selective deficit during semantic word retrieval [Dickinson et al., 2007; Heinrichs and Zakzanis, 1998]. Brain regions associated with this impairment include the left IFG, ACC [Fletcher et al., 1996], and left posterior MTG [Hall et al., 2006; Ragland et al., 2007]. For all these investigations, similar behavioral performance in patients versus controls was apparent despite differential brain activation (see also Kircher et al. [2008]).
Several studies showed structural changes in the MTG, bilaterally, for patients with schizophrenia compared to controls [Honea et al., 2005; Shenton et al., 2001]. It was found that the volume of this region is reduced in patients. In addition, functional differences have been found between patients and high‐risk subjects compared to controls [Kircher et al., 2001; Li et al., 2007; Ragland et al., 2007]. Activation of this temporal region was found to be elevated in patients or subjects at high‐risk compared to controls.
The volume of the right middle temporal gyrus has been found to be correlated with psychotic symptoms in subjects at high risk for psychosis [Spencer et al., 2007]. In addition, the right MTG and the right precuneus were found to show higher activations during speech production in patients with formal thought disorder compared to healthy subjects [Kircher et al., 2002]. As such, these regions are prominent areas in speech production and psychosis.
These findings imply that although no differences emerged on the behavioral level, brain activation is altered not only in schizophrenia patients but also in healthy subjects with a genotype that has been found associated with schizophrenia.
Our findings are in line with a previous study testing the influence of G72 on cognition and brain activation [Goldberg et al., 2006]. It was shown that M24 genotype exerts an influence on cognition as measured with the CPT and BOLD signal during encoding of neutral or aversive scenes. It could be demonstrated that healthy individuals homozygous for the minor allele (T/T) showed a lower activation during encoding than A‐allele carriers in hippocampal and parahippocampal regions. In another study [Hall et al., 2008], examining individuals at high risk for psychosis, minor allele carriers of M23 showed increased activation in the right inferior frontal gyrus while showing decreased activation in the hippocampus during a sentence‐completion task. These results show that depending on the brain region and the task, differential activation might be observed within the same group of allele carriers. The differences in brain regions activated in these studies may be explained by two factors: first, all three studies applied different neurocognitive paradigms and second, opposed to the study of Hall et al. 2008, in this study, only healthy individuals without a family history of psychosis were examined. All in all, these issues make the studies difficult to compare.
One limitation is that our sample mainly consisted of university students. This makes generalizations on a population level more difficult. A second limitation is that, so far, there is only one study to report expression levels of G72 [Korostishevsky et al., 2004] in human dorsolateral prefrontal cortex but not the temporal gyrus. Because these are the first reported correlations of G72 (M23 and M24) with semantic verbal fluency, they should be confirmed in independent samples. It is of note that the results of the between group comparisons would not have withstood FWE or FDR comparisons on the whole brain level.
For this study, we have concentrated on the investigation of the polymorphisms M23 and M24 in G72. This choice was based on the fact that we have previously undertaken extensive genetic studies in samples of German patients with various psychiatric diseases and have obtained consistent evidence for an involvement of markers M23 and M24 across diagnostic boundaries including schizophrenia [Rietschel et al., 2008; Schulze et al., 2005; Schumacher et al., 2004, 2005]. By concentrating on two polymorphisms, we have tried to minimize the problem of multiple testing, which can dramatically reduce power when multiple polymorphisms are investigated and conservative correction is performed.
In summary, we found an allele dependent effect of G72 on brain activation during a semantic verbal fluency task in the right middle temporal gyrus (BA 39). The increase in BOLD response was correlated with the number of minor alleles in M24. The data point to an influence of G72 onto brain activation during semantic verbal fluency in two key regions for schizophrenia, even though this increase in activation, does not necessarily surface on the behavioral level. With regard to neurocognitive profiles, these findings show that neural systems underlying cognitive deficits found in psychoses and other disorders might be impaired before manifest disorders emerge and before these deficits might be detectable on a behavioral level. It is noteworthy that G72 influences neural activation in our sample of healthy individuals in the right MTG in a way that has been found previously in patients with schizophrenia.
REFERENCES
- Abou Jamra R, Schmael C, Cichon S, Rietschel M, Schumacher J, Nothen MM ( 2006): The G72/G30 gene locus in psychiatric disorders: A challenge to diagnostic boundaries? Schizoph Bull 32: 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington AM, Gornick M, Sporn AL, Gogtay N, Greenstein D, Lenane M, Gochman P, Baker N, Balkissoon R, Vakkalanka RK, Weinberger DR, Straub RE, Rapoport JL ( 2004): Polymorphisms in the 13q33.2 gene G72/G30 are associated with childhood‐onset schizophrenia and psychosis not otherwise specified. Biol Psychiatry 55: 976–980. [DOI] [PubMed] [Google Scholar]
- Antila M, Tuulio‐Henriksson A, Kieseppa T, Soronen P, Palo OM, Paunio T, Haukka J, Partonen T, Lonnqvist J ( 2007): Heritability of cognitive functions in families with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet 144: 802–808. [DOI] [PubMed] [Google Scholar]
- Axelrod SR, Grilo CM, Sanislow C, McGlashan TH ( 2001): Schizotypal Personality Questionnaire‐Brief: Factor structure and convergent validity in inpatient adolescents. J Pers Disord 15: 168–179. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Fujita F ( 1994): Schizophrenia and personality: Exploring the boundaries and connections between vulnerability and outcome. J Abnorm Psychol 103: 148–158. [DOI] [PubMed] [Google Scholar]
- Boksman K, Theberge J, Williamson P, Drost DJ, Malla A, Densmore M, Takhar J, Pavlosky W, Menon RS, Neufeld RW ( 2005): A 4.0‐T fMRI study of brain connectivity during word fluency in first‐episode schizophrenia. Schizophr Res 75: 247–263. [DOI] [PubMed] [Google Scholar]
- Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, Bougueleret L, Barry C, Tanaka H, La Rosa P, Puech A, Tahri N, Cohen‐Akenine A, Delabrosse S, Lissarrague S, Picard FP, Maurice K, Essioux L, Millasseau P, Grel P, Debailleul V, Simon AM, Caterina D, Dufaure I, Malekzadeh K, Belova M, Luan JJ, Bouillot M, Sambucy JL, Primas G, Saumier M, Boubkiri N, Martin‐Saumier S, Nasroune M, Peixoto H, Delaye A, Pinchot V, Bastucci M, Guillou S, Chevillon M, Sainz‐Fuertes R, Meguenni S, Aurich‐Costa J, Cherif D, Gimalac A, Van Duijn C, Gauvreau D, Ouellette G, Fortier I, Raelson J, Sherbatich T, Riazanskaia N, Rogaev E, Raeymaekers P, Aerssens J, Konings F, Luyten W, Macciardi F, Sham PC, Straub RE, Weinberger DR, Cohen N, Cohen D ( 2002): Genetic and physiological data implicating the new human gene G72 and the gene for d‐amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA 99: 13675–13680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis VA, Bullmore ET, Brammer MJ, Wright IC, Williams SC, Morris RG, Sharma TS, Murray RM, McGuire PK ( 1998): Attenuated frontal activation during a verbal fluency task in patients with schizophrenia. Am J Psychiatry 155: 1056–1063. [DOI] [PubMed] [Google Scholar]
- Detera‐Wadleigh SD, McMahon FJ ( 2006): G72/G30 in schizophrenia and bipolar disorder: Review and meta‐analysis. Biol Psychiatry 60: 106–114. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM ( 2007): Overlooking the obvious: A meta‐analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry 64: 532–542. [DOI] [PubMed] [Google Scholar]
- Dinzeo TJ, Docherty NM ( 2007): Normal personality characteristics in schizophrenia: A review of the literature involving the FFM. J Nerv Ment Dis 195: 421–429. [DOI] [PubMed] [Google Scholar]
- Dye SM, Spence SA, Bench CJ, Hirsch SR, Stefan MD, Sharma T, Grasby PM ( 1999): No evidence for left superior temporal dysfunction in asymptomatic schizophrenia and bipolar disorder. PET study of verbal fluency. Br J Psychiatry 175: 367–374. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Grasby PM, Friston KJ, Dolan RJ ( 1996): Local and distributed effects of apomorphine on fronto‐temporal function in acute unmedicated schizophrenia. J Neurosci 16: 7055–7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT ( 2002): Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry 51: 485–492. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston KJ, Herold S, Silbersweig D, Fletcher P, Cahill C, Dolan RJ, Frackowiak RS, Liddle PF ( 1995): Regional brain activity in chronic schizophrenic patients during the performance of a verbal fluency task. Br J Psychiatry 167: 343–349. [DOI] [PubMed] [Google Scholar]
- Fu CH, Suckling J, Williams SC, Andrew CM, Vythelingum GN, McGuire PK ( 2005): Effects of psychotic state and task demand on prefrontal function in schizophrenia: An fMRI study of overt verbal fluency. Am J Psychiatry 162: 485–494. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Almasy L, Blangero J, Burk GM, Estrada J, Peralta JM, Meyenberg N, Castro MP, Barrett J, Nicolini H, Raventos H, Escamilla MA ( 2007): Adjudicating neurocognitive endophenotypes for schizophrenia. Am J Med Genet B Neuropsychiatr Genet 144B: 242–249. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Straub RE, Callicott JH, Hariri A, Mattay VS, Bigelow L, Coppola R, Egan MF, Weinberger DR ( 2006): The G72/G30 gene complex and cognitive abnormalities in schizophrenia. Neuropsychopharmacology 31: 2022–2032. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Mintz J, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Schork NJ ( 2007): Initial heritability analyses of endophenotypic measures for schizophrenia: The consortium on the genetics of schizophrenia. Arch Gen Psychiatry 64: 1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Whalley HC, Job DE, Baig BJ, McIntosh AM, Evans KL, Thomson PA, Porteous DJ, Cunningham‐Owens DG, Johnstone EC, Lawrie SM ( 2006): A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nat Neurosci 9: 1477–1478. [DOI] [PubMed] [Google Scholar]
- Hall J, Whalley HC, Moorhead TW, Baig BJ, McIntosh AM, Job DE, Owens DG, Lawrie SM, Johnstone EC ( 2008): Genetic variation in the DAOA (G72) gene modulates hippocampal function in subjects at high risk of schizophrenia. Biol Psychiatry 64: 428–433. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK ( 1998): Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology 12: 426–445. [DOI] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE ( 2005): Regional deficits in brain volume in schizophrenia: A meta‐analysis of voxel‐based morphometry studies. Am J Psychiatry 162: 2233–2245. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ ( 2004): The spatial and temporal signatures of word production components. Cognition 92: 101–144. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL ( 2006): Personality and major depression: A Swedish longitudinal, population‐based twin study. Arch Gen Psychiatry 63: 1113–1120. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Bulimore ET, Brammer MJ, Williams SC, Broome MR, Murray RM, McGuire PK ( 2001): Differential activation of temporal cortex during sentence completion in schizophrenic patients with and without formal thought disorder. Schizophr Res 50: 27–40. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Liddle PF, Brammer MJ, Williams SC, Murray RM, McGuire PK ( 2002): Reversed lateralization of temporal activation during speech production in thought disordered patients with schizophrenia. Psychol Med 32: 439–449. [DOI] [PubMed] [Google Scholar]
- Kircher T, Whitney C, Krings T, Huber W, Weis S ( 2008): Hippocampal dysfunction during free word association in male patients with schizophrenia. Schizophr Res 101: 242–255. [DOI] [PubMed] [Google Scholar]
- Kirov G, O'Donovan MC, Owen MJ ( 2005): Finding schizophrenia genes. J Clin Invest 115: 1440–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korostishevsky M, Kaganovich M, Cholostoy A, Ashkenazi M, Ratner Y, Dahary D, Bernstein J, Bening‐Abu‐Shach U, Ben‐Asher E, Lancet D, Ritsner M, Navon R ( 2004): Is the G72/G30 locus associated with schizophrenia? Single nucleotide polymorphisms, haplotypes, and gene expression analysis. Biol Psychiatry 56: 169–176. [DOI] [PubMed] [Google Scholar]
- Lehrl S, Triebig G, Fischer B ( 1995): Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol Scand 91: 335–345. [DOI] [PubMed] [Google Scholar]
- Li D, He L ( 2007): G72/G30 genes and schizophrenia: A systematic meta‐analysis of association studies. Genetics 175: 917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Branch CA, Bertisch HC, Brown K, Szulc KU, Ardekani BA, DeLisi LE ( 2007): An fMRI study of language processing in people at high genetic risk for schizophrenia. Schizophr Res 91: 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A ( 2003): The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry 60: 497–502. [DOI] [PubMed] [Google Scholar]
- Moghaddam B ( 2003): Bringing order to the glutamate chaos in schizophrenia. Neuron 40: 881–884. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Otte DM, Bilkei‐Gorzo A, Filiou MD, Turck CW, Yilmaz O, Holst MI, Schilling K, Abou‐Jamra R, Schumacher J, Benzel I, Kunz WS, Beck H, Zimmer A ( 2009): Behavioral changes in G72/G30 transgenic mice. Eur Neuropsychopharmacol 19: 339–348. [DOI] [PubMed] [Google Scholar]
- Quraishi S, Frangou S ( 2002): Neuropsychology of bipolar disorder: A review. J Affect Disord 72: 209–226. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Moelter ST, Bhati MT, Valdez JN, Kohler CG, Siegel SJ, Gur RC, Gur RE ( 2007): Effect of retrieval effort and switching demand on fMRI activation during semantic word generation in schizophrenia. Schizophr Res 99: 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Weiser M, Rabinowitz J, Caspi A, Schmeidler J, Mark M, Kaplan Z, Davidson M ( 2002): A population‐based cohort study of premorbid intellectual, language, and behavioral functioning in patients with schizophrenia, schizoaffective disorder, and nonpsychotic bipolar disorder. Am J Psychiatry 159: 2027–2035. [DOI] [PubMed] [Google Scholar]
- Rietschel M, Beckmann L, Strohmaier J, Georgi A, Karpushova A, Schirmbeck F, Boesshenz KV, Schmal C, Burger C, Jamra RA, Schumacher J, Hofels S, Kumsta R, Entringer S, Krug A, Markov V, Maier W, Propping P, Wust S, Kircher T, Nothen MM, Cichon S, Schulze TG ( 2008): G72 and its association with major depression and neuroticism in large population‐based groups from Germany. Am J Psychiatry 165: 753–762. [DOI] [PubMed] [Google Scholar]
- Sacchi S, Bernasconi M, Martineau M, Mothet JP, Ruzzene M, Pilone MS, Pollegioni L, Molla G ( 2008): pLG72 modulates intracellular D‐serine levels through its interaction with d‐amino acid oxidase: Effect on schizophrenia susceptibility. J Biol Chem 283: 22244–22256. [DOI] [PubMed] [Google Scholar]
- Schulze TG, Ohlraun S, Czerski PM, Schumacher J, Kassem L, Deschner M, Gross M, Tullius M, Heidmann V, Kovalenko S, Jamra RA, Becker T, Leszczynska‐Rodziewicz A, Hauser J, Illig T, Klopp N, Wellek S, Cichon S, Henn FA, McMahon FJ, Maier W, Propping P, Nothen MM, Rietschel M ( 2005): Genotype‐phenotype studies in bipolar disorder showing association between the DAOA/G30 locus and persecutory delusions: A first step toward a molecular genetic classification of psychiatric phenotypes. Am J Psychiatry 162: 2101–2108. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Jamra RA, Freudenberg J, Becker T, Ohlraun S, Otte AC, Tullius M, Kovalenko S, Bogaert AV, Maier W, Rietschel M, Propping P, Nothen MM, Cichon S ( 2004): Examination of G72 and d‐amino‐acid oxidase as genetic risk factors for schizophrenia and bipolar affective disorder. Mol Psychiatry 9: 203–207. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Abou Jamra R, Becker T, Klopp N, Franke P, Jacob C, Sand P, Fritze J, Ohlraun S, Schulze TG, Rietschel M, Illig T, Propping P, Cichon S, Deckert J, Nothen MM ( 2005): Investigation of the DAOA/G30 locus in panic disorder. Mol Psychiatry 10: 428–429. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW ( 2001): A review of MRI findings in schizophrenia. Schizophr Res 49: 1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J Jr ( 2003): Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res Cogn Brain Res 17: 75–82. [DOI] [PubMed] [Google Scholar]
- Spence SA, Liddle PF, Stefan MD, Hellewell JS, Sharma T, Friston KJ, Hirsch SR, Frith CD, Murray RM, Deakin JF, Grasby PM ( 2000): Functional anatomy of verbal fluency in people with schizophrenia and those at genetic risk. Focal dysfunction and distributed disconnectivity reappraised. Br J Psychiatry 176: 52–60. [DOI] [PubMed] [Google Scholar]
- Spencer MD, Moorhead TW, McIntosh AM, Stanfield AC, Muir WJ, Hoare P, Owens DG, Lawrie SM, Johnstone EC ( 2007): Grey matter correlates of early psychotic symptoms in adolescents at enhanced risk of psychosis: A voxel‐based study. Neuroimage 35: 1181–1191. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC ( 2003): Schizophrenia as a complex trait: Evidence from a meta‐analysis of twin studies. Arch Gen Psychiatry 60: 1187–1192. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐Planar Stereotaxic Atlas of the Human Brain. New York: Thieme. [Google Scholar]
- Tiihonen J, Haukka J, Henriksson M, Cannon M, Kieseppa T, Laaksonen I, Sinivuo J, Lonnqvist J ( 2005): Premorbid intellectual functioning in bipolar disorder and schizophrenia: Results from a cohort study of male conscripts. Am J Psychiatry 162: 1904–1910. [DOI] [PubMed] [Google Scholar]
- Yurgelun‐Todd DA, Waternaux CM, Cohen BM, Gruber SA, English CD, Renshaw PF ( 1996): Functional magnetic resonance imaging of schizophrenic patients and comparison subjects during word production. Am J Psychiatry 153: 200–205. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Leach L, Kaplan E ( 1998): On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry Neuropsychol Behav Neurol 11: 111–119. [PubMed] [Google Scholar]


