Abstract
Previously, multi‐voxel pattern analysis has been used to decode words referring to concrete object categories. In this study we investigated if single‐trial‐based brain activity was sufficient to distinguish abstract (e.g., mercy) versus concrete (e.g., barn) concept representations. Multiple neuroimaging studies have identified differences in the processing of abstract versus concrete concepts based on the averaged activity across time by using univariate methods. In this study we used multi‐voxel pattern analysis to decode functional magnetic resonance imaging (fMRI) data when participants perform a semantic similarity judgment task on triplets of either abstract or concrete words with similar meanings. Classifiers were trained to identify individual trials as concrete or abstract. Cross‐validated accuracies for classifying trials as abstract or concrete were significantly above chance (P < 0.05) for all participants. Discriminating information was distributed in multiple brain regions. Moreover, accuracy of identifying single trial data for any one participant as abstract or concrete was also reliably above chance (P < 0.05) when the classifier was trained solely on data from other participants. These results suggest abstract and concrete concepts differ in representations in terms of neural activity patterns during a short period of time across the whole brain. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: fMRI, MVPA, language
INTRODUCTION
Representations of concrete and abstract concepts in the brain are relevant to understanding language function in both healthy and clinical populations [Eviatar et al., 1990; Kuperberg et al., 2008; Mervis and John, 2008]. A series of behavioral advantages of processing concrete compared to abstract concepts have been well documented and referred to as the concreteness effect: concrete words are acquired earlier during development, remembered and recognized more rapidly and accurately, and are less vulnerable to brain damage than abstract words [Kroll and Merves, 1986; Marschark and Cornoldi, 1991; Schwanenflugel, 1991].
Numerous neuroimaging studies have contributed evidence for distinct neural substrates of abstract versus concrete representation. These studies have examined representational differences by using a statistical parametric mapping approach based on data averaged across time. Whether the differences between abstract and concrete concepts can be detected in a single trial remains an open question. A complementary approach to statistical parametric mapping is to use multi‐voxel pattern analysis (MVPA), a pattern‐based approach that detects neural response by jointly investigating information in multiple voxels. This method is more sensitive compared to univariate statistical parametric mapping that localizes the differences of activation averaged across trials [Haynes and Rees, 2006; Norman et al., 2006; O'Toole et al., 2007]. MVPA allows focusing on single trials of data, which has a potential future application in brain‐computer interface.
MVPA has been successfully used to investigate how semantic information about objects is represented in the brain. Previous studies on concept representation were able to detect the neural responses associated with viewing categories of objects [Carlson et al., 2003; Chan et al., 2011; Cox and Savoy, 2003; Hanson and Halchenko, 2007; Hanson et al., 2004; Haxby et al., 2001; Just et al., 2010; O'Toole et al., 2005; Polyn et al., 2005; Shinkareva et al., 2011; Shinkareva et al., 2008]. Moreover, the category of an object that a participant was viewing [Shinkareva et al., 2008] or a concrete noun that a participant was reading [Just et al., 2010; Shinkareva et al., 2011] can be identified based only on other participants' characteristic neural activation patterns, establishing the commonality in how different people's brains represent the same object.
Most MVPA studies on concept representation used pictorial stimuli [Carlson et al., 2003; Cox and Savoy, 2003; Hanson and Halchenko, 2007; Hanson et al., 2004; Haxby et al., 2001; O'Toole et al., 2005; Polyn et al., 2005; Shinkareva et al., 2008]. Only a few studies have applied MVPA to decode semantic concept representations of concrete objects based on verbal stimuli [Chan et al., 2011; Just et al., 2010; Shinkareva et al., 2011]. Compared to visual depictions of objects, verbal stimuli are more independent of visual perception and can refer to abstract concepts. Whether representation of abstract concepts can be distinguished from concrete concepts using MVPA methods is unclear. In this work we extend the previous MVPA findings on concept representation by including the abstract category that is less dependent on perceptual or motor experiences [for an alternate explanation see Barsalou, 1999; Lakoff and Johnson, 1980]. The purpose of this study was twofold. First, we explored whether MVPA methods could be used to identify single trials as abstract or concrete within each individual by decoding functional patterns of whole brain activity, thus extending previous MVPA studies of concept representation to abstract concepts. We also examined where the discriminating information between abstract and concrete concepts is located in the brain by focusing on the spatially localized anatomical brain regions that contained sufficient information for identification of abstract or concrete concepts on average across participants. Second, we investigated whether the representations of abstract and concrete concepts are similar across individuals by training the classifier on all but one participant and then predicting single trials as abstract or concrete in the left out participant.
METHODS
Participants
Thirteen participants (six female) from the University of South Carolina community participated in this experiment and gave written informed consent in accordance with the Institutional Review Board at the University of South Carolina. Participants were right‐handed, healthy adults and native English‐speakers.
Materials
Stimuli were word triplets comprised of semantically similar nouns from two concrete (tools and dwellings) and two abstract (cognition and emotion) categories (Supporting Information Table S1). Each category contained four exemplars, with four different words in each exemplar. For instance, the words knife, scalpel, razorblade, and cutlass composed the exemplar cutting object within the concrete category tools. For each exemplar, six different triplets were selected from all possible permutations of the four words. Because the six triplets in each exemplar referred to the same semantic concept, these triplets were regarded as repetitions of the same exemplar. The 16 exemplars were each presented six times, with each repetition composed of a unique list of triplets, generating 96 triplets in total (4 categories × 4 exemplars × 6 repetitions). Triplets were balanced between the abstract and concrete categories on word frequency [M Abstract = 27.86 and M Concrete = 31.98, t(94) = −0.53, P = 0.60] and word length [M Abstract = 7.25 and M Concrete = 6.83, t(94) = 1.84, P = 0.07].
Experimental Paradigm
While being scanned, the participants were asked to make judgments on semantically similar written words, analogous to the synonym judgment paradigm [Breedin et al., 1994; Noppeney and Price, 2004; Sabsevitz et al., 2005]. In each trial, a word triplet was presented for three seconds, followed by a seven‐second fixation period. For each triplet, participants were asked to decide during the three‐second triplet presentation which of two words at the bottom of the display was more similar to the word shown at the top. During the presentation of the seven‐second fixation, the participant was instructed to clear the mind and fixate on the cross at the center of the screen. The task was designed to prompt careful evaluation of each item and its properties, thus implicitly eliciting the semantic representation of the presented exemplar. A long fixation trial of 24 seconds was presented after each repetition of the 16 exemplars. Participants were prompted by the word “Ready?” following the long fixation to indicate the beginning of the next repetition. The whole experiment was completed in two scanning sessions, with three repetitions of the 16 exemplars in each session.
MRI Acquisition
Functional images were acquired with gradient echo EPI on a Siemens 3T Trio scanner at the McCausland Brain Imaging Center at the University of South Carolina with the following parameters: TR = 2200 ms, TE = 30 ms, and 90° flip angle. Thirty‐six oblique‐axial slices were imaged with no gap. The acquisition matrix was 64 × 64 pixels with 3 × 3 × 3 mm voxels.
fMRI Data Preprocessing
Data were corrected for head movement and then normalized into a standard template in SPM5 (http://www.fil.ion.ucl.ac.uk/spm). First, head‐movement artifacts were corrected based on a six‐parameter rigid body transformation. The head movement in any direction of any participant was smaller than 1.5 mm. The first image was used as the reference volume for realignment. The mean functional image was created and coregistered to a standard stereotactic space using the EPI‐derived Montreal Neurological Institute (MNI) template, and the registration parameters were used to normalize the realigned functional images.
MVPA Methods
The MVPA analysis steps employed in this work are similar to those that have been successfully used in other MVPA studies [Mitchell et al., 2008; Shinkareva et al., 2011; Shinkareva et al., 2008]. Classifiers were trained on the mean percent signal change (PSC) of functional activity for each word triplet in the training set to identify the cognitive states associated with processing abstract and concrete concepts. For each participant's data, the mean PSC of each voxel was the ratio of signal difference between word triplets and the baseline to the baseline signal. The baseline was computed from the averaged signal in the long fixation trials. The signal of each triplet was computed by averaging two volumes offset 4.4 s away from the stimulus onset (the third and fourth volumes of one trial) to account for the delay of hemodynamic response function. Furthermore, the PSCs in each voxel were normalized across triplets to have mean 0, and variance 1, to equate variations in different voxels [Pereira et al., 2009].
Feature selection
To reduce the size of the data prior to classification, relevant features were extracted by using voxels with the most consistent responses toward different conditions across cross‐validation folds [Pereira et al., 2009]. Response stability was computed by averaging pairwise correlation coefficients between vectors of repetitions of all exemplars [Shinkareva et al., 2011]. The voxels with lowest response stability were removed. The rationale of stability‐based feature selection was that if a voxel responded unsystematically between repetitions across conditions, it was unlikely to contain information that is associated with different conditions. This procedure was based on training data only to avoid over‐fitting. We explored different numbers of voxels retained by feature selection instead of deciding upon an arbitrary threshold.
Classification within participants
A logistic regression classifier was used for abstract versus concrete two‐way classification. As a commonly used classifier, logistic regression directly estimates its parameters from the training data [Bishop, 2006]. This classifier was chosen because it is simple, less likely to generate over‐fitting compared to non‐linear classifiers, and has been successfully applied in previous studies [Mitchell et al., 2004; Pereira et al., 2009]. To ensure the evaluation of classification performance was unbiased, classification accuracy was evaluated using six‐fold cross validation procedure, where each fold corresponded to one repetition of all exemplars. The repetitions were separated by the long fixation period, thus the independence between training and test sets was ensured.
For each cross‐validation fold, the trained classifiers were applied to each trial in the test set to classify it as abstract or concrete, and the proportion of trials that were correctly classified was reported. For each participant, the obtained accuracy was compared to an empirically generated null distribution, formed by 1000 classification accuracies obtained from the same dataset, but with randomly permuted labels.
To locate the voxels that contributed most to classifying individual trials as abstract or concrete (henceforth, informative voxels), voxels with the highest and lowest five percent of logistic regression weights were identified for each cross‐validation fold. A union of such voxels across cross‐validation folds was visualized for each participant. To investigate the consistency of informative voxel locations across individuals, a voxel location probability map was generated across participants after convolving each voxel with a 4 mm Gaussian kernel [Kober et al., 2008]. The probability map was further thresholded by a simulated null hypothesis distribution at P = 0.05 (FWE corrected).
In addition, the multinomial logistic regression classifiers were used to identify each of the 16 exemplars. For simplicity, the number of voxels from a feature selection step in this analysis was set to 400. Feature selection, cross‐validation, and significance testing were as described above.
Region of interest (ROI) analysis
To investigate how the discriminating information is distributed in the brain, the classifiers were trained on data from one of the 90 anatomically defined regions at a time [Shinkareva et al., In press]. ROIs were defined by Automated Anatomical Labeling [AAL; Tzourio‐Mazoyer et al., 2002]. Mean PSC in all gray matter voxels in each ROI was used to train the logistic regression classifiers. To access if an anatomical region contained sufficient information to decode abstract or concrete concepts on average across participants, the classification accuracy for each region was compared to a binomial distribution B(n, p), where n is the number of triplets, and p is the probability of successfully identifying a triplet as abstract or concrete under the hypothesis that triplets are randomly assigned to the two categories [Pereira et al., 2009]. P‐values (computed using a normal approximation) were obtained for the mean classification accuracy, computed across participants for each region. The P‐values for anatomically defined regions were compared to 0.05 level of significance using the Bonferroni correction for multiple comparisons.
Classification across participants
To test for a commonality in the neural representation of abstract and concrete concepts across individuals, classifiers were trained on data from all but one participant to identify trials as abstract or concrete in the left‐out participant. An entropy‐based feature selection was applied to retain the voxels containing most stable information across individuals. For each voxel, the Shannon entropy was computed from the data of twelve individuals in the training set ordered by individual exemplars within abstract and concrete categories. Entropy‐based feature selection has been validated as an efficient index of the voxel sensitivity toward the variation of conditions [Poldrack et al., 2009]. For simplicity, the top 20% of most stable voxels, that is, voxels with the lowest entropy values, were selected. For each cross‐validation fold, the classifier was trained on the PSC data from all but one participant, which was the test dataset. This procedure was repeated for all participants. Classification accuracy was compared to the empirically generated distribution, formed by 1000 classification accuracies obtained from the same dataset, but with randomly permuted labels. Accuracies with P‐values smaller than 0.05 were considered significant.
RESULTS
Behavioral Results
There were no significant differences in the mean reaction times across participants between judgments on abstract and concrete triplets [M Abstract = 1.66 and M Concrete = 1.69, t(12) = −0.85, P = 0.41]. Moreover, none of the individual participants showed significantly different reaction times between abstract and concrete triplets (p ranged from 0.08 to 0.94). These results suggest making judgments on abstract or concrete triplets did not differ in difficulty.
Within‐Participant Classification Based on the Whole Brain
When classifiers were trained to identify word triplets as abstract or concrete, the mean accuracies across participants were significantly greater than chance (P < 0.05) for all threshold levels (Fig. 1). Classification accuracies for one participant were as high as 90.62% (87 out of 96 triplets correctly identified as abstract or concrete). The classification accuracies were highest when the numbers of voxels used for classification ranged from 50 to 3000. The accuracies were reliably above chance for most participants even when all the voxels were included in the analysis.
Figure 1.
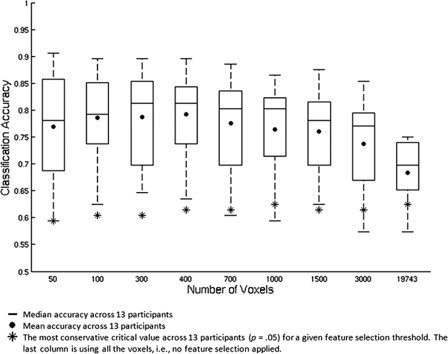
Within‐participant classification accuracies for identifying trials as abstract or concrete, summarized across 13 participants by box plots, are shown as a function of different number of voxels.
The locations of voxels with largest classifier weights for identifying trials as abstract or concrete were distributed in multiple areas in the brain and were similar across participants. For example, when feature selection retained 400 voxels, the most informative voxels for identifying abstract concepts, consistently detected across participants, were located in the left inferior frontal gyrus, middle temporal gyrus, and posterior cingulate cortex; the most consistent informative voxels for identifying concrete concepts were located in the left angular gyrus, fusiform gyrus, inferior temporal gyrus, middle frontal gyrus, posterior cingulate cortex, and precuneus (Fig. 2).
Figure 2.
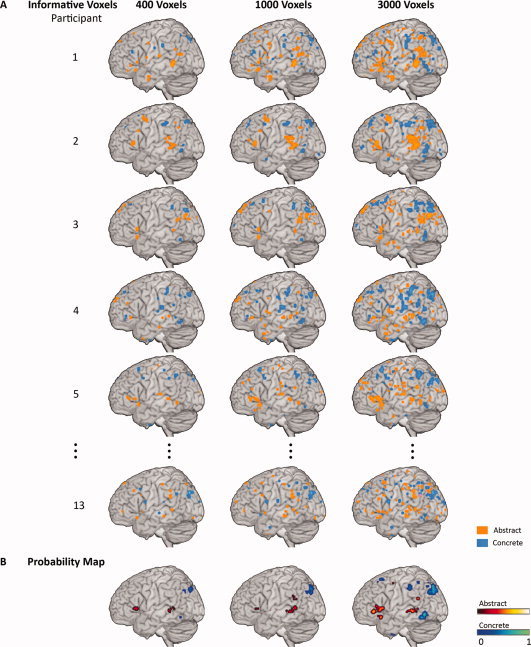
Consistency of informative voxels across participants. Panel A: Most informative voxels for decoding abstract versus concrete concepts representation within participants are shown on a surface rendering at three feature selection thresholds: retaining 400, 1000, or 3000 voxels. Participants were ordered by within‐participant classification accuracy. The warm color indicates the top 5% of voxels that were most informative for identifying abstract trials. The cool color indicates the top 5% of voxels that were most informative for identifying concrete trials. Panel B: The thresholded probability maps (P = 0.05, FWE corrected) of the informative voxels that were consistently identified across all 13 participants. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In addition, classifiers were trained to identify a specific exemplar about which a participant was making similarity judgments. Classification reached mean accuracy of 14.4% across participants for classifying an exemplar into one of the 16 categories (compared to 9.38% at P = 0.05 level of significance). Exemplars were reliably (P < 0.05) identified for 11 out of 13 participants. Most of the mistakes that the classifier was making were within the same abstract or concrete category (Fig. 3). Thus, the brain activity patterns associated with making similarity judgments with either abstract or concrete concepts can be decoded on a single‐trial basis, suggesting the distinct representations of abstract and concrete concepts.
Figure 3.
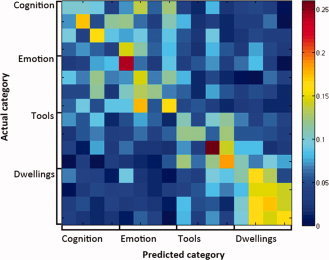
Exemplar classification confusion matrix averaged across participants. The value of each element indicates the proportion of exemplars identified as the corresponding label. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Within‐Participant Classification Based on Single ROIs
To investigate whether individual regions contain sufficient information for decoding abstract and concrete concepts, classifiers were trained using voxels from only one anatomical region at a time. Fifty‐two out of the 90 ROIs showed reliable (P < 0.05) classification accuracies on average across participants. These regions were distributed across temporal, frontal, parietal, and occipital lobes bilaterally, whereas the regions with the highest accuracies were mostly in the left hemisphere (Fig. 4). Out of the 52 informative ROIs, 30 were in the left hemisphere, including the top 15 ROIs with highest average accuracies across participants. For all informative right‐hemisphere ROIs the left homologues also contained information for successful identification. Among these bilateral region pairs, the average classification accuracies across participants were higher in the left hemisphere, with an exception of the lingual gyrus. Five ROIs, including left middle temporal gyrus, left precuneus, left angular gyrus, left middle occipital gyrus, and left precentral gyrus, showed significant accuracy for all of the participants (Fig. 5). These results were consistent the locations of the informative voxels from the whole brain analysis (Fig. 2). Thus multiple brain regions contain information sufficient to decode abstract versus concrete concept representation.
Figure 4.
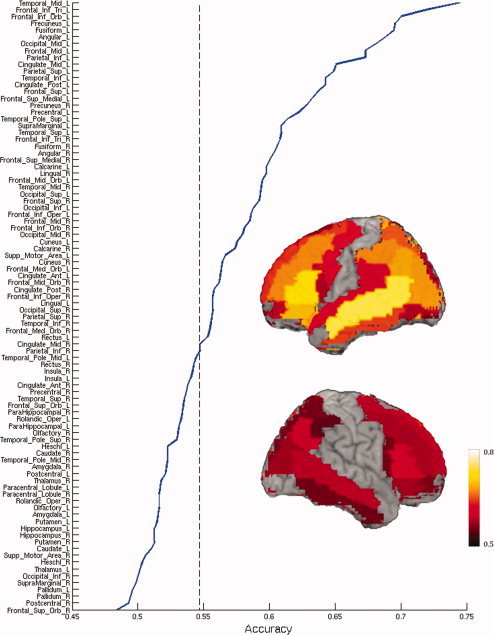
Mean classification accuracies across participants, for trial identification as abstract or concrete, are shown for each anatomically defined ROI. Regions with significant mean accuracy across participants (P = 0.05) are shown on a surface rendering of a brain template (http://www.cabiatl.com/mricro/mricron/index.html). ROIs are ordered by the mean classification accuracy across participants. The dashed line indicates the threshold of significant accuracy. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 5.
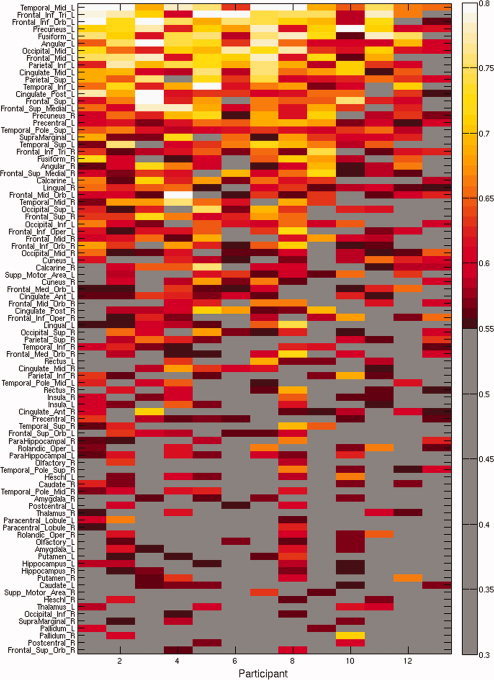
Classification accuracies for identification of trials as abstract or concrete are shown for each ROI and each participant. Significant accuracies (P = 0.05) are shown in color. ROIs are ordered by the mean classification accuracy across participants. Participants are ordered by within‐participant accuracy based on 400 voxels. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Across‐Participant Classification Based on the Whole Brain
Classifiers were trained on data from 12 participants to determine if it was possible to identify individual trials as abstract or concrete in the left‐out participant. The average accuracy across participants of identifying triplets as abstract or concrete when the classifier was trained on data from other participants was 84.13% (P < 0.001). Word triplets were reliably (P < 0.05) identified for all 13 participants, with the accuracies ranging from 62.50% to 93.75% (Fig. 6). This result indicates the commonality of abstract versus concrete concept representation across individuals.
Figure 6.
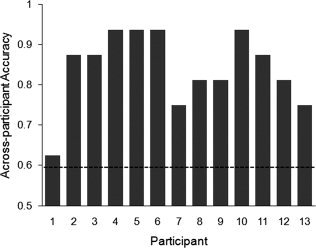
High across‐participants classification accuracies for identifying single trials as abstract or concrete based on data from other participants. Dashed line indicates P = 0.05 level of significance. Participants are ordered by within‐participant accuracy based on 400 voxels.
DISCUSSION
We were able to successfully identify brain activity patterns as abstract or concrete based on single‐trial data. This study has extended previous results on concrete words representation [Chan et al., 2011; Just et al., 2010; Shinkareva et al., 2011] to abstract concepts. Compared with studies that examined activation differences in abstract and concrete concept representation, this study suggests neural responses during abstract and concrete semantic concepts processing can be identified from distributed patterns of activity on an individual trial basis.
Moreover, whether a participant was making similarity judgments about abstract or concrete concepts was identifiable solely based on data from other participants, in spite of the anatomical and functional variability across individual brains [Fedorenko and Kanwisher, 2009]. It supports the cross‐individual principles of processing semantic concepts. Classification of mental states across individuals has been previously shown for visually depicted objects [Shinkareva et al., 2008], concrete nouns referring to physical objects [Just et al., 2010; Shinkareva et al., 2011], lie detection [Davatzikos et al., 2005], attentional tasks [Mourão‐Miranda et al., 2005], cognitive tasks [Poldrack et al., 2009], and voxel‐by‐voxel correspondence across individuals has been demonstrated during movie‐watching [Hasson et al., 2004]. The current study for the first time demonstrates the ability to identify the mental states of a participant as processing abstract or concrete concepts based on neural activation data from other participants.
Classification within individual anatomically defined regions showed that activity patterns in even single regions were sufficient for identifying trials as abstract or concrete. The present results of regions with discriminating information show considerable overlap with the meta‐analysis results based on previous statistical parametric mapping studies locating the differences between abstract versus concrete semantic concept representation [Binder et al., 2009; Wang et al., 2010]. All but one region that were previously identified by the meta‐analysis (Table I) were also found to contain information sufficient for identification of trials as abstract or concrete in the current study (Fig. 4). The top six ROIs with the highest average accuracy were also identified by the meta‐analyses results. However, this single study identified more informative areas compared to the combined results of early lesion and neuroimaging studies. In fact, the current results are more comparable to the collection of previous univariate results (Fig. 7).
Table I.
Consistent brain regions of abstract versus concrete distinction (based on Wang et al., 2010)
| Abstract > Concrete |
| Left inferior frontal gyrus |
| Left middle temporal gyrus |
| Left superior temporal gyrus |
| Concrete > Abstract |
| Left fusiform |
| Left parahippocampalgyrus |
| Left posterior cingulate gyrus |
| Left precuneus |
Figure 7.
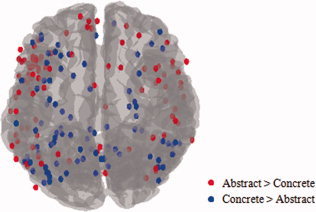
Activation peaks for abstract versus concrete representation from 19 studies are shown on the brain template (See Wang et al., 2010 for the list of studies and meta‐analysis results). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The left hemisphere was engaged in abstract versus concrete concept identification to a very large extent. Thirty out of the 45 left hemisphere ROIs showed significant accuracies on average across participants. A number of right hemisphere regions also held information of abstract versus concrete differentiation. Previous studies have found the activation differences in some of these right hemisphere regions but with low cross‐study consistency [Binder et al., 2005; D'Esposito et al., 1997; Fliessbach et al., 2006; Grossman et al., 2002; Harris et al., 2006; Jessen et al., 2000; Mellet et al., 1998; Perani et al., 1999a; Sabsevitz et al., 2005; Tettamanti et al., 2008; Wallentin et al., 2005; Whatmough et al., 2004]. A specific investigation of the informative ROIs shows some of these areas have been considered typical for differences between abstract versus concrete concept representations, whereas other regions were not consistently found in previous literature. For example, the results of at least one of the quantitative meta‐analyses on semantic processing have identified the left inferior frontal gyrus, left middle temporal gyrus and left superior temporal gyrus and sulcus for being engaged more in abstract than concrete concept processing; whereas the bilateral angular gyrus, left fusiform gyrus, left parahippocampal gyrus, left posterior cingulate, and left precuneus have been identified to be consistently more engaged in concrete than in abstract concept processing [Binder et al., 2009; Wang et al., 2010].
This is the first time that such a large number of informative brain areas for abstract versus concrete concept representation were identified in a single experiment. The extensive spatial distribution of discriminating information may reflect the lack of semantic context restriction during single word processing. Compared to the word specified in a meaningful sentence, single word processing in a semantics‐related task may stimulate the rich contexts of the word more extensively [Price, 2010]. However the MVPA results do not directly reveal the properties of the information reflected in the data [Hanke et al., 2010]. What do these results suggest about the underlying processes driving the neural differences between abstract and concrete concept processing? The following sections will discuss this question first based on the most consistently identified brain regions, then based on the areas that were less consistently reported.
Left middle temporal gyrus
Classification accuracy of this single region reached 74.52% across participants, which is striking considering the 79.17% accuracy based on voxels distributed across the whole brain gray matter. A number of studies have reported greater activation in the left middle temporal gyrus for abstract than concrete concept representations [Grossman et al., 2002; Harris et al., 2006; Noppeney and Price, 2004; Pexman et al., 2007; Sabsevitz et al., 2005; Tettamanti et al., 2008; Wallentin et al., 2005]. Multiple aspects of language processing have been shown to relate to activity in this area, including explicit or implicit word reading [Paulesu et al., 2001], speech comprehension [Binder, et. al., 2000; Crinion et al., 2003; Davis et al., 2007; Davis and Johnsrude, 2003], word memorization [Ojemann et al., 1988], and executively demanding semantic judgment [Whitney et al., 2011]. Increasing activation in this area is also related to processing higher lexical frequency words in implicit semantic processing [Graves et al., 2010]. One of the most likely associations of this region with the current results is its pivotal role in mapping semantic concepts to words, which is usually reflected in the object naming or picture identification tasks [Boatman et al., 2000; Perani et al., 1999b; Schwartz et al., 2009]. Moreover, the left middle temporal gyrus is also engaged in retrieving conceptual knowledge related to object manipulation [Jastorff et al., 2010], suggesting a property‐based, “transmodal” way of encoding or retrieving concepts of this area [Binder et al., 2009; Mesulam, 1998]. Thus, the highly discriminating activity patterns in this region may be due to the different association strengths with physical objects, or concreteness, between abstract and concrete concepts.
Another probable reason for the middle temporal gyrus to be exceptionally informative for classification is its involvement in context‐dependent encoding of word meanings. The left middle temporal gyrus, along with inferior frontal gyrus and parahippocampal gyrus, has been identified in acquiring new word meaning [Mestres‐Missé et al., 2008]. Studies manipulating syntactic ambiguities suggest this region may be involved in disambiguating word meanings based on sentence contexts [Gennari et al., 2007; Rodd et al., 2010]. Because the similarity judgment requires the participant to make subtle distinctions in the context of synonymous triplets, the left middle temporal gyrus might be one of the most important areas to represent semantic information, thus being more sensitive to the abstract versus concrete differences.
Left inferior frontal gyrus
Pars triangularis and pars orbitalis of the left inferior frontal gyrus are highly informative for abstract versus concrete decoding. This result is not surprising given the fact that left inferior frontal gyrus is one of the most canonical regions found in abstract > concrete contrast in previous literature [Binder et al., 2005; Fiebach and Friederici, 2004; Fliessbach et al., 2006; Friederici et al., 2000; Jessen et al., 2000; Noppeney and Price, 2004; Perani et al., 1999a; Pexman et al., 2007; Sabsevitz et al., 2005; Tettamanti et al., 2008; Wallentin et al., 2005]. The roles of the left inferior frontal gyrus are suggested to be multifold. Left pars triangularis and pars orbitalis along with other prefrontal areas have been found to be sensitive to increasing abstractness of a concept in a semantic decision task [Goldberg et al., 2007] and explicit requirement of semantic retrieval [Friederici et al., 2000; Petersen et al., 1988; Wagner et al., 2001]. Several studies suggest that the left inferior frontal gyrus activity does not reflect a semantic retrieval process per se, but rather reflects a specific executive for the demand of semantic selection [Demb et al., 1995; Nagel et al., 2008; Thompson‐Schill et al., 1997]. According to these conclusions, the successful classification within the left inferior frontal gyrus in the current study may be attributed to strategic verbal retrieval in abstract concept representation. Verbal representation of word meanings is not given priority during semantic processing, but when the available prior information, for example, perceptual and imagery details, is insufficient, the verbal representation will step in to facilitate the semantic processing.
Another line of evidence suggests the importance of phonological processing in the left inferior frontal gyrus for the abstract versus concrete differentiation. Left pars opercularis, an area with a moderate but still significant classification accuracy, has been associated with phonological working memory [Binder et al., 2005; Burton, 2001; Zatorre et al., 1992], and sequencing of phonemes and hummed notes [Gelfand and Bookheimer, 2003]. The activity pattern differences in this region may relate to how long the information is held in the phonological loop. This is in line with the hypothesis that processing abstract words occupies the working memory to a greater extent than concrete words, because it requires additional semantic processing [Binder et al., 2005]. Thus, this area may implicate a natural difficulty of representing abstract concepts even when no difference is found in behavioral responses.
Precuneus and posterior cingulate
These two structures in the left hemisphere have been consistently identified in the concrete > abstract contrast in previous literature [e.g., Binder et al., 2005; Harris et al., 2006; Mellet et al., 1998; Pexman et al., 2007; Sabsevitz et al., 2005]. Due to their adjacency in location as well as their structural and functional connectivity [Castellanos et al., 2008; Cavanna and Trimble, 2006; Fransson and Marrelec, 2008; Mizelle and Wheaton, 2010; Vogt et al., 2006], we discuss them in a combined section. The precuneus has been shown to be involved in different tasks that require mental image generation, such as mental rotation [Butler et al., 2006; Cohen et al., 1996; Gauthier et al., 2002] and visuospatial episodic memory encoding and retrieval [Aggleton and Pearce, 2001; Fletcher et al., 1995; Ghaem et al., 1997; Mellet et al., 2000; but also see Krause et. al. 1999]. Bilateral precuneus has also been associated with motor imagery [Hanakawa et al., 2003; Malouin et al., 2003]. Similarly, the posterior cingulate has been found in spatial representation and episodic retrieval of places [Sugiura et al., 2005], happy event imagery [Mantani et al., 2005], name recognition [Sugiura et al., 2009], and memorizing route [Katayama et al., 1999], suggesting its engagement in imagery generation during memory tasks. These findings, together with the results of abstract versus concrete word classification, are in agreement with the dual‐coding hypothesis: the concrete concepts representation is facilitated by an additional imagery coding system because of the more detailed perceptual information compared to the abstract concepts.
Left fusiform gyrus
Left fusiform gyrus has been associated with object recognition, naming colors and reading words in visual and auditory forms [see Price and Devlin, 2003 for a review]; the functions of representing specific categories of objects have been finely localized within subareas of this region [Martin and Chao, 2001]. The frequent identification of the fusiform gyrus in the contrast of concrete > abstract representation has been attributed to the easiness of mental generation of object features represented by concrete concepts [D'Esposito et al., 1997; Mellet et al., 1998; Mestres‐Missé et al., 2009; Wise et al., 2000]. In addition to the previous assumption of modality‐specific area for visual input [e.g., Cohen et al., 2002; Kanwisher et al., 1997], the left fusiform gyrus has recently also been recognized to integrate sensory information from other input modalities, and even associate visual form stimuli with higher‐order properties [Devlin et al., 2006; Doehrmann et al., 2010]. The left posterior portion of fusiform gyrus has been characterized as semantically processing words representing objects [Wheatley et al., 2005]. The involvement of perceptual areas in distinguishing abstract and concrete word processing implicates the perceptual grounding of representing concrete semantic concepts.
Angular gyrus and inferior parietal lobule
The bilateral angular gyrus is another region with high classification accuracy that has been consistently identified by neuroimaging studies for greater activation in concrete compared to abstract word representations [Binder et al., 2005; Fliessbach et al., 2006; Sabsevitz et al., 2005]. It is noteworthy that most of its surrounding areas in the left hemisphere, including the superior temporal gyrus, middle temporal gyrus and inferior parietal lobule, are among the most informative regions of abstract versus concrete classification. The left angular gyrus has been suggested to be critical to the transfer and organization of multi‐modal sensory‐motor information for higher‐level conceptualizations [Geschwind, 1965] and to the assembly of verbal information in auditory working memory for integrative comprehension tasks [Dronkers et al., 2004; Pugh et al., 2000], thereby it is not surprising that the angular gyrus is one of the centers for integrative semantic processing and knowledge retrieval [Binder et al., 2009]. The left inferior parietal lobule has been linked with integrating features for semantic categorization [Koenig et al., 2005]. The information content sufficient for decoding abstract versus concrete concept representations in these regions, especially in the left hemisphere, may reflect the abstract versus concrete distinction on a semantic comparison level, which is a consequence of the differences in either perceptual‐motor information from mental imagery or associate verbal contexts.
Left superior temporal gyrus
The left superior temporal gyrus has been recognized for greater activation in abstract than concrete concept representation in several previous studies [Grossman et al., 2002; Perani et al., 1999a; Pexman et al., 2007]. This region has been linked to the assembly of phonology in perception [Booth et al., 2004; Scott et al., 2000] and production [Buchsbaum et al., 2001; Hickok et al., 2000]. Activity in the bilateral superior temporal gyri has also been associated with the effect of semantic context [Friederici et al., 2003; Van Petten and Luka, 2006] and semantic judgment task [D'Esposito et al., 1995]. Therefore, the significant classification accuracy of this area might be caused by the longer processing of abstract than concrete concepts in the phonological loop, or the stronger reliance of abstract concepts on semantic context.
It is quite striking that single regions contain, on their own, enough information to decode the presented concepts. It is likely to be the case that sufficient information for category identification is represented in several different regions. Several of the discriminating regions, as discussed above, have been associated with functions other than semantic processing, thus raising questions of whether the successful classification results were driven solely by the representational differences of abstract and concrete concepts. The balanced lexical features of the stimuli and the behavioral results suggest that task difficulty is unlikely to be the confounder. We believe that this wide involvement of regions is due to the multiple mechanisms engaged in processing semantic concepts. The processing of abstract and concrete concepts may differ on several aspects, such as richness of semantic context, coding system, retrieval strategy, or the occupation of working memory. A number of regions identified in the current study have been shown in previous studies using statistical parametric mapping, but not in the same experiment, for a single task and a limited number of stimuli. One of the reasons, based on the current results, may be the lack of sensitivity in detecting the differences. These results suggest that the representation of abstract and concrete concepts were differentiated on various aspects rather than a single mechanism. Further studies may help illuminate the representational content in regions that support category identification across stimulus formats, such as studies using item‐repetition priming [Grill‐Spector et al., 1999; James et al., 2002; Vuilleumier et al., 2002] or Dynamically Adaptive Imaging [Cusack et al., 2011].
By using multi‐voxel pattern analysis, this study successfully identified brain activity patterns as abstract or concrete based on single‐trial data, suggesting participants' mental states during processing of abstract and concrete semantic concepts were identifiable from distributed patterns of activity on an individual trial basis. The ability to identify whether a participant was representing abstract or concrete concepts solely from other participants' data suggests the cross‐individual principles of processing this type of knowledge are similar.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
REFERENCES
- Aggleton JP, Pearce JM ( 2001): Neural systems underlying episodic memory: Insights from animal research. Philos Trans R Soc Lond Series B: Biol Sci 356: 1467–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalou LW ( 1999): Perceptual symbol systems. Behav Brain Sci 22: 577–660. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL ( 2009): Where is the semantic system? A critical review and meta‐analysis of 120 functional neuroimaging studies. Cerebral Cortex 19: 2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Springer JA, Kaufman JN, Possing ET ( 2000): Human temporal lobe activation by speech and nonspeech sounds. Cerebral Cortex 10: 512–528. [DOI] [PubMed] [Google Scholar]
- Binder JR, Westbury CF, McKiernan KA, Possing ET, Medler DA ( 2005): Distinct brain systems for processing concrete and abstract concepts. J Cogn Neurosci 17: 905–917. [DOI] [PubMed] [Google Scholar]
- Bishop CM ( 2006): Pattern Recognition and Machine Learning. New York: Springer. [Google Scholar]
- Boatman D, Gordon B, Hart J, Selnes O, Miglioretti D, Lenz F ( 2000): Transcortical sensory aphasia: Revisited and revised. Brain 123: 1634–1642. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM ( 2004): Development of brain mechanisms for processing orthographic and phonologic representations. J Cogn Neurosci 16: 1234–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedin SD, Saffran EM, Coslett HB ( 1994): Reversal of the concreteness effect in a patient with semantic dementia. Cogn Neuropsychology 11: 617–660. [Google Scholar]
- Buchsbaum BR, Hickok G, Humphries C ( 2001): Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cogn Sci 25: 663–678. [Google Scholar]
- Burton MW ( 2001): The role of inferior frontal cortex in phonological processing. Cogn Sci 25: 695–709. [Google Scholar]
- Butler T, Imperato‐McGinley J, Pan H, Voyer D, Cordero J, Zhu Y‐S, Stern E, Silbersweig D ( 2006): Sex differences in mental rotation: Top‐down versus bottom‐up processing. Neuroimage 32: 445–456. [DOI] [PubMed] [Google Scholar]
- Carlson TA, Schrater P, He S ( 2003): Patterns of activity in the categorical representations of objects. J Cogn Neurosci 15: 704–717. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B and others ( 2008): Cingulate‐precuneus interactions: A new locus of dysfunction in adult attention‐deficit/hyperactivity disorder. Biol Psychiatry 63: 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR ( 2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129: 564–583. [DOI] [PubMed] [Google Scholar]
- Chan AM, Halgren E, Marinkovic K, Cash SS ( 2011): Decoding word and category‐specific spatiotemporal representations from MEG and EEG. Neuroimage 54: 3028–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Lehéricy S, Chochon F, Lemer C, Rivaud S, Dehaene S ( 2002): Language–specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain 125: 1054–1069. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Kosslyn SM, Breiter HC, DiGirolamo GJ, Thompson WL, Anderson AK, Bookheimer SY, Rosen BR, Belliveau JW ( 1996): Changes in cortical activity during mental rotation: A mapping study using functional MRI. Brain 119: 89–100. [DOI] [PubMed] [Google Scholar]
- Cox DD, Savoy RL ( 2003): Functional magnetic resonance imaging (fMRI) "brain reading": Detecting and classifying distributed patterns of fMRI activity in human visual cortex. Neuroimage 19: 261–270. [DOI] [PubMed] [Google Scholar]
- Crinion JT, Lambon‐Ralph MA, Warburton EA, Howard D, Wise RJS ( 2003): Temporal lobe regions engaged during normal speech comprehension. Brain 126: 1193–1201. [DOI] [PubMed] [Google Scholar]
- Cusack R, Veldsman M, Naci L, Mitchell DJ, Linke AC ( 2011): Seeing different objects in different ways: Measuring ventral visual tuning to sensory and semantic features with dynamically adaptive imaging. Hum Brain Mapp. doi: 10.1002/hbm.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Aguirre GK, Stallcup M, Alsop D, Tippet LJ, Farah MJ ( 1997): A functional MRI study of mental image generation. Neuropsychologia 35: 725–730. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M ( 1995): The neural basis of the central executive system of working memory. Nature 378: 279–281. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Ruparel K, Fan Y, Shen DG, Acharyya M, Loughead JW, Gur RC, Langleben DD ( 2005): Classifying spatial patterns of brain activity with machine learning methods: Application to lie detection. Neuroimage 28: 663–668. [DOI] [PubMed] [Google Scholar]
- Davis MH, Coleman MR, Absalom AR, Rodd JM, Johnsrude IS, Matta BF, Owen AM, Menon DK ( 2007): Dissociating speech perception and comprehension at reduced levels of awareness. Proc Natl Acad Sci USA 104: 16032–16037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH, Johnsrude IS ( 2003): Hierarchical processing in spoken language comprehension. J Neurosci 23: 3423–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD ( 1995): Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task difficulty and process specificity. J Neurosci 15: 5870–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Jamison HL, Gonnerman LM, Matthews PM ( 2006): The role of the posterior fusiform gyrus in reading. J Cogn Neurosci 18: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehrmann O, Weigelt S, Altmann CF, Kaiser J, Naumer MJ ( 2010): Audiovisual functional magnetic resonance imaging adaptation reveals multisensory integration effects in object‐related sensory cortices. J Neurosci 30: 3370–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Redfern BB, Jaeger JJ ( 2004): Lesion analysis of the brain areas involved in language comprehension. Cognition 92: 145–177. [DOI] [PubMed] [Google Scholar]
- Eviatar Z, Menn L, Zaidel E ( 1990): Concreteness: Nouns, verbs, and hemispheres. Cortex 26: 611–624. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Kanwisher N ( 2009): Neuroimaging of language: Why hasn't a clearer picture emerged? Lang Ling Compass 3: 839–865. [Google Scholar]
- Fiebach CJ, Friederici AD ( 2004): Processing concrete words: fMRI evidence against a specific right‐hemisphere involvement. Neuropsychologia 42: 62–70. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RSJ, Dolan RJ ( 1995): The mind's eye—Precuneus activation in memory‐related imagery. Neuroimage 2: 195–200. [DOI] [PubMed] [Google Scholar]
- Fliessbach K, Weis S, Klaver P, Elger CE, Weber B ( 2006): The effect of word concreteness on recognition memory. Neuroimage 32: 1413–1421. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G ( 2008): The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage 42: 1178–1184. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Opitz B, von Cramon DY ( 2000): Segregating semantic and syntactic aspects of processing in the human brain: An fMRI investigation of different word types. Cerebral Cortex 10: 698–705. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Rüschemeyer S‐A, Hahne A, Fiebach CJ ( 2003): The role of left inferior frontal and superior temporal cortex in sentence comprehension: Localizing syntactic and semantic processes. Cerebral Cortex 13: 170–177. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Hayward WG, Tarr MJ, Anderson AW, Skudlarski P, Gore JC ( 2002): BOLD activity during mental rotation and viewpoint‐dependent object recognition. Neuron 34: 161–171. [DOI] [PubMed] [Google Scholar]
- Gelfand JR, Bookheimer SY ( 2003): Dissociating neural mechanisms of temporal sequencing and processing phonemes. Neuron 38: 831–842. [DOI] [PubMed] [Google Scholar]
- Gennari SP, MacDonald MC, Postle BR, Seidenberg MS ( 2007): Context‐dependent interpretation of words: Evidence for interactive neural processes. Neuroimage 35: 1278–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N ( 1965): Disconnection syndromes in animals and man. Brain 88: 237–294. [DOI] [PubMed] [Google Scholar]
- Ghaem O, Mellet E, Crivello F, Tzourio N, Mazoyer B, Berthoz A, Denis M ( 1997): Mental navigation along memorized routes activates the hippocampus, precuneus, and insula. Neuroreport 8: 739–744. [DOI] [PubMed] [Google Scholar]
- Goldberg RF, Perfetti CA, Fiez JA, Schneider W ( 2007): Selective retrieval of abstract semantic knowledge in left prefrontal cortex. J Neurosci 27: 3790–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves WW, Binder JR, Desai RH, Conant LL, Seidenberg MS ( 2010): Neural correlates of implicit and explicit combinatorial semantic processing. Neuroimage 53: 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill‐Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y ( 1999): Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron 24: 187–203. [DOI] [PubMed] [Google Scholar]
- Grossman M, Koenig P, DeVita C, Glosser G, Alsop D, Detre J, Gee J ( 2002): The neural basis for category‐specific knowledge: An fMRI study. Neuroimage 15: 936–948. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Immisch I, Toma K, Dimyan MA, Van Gelderen P, Hallett M ( 2003): Functional properties of brain areas associated with motor execution and imagery. J Neurophysiology 89: 989–1002. [DOI] [PubMed] [Google Scholar]
- Hanke M, Halchenko YO, Haxby JV, Pollmann S ( 2010): Statistical learning analysis in neuroscience: Aiming for transparency. Front Neurosci 3: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SJ, Halchenko YO ( 2007): Brain reading using full brain support vector machines for object recognition: There is no ‘face’ identification area. Neural Comput 20: 486–503. [DOI] [PubMed] [Google Scholar]
- Hanson SJ, Matsuka T, Haxby JV ( 2004): Combinatorial codes in ventral temporal lobe for object recognition: Haxby (2001) revisited: Is there a "face" area? Neuroimage 23: 156–166. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Chabris CF, Clark J, Urban T, Aharon I, Steele S, McGrath L, Condouris K, Tager‐Flusberg H ( 2006): Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cogn 61: 54–68. [DOI] [PubMed] [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R ( 2004): Intersubject synchronization of cortical activity during natural vision. Science 303: 1634–1640. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P ( 2001): Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science 293: 2425–2430. [DOI] [PubMed] [Google Scholar]
- Haynes JD, Rees G ( 2006): Decoding mental states from brain activity in humans. Nat Rev Neurosci 7: 523–34. [DOI] [PubMed] [Google Scholar]
- Hickok G, Erhard P, Kassubek J, Helms‐Tillery AK, Naeve‐Velguth S, Strupp JP, Strick PL, Ugurbil K ( 2000): A functional magnetic resonance imaging study of the role of left posterior superior temporal gyrus in speech production: Implications for the explanation of conduction aphasia. Neurosci Lett 287: 156–160. [DOI] [PubMed] [Google Scholar]
- James TW, Humphrey GK, Gati JS, Menon RS, Goodale MA ( 2002): Differential effects of viewpoint on object‐driven activation in dorsal and ventral streams. Neuron 35: 793–801. [DOI] [PubMed] [Google Scholar]
- Jastorff J, Clavagnier S, Gergely G, Orban GA ( 2010): Investigating action understanding: Activation of the middle temporal gyrus by irrational actions. J Vision 10: 1092. [Google Scholar]
- Jessen F, Heun R, Erb M, Granath D, Klose U, Papassotiropoulos A ( 2000): The concreteness effect: Evidence for dual‐coding and context availability. Brain Lang 74: 103–112. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Aryal S, Mitchell TM ( 2010): A Neurosemantic theory of concrete noun representation based on the underlying brain codes. PLoS ONE 5: e8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM ( 1997): The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama K, Takahashi N, Ogawara K, Hattori T ( 1999): Pure topographical disorientation due to right posterior cingulate lesion. Cortex 35: 279–282. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss‐Moreau E, Lindquist K, Wager TD ( 2008): Functional grouping and cortical–subcortical interactions in emotion: A meta‐analysis of neuroimaging studies. Neuroimage 42: 998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig P, Smith EE, Glosser G, DeVita C, Moore P, McMillan C, Gee J, Grossman M ( 2005): The neural basis for novel semantic categorization. Neuroimage 24: 369–383. [DOI] [PubMed] [Google Scholar]
- Krause BJ, Schmidt D, Mottaghy FM, Taylor J, Halsband U, Herzog H, Tellmann L, Müller‐Gärtner H‐W ( 1999): Episodic retrieval activates the precuneus irrespective of the imagery content of word pair associates. Brain 122: 255–263. [DOI] [PubMed] [Google Scholar]
- Kroll JF, Merves JS ( 1986): Lexical access for concrete and abstract words. J Exp Psychology: Learn Memory Cogn 12: 92–107. [Google Scholar]
- Kuperberg GR, West WC, Lakshmanan BM, Goff D ( 2008): Functional magnetic resonance imaging reveals neuroanatomical dissociations during semantic integration in schizophrenia. Biol Psychiatry 64: 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakoff G, Johnson M ( 1980): Metaphors we Live by. Chicago: University of Chicago Press. [Google Scholar]
- Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J ( 2003): Brain activations during motor imagery of locomotor‐related tasks: A PET study. Hum Brain Mapp 19: 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantani T, Okamoto Y, Shirao N, Okada G, Yamawaki S ( 2005): Reduced activation of posterior cingulate cortex during imagery in subjects with high degrees of alexithymia: A functional magnetic resonance imaging study. Biol Psychiatry 57: 982–990. [DOI] [PubMed] [Google Scholar]
- Marschark M, Cornoldi C ( 1991): Imagery and verbal memory In: Cornoldi C, McDaniel A, editors. Imagery and Cognition. New York: Springer‐Verlag; pp 133–182. [Google Scholar]
- Martin A, Chao LL ( 2001): Semantic memory and the brain: Structure and processes. Curr Opin Neurobiol 11: 194–201. [DOI] [PubMed] [Google Scholar]
- Mellet E, Tzourio‐Mazoyer N, Bricogne S, Mazoyer B, Kosslyn SM, Denis M ( 2000): Functional anatomy of high‐resolution visual mental imagery. J Cogn Neurosci 12: 98–109. [DOI] [PubMed] [Google Scholar]
- Mellet E, Tzourio N, Denis M, Mazoyer B ( 1998): Cortical anatomy of mental imagery of concrete nouns based on their dictionary definition. Neuro Report 9: 803–808. [DOI] [PubMed] [Google Scholar]
- Mervis CB, John AE ( 2008): Vocabulary abilities of children with Williams syndrome: Strengths, weaknesses, and relation to visuospatial construction ability. J Speech Lang Hear Res 51: 967–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestres‐Missé A, Càmara E, Rodriguez‐Fornells A, Rotte M, Münte TF ( 2008): Functional neuroanatomy of meaning acquisition from context. J Cogn Neurosci 20: 2153–2166. [DOI] [PubMed] [Google Scholar]
- Mestres‐Missé A, Münte TF, Rodriguez‐Fornells A ( 2009): Functional neuroanatomy of contextual acquisition of concrete and abstract words. J Cogn Neurosci 21: 2154–2171. [DOI] [PubMed] [Google Scholar]
- Mesulam MM ( 1998): From sensation to cognition. Brain 121: 1013–1052. [DOI] [PubMed] [Google Scholar]
- Mitchell TM, Hutchinson R, Niculescu RS, Pereira F, Wang X, Just M, Newman S ( 2004): Learning to decode cognitive states from brain images. Mach Learn 57: 145–175. [Google Scholar]
- Mitchell TM, Shinkareva SV, Carlson A, Chang K‐M, Malave VL, Mason RA, Just MA ( 2008): Predicting human brain activity associated with the meanings of nouns. Science 320: 1191–1195. [DOI] [PubMed] [Google Scholar]
- Mizelle JC, Wheaton LA ( 2010): Neural activation for conceptual identification of correct versus incorrect tool‐object pairs. Brain Res 1354: 100–112. [DOI] [PubMed] [Google Scholar]
- Mourão‐Miranda J, Bokde AL, Born C, Hampel H, Stetter M ( 2005): Classifying brain states and determining the discriminating activation patterns: Support vector machine on functional MRI data. Neuroimage 28: 980–995. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Schumacher EH, Goebel R, D'Esposito M ( 2008): Functional MRI investigation of verbal selection mechanisms in lateral prefrontal cortex. Neuroimage 43: 801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppeney U, Price CJ ( 2004): Retrieval of abstract semantics. Neuroimage 22: 164–170. [DOI] [PubMed] [Google Scholar]
- Norman KA, Polyn SM, Detre GJ, Haxby JV ( 2006): Beyond mind‐reading: Multi‐voxel pattern analysis of fMRI data. Trends Cogn Sci 10: 424–430. [DOI] [PubMed] [Google Scholar]
- O'Toole A, Jiang F, Abdi H, Haxby JV ( 2005): Partially distributed representations of objects and faces in ventral temporal cortex. J Cogn Neurosci 17: 580–590. [DOI] [PubMed] [Google Scholar]
- O'Toole AJ, Jiang F, Abdi H, Penard N, Dunlop JP, Parent MA ( 2007): Theoretical, statistical, and practical perspectives on pattern‐based classification approaches to the analysis of functional neuroimaging data. J Cogn Neurosci 19: 1735–1752. [DOI] [PubMed] [Google Scholar]
- Ojemann GA, Creutzfeldt O, Lettich E, Haglund MM ( 1988): Neuronal activity in human lateral temporal cortex related to short‐term verbal memory, naming and reading. Brain 111: 1383–1404. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Demonet J‐F, Fazio F, McCrory E, Chanoine V, Brunswick N, Cappa SF, Cossu G, Habib M, Frith CD ( 2001): Dyslexia: Cultural diversity and biological unity. Science 291: 2165–2167. [DOI] [PubMed] [Google Scholar]
- Perani D, Cappa SF, Schnur T, Tettamanti M, Collina S, Rosa MM, Fazio F ( 1999a): The neural correlates of verb and noun processing: A PET study. Brain 122: 2337–2344. [DOI] [PubMed] [Google Scholar]
- Perani D, Schnur T, Tettamanti M, Gorno‐Tempini M, Cappa SF, Fazio F ( 1999b): Word and picture matching: A PET study of semantic category effects. Neuropsychologia 37: 293–306. [DOI] [PubMed] [Google Scholar]
- Pereira F, Mitchell T, Botvinick M ( 2009): Machine learning classifiers and fMRI: A tutorial overview. Neuroimage 45: S199–S209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME ( 1988): Positron emission tomographic studies of the cortical anatomy of single‐word processing. Nature 331: 585–589. [DOI] [PubMed] [Google Scholar]
- Pexman PM, Hargreaves IS, Edwards JD, Henry LC, Goodyear BG ( 2007): Neural correlates of concreteness in semantic categorization. J Cogn Neurosci 19: 1407–1419. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Halchenko YO, Hanson SJ ( 2009): Decoding the large‐scale structure of brain function by classifying mental states across individuals. Psychol Sci 20: 1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyn SM, Natu VS, Cohen JD, Norman KA ( 2005): Category‐specific cortical activity precedes retrieval during memory search. Science 310: 1963–1966. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT ( 2003): The myth of the visual word form area. Neuroimage 19: 473–481. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Shaywitz BA, Shaywitz SE, Fulbright RK, Constable RT, Skudlarski P, Marchione KE, Jenner AR, Fletcher JM ( 2000): The angular gyrus in developmental dyslexia: Task‐specific differences in functional connectivity within posterior cortex. Psychol Sci 11: 51–56. [DOI] [PubMed] [Google Scholar]
- Rodd JM, Longe OA, Randall B, Tyler LK ( 2010): The functional organisation of the fronto‐temporal language system: Evidence from syntactic and semantic ambiguity. Neuropsychologia 48: 1324–1335. [DOI] [PubMed] [Google Scholar]
- Sabsevitz DS, Medler AA, Seidenberg M, Binder JR ( 2005): Modulation of the semantic system by word imageability. Neuroimage 27: 188–200. [DOI] [PubMed] [Google Scholar]
- Schwanenflugel PJ ( 1991): Why are abstract concepts hard to understand? In: Schwanenflugel PJ, editor. The Psychology of Word Meanings. Hillsdale, NJ: Erlbaum; pp 223–250. [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, Coslett HB ( 2009): Anterior temporal involvement in semantic word retrieval: Voxel‐based lesion‐symptom mapping evidence from aphasia. Brain 132: 3411–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJS ( 2000): Identification of a pathway for intelligible speech in the left temporal lobe. Brain 123: 2400–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkareva SV, Malave VL, Just MA, Mitchell TM (In press): Commonalities across participants in the neural representation of objects. Hum Brain Mapp, doi: 10.1002/hbm.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkareva SV, Malave VL, Mason RA, Mitchell TM, Just MA ( 2011): Commonality of neural representations of words and pictures. Neuroimage 54: 2418–2425. [DOI] [PubMed] [Google Scholar]
- Shinkareva SV, Mason RA, Malave VL, Wang W, Mitchell TM, Just MA ( 2008): Using fMRI brain activation to identify cognitive states associated with perception of tools and dwellings. PLoS ONE 3: e1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M, Sassa Y, Watanabe J, Akitsuki Y, Maeda Y, Matsue Y, Kawashima R ( 2009): Anatomical segregation of representations of personally familiar and famous people in the temporal and parietal cortices. J Cogn Neurosci 21: 1855–1868. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Shah NJ, Zilles K, Fink GR ( 2005): Cortical representations of personally familiar objects and places: Functional organization of the human posterior cingulate cortex. J Cogn Neurosci 17: 183–198. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Manenti R, Rosa PAD, Falini A, Perani D, Cappa SF, Moro A ( 2008): Negation in the brain: Modulating action representations. Neuroimage 43: 358–367. [DOI] [PubMed] [Google Scholar]
- Thompson‐Schill SL, D'Esposito M, Aguirre GK, Farah MJ ( 1997): Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc Natl Acad Sci USA 94: 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Luka BJ ( 2006): Neural localization of semantic context effects in electromagnetic and hemodynamic studies. Brain Lang 97: 279–293. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S ( 2006): Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage 29: 452–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Henson RN, Driver J, Dolan RJ ( 2002): Multiple levels of visual object constancy revealed by event‐related fMRI of repetition priming. Nat Neurosci 5: 491–499. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Paré‐Blagoev EJ, Clark J, Poldrack RA ( 2001): Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron 31: 329–338. [DOI] [PubMed] [Google Scholar]
- Wallentin M, Østergaard S, Lund TE, Østergaard L, Roepstorff A ( 2005): Concrete spatial language: See what I mean? Brain Lang 92: 221–233. [DOI] [PubMed] [Google Scholar]
- Wang J, Conder JA, Blitzer DN, Shinkareva SV ( 2010): Neural representation of abstract and concrete concepts: A meta‐analysis of neuroimaging studies. Hum Brain Mapp 31: 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatmough C, Verret L, Fung D, Chertkow H ( 2004): Common and contrasting areas of activation for abstract and concrete concepts: An H2 15O PET study. J Cogn Neurosci 16: 1211–1226. [DOI] [PubMed] [Google Scholar]
- Wheatley T, Weisberg J, Beauchamp MS, Martin A ( 2005): Automatic priming of semantically related words reduces activity in the fusiform gyrus. J Cogn Neurosci 17: 1871–1885. [DOI] [PubMed] [Google Scholar]
- Whitney C, Kirk M, O'Sullivan J, Lambon Ralph MA, Jefferies E ( 2010): The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cerebral Cortex 21: 1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RJS, Howard D, Mummery CJ, Fletcher P, Leff A, Büchel C ( 2000): Noun imageability and the temporal lobes. Neuropsychologia 38: 985–994. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E, Gjedde A ( 1992): Lateralization of phonetic and pitch discrimination in speech processing. Science 256: 846–849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


