Abstract
Somatoform disorder patients suffer from impaired emotion recognition and other emotional deficits. Emotional empathy refers to the understanding and sharing of emotions of others in social contexts. It is likely that the emotional deficits of somatoform disorder patients are linked to disturbed empathic abilities; however, little is known so far about empathic deficits of somatoform patients and the underlying neural mechanisms. We used fMRI and an empathy paradigm to investigate 20 somatoform disorder patients and 20 healthy controls. The empathy paradigm contained facial pictures expressing anger, joy, disgust, and a neutral emotional state; a control condition contained unrecognizable stimuli. In addition, questionnaires testing for somatization, alexithymia, depression, empathy, and emotion recognition were applied. Behavioral results confirmed impaired emotion recognition in somatoform disorder and indicated a rather distinct pattern of empathic deficits of somatoform patients with specific difficulties in “empathic distress.” In addition, somatoform patients revealed brain areas with diminished activity in the contrasts “all emotions”–“control,” “anger”–“control,” and “joy”–“control,” whereas we did not find brain areas with altered activity in the contrasts “disgust”–“control” and “neutral”–“control.” Significant clusters with less activity in somatoform patients included the bilateral parahippocampal gyrus, the left amygdala, the left postcentral gyrus, the left superior temporal gyrus, the left posterior insula, and the bilateral cerebellum. These findings indicate that disturbed emotional empathy of somatoform disorder patients is linked to impaired emotion recognition and abnormal activity of brain regions responsible for emotional evaluation, emotional memory, and emotion generation. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: fMRI, somatoform disorder, emotion, empathy
INTRODUCTION
Somatoform disorders, a group of complex diseases consisting of medically unexplained somatic symptoms [Hiller et al., 2010; Kirmayer et al., 1994; Pedrosa Gil et al., 2009; Stein and Muller, 2008], are linked to alexithymia‐associated emotional dysfunctions as underlying psychological basis [Bach and Bach, 1996; Bailey and Henry, 2007; Bankier et al., 2001; Burba et al., 2006; Duddu et al., 2003; Grabe et al., 2004; Mattila et al., 2008; Pedrosa Gil et al., 2008; Wood et al., 2009].
In addition to alexithymia, which means diminished emotional awareness, somatoform patients also exhibit problems in emotion recognition (i.e., the correct labeling of emotions). Pedrosa Gil et al. [2009] found significant problems in the overall recognition of emotional faces in somatoform patients. Psychodynamic concepts of somatoform disorders suggest, that impaired emotion recognition in somatoform disorder is caused by the (unconscious) repression of emotions to avoid interpersonal conflicts [Bowlby, 1973; Maunder and Hunter, 2004; Waller and Scheidt, 2006].
Empathy refers to the ability of understanding and sharing emotions in social contexts [Decety and Jackson, 2004; Decety and Moriguchi, 2007; Lamm et al., 2007; Preston and de Waal, 2002]. According to a recent concept provided by Shamay‐Tsoory [2011], two underlying systems can be distinguished: “emotional empathy” (i.e., the sharing of another's emotional state) and “cognitive empathy” (i.e., “the adoption of another's psychological point of view”). Regarding this, emotional empathy overlaps with other emotional processes such as “emotional contagion” (i.e., the sharing of another's emotional state without awareness that the own emotional state was induced by another individual, which differs from “emotional empathy,” because in the latter a distinction between self and other is possible [Lamm et al., 2007; Preston and de Waal, 2002]) and “emotion recognition” [Nomi et al., 2008; Shamay‐Tsoory, 2011].
With reference to the study of Pedrosa Gil et al. [2009], which demonstrated disturbed emotion recognition in somatoform disorder patients (see above), empathic deficits of somatoform disorder patients are likely. Even more, if one considers the connections between alexithymia and impaired emotional empathy [Decety and Moriguchi, 2007; Guttman and Laporte, 2002; Williams and Wood, 2010]. However, empathic deficits in somatoform patients have not been investigated so far.
Furthermore, little is known about the neural mechanisms underlying the disturbed emotion processing (and empathy) of somatoform disorder patients. To our knowledge, only one recent study investigated brain activity of Korean patients suffering from Hwa‐Byung, a Korean, culture‐bound disorder, which resembles somatoform disorder with regard to medically unexplained symptoms [Lee et al., 2009; Min, 2008; Min and Suh, 2010; Min et al., 2009]. The authors reported increased activity in patients' lingual and fusiform gyrus during the perception of neutral, sad, and angry stimuli, and lower activity in the thalamus [Lee et al., 2009]. It is not known, whether the same mechanisms also apply to somatoform disorders.
However, emotional empathy and cognitive empathy have been investigated intensively in healthy subjects, and sets of relevant brain regions have been identified: During emotional empathy a brain network consisting of anterior cingulate cortex, anterior insula, superior temporal cortex, amygdala, and inferior frontal cortex is reliably involved [Carr et al., 2003; de Greck et al., 2011a; Fan et al., 2011; Jabbi et al., 2007; Wicker et al., 2003]. During cognitive empathy, the superior temporal sulcus, medial prefrontal cortex, the temporal poles, and the inferior frontal cortex are active [Hooker et al., 2008, 2010; Ochsner et al., 2004]. In addition, the neural correlates of emotion recognition have been investigated in healthy subjects. Here, a similar network including the amygdala, insula, inferior frontal cortex, but also the putamen and somatosensory cortex is active [Adolphs et al., 2000; Derntl et al., 2009; Gur et al., 2002; Sprengelmeyer et al., 1998]. As the study by Nomi et al. [2008] showed, both processes (i.e., emotion recognition and emotional empathy) induce neuronal activity in the same regions (including the inferior frontal gyrus, middle frontal gyrus, superior frontal gyrus, and others), underlining the close link between the emotion recognition and emotional empathy.
Since empathy is important for our survival and success in social environments [Blair, 2003; Decety and Moriguchi, 2007; Gallese et al., 2004] and deficits in empathy have been associated to impaired social functioning [Henry et al., 2008], it is crucial to learn more about the exact relationship between disturbed emotion processing and empathic deficits in somatoform disorder. This study aimed to investigate the exact pattern of empathic dysfunctions in somatoform disorder patients and the underlying neural substrates. We applied an empathy task in which subjects were instructed to share the affective state of presented emotional faces. Facial stimuli displaying anger, disgust, joy, and a neutral state were applied; in addition, trial based evaluations of the subjective impression of empathic abilities were acquired. Moreover, fMRI allowed for simultaneously monitoring of empathy related neural responses. Further, clinical scales (Symptom Check List 90‐R, Toronto Alexithymia Scale, Beck Depression Inventory), an empathy test (Interpersonal Reactivity Index), and an emotion recognition test (Tübinger Affekt Batterie, the German version of the Florida Affect Battery) were applied.
Hypotheses
We hypothesized that the applied behavioral questionnaires revealed disabilities in emotion recognition and emotional empathy of somatoform patients. In addition, we expected diminished neuronal activity in brain regions involved in emotion recognition such as insula, inferior frontal cortex, putamen, somatosensory cortex. Moreover, we were prepared to find diminished activity in brain regions involved in emotional empathy (such as anterior cingulate cortex, anterior insula, superior temporal cortex, amygdala, and inferior frontal cortex) or cognitive empathy (such as superior temporal sulcus, medial prefrontal cortex, the temporal poles, and the inferior frontal cortex). With regard to psychodynamic explanations of somatoform disorder, we hypothesized impaired empathic abilities and altered neural activity for specific emotions, rather than overall emotional deficits. In particular, we expected the largest problems in social emotions, such as anger and joy, whereas non‐social emotions such as disgust or neutral expression should lead to fewer differences.
METHODS
Ethical Approval
The study was ethically approved by the Institutional Review Board of the Otto‐von‐Guericke University of Magdeburg/Germany. After a detailed explanation of the study, all subjects gave informed consent. The study was conducted at the Otto‐von‐Guericke University of Magdeburg/Germany.
Participants
We investigated 20 patients (gender: 12 females, 8 males; handedness: 19 right‐handed, 1 left‐handed; age: mean = 42.5, s.d. = 14.0). All patients suffered from a somatoform disorder as ascertained by a trained psychologist using the Structured Clinical Interview for DSM‐IV (German version: SKID, [Wittchen et al., 1997]). More precise, 13 of the 20 patients fulfilled criteria of an undifferentiated somatoform disorder (DSM‐IV: 300.81), 5 of the 20 patients had a pain disorder (DSM‐IV: 307.80), and 2 of the 20 patients had a somatization disorder (DSM‐IV: 300.81). All patients were recruited at the start of an inpatient psychotherapy. Patients were recruited from the Department of Psychosomatic Medicine and Psychotherapy of the Otto‐von‐Guericke‐University Hospital in Magdeburg (11/20), from the Department of Psychotherapeutic Medicine of the Fachklinikum Uchtspringe (4/20), and from the Department of Psychosomatic Medicine and Psychotherapy of the AWO Hospital Jerichow (5/20). Six of the 20 patients were on psychotropic medication with duloxetine (1/20), duloxetine and trimipramine (1/20), opipramol (1/20), opipramol and paroxetine (1/20), doxepine (1/20), and hypericum (1/20) during the time point of the fMRI session.
Twenty gender and age matched healthy controls were also recruited in this study (gender: 12 females, 8 males; handedness: 16 right‐handed, 2 left‐handed, 2 both handed; age: mean = 37.0, s.d. = 10.6, t(38) = 1.387; P [two‐tailed] = 0.173). All subjects received financial compensation for their participation in the study.
Psychological Measures
We applied the following psychological measurements to control for differences between the patient group and the group of healthy subjects.
Somatization
Somatization was assessed using a German edition of the “Symptom Check List 90‐Revised Version” (SCL‐90‐R, [Derogatis, 1977; Franke, 2002]). The SCL‐90‐R is a 90 item self‐report questionnaire, which contains a number of subscales such as somatization, depression, and anxiety. Here, we focused on the somatization subscale. The SCL‐90‐R somatization score was collected from 16 control subjects and 19 somatoform patients.
Emotional awareness
To test for emotional comprehension and awareness, we applied a German edition of the “Toronto Alexithymia Scale–20” (TAS‐20, [Bagby et al., 1994; Bressi et al., 1996]), a well‐established self‐descriptive questionnaire. TAS‐20 scores were recorded for 19 healthy subjects and 20 somatoform patients.
Mood state
Subjective experience of depressive symptoms was assessed with a German edition of the “Beck Depression Inventory” (BDI, [Beck et al., 1961]). BDI scores were obtained from 18 healthy subjects and 20 somatoform patients.
Empathy
Empathy was assessed using the Interpersonal Reactivity Index (IRI, [Davis, 1983]). The IRI is a well‐established questionnaire, which allows to test for four different empathy categories: “empathic fantasy,” “empathic concern,” “empathic distress,” and “perspective taking.” IRI scores were collected from 19 healthy subjects and 20 somatoform patients.
Emotion recognition abilities
We used the “Tübinger Affekt Batterie” (TAB, [Breitenstein et al., 1996]), the German version of the “Florida Affect Battery” (FAB, [Bowers et al., 1999]) to test for emotion recognition abilities. We applied four subtests of the TAB: the TAB 3, which uses emotional face stimuli to test the ability to visually recognize different emotions; the TAB 5, which uses emotional face stimuli to check the ability to associate one emotional stimulus to a congruent other emotional stimulus; the TAB 8a, which uses spoken emotional sentences to test for the ability identify prosody and semantic content; and the TAB 8b, which uses spoken sentences as the TAB 8a, but applies a number of incongruent auditory stimuli (i.e., sentences with different prosody and emotional content). The emotion conditions applied in the TAB are: anger, joy, fear, sadness, and neutral. TAB scores were obtained from 14 healthy subjects and 20 somatoform patients.
Paradigm
We applied a paradigm that contained a combination of two tasks: a reward anticipation task and an empathy task. The tasks were separated from each other in a block wise manner. Due to the complexity of the study, here we report only results obtained from the empathy blocks. (See [de Greck et al., 2011b]) for the results obtained during the reward anticipation paradigm).
Experimental design
The fMRI experiment consisted of 6 blocks of 630s. Blocks 1, 3, and 5 were reward blocks; blocks 2, 4, and 6 were empathy blocks. Prior to the scanning subjects read detailed information about the paradigm with all the tasks and completed a couple of trial runs in order to familiarize them with the experiment. While lying in the scanner, the stimuli were displayed using the “Presentation” software package (Neurobehavioral Systems, Albany, CA), and were projected onto a matt screen via an LCD projector, which was visible through a mirror mounted on the head coil.
Empathy blocks
Every empathy block started with a short finger‐tapping task. This task was included to have the later option of identifying each subject's hand field of the primary motor cortex (due to complexity reasons, the results of this task cannot be reported here). This was directly followed by the actual empathy session, which started with the presentation of a short instruction lasting for 6 s. A total number of 40 empathy trials were presented in a random order. Figure 1 illustrates the task. After every eight empathy trials, a short pause of 6, 7, or 8 s duration occurred; during pauses the fixation cross was presented. At the end of each block, subjects were asked to evaluate their present feeling for contentedness as well as their impression of engagement in the empathy task, by virtually moving a bar on a visual analogue scale.
Figure 1.
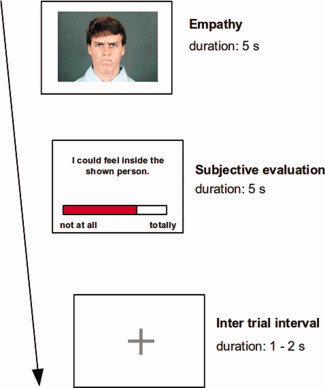
Paradigm of the fMRI study. Every trial began with a 5 s lasting sole display of an emotional face or the presentation of a control stimulus. Subjects were instructed to empathize with the presented emotional face, which was expressed by the instruction phrase “please try to share the emotional state of the shown person.” Immediately after the presentation of the emotional face a subjective evaluation task was presented and subjects were asked to rate their ability to empathize with the preceding picture. For this, the subjects were trained to move a virtual bar of a visual analogue scale. Prior to the following empathy trial a short inter trial interval was presented, which lasted for 2 or 3 s. The facial stimuli included the emotion conditions anger, disgust, joy, and neutral emotional state. As control, stimuli served smoothed pictures with unrecognizable contents. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Stimuli
The emotional face stimuli were taken from the “Japanese and Caucasian Facial Expressions of Emotion (JACFEE) and Neutral Faces (JACNeuF)”‐battery provided by Matsumoto and Ekman [Matsumuto and Ekman, 1988]. Of every emotion (namely anger, disgust, joy, and neutral) eight different facial stimuli were shown, resulting in a total number of 32 different facial stimuli. The emotions were presented by the same number of Caucasian and Japanese actors (Japanese face stimuli were included to keep the number of repeated presentations of each face stimulus as small as possible), half of them female, half of them male. As control stimuli served eight smoothed pictures with unrecognizable contents. Relying on a previous study, which implemented unrecognizable stimuli [Gerlach et al., 2002], we produced the control stimuli by transformation of the neutral stimuli using a smoothing procedure. We decided to implement unrecognizable stimuli (and not for instance neutral stimuli) to avoid any (even automatic) empathic responses. Since empathic responses might be induced by the mere presentation of facial stimuli, even without emotional expressions [de Greck et al., 2011a], and even without a specific instruction to empathize [Yamada and Decety, 2009], we favored unrecognizable stimuli over neutral stimuli. Another reason for us not to implement neutral stimuli was the possibility that the somatoform patients might have misrecognized neutral stimuli. The tendency to misrecognize facial stimuli is not only known for somatoform patients [Pedrosa Gil et al., 2009], but also for depressed patients [Csukly et al., 2009]. Subjects were instructed to rate the smallest empathy amount (zero) after the presentation of control stimuli. During the whole experiment, every emotional face stimulus was presented once in each block, and for three times during the entire experiment.
fMRI Data Acquisition
The fMRI data were collected in a 1.5T MR scanner (General Electric Sigma Horizon) via a standard circular polarized head coil. Using a midsagittal scout image, a stack of 23 slices was aligned parallel to the bicomissural plane. During each functional run 320 whole brain volumes were acquired (gradient echo EPI, TR = 2 s; TE = 35 ms; flip angle = 80°; Field of View = 200 × 200 mm2; slice thickness = 5 mm, inter‐slice gap = 1 mm, spatial resolution = 3.125 × 3.125 × 5 mm3). Additionally, a T1 weighted image of every subject was acquired.
fMRI Data Analysis
Image processing and statistical analyses were carried out using the software package AFNI (http://afni.nimh.nih.gov/afni/, [Cox, 1996]). The first five volumes were discarded to compensate for saturation effects. All functional images were slice‐time corrected with reference to the acquisition time of the first slice and corrected for motion artifacts by realignment to the first volume. The images were spatially normalized to a standard EPI‐template provided by AFNI (“TT_EPI”) and resampled to 3 × 3 × 3 mm3. Finally, all functional images were smoothed with an isotropic 6 mm full‐width half maximum Gaussian kernel. Only runs 2, 4, and 6 were included in the statistical analysis. T1‐weighted images were normalized to a standard T1‐template provided by AFNI (“TT_avg152T1”).
For each subject, regressors of interest were created by the convolution of a gamma response function with the according stimulus time functions [Josephs et al., 1997]. At this, all relevant periods (namely all empathy periods, all evaluation periods, the pauses, and the free interval at the end of each session) were included in the model. In addition, six movement parameters resulting from the motion correction procedure, as well as nine regressors for the third degree polynomial model of the baseline of each block were included as regressors to account for any residual effects of head motion and baseline fluctuations, respectively.
Contrast images were calculated by employing linear contrasts to the parameter estimates for the regressors of each event. The resulting contrast images were then submitted to a second level random‐effects analysis. Here, one‐sample t‐tests (including the 20 healthy subjects) and independent two sample t‐tests (comparing the 20 somatoform patients and the 20 healthy subjects) were applied [Friston et al., 1995]. To control for the multiple testing problem, we performed a false discovery rate correction [Nichols and Hayasaka, 2003] and calculated family wise error probabilities. The anatomical localization and labeling of significant activations were assessed with reference to the standard stereotactic atlas of Talairach and Tournoux [1988] and by superimposition of the group contrast images on a mean brain generated by an average of each subject's normalized T1‐weighted image.
In a second step, we performed a statistical analysis of the raw fMRI signals. Using the significant clusters from the different contrasts as regions of interest, we extracted fMRI signals from activations found in the second level analysis using sphere shaped regions of interest with a radius of 5 mm. fMRI raw data timecourses were processed using the software package PERL (http://www.perl.org). The timecourses were linearly interpolated and normalized with respect to a time window ranging from −6 to 30 s before and after the onset of each event. fMRI signal changes of every event were calculated with regard to the fMRI signal value of the onset of the according event. Mean normalized fMRI signal values from two following time steps (6–8 s after onset of the according event) were included in the statistical analysis. We used Repeated Measurements ANOVAs and independent t‐tests to test for differences; Spearman correlations were applied to analyze the association of different clinical and emotional scores (namely the SCL‐90‐R–somatization score, the TAS‐20 score, the BDI score, the IRI–empathic distress score, and the TAB–error rates for anger and joy, as well as overall TAB–error rates) with hemodynamic responses of our regions of interest.
Since six of the patients were on psychotropic medication with drugs, which might have affected their hemodynamic responses, we included additional statistical analyses using independent t‐tests, which included the 20 healthy subjects and the 14 patients without medication.
RESULTS
Behavioral Results
Clinical results
As presented in Table I, somatoform patients exhibited increased somatization symptoms (SCL‐90‐R ‐ somatization), increased alexithymia scores (TAS‐20), and elevated depression scores (BDI).
Table I.
Behavioral results
| Group | n | Mean | 95%‐CI | t | P [one‐tailed] | |
|---|---|---|---|---|---|---|
| Somatization (Symptom‐Checklist‐90‐R‐somatization) | ||||||
| h | 16 | 42.2 | 37.8–46.6 | 4.718 | <0.001** | |
| p | 19 | 65.3 | 56.6–74.0 | |||
| Alexithymia (Toronto‐Alexithymia‐Scale‐20) | ||||||
| h | 19 | 36.7 | 32.8–40.6 | 6.115 | <0.001** | |
| p | 20 | 54.4 | 49.7–59.0 | |||
| Depression (Beck‐Depression‐Inventory) | ||||||
| h | 18 | 3 | 0.7–5.3 | 8.217 | <0.001** | |
| p | 20 | 19.6 | 16.1–23.1 | |||
| Empathy (Interpersonal‐Reactivity‐Index) | ||||||
| Empathic fantasy | h | 19 | 23.1 | 20.7–25.5 | 0.233 | 0.816 |
| p | 20 | 22.7 | 20.0–25.5 | |||
| Empathic concern | h | 19 | 26.7 | 24.8–28.6 | 0.550 | 0.586 |
| p | 20 | 25.9 | 23.6–28.2 | |||
| Empathic distress | h | 19 | 19.3 | 16.7–22.0 | 6.483 | <0.001** |
| p | 20 | 31.6 | 28.6–34.5 | |||
| Perspective taking | h | 19 | 24.2 | 21.8–26.5 | 1.288 | 0.207 |
| p | 20 | 22.4 | 20.7–24.1 | |||
| Emotion recognition (Tübinger‐Affekt‐Batterie)‐Error rates | ||||||
| Total | h | 14 | 14.0% | 9.5–18.6% | 3.006 | 0.003** |
| p | 20 | 23.1% | 18.6–27.7% | |||
| Neutral | h | 14 | 7.1% | 2.8–11.5% | 1.248 | 0.111 |
| p | 20 | 10.7% | 6.6–14.8% | |||
| Anger | h | 14 | 10.3% | 6.4–14.2% | 2.440 | 0.011* |
| p | 20 | 19.4% | 12.5–26.2% | |||
| Sadness | h | 14 | 11.0% | 5.2–16.7% | 2.302 | 0.014* |
| p | 20 | 20.3% | 13.9–26.8% | |||
| Fear | h | 14 | 20.1% | 14.2–26.0% | 2.242 | 0.016* |
| p | 20 | 30.3% | 22.7–38.0% | |||
| Joy | h | 14 | 21.0% | 11.3–30.6% | 2.176 | 0.019* |
| p | 20 | 34.1% | 25.7–42.5% | |||
| Intra‐scanner empathy ratings‐trial based | ||||||
| Total | h | 20 | 63.6 | 56.2–71.0 | [due to a lack of significant interactions in the ANOVA, t‐tests were not calculated] | |
| p | 20 | 68.0 | 60.6–75.5 | |||
| Neutral | h | 20 | 57.5 | 49.3–65.7 | ||
| p | 20 | 70.2 | 63.1–77.3 | |||
| Anger | h | 20 | 59.2 | 48.6–69.8 | ||
| p | 20 | 62.3 | 50.9–73.7 | |||
| Joy | h | 20 | 80.5 | 73.5–87.5 | ||
| p | 20 | 83.9 | 77.2–90.7 | |||
| Disgust | h | 20 | 57.2 | 46.0–68.4 | ||
| p | 20 | 55.0 | 42.9–67.1 | |||
| Intra‐scanner ratings‐block based | ||||||
| Engagement | h | 20 | 68.0 | 61.1–74.9 | 0.809 | 0.424 |
| p | 20 | 73.3 | 61.6–84.9 | |||
| Contentedness | h | 20 | 63.6 | 54.8–72.3 | 0.429 | 0.670 |
| p | 20 | 69.6 | 57.6–81.4 | |||
Behavioral results of healthy subjects (h) and somatoform patients (p).
Thirty‐five percent of the acute patients (7/20) and none of the patients after psychotherapy had TAS‐20 scores greater than 60; TAS‐20 scores greater than 60 indicate alexithymia [Kooiman et al., 2000]. Moreover, 45% of the acute patients (9/20) and none of the patients after psychotherapy had BDI scores above 18; BDI scores above 18 indicate moderate or severe depression [Beck et al., 1961].
Empathy
Using an ANOVA, we found a significant effect of the factor group (F(1,37) = 5.879, P = 0.020*), as well as a significant effect for the factor IRI‐subcategory (F(3, 111) = 4.957, P = 0.003**). In addition, we found a significant group × IRI‐subcategory interaction (F(3, 111) = 19.2786, P > 0.001**). Somatoform patients showed a significant difference in the “empathic distress” subcategory of the IRI, whilst all other subcategories prevailed no significant differences (see Table I and Fig. 2a).
Figure 2.
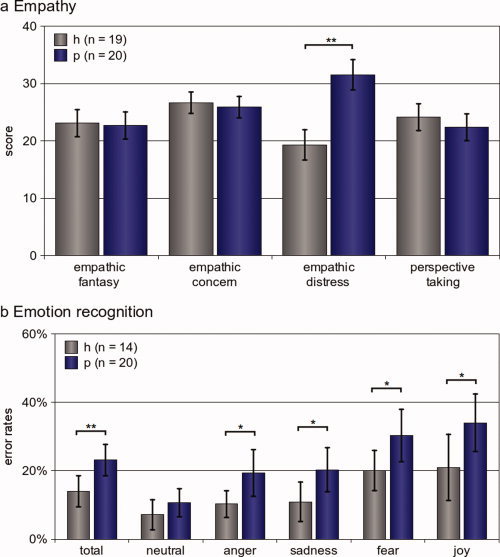
Behavioral results. (a) Empathy: Empathy was assessed using the Interpersonal Reactivity Index (IRI), which provides the four categories “empathic fantasy,” “empathic concern,” “empathic distress,” and “perspective taking.” We found a significant difference between healthy subjects and somatoform patients only in the “empathic distress” subcategory (t(37) = 6.483, P[two‐tailed] > 0.001**). (b) Emotion recognition Overall performance in the Tübinger Affekt Batterie (TAB, subtests 3, 5, 8a, and 8b). Somatoform patients (P, n = 20) made significantly more errors compared to healthy controls (h, n = 14) in all emotions taken together as well as in the emotional subconditions “anger,” “sadness,” “fear,” and “joy.” We did not find differences between somatoform patients and healthy subjects for the subcondition “neutral,” although. When looking for further differences between somatoform patients and controls (applying a post‐hoc approach), we found that patients (but not controls) made significantly more mistakes in the subcondition “anger” compared to “neutral” (healthy: t(13) = 1.162; P[two‐tailed] = 0.266; somatoform patients: t(19) = 3.693; P[two‐tailed] = 0.002**). The same was the case for “neutral” and “sadness” (healthy: t(13) = 1.421; P[two‐tailed] = 0.179; somatoform patients: t(19) = 2.759; P[two‐tailed] < 0.012*). The error bars reflect the 95% confidence interval. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Emotion recognition
The ANOVA showed a significant effect for the factors group (F(1,32) = 8.4265, P = 0.007**) and TAB‐subcategory (F(4,128) = 18.514, P < 0.001**), whilst the interaction of group and TAB‐subcategory failed significance (F(4,128) = 0.841, P = 0.502). Somatoform patients made overall significantly more mistakes in the TAB; with regard to the single emotional conditions, this was also the case for “anger,” “joy,” “sadness,” and “fear.” Somatoform patients performed as good as healthy subjects for the emotional condition “neutral,” although (see Table I and Fig. 2b).
Trial‐based intra‐scanner ratings
After each empathy trial, subjects evaluated their subjective impression of empathy capability with regard to the stimulus presented in the preceding trial. The ANOVA did not show a significant effect for the factor group (F(1,38) = 0.680, P = 0.4148), but a significant effect for the factor emotion (F(3,114) = 26.843, P < 0.001). The interaction of group and emotion was not significant either (F(3,114) = 1.9691, P = 0.1226). (See Table I for details.)
Block‐based intra‐scanner ratings
At the end of each block, participants were asked to evaluate their subjective impression of engagement in the empathy tasks as well as their general contentedness. Statistical analysis revealed no significant differences between healthy subject and somatoform patients for intra scanner ratings of engagement in the empathy task and general contentedness. (See Table I for details.)
fMRI Results
Empathy regions of healthy subjects
The contrast of all emotions together compared with the control condition ([“anger” + “disgust” + “joy” + “neutral expression”] – “control”) showed increased hemodynamic responses in a network of brain regions including the bilateral inferior frontal cortex, the right middle temporal cortex, bilateral amygdala, bilateral occipital cortex and bilateral cerebellum. The contrasts of each single emotion compared to the control condition led to increased hemodynamic responses in several regions, including the inferior frontal gyrus, amygdala, and occipital cortex (see Table II for details).
Table II.
Empathy regions of healthy subjects
| Region | x | y | z | T | n | P [FWE] | |
|---|---|---|---|---|---|---|---|
| All emotions ([“anger” + “disgust” + “happy” + “neutral”] – “control”) P[FDR] − 0.05, minimum cluster size 50 voxel | |||||||
| Left | Inferior frontal cortex | −45 | −12 | 24 | 5.690 | 345 | <0.001 |
| Right | Inferior frontal cortex | 45 | −6 | 15 | 4.562 | 223 | 0.016 |
| Right | Dorsomedial prefrontal cortex | 6 | −54 | 21 | 4.878 | 53 | 0.999 |
| Right | Middle temporal gyrus | 45 | 39 | 0 | 5.989 | 55 | 0.998 |
| Left | Precentral gyrus | −48 | 9 | 48 | 5.299 | 73 | 0.954 |
| Right | Precentral gyrus | 51 | 6 | 39 | 5.132 | 85 | 0.840 |
| Right | Supplementary motor area | 6 | 0 | 60 | 3.437 | 302 | 0.001 |
| Right | Postcentral gyrus | 21 | 45 | 66 | 5.212 | 88 | 0.807 |
| Left | Amygdala | −12 | 12 | −18 | 5.464 | 321 | < 0.001 |
| Right | Amygdala | 21 | 6 | −18 | 6.067 | 370 | < 0.001 |
| Right | Ventral striatum | 15 | −12 | 0 | 5.372 | 163 | 0.130 |
| Left | Occipital cortex | −23 | 91 | −19 | 7.051 | 543 | < 0.001 |
| Right | Occipital cortex | 31 | 87 | −19 | 6.476 | 618 | < 0.001 |
| Left | Cerebellum | −28 | 87 | −23 | 7.010 | 543 | < 0.001 |
| Left | Cerebellum | −39 | 47 | −29 | 5.524 | 543 | < 0.001 |
| Right | Cerebellum | 44 | 75 | −23 | 6.389 | 618 | <0.001 |
| Right | Cerebellum | 46 | 51 | −27 | 4.873 | 618 | < 0.001 |
| Anger (“anger” – “control”) P [FDR] ≤ 0.05, minimum cluster size 50 voxel | |||||||
| Left | Inferior frontal gyrus | −48 | −24 | 18 | 4.942 | 94 | 0.096 |
| Right | Inferior frontal gyrus | 45 | −6 | 18 | 4.758 | 157 | < 0.001 |
| Right | Supplementary motor area | 6 | 3 | 60 | 5.866 | 144 | 0.004 |
| Right | Precentral gyrus | 51 | 6 | 42 | 6.219 | 83 | 0.160 |
| Left | Postcentral gyrus | −48 | 6 | 48 | 5.854 | 50 | 0.741 |
| Right | Postcentral gyrus | 18 | 48 | 69 | 4.949 | 70 | 0.332 |
| Left | Amygdala | −18 | 0 | −18 | 7.259 | 120 | 0.019 |
| Right | Amygdala | 21 | 6 | −18 | 5.249 | 96 | 0.084 |
| Left | Occipital cortex | −39 | 81 | −21 | 9.752 | 403 | < 0.001 |
| Right | Occipital cortex | 33 | 87 | −24 | 6.524 | 291 | < 0.001 |
| Right | Cerebellum | 39 | 51 | −33 | 5.018 | 56 | 0.600 |
| Disgust (“disgust” – “control”) P [FDR] ≤ 0.05, minimum cluster size 50 voxel | |||||||
| Left | Inferior frontal gyrus | −45 | −27 | 0 | 5.855 | 187 | 0.002 |
| Left | Supplementary motor area | −4 | 4 | 59 | 4.941 | 134 | 0.005 |
| Right | Supplementary motor area | 3 | 2 | 51 | 5.547 | 134 | 0.005 |
| Left | Occipital cortex | −39 | 81 | −21 | 10.037 | 273 | 0.002 |
| Right | Occipital cortex | 27 | 87 | −21 | 7.625 | 281 | 0.002 |
| Right | Cerebellum | 39 | 45 | −30 | 5.046 | 69 | 0.330 |
| Joy (“joy” – “control”) P [FDR] ≤ 0.05, minimum cluster size 50 voxel | |||||||
| Right | Superior temporal gyrus | 28 | −5 | −19 | 3.911 | 263 | < 0.001 |
| Right | Supplementary motor area | 9 | 0 | 60 | 4.852 | 64 | 0.344 |
| Left | Amygdala | −24 | 15 | −18 | 4.805 | 83 | 0.114 |
| Right | Amygdala | 19 | 10 | −19 | 4.505 | 263 | < 0.001 |
| Left | Occipital cortex | −36 | 81 | −21 | 8.171 | 273 | < 0.001 |
| Right | Occipital cortex | 30 | 84 | −24 | 6.724 | 413 | < 0.001 |
| Left | Cerebellum | −36 | 48 | −27 | 5.437 | 60 | 0.406 |
| Neutral (“neutral” – “control”) P [FDR] ≤ 0.05, minimum cluster size 50 voxel | |||||||
| Left | Inferior frontal gyrus | −48 | −21 | 12 | 5.234 | 164 | 0.012 |
| Right | Supplementary motor area | 3 | 0 | 60 | 6.564 | 193 | 0.003 |
| Right | Amygdala | 21 | 8 | −15 | 5.166 | 57 | 0.901 |
| Left | Occipital cortex | −39 | 81 | −21 | 10.762 | 410 | < 0.001 |
| Right | Occipital cortex | 39 | 75 | −24 | 6.472 | 402 | < 0.001 |
Active clusters of healthy subjects (n = 20). x, y, and z coordinates belong to the peak voxel of the cluster and refer to the Talairach and Tournoux stereotactical space. T‐values refer to the peak voxel; n represents the number of voxels in the cluster; P [FWE] describes the family wise error of a cluster of the given size.
Empathy regions with diminished activity in somatoform patients
Comparing hemodynamic responses of somatoform patients and healthy controls, we found two regions with diminished hemodynamic responses for the contrast [“anger” + “disgust” + “joy” + “neutral expression”] – “control”: the right parahippocampal gyrus and the left amygdala (see Table III and Fig. 3)
Table III.
Empathy regions with different activity in somatoform disorder patients
| All emotions ([“anger”+ “disgust” + “happy” + “neutral”] – “control”) | ||||||||
| Somatoform patients < healthy subjects, P [uncorrected] ≤ 0.001, minimum cluster size 10 voxel | ||||||||
| Region | x | y | z | T | n | P [FWE] | T [20/14]* | |
| Right Parahippocampal gyrus | 30 | 54 | −3 | 4.851 | 22 | 0.664 | 4.149 | |
| Left Amygdala | −24 | −3 | −24 | 5.051 | 19 | 0.828 | 4.129 | |
| *All T [20/14] values correspond to P [uncorrected] ≤ 0.001 | ||||||||
| Somatoform patients > healthy subjects, P [uncorrected] ≤ 0.001, minimum cluster size 10 voxel no region | ||||||||
| Anger (“anger” – “control”) | ||||||||
| somatoform patients < healthy subjects, P [uncorrected] ≤ 0.001, minimum cluster size 10 voxel | ||||||||
| Region | x | y | z | T | n | P [FWE] | T [20/14]* | |
| Left postcentral gyrus | −15 | 39 | 66 | 4.294 | 21 | 0.899 | 3.223 | |
| Left superior temporal gyrus | −33 | −15 | −27 | 4.165 | 15 | 0.998 | 3.616 | |
| Left parahippocampal gyrus | −33 | 18 | −24 | 4.319 | 15 | 0.998 | 3.656 | |
| Right parahippocampal gyrus | 18 | 21 | −15 | 4.736 | 13 | 0.999 | 4.972 | |
| Left posterior insula | −36 | 33 | 15 | 4.653 | 13 | 0.999 | 4.375 | |
| Left Amygdala | −21 | −3 | −21 | 4.913 | 17 | 0.978 | 5.047 | |
| Left Cerebellum | −36 | 81 | −24 | 4.635 | 14 | 0.999 | 4.646 | |
| *All T [20/14] values correspond to P [uncorrected] ≤ 0.0029 | ||||||||
| Somatoform patients > healthy subjects, P [uncorrected] ≤ 0.001, minimum cluster size 10 voxel no region | ||||||||
| Disgust (“disgust” – “control”) | ||||||||
| Somatoform patients < healthy subjects, P [uncorrected] ≤ 0.001, minimum cluster size 10 voxel no region | ||||||||
| Somatoform patients > healthy subjects, P [uncorrected] ≤ 0.001, minimum cluster size 10 voxel no region | ||||||||
| Joy (“joy” – “control”) | ||||||||
| Somatoform patients < healthy subjects, P [uncorrected] ≤ 0.001, minimum cluster size 10 voxel | ||||||||
| Region | x | y | z | T | n | P [FWE] | T [20/14]* | |
| Right Parahippocampal gyrus | 30 | 54 | −3 | 4.533 | 22 | 0.472 | 3.601 | |
| Right Cerebellum | 33 | 84 | −27 | 4.811 | 11 | 0.997 | 4.455 | |
| Right Cerebellum | 21 | 87 | −30 | 4.131 | 10 | 0.999 | 5.061 | |
| *All T [20/14] values correspond to P [uncorrected] ≤ 0.0011 | ||||||||
| Somatoform patients > healthy subjects, P [uncorrected] ≤ 0.001, minimum cluster size 10 voxel no region | ||||||||
| Neutral (“neutral” – “control”) | ||||||||
| Somatoform patients < healthy subjects, P [uncorrected] ≤ 0.001, minimum cluster size 10 voxel no region | ||||||||
| Somatoform patients > healthy subjects, P [uncorrected] ≤ 0.001, minimum cluster size 10 voxel no region | ||||||||
Active clusters of the contrasts “all emotions” – “control,” “anger” – “control,” “disgust” – “control,” “joy” – “control,” and “neutral” – “control”; voxel‐based independent samples t‐test comparing healthy subjects (n = 20) and somatoform patients (n = 20). x, y, and z coordinates belong to the peak voxel of the cluster and refer to the Talairach and Tournoux stereotactical space. T‐values refer to the peak voxel; n represents the number of voxels in the cluster; P [FWE] describes the family‐wise error of a cluster of the given size; T [20/14] reflects the T score of the same peak voxel using a contrast, which included the 20 healthy controls and the 14 nonmedicated patients.
Figure 3.
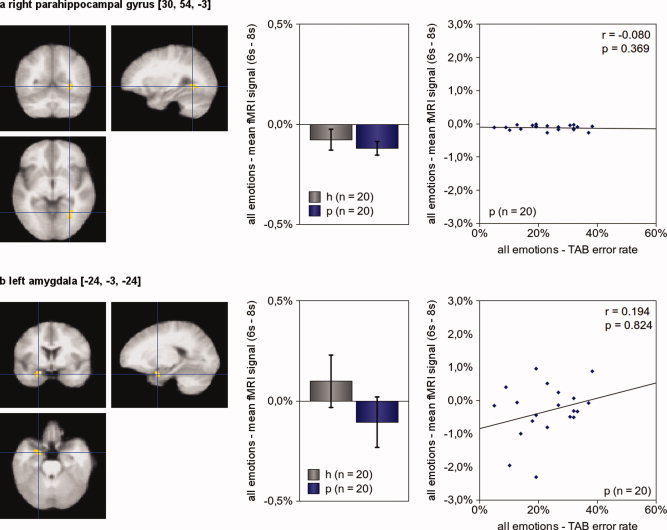
Brain activity during empathy. The comparison of healthy subjects and somatoform patients revealed two brain regions with significant differences in hemodynamic responses for the contrast [“anger” + “disgust” + “joy” + “neutral expression”] – “control” (P [uncorrected] = 0.001, minimum cluster size 10 voxel): the right parahippocampal gyrus (a) and the left amygdala (b). The left picture illustrates the exact location of the region of interest. The center picture shows the mean hemodynamic responses of healthy subjects (h, n = 20) and patients (p, n = 20) during empathy. In the right picture, each patient is presented by a dot; its x‐value representing his average error rate in the TAB and its y‐value representing his mean fMRI signal during empathy. Activation maps are superimposed on a normalized mean image of all 40 participants (patients and healthy control subjects). The figure shows disturbed neuronal activity of somatoform patients in the right parahippocampal gyrus and the left amygdala. The figure also suggests that there is no association of overall emotion recognition deficits and brain activity during empathy in both regions. (The error bars reflect the 95% confidence interval; P‐values are “one‐tailed.”) [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
When looking for differences between both groups for empathy with single emotions, we found regions with significantly greater hemodynamic responses in controls when compared to patients only for “anger” – “control” (left postcentral gyrus, left superior temporal gyrus, bilateral parahippocampal gyrus, left posterior insula, left amygdala, left cerebellum; see Table III and Fig. 4) and “joy” – “control” (right parahippocampal gyrus, right cerebellum; see Table III and Fig. 5). We did not find brain regions with significant differences in hemodynamic activity for “disgust” – “control” and “neutral” – “control”. None of the five contrasts revealed brain regions with increased hemodynamic responses in somatoform disorder patients.
Figure 4.
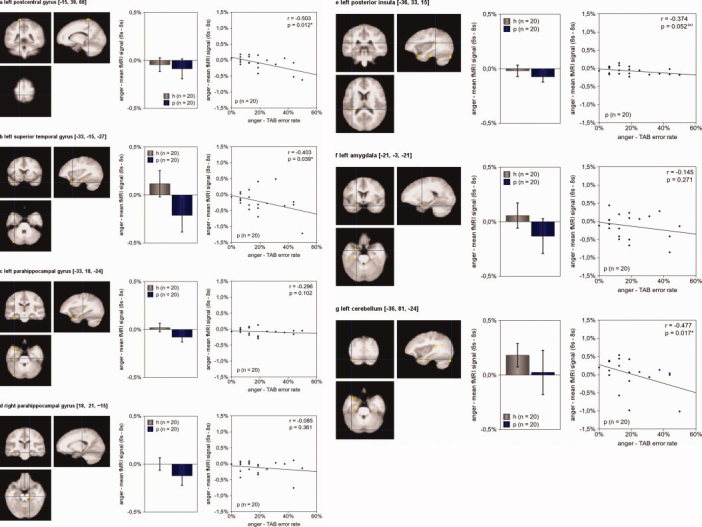
Brain activity during empathy with angry faces Seven brain regions of somatoform disorder patients showed diminished hemodynamic responses compared to healthy subjects for the contrast “anger” – “control” (P [uncorrected] = 0.001, minimum cluster size 10 voxel): the left postcentral gyrus (a), the left superior temporal gyrus (b), the bilateral parahippocampal gyrus (c, d), the left posterior insula (e), the left amygdala (f), and the left cerebellum (g). Alike Figure 3, the left picture illustrates the exact location of the region of interest. The center picture shows the mean hemodynamic responses of healthy subjects (h, n = 20) and patients (p, n = 20) during empathy with anger. In the right picture, each patient is presented by a dot; its x‐value representing his average error rate in the TAB during anger and its y‐value representing his mean fMRI signal during empathy with anger. Activation maps are superimposed on a normalized mean image of all 40 participants (patients and healthy control subjects). Somatoform patients' TAB error rates during anger predicted diminished hemodynamic responses during anger of three brain regions: the left postcentral gyrus, the left superior temporal gyrus, and the left cerebellum. The more problems a patient had in recognizing anger, the smaller his hemodynamic activity during empathy with anger in those three regions. (The error bars reflect the 95% confidence interval; P‐values are “one‐tailed.”) [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 5.
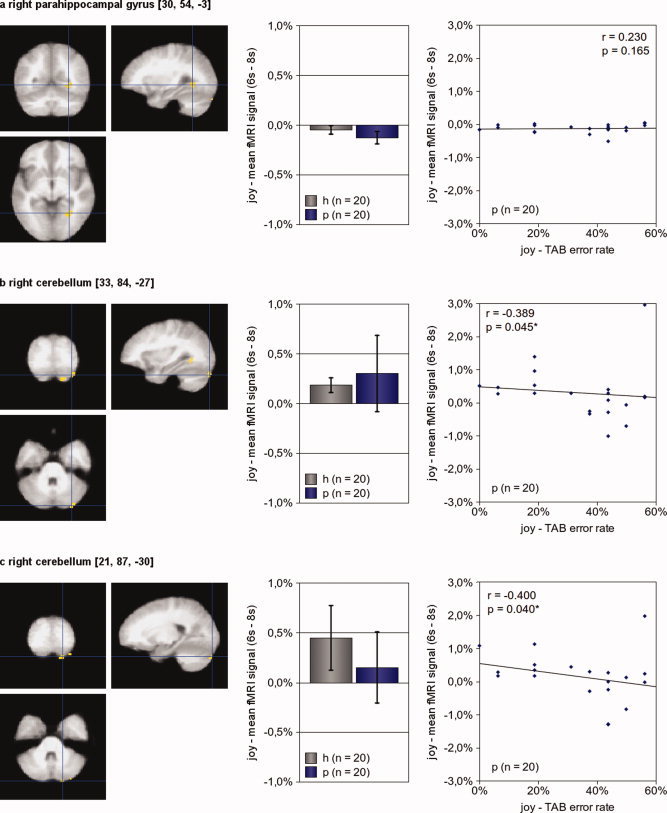
Brain activity during empathy with joyful faces Three brain regions showed diminished hemodynamic responses of somatoform disorder patients compared to healthy subjects during the contrast “joy” – “control” (P[uncorrected] = 0.001, minimum cluster size 10 voxel): the right parahippocampal gyrus (a), and two regions of the left cerebellum (b, c). Alike Figure 3 and 4, the left picture illustrates the exact location of the region of interest. The center picture shows the mean hemodynamic responses of healthy subjects (h, n = 20) and patients (p, n = 20) during empathy with joy. In the right picture, each patient is presented by a dot; its x‐value representing his average error rate in the TAB during joy and its y‐value representing his mean fMRI signal during empathy with joy. Activation maps are superimposed on a normalized mean image of all 40 participants (patients and healthy control subjects). Somatoform patients' TAB error rates during joy predicted diminished hemodynamic responses during joy of both cerebellar regions. The worse a patient's performance during the detection of joy, the smaller his hemodynamic activity during empathy with joy. (The error bars reflect the 95% confidence interval; P‐values are “one‐tailed.”)
Correlation of clinical scores and hemodynamic responses
We found a significant negative correlation of SCL‐90‐R‐ somatization scores and hemodynamic responses for the contrast “anger” – “control” in the left superior temporal gyrus (−33, −15, −27; r [Spearman] = −0.428, P [one‐tailed] = 0.034*). All other correlations of SCL‐90‐R, TAS‐20, and BDI scores with hemodynamic responses of any of the regions of interest did not reveal significant results. In addition, we did not find significant correlations of IRI empathic distress scores and hemodynamic responses for one of the regions of interest.
Correlation of emotion recognition error rates and hemodynamic responses in somatoform patients
We found significant negative correlations of TAB error rates for anger with hemodynamic responses of the contrast “anger” – “control” in somatoform patients in three regions: the left postcentral gyrus, the left superior temporal gyrus, and the left cerebellum (see Fig. 4 for details). TAB error rates for joy correlated negatively with hemodynamic responses of the contrast “joy” – “control” in two regions, both located in the right cerebellum (see Fig. 5 for details). No positive correlations were found.
DISCUSSION
Summary of Findings
Behaviorally, somatoform patients showed abnormal scores in several self‐report questionnaires, including the Symptom‐Checklist‐90‐R (SCL‐90‐R), the Toronto‐Alexithymia‐Scale 20 (TAS‐20), and the Beck‐Depression‐Inventory (BDI). Moreover, somatoform patients scored higher in the “empathic distress” subscale of the Interpersonal‐Reactivity‐Index (IRI) when compared to the healthy control group. Scores of somatoform patients in the “empathic fantasy,” “empathic concern,” and “perspective taking” subscale of the IRI did not differ significantly from scores of the healthy control group. Emotion recognition of somatoform patients in the Tübinger Affekt Batterie (TAB) was impaired when compared to healthy subject with regard to all tested emotions except “neutral.” Noteworthy, somatoform patients but not healthy subjects performed worse in trials using anger and sad stimuli compared to neutral stimuli. In addition, somatoform patients reported equal subjective impression of empathic capabilities during the fMRI experiment.
The fMRI analysis revealed diminished responsiveness of brain regions during empathy in three of the five contrasts: “all emotions” − “control,” “anger” − “control,” and “joy” − “control,” whereas we found no significant regions for the contrasts “disgust” − “control” and “neutral expression” − “control.” Two regions showed diminished activity in somatoform patients in the contrast “all emotions” − “control”: the right parahippocampal gyrus and the left amygdala. Hemodynamic responses in these regions did, however, not correlate with the overall error rates in the TAB. Several regions showed diminished neuronal activity in the contrast “anger” − “control,” including the left postcentral gyrus, the left superior temporal gyrus, the bilateral parahippocampal gyrus, the left posterior insula, the left amygdala and the left cerebellum. TAB error rates for anger correlated with hemodynamic responses during empathy with anger in the left postcentral gyrus, the left superior temporal gyrus and the left cerebellum. During empathy with joyful faces, somatoform patients showed diminished responsiveness in three regions: the right parahippocampal gyrus and two regions in the right cerebellum. TAB error rates for joy correlated with hemodynamic responses during empathy with joy in both cerebellar regions.
Brain Activity During Empathy in Healthy Subjects
Empathy lead to increased neuronal activity in a number of brain regions in healthy subjects, which included the bilateral inferior frontal cortex, bilateral amygdala, right supplementary motor area, right postcentral gyrus, and other regions. These results are in accordance with previous studies investigating the neural correlates of empathy [Blair et al., 1999; Breiter et al., 1996; Carr et al., 2003; de Greck et al., 2011a; Jabbi et al., 2007; Morris et al., 1996; Nomi et al., 2008; Phillips et al., 1997; Sprengelmeyer et al., 1998].
Comorbidity of Somatoform Patients
Somatoform patients not only exhibited increased somatization scores (as ascertained by the SCL‐90‐R), but also reported more depressive (BDI) and alexithymic symptoms (TAS‐20). This finding is in line with several studies about somatoform disorder, which concordantly reported a high comorbidity of somatoform symptoms, depressive symptoms and alexithymic symptoms [Bailey and Henry, 2007; Hanel et al., 2009; Waller and Scheidt, 2004]. Whilst we found significant correlations of hemodynamic responses of several regions and error rates in the TAB, as well as a significant correlation of SCL‐90‐R scores with hemodynamic responses of the left superior temporal gyrus during anger, we did not find any significant correlations of depressive or alexithymic symptom scores with hemodynamic responses. This supports the conclusion that our results are associated with impaired emotion recognition and somatization, rather than depression and alexithymia.
Diminished Neuronal Modulation in Somatoform Disorder
Our results differ from those of a previous study conducted by Lee et al. [2009], who investigated patients suffering from Hwa‐Byung, a mental disorder bound to the Korean culture, which resembles somatoform disorder with regard to medically unexplained symptoms. In their fMRI study using emotional faces, Lee et al. found several brain regions with stronger activity in Hwa‐Byung patients (compared to a group of healthy controls); these regions included the lingual gyrus, the fusiform gyrus and the inferior occipital gyrus. The authors described only one region with stronger activity in healthy controls during angry faces, the right thalamus. Besides several differences with regard to the study design (for instance Lee et al. used an epoch‐related design and their participants were instructed to merely watch the facial stimuli), differences in the psychodynamic mechanisms underlying somatoform disorder and Hwa‐Byung could explain the differences in brain activity: in Hwa‐Byung, patients suffer from medically unexplained symptoms which are caused by the initial suppression of anger, followed by an exaggerated anger response [Lee et al., 2009; Lin, 1983; Min, 2008]. Lee et al. [2009] suggest that a lack of emotional control is responsible for the increased activity in the lingual gyrus, fusiform gyrus, and inferior occipital gyrus (and the decreased activity of the right thalamus) during the processing of emotional faces. The psychodynamic mechanisms underlying somatoform disorders are different, however, since somatoform disorders are explained by a chronic suppression of specific emotions in order to protect relevant relationships [Bowlby, 1973; Maunder and Hunter, 2004; Waller and Scheidt, 2006]. Diminished modulation of brain activity in several regions of somatoform disorder patients in our study is in accordance with this concept.
Empathic Deficits, Impaired Emotion Recognition and Abnormal Brain Activity in Somatoform Disorder
The intra‐scanner ratings of subjective empathy experience and the IRI results suggest that somatoform patients do rather not present a global alteration of empathic disabilities but a distinct problem with a specific empathic process: “empathic distress”. Individuals, who score high on “empathic distress” in the IRI, describe themselves as to be easily affected and overwhelmed by negative emotional states of others. The TAB results show that somatoform patients have more difficulties in the recognition of all applied emotions except “neutral” (when compared to healthy control subjects).
The fMRI results show that brain activity of somatoform patients is abnormal for anger and joy, whilst we found no differences for the emotions disgust and neutral expression. These findings are in accordance with our initial hypothesis based on psychodynamic concepts of somatoform disorder, which explain somatoform disorder by the repression of emotions to avoid interpersonal conflicts [Bowlby, 1973; Maunder and Hunter, 2004; Waller and Scheidt, 2006]: somatoform patients showed abnormal brain activity only during social emotions such as anger and joy, whereas brain activity was normal during disgust and neutral expression. However, we are cautious with this interpretation because the behavioral results indicate that both groups felt better able to empathize with angry and happy faces compared to faces showing disgust and neutral expression.
Nevertheless, our findings fit into the psychodynamic concept of somatoform disorder in additional ways: (i) Somatoform patients showed normal empathic abilities in most of the applied behavioral empathy tests and only exhibited distinct changes in their empathic abilities connected to empathic distress. Negative emotions of others are experienced comparatively stronger and somatoform patients tend to experience them as overwhelming, which might trigger suppressing mechanisms [Waller and Scheidt, 2004]. (ii) Brain regions which showed diminished neural activity in somatoform patients included brain regions which are associated to emotional experience (amygdala), evaluation of social emotions (superior temporal gyrus), and emotional memory (parahippocampal gyrus). Diminished neuronal activity in these regions might reflect inhibition of emotion processing in these regions.
What can we infer from the regions, which showed altered neuronal activity in somatoform patients? The location of these regions (i.e., parahippocampal gyrus, amygdala, postcentral gyrus, superior temporal gyrus, posterior insula, cerebellum) is in accordance with the conclusion that somatoform patients do not only suffer from disturbed emotion recognition—a process which is associated with activity in amygdala and somatosensory cortex [Adolphs et al., 2000; Derntl et al., 2009; Gur et al., 2002] —but also from disturbances of other processes such as emotional empathy and emotional memory. Emotional empathy is known to induce activity in the superior temporal sulcus region [Carr et al., 2003; de Greck et al., 2011a; Hoekert et al., 2008; Hooker et al., 2008], this region showed disturbed neuronal activity in somatoform patients. Emotional memory is associated with neuronal activity in the parahippocampal gyrus [Smith et al., 2004; Sterpenich et al., 2006], another region, which showed diminished modulation in somatoform patients. Interestingly, in a recent study by Loughead et al., activity in the parahippocampal gyrus was positively correlated with the degree of conflict related to autobiographical episodes [Loughead et al., 2010]. Disturbed activity in the parahippocampal gyrus in somatoform patients is hence in line with the assumption that somatoform patients suffer from disturbed processing of emotional memory, a conclusion which is in accordance with psychodynamic concepts concerning somatoform disorder. However, since we did not investigate emotional memory in this study, this conclusion has to be preliminary.
Whilst we found negative correlations of emotion recognition error rates and hemodynamic responses of the left postcentral gyrus, left superior temporal gyrus, left posterior insula, and the bilateral cerebellum, other regions including the bilateral parahippocampal gyrus and the amygdala lacked this correlation. These results support our conclusion that the empathic deficits of somatoform patients cannot be completely reduced to impaired emotion recognition.
Our results suggest further that the cerebellum plays a role in the empathic dysfunctions of somatoform patients during anger and joy. A region in the left cerebellum showed diminished activity the contrast “anger” – “control,” and two regions in the right cerebellum showed diminished activation in the contrast “joy” – “control.” Hemodynamic responses of all three cerebellar regions correlated negatively with error rates for the according emotions in the TAB. The cerebellum is known to play a role in emotion regulation [Schutter and van Honk, 2005] and is involved in the processing of emotional faces [Fusar‐Poli et al., 2009] and emotional stimuli in general [Bermpohl, et al., 2006]. Furthermore, the cerebellum plays a role in the processing of autobiographic memory [Svoboda et al., 2006].
What new insights do our results reveal about the relationship of empathy and emotion recognition and their impairment in somatoform disorder? Empathy describes the induction of a congruent emotion in one individual caused by the emotional state of another individual [Decety and Jackson, 2004; Preston and de Waal, 2002]. Emotion recognition means the correct labeling of an emotion presented by a target individual [Pedrosa Gil et al., 2009]. For both processes, the generation of a specific emotional state (congruent to the emotional state of the target) is essential [Adolphs et al., 2000; Preston and de Waal, 2002]. Regarding this, the generation of an emotional state can be induced (i) by external stimuli—for instance by emotional contagion, a process which involves neuronal activity in particular in the inferior frontal gyrus and the inferior parietal cortex [Keysers and Gazzola, 2006; Kramer et al., 2010; Nummenmaa et al., 2008; Shamay‐Tsoory, 2011] —or (ii) by a stimulus from the internal world ‐ for instance the induction of emotion by autobiographic memory, which involves neuronal activity in particular in the parahippocampal gyrus [Damasio et al., 2000; Smith et al., 2004; Sterpenich et al., 2006]. The two processes have in common that they engage further activity in somatosensory cortices, such as the postcentral gyrus and insula to generate a full blown emotional response [Adolphs et al., 2000; Banissy et al., 2010; Damasio et al., 2000; Immordino‐Yang et al., 2009; Nummenmaa et al., 2008; Rudrauf et al., 2009].
Relying on these concepts, the following interpretation is consistent with our data: Somatoform disorder patients suffer from deficits in emotion recognition and show abnormal brain activity during empathy, because they cannot generate the congruent emotional state within themselves. Why are somatoform patients not able to do so and how can we support this conclusion? Relying on psychodynamic concepts, a possible explanation for impaired emotion generation of somatoform disorder patients is that these patients have learnt to unconsciously suppress specific emotions in order to protect relevant relationships [Bowlby, 1973; Maunder and Hunter, 2004; Waller and Scheidt, 2006]. In addition, as our behavioral results show, somatoform disorder patients describe more empathic distress, they are afraid to be overwhelmed by emotions, which probably inhibits emotion generation processes. With regard to our fMRI findings, another explanation for impaired emotion generation of somatoform patients is the disturbed activity of the parahippocampal gyrus, which does not provide the necessary biographical memory to generate the emotional state. In addition, our study showed diminished modulation of the somatoform patients' postcentral gyrus and insula; both regions do not provide the somatosensory information necessary for sufficient emotion generation and emotional experience.
Limitations
We would also like to address a few limitations of our study. The group of patients was rather heterogeneous, consisting of different forms of somatoform disorders. In addition, we are aware of the discussion about the heterogeneous concept of somatoform disorders in general [Kroenke, 2006; Mayou et al., 2005; Voigt et al., 2010]. However, since we focused on the empathic processing of emotional stimuli, which is independent of the individual symptoms of each patient, we decided to include any patient who fulfilled the criteria of a somatoform disorder. Further, in the fMRI contrasts comparing somatoform patients and healthy subjects, we did not apply corrections for multiple comparisons. Our results are thus preliminary and await replication in other studies. Nonetheless, we found significant correlations of hemodynamic responses and error rates in the TAB in several regions (left postcentral gyrus, left superior temporal gyrus, bilateral cerebellum), which confirm the involvement of these regions. Moreover, six of our patients were on psychotropic medication. However, we implemented additional voxel based contrasts comparing the 14 nonmedicated patients and the 20 healthy controls, in which we found comparable T scores of the peak voxels of the regions of interest. These results strongly suggest that differences in neuronal activity between healthy controls and somatoform patients are not due to medication effects; nonetheless, we can not completely exclude a bias caused by the psychotropic medication. Another limitation regards the empathy task of our study. We applied an empathy task, which focused on intentional emotional empathy, which is only a small aspect within the range of empathic responses. In future studies it might be of interest to investigate further empathic processes, such as emotional contagion or in particular empathy for pain. Finally, due to time restraints, the variety of emotions applied in our study was limited. In addition, our paradigm did not allow disentangle empathic processes from other emotional processes such as emotion recognition or emotion generation; such a comparison would be another promising endeavor. In future studies it might also be interesting to explore brain function of somatoform patients during empathy with other emotions, such as fear or in particular sadness, which were not included in our study.
CONCLUSIONS
Impaired emotion recognition of somatoform disorder patients is linked to abnormal brain activity during emotional empathy in brain regions responsible for emotional evaluation, emotional memory, and emotion generation.
Acknowledgements
The authors thank the staff of the Department of Neurology of the Otto‐von‐Guericke‐University of Magdeburg for their support. The authors also thank Jennifer Dent and Niall Duncan for their helpful propositions concerning the script. M.G. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR ( 2000): A role for somatosensory cortices in the visual recognition of emotion as revealed by three‐dimensional lesion mapping. J Neurosci 20: 2683–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M, Bach D ( 1996): Alexithymia in somatoform disorder and somatic disease: A comparative study. Psychother Psychosom 65: 150–152. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Taylor GJ, Parker JD ( 1994): The Twenty‐item Toronto Alexithymia Scale‐II. Convergent, discriminant, and concurrent validity. J Psychosom Res 38: 33–40. [DOI] [PubMed] [Google Scholar]
- Bailey PE, Henry JD ( 2007): Alexithymia, somatization and negative affect in a community sample. Psychiatry Res 150: 13–20. [DOI] [PubMed] [Google Scholar]
- Banissy MJ, Sauter DA, Ward J, Warren JE, Walsh V, Scott SK ( 2010): Suppressing sensorimotor activity modulates the discrimination of auditory emotions but not speaker identity. J Neurosci 30: 13552–13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankier B, Aigner M, Bach M ( 2001): Alexithymia in DSM‐IV disorder: Comparative evaluation of somatoform disorder, panic disorder, obsessive‐compulsive disorder, and depression. Psychosomatics 42: 235–240. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J ( 1961): An inventory for measuring depression. Arch Gen Psychiatry 4: 561–571. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Pascual‐Leone A, Amedi A, Merabet LB, Fregni F, Gaab N, Alsop D, Schlaug G, Northoff G ( 2006): Dissociable networks for the expectancy and perception of emotional stimuli in the human brain. Neuroimage 30: 588–600. [DOI] [PubMed] [Google Scholar]
- Blair RJ ( 2003): Facial expressions, their communicatory functions and neuro‐cognitive substrates. Philos Trans R Soc Lond B Biol Sci 358: 561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ ( 1999): Dissociable neural responses to facial expressions of sadness and anger. Brain 122( Pt 5): 883–893. [DOI] [PubMed] [Google Scholar]
- Bowers D, Blonder LX, Heilman KM ( 1999): Florida Affect Battery: Center for Neuropsychological Studies, Cognitive Neuroscience Laboratory. Florida: University of Florida. [Google Scholar]
- Bowlby J ( 1973): Attachment and Loss: Vol. 2. Separation: Anger and Anxiety. The Travistock Institute of Human Relations, London. [Google Scholar]
- Breitenstein C, Daum I, Ackermann H, Lütgehetmann R, Müller E ( 1996): Erfassung der Emotionswahrnehmung bei zentralnervösen Läsionen und Erkrankungen: Psychometrische Gütekriterien der “Tübinger Affekt Batterie”. Neurol Rehabil 2: 9. [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR ( 1996): Response and habituation of the human amygdala during visual processing of facial expression. Neuron 17: 875–887. [DOI] [PubMed] [Google Scholar]
- Bressi C, Taylor G, Parker J, Bressi S, Brambilla V, Aguglia E, Allegranti I, Bongiorno A, Giberti F, Bucca M, Todarello O, Callegari C, Vender S, Gala C, Invernizzi G. ( 1996): Cross validation of the factor structure of the 20‐item Toronto Alexithymia Scale: An Italian multicenter study. J Psychosom Res 41: 551–559. [DOI] [PubMed] [Google Scholar]
- Burba B, Oswald R, Grigaliunien V, Neverauskiene S, Jankuviene O, Chue P ( 2006): A controlled study of alexithymia in adolescent patients with persistent somatoform pain disorder. Can J Psychiatry 51: 468–471. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL ( 2003): Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A 100: 5497–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW ( 1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- Csukly G, Czobor P, Szily E, Takacs B, Simon L ( 2009): Facial expression recognition in depressed subjects: the impact of intensity level and arousal dimension. J Nerv Ment Dis 197: 98–103. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD ( 2000): Subcortical and cortical brain activity during the feeling of self‐generated emotions. Nat Neurosci 3: 1049–1056. [DOI] [PubMed] [Google Scholar]
- Davis MH ( 1983): Measuring individual differences in empathy: Evidence for a multidimensional approach. J Personality Soc Psychol 44: 14. [Google Scholar]
- de Greck M, Gang W, Yang X, Wang X, Northoff G, Han S: Neural substrates underlying intentional empathy. Soc Cogn Affect Neurosci (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Greck M, Scheidt L, Bolter AF, Frommer J, Ulrich C, Stockum E, Enzi B, Tempelmann C, Hoffmann T, Northoff G ( 2011): Multimodal psychodynamic psychotherapy induces normalization of reward related activity in somatoform disorder. World J Biol Psychiatry 12: 296–308. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL ( 2004): The functional architecture of human empathy. Behav Cogn Neurosci Rev 3: 71–100. [DOI] [PubMed] [Google Scholar]
- Decety J, Moriguchi Y ( 2007): The empathic brain and its dysfunction in psychiatric populations: implications for intervention across different clinical conditions. Biopsychosoc Med 1: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B, Habel U, Windischberger C, Robinson S, Kryspin‐Exner I, Gur RC, Moser E ( 2009): General and specific responsiveness of the amygdala during explicit emotion recognition in females and males. BMC Neurosci 10: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR ( 1977): SCL‐90‐R, administration, scoring and procedures manual‐I for the R(evised) version. Baltimore, MD: Johns Hopkins University School of Medicine. [Google Scholar]
- Duddu V, Isaac MK, Chaturvedi SK ( 2003): Alexithymia in somatoform and depressive disorders. J Psychosom Res 54: 435–438. [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G ( 2011): Is there a core neural network in empathy? An fMRI based quantitative meta‐analysis. Neurosci Biobehav Rev 35: 903–911. [DOI] [PubMed] [Google Scholar]
- Franke GH ( 2002): Die Symptom‐Checkliste von Derogatis‐Deutsche version‐Manual. Göttingen, Germany: Beltz. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RS ( 1995): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 12. [Google Scholar]
- Fusar‐Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P ( 2009): Functional atlas of emotional faces processing: A voxel‐based meta‐analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci 34: 418–432. [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G ( 2004): A unifying view of the basis of social cognition. Trends Cogn Sci 8: 396–403. [DOI] [PubMed] [Google Scholar]
- Gerlach C, Aaside CT, Humphreys GW, Gade A, Paulson OB, Law I ( 2002): Brain activity related to integrative processes in visual object recognition: bottom‐up integration and the modulatory influence of stored knowledge. Neuropsychologia 40: 1254–1267. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Spitzer C, Freyberger HJ ( 2004): Alexithymia and personality in relation to dimensions of psychopathology. Am J Psychiatry 161: 1299–1301. [DOI] [PubMed] [Google Scholar]
- Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, Alsop D, Maldjian J, Gur RE ( 2002): Brain activation during facial emotion processing. Neuroimage 16( 3 Pt 1): 651–662. [DOI] [PubMed] [Google Scholar]
- Guttman H, Laporte L ( 2002): Alexithymia, empathy, and psychological symptoms in a family context. Compr Psychiatry 43: 448–455. [DOI] [PubMed] [Google Scholar]
- Hanel G, Henningsen P, Herzog W, Sauer N, Schaefert R, Szecsenyi J, Lowe B ( 2009): Depression, anxiety, and somatoform disorders: vague or distinct categories in primary care? Results from a large cross‐sectional study. J Psychosom Res 67: 189–197. [DOI] [PubMed] [Google Scholar]
- Henry JD, Bailey PE, Rendell PG ( 2008): Empathy, social functioning and schizotypy. Psychiatry Res 160: 15–22. [DOI] [PubMed] [Google Scholar]
- Hiller W, Cebulla M, Korn HJ, Leibbrand R, Roers B, Nilges P ( 2010): Causal symptom attributions in somatoform disorder and chronic pain. J Psychosom Res 68: 9–19. [DOI] [PubMed] [Google Scholar]
- Hoekert M, Bais L, Kahn RS, Aleman A ( 2008): Time course of the involvement of the right anterior superior temporal gyrus and the right fronto‐parietal operculum in emotional prosody perception. PLoS One 3: e2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Germine LT, Knight RT, D'Esposito M ( 2008): Mentalizing about emotion and its relationship to empathy. Soc Cogn Affect Neurosci 3: 204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Germine LT, Knight RT, D'Esposito M ( 2010): Neural activity during social signal perception correlates with self‐reported empathy. Brain Res 1308: 100–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immordino‐Yang MH, McColl A, Damasio H, Damasio A ( 2009): Neural correlates of admiration and compassion. Proc Natl Acad Sci U S A 106: 8021–8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, Swart M, Keysers C ( 2007): Empathy for positive and negative emotions in the gustatory cortex. Neuroimage 34: 1744–1753. [DOI] [PubMed] [Google Scholar]
- Josephs O, Turner R, Friston K ( 1997): Event‐related f MRI. Hum Brain Mapp 5: 243–248. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V ( 2006): Towards a unifying neural theory of social cognition. Prog Brain Res 156: 379–401. [DOI] [PubMed] [Google Scholar]
- Kirmayer LJ, Robbins JM, Paris J ( 1994): Somatoform disorders: personality and the social matrix of somatic distress. J Abnorm Psychol 103: 125–136. [DOI] [PubMed] [Google Scholar]
- Kooiman CG, Bolk JH, Brand R, Trijsburg RW, Rooijmans HG ( 2000): Is alexithymia a risk factor for unexplained physical symptoms in general medical outpatients? Psychosom Med 62: 768–778. [DOI] [PubMed] [Google Scholar]
- Kramer UM, Mohammadi B, Donamayor N, Samii A, Munte TF ( 2010): Emotional and cognitive aspects of empathy and their relation to social cognition‐‐an fMRI‐study. Brain Res 1311: 110–120. [DOI] [PubMed] [Google Scholar]
- Kroenke K ( 2006): Physical symptom disorder: A simpler diagnostic category for somatization‐spectrum conditions. J Psychosom Res 60: 335–339. [DOI] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J ( 2007): The neural substrate of human empathy: Effects of perspective‐taking and cognitive appraisal. J Cogn Neurosci 19: 42–58. [DOI] [PubMed] [Google Scholar]
- Lee BT, Paik JW, Kang RH, Chung SY, Kwon HI, Khang HS, Lyoo IK, Chae JH, Kwon JH, Kim JW, Lee MS, Ham BJ ( 2009): The neural substrates of affective face recognition in patients with Hwa‐Byung and healthy individuals in Korea. World J Biol Psychiatry 10: 552–559. [DOI] [PubMed] [Google Scholar]
- Lin KM ( 1983): Hwa‐Byung: A Korean culture‐bound syndrome? Am J Psychiatry 140: 105–107. [DOI] [PubMed] [Google Scholar]
- Loughead JW, Luborsky L, Weingarten CP, Krause ED, German RE, Kirk D, Gur RC ( 2010): Brain activation during autobiographical relationship episode narratives: A core conflictual relationship theme approach. Psychother Res 20: 321–336. [DOI] [PubMed] [Google Scholar]
- Matsumuto D, Ekman P ( 1988): Japanese and Caucasian Facial Expressions of Emotion (JACFEE) and Neutral Faces JACNeuF. San Francisco: University of California. [Google Scholar]
- Mattila AK, Kronholm E, Jula A, Salminen JK, Koivisto AM, Mielonen RL, Joukamaa M ( 2008): Alexithymia and somatization in general population. Psychosom Med 70: 716–722. [DOI] [PubMed] [Google Scholar]
- Maunder R, Hunter J ( 2004): An integrated approach to the formulation and psychotherapy of medically unexplained symptoms: Meaning‐and attachment‐based intervention. Am J Psychother 58: 17–33. [DOI] [PubMed] [Google Scholar]
- Mayou R, Kirmayer LJ, Simon G, Kroenke K, Sharpe M ( 2005): Somatoform disorders: Time for a new approach in DSM‐V. Am J Psychiatry 162: 847–855. [DOI] [PubMed] [Google Scholar]
- Min SK ( 2008): Clinical correlates of hwa‐byung and a proposal for a new anger disorder. Psychiatry Investig 5: 125–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SK, Suh SY ( 2010): The anger syndrome hwa‐byung and its comorbidity. J Affect Disord 124: 211–214. [DOI] [PubMed] [Google Scholar]
- Min SK, Suh SY, Song KJ ( 2009): Symptoms to use for diagnostic criteria of hwa‐byung, an anger syndrome. Psychiatry Investig 6: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ ( 1996): A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383: 812–815. [DOI] [PubMed] [Google Scholar]
- Nichols T, Hayasaka S ( 2003): Controlling the familywise error rate in functional neuroimaging: A comparative review. Stat Methods Med Res 12: 419–446. [DOI] [PubMed] [Google Scholar]
- Nomi JS, Scherfeld D, Friederichs S, Schafer R, Franz M, Wittsack HJ, Azari NP, Missimer J, Seitz RJ ( 2008): On the neural networks of empathy: A principal component analysis of an fMRI study. Behav Brain Funct 4: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Hirvonen J, Parkkola R, Hietanen JK ( 2008): Is emotional contagion special? An fMRI study on neural systems for affective and cognitive empathy. Neuroimage 43: 9. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC ( 2004): Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci 16: 1746–1772. [DOI] [PubMed] [Google Scholar]
- Pedrosa Gil F, Scheidt CE, Hoeger D, Nickel M ( 2008): Relationship between attachment style, parental bonding and alexithymia in adults with somatoform disorders. Int J Psychiatry Med 38: 437–451. [DOI] [PubMed] [Google Scholar]
- Pedrosa Gil F, Ridout N, Kessler H, Neuffer M, Schoechlin C, Traue HC, Nickel M ( 2009): Facial emotion recognition and alexithymia in adults with somatoform disorders. Depress Anxiety 26: E26–33. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SC, Gray JA, David AS ( 1997): A specific neural substrate for perceiving facial expressions of disgust. Nature 389: 495–498. [DOI] [PubMed] [Google Scholar]
- Preston SD, de Waal FB ( 2002): Empathy: Its ultimate and proximate bases. Behav Brain Sci 25: 1–20; discussion 20–71. [DOI] [PubMed] [Google Scholar]
- Rudrauf D, Lachaux JP, Damasio A, Baillet S, Hugueville L, Martinerie J, Damasio H, Renault B ( 2009): Enter feelings: Somatosensory responses following early stages of visual induction of emotion. Int J Psychophysiol 72: 13–23. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, van Honk J ( 2005): The cerebellum on the rise in human emotion. Cerebellum 4: 290–294. [DOI] [PubMed] [Google Scholar]
- Shamay‐Tsoory SG ( 2011): The Neural Bases for Empathy. Neuroscientist 17: 18–24. [DOI] [PubMed] [Google Scholar]
- Smith AP, Henson RN, Dolan RJ, Rugg MD ( 2004): fMRI correlates of the episodic retrieval of emotional contexts. Neuroimage 22: 868–878. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H ( 1998): Neural structures associated with recognition of facial expressions of basic emotions. Proc Biol Sci 265: 1927–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ, Muller J ( 2008): Cognitive‐affective neuroscience of somatization disorder and functional somatic syndromes: Reconceptualizing the triad of depression‐anxiety‐somatic symptoms. CNS Spectr 13: 379–384. [DOI] [PubMed] [Google Scholar]
- Sterpenich V, D'Argembeau A, Desseilles M, Balteau E, Albouy G, Vandewalle G, Degueldre C, Luxen A, Collette F, Maquet P ( 2006): The locus ceruleus is involved in the successful retrieval of emotional memories in humans. J Neurosci 26: 7416–7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B ( 2006): The functional neuroanatomy of autobiographical memory: A meta‐analysis. Neuropsychologia 44: 2189–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Voigt K, Nagel A, Meyer B, Langs G, Braukhaus C, Lowe B ( 2010): Towards positive diagnostic criteria: A systematic review of somatoform disorder diagnoses and suggestions for future classification. J Psychosom Res 68: 403–414. [DOI] [PubMed] [Google Scholar]
- Waller E, Scheidt CE ( 2004): Somatoform disorders as disorders of affect regulation: A study comparing the TAS‐20 with non‐self‐report measures of alexithymia. J Psychosom Res 57: 239–247. [DOI] [PubMed] [Google Scholar]
- Waller E, Scheidt CE ( 2006): Somatoform disorders as disorders of affect regulation: A development perspective. Int Rev Psychiatry 18: 13–24. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G ( 2003): Both of us disgusted in My insula: The common neural basis of seeing and feeling disgust. Neuron 40: 655–664. [DOI] [PubMed] [Google Scholar]
- Williams C, Wood RL ( 2010): Alexithymia and emotional empathy following traumatic brain injury. J Clin Exp Neuropsychol 32: 259–267. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Zaudig M, Fydrich T ( 1997): SKID. Strukturiertes Klinisches Interview für DSM‐IV. Göttingen, Germany: Hogrefe, Verlag für Psychologie. [Google Scholar]
- Wood RL, Williams C, Kalyani T ( 2009): The impact of alexithymia on somatization after traumatic brain injury. Brain Inj 23: 649–654. [DOI] [PubMed] [Google Scholar]
- Yamada M, Decety J ( 2009): Unconscious affective processing and empathy: An investigation of subliminal priming on the detection of painful facial expressions. Pain 143: 71–75. [DOI] [PubMed] [Google Scholar]


