Abstract
Functional magnetic resonance imaging (fMRI) is able to detect changes in blood oxygenation level associated with neuronal activity throughout the brain. For more than a decade, fMRI alone or in combination with simultaneous EEG recording (EEG‐fMRI) has been used to investigate the hemodynamic changes associated with interictal and ictal epileptic discharges. This is the first literature review to focus on the various fMRI acquisition and data analysis methods applied to map epileptic seizure‐related hemodynamic changes from the first report of an fMRI scan of a seizure to the present day. Two types of data analysis approaches, based on temporal correlation and data driven, are explained and contrasted. The spatial and temporal relationship between the observed hemodynamic changes using fMRI and other non‐invasive and invasive electrophysiological and imaging data is considered. We then describe the role of fMRI in localizing and exploring the networks involved in spontaneous and triggered seizure onset and propagation. We also discuss that fMRI alone and combined with EEG hold great promise in the investigation of seizure‐related hemodynamic changes non‐invasively in humans. We think that this will lead to significant improvements in our understanding of seizures with important consequences for the treatment of epilepsy. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: haemodynamic, seizure, BOLD, EEG‐fMRI
INTRODUCTION
Epilepsy affects 5 to 10 persons per thousand [Sander,2003], and 30% to 40% of patients continue to experience refractory seizures despite medical treatment [Kwan and Sander,2004]. Functional MRI (fMRI) works on the principle that neuronal activity causes regional changes in cerebral blood flow, cerebral blood volume, and blood oxygenation resulting in variations in the ratio of diamagnetic oxyhemoglobin and paramagnetic deoxyhemoglobin which can be detected as the blood‐oxygen‐level‐dependent (BOLD) contrast [Kwong et al.,1992; Ogawa et al.,1990]. This intrinsic contrast mechanism provides a means of mapping function‐related hemodynamic changes non‐invasively over the entire brain with good spatial resolution. In addition to the study of task‐modulated effects which are the subject of the majority of fMRI studies, the technique has been used to map brain state‐related hemodynamic changes in the resting state [Fox et al.,2009; Gusnard et al.,2001; Laufs et al.,2006] and during sleep [Czisch et al.,2004; Laufs et al.,2007]. EEG was incorporated in the fMRI set up for the first time in 1993 by Ives [Ives et al.,1993], with the later development of EEG‐triggered fMRI in epilepsy [Warach et al.,1996]. Simultaneous and continuous recording of good quality EEG and fMRI data in 2001 [Lemieux et al.,2001] further broadened the range of neural events, effects and states that could be studied using fMRI, particularly in the resting state.
The literature on EEG‐fMRI has expanded rapidly in the last decade, especially on the study of interictal epileptic discharges (IED) related hemodynamic changes in focal [Archer et al.,2003; Bagshaw et al.,2005; Jacobs et al.,2009; Kobayashi et al.,2006a; LeVan et al.,2010a; Salek‐Haddadi et al.,2006] and generalized epilepsy [Hamandi et al.,2006]. This has led to new insights on the effects of epilepsy on neurovascular coupling [Carmichael et al.,2008], localization of epileptogenic zone in focal epilepsy [Federico et al.,2005b; Jacobs et al.,2008; Salek‐Haddadi et al.,2006; Thornton et al.,2010a; Zijlmans et al.,2007] and the involvement of epileptic networks for 3 Hz generalized spike wave discharges (GSWDs) [Gotman et al.,2005; Hamandi et al.,2006; Kobayashi et al.,2006b]. However, the delineation of the brain areas producing interictal spikes, known as the “irritative zone” (IZ), may not suffice to localize the seizure onset zone (SOZ), which is clinically more important [Luders et al.,2006]. Thus there has been an increasing interest in investigating specific seizure‐related hemodynamic changes using fMRI. Due to methodological and practical issues the data on seizure‐related hemodynamic changes is not as extensive as for the interictal state. Our aim is to provide a detailed review of the published literature on mapping seizure‐related hemodynamic changes using fMRI alone or combined with EEG. We have described the main methodological constraints, followed by clinical applications, and have discussed the possible future role of the technique in investigating seizures. We will not discuss in detail the technical aspects of EEG recording during fMRI which have been comprehensively discussed in a number of reviews recently [Laufs and Duncan,2007; Laufs et al.,2008].
For the purpose of this work, we performed a comprehensive search of English literature on Medline Pubmed database with the keywords; “functional imaging AND epilepsy,” “functional imaging AND seizures,” “ictal fMRI,” “EEG‐fMRI,” “EEG‐fMRI AND epilepsy,” and “EEG‐fMRI AND seizures” from 1990 to 2010. In addition we also searched through the bibliographies of the relevant original articles. We selected all original research articles investigating seizure related hemodynamic changes using fMRI alone or combined EEG‐fMRI (Table I).
Table I.
Publications on mapping seizure‐related BOLD changes using fMRI or EEG‐fMRI
| Study | Syndrome | Subjects | EEG/ channels | Motion Cut‐off (mm) | Seizure activation procedure | Seizure detection | Data analysis approach clinic/results |
|---|---|---|---|---|---|---|---|
| Adults: fMRI only studies | |||||||
| [Detre et al.,1995] | FE | 1 | N | — | N | OBN | Δ Signal Intensity |
| [Detre et al.,1996] | FE | 1 | N | — | N | NM | Δ Signal Intensity |
| [Krakow et al.,2000] | FOS | 1 | N | — | F | ETS | GLM |
| [Kubota et al.,2000] | FE | 1 | N | — | N | OBN | Δ Signal Intensity |
| [Krings et al.,2000] | FE | 1 | N | — | N | OBN, VR | Δ Signal Intensity |
| [Morocz et al.,2003] | ME | 1 | N | — | M | PRB | GLM, ICA |
| [Archer et al.,2006] | FE | 1 | N | — | N | VR | GLM |
| [Auer et al.,2008] | FE | 1 | N | 0.2 | N | OBN | Δ Signal Intensity |
| [Donaire et al., 2009] | FE | 1 | N | 0.5 | N | OBN | Seq.GLM, ICA |
| Adults: EEG‐fMRI studies | |||||||
| [Iannetti et al.,2002] | FOS | 3 | Y/32 | — | F | EEG | GLM |
| [Salek‐haddadi et al.,2002] | FE | 1 | Y/11 | — | N | EEG | GLM |
| [Salek‐haddadi et al.,2003] | JAE | 1 | Y/nm | — | N | EEG | GLM |
| [Aghakhani et al.,2004] | IGE | 25 | Y/21 | — | N | EEG | GLM |
| [Gotman et al.,2005] | IGE | 25 | Y/21 | 1 | N | EEG | GLM |
| [Federico et al., 2005] | FE | 3 | a | — | SD | VR | GLM |
| [Di Bonaventura et al.,2005] | FOS | 3 | Y/nm | — | F | EEG | GLM |
| [Di Bonaventura et al.,2006] | RE | 1 | Y/nm | — | N | EEG | GLM |
| [Di Bonaventura et al.,2006] | FE/IGE | 32/11 | Y/18 | — | N | EEG, VR, PRB | GLM |
| [Kobayashi et al., 2006] | FE | 1 | Y/27 | 1 | N | EEG | GLM |
| [Hamandi et al.,2006] | IGE/SGE | 30/16 | Y/12 | — | N | EEG | GLM |
| [Laufs et al.,2006] | JAE | 1 | Y/29 | — | N | EEG | GLM |
| [Hamandi et al.,2008] | IGE/SGE | 2/2 | Y/32 | — | N | EEG | GLM |
| [Carmichael et al.,2008] | IGE/SGE | 2/2 | Y/32 | — | N | EEG | GLM |
| [Salek‐haddadi et al., 2008] | ReE | 9 | Y/11 | — | Re | EEG, SR, EMG, PRB | GLM |
| [Tyvaert et al.,2008] | FE | 8 | Y/25 | — | N | EEG | GLM |
| [Donaire et al., 2009] | FE | 10 | Y/27 | — | N | OBN | Seq.GLM |
| [Tyvaert et al.,2009] | FE | 17 | Y/25 | 1 | N | EEG, VR | Seq.GLM |
| [LeVan et al., 2009] | FE | 15 | Y/25 | — | N | EEG | ICA |
| [Chassagnon et al.,2009] | FE | 1 | Y/27 | — | N | EEG | GLM |
| [Marrosu et al.,2009] | ME | 1 | Y/nm | — | N | EEG | GLM |
| [Thornton et al., 2010] | FE | 83 | Y/32–64 | — | N | EEG | GLM, ICA |
| Children | |||||||
| [Jackson et al.,1994] | FE | 1 | N | — | N | Δ Signal Intensity | |
| [Labate et al., 2005] | IGE | 1 | Y/18 | — | N | EEG | GLM |
| [Liu et al.,2008] | EMA | 4 | Y/21 | — | N | EEG | GLM |
| [Moeller et al., 2008] | CAE | 10 | Y/30 | — | N | EEG | GLM |
| [Moeller et al., 2008] | IGE | 10 | Y/30 | — | N | EEG | GLM |
| [Moeller et al., 2009] | IGE/PPR | 16/14 | Y/30 | — | Ph | EEG | GLM |
| [Li et al.,2009] | CAE | 15 | Y/34 | — | N | EEG | GLM |
| [Moeller et al., 2009] | IGE | 1 | Y/30 | — | Ph | EEG | GLM |
| [Bai et al.,2010] | CAE | 42 | Y/21 | >3 | DR,SD | EEG | GLM |
| [Moeller et al., 2010] | IGE | 12 | Y/25 | — | N | EEG | GLM, ICA |
| [Berman et al.,2010] | CAE | 37 | Y/21–32 | — | DR | EEG | GLM |
| [Moeller et al., 2010] | ?IGE | 1 | Y/32 | — | — | EEG | GLM |
| [Moeller et al., 2010] | CAE/JAE | 14 | Y/25–30 | 1 | — | EEG | Seq.GLM |
| [Carney et al.,2010] | CAE | 11 | Y/18 | — | DR | EEG | GLM |
One patient had simultaneously recorded EEG.
FE = focal epilepsy, FOS = fixation‐off sensitivity, ME = musicogenic epilepsy, JAE = juvenile absence epilepsy, IGE = idiopathic generalized epilepsy, RE = Rasmussan's encephalitis, SGE = secondary generalized epilepsy, ReE = reading epilepsy, EMA = eyelid myoclonia with absences, CAE = childhood absence epilepsy, PPR = photoparoxysmal response, N = none, Y = yes, F = fixation, M = music, SD = sleep deprivation, Re = reading, Ph = photic stimulation, DR = drug reduction, OBN = observed by neurologist, ETS = eye tracking system, VR = video recording, PRB = patient response button, SR = sound/voice recording, EMG = electromyography, Δ = change, GLM = general linear model, ICA = independent component analysis, Seq. = sequential, EEG = electroencephalography.
METHODOLOGICAL CONSIDERATIONS FOR MAPPING SEIZURES USING fMRI
Studies using fMRI and/or EEG‐fMRI as an investigation tool face a number of methodological issues pertaining to patient recruitment, seizure detection, and data analysis which makes seizure‐related fMRI data acquisition complex. Here we will discuss these issues within the specific context of their demonstrated application.
Patient Selection
Patient recruitment for ictal studies using fMRI has been either fortuitous, in cases when seizures have occurred during studies of the interictal state, or targeted. The majority of series published to date have resulted from the former. The targeted selection of patients based on the specific aim of capturing seizures has generally been when seizures were provoked by certain triggers such as fixation‐off sensitivity (FOS) [Di Bonaventura et al.,2005; Iannetti et al.,2002; Krakow et al.,2000], music [Morocz et al.,2003], reading [Salek‐Haddadi et al.,2009] and photoparoxysmal response (PPR) [Moeller et al.,2009a,b]. Most studies have been single case reports or small series of patients with specific subgroups of epilepsy (Table I). The unpredictable nature of seizures (except when seizures can be triggered) explains this situation.
Vigorous head or body movements inside an MRI scanner may be hazardous from the patient safety perspective [Lemieux et al.,1997] and also adversely affect data quality [Lemieux et al.,2007]. Patient recruitment has, therefore, been limited to specific seizure types which can be scanned without patient safety and data quality concerns.
Seizure Identification Methods
Various means of seizure identification have been applied in fMRI studies including: online and retrospective review of EEG, Electromyography, video recording, observation of ictal semiology (for example: by person inside the scanner room), eye tracking technology, patient response button, and sound/voice recording (Table I). Using simultaneous video‐EEG recording is a well established method of seizure identification and video recording can provide additional valuable information to identify seizures when EEG is not helpful [Binnie et al.,1981; Smith,2005]. The synchronization of EEG and video is pivotal, as there may be a time delay for the electrical activity to propagate from the SOZ to the symptomatogenic zone to produce clinical symptoms [Luders et al.,2006]. Thus depending solely on video or sound recording may be inaccurate or suboptimal. Similarly, if consciousness is affected immediately at the beginning of a seizure, the use of patient signal (e.g. via a button press) may not be a reliable way of identifying seizure onset because the patient may not be able to press the button at all due to impairment of consciousness. Recently a system for simultaneous and synchronized video recording during EEG‐fMRI (vEEG‐fMRI) has been successfully demonstrated without significant detrimental effects on image quality [Chaudhary et al.,2010]. The combined information from video‐EEG can help to identify seizures semiologically and electrophysiologically. Electromyography recordings may also prove helpful during EEG‐fMRI studies for atonic/tonic seizures and myoclonic jerks.
Reducing Artifacts in EEG Data Recorded During fMRI
EEG recordings inside an MRI scanner are obscured by two main types of artifacts: gradient artifact (GA) and pulse artifact (PA). For most applications, in particular those relying on the quantitative EEG analysis, it is necessary to remove these artifacts subsequently before any further processing. The GA is produced by the rapidly changing magnetic field used for fMRI acquisition. A number of methods have been devised over the years with varying degrees of efficacy including: frequency domain method [Hoffmann et al.,2000], average image artifact subtraction, average image artifact subtraction and adaptive noise cancellation [Allen et al.,2000], and principal component analysis (PCA) [Negishi et al.,2004; Niazy et al.,2005]. To date the most commonly used method is average image artifact subtraction and adaptive noise cancellation [Allen et al.,2000] which is used both for online and offline EEG artifact removal.
The PA is also often called the ballistocardiographic artifact (BCG). This effect results at least in part from the pulsatile movement of the head and scalp in relation to the cardiac cycle which results in induced voltages (artifacts) in the EEG recording circuit. This effect may make the identification of epileptic events difficult in most patients. Here again methods based on the average artifact subtraction principle [Allen et al.,1998] are the most commonly applied technique for removing PA. Other methods include applying adaptive filtering [Bonmassar et al.,2002], weighted average subtraction [Goldman et al.,2000], median filter template [Ellingson et al.,2004] or statistical algorithms in independent component analysis (ICA) [Srivastava et al.,2005]. All these methods have been validated in a number of studies and provide adequate solutions facilitating the identification of epileptic discharges.
Artifacts in the fMRI Data
The quality of the fMRI data acquired during EEG recording in general and seizures in particular can be severely degraded by a number of factors, namely electromagnetic interference caused by the EEG recording system (passive: distortion from metallic electrodes and leads; active: contamination of MR signal by radio‐frequency waves generated by the EEG electronics), head motion, and pulse and respiration related artifacts.
Head motion‐related artifact
Head and body movements as small as a fraction of a millimeter can affect fMRI data quality adversely and may make them unusable or impossible to interpret [Hajnal et al.,1994], unless special postprocessing steps are utilized [Friston et al.,1995a]. Motion is integral to most seizure types. Therefore physical means to limit motion such as vacuum cushions can play an important role at the acquisition stage [Benar et al.,2003].
The most common and widely applied motion correction method in fMRI is postacquisition realignment of the fMRI time‐series using six rigid body realignment parameters (RP). The realignment parameters are the three rotation angles and three translation shifts that describe the rigid‐body relationship between an individual volume in the fMRI time series and the first volume in the series, estimated using a least squares approach and expressed as six time series [Friston et al.,1995a]. Some investigators [Gotman et al.,2005] have used a cut off limit of 1 mm and 1 degree inter‐scan motion above which the whole data‐set is discarded from the analysis. Each seizure‐related fMRI dataset being considered in this review is potentially unique, in contrast to most cognitive studies in healthy subjects; therefore, another approach is to systematically attempt to extract as much information as possible from every dataset through adapted signal modeling strategies. For example, residual motion‐related signal may be treated as confound within the general linear modeling framework. These effects can be modeled based on the motion RPs derived from the scan realignment procedure [Friston et al.,1995a] and by calculating additional linear and nonlinear motion‐related effects (using Volterra‐expansion of motion RPs) [Hamandi et al.,2006; Salek‐Haddadi et al.,2003].
For large motion effects, the inclusion of “scan nulling” regressors has also been proposed [Lemieux et al.,2007], instead of discarding the scans, on the basis of the principle that inclusion of nuisance effects into models of the fMRI signal, in addition to the effects of interest, can increase sensitivity [Friston et al.,1995b]. These regressors attempt to explain the signal variation due to severe head motion events (i.e. above a prefixed cut‐off value of the inter‐scan motion derived from the RP), by representing the effect as a series of four regressors each consisting of a one TR duration Heaviside function corresponding to the scan affected by head motion and the 3 subsequent scans [Lemieux et al.,2007].
Cardiac pulse and respiration related effects: Physiological noise
The cyclical variability of the pulse causes changes in oxygenated blood volume in the brain, and small displacements of the brain tissue from compression and decompression during the cardiac cycle. Similarly, respiration‐related physiological noise also affects fMRI data quality through changes in magnetic field secondary to chest movements and due to changes in pCO2 (partial pressure of CO2 in blood) levels causing fluctuations in BOLD. Though these signal variations are present in all fMRI studies, their effect becomes more significant for EEG‐fMRI studies of epilepsy in view of the greater imperative of extracting as much information as possible from individual datasets and due to the possible clinical implications of the result for the patient's management in some cases; this is in contrast with most fMRI studies in which a dataset of suboptimal quality can be replaced with a better one, possibly from a different subject.
Image data correction and regression methods based on the phase of the cardiac cycle relative to the scan acquisition [Glover et al.,2000; Liston et al.,2006] and independent component analysis (ICA) based identification of physiological noise related components and removal [Perlbarg et al.,2007] have been used. For respiration related signals, modeling of respiratory volume per minute and changes in depth and height of respiration as confounds [Birn et al.,2006; van Houdt et al.,2010] have been employed. It has been shown in EEG‐fMRI studies of IEDs [Glover et al.,2000; Liston et al.,2006] and alpha rhythms [van Houdt et al.,2010] that inclusion of these additional regressors as confounds improves sensitivity. In addition cardiac effects have also been modeled in fMRI analysis of seizures [Thornton et al.,2010b].
The inclusion of as complete and thorough a model of the effects of motion and physiological confounds such as pulse and respiration on the fMRI signal is the best possible means of avoiding false‐positive findings and therefore guaranteeing maximum specificity of the observed BOLD signal changes in relation to neural activity.
fMRI Data Analysis: Seizure‐Related BOLD Effects
The overall aim of the analysis is to identify hemodynamic patterns reflecting the epileptic activity. Three types of data analysis approaches have been proposed for this purpose: the identification of regions showing particular patterns of signal fluctuations; the general linear model (GLM); and data‐driven such as ICA. Within the GLM framework, the choice and specification of the model embody the investigator's hypotheses. These differ in their underlying assumptions and in particular the degree to which they rely on the use of other data such as synchronously‐recorded EEG to reveal these patterns.
Identification of variations in MR signal intensity without reference to EEG or other event marker
Early studies investigating hemodynamic changes during seizures used fMRI without simultaneous EEG or video. In these, fluctuations in signal intensity relative to the mean signal [Detre et al.,1995; Jackson et al.,1994] or the baseline signal [Krings et al.,2000; Kubota et al.,2000] were identified to produce maps of ictal fMRI change. Detre et al. performed a cross‐correlation analysis between regions of the earliest signal change including the well characterized focus and the rest of the brain [Detre et al.,1996]. More recently, Auer et al. correlated the signal at every voxel in the brain to an internal reference curve defined by visually examining the signal alterations of the voxels in the area responsible for seizures to map changes in other regions [Auer et al.,2008].
Detection of variation in signal intensity, as applied in the earlier studies, lacks the concurrent information from EEG on seizure onset and evolution; in addition, mapping of putative ictal hemodynamic changes based on visual identification of regions of raw signal intensity changes may be particularly unreliable due to the effects of motion and MR signal drift.
Correlation analysis: General linear model
The analysis of most fMRI time series data relies on the application of the GLM which is a correlation based technique in a mass univariate (voxel‐by‐voxel) approach where the signal is expressed as a weighted sum of effects, fitted to the data at each voxel and subsequent mapping of the fitted weight statistics [Friston,1996]. In practice, it is most commonly implemented in the form of a design matrix encompassing all known effects on the signal either as effects of interest (EOI) or confounds. This is also the case in most EEG‐fMRI applications, where the effects associated with epileptic discharges identified on EEG are incorporated into the GLM as a regressor of interest, to map the degree of correlation of this regressor with the measured signal across all voxels. Each EOI to be included as a regressor in a GLM design requires the choice of a mathematical representation, and is convolved with hemodynamic response function (HRF).
A number of questions needs to be considered and explored further in detail to find an appropriate and more realistic way of modeling seizures in fMRI data analysis. For example, can we presume that the hemodynamic changes that take place during a seizure that lasts 10 s correspond to a neuronal event with fixed intensity over that duration? More specifically, can a seizure be realistically represented as an event block analogous to those used in block design cognitive fMRI studies? There is also the issue of the seizure onset and the preictal state; do they correspond to distinct states that fundamentally differ from the baseline or is there a gradual hemodynamic transition from interictal to ictal state? Similarly, can multiple seizures be considered as the same phenomenon and therefore be grouped as a single effect for the purpose of modeling?
Event representation
The EOI are usually represented as box‐car functions for example in the fMRI studies following a paradigm such as cognitive studies, or idealized zero‐duration stick functions for example in the fMRI studies of random brief events such as IEDs. However, seizures are dynamic in nature, potentially involving a complex sequence of various combinations of brain regions over time. This complexity makes their mathematical representation for GLM model building much more difficult, which is reflected by the variety of approaches used to analyze ictal fMRI data.
In the case of provoked seizures this is somewhat simplified by the possibility of identifying a clear seizure onset using the timing of provoking factor such as: eye closure in FOS [Di Bonaventura et al.,2006; Iannetti et al.,2002; Krakow et al.,2000], music in musicogenic seizures [Marrosu et al.,2009; Morocz et al.,2003], and reading in reading epilepsy [Salek‐Haddadi et al.,2009]. In some case it may be possible to provoke repeated seizures, separated by periods of rest which may therefore be considered repetitions of the same type of event (similar to the block design of conventional cognitive fMRI studies) and represented mathematically as box‐car [Iannetti et al.,2002; Krakow et al.,2000; Marrosu et al.,2009; Morocz et al.,2003] or as series of stick functions [Salek‐Haddadi et al.,2009]. An important issue which has not been taken into account for provoked seizures is the possible time lag between stimulus presentation and seizure onset, which could allow further exploration of the mechanisms of seizure generation.
In contrast, spontaneous seizures such as focal or absence seizures which occur at random may have uncertainty in the identification of the event onset given the available means. Ictal events must be identified employing different methods (Seizure Identification Methods and Table I), and categorized using the same data. Onset and offset timings of single or multiple seizures or preictal states have been used to define the EOI to be modeled and represented as variable duration blocks (depending upon the seizure duration) to be included in the design matrix as regressors [Salek‐Haddadi et al.,2002,2009; Tyvaert et al.,2008].
Seizures are an evolving phenomena and when seizures are modeled as a single block, though they reveal BOLD changes, the ability to differentiate seizure onset from propagated activity is diminished. Based on this hypothesis, peri‐ictal hemodynamic changes have been mapped sequentially; from before the seizure onset (preictal: 120 s before clinical seizure onset [Donaire et al.,2009a], 9 s before the ictal discharge onset on EEG [Tyvaert et al.,2009]) up to prespecified time following the seizure offset (postictal: 60 s after clinical seizure end [Donaire et al.,2009a], 10 s after the EEG discharge offset [Tyvaert et al.,2009]). Donaire et al. divided the preictal, ictal, and postictal blocks into sequential fixed width 10 s blocks each representing a different effect (i.e. independent regressors in the GLM) and compared with a baseline period (20 s temporally unrelated to the seizure). Secondly, the signals in each 10 s block were also compared with that in the contiguous 10 s block within the GLM framework [Donaire et al.,2009a,b]. This approach provides the ability to map and differentiate seizure onset and seizure propagation; however it makes minimum use of the simultaneously recorded EEG. More recently, Thornton and co‐workers have modeled seizures by dividing each seizure into three distinct and consecutive phases (representing each as a block of variable durations): early ictal, clinical ictal, and late ictal, based on the onset, duration, and electroclinical evolution over time. The authors argued that this modeling approach makes better use of the available physiological information and allows for the better understanding of propagation of hemodynamic effects between phases [Thornton et al.,2010b].
Based on the principle that all quantifiable effects should be included in the GLM design matrix to reduce the amount of residual signal variance, IEDs and confounds: RP and pulse regressors have also been represented in the same design matrix as separate regressors and confounds respectively (Head motion‐related artifact and Cardiac pulse and respiration related effects: Physiological noise) [Chassagnon et al.,2009; Gotman et al.,2005; Hamandi et al.,2006; Laufs et al.,2006; Salek‐Haddadi et al.,2002,2009; Thornton et al.,2010b; Tyvaert et al.,2008]. As yet, however, there is no consensus on the degree to which such confounds must be modeled.
An important question here is; how should one represent multiple seizures in the design matrix? This is of utmost importance because; unless all seizures recorded are electroclinically stereotyped, they may be manifestations of a variety of underlying epileptogenic networks, in which case they must be represented as different effects (different regressors) in the GLM. Three different approaches have been applied:
-
1
Similar repeated seizures as variable duration blocks in a single regressor [Tyvaert et al.,2008].
-
2
Each epileptic event of variable duration represented as a separate regressor and the event with the longest duration and clear electrical and spatial propagation represented as seizure [Tyvaert et al.,2009].
-
3
Ictal phase‐specific effects represented as variable duration blocks across seizures [Thornton et al.,2010b].
Epilepsy‐related HRF
Once the events are identified and represented mathematically they must convolve with an informed basis set representing the shape of the HRF1 for the purpose of inclusion in the GLM. The creation of regressors to represent the ictal events themselves (Event representation) and the choice of HRF for ictal events is particularly problematic given that ictal events can last many minutes, and may involve multiple brain regions at different times as demonstrated by seizure semiology and electrophysiology. In addition, we do not know if the BOLD changes associated with ictal events remain stationary in a particular brain region once started, or they evolve and diminish before propagating to other brain areas. Thus, longer duration and dynamic nature of seizures increase the difficulty to select a basis set or shape of HRF for seizures.
On the other hand, an important consequence of the univariate aspect of the GLM approach and the choice of basis set is the possibility of accounting for spatial variability in the shape of HRF as it has been shown in interictal and ictal studies that the shape and timing of the peak HRF may deviate from the canonical shape in relation to generalized activity [Bagshaw et al.,2004; Benar et al.,2002; Grouiller et al.,2010; Hawco et al.,2007; Jacobs et al.,2009; Masterton et al.,2010; Moeller et al.,2008a,2010a; Siniatchkin et al.,2010]. It has also been shown that the shape of HRF may be epilepsy syndrome specific and the use of syndrome‐specific HRF may improve the localization of BOLD signal changes [Masterton et al.,2010]. The lesser the flexibility of the basis set the fewer the questions that can be addressed related to the time course of the ictal related changes. For single‐term basis sets, such as the canonical HRF, the only question that can be addressed is the sign of the putative BOLD changes. Therefore, inter‐dependence between the temporal and spatial aspects of the analysis is embodied in the necessary choice of HRF for GLM model building, which reflects the investigator's specific hypotheses about the inter‐relationship between the ictal phenomenology and the fMRI signals [Archer et al.,2006; Donaire et al.,2009a,b; Hamandi et al.,2006,2008; Laufs et al.,2006; Salek‐Haddadi et al.,2002,2009; Thornton et al.,2010b].
For more extensive basis sets such as the Fourier set consisting of a linear combination of n sine and n cosine functions, the time course can be evaluated over the specified time window [Salek‐Haddadi et al.,2002; Thornton et al.,2010b]. Multiple GLMs each with different gamma functions (time to peak: 3, 5, 7, and 9 s) have also been used to account for some variability in the timing of the HRF [Aghakhani et al.,2004; Chassagnon et al.,2009; Kobayashi et al.,2006b; Tyvaert et al.,2008].
Common features: Mapping group effects
The GLM framework has been used to explore group effects by combining the single‐subject analyses using spatial normalization [Penny and Holmes,2004] for the purpose of making population level inferences [Friston et al.,1999; Worsley et al.,2002]. This is particularly useful to identify shared features and is probably most relevant in stereotypical syndromes such as idiopathic generalized epilepsy (IGE) [Gotman et al.,2005], childhood absence epilepsy (CAE) [Moeller et al.,2008b], and some types of secondary generalized epilepsy (SGE) [Hamandi et al.,2006].
Independent component analysis
ICA is a well‐established and powerful data‐driven approach based on basic assumptions regarding the properties of the sources responsible for the observed signals, particularly their statistical independence. It can be used to decompose multichannel time series data into spatially or temporally independent components by maximizing their statistical independence. If the signal satisfies the necessary conditions, each independent component represents the activity of one of the sources of the signal [Calhoun et al.,2001; McKeown et al.,2003]. A common assumption in ICA algorithms is that the sources are spatially stationary [McKeown et al.,1998,1999] which is helpful in separating the signal related sources from noise sources which are presumed to be nonstationary [DeMartino et al.,2007]. However, seizures involve complex spatial and temporal propagation; therefore, the applicability of ICA to such a dynamic process must be questioned in principle. Nonetheless, ICA has been used to study ictal hemodynamic patterns with some interesting results as discussed in section Applications of ICA in Epilepsy, making this assumption of stationarity less relevant.
The components identified by ICA cannot be ranked by degree of importance, in contrast to principal component analysis (PCA). Furthermore for spatial ICA of fMRI time series data, the number of components can be up to the number of scans. Therefore, some form of data dimensionality reduction step is commonly employed either by prespecifying or calculating (using PCA [McKeown et al.,1998]) or using a model of the noise [Beckmann and Smith,2004] and the number of sources are identified. Furthermore, spatiotemporal characterization of BOLD signals on one hand and fMRI artifacts on the other combined with a pattern recognition technique has also been used to automatically classify components [DeMartino et al.,2007; Rodionov et al.,2007].
Applications of ICA in epilepsy
Morocz et al. [2003] and Donaire et al. [2009b] applied ICA on ictal fMRI time‐series data, and visually compared the activity maps with the GLM based statistical maps of BOLD changes. LeVan et al. [2010b] have used an iterative fixed‐point method [Hyvarinen and Oja,2000] to compute independent components (IC), and repeated the decomposition 20 times to identify stable and reproducible sources. They separated ictal components from physiological components, artifacts, residual motion, and cerebral BOLD activity by applying GLM based modeling assumptions whereby: HRF convolved with seizures was fitted to IC time courses in an auto‐regressive model and activation maps for ictal components were generated. For multiple seizures in the same patient, single HRF shape was calculated presuming it remains the same for each seizure [LeVan et al.,2010b]. It is also argued that although ICA can reveal BOLD changes and noise separately [McKeown and Sejnowski,1998; McKeown et al.,1998] it cannot specifically separate the different types of structured noise [LeVan et al.,2010b]. Thornton and colleagues applied cortex based spatial ICA with automated component classification to identify seizure‐related ICs spatially concordant with the SOZ [Thornton et al.,2010b].
What are the respective roles of the GLM or ICA‐based analyses?
The GLM embodies an approach to fMRI data analysis based on fitting a postulated model of the fMRI time series to the data. It therefore relies entirely on the ability of the investigator to devise a suitable model to allow the identification of the brain regions for which the degree of fit is sufficiently high (in relation to the level of unexplained variance and given the number of voxels at which the degree of fitness is tested). In other words, this approach can only be used to answer questions of the type: in which parts of the brain does the fMRI signal match a prespecified pattern? Assuming the availability of suitable models for each effect, this approach can be used to account for multiple effects simultaneously such as the BOLD changes related to seizure and interictal activity on one hand [Chassagnon et al.,2009; Salek‐Haddadi et al.,2009; Thornton et al.,2010b; Tyvaert et al.,2008] and various sources of physiological noise through the inclusion of pulse [Liston et al.,2006], respiration [van Houdt et al.,2010], and motion effects [Salek‐Haddadi et al.,2009]. Therefore, this requires the proper identification of events of interest, the mathematical representation of these events in the GLM framework and a choice of hemodynamic kernel as discussed above. Given that the aim of such studies is to reveal BOLD changes specifically related to seizures in any part of the brain and that this depends on the separation between the ictal and interictal states, it is crucial to realize that the GLM approach effectively attempts to explain all fMRI signal variations across the entire brain based on EEG (and possibly video), which is known to have limited sensitivity.
In comparison, ICA of fMRI by not relying on a postulated specific model of the signal time‐course, can be seen as a way of freeing the analysis from the bias of scalp EEG. However, this lack of prior model shifts the problem to the interpretation of the often very numerous components. Nonetheless, ICA of fMRI can reveal BOLD patterns that can be associated with seizures through post hoc comparison with EEG [Thornton et al.,2010b]. We envisage that simultaneous intracranial EEG‐fMRI will provide important validation data [Vulliemoz et al.,2011].
Validation of the fMRI findings
Given that the aim of fMRI studies of ictal activity is localization, validation requires two elements: a gold standard (intracranial EEG is used as gold standard in most epilepsy surgery programmes) and a method to compare the fMRI maps with the gold standard. Notwithstanding the precise nature of the gold standard, the latter usually requires the application of coregistration of the fMRI maps and gold standard onto a structural MR image of the subject's brain. Because of the complex nature of the fMRI‐maps, summarizing the level of spatial agreement can be challenging and various criteria (Table II) have been applied which can be grouped into the following broad categories:
-
1
Localization of the most statistically significant BOLD change cluster, and also taking into account the localization of other BOLD change clusters [Salek‐Haddadi et al.,2006; Thornton et al.,2010b].
-
2
Localization of the cluster corresponding to the earliest BOLD increase, with a cut‐off threshold for statistical significance and spatial extent [Donaire et al.,2009a].
-
3
Localization of the most statistically significant BOLD change cluster with a cut‐off threshold for spatial extent (greatest number of voxels) [Di Bonaventura et al.,2006].
-
4
Localization of the most statistically significant BOLD change cluster [Tyvaert et al.,2008].
-
5
Localization of all clusters of BOLD change above predefined statistical and spatial threshold [Gotman et al.,2005].
-
6
Localization of the most statistically significant BOLD cluster, and also the most statistically significant cluster in the individual lobes [Hamandi et al.,2006].
Table II.
Sensitivity, Criteria for Concordance, and Degree of Concordance from Previous Publications on Seizures Using fMRI
| Study | Subjects | Selection Criteria | Subjects with Seizures | Subjects with fMRI Results | Criteria for Concordance of BOLD Clusters in Focal Epilepsy / Criteria for Localization of BOLD Clusters in Generalized Epilepsy | Degree of Concordance |
|---|---|---|---|---|---|---|
| [Iannetti et al.,2002] | 3 | Refractory epilepsy triggered by fixation off sensitivity | 3 | 3 | Localization of all BOLD clusters according to coordinates system in MNI space at sublobar level | NA |
| [Aghakhani et al.,2004] | 25 | IGE patients with 2–4 Hz GSWDs | 15 | 15 | Coregistration and localization of all BOLD clusters with individual anatomical scans | NA |
| [Gotman et al.,2005] | 25 | IGE patients with 2–4 Hz GSWDs | 15 | 15 | Localization of all BOLD clusters according to coordinates system in MNI space at lobar level | NA |
| [Federico et al., 2005] | 3 | FE patients undergoing presurgical evaluation | 3 | 3 | Visual comparison of the statistically most significant BOLD cluster with; icEEG based (cases:1/3) or electroclinical, MRI and ictal SPECT based (cases:2/3) SOZ | 1/3 |
| [Di Bonaventura et al.,2006] | 43 | FE/ IGE patients with well‐defined epileptic syndrome, easily recognizable EEG activity and without extensive motion during seizures | 13 | 9 | Visual comparison of the location of all BOLD clusters (thresholded for amplitude (P < 0.05) and extent (350 mm3)) with electroclinical and MRI based SOZ. All clusters were labelled using Talairach atlas according to coordinates system in MNI space at lobar | 8/9 |
| [Hamandi et al.,2006] | 46 | IGE /SGE patients with frequent GSWDs | 33 | 22 | Localization of all BOLD clusters according to coordinates system in MNI space at lobar level | NA |
| [Salek‐Haddadi 2008] | 9 | Reading epilepsy patients | 6 | 6 | Visual identification and localization of the statistically most significant and other BOLD clusters for individual patients | |
| [Tyvaert et al.,2008] | 8 | From the EEG‐fMRI data‐base, patients with MCD and interictal and ictal Events | 8 | 8 | Visual comparison of the location of the most statistically significant BOLD clusters with; icEEG (Cases:2/8), or electroclinical and lesions on MRI based (Cases:6/8) SOZ | 8/8 |
| [Donaire et al., 2009] | 10 | From the EEG‐fMRI data‐base, patients with at least one clinical seizure during fMRI, with Ictal SPECT and with icEEG Evaluation | 10 | 5 | Coregistration and comparison of the earliest BOLD cluster (thresholded for extent (minimum five contagious voxels) and statistical significance (t > 3)) with; icEEG (cases:2/5) or electroclinical, MRI and Ictal SPECT based (cases:3/5) SOZ | 4/5 |
| [Tyvaert et al.,2009] | 17 | From the EEG‐fMRI database, patients; (a) with spontaneous seizure during fMRI | 10 | 10 | Visual comparison of the location of the earliest BOLD cluster (thresholded for extent and statistical significance) with; icEEG (cases: 3/10) or electroclinical, lesion on MRI based (cases: 7/10) SOZ | 9/10 |
| [LeVan et al., 2009] | 15 | From the EEG‐fMRI database, patients with seizure during EEG‐fMRI | 15 | 14 | Visual comparison of the location of the statistically most significant BOLD cluster (thresholded for extent (minimum five contagious voxels) and statistical significance (t > 3)) or ICA based clusters correlated with GLM, with; icEEG (cases: 4/15) or electroclinical and MRI based (cases: 11/15) SOZ | 13/15 |
| [Thornton et al., 2010] | 83 | Refractory focal epilepsy patients undergoing presurgical evaluation | 9 | 7 | Coregistration and comparison of all the statistically significant BOLD clusters with icEEG (cases: 8/9) based SOZ, and defined as, concordant; when all significant BOLD clusters were concordant with the SOZ and the area of maximal signal change was in the same gyrus and within 2 cm of the icEEG electrode, or concordant plus when the statistically most significant BOLD cluster was concordant but additional discordant clusters were also present | 4/9 |
| [Liu et al.,2008] | 4 | Eye lid myoclonia associated with absences patients | 4 | 4 | Visual identification and localization of all BOLD Clusters thresholded for extent (minimum five contagious voxels) and statistical significance (t > 3) for individual patients at lobar and sublobar level | NA |
| [Moeller et al., 2008] | 10 | Newly diagnosed CAE patients | 6 | 6 | Visual identification and localization of all BOLD clusters, coregistered with individual MRI (MNI space), thresholded for extent (minimum five contagious voxels) and statistical significance (t > 4.7) | NA |
| [Li et al.,2009] | 15 | CAE patients with frequent GSWDs | 6 | 6 | Visual identification and localization of all BOLD clusters thresholded for extent (minimum five contagious voxels) and statistical significance (t > 3) for individual patients at lobar and sublobar level | NA |
| [Moeller et al., 2009] | 16/14 | IGE patients/healthy subjects with PPR | 6 | 6 | Visual identification and localization of all BOLD clusters, coregistered with individual MRI (MNI space), thresholded for extent (minimum five contagious voxels) and statistical significance (t > 4.7) | NA |
| [Bai et al.,2010] | 42 | CAE patients with 3–4 Hz GSWDs | 9 | 9 | Visual identification and localization of all BOLD clusters, coregistered with individual MRI (MNI space), thresholded for statistical significance (P < 0.05, FDR) | NA |
| [Berman et al.,2010] | 37 | CAE patients with 3–4 Hz GSWDs, without additional seizure types or structural brain abnormality or neurologic disorders | 9 | 9 | Visual identification and localization of all BOLD clusters, coregistered with individual MRI (MNI space), thresholded for extent (minimum three contagious voxels) and statistical significance (P < 0.05, FDR) | NA |
| [Moeller et al., 2010] | 14 | CAE patients with 3 Hz GSWDs (3–30 s, inducible by hyperventilation), transient impairment of consciousness | 9 | 9 | Visual identification and localization of all BOLD clusters, coregistered with individual MRI (MNI space), thresholded for extent and statistical significance (F > 5.7) | NA |
| [Carney et al.,2010] | 11 | Children with absences as exclusive seizure type, not on medications, with 3–3.5 Hz GSWDs inducible with hyperventilation, with normal early development and normal structural imaging | 11 | 11 | Visual identification and localization of all BOLD clusters, coregistered with individual MRI (MNI space), thresholded for statistical significance (P < 0.001) | NA |
SOZ = seizure onset zone, BOLD = blood oxygen level dependent, NA = not applicable, IGE = idiopathic generalized epilepsy, GSWDs = generalized spike wave discharges, FE = focal epilepsy, MNI = Montreal Neuroimaging Institute, SGE = secondary generalized epilepsy, MCD = malformations of cortical development, icEEG = intracranial EEG, CAE = childhood absence epilepsy, PPR = photoparoxysmal response, FDR = false discovery rate.
In some instances concordance relative to the gold standard is defined (Table II) in terms of Cartesian [Carney et al.,2010; Hamandi et al.,2006; Iannetti et al.,2002; Salek‐Haddadi et al.,2003] or cortically informed [Thornton et al.,2010b] distance, while in others it is defined at the lobar level [Salek‐Haddadi et al.,2006; Salek‐Haddadi et al.,2009; Tyvaert et al.,2009].
CLINICAL APPLICATIONS
EEG‐fMRI has been shown to provide new and unique information on the brain networks involved in relation to epileptic seizures. Evidence relevant to assessing the potential clinical value of EEG‐fMRI applied to ictal activity, either in relation to localization of the SOZ or syndrome classification, is very limited. To the best of our knowledge, no systematic comparison of the findings of EEG‐fMRI with ictal SPECT or PET has been performed to date. Validation in individual cases by comparison with icEEG and or ictal SPECT or PET, is generally encouraging while keeping in mind the limitations of these techniques and of our general understanding of seizure initiation and propagation. No formal assessment of sensitivity and specificity has been done to date, possibly reflecting both the technique's novelty and an early realization of the difficulties in modeling the relationship between EEG and semiology on one hand and fMRI on the other and interpreting the resulting maps. Therefore, we are limited to relatively anecdotal observations. In the published literature the sensitivity in terms of patients who will have a seizure during EEG‐fMRI acquisition varies from 10% to 100%, and the sensitivity in terms of patients revealing BOLD changes out of patients with seizures during EEG‐fMRI acquisition varies from 66% to 100%. This wide‐range of sensitivity largely depends on the variable patient selection criteria used in different studies (Table II). Similarly, the ability of EEG‐fMRI studies to localize SOZ in focal epilepsy is also quite variable depending upon the various concordance criteria applied (Table II).
Localization of Seizure Onset in Focal Epilepsy
Early case‐reports with fMRI alone showed MR signal build up and recovery to baseline in the presumed SOZ (PSOZ) for clinical/subclinical seizures [Detre et al.,1995; Jackson et al.,1994; Kubota et al.,2000]. In another case‐report, Krings et al. observed, temporally and spatially distinct, early MR signal change in perilesional cortex before the clinical seizure and an increase in MR signal intensity in eloquent cortex during clinical seizure (symptomatogenic zone) [Krings et al.,2000].
In two case‐reports [Kobayashi et al.,2006b; Salek‐Haddadi et al.,2002] and a case‐series [Donaire et al.,2009a], using EEG‐fMRI and GLM‐based data analysis approach, time‐locked BOLD increase within the PSOZ was revealed during seizures. The amplitude of BOLD signal increase was found to be generally higher in PSOZ in comparison with other areas. In a series of malformations of cortical development [Tyvaert et al.,2008], using GLM based data analysis approach, the spatial localization of seizure‐related hemodynamic response was shown to be different in various pathological lesions. In nodular heterotopia (four of four cases) the maximum BOLD increase was observed in the overlying cortex of heterotopia whereas in focal cortical dysplasia (FCD) (two of two cases) and band heterotopia (two of two cases) maximum BOLD increase was found within the structural lesion, however, other smaller and less significant clusters of BOLD signal change were also observed remotely [Tyvaert et al.,2008]. Thornton et al. [2010b] investigated seizures by dividing into three phases (see Event representation section for details). Using GLM based data analysis approach they showed significant BOLD changes in seven of nine cases, and the concordance of BOLD localization with the SOZ (defined using icEEG) was found to be greatest for the early ictal phase and the combined phases (see Fig. 1). Moreover, they argued that as the ictal activity spreads on EEG, the BOLD changes should also evolve temporally. Therefore, BOLD changes during clinical ictal and late ictal phases discordant with SOZ may represent propagated activity. Using ICA on the data from the same group of patients, it was demonstrated that ICs were spatially concordant with SOZ in all cases (confirmed in eight of nine cases with icEEG), making an argument that ICA is helpful to reveal ictal BOLD changes when GLM based approach fails [Thornton et al.,2010b].
Figure 1.
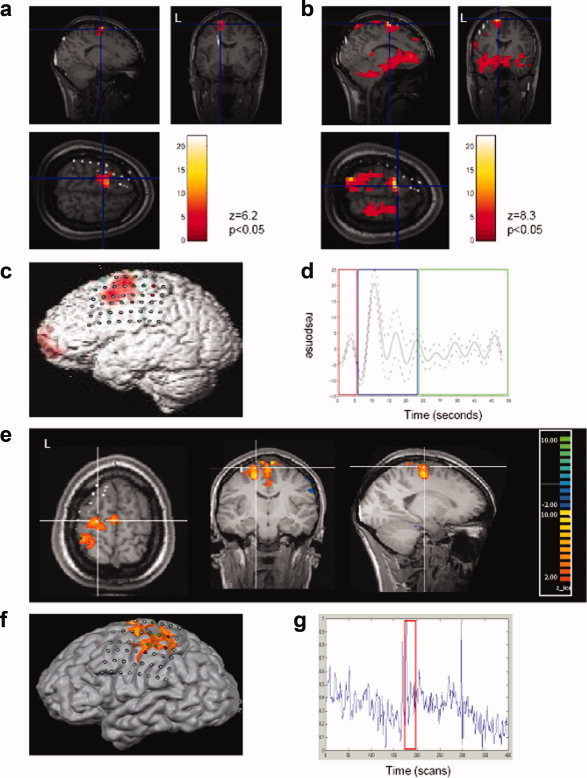
Hemodynamic changes revealed by EEG‐fMRI for a single seizure in a patient with FCD in left superior frontal gyrus. (a) BOLD clusters overlaid on T1‐weighted MRI fused with a CT‐scan containing subdural grids, (canonical GLM analysis). (b) BOLD clusters overlaid on T1‐weighted MRI fused with a CT‐scan containing subdural grids, (fourier basis set GLM analysis). (c) BOLD clusters overlaid on a surface rendering of T1‐weighted MRI with subdural grids, (canonical GLM based approach). (d) Time‐course of the BOLD signal change over the course of the seizure. (e) ICA clusters overlaid on T1‐weighted MRI fused with a CT containing subdural grids. (f) ICA component illustrated in (e) overlaid on a surface rendering of the T1‐weighted MRI. (g) Time‐course of the component illustrated in e) over the whole recording with the seizure indicated by the red box [Thornton et al.,2010b]. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
It has also been shown that the spatial extent of BOLD signal change was larger for the combined effect of multiple seizures than single seizures [Kobayashi et al.,2006b], and the BOLD changes for seizures were widespread in comparison to the BOLD changes for IEDs [Kobayashi et al.,2006b; Tyvaert et al.,2008]. The significance of BOLD signal decrease is not clearly known, however, additional areas of BOLD signal decrease during seizures were observed; within perilesional cortex and opposite hemisphere of brain [Kobayashi et al.,2006b]; within PSOZ [Donaire et al.,2009a]; within surrounding areas of BOLD signal increase and remotely from the PSOZ/SOZ [Thornton et al.,2010b; Tyvaert et al.,2008]. These remote areas were often part of the so‐called default mode network [Raichle et al.,2001], in common with many studies of IEDs [Laufs et al.,2006; Salek‐Haddadi et al.,2006].
Preictal Changes, Seizure Evolution, and Propagation in Focal Epilepsy
Federico et al. focused specifically on preictal BOLD changes in three cases and revealed BOLD increase (two of three cases) and decrease (one of three cases) starting before the clinical seizure, however, preictal BOLD increase (∼4 min) was concordant with SOZ in only one case [Federico et al.,2005a]. Using series of sequential fixed‐width block model for seizure representation in the GLM framework, Donaire et al. [2009a] described widespread BOLD decrease 10 to 26 s before the first BOLD increase which in itself preceded the first clinical (6–52 s) or electrographic change (8–12 s) in 5 of 10 cases. In contrast, using a similar model to Donaire et al.'s, Tyvaert et al. [2009] found that on average BOLD changes started 5.2 ± 2.6 s after EEG‐onset of seizure and returned to baseline 28.8 ± 12.9 s after EEG onset in 10/17 cases, roughly in line with what would be expected in response to a physiological block stimulus. The initial area of BOLD increase was found to be concordant with the SOZ/PSOZ (Table II) [Donaire et al.,2009a; Tyvaert et al.,2009]. Moreover, by relaxing the statistical threshold additional BOLD change clusters concordant with SOZ/PSOZ were noticed in 2 of 17 cases before ictal onset on EEG. [Tyvaert et al.,2009]. They argued that other areas of BOLD signal increase demonstrated propagation of seizures as it spreads in time. Though these observations were made in small studies, this may point towards EEG‐fMRI's ability to identify preictal hemodynamic changes even when epileptic discharges are not yet visible on scalp EEG.
Seizure‐Related Networks in Idiopathic Generalized Epilepsy
Salek‐Haddadi et al. [2003], using GLM based data analysis approach, described hemodynamic involvement of deeper structures of the brain during absence seizures, identifying two patterns of simultaneous BOLD change; BOLD increase (+3% from baseline) in thalamic nuclei and symmetrical, widespread decreases in cerebrum, maximum in the frontal lobes (−8% from baseline) in relation to four long (mean duration: 30 s) absence seizures. Subsequent group studies of IGE patients [Aghakhani et al.,2004; Gotman et al.,2005; Hamandi et al.,2006; Laufs et al.,2006] also revealed common patterns of BOLD decrease in the frontal and parietal cortices, posterior cingulate, caudate and precuneus, and predominant BOLD increase in the thalamus, mesial frontal region, and insulae (see Fig. 2).
Figure 2.
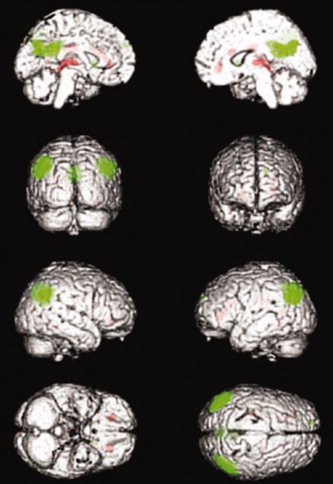
BOLD clusters correlated with 3 Hz GSWDs in IGE patients revealed by group analysis. Red cluster reveal activations and green clusters reveal deactivations [Hamandi et al.,2006]. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The areas involved in GSWD‐related cortical BOLD decrease resemble the default mode network [Raichle et al.,2001]. The BOLD signal decrease in these areas, in relation to GSWDs, can be argued to represent the suspension of physiologic conscious rest or an fMRI signature of the negative clinical phenomenology of absences during which cognitive processes are impaired [Laufs et al.,2006]. The studies of absence seizures in animal models [Danober et al.,1998] and in humans using transcranial Doppler, H2(15)O and PET [Diehl et al.,1998; Prevett et al.,1995] and invasive recordings have also pointed towards the involvement of corticothalamic network in the generation of absence seizures [Avoli and Gloor,1981,1982a,b; Avoli and Kostopoulos,1982; Avoli et al.,2001]. However, EEG‐fMRI noninvasively provides the evidence for the association of the cortico‐subcortical/striatocorticothalamic network with GSWD.
The effect of duration of GSWDs on the spatial distribution of BOLD changes is complex. Aghakhani et al. showed that the BOLD changes for shorter (<3 s) and longer (>3 s) bursts of GSWDs were similar in three of four cases and one case did not reveal any BOLD change for shorter discharges [2004]. On the contrary, it has also been discussed that BOLD changes were of greater magnitude [Carney et al.,2010] or tended to be more frequent in the thalamus [Hamandi et al.,2006; Li et al.,2009] when GSWDs were of longer duration and higher in number. This may be due to improved signal to noise ratio or may reflect the role of thalamus in maintaining the GSWD [Avoli et al.,2001] for longer duration discharges.
Studies using simultaneous EEG‐fMRI and perfusion mapping techniques (arterial spin labeling) have shown a positive correlation between BOLD changes and cerebral blood flow irrespective of the sign of BOLD change. This association is stronger during GSWDs than during background activity suggesting preserved (i.e. within normal limits) neurovascular coupling [Carmichael et al.,2008; Hamandi et al.,2008].
Timing of the BOLD changes relative to the EEG onset of GSWD is also under‐debate. Using conventional GLM based data analysis approach: GSWDs modeled as box‐car and convolved with a standard HRF [Glover et al.,2000], it was shown that BOLD increase in the thalamus, occipital cortex, cerebellum, temporal lobes, insula, caudate, and pons; and decrease in the parietal cortex, precuneus, cingulate gyrus, basal ganglia, and pons started after EEG onset, and BOLD increases peaked earlier than BOLD decreases [Aghakhani et al.,2004; Bai et al.,2010; Berman et al.,2010; Carney et al.,2010; Hamandi et al.,2006; Moeller et al.,2008b]. However, it is argued that this analysis approach may not be perfect physiologically; assuming electrophysiological and hemodynamic changes during seizures start and end abruptly. Therefore, by modeling the HRF before the EEG onset of GSWD in the GLM framework [Moeller et al.,2008a], and by investigating the mean time course of GSWD related hemodynamic change in specific areas (selected on the basis of location of significant BOLD cluster) [Bai et al.,2010; Carney et al.,2010], it was shown that temporal pattern of BOLD changes was different from the conventionally reported pattern. The BOLD increase in the thalamus, [Moeller et al.,2008a], parietal cortex and precuneus [Carney et al.,2010] preceded the GSWD onset on EEG. Bai and colleagues also suggested that the BOLD increase in orbital frontal cortex, frontal polar, cingulate, parietal cortex, precuneus, and occipital cortex preceded the GSWD onset on EEG followed by BOLD decrease in the same areas lasting 20 s after GSWD [Bai et al.,2010]. Animal models of absence seizures investigated with optical imaging also showed changes in concentration of oxy/deoxy‐hemoglobin before the onset of GSWD [Roche‐Labarbe et al.,2010], illustrating all the more need to correlate the findings of animal models with human studies. The inconsistency of temporal evolution of GSWD‐related BOLD changes in IGE may result from variable modeling strategies applied [Bai et al.,2010; Moeller et al.,2008a], different types of epileptic discharges (polyspikes and waves and GSWDs) and age‐related biological differences between adults [Hamandi et al.,2006] and children [Moeller et al.,2008a]. The pre‐GSWD BOLD changes may reflect early electrophysiological changes which are not evident on scalp EEG, however, this needs to be investigated further.
Moeller et al. investigated the patient specific BOLD changes for absence seizure, using series of sequential fixed width blocks to represent seizures in the GLM framework, and found that BOLD changes were consistent for several absences within one patient but varied across patients [Moeller et al.,2010a]. Moreover, the BOLD changes in various anatomical areas; thalamus, cortex and caudate, were dynamic (evolving at different time points) throughout the duration of absence seizure rather than being static [Moeller et al.,2010a]. They also showed that the BOLD increase in cortex (six of nine cases) and decrease in caudate (five of nine cases) preceded the BOLD increase in the thalamus (nine of nine cases) [Moeller et al.,2010a]. These cortical, absence seizure‐related, focal BOLD increases [Bai et al.,2010; Carney et al.,2010; Moeller et al.,2010a] are consistent with the cortical focus theory of initiation of absences [Meeren et al.,2002; Polack et al.,2007; Vaudano et al.,2009; Westmijse et al.,2009].
Seizure‐Related Networks in Other Forms of Epilepsy
Seizure‐related networks explored in fixation‐off sensitivity using EEG‐fMRI have revealed BOLD increase in parieto‐occipital regions correlated with 2.5 to 3 Hz activity triggered by eye‐closure [Di Bonaventura et al.,2005; Iannetti et al.,2002; Krakow et al.,2000]. In two case reports on musicogenic epilepsy, it was shown that BOLD networks involved PSOZ: right gyrus rectus and left anterior temporal lobe [Morocz et al.,2003], and right dorsal frontal cortex and right temporal lobe [Marrosu et al.,2009] during presentation of epileptogenic music. Morocz et al. argued that the BOLD changes in the left anterior temporal lobe represented the seizure focus (confirmed by ictal‐EEG and ictal‐SPECT) and that the BOLD changes in the ventral frontal lobe and right gyrus rectus were related to presentation and listening of epileptogenic music, in line with previous PET studies [Morocz et al.,2003].
For pseudo‐absences (three cases) in frontal lobe epilepsy, focal BOLD increase was revealed in the mesial frontal cortex correlated with GSWDs which was not seen in typical absences (six cases) in the same study [Di Bonaventura et al.,2006]. However, more recent studies on absences in children have also shown focal BOLD increases in the cortex (Seizure‐Related Networks in Idiopathic Generalized Epilepsy), therefore, it needs to be further investigated as to whether these different hemodynamic changes between typical and pseudo‐absences can be applied as a differentiating point. Similarly, comparison of the GSWD‐related BOLD patterns in patients with primary and secondarily generalized epilepsies revealed many similarities in the distribution but greater inter‐individual variability in cases of secondary generalization, and did not lead to reliable classification of individual cases [Hamandi et al.,2006].
In patients with reading epilepsy, BOLD networks involving the motor and premotor cortex, striatum, mesial temporal lobe, and thalamus were revealed in correlation with orofacial reflex myoclonus and EEG discharges triggered by reading. These changes were also differentiated from the BOLD activations observed during language and facial motor tasks [Salek‐Haddadi et al.,2009]. EEG‐fMRI has also helped to demonstrate the coexistence of separate epileptic networks for GSWDs and for focal facial twitching in a single patient, and the BOLD changes for the latter helped to ascertain that the FCD previously seen on MRI was epileptogenic [Chassagnon et al.,2009].
In children with photoparoxysmal response (PPR) (six cases; 4 = IGE/CAE, 2 = PPR only) [Moeller et al.,2009a], focal BOLD increases were observed in premotor and parietal cortex (adjacent to intraparietal sulcus) 3 s before PPR (corresponding to gamma activity on EEG), followed by BOLD decrease in the same areas during PPR. It is possible that the early BOLD changes in parietal cortex indicate its possible role in the generation of PPR, or may reflect it being part of the frontoparietal visual networks responsible for saccades and visual attention, which needs to be elucidated further [Moeller et al.,2009a]. Additionally, most of the subjects (five cases) did not show any BOLD changes in the thalamus [Moeller et al.,2009a], and it can be argued that the thalamus may play a less important role in the generation of PPR in comparison to typical GSWD in IGE for which an intact thalamocortical network is deemed necessary [Avoli et al.,2001].
Liu and colleagues, investigating children with eyelid myoclonia associated with absences (EMA) showed BOLD increase in thalamus, temporal lobes, midline structures and BOLD decrease in parietal and frontal cortex. This epileptic network in EMA is similar to the epileptic network identified in IGE, which can be explained by the presence of GSWDs both in EMA and IGE [Liu et al.,2008].
Hemodynamic Mapping of Cognitive Networks During GSWDs
The hemodynamic changes for GSWDs associated with cognitive impairment have been investigated only recently using various attention tasks during EEG‐fMRI [Bai et al.,2010; Berman et al.,2010; Moeller et al.,2010b]. BOLD increases in thalamus, frontal cortex, primary visual, auditory, somatosensory, and motor cortex, and BOLD decreases in the lateral and medial parietal cortex, cingulate gyrus, and basal ganglia have been revealed for GSWDs during which behavioral performance was poor (four of nine cases, 100% error rate). In comparison, for GSWDs during which behavioral performance was good (two of nine cases, 0% error rate), no significant GSWD‐related BOLD changes were observed [Berman et al.,2010]. These findings provide some evidence that activity in structures thought to be important during attention tasks [Riccio et al.,2002] can be affected during GSWDs. The impairment of consciousness during GSWDs and the associated BOLD changes is a complex question which needs to be explored systematically in the future.
The Role of EEG‐fMRI on Seizures in Diagnosis and Management of Epilepsy
In single case reports, EEG‐fMRI has also demonstrated the ability to help in diagnosis and management of epilepsy. Spatial location of BOLD changes associated with myoclonic jerks of right foot helped to localize an FCD from left frontal lobe which was confirmed with intraoperative cortical mapping as SOZ and resected resulting in complete abolition of seizures [Archer et al.,2006]. In another patient with refractory epilepsy, who had undergone epilepsy surgery twice without any appreciable success, fMRI showed widespread BOLD changes involving the cortex, caudate nucleus, thalamus and other areas during the seizure (a pattern known to be present in generalized epilepsy). Consequently, the antiepileptic drugs were changed to control generalized epilepsy and seizure frequency reduced from 10/day to 1/month [Auer et al.,2008].
PERSPECTIVES AND CONCLUSIONS
What is the future role of EEG‐fMRI? Given that seizures happen randomly with likely head or body motion, it would seem that the application of ictal EEG‐fMRI will be limited to a subset of cases with very carefully defined selection criteria. However, we believe that such studies will provide crucial new insights into the spontaneous transition from the interictal to ictal state in humans, complementing invasive EEG studies with their limited spatial coverage.
The most suitable candidates for such investigations are patients with daily seizures and with seizure types: absence seizures, myoclonic jerks localized to limbs, simple motor/sensory seizures, subclinical/electrographic seizures and complex partial seizures without large head movements (Table II). To identify seizures, synchronized video‐EEG inside the MRI scanner along with simultaneous testing for awareness by performing cognitive tasks would provide the most valuable data.
Methodologically robust and consistent strategy which can separate seizure‐related BOLD changes from artifactual changes need to be applied to analyze seizure‐related fMRI data. Considering the clinical importance of EEG to identify seizures, we suggest the use of EEG‐based GLM, using a system of event representation of seizures as dynamic entities [Thornton et al.,2010b], as a first line approach. Further exploration could proceed using a more flexible model, such as Fourier expansion [Thornton et al.,2010b] or series of short, uniform blocks spanning the seizure or beyond [Donaire et al.,2009a]. The value of purely data‐driven approaches such as ICA for the identification of ictal BOLD patterns will probably require a much better characterization of ictal hemodynamic patterns.
EEG‐fMRI studies in focal epilepsy have demonstrated the technique's ability to localize the SOZ confirmed by icEEG [Thornton et al.,2010b]. The future potential of EEG‐fMRI to provide a noninvasive way of better planning the placement of subdural grids and intracranial electrodes to outline SOZ has also been proposed [Thornton et al.,2010b]. However, the clinical value in the context of technique's sensitivity and specificity remains to be assessed prospectively which can be very complex considering the way multiple test are done [Knowlton,2006]. EEG‐fMRI studies on GSWDs have progressed more rapidly, possibly due to the presumed more uniform underlying phenomenology. The identification of preictal BOLD changes and BOLD networks for various seizure types using EEG‐fMRI has improved our knowledge of the underlying epileptic networks such as cortico‐thalamic network in IGE. However, the effects of seizures on the networks involved in cognition and maintaining awareness remains to be explored properly.
Future studies exploiting fMRIs capability to map activity patterns over the entire brain at the second time scale may improve our understanding of seizure initiation and propagation and help us answer clinically relevant questions.
Footnotes
In this work, the use of the conventional “response function” terminology (as in HRF, which is derived from cognitive experiments with clear causality) does not imply a causal relationship between epileptic events and putative BOLD signal change. In the context of resting‐state EEG‐fMRI experiments, the use of features identified on scalp EEG does not guarantee that any fMRI signal change associated with these events will occur after the event. This is due to the possibility of a degree of decoupling due to the limited sensitivity of scalp EEG: for example, one could imagine physiological changes (associated with hemodynamic changes) taking place in the brain before they appear on the scalp and in a time‐locked fashion. In the same spirit, we use the “epileptic event related BOLD signal change” terminology in preference to “BOLD response.”
REFERENCES
- Aghakhani Y, Bagshaw AP, Benar CG, Hawco C, Andermann F, Dubeau F, Gotman J ( 2004): fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain 127: 1127–1144. [DOI] [PubMed] [Google Scholar]
- Allen PJ, Josephs O, Turner R ( 2000): A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage 12: 230–239. [DOI] [PubMed] [Google Scholar]
- Allen PJ, Polizzi G, Krakow K, Fish DR, Lemieux L ( 1998): Identification of EEG events in the MR scanner: The problem of pulse artifact and a method for its subtraction. Neuroimage 8: 229–239. [DOI] [PubMed] [Google Scholar]
- Archer JS, Briellman RS, Abbott DF, Syngeniotis A, Wellard RM, Jackson GD ( 2003): Benign epilepsy with centro‐temporal spikes: Spike triggered fMRI shows somato‐sensory cortex activity. Epilepsia 44: 200–204. [DOI] [PubMed] [Google Scholar]
- Archer JS, Waites AB, Abbott DF, Federico P, Jackson GD ( 2006): Event‐related fMRI of myoclonic jerks arising from dysplastic cortex. Epilepsia 47: 1487–1492. [DOI] [PubMed] [Google Scholar]
- Auer T, Veto K, Doczi T, Komoly S, Juhos V, Janszky J, Schwarcz A ( 2008): Identifying seizure‐onset zone and visualizing seizure spread by fMRI: A case report. Epileptic Disord 10: 93–100. [DOI] [PubMed] [Google Scholar]
- Avoli M, Gloor P ( 1981): The effects of transient functional depression of the thalamus on spindles and on bilateral synchronous epileptic discharges of feline generalized penicillin epilepsy. Epilepsia 22: 443–452. [DOI] [PubMed] [Google Scholar]
- Avoli M, Gloor P ( 1982a): Interaction of cortex and thalamus in spike and wave discharges of feline generalized penicillin epilepsy. Exp Neurol 76: 196–217. [DOI] [PubMed] [Google Scholar]
- Avoli M, Gloor P ( 1982b): Role of the thalamus in generalized penicillin epilepsy: Observations on decorticated cats. Exp Neurol 77: 386–402. [DOI] [PubMed] [Google Scholar]
- Avoli M, Kostopoulos G ( 1982): Participation of corticothalamic cells in penicillin‐induced generalized spike and wave discharges. Brain Res 247: 159–163. [DOI] [PubMed] [Google Scholar]
- Avoli M, Rogawski MA, Avanzini G ( 2001): Generalized epileptic disorders: An update. Epilepsia 42: 445–457. [DOI] [PubMed] [Google Scholar]
- Bagshaw AP, Aghakhani Y, Benar CG, Kobayashi E, Hawco C, Dubeau F, Pike GB, Gotman J ( 2004): EEG‐fMRI of focal epileptic spikes: analysis with multiple haemodynamic functions and comparison with gadolinium‐enhanced MR angiograms. Hum Brain Mapp 22: 179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw AP, Hawco C, Benar CG, Kobayashi E, Aghakhani Y, Dubeau F, Pike GB, Gotman J ( 2005): Analysis of the EEG‐fMRI response to prolonged bursts of interictal epileptiform activity. Neuroimage 24: 1099–1112. [DOI] [PubMed] [Google Scholar]
- Bai X, Vestal M, Berman R, Negishi M, Spann M, Vega C, Desalvo M, Novotny EJ, Constable RT, Blumenfeld H ( 2010): Dynamic time course of typical childhood absence seizures: EEG, behavior, and functional magnetic resonance imaging. J Neurosci 30: 5884–5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM ( 2004): Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging 23: 137–152. [DOI] [PubMed] [Google Scholar]
- Benar C, Aghakhani Y, Wang Y, Izenberg A, Al‐Asmi A, Dubeau F, Gotman J ( 2003): Quality of EEG in simultaneous EEG‐fMRI for epilepsy. Clin Neurophysiol 114: 569–580. [DOI] [PubMed] [Google Scholar]
- Benar CG, Gross DW, Wang Y, Petre V, Pike B, Dubeau F, Gotman J ( 2002): The BOLD response to interictal epileptiform discharges. Neuroimage 17: 1182–1192. [DOI] [PubMed] [Google Scholar]
- Berman R, Negishi M, Vestal M, Spann M, Chung MH, Bai X, Purcaro M, Motelow JE, Danielson N, Dix‐Cooper L, Enev M, Novotny EJ, Constable RT, Blumenfeld H ( 2010): Simultaneous EEG, fMRI, and behavior in typical childhood absence seizures. Epilepsia 51: 2011–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnie CD, Rowan AJ, Overweg J, Meinardi H, Wisman T, Kamp A, Lopes da SF ( 1981): Telemetric EEG and video monitoring in epilepsy. Neurology 31: 298–303. [DOI] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA ( 2006): Separating respiratory‐variation‐related fluctuations from neuronal‐activity‐related fluctuations in fMRI. Neuroimage 31: 1536–1548. [DOI] [PubMed] [Google Scholar]
- Bonmassar G, Purdon PL, Jaaskelainen IP, Chiappa K, Solo V, Brown EN, Belliveau JW ( 2002): Motion and ballistocardiogram artifact removal for interleaved recording of EEG and EPs during MRI. Neuroimage 16: 1127–1141. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ ( 2001): Spatial and temporal independent component analysis of functional MRI data containing a pair of task‐related waveforms. Hum Brain Mapp 13: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael DW, Hamandi K, Laufs H, Duncan JS, Thomas DL, Lemieux L ( 2008): An investigation of the relationship between BOLD and perfusion signal changes during epileptic generalised spike wave activity. Magn Reson Imaging 26: 870–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney PW, Masterton RA, Harvey AS, Scheffer IE, Berkovic SF, Jackson GD ( 2010): The core network in absence epilepsy. Differences in cortical and thalamic BOLD response. Neurology 75: 904–911. [DOI] [PubMed] [Google Scholar]
- Chassagnon S, Hawko CS, Bernasconi A, Gotman J, Dubeau F ( 2009): Coexistence of symptomatic focal and absence seizures: Video‐EEG and EEG‐fMRI evidence of overlapping but independent epileptogenic networks. Epilepsia 50: 1821–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary UJ, Kokkinos V, Carmichael DW, Rodionov R, Gasston D, Duncan JS, Lemieux L ( 2010): Implementation and evaluation of simultaneous video‐electroencephalography and functional magnetic resonance imaging. Magn Reson Imaging 28: 1192–1199. [DOI] [PubMed] [Google Scholar]
- Czisch M, Wehrle R, Kaufmann C, Wetter TC, Holsboer F, Pollmacher T, Auer DP ( 2004): Functional MRI during sleep: BOLD signal decreases and their electrophysiological correlates. Eur J Neurosci 20: 566–574. [DOI] [PubMed] [Google Scholar]
- Danober L, Deransart C, Depaulis A, Vergnes M, Marescaux C ( 1998): Pathophysiological mechanisms of genetic absence epilepsy in the rat. Prog Neurobiol 55: 27–57. [DOI] [PubMed] [Google Scholar]
- DeMartino F, Gentile F, Esposito F, Balsi M, Di SF, Goebel R, Formisano E ( 2007): Classification of fMRI independent components using IC‐fingerprints and support vector machine classifiers. Neuroimage 34: 177–194. [DOI] [PubMed] [Google Scholar]
- Detre JA, Alsop DC, Aguirre GK, Sperling MR ( 1996): Coupling of cortical and thalamic ictal activity in human partial epilepsy: Demonstration by functional magnetic resonance imaging. Epilepsia 37: 657–661. [DOI] [PubMed] [Google Scholar]
- Detre JA, Sirven JI, Alsop DC, O'Connor MJ, French JA ( 1995): Localization of subclinical ictal activity by functional magnetic resonance imaging: correlation with invasive monitoring. Ann Neurol 38: 618–624. [DOI] [PubMed] [Google Scholar]
- Di Bonaventura C, Vaudano AE, Carni M, Pantano P, Nucciarelli V, Garreffa G, Maraviglia B, Prencipe M, Bozzao L, Manfredi M, Giallonardo AT ( 2005): Long‐term reproducibility of fMRI activation in epilepsy patients with Fixation Off Sensitivity. Epilepsia 46: 1149–1151. [DOI] [PubMed] [Google Scholar]
- Di Bonaventura C, Vaudano AE, Carni M, Pantano P, Nucciarelli V, Garreffa G, Maraviglia B, Prencipe M, Bozzao L, Manfredi M, Giallonardo AT ( 2006): EEG/fMRI study of ictal and interictal epileptic activity: Methodological issues and future perspectives in clinical practice. Epilepsia 47( Suppl 5): 52–58. [DOI] [PubMed] [Google Scholar]
- Diehl B, Knecht S, Deppe M, Young C, Stodieck SR ( 1998): Cerebral hemodynamic response to generalized spike‐wave discharges. Epilepsia 39: 1284–1289. [DOI] [PubMed] [Google Scholar]
- Donaire A, Bargallo N, Falcon C, Maestro I, Carreno M, Setoain J, Rumia J, Fernandez S, Pintor L, Boget T ( 2009a): Identifying the structures involved in seizure generation using sequential analysis of ictal‐fMRI data. Neuroimage 47: 173–183. [DOI] [PubMed] [Google Scholar]
- Donaire A, Falcon C, Carreno M, Bargallo N, Rumia J, Setoain J, Maestro I, Boget T, Pintor L, Agudo R, Falip M, Fernandez S ( 2009b): Sequential analysis of fMRI images: A new approach to study human epileptic networks. Epilepsia 50: 2526–2537. [DOI] [PubMed] [Google Scholar]
- Ellingson ML, Liebenthal E, Spanaki MV, Prieto TE, Binder JR, Ropella KM ( 2004): Ballistocardiogram artifact reduction in the simultaneous acquisition of auditory ERPS and fMRI. Neuroimage 22: 1534–1542. [DOI] [PubMed] [Google Scholar]
- Federico P, Abbott DF, Briellmann RS, Harvey AS, Jackson GD ( 2005a): Functional MRI of the pre‐ictal state. Brain 128: 1811–1817. [DOI] [PubMed] [Google Scholar]
- Federico P, Archer JS, Abbott DF, Jackson GD ( 2005b): Cortical/subcortical BOLD changes associated with epileptic discharges: An EEG‐fMRI study at 3 T. Neurology 64: 1125–1130. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME ( 2009): The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101: 3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K.J. , 1996. Statistical parametric mapping and other analyses of functional imaging data In: Toga AW, Mazziotta JC, editors. Brain Mapping: The Methods. San Diego: Academic Press; pp 363–396. [Google Scholar]
- Friston KJ, Ashburner J, Frith C, Poline JB, Heather JD, Frackowiak RSJ ( 1995a): Spatial registration and normalization of images. Hum Brain Mapp 3: 165–189. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ ( 1999): How many subjects constitute a study? Neuroimage 10: 1–5. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Firth CD, Frackowiak RSJ ( 1995b): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Glover GH, Li TQ, Ress D ( 2000): Image‐based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 44: 162–167. [DOI] [PubMed] [Google Scholar]
- Goldman RI, Stern JM, Engel J Jr, Cohen MS ( 2000): Acquiring simultaneous EEG and functional MRI. Clin Neurophysiol 111: 1974–1980. [DOI] [PubMed] [Google Scholar]
- Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F ( 2005): Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci USA 102: 15236–15240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grouiller F, Vercueil L, Krainik A, Segebarth C, Kahane P, David O ( 2010): Characterization of the hemodynamic modes associated with interictal epileptic activity using a deformable model‐based analysis of combined EEG and functional MRI recordings. Hum Brain Mapp 31: 1157–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME ( 2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal JV, Myers R, Oatridge A, Schwieso JE, Young IR, Bydder GM ( 1994): Artifacts due to stimulus correlated motion in functional imaging of the brain. Magn Reson Med 31: 283–291. [DOI] [PubMed] [Google Scholar]
- Hamandi K, Laufs H, Noth U, Carmichael DW, Duncan JS, Lemieux L ( 2008): BOLD and perfusion changes during epileptic generalised spike wave activity. Neuroimage 39: 608–618. [DOI] [PubMed] [Google Scholar]
- Hamandi K, Salek‐Haddadi A, Laufs H, Liston A, Friston K, Fish DR, Duncan JS, Lemieux L ( 2006): EEG‐fMRI of idiopathic and secondarily generalized epilepsies. Neuroimage 31: 1700–1710. [DOI] [PubMed] [Google Scholar]
- Hawco CS, Bagshaw AP, Lu Y, Dubeau F, Gotman J ( 2007): BOLD changes occur prior to epileptic spikes seen on scalp EEG. Neuroimage 35: 1450–1458. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Jager L, Werhahn KJ, Jaschke M, Noachtar S, Reiser M ( 2000): Electroencephalography during functional echo‐planar imaging: Detection of epileptic spikes using post‐processing methods. Magn Reson Med 44: 791–798. [DOI] [PubMed] [Google Scholar]
- Hyvarinen A, Oja E ( 2000): Independent component analysis: Algorithms and applications. Neural Netw 13: 411–430. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Di BC, Pantano P, Giallonardo AT, Romanelli PL, Bozzao L, Manfredi M, Ricci GB ( 2002): fMRI/EEG in paroxysmal activity elicited by elimination of central vision and fixation. Neurology 58: 976–979. [DOI] [PubMed] [Google Scholar]
- Ives JR, Warach S, Schmitt F, Edelman RR, Schomer DL ( 1993): Monitoring the patient's EEG during echo planar MRI. Electroencephalogr Clin Neurophysiol 87: 417–420. [DOI] [PubMed] [Google Scholar]
- Jackson GD, Connelly A, Cross JH, Gordon I, Gadian DG ( 1994): Functional magnetic resonance imaging of focal seizures. Neurology 44: 850–856. [DOI] [PubMed] [Google Scholar]
- Jacobs J, LeVan P, Moeller F, Boor R, Stephani U, Gotman J, Siniatchkin M ( 2009): Hemodynamic changes preceding the interictal EEG spike in patients with focal epilepsy investigated using simultaneous EEG‐fMRI. Neuroimage 45: 1220–1231. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Rohr A, Moeller F, Boor R, Kobayashi E, LeVan MP, Stephani U, Gotman J, Siniatchkin M ( 2008): Evaluation of epileptogenic networks in children with tuberous sclerosis complex using EEG‐fMRI. Epilepsia 49: 816–825. [DOI] [PubMed] [Google Scholar]
- Knowlton RC ( 2006): The role of FDG‐PET, ictal SPECT, and MEG in the epilepsy surgery evaluation. Epilepsy Behav 8: 91–101. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Bagshaw AP, Grova C, Gotman J, Dubeau F ( 2006a): Grey matter heterotopia: What EEG‐fMRI can tell us about epileptogenicity of neuronal migration disorders. Brain 129: 366–374. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Hawco CS, Grova C, Dubeau F, Gotman J ( 2006b): Widespread and intense BOLD changes during brief focal electrographic seizures. Neurology 66: 1049–1055. [DOI] [PubMed] [Google Scholar]
- Krakow K, Baxendale SA, Maguire EA, Krishnamoorthy ES, Lemieux L, Scott CA, Smith SJ ( 2000): Fixation‐off sensitivity as a model of continuous epileptiform discharges: Electroencephalographic, neuropsychological and functional MRI findings. Epilepsy Res 42: 1–6. [DOI] [PubMed] [Google Scholar]
- Krings T, Topper R, Reinges MH, Foltys H, Spetzger U, Chiappa KH, Gilsbach JM, Thron A ( 2000): Hemodynamic changes in simple partial epilepsy: A functional MRI study. Neurology 54: 524–527. [DOI] [PubMed] [Google Scholar]
- Kubota F, Kikuchi S, Ito M, Shibata N, Akata T, Takahashi A, Sasaki T, Oya N, Aoki J ( 2000): Ictal brain hemodynamics in the epileptic focus caused by a brain tumor using functional magnetic resonance imaging (fMRI). Seizure 9: 585–589. [DOI] [PubMed] [Google Scholar]
- Kwan P, Sander JW ( 2004): The natural history of epilepsy: an epidemiological view. J Neurol Neurosurg Psychiatry 75: 1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R ( 1992): Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA 89: 5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Daunizeau J, Carmichael DW, Kleinschmidt A ( 2008): Recent advances in recording electrophysiological data simultaneously with magnetic resonance imaging. Neuroimage 40: 515–528. [DOI] [PubMed] [Google Scholar]
- Laufs H, Duncan JS ( 2007): Electroencephalography/functional MRI in human epilepsy: What it currently can and cannot do. Curr Opin Neurol 20: 417–423. [DOI] [PubMed] [Google Scholar]
- Laufs H, Lengler U, Hamandi K, Kleinschmidt A, Krakow K ( 2006): Linking generalized spike‐and‐wave discharges and resting state brain activity by using EEG/fMRI in a patient with absence seizures. Epilepsia 47: 444–448. [DOI] [PubMed] [Google Scholar]
- Laufs H, Walker MC, Lund TE ( 2007): 'Brain activation and hypothalamic functional connectivity during human non‐rapid eye movement sleep: An EEG/fMRI study—Its limitations and an alternative approach. Brain 130: e75. [DOI] [PubMed] [Google Scholar]
- Lemieux L, Allen PJ, Franconi F, Symms MR, Fish DR ( 1997): Recording of EEG during fMRI experiments: Patient safety. Magn Reson Med 38: 943–952. [DOI] [PubMed] [Google Scholar]
- Lemieux L, Salek‐Haddadi A, Josephs O, Allen P, Toms N, Scott C, Krakow K, Turner R, Fish DR ( 2001): Event‐related fMRI with simultaneous and continuous EEG: Description of the method and initial case report. Neuroimage 14: 780–787. [DOI] [PubMed] [Google Scholar]
- Lemieux L, Salek‐Haddadi A, Lund TE, Laufs H, Carmichael D ( 2007): Modelling large motion events in fMRI studies of patients with epilepsy. Magn Reson Imaging 25: 894–901. [DOI] [PubMed] [Google Scholar]
- LeVan P, Tyvaert L, Gotman J ( 2010a): Modulation by EEG features of BOLD responses to interictal epileptiform discharges. Neuroimage 50: 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVan P, Tyvaert L, Moeller F, Gotman J ( 2010b): Independent component analysis reveals dynamic ictal BOLD responses in EEG‐fMRI data from focal epilepsy patients. Neuroimage 49: 366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Luo C, Yang T, Yao Z, He L, Liu L, Xu H, Gong Q, Yao D, Zhou D ( 2009): EEG‐fMRI study on the interictal and ictal generalized spike‐wave discharges in patients with childhood absence epilepsy. Epilepsy Res 87: 160–168. [DOI] [PubMed] [Google Scholar]
- Liston AD, Lund TE, Salek‐Haddadi A, Hamandi K, Friston KJ, Lemieux L ( 2006): Modelling cardiac signal as a confound in EEG‐fMRI and its application in focal epilepsy studies. Neuroimage 30: 827–834. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yang T, Liao W, Yang X, Liu I, Yan B, Chen H, Gong Q, Stefan H, Zhou D ( 2008): EEG‐fMRI study of the ictal and interictal epileptic activity in patients with eyelid myoclonia with absences. Epilepsia 49: 2078–2086. [DOI] [PubMed] [Google Scholar]
- Luders HO, Najm I, Nair D, Widdess‐Walsh P, Bingman W ( 2006): The epileptogenic zone: general principles. Epileptic Disord 8( Suppl 2): S1–S9. [PubMed] [Google Scholar]
- Marrosu F, Barberini L, Puligheddu M, Bortolato M, Mascia M, Tuveri A, Muroni A, Mallarini G, Avanzini G ( 2009): Combined EEG/fMRI recording in musicogenic epilepsy. Epilepsy Res 84: 77–81. [DOI] [PubMed] [Google Scholar]
- Masterton RA, Harvey AS, Archer JS, Lillywhite LM, Abbott DF, Scheffer IE, Jackson GD ( 2010): Focal epileptiform spikes do not show a canonical BOLD response in patients with benign rolandic epilepsy (BECTS). Neuroimage 51: 252–260. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Hansen LK, Sejnowsk TJ ( 2003): Independent component analysis of functional MRI: What is signal and what is noise? Curr Opin Neurobiol 13: 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown MJ, Humphries C, Iragui V, Sejnowski TJ ( 1999): Spatially fixed patterns account for the spike and wave features in absence seizures. Brain Topogr 12: 107–116. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, Jung TP, Kindermann SS, Bell AJ, Sejnowski TJ ( 1998): Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp 6: 160–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown MJ, Sejnowski TJ ( 1998): Independent component analysis of fMRI data: Examining the assumptions. Hum Brain Mapp 6: 368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeren HK, Pijn JP, Van Luijtelaar EL, Coenen AM, Lopes da Silva FH ( 2002): Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci 22: 1480–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller F, LeVan P, Muhle H, Stephani U, Dubeau F, Siniatchkin M, Gotman J ( 2010a): Absence seizures: Individual patterns revealed by EEG‐fMRI. Epilepsia 51: 2000–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller F, Muhle H, Wiegand G, Wolff S, Stephani U, Siniatchkin M ( 2010b): EEG‐fMRI study of generalized spike and wave discharges without transitory cognitive impairment. Epilepsy Behav 18: 313–316. [DOI] [PubMed] [Google Scholar]
- Moeller F, Siebner HR, Ahlgrimm N, Wolff S, Muhle H, Granert O, Boor R, Jansen O, Gotman J, Stephani U, Siniatchkin M ( 2009a): fMRI activation during spike and wave discharges evoked by photic stimulation. Neuroimage 48: 682–695. [DOI] [PubMed] [Google Scholar]
- Moeller F, Siebner HR, Wolff S, Muhle H, Boor R, Granert O, Jansen O, Stephani U, Siniatchkin M ( 2008a): Changes in activity of striato‐thalamo‐cortical network precede generalized spike wave discharges. Neuroimage 39: 1839–1849. [DOI] [PubMed] [Google Scholar]
- Moeller F, Siebner HR, Wolff S, Muhle H, Granert O, Jansen O, Stephani U, Siniatchkin M ( 2008b): Simultaneous EEG‐fMRI in drug‐naive children with newly diagnosed absence epilepsy. Epilepsia 49: 1510–1519. [DOI] [PubMed] [Google Scholar]
- Moeller F, Siebner HR, Wolff S, Muhle H, Granert O, Jansen O, Stephani U, Siniatchkin M ( 2009b): Mapping brain activity on the verge of a photically induced generalized tonic‐clonic seizure. Epilepsia 50: 1632–1637. [DOI] [PubMed] [Google Scholar]
- Morocz IA, Karni A, Haut S, Lantos G, Liu G ( 2003): fMRI of triggerable aurae in musicogenic epilepsy. Neurology 60: 705–709. [DOI] [PubMed] [Google Scholar]
- Negishi M, Abildgaard M, Nixon T, Constable RT ( 2004): Removal of time‐varying gradient artifacts from EEG data acquired during continuous fMRI. Clin Neurophysiol 115: 2181–2192. [DOI] [PubMed] [Google Scholar]
- Niazy RK, Beckmann CF, Iannetti GD, Brady JM, Smith SM ( 2005): Removal of FMRI environment artifacts from EEG data using optimal basis sets. Neuroimage 28: 720–737. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW ( 1990): Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 87: 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny, W. , Holmes, A. , 2004. Random‐Effects Analysis In: Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Price CJ, Zeki S, Ashburner J, Penny W, editors. Human Brain Function, 2nd ed. London: Academic Press; pp 843–850. [Google Scholar]
- Perlbarg V, Bellec P, Anton JL, Pelegrini‐Issac M, Doyon J, Benali H ( 2007): CORSICA: Correction of structured noise in fMRI by automatic identification of ICA components. Magn Reson Imaging 25: 35–46. [DOI] [PubMed] [Google Scholar]
- Polack PO, Guillemain I, Hu E, Deransart C, Depaulis A, Charpier S ( 2007): Deep layer somatosensory cortical neurons initiate spike‐and‐wave discharges in a genetic model of absence seizures. J Neurosci 27: 6590–6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevett MC, Duncan JS, Jones T, Fish DR, Brooks DJ ( 1995): Demonstration of thalamic activation during typical absence seizures using H2(15)O and PET. Neurology 45: 1396–1402. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio CA, Reynolds CR, Lowe P, Moore JJ ( 2002): The continuous performance test: A window on the neural substrates for attention? Arch Clin Neuropsychol 17: 235–272. [PubMed] [Google Scholar]
- Roche‐Labarbe N, Zaaimi B, Mahmoudzadeh M, Osharina V, Wallois A, Nehlig A, Grebe R, Wallois F ( 2010): NIRS‐measured oxy‐ and deoxyhemoglobin changes associated with EEG spike‐and‐wave discharges in a genetic model of absence epilepsy: the GAERS. Epilepsia 51: 1374–1384. [DOI] [PubMed] [Google Scholar]
- Rodionov R, De MF, Laufs H, Carmichael DW, Formisano E, Walker M, Duncan JS, Lemieux L ( 2007): Independent component analysis of interictal fMRI in focal epilepsy: Comparison with general linear model‐based EEG‐correlated fMRI. Neuroimage 38: 488–500. [DOI] [PubMed] [Google Scholar]
- Salek‐Haddadi A, Diehl B, Hamandi K, Merschhemke M, Liston A, Friston K, Duncan JS, Fish DR, Lemieux L ( 2006): Hemodynamic correlates of epileptiform discharges: An EEG‐fMRI study of 63 patients with focal epilepsy. Brain Res 1088: 148–166. [DOI] [PubMed] [Google Scholar]
- Salek‐Haddadi A, Lemieux L, Merschhemke M, Friston KJ, Duncan JS, Fish DR ( 2003): Functional magnetic resonance imaging of human absence seizures. Ann Neurol 53: 663–667. [DOI] [PubMed] [Google Scholar]
- Salek‐Haddadi A, Mayer T, Hamandi K, Symms M, Josephs O, Fluegel D, Woermann F, Richardson MP, Noppeney U, Wolf P, Koepp MJ ( 2009): Imaging seizure activity: A combined EEG/EMG‐fMRI study in reading epilepsy. Epilepsia 50: 256–264. [DOI] [PubMed] [Google Scholar]
- Salek‐Haddadi A, Merschhemke M, Lemieux L, Fish DR ( 2002): Simultaneous EEG‐Correlated Ictal fMRI. Neuroimage 16: 32–40. [DOI] [PubMed] [Google Scholar]
- Sander JW ( 2003): The epidemiology of epilepsy revisited. Curr Opin Neurol 16: 165–170. [DOI] [PubMed] [Google Scholar]
- Siniatchkin M, Groening K, Moehring J, Moeller F, Boor R, Brodbeck V, Michel CM, Rodionov R, Lemieux L, Stephani U ( 2010): Neuronal networks in children with continuous spikes and waves during slow sleep. Brain 133: 2798–2813. [DOI] [PubMed] [Google Scholar]
- Smith SJ ( 2005): EEG in the diagnosis, classification, and management of patients with epilepsy. J Neurol Neurosurg Psychiatry 76( Suppl 2): ii2–ii7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava G, Crottaz‐Herbette S, Lau KM, Glover GH, Menon V ( 2005): ICA‐based procedures for removing ballistocardiogram artifacts from EEG data acquired in the MRI scanner. Neuroimage 24: 50–60. [DOI] [PubMed] [Google Scholar]
- Thornton R, Laufs H, Rodionov R, Cannadathu S, Carmichael DW, Vulliemoz S, Salek‐Haddadi A, McEvoy AW, Smith SM, Lhatoo S, Elwes RD, Guye M, Walker MC, Lemieux L, Duncan JS ( 2010a): EEG correlated functional MRI and postoperative outcome in focal epilepsy. J Neurol Neurosurg Psychiatry 81: 922–927. [DOI] [PubMed] [Google Scholar]
- Thornton RC, Rodionov R, Laufs H, Vulliemoz S, Vaudano A, Carmichael D, Cannadathu S, Guye M, McEvoy A, Lhatoo S, Bartolomei F, Chauvel P, Diehl B, De MF, Elwes RD, Walker MC, Duncan JS, Lemieux L ( 2010b): Imaging haemodynamic changes related to seizures: Comparison of EEG‐based general linear model, independent component analysis of fMRI and intracranial EEG. Neuroimage 53: 196–205. [DOI] [PubMed] [Google Scholar]
- Tyvaert L, Hawco C, Kobayashi E, LeVan P, Dubeau F, Gotman J ( 2008): Different structures involved during ictal and interictal epileptic activity in malformations of cortical development: An EEG‐fMRI study. Brain 131: 2042–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyvaert L, LeVan P, Dubeau F, Gotman J ( 2009): Noninvasive dynamic imaging of seizures in epileptic patients. Hum Brain Mapp 30: 3993–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Houdt PJ, Ossenblok PP, Boon PA, Leijten FS, Velis DN, Stam CJ, de Munck JC ( 2010): Correction for pulse height variability reduces physiological noise in functional MRI when studying spontaneous brain activity. Hum Brain Mapp 31: 311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudano AE, Laufs H, Kiebel SJ, Carmichael DW, Hamandi K, Guye M, Thornton R, Rodionov R, Friston KJ, Duncan JS, Lemieux L ( 2009): Causal hierarchy within the thalamo‐cortical network in spike and wave discharges. PLoS One 4: e6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulliemoz S, Carmichael DW, Rosenkranz K, Diehl B, Rodionov R, Walker MC, McEvoy AW, Lemieux L ( 2011): Simultaneous intracranial EEG and fMRI of interictal epileptic discharges in humans. Neuroimage 54: 182–190. [DOI] [PubMed] [Google Scholar]
- Warach S, Ives JR, Schlaug G, Patel MR, Darby DG, Thangaraj V, Edelman RR, Schomer DL ( 1996): EEG‐triggered echo‐planar functional MRI in epilepsy. Neurology 47: 89–93. [DOI] [PubMed] [Google Scholar]
- Westmijse I, Ossenblok P, Gunning B, van LG ( 2009): Onset and propagation of spike and slow wave discharges in human absence epilepsy: A MEG study. Epilepsia 50: 2538–2548. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC ( 2002): A general statistical analysis for fMRI data. Neuroimage 15: 1–15. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Huiskamp G, Hersevoort M, Seppenwoolde JH, van Huffelen AC, Leijten FS ( 2007): EEG‐fMRI in the preoperative work‐up for epilepsy surgery. Brain 130: 2343–2353. [DOI] [PubMed] [Google Scholar]


