Abstract
Treatment‐refractory depression (TRD) represents a large proportion of the depressive population, yet has seldom been investigated using advanced imaging techniques. To characterize brain dysfunction in TRD, we performed resting‐state functional MRI (rs‐fMRI) on 22 TRD patients, along with 26 matched healthy subjects and 22 patients who were depressed but not treatment‐refractory (NDD) as comparison groups. Results were analyzed using a data‐driven approach known as Regional Homogeneity (ReHo) analysis which measures the synchronization of spontaneous fMRI signal oscillations within spatially neighboring voxels. Relative to healthy controls, both depressed groups showed high ReHo primarily within temporo‐limbic structures, and more widespread low ReHo in frontal, parietal, posterior fusiform cortices, and caudate. TRD patients showed more cerebral regions with altered ReHo than did NDD. Moderate but significant correlations between the altered regional ReHo and measures of clinical severity were observed in some identified clusters. These findings shed light on the pathophysiological mechanisms underlying TRD and demonstrate the feasibility of using ReHo as a research and clinical tool to monitor persistent cerebral dysfunction in depression, although further work is necessary to compare different measures of brain function to elucidate the neural substrates of these ReHo abnormalities. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: treatment‐refractory depression, functional magnetic resonance imaging, resting‐state, regional homogeneity, major depressive disorder, not‐refractory MDD
INTRODUCTION
Despite advances in the therapy of depressive disorders, some 15–30% of depressed patients are resistant to treatment, leading to significant personal, social, and economic burden [Berlim and Turecki,2007]. Development of more effective treatment strategies for this condition will require better knowledge of its neurobiological basis. Functional imaging studies using positron emission tomography (PET) or blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) have focused on major depressive disorder (MDD), [Drevets,2000; Sheline,2003], revealing functional abnormalities in specific cerebral regions including anterior cingulate cortex (ACC), hippocampus, amygdala, basal ganglia, and frontal lobes [Bonelli et al.,2006; Davidson et al.,1999; Drevets,2000; Goodwin,1997; Videbech and Ravnkilde,2004]. In treatment‐refractory depression (TRD), increased regional cerebral blood flow (rCBF) at medication‐free baseline has been reported in limbic‐cortical regions using PET [Mayberg et al.,2005] and single photon emission computed tomography (SPECT), [Hornig et al.,1997]; this increased metabolism is reduced by deep brain stimulation (DBS), [Mayberg et al.,2005] and vagus nerve stimulation (VNS), [Pardo et al.,2008]. These observations in TRD were of brain activity in the resting state. Similarly, resting‐state fMRI (rs‐fMRI) has recently been suggested as a promising approach to studying MDD because of the persistent and pervasive nature of depressive symptoms [Greicius et al.,2007]. Anand et al. found significantly decreased resting state functional connectivity (FC) between dorsal‐ and pre‐genual ACC and limbic regions in medication‐free depressed patients compared to healthy subjects [Anand et al.,2005a], which was normalized after antidepressant treatment [Anand et al.,2005b,2007]. Greicius et al. found increased resting state subgenual cingulate and thalamic FC with the default‐mode network [Greicius et al.,2007]. Both results implicate cortical‐limbic circuit dysregulation in depression.
Recently a new analytical method, Regional Homogeneity (ReHo), was developed to characterize the synchronization of BOLD signal fluctuation among neighboring voxels within a single region, and this can complement the earlier FC analysis in analyzing disease‐related rs‐fMRI data [Zang et al.,2004]. Based on the hypothesis that spatially neighboring voxels should have similar temporal patterns, ReHo calculates the Kendall's coefficient of concordance (KCC), [Kendall and Gibbons,1990] between the time series of a given voxel and those of its neighbors in a voxel‐wise manner. ReHo has been applied to the investigation of brain function in healthy subjects [He et al.,2004; Zang et al.,2004] and has identified cerebral regions, notably the posterior cingulate, medial prefrontal and bilateral inferior parietal areas, whose KCC is larger than that of the rest of the brain. This finding is in line with an early resting PET study that found higher metabolism in these cerebral regions relative to the whole brain [Raichle et al.,2001]. A number of rs‐fMRI studies of psychiatric and neurological disorders have also employed ReHo as an analytical approach [Bai et al.,2008; He et al.,2007; Liu et al.,2006; Paakki et al.,2009; Wu and Hallett,2008; Yuan et al.,2008; Zang et al.,2004]. Notably, the recent application of ReHo to remitted geriatric depression [Yuan et al.,2008] and MDD [Yao et al.,2008] demonstrates its capability to detect regional functional changes in the resting state which are associated with depression.
Given the paucity of studies applying advanced imaging techniques specifically to TRD and the potential importance of such studies for the development of more effective treatment strategies, we performed rs‐fMRI on a group of TRD patients along with two comparison groups: nonrefractory MDD (NDD) patients and healthy controls. With the aim of identifying imaging markers that distinguish these groups, ReHo was employed to compare their regional brain functions in the resting state. We hypothesized that regional brain dysfunction would be detected in TRD relative to both NDD and healthy control subjects, particularly in cerebral regions which have been implicated in depression by previous studies.
MATERIALS AND METHODS
Participants
Depressed patients were originally recruited for a clinical trial from the Mental Health Center of our university‐affiliated hospital. Patients were diagnosed by two experienced psychiatrists using the Structured Clinical Interview according to DSM‐IV criteria [First et al.,1997] and the Research Diagnostic Criteria for major depressive disorder. Exclusion criteria for patients included: bipolar disorder or psychiatric disorders other than depression; any history of head trauma with loss of consciousness; any cardiovascular, renal, or other major medical illness; age less than 18 or more than 60 years; not being right‐handed; and any psychiatric treatment or substance misuse during 2 months prior to inclusion in the study. In view of the high comorbidity of depression with anxiety, we specifically assessed this in depressed subjects, and excluded those who also met the diagnostic criteria for anxiety disorder. For each subject, MRI scan was performed just once before the start of the trial. Depression severity was assessed on the day of MRI scan using the 17‐item Hamilton Rating Scale for Depression (HRSD), [Hamilton,1967] and inclusion required a total HRSD score no less than 18. Antidepressant treatments were applied according to standard local clinical protocols immediately following the MRI examination. Three classes of antidepressants were used: tricyclic, typical serotonin‐norepinephrine reuptake inhibitors, and typical selective serotonin reuptake inhibitors. After the treatment trials, MDD patients were separated into a TRD group and a NDD group based on responses to treatment: the TRD group was defined by nonresponsiveness to at least two adequate trials [in terms of dosage, duration (6 weeks for each trial), and compliance] with antidepressants from different pharmacologic classes [Berlim and Turecki,2007; World Psychiatry Association,1974]; nonresponsiveness was defined as less than 50% reduction in HRSD scores after antidepressant treatments at a minimum dose of 150 mg day−1 of imipramine equivalents (dose converted using a conversion table [Iidaka et al.,1997]). The assignment of patients was made blind to any MRI results. Right‐handed healthy control subjects were recruited by advertisement. Nonpatient Version Structured Interview from the Diagnostic and Statistical Manual of Mental Disorders IV was used to screen the healthy subjects to confirm the absence of a history of psychiatric or neurologic illness. All the study procedures were approved by the local Ethics Committee, and written informed consent was obtained from all participants.
MRI Data Acquisition
MRI data from all participants were acquired using a 3T GE scanner (EXCITE, GE Signa, Milwaukee, USA) with an 8‐channel phase‐array head coil as MR signal receiver. After a localizer scan and conventional structural imaging, resting‐state functional images were obtained with an echo‐planar imaging (EPI) sequence. Sequence parameters were as follows: 30 contiguous slices with a slice thickness = 5 mm, repetition time (TR) = 2,000 ms, echo time (TE) = 30 ms, flip angle (FA) = 90°, field of view (FOV) = 24 × 24 cm2, data matrix = 64 × 64, and total volumes = 200. During the rs‐fMRI scan, the head was positioned carefully with comfortable support, and ear plugs were used against scanning noise. Subjects were instructed to relax with eyes closed, but without falling asleep and without directed, systematic thought. This was confirmed after completion of scanning.
Data Processing
None of the participants showed brain abnormalities on conventional MRI as assessed by two experienced radiologists. After an inspection of the quality of raw functional images, data preprocessing was performed in SPM5 (http://www.fil.ion.ucl.ac.uk/spm), including slice timing, realignment, and normalization. The first five volumes (10 s) of the data were discarded to ensure stable magnetization and allow the participants to adapt to the EPI scanning environment. In total, 9 subjects were excluded for flaws on raw images or excessive head motion (>1 mm of translation or 1° of rotation), resulting in a final sample of 26 healthy subjects, 22 TRD patients and 22 NDD patients. We performed ANOVA on the mean absolute estimated motion parameters of the final subjects: specifically, individual maximum translation along x, y, and z axes and the square root of the sum of their squares (RSS), as well as the maximum rotation angles, were examined among groups.
ReHo analysis was performed using the in‐house software REST (http://resting-fmri.sourceforge.net). The linear trend of the time series was removed and band‐pass filtering (0.01 Hz < f < 0.08 Hz) was performed to reduce the influence of physiological noise, such as the respiratory and cardiac rhythms. Individual ReHo maps were generated by assigning each voxel a value corresponding to the KCC of its time series with its nearest 26 neighboring voxels [Kendall and Gibbons,1990; Zang et al.,2004]. Then a mask (made from the MNI template to assure matching with the normalization step) was used to remove non‐brain tissues and noise on the ReHo maps, and for standardization purposes the individual ReHo maps were divided by their own mean KCC within the mask [Paakki et al.,2009; Yuan et al.,2008]. To avoid introducing bias, we first compared the mean within‐mask KCC values of the three groups with ANOVA, and found no group differences (P = 0.702).
Statistical Analysis
Distributions of age and gender among groups were compared using one‐way analysis of variance (ANOVA) and chi‐square test, respectively. A two‐sample t‐test was performed to compare the disease duration and HRSD score between TRD and NDD groups (Table I).
Table I.
Demographic information and disease severity for three groups
| Variables (Mean ± SD) | Control (n = 26) | NDD (n = 22) | TRD (n = 22) | P value |
|---|---|---|---|---|
| Gender (M:F) | 16:10 | 10:12 | 15:07 | 0.288# |
| Age (yrs) | 33 ± 8 | 35 ± 13 | 35 ± 13 | 0.297* |
| Age range | 18–49 | 18–60 | 18–61 | |
| Disease duration (months) | — | 32 ± 64 | 103 ± 65 | 0.001+ |
| HRSD | — | 23.2 ± 4.8 | 22.0 ± 3.5 | 0.378+ |
#, * and + indicate P values for chi‐square test, one‐way ANOVA, and 2 sample t‐test, respectively.
Voxel‐based comparison of whole brain ReHo maps was conducted in SPM5 using a full factorial model, followed by post hoc t‐tests to identify differences between each pair of groups. Although age was not significantly different between groups, it was included as a covariate to avoid any undetected age effect. Cerebral regions showing significant differences between two groups were identified and used as regions of interest (ROI) to extract regional mean ReHo values for correlation analysis with the clinical measures, namely, disease duration and HRSD score. Of note, for each activated cluster (ROI) the correlation analysis was restricted within the depressed group(s) from whose comparison the cluster was derived.
In addition to voxel based analysis (VBA), we performed ROI‐based analyses for defined small structures which are thought to be important in mood modulation. ROIs of bilateral amygdala, hippocampus, insula, putamen, caudate, globus pallidus and thalamus were adopted from the Anatomical Automatic Labeling (AAL) template. In a plugin for SPM, MarsBaR (http://marsbar.sourceforge.net), ANOVA and correlative analyses were implemented on regional ReHo values to examine group differences and relationships with clinical measures.
RESULTS
The demographic and clinical data are presented in Table I. Gender and age did not differ significantly among the three subject groups. There was no significant difference in pretreatment HRSD score between NDD and TRD groups, although their post‐treatment HRSD scores were significantly different (NDD: 8.4 ± 3.1; TRD: 16.9 ± 4.1; P < 0.001). Disease duration was significantly greater in the TRD group compared to NDD (P = 0.001). ANOVA of head motion parameters demonstrated no significant differences between groups (translation (RSS): P = 0.750; rotation: P = 0.668).
In the voxel‐based group comparisons of ReHo maps, we chose a statistical threshold at voxel level P < 0.005 (uncorrected) with a cluster extent of κ > 20 voxels to reduce Type I error, which resulted in a cluster level corrected P < 0.001. When the NDD and TRD groups were pooled into a whole MDD group and compared to healthy controls, MDD subjects (Fig. 1a) showed higher ReHo in limbic structures including bilateral anterior cingulate cortex (ACC) and adjacent medial prefrontal cortex, right insula, and right parahippocampal gyrus, as well as lower ReHo in several left‐sided cerebral regions including the posterior fusiform gyrus (PFG), the inferior frontal area, inferior parietal lobule (IPL), caudate body, and a small area in rectal gyrus. When compared to NDD, TRD subjects (Fig. 1d) showed higher ReHo in the right middle temporal gyrus, right insula and middle cingulate, and lower ReHo in the left precuneus and left inferior frontal gyrus. Compared to healthy controls, the NDD group (Fig. 1b) showed higher ReHo in small clusters in the medial prefrontal and parahippocampal areas, and lower ReHo in the left PFG. The TRD group (Fig 1c) showed more widespread brain regions with altered ReHo relative to the healthy group than did the NDD group (compare Fig. 1c with Fig. 1b). Regions with higher ReHo in TRD (Fig. 1c) included bilateral ACC and adjacent medial prefrontal gyrus, right insula and connected transverse temporal gyrus, superior and middle temporal gyrus, and left‐sided posterior medial frontal gyrus. Clusters with lower ReHo were all found in the left hemisphere and included lateral inferior frontal gyrus, PFG, IPL, superior parietal lobule and precuneus. More detailed information about the clusters in all the group comparisons is presented in Table II.
Figure 1.
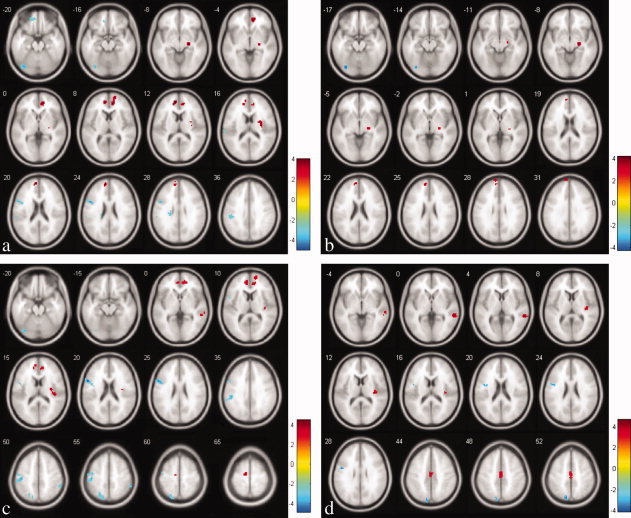
Statistical maps showing ANOVA results of ReHo maps amongst three subject groups: (a) the pooled MDD group compared with the healthy controls; (b) NDD subjects compared with healthy controls; (c) TRD subjects compared with healthy controls; and (d) comparison between TRD and NDD groups. Red and blue denote increased and decreased ReHo respectively and the color bars indicate the t value from post hoc analysis between each pair of groups. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table II.
Clusters derived from the voxel based comparisons between participant groups
| Contrasts and brain regions (BA areas) | MNI coordinates (cluster maxima) | t (cluster maxima) | P (cluster level, corrected) | Cluster size | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Dep > control | ||||||
| *ACC, MFG_R, (32, 10) | 12 | 45 | 9 | 3.99 | <0.001 | 85 |
| ACC, MFG_L, (10) | −9 | 48 | 12 | 3.90 | <0.001 | 48 |
| *Insula_R, (13) | 33 | −12 | 18 | 3.87 | <0.001 | 20 |
| *Parahippocampal gyrus_R, (28) | 24 | −18 | −9 | 3.66 | <0.001 | 22 |
| Dep < control | ||||||
| *PFG_L, (19) | −33 | −78 | −18 | 4.87 | <0.001 | 42 |
| Lateral inferior frontal gyrus_L, (44) | −48 | 9 | 24 | 4.28 | <0.001 | 36 |
| Caudate body and adjacent white matter_L | −21 | −24 | 27 | 3.56 | <0.001 | 20 |
| IPL_L, (40, 2) | −48 | −27 | 39 | 3.53 | <0.001 | 52 |
| *Inferior medial prefrontal cortex_L, (10, 11) | −15 | 45 | −21 | 3.19 | <0.001 | 30 |
| TRD > NDD | ||||||
| Middle temporal gyrus_R, (22, 21) | 57 | −39 | 0 | 4.67 | <0.001 | 41 |
| Insula_R, (13) | 36 | −21 | 12 | 3.97 | <0.001 | 23 |
| Middle cingulate, MFG, (24, 31) | −3 | −15 | 45 | 3.85 | <0.001 | 66 |
| TRD < NDD | ||||||
| *Precuneus_L, (7) | −12 | −78 | 54 | 4.03 | <0.001 | 40 |
| Inferior frontal gyrus_L, (9, 6) | −48 | 0 | 27 | 3.49 | <0.001 | 31 |
| NDD > control | ||||||
| Medial prefrontal gyrus_L,(9) | −9 | 54 | 24 | 3.79 | <0.001 | 29 |
| Parahippocampal gyrus_R, (28) | 24 | −21 | −9 | 3.69 | <0.001 | 24 |
| NDD < control | ||||||
| PFG_L, (19) | −33 | −78 | −18 | 4.18 | <0.001 | 28 |
| TRD > control | ||||||
| Insula, Transverse temporal gyrus_R, (13, 41) | 33 | −12 | 18 | 4.49 | <0.001 | 31 |
| ACC, MFG_L, (9, 32) | −9 | 48 | 12 | 4.17 | <0.001 | 50 |
| MFG_L, (6) | −12 | −12 | 66 | 3.94 | <0.001 | 21 |
| ACC, MFG_R, (32, 10) | 15 | 48 | 0 | 3.46 | <0.001 | 86 |
| Superior and middle temporal gyrus_R, (21, 22) | 57 | −39 | 3 | 3.37 | <0.001 | 21 |
| TRD < control | ||||||
| Lateral inferior frontal gyrus_L, (45, 9) | −54 | 9 | 21 | 4.79 | <0.001 | 73 |
| *PFG_L, (19) | −33 | −78 | −18 | 3.98 | <0.001 | 20 |
| IPL_L, (3, 40) | −45 | −24 | 51 | 3.95 | <0.001 | 166 |
| IPL_R, (40) | 54 | −42 | 48 | 3.46 | <0.001 | 23 |
| Superior parietal lobule, Precuneus_L, (7) | −24 | −66 | 54 | 3.38 | <0.001 | 48 |
Abbreviations: ACC, anterior cingulate cortex; PFG, posterior fusiform gyrus; IPL, inferior parietal lobule; MFG, medial frontal gyrus. Labels * indicate clusters which show significant correlations between ReHo values and disease severity measures.
Average ReHo values within these identified clusters were extracted for correlation analysis in MarsBaR. If a region was identified as activated in more than one comparison, e.g., the left PFG, the cluster with highest t value was chosen as the ROI. As a result, 18 regions were entered into the analysis and 36 tests were performed. When the statistical threshold was set at P < 0.05 uncorrected, a series of moderate correlations were observed between regional ReHo values and clinical measures (Table III; Fig. 2). The left PFG and the left precuneus showed negative correlation with disease duration, while the right insular area showed positive correlation. For the HRSD score, the right ACC and the right parahippocampal gyrus demonstrated positive correlations, while the left medial inferior frontal gyrus showed negative correlations. When corrected using the Benjamini and Hochberg False Discovery Rate method, only one correlation (between KCC and disease duration within the precuneus) survives at the corrected P < 0.05 threshold (Table III).
Table III.
Significant correlations between cluster‐ReHo and disease severity measures
| Brain regions (BA areas) | r (Pearson correlation) | P uncorrected | P corrected |
|---|---|---|---|
| Significant correlations with disease duration | |||
| Posterior fusiform gyrus_L, (19) | −0.391 | 0.009 | 0.154 |
| Precuneus_L, (7) | −0.535 | <0.001 | 0.007 |
| Insula_R, (13) | 0.328 | 0.03 | 0.214 |
| Significant correlations with HRSD score | |||
| Anterior cingulate, Medial frontal gyrus_R, (32, 10) | 0.344 | 0.022 | 0.267 |
| Parahippocampal gyrus_R, (28) | 0.316 | 0.037 | 0.219 |
| Inferior medial prefrontal cortex_L, (10, 11) | −0.306 | 0.043 | 0.222 |
Figure 2.
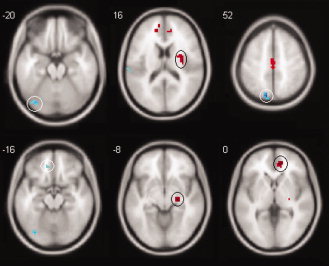
Brain slices chosen from Figure 1 showing circled clusters within which significant correlations were identified between ReHo and clinical measures. The upper row clusters show correlations with total disease duration, the bottom row clusters show correlations with HRSD score; note that the signs of the correlations are in line with the increase and decrease of ReHo in the corresponding clusters from which the correlations were derived. Details of the correlations are presented in Table III. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In the additional ROI analyses on the seven limbic and basal structures, neither ANOVA nor the correlative analyses revealed any significant findings.
DISCUSSION
By using BOLD rs‐fMRI and the ReHo analytical method, we have identified brain regions showing differences in spontaneous BOLD signal activity between healthy control, NDD and TRD groups. In general, TRD patients demonstrated more widely distributed cerebral regions with altered ReHo than did NDD. Relative to healthy controls, depressed groups showed regional high ReHo mainly in temporo‐limbic structures, and more widespread low ReHo in frontal, parietal, and posterior fusiform cortices. Moderate but significant correlations between the altered regional ReHo values and patients' clinical scores were also observed in several clusters.
The results of comparison between pooled MDD patients and controls are consistent with four seminal earlier rs‐fMRI studies in depression [Anand et al.,2005a,b,2007; Greicius et al.,2007]. In brief, Anand et al. found that at resting state, MDD patients showed decreased connectivity between dorsal ACC and some other limbic regions, while Greicius et al. found higher resting‐state subgenual cingulate and thalamic FC with the default mode network in depressed subjects. Both results are consistent with the limbic‐cortical dysregulation model [Mayberg,1997], one showing reduced connectivity to the ‘cognitive’ dorsal ACC and the other excessive connectivity to the “affective” subgenual cingulate. In contrast, we identified a pattern of high ReHo in the temporo‐limbic structures and low ReHo in the cortical regions; this may also suggest decreased coupling between limbic and cortical systems, although the deduction is not as direct as the earlier inter‐regional connectivity analyses. Another consistent finding of the earlier work is correlation of ACC function with clinical severity. Our findings confirm the role of resting‐state function at this site as the marker for disease refractoriness to treatment. More detailed comparison with these earlier results is limited by the methodological differences.
The pattern of high ReHo in temporo‐limbic and low ReHo in cortical regions was also identified in TRD in comparison with NDD. Although this is less marked than when compared with normal controls, the TRD group exhibited far more functional alterations than did NDD. Few direct comparisons have been made of carefully defined TRD, NDD and healthy controls. The results of two early studies using SPECT and morphometric MRI [Hornig et al.,1997; Shah et al.,2002] identified metabolic and structural abnormalities in TRD which were consistent with the pattern of our present findings. In particular, the MRI study found that TRD exhibited volumetric reductions in frontal‐striatal structures compared to both controls and NDD, but found no differences between recovered MDD and healthy subjects [Shah et al.,2002]. Although not assessed, volumetric abnormalities may also exist in our TRD cohort, as well as the functional alterations revealed by ReHo analysis, and the more widely abnormal regional ReHo in the TRD group may be partially attributed to the significantly greater disease duration of this group compared to NDD (Table I). In Shah's work, the TRD group also had significantly greater lifetime illness duration than NDD [Shah et al.,2002].
For TRD, high ReHo was observed in ACC, insular, temporal and frontal areas; while in NDD only small clusters in the parahippocampal gyrus and frontal pole had higher ReHo values than the healthy group. The involvement of these limbic and fronto‐temporal structures in the neuropathology of TRD has been suggested by results in a number of studies [Lui et al.,2009]. Shan et al. have identified gray matter density or volume reductions in ACC, frontal, temporal, and parahippocampal areas [Shah et al.,2002]. In our previous study of TRD using magnetization transfer imaging [Zhang et al.,2009], reduced magnetization transfer ratio, indicative of abnormalities of brain tissue composition, was observed in the ACC, insular and parahippocampal areas.
Other attempts to find metabolic or functional alterations in TRD have revealed increased blood flow in hippocampus‐amygdala in TRD compared to both NDD and healthy controls [Hornig et al.,1997], and hypometabolism in cingulate, insula, frontal, and temporal areas in TRD, in association with different components of the severity and course of illness [Kimbrell et al.,2002]. In addition, treatments for refractory depression such as VNS [Pardo et al.,2008; Zobel et al.,2005], transcranial magnetic stimulation (TMS), [Conca et al.,2002] and DBS [Mayberg et al.,2005] appeared to change the rCBF or glucose uptake in relevant cerebral structures. Of note, in the present study, the high ReHo in the parahippocampal area was observed in the comparisons between NDD/MDD and healthy controls, correlated with HRSD score, but not in that of TRD vs. controls. Unlike generally defined depression‐related hypometabolism in ACC and frontal, temporal, insular regions [Mayberg et al.,1994], the hippocampal area showed hyperactivity in TRD compared to normal controls [Hornig et al.,1997]. These collectively reflect a different pattern of hippocampal activity in refractory depression.
In comparison with healthy controls, PFG was the only area which was identified with low ReHo in both NDD and TRD groups. ReHo values within this region also showed a negative correlation with disease duration amongst all depressed subjects. The PFG is reportedly involved in depression‐associated emotional information processing [Chan et al.,2009; Demaree et al.,2009]. Of interest, a PET study on healthy subjects [Geday et al.,2003] revealed that upon presentation of emotional stimuli (pictures) rCBF was altered not only in the right PFG, but also in the inferior medial prefrontal cortex (MPC). The latter region also showed low ReHo in the present work in the pooled MDD group compared to healthy controls, and ReHo values within this region were negatively correlated with HRSD score. As has been argued [Geday et al.,2003], this may implicate a neural network which exists to convey emotionally important visual message from the PFG to the MPC, which is then evaluated in terms of relevance for attention. Our findings suggest that in depression this network is disrupted in a way which worsens with progress of disease.
Other regions in TRD or MDD groups showing lower ReHo compared to healthy controls included the left lateral inferior frontal gyrus, superior and inferior parietal area, the left precuneus, and caudate (see Fig 1). Mayberg et al. observed greatly decreased rCBF in inferior frontal cortex of patients with resistant depression [Mayberg et al.,1994]. A study of depressed patients with TMS treatment also found, at medication‐free baseline, lower blood flow in non‐responders than responders [Teneback et al.,1999]. Abnormal regional metabolic response in the parietal lobe has been implicated in both MDD and TRD [Anderson et al.,2004; Conway et al., 2006; Goldapple et al.,2004; Teneback et al.,1999]. In particular the IPL is thought to play a role in emotional modulation. In MDD patients, it has been identified with enhanced activation in response to stimuli consisting of sad words compared to controls [Canli et al.,2004], and in remitted geriatric depression activation to attentional targets was attenuated in this region [Wang et al.,2008]. As a part of the parietal lobe, the precuneus has been reported to show gray matter volumetric abnormality in TRD [Shah et al.,2002], and increased activity after TMS in nonresponders was observed in this region [Teneback et al.,1999]. In the current study the precuneus also exhibited low ReHo in TRD compared to NDD, and showed a negative correlation with disease duration, suggesting its relevance to differing antidepressant effects.
Previous studies have proposed that the functions of specific anatomical structures may serve as key factors which differentiate TRD from NDD patients. For instance, in an early PET study [Mayberg et al.,1997] the only observation at pretreatment baseline to predict antidepressant effects was the ACC function, specifically, hypermetabolism in treatment responders and hypometabolism in nonresponders. The amygdala‐hippocampal area has been identified as another such site [Hornig et al.,1997]. However, the present study revealed widespread differences in regional function between TRD and NDD, although direct comparison yielded fewer clusters than their separate comparisons versus the healthy group. Take together with the extensive structural differences identified by previous morphometric analysis [Shah et al.,2002], this suggests that treatment refractoriness is a result of disruption of complex cortico‐limbic networks. The observation of a single structure as a distinguishing site may arise from the difference in patient classification and imaging modalities employed.
Direct comparison of our findings to those of studies employing other approaches to measuring brain function is difficult. First, comparison amongst studies is impeded by the diverse stimulation tasks used, and, even for resting‐state studies, by varying definitions of treatment resistance. For example, in published trials [Mayberg et al.,2005; Pardo et al.,2008; Teneback et al.,1999; Zobel et al.,2005], regardless of the response to stimulation therapies, all the depressed subjects recruited were refractory to other antidepressant treatments. Second, such comparisons are hampered by the current lack of any clear theory of the relationship between spontaneous brain function measured by ReHo and functional information obtained by other means. Presumably increased temporal correlation between spontaneous signals in neighboring voxels might be associated with high regional metabolism, but increased metabolism would be the result of any local increased activity, whether or not temporally correlated. The relationship between ReHo and fMRI analysis based on the general linear model (GLM) also remains unclear. For example, in unilateral finger movement compared to the resting condition [Zang et al.,2004], ReHo was greater in the M1 area of both hemispheres; however, it was higher in the ipsilateral M1 than the contralateral area, which observation is contrary to the results of GLM analysis, which shows greater activation in the contralateral M1. Another methodological issue is the effect of physiologic noise on the functional data analysis. Noise of cardiac and respiratory origin cannot be completely filtered given the relatively low sampling rate (TR = 2 s). These aliasing physiologic noises might reduce the specificity of the regional signal synchronization, or conceivably confound the group comparison. In the present study, depressed patients and healthy controls might have different physiologic responses to the scanning environment; however, it seems unlikely that responses would differ between the TRD and NDD groups, given the similar disease severities as assessed by the HRSD score. In future work it would be useful to estimate these effects by recording respiratory and cardiac rate during the data acquisition.
CONCLUSION
In summary, rs‐fMRI and ReHo analysis reveals differentiated anatomical patterns of abnormal brain function in TRD versus NDD. A robust implication is that these regions are relevant to the neural substrates underlying treatment refractoriness, and one possibility is that they are involved in a disruption of complex cortico‐limbic networks. In any case ReHo has demonstrated the capability to characterize spontaneous brain dysfunction in depression. Further analysis of the implications of the findings will require more work directed at understanding the neural mechanisms accounting for positive and negative alterations in ReHo. For example, combination of rs‐fMRI and PET/SPECT to study the same depressed group would be illuminating. Finally, due to its ease of implementation, noninvasive nature, and its ability to localize single abnormal sites, ReHo in combination with rs‐fMRI may be a useful tool for studying depressive disorders, and hopefully to help both clinical monitoring and antidepressant development.
Acknowledgements
The authors thank Ms. He‐Han Tang and Qin Ouyang for their contribution in the MRI data acquisition.
REFERENCES
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ ( 2005a): Activity and connectivity of brain mood regulating circuit in depression: A functional magnetic resonance study. Biol Psychiatry 57: 1079–1088. [DOI] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ ( 2005b): Antidepressant effect on connectivity of the mood‐regulating circuit: An fMRI study. Neuropsychopharmacology 30: 1334–1344. [DOI] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Gardner K, Lowe MJ ( 2007): Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: An fMRI study. J Neuropsychiatry Clin Neurosci 19: 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AD, Oquendo MA, Parsey RV, Milak MS, Campbell C, Mann JJ ( 2004): Regional brain responses to serotonin in major depressive disorder. J Affect Disord 82: 411–417. [DOI] [PubMed] [Google Scholar]
- Bai F, Zhang Z, Yu H, Shi Y, Yuan Y, Zhu W, Zhang X, Qian Y ( 2008): Default‐mode network activity distinguishes amnestic type mild cognitive impairment from healthy aging: A combined structural and resting‐state functional MRI study. Neurosci Lett 438: 111–115. [DOI] [PubMed] [Google Scholar]
- Berlim MT, Turecki G ( 2007): Definition, assessment, and staging of treatment‐resistant refractory major depression: A review of current concepts and methods. Can J Psychiatry 52: 46–54. [DOI] [PubMed] [Google Scholar]
- Bonelli RM, Kapfhammer HP, Pillay SS, Yurgelun‐Todd DA ( 2006): Basal ganglia volumetric studies in affective disorder: What did we learn in the last 15 years? J Neural Transm 113: 255–268. [DOI] [PubMed] [Google Scholar]
- Canli T, Sivers H, Thomason ME, Whitfield‐Gabrieli S, Gabrieli JD, Gotlib IH ( 2004): Brain activation to emotional words in depressed vs. healthy subjects. Neuroreport 15: 2585–2588. [DOI] [PubMed] [Google Scholar]
- Chan SW, Norbury R, Goodwin GM, Harmer CJ ( 2009): Risk for depression and neural responses to fearful facial expressions of emotion. Br J Psychiatry 194: 139–145. [DOI] [PubMed] [Google Scholar]
- Conca A, Peschina W, Konig P, Fritzsche H, Hausmann A ( 2002): Effect of chronic repetitive transcranial magnetic stimulation on regional cerebral blood flow and regional cerebral glucose uptake in drug treatment‐resistant depressives. A brief report. Neuropsychobiology 45: 27–31. [DOI] [PubMed] [Google Scholar]
- Conway CR, Sheline YI, Chibnall JT, George MS, Fletcher JW, Mintun MA ( 2006): Cerebral blood flow changes during vagus nerve stimulation for depression. Psychiatry Res 146: 179–184. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Abercrombie H, Nitschke JB, Putnam K ( 1999): Regional brain function, emotion and disorders of emotion. Curr Opin Neurobiol 9: 228–234. [DOI] [PubMed] [Google Scholar]
- Demaree HA, Pu J, Jesberger J, Feeny N, Jeng L, Everhart DE, Duerk J, Tkach J ( 2009): 5HTTLPR predicts left fusiform gyrus activation to positive emotional stimuli. Magn Reson Imaging 27: 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC ( 2000): Neuroimaging studies of mood disorders. Biol Psychiatry 48: 813–829. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW ( 1997): Structured Clinical Interview for DSM‐IV Axis I Disorders. American Psychiatric Press, Washington, DC. [Google Scholar]
- Geday J, Gjedde A, Boldsen AS, Kupers R ( 2003): Emotional valence modulates activity in the posterior fusiform gyrus and inferior medial prefrontal cortex in social perception. Neuroimage 18: 675–684. [DOI] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H ( 2004): Modulation of cortical‐limbic pathways in major depression: Treatment‐specific effects of cognitive behavior therapy. Arch Gen Psychiatry 61: 34–41. [DOI] [PubMed] [Google Scholar]
- Goodwin GM ( 1997): Neuropsychological and neuroimaging evidence for the involvement of the frontal lobes in depression. J Psychopharmacol 11: 115–122. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF ( 2007): Resting‐state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M ( 1967): Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 6: 278–296. [DOI] [PubMed] [Google Scholar]
- He Y, Zang Y, Jiang T, Lu Y, Weng X ( 2004): Detection of Functional Networks in the Resting Brain. 2nd IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Arlington, VA, USA, April 15‐18, 2004 (ISBI'04).
- He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, Jiang T ( 2007): Regional coherence changes in the early stages of Alzheimer's disease: A combined structural and resting‐state functional MRI study. Neuroimage 35: 488–500. [DOI] [PubMed] [Google Scholar]
- Hornig M, Mozley PD, Amsterdam JD ( 1997): HMPAO SPECT brain imaging in treatment‐resistant depression. Prog Neuropsychopharmacol Biol Psychiatry 21: 1097–1114. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Nakajima T, Suzuki Y, Okazaki A, Maehara T, Shiraishi H ( 1997): Quantitative regional cerebral flow measured by Tc‐99M HMPAO SPECT in mood disorder. Psychiatry Res 68: 143–154. [DOI] [PubMed] [Google Scholar]
- Kendall M, Gibbons JD ( 1990): Rank Correlation Methods. Oxford: Oxford University Press. [Google Scholar]
- Kimbrell TA, Ketter TA, George MS, Little JT, Benson BE, Willis MW, Herscovitch P, Post RM ( 2002): Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biol Psychiatry 51: 237–252. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu Z, Liang M, Hao Y, Tan L, Kuang F, Yi Y, Xu L, Jiang T ( 2006): Decreased regional homogeneity in schizophrenia: A resting state functional magnetic resonance imaging study. Neuroreport 17: 19–22. [DOI] [PubMed] [Google Scholar]
- Lui S, Parkes LM, Huang XQ, Zou K, Chan RCK, Yang H, Zou L, Li DM, Tang HH, Zhang TJ, Li XL, Wei Y, Chen L, Sun XL, Kemp GJ, Gong QY ( 2009): Depressive disorders: Focally altered cerebral perfusion measured with arterial spin‐labeled MR imaging. Radiology 251: 476–484. [DOI] [PubMed] [Google Scholar]
- Mayberg HS ( 1997): Limbic‐cortical dysregulation: A proposed model of depression. J Neuropsychiatry Clin Neurosci 9: 471–481. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lewis PJ, Regenold W, Wagner HN Jr. ( 1994): Paralimbic hypoperfusion in unipolar depression. J Nucl Med 35: 929–934. [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT ( 1997): Cingulate function in depression: A potential predictor of treatment response. Neuroreport 8: 1057–1061. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH ( 2005): Deep brain stimulation for treatment‐resistant depression. Neuron 45: 651–660. [DOI] [PubMed] [Google Scholar]
- Paakki JJ, Rahko J, Long X, Moilanen I, Tervonen O, Nikkinen J, Starck T, Remes J, Hurtig T, Haapsamo H, Jussila K, Kuusikko‐Gauffin S, Mattila ML, Zang Y, Kiviniemi V ( 2009): Alterations in regional homogeneity of resting‐state brain activity in autism spectrum disorders. Brain Res 1321: 169–179. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Sheikh SA, Schwindt GC, Lee JT, Kuskowski MA, Surerus C, Lewis SM, Abuzzahab FS, Adson DE, Rittberg BR ( 2008): Chronic vagus nerve stimulation for treatment‐resistant depression decreases resting ventromedial prefrontal glucose metabolism. Neuroimage 42: 879–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PJ, Glabus MF, Goodwin GM, Ebmeier KP ( 2002): Chronic, treatment‐resistant depression and right fronto‐striatal atrophy. Br J Psychiatry 180: 434–440. [DOI] [PubMed] [Google Scholar]
- Sheline YI ( 2003): Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry 54: 338–352. [DOI] [PubMed] [Google Scholar]
- Teneback CC, Nahas Z, Speer AM, Molloy M, Stallings LE, Spicer KM, Risch SC, George MS ( 1999): Changes in prefrontal cortex and paralimbic activity in depression following two weeks of daily left prefrontal TMS. J Neuropsychiatry Clin Neurosci 11: 426–435. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B ( 2004): Hippocampal volume and depression: A meta‐analysis of MRI studies. Am J Psychiatry 161: 1957–1966. [DOI] [PubMed] [Google Scholar]
- Wang L, Krishnan KR, Steffens DC, Potter GG, Dolcos F, McCarthy G ( 2008): Depressive state‐ and disease‐related alterations in neural responses to affective and executive challenges in geriatric depression. Am J Psychiatry 165: 863–871. [DOI] [PubMed] [Google Scholar]
- World Psychiatry Association ( 1974): Symposium on therapy resistant depression. Pharmacopsychiatry 7: 69–224. [Google Scholar]
- Wu T, Hallett M ( 2008): Neural correlates of dual task performance in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 79: 760–766. [DOI] [PubMed] [Google Scholar]
- Yao Z, Wang L, Lu Q, Liu H, Teng G ( 2008): Regional homogeneity in depression and its relationship with separate depressive symptom clusters: A resting‐state fMRI study. J Affect Disord 115: 430–438. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zhang Z, Bai F, Yu H, Shi Y, Qian Y, Liu W, You J, Zhang X, Liu Z ( 2008): Abnormal neural activity in the patients with remitted geriatric depression: A resting‐state functional magnetic resonance imaging study. J Affect Disord 111: 145–152. [DOI] [PubMed] [Google Scholar]
- Zang Y, Jiang T, Lu Y, He Y, Tian L ( 2004): Regional homogeneity approach to fMRI data analysis. Neuroimage 22: 394–400. [DOI] [PubMed] [Google Scholar]
- Zhang TJ, Wu QZ, Huang XQ, Sun XL, Zou K, Lui S, Liu F, Hu JM, Kuang WH, Li DM, Li F, Chen HF, Chan RCK, Mechelli A, Gong QY ( 2009): Magnetization transfer imaging reveals the brain deficit in patients with treatment‐refractory depression. J Affect Disord 117: 157–161. [DOI] [PubMed] [Google Scholar]
- Zobel A, Joe A, Freymann N, Clusmann H, Schramm J, Reinhardt M, Biersack HJ, Maier W, Broich K ( 2005): Changes in regional cerebral blood flow by therapeutic vagus nerve stimulation in depression: An exploratory approach. Psychiatry Res 139: 165–179. [DOI] [PubMed] [Google Scholar]


