Abstract
We used fMRI to explore the extent of the anatomical overlap of three neural systems that the literature on developmental dyslexia associates with reading: the auditory phonological, the visual magnocellular, and the motor/cerebellar systems. Twenty‐eight normal subjects performed four tasks during fMRI scans: word and pseudoword reading, auditory rhyming for letter names, visual motion perception, and a motor sequence learning task. We found that the left occipitotemporal cortex (OTC), which previous studies reported to be dysfunctional in dyslexia, can be fractionated into different functional areas: an anterior and lateral area that was activated by both reading and auditory rhyming tasks; a posterior area that was commonly activated by both the reading and the motion perception task and a medial/intermediate area, including the so‐called Visual Word Form Area, which was specifically activated by the reading task. These results show that the left OTC is an area of segregated convergence of different functional systems. We compared our results with the hypoactivation pattern reported for reading in a previous cross‐cultural PET study on 36 dyslexic subjects from three countries. The region of decreased activation in dyslexia overlapped with regions that are specific for reading and those activated during both the auditory rhyming task and the single word and pseudoword reading task described in the present fMRI study. No overlap was found with the activation patterns for the visual motion perception task or for the motor sequence learning task. These observations challenge current theories of dyslexia. Hum Brain Mapp 34:2669–2687, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: reading, developmental dyslexia, occipitotemporal cortex, cerebellum, fMRI
Abbreviations
- BOLD
blood oxygen level‐dependent
- DTI
diffusion tensor imaging
- fMRI
functional magnetic resonance imaging
- FWE
family‐wise error
- LIMA
lateral inferior multimodal area
- OTC
occipitotemporal cortex
- PET
positron emission tomography
- ROI
region of interest
- VBM
voxel‐based morphometry
- VOT
voice onset time
- VWFA
visual word form area
INTRODUCTION: PHYSIOLOGICAL MODELS OF READING AND THE INTERPRETATION OF DYSLEXIA
Reading is a multicomponent task that involves basic visuoperceptive, oculomotor, and attentional skills [Aghababian and Nazir, 2000; Levy, 2010; Rayner, 1986; Shaywitz and Shaywitz, 2008], together with access to symbolic orthographic and lexico‐semantic knowledge and the activation of phonological representations [Coltheart et al., 1997; Plaut et al., 1996; Zorzi et al., 1998].
During the last 20 years, more than 40 studies investigating the neural network involved in reading single words or pseudowords (i.e., legally spelled neologisms) have been published [for reviews, see Fiez and Petersen, 1998; Price, 2000; Price and Devlin, 2003]. On the basis of these studies there is little mystery about which brain regions are involved, at least loosely, with the task of reading [Fiez and Petersen, 1998; Graves et al., 2010]. However, there is still considerable controversy regarding the functional contributions of the individual brain areas or about the possibility of supporting a specific cognitive model of reading [Fiez and Petersen, 1998; Graves et al., 2010; Price, 2000; Price and Devlin, 2003; Wandell, 2011].
Indeed, to‐date no conclusive evidence has emerged in support of a dual route model for reading rather than a single process connectionist model [Binder et al., 2005; Jobard et al., 2003; Levy et al., 2009].
To make another more anatomically oriented example, the activation of the so‐called Visual Word Form Area (VWFA), a must‐find for imaging experiments on normal word and pseudoword reading, has been interpreted by some authors in terms of specialized orthographic recognition [Cohen et al., 2002; Kronbichler et al., 2009], whereas other authors deny this cortical region has such a level of specialization [Price and Devlin, 2003].
Matters would become even more complex for anyone daring to venture into the interactions between the decoding process of reading and the oculomotor and attentional control needed for the ecologically more relevant reading of sentences rather than the reading of isolated single words [Cutting et al., 2006; Weger and Inhoff, 2006].
The lack of a shared interpretation of the functional role of the different regions of the reading brain network also has implications for the interpretation of the abnormalities found in reading disorders, including developmental dyslexia, a syndrome that has been interpreted in several, sometimes contrasting, ways [Frith, 1999; Nicolson et al., 2001; Snowling, 2001; Stein, 2001].
Among the many competing theories, there are three major hypotheses on dyslexia which postulate: (i) a phonological language disorder affecting the decoding system, the phonological hypothesis [Frith, 1999; Ramus et al., 2003; Snowling, 2001], (ii) a disorder of the magnocellular visual system implicated in visual motion perception, oculomotor control, and spatial attention [Eden et al., 1996; Galaburda, 1993; Hari and Renvall, 2001; Stein, 2001]1, and (iii) a defective functioning of the cerebellum and of the skill‐learning system [Nicolson et al., 2001].
The phonological hypothesis is more specific than the others because it assumes that dyslexia is caused by a functionally independent deficit [Frith, 1999]; dyslexic subjects would suffer from a selective deficit of the “phonological system”, with the implication that additional deficits, including more elementary auditory ones [Tallal, 1980], do not belong to the core of the syndrome in its pure form [Ramus et al., 2003]. This deficit would provoke delayed and imperfect development of the mappings between the sounds of speech and their corresponding orthographic representations [Frith, 1999; Ramus et al., 2003; Snowling, 2001]. Even though the exact aspect of the phonological processing compromised in dyslexia remains to be identified (phoneme to grapheme, orthographic and phonological lexicon), pathological observations and functional anatomical studies report neural abnormalities in brain regions typically involved in sublexical phonological processing [e.g., the planum temporale, opercular Broca's area; Paulesu et al., 1996; Rumsey et al., 1992], as identified by phonological awareness tasks at the syllabic or phonological level, but also in brain regions involved in the phonological retrieval of reading, in object naming and in orthographic decoding [e.g., the left inferior temporal and the fusiform gyri; McCrory et al., 2005a; Paulesu et al., 2001; Salmelin et al., 1996; Shaywitz et al., 2002].
The two other major theories predicate a more basic neurocognitive disorder that, as a result of a cascade effect, may also explain the phonological deficit. Therefore, these theories are more general because they attempt to explain the broader spectrum of symptoms sometimes associated with dyslexia.
In particular, the visual magnocellular hypothesis suggests that developmental dyslexia is the consequence of the dysfunction/lack of development of the magnocells of the visual system. These neurons have a transient response to stimuli and are therefore suited to capture stimulations in the high‐frequency range [see Stein and Walsh, 1997]. This neural system is capable of processing fast flashing stimuli or moving stimuli in the low spatial frequency domain, like the Gabor patches used as stimuli in our experiment. The magnocellular hypothesis may also explain visuo‐perceptual problems such as the results of deficits of oculomotor control [Cornelissen et al., 1995, 1998; Stein and Walsh, 1997]. According to Stein 1997, “a visual magnocellular deficit may explain why dyslexics often complain that small letters appear to blur and move around when they are trying to read”. Once again in Stein's words 2001, “the visual magnocellular system is responsible for timing visual events when reading. It therefore signals any visual motion that occurs if unintended movements lead to images moving off the fovea ('retinal slip'). These signals are then used to bring the eyes back on target”. Another visual magnocellular concept, sometimes called into play for dyslexia, is saccadic suppression [Breitmeyer, 1993; for a review of this process in normal subjects, see Ross et al., 2001]. However, so far no definitive demonstration that saccadic suppression might be deficient in dyslexia is available [Boden and Giaschi, 2007].
Finally, according to Galaburda et al. 1994, a deficit of the magnocellular subdivision of the auditory thalamus would be consistent with the left hemisphere‐based phonological deficits observed in dyslexics at the behavioral level.
In contrast, the “cerebellar” hypothesis suggests that reading disorders in children with developmental dyslexia would be due both to a deficit in skill learning and to an inner speech production disorder [Nicolson and Fawcett 1990; Nicolson et al., 2001] caused either by diffuse damage or by a faulty development of the cerebellum. At the behavioral level, the cerebellar dysfunction may explain the difficulties of dyslexics in information processing speed, time estimation, motor skill and motor memory, and in balance tasks [Nicolson and Fawcett, 1990; Nicolson et al., 1995; Stoodley et al., 2005]. The cerebellar hypothesis has the potential to explain the phonological deficits of dyslexics if it is postulated that these are articulatory in nature [Nicolson et al., 2001].2
Aims of the Study
In principle, several benefits should arise from a well‐defined characterization of the physiology of reading and a detailed functional cartography of the cortices that provide information about the level of anatomical convergence between the different systems called into play by the theories on dyslexia. On the one hand, more would be learnt about reading, a noninnate skill that implies the interactions and possibly the intersections of different systems; do these systems overlap anatomically in the adult brain or do they interact from a distance? On the other hand, a detailed cartography of the reading system should facilitate examination of the physiological and cognitive deficits of dyslexia, permitting a more explicit assessment of the explanatory power of the competing theories on dyslexia, both at a population level and when considering single dyslexic subjects. However, despite the phenomenal experimental effort documented by the literature, to the best of our knowledge no experiments have assessed the particular perspective taken here.
With the long‐term goal of evaluating the functional anatomical abnormalities observed in dyslexia through a detailed characterization of the properties of the cortices involved in reading, we initially examined the typical fMRI patterns associated with word and pseudoword reading, and mapped their anatomical overlap with the fMRI patterns associated with auditory phonological discrimination, visual motion perception [a classical visual magnocellular task; Demb et al., 1998], and motor learning, a task reliably associated with the activation of the motor system, including the cerebellum [Friston et al., 1991; Jenkins et al., 1994; Nicolson et al., 2001].3
With a view to provide an initial assessment of the relevance of these new fMRI observations for the interpretation of the dysfunctional anatomy of reading described in dyslexia, we measured the degree of overlap of this new mapping study with the pattern of reduced activation for reading described by Paulesu et al. 2001 in a PET study on a sample of 36 dyslexic subjects and 36 controls from three countries.
MATERIALS AND METHODS
Participants
Twenty‐eight healthy, right‐handed Italian university students (F = 13, M = 15; agemean = 21; agest.dev.= 2.29) with at least 13 years of schooling participated in this study. The subjects had no history of neurological and psychiatric disorders. Informed consent was obtained from all subjects prior to the experiment.
Neuropsychological tests performed before the fMRI scanner
All subjects had a normal IQ, as measured by the Wechsler Adult Intelligence Scale‐Revised [WAIS‐R; Wechsler, 1981]. Moreover, we measured the reading times and error rates for 20 words (concrete and familiar nouns) and 20 pseudowords4 created from the words by maintaining the “word envelope” but changing the internal consonants. Each stimulus was displayed for a maximum of 1.5 s and the participants were asked to read it as soon as it appeared on the screen. The voice onset times (VOT) for the detection of a single dot was used as a baseline measure to distinguish any generic lengthening of visuo‐vocal reaction times from more specific reading disorders [Paulesu et al., 2000]. Table 1 reports the demographic data and the results of the neuropsychological testing.
Table 1.
Demographic and neuropsychological data
| Demographic data | Age | 21.3 (2.52) |
| Education | 14 (2.73) | |
| Female/Male | 13/15 | |
| Psychological testing outside the scanner | IQ | 119.6 (7.58) |
| Verbal IQ | 116.3 (8.8) | |
| Performance IQ | 121.1 (8.43) | |
| V.O.T. | 364.5 (60.6) | |
| Word reading (time) | 464.1 (51.14) | |
| Word reading (errors) | 0.06 (0.25) | |
| Pseudoword reading (time) | 529.2 (66.5) | |
| Pseudoword reading (errors) | 0.42 (0.72) | |
| Performance during fMRI | Pure tone discrimination (hits) | 14.08 (1.47) |
| Letter names rhyming task (hits) | 13.22 (0.04) | |
| Motor sequence learning: longest sequence of taps learned of a fixed 8‐taps target sequence | 4.63 (1.01) | |
| Motor sequence learning: attempts | 15.41 (2.82) |
For each variable we report the mean and the standard deviation values.
Experimental Tasks
The participants performed five tasks during the fMRI: (1) a word reading task, (2) a pseudoword reading task, (3) an auditory rhyming task, (4) a visual motion perception task and (5) a motor sequence learning task.
The activation patterns of the reading tasks served as a reference where we evaluated the degree of spatial overlap of the patterns of activation of tasks 3, 4, and 5: these were designed to challenge the auditory phonological system, the visual magnocellular system, and the cerebellar/motor system, respectively.
Word and pseudoword reading
The participants were asked to silently read blocks of single words or blocks of pseudowords during a 6‐min fMRI scanning session. These stimuli were presented one at a time, in the center of a computer screen, and were alternated with blocks of baseline stimuli made of strings of differently oriented lines matching the visual angle covered by the words and the pseudowords. Each block lasted 30 s. The participants were also asked to press a response button after the presentation of each stimulus during both the reading and the baseline blocks [Paulesu et al., 2000].
A total of 45 high‐ and low‐frequency, bi‐, tri‐, and quadri‐syllabic Italian words were presented in three blocks.
Pseudowords were generated by changing the internal consonants of Italian words, while maintaining the word shape. As for words, a total of 45 pseudowords were presented in three experimental blocks.
Our objective, in using this protocol and subtracting only the most peripheral aspects of visual perception for simple line patterns, was to measure the brain response from the stage of visual discrimination of visual patterns typical of printed letters, to the mental phonological retrieval for the printed stimuli and, in the case of real words, to the inevitable access to meaning.
Auditory rhyming task
The participants were presented with 30 s of alternating blocks either of pairs of pure tones or pairs of letters; they were instructed to press a key on a response box using the right index finger when letters rhymed and when pure tones matched. The presentation rate was one stimulus every two and a half seconds. The target rate was 5/12 for each block. We adopted an auditory, instead of a visual, rhyming task to characterize the functional correlates of auditory phonological processing while excluding the visual‐to‐phonological conversion processes. We reasoned that any overlap between these activation patterns and those for reading would be even stronger evidence for regions of convergence of representations from multiple domains. The discrimination of pure tones was chosen as a baseline for the rhyming task to isolate the neurofunctional activation associated with phonological processes, while controlling for lower‐level auditory processing.
Visual motion perception task
A low spatial frequency Gabor patch was presented on the computer screen as either stationary (control epochs) or moving randomly across the screen in four directions (horizontally from left to right, vertically from the top to the bottom, diagonally from the lower left to the upper right and vice versa for the other diagonal) for a total of 30 times in each block. Thus there was a direction‐shift every 500 ms. During the baseline and the experimental epochs, the participants were instructed to focus on a fixation cross at the centre of the screen for the entire scanning session, and not to follow the moving stimuli with their eyes during the experimental epochs. No explicit response was requested from the participants. We adopted a low spatial frequency Gabor patch, as described in Ramus et al. 2003, to isolate the visual processes associated with the magnocellular pathway.
Motor sequence “cerebellar” learning task
The participants were instructed to identify and learn a sequence of eight taps using a four key response‐box; they received a high‐pitch acoustic feedback for correct taps and a low pitch feedback for wrong taps. The high‐pitch/correct‐response and low‐pitch/wrong‐response rule was explained prior to the task. After each wrong key‐press, the participant returned to the beginning of the tap sequence. In the baseline condition, they were instructed to listen passively to the same sounds used as feedback during the motor learning task. They were presented with 30‐s of alternating blocks of the baseline and the motor sequence learning task. The rate of the task was set so that participants had to press a key every two seconds (15 keys were pressed for each learning block). This motor learning task was adopted because of its well‐documented association with cerebellar activation in normal subjects [Jenkins et al., 1994] and the dysfunctional patterns described in dyslexics [Nicolson et al., 1999].5
Summarizing, fMRI patterns were measured for (1) real word or (2) pseudoword reading versus observation of strings of lines in different orientations; (3) auditory letter rhyme detection versus discrimination of pure tones; (4) motion perception (moving low frequency Gabor patch) versus baseline (stationary low frequency Gabor patch); (5) motor sequence learning versus an acoustically matched baseline.
fMRI Data Acquisition
MRI scans were performed with a 1.5 T Marconi‐Philips Infinion Scanner (17 subjects) or with a General Electric Signa HD‐XT scanner (11 subjects), using an Echo Planar Imaging (EPI) gradient echo sequence (Flip angle = 90°; T.E. = 60 ms; TR = 3,000 ms; FOV = 240 × 240; matrix = 64 × 64). The selected volume consisted of 26 contiguous transverse images (thickness = 5 mm; gap = 0 mm).
All of the fMRI tasks involved 60 fMRI scans collected in alternating blocks of 10 scans of the baseline condition and 10 of the experimental task; thus, we had three blocks of baseline and three blocks of the experimental condition for each task.
fMRI Data Analysis
For all participants, the sampled anatomical space included the entire cerebral hemispheres and the cerebellum down to −40 mm below the bicommissural plane. After the standard preprocessing, and after high‐pass filtering and proportional scaling, conditions were modeled in a block‐design, and the condition‐specific effects were estimated using the General Linear Model as implemented in SPM2 [Friston et al., 1995]. The BOLD signal associated with each experimental condition was analyzed by a convolution with a canonical hemodynamic response function [Worsley and Friston, 1995]. The individual effects were then submitted to a random effect analysis using the contrast images estimated in the individual fixed effects analyses [Holmes and Friston, 1998; Penny and Holmes, 2004].
The analyses conformed to a second‐level ANOVA, for which were entered the contrast images of the (1) word reading versus baseline, (2) pseudoword reading versus baseline, (3) auditory rhyme detection versus baseline, (4) motion perception versus baseline, and (5) motor sequence learning versus baseline.
Activation patterns of each task
Firstly, the simple effects of each task were assessed. These effects were thresholded at P < 0.05 and corrected for multiple comparisons [FWE, family wise error; Worsley et al., 1996].
Because the topography of the activations for word reading and pseudoword reading coincided, with a marginal and yet already well‐known larger activation for pseudoword reading in the left inferior frontal cortex [see for example, Paulesu et al., 2000], all further analyses aiming at a characterization of the topographical congruency between the reading system and the other three systems were exclusively based on the pseudoword reading pattern.
Intersections between word reading and phonological, visual magnocellular and motor systems
To characterize the nature of the brain regions involved in word and pseudoword reading, we performed three pair‐wise conjunction analyses between the reading pattern and the patterns involved in auditory phonological discrimination, visual motion perception, and motor learning [Friston et al., 1999; Worsley and Friston, 2000]. Each of these pair‐wise conjunction analyses were exclusively masked6 in such a way that voxels belonging to a system not assessed by a particular analysis were excluded from the test, e.g., the pseudoword reading and auditory rhyming conjunction images were exclusively masked for both the visual motion perception and motor learning patterns. The maps for the exclusive masks were generated by using a very low threshold (P < 0.05 uncorrected); this ensured that the conjunction analyses did not consider voxels showing even the weakest trend for activation in tasks not under consideration. The conjunction effects were thresholded at P < 0.001. These effects were called “first‐order intersections”.
Three‐way and four‐way conjunction analyses (second and third order intersections)
Higher level conjunction analyses were also conducted, using the logic and procedures adopted for the first‐order intersections. As an example, the spatial overlap between the reading, the phonological and the motor learning system (a “second‐order intersection”) was assessed by conducting a conjunction analysis of these three patterns after exclusion of all voxels activated by the visual magnocellular task. The same logic was applied to assess the overlap between reading, phonological and magnocellular systems and between reading, magnocellular, and cerebellar systems.
Reading per‐se
The brain regions activated for reading per‐se were identified by explicitly masking all voxels that showed even the weakest trend (P < 0.05 uncorrected) of activation in the other three tasks from the statistical map of pseudoword reading.
Relevance of the present fMRI findings for dyslexia
Whether and how the intersection areas overlapped with the hypoactivations frequently observed in dyslexics during word and pseudoword reading was investigated by comparing the present results with the hypoactivations reported by Paulesu et al. 2001 in a PET activation experiment on a sample of 36 dyslexic subjects and 36 controls from three countries (France, Italy and UK). Materials, methods and the results of that study are described in Paulesu et al. 2001. We regard this comparison as a form of explicit meta‐analysis in which the functional properties of the potentially less resolved dyslexia PET patterns are assessed with the potentially better resolved fMRI data.7 The statistical maps of the present study and those of the 2001 study fall in the same stereotactic space; the anatomical congruity of the two sets of results was assessed by transforming the statistical maps into regions of interest (ROIs) using the MRIcro software [Rorden and Brett, 2000] and by assessing the level of spatial overlap between the ROIs.
RESULTS
Behavioral and fMRI Activation Patterns for Each Task
Behavioral results
In the auditory rhyming task for letter names, the participants performed at ceiling level (meanhits = 13.22 out of 15 targets; s.d.hits = 0.04).
In the motor learning task, participants learned, on average, a sequence of 4.63 taps (s.d. = 1.01), out of eight targets; on average, during the three learning blocks they made 15.41 (s.d. = 2.82) attempts. None of the subjects had managed to learn the complete sequence by the end of the three blocks, showing that throughout the entire duration of the task the process of learning was captured by trial and error, rather than by the rehearsal of a fully learned sequence.
The participants were not required to give an explicit response in either the reading task or the visual motion perception task; however after the scans they confirmed that they had read the stimuli consistently, had noticed the moving Gabor patch and had kept their attention on the fixation marker during the moving or stationary Gabor patch trials.
The results of the analyses of the behavioral data from the fMRI scans are summarized in Table 1.
fMRI data
The fMRI results are reported in Table 2 and in Figure 1.
Table 2.
Activations in normal readers during the fMRI tasks
| Brain region | Left hemisphere | Right hemisphere | ||||||
|---|---|---|---|---|---|---|---|---|
| MNI coordinates | ||||||||
| x | y | z | Z score | x | y | z | Z score | |
| A. Pseudoword reading | ||||||||
| Inf. frontal gyrus, pars triangularis | −52 | 34 | 12 | 5.3a,ba | ||||
| Inf. frontal gyrus, pars opercularis | −44 | 14 | 24 | 6.1a,ba | ||||
| Precentral gyrus | −46 | 4 | 32 | 6.2a,ba | ||||
| Sup. temporal pole | −54 | 12 | −6 | 6.4a,ba | ||||
| Mid. temporal gyrus | −64 | −38 | −6 | 5.6a,bd | ||||
| Inf. temporal gyrus (LIMA) | −50 | −54 | −18 | 7.0a,bb | ||||
| Mid. occipital gyrus | −26 | −98 | −6 | Inf. a,bb | ||||
| Fusiform gyrus | −44 | −56 | −20 | Inf. a,bb | ||||
| Fusiform gyrus (VWFA) | −40 | −56 | −16 | 7.5a,bb | ||||
| Inf. occipital gyrus | 26 | −100 | 0 | 7.8a,bc | ||||
| Cerebellum | −42 | −48 | −28 | Inf. a,bb | 34 | −76 | −22 | 5.5a,bc |
| B. Word reading | ||||||||
| Inf. frontal orb. gyrus | −46 | 32 | −2 | 5.5a,bb | ||||
| −42 | 30 | −8 | 5.3a,bb | |||||
| Inf. frontal tri. gyrus | −50 | 24 | 8 | 4.9a,bb | 58 | 28 | 0 | 4.9a,bf |
| Inf. frontal op. gyrus | −46 | 16 | 24 | 5.0a,be | ||||
| Mid. temporal gyrus | −58 | −40 | −2 | 5.3a,bc | ||||
| −60 | −36 | −2 | 5.3a,bc | |||||
| Inf. temporal gyrus (LIMA) | −50 | −54 | −18 | 5.0a,ba | ||||
| Fusiform gyrus | −44 | −52 | −22 | 6.8a,ba | ||||
| Fusiform gyrus (VWFA) | −40 | −56 | −16 | 5.7a,ba | ||||
| Mid. occipital gyrus | −26 | −98 | −6 | Infa,ba | ||||
| −22 | −100 | −4 | Infa,ba | |||||
| Inf. occipital gyrus | 34 | −94 | −4 | 4.5a,bd | ||||
| Calcarine fissure | 22 | −100 | 0 | Infa,bd | ||||
| Cerebellum | −40 | −48 | −26 | 6.8a,ba | ||||
| −40 | −70 | −20 | 5.6a,ba | |||||
| C. Auditory rhyming | ||||||||
| Inf. frontal gyrus, pars triangularis | −48 | 20 | 24 | 5.6a,bb | ||||
| Sup. parietal gyrus | −26 | −74 | 46 | 5.3a,bd | ||||
| Mid. temporal gyrus | −60 | −16 | −12 | 7.7a,ba | 62 | −12 | −14 | 5.5a,be |
| −58 | −34 | −4 | 6.0a,ba | 58 | −36 | −2 | 5.5a,bc | |
| Inf. temporal gyrus | −50 | −54 | −22 | 5.5a,ba | ||||
| D. Visual motion perception | ||||||||
| Mid. frontal gyrus | 48 | −2 | 56 | 5.5a,be | ||||
| Sup. parietal gyrus | −20 | −62 | 64 | Inf. a,ba | 22 | −66 | 64 | 7.8a,ba |
| −28 | −54 | 62 | Inf. a,ba | |||||
| Precuneus | 8 | −64 | 66 | 6.9a,ba | ||||
| Mid. temporal gyrus | 50 | −70 | 0 | Inf. a,bc | ||||
| Sup. occipital gyrus | −24 | −88 | 28 | 7.6a,bb | 28 | −88 | 28 | 6.1a,bd |
| Mid. occipital gyrus | −48 | −78 | −2 | Inf. a,bb | ||||
| Lingual gyrus | −14 | −82 | −14 | 5.7a,bb | ||||
| Cerebellum | −40 | −70 | −20 | 6.6a,bb | 42 | −70 | −20 | 6.2a,bc |
| 22 | −80 | −16 | 4.8a,bf | |||||
| E. Motor sequence learning | ||||||||
| Sup. frontal gyrus | 26 | 0 | 60 | 7.7a,bf | ||||
| Sup. frontal med. Gyrus | 0 | 24 | 40 | 5.0a,bd | ||||
| Mid. frontal gyrus | 40 | 40 | 32 | 7.0a,be | ||||
| Inf. frontal oper. Gyrus | 58 | 12 | 30 | 5.8a,bb | ||||
| 54 | 12 | 20 | 5.5a,bb | |||||
| Rolandic oper. | 58 | 14 | 2 | 6.7a,bb | ||||
| Precentral gyrus | −58 | 10 | 24 | 5.7a,bh | ||||
| −54 | 6 | 40 | 5.2a,bh | |||||
| SMA | −2 | 0 | 58 | 6.9a,bd | ||||
| Insula | −34 | 20 | −2 | 4.9a,bg | ||||
| Sup. parietal gyrus | −40 | −44 | 62 | Inf. a,ba | ||||
| Inf. parietal gyrus | 52 | −38 | 54 | Inf. a,ba | ||||
| Precuneus | 8 | −66 | 62 | Inf. a,ba | ||||
| Sup. temporal pole | −56 | 10 | −6 | 5.8a,bg | ||||
| Inf. temporal gyrus | 58 | −52 | −16 | 6.2a,bj | ||||
| 54 | −56 | −20 | 6.0a,bj | |||||
| Cerebellum | −36 | −62 | −30 | 6.4bi | 2 | −82 | −18 | 8.8a,bc |
| −30 | −70 | −26 | 5.9bi | 24 | −58 | −28 | 6.5a,bc | |
| Thalamus | −8 | −18 | 4 | 4.5a,bk | 6 | −10 | 6 | 5.5a,bl |
aFDR corrected.
bFWE corrected.
Cluster‐size: (A) a = 3,054 voxels; b = 1,822 voxels; c = 256 voxels; d = 187 voxels; (B) a = 1,184 voxels; b = 364 voxels; c = 159 voxels; d = 158 voxels; e = 62 voxels; f = 17 voxels; (C) a = 906 voxels; b = 297 voxels; c = 198 voxels; d = 114 voxels; e = 51 voxels; (D) a = 2,603 voxels; b = 2,100 voxels; c = 1,093 voxels; d = 342 voxels; e = 88 voxels; f = 20 voxels; (E) a= 9,409 voxels; b = 1,040 voxels; c = 1,003 voxels; d = 838 voxels; e = 517 voxels; f = 344 voxels; g = 333 voxels; h = 191 voxels; i = 140 voxels; j = 134 voxels; k = 110 voxels; l = 55 voxels.
Figure 1.
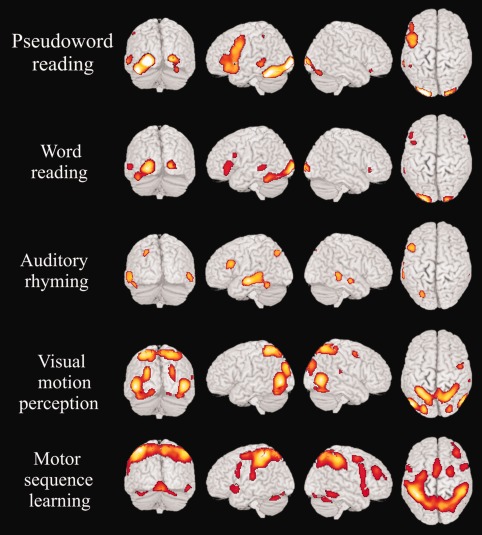
fMRI activation patterns for pseudoword reading word reading, auditory rhyming for letter names, visual motion perception for a moving Gabor patch and motor sequence learning. Baselines were observation of strings of lines with different orientations for (1) pseudoword or (2) word reading, (3) discrimination of tone pairs for the rhyming task, (4) a stationary Gabor patch for the visual motion perception task and (5) an acoustically matched baseline for the new motor sequence learning task (see also the Methods section). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Word and pseudoword reading
Given the outcome of the comparison with the passive viewing of strings of lines, these were associated with the activation of the left inferior frontal and precentral gyri, of the left superior temporal pole, of the left middle and inferior temporal and occipital gyri, and of the left fusiform gyrus. Occipital activations were also detected in the right hemisphere. The cerebellum was bilaterally activated. As the topography of the two patterns did not differ significantly, with the exception of a greater activation of the left precentral gyrus during the pseudoword reading, the intersection/conjunction analyses were performed with reference to the pseudoword reading data.
Auditory rhyming task
Phonological awareness, tested by means of an auditory rhyming task for letter names compared with a simple tone discrimination task, was associated with a large vast activation of the left frontal, temporal and parietal cortices. A right‐sided activation was observed in the middle temporal gyrus.
Visual motion perception
The comparison of the fMRI signal elicited by the moving Gabor patch with that elicited by a stationary Gabor patch, showed a significant bilateral activation in the dorso‐ and lateral‐occipital (including the V5/MT area) and in the ventral occipitotemporal extrastriate visual areas. In addition, we also found a strong activation of the dorsal posterior‐parietal and of the dorsal‐premotor cortices, typically involved in eye movement control [Thiele et al., 2002].
Motor sequence learning
The process of learning a new motor sequence, compared with its acoustically matched baseline, was associated with an extensive cortico‐subcortical activation involving the fronto‐parietal cortices, right prefrontal cortices, and the cerebellum bilaterally. There were also bilateral thalamic activations in the region of the centro‐medial/dorso‐lateral nuclei.
Conjunction Effects Across Tasks
First‐order intersections
Intersections between pseudoword reading and auditory rhyming tasks
Areas of anatomical overlap were found in the inferior frontal gyrus, in the middle and in the inferior temporal gyri of the left hemisphere (see Table 3 and Fig. 2 for more details).
Table 3.
Brain areas normally involved in pseudoword reading: conjunctions of two tasks
| Brain region | Left hemisphere | Right hemisphere | ||||||
|---|---|---|---|---|---|---|---|---|
| MNI coordinates | ||||||||
| x | y | z | Z score | x | y | z | Z score | |
| A. Conjunction of the activations of pseudoword reading and auditory rhyming tasks | ||||||||
| Inf. frontal gyrus, pars orbitalis | −42 | 30 | −14 | 3.5ad | ||||
| −44 | 28 | −10 | 3.4ad | |||||
| Inf. frontal gyrus, pars triangularis | −48 | 18 | 24 | 6.0a, ba | ||||
| −48 | 28 | 14 | 4.6a, ba | |||||
| Mid. temporal gyrusc | −62 | −36 | −4 | 5.4a, bb | ||||
| −62 | −22 | −8 | 3.8ab | |||||
| Inf. temporal gyrus (LIMA)c | −50 | −54 | −18 | 5.0a, bc | ||||
| B. Conjunction of the activations of pseudoword reading and visual motion perception tasks | ||||||||
| Mid. occipital gyrus | −40 | −82 | −8 | 6.3a, be | 32 | −94 | 6 | 3.5ag |
| −42 | −76 | −10 | 6.1a, be | 38 | −90 | 2 | 3.3g | |
| Inf. occipital gyrus | 28 | −86 | −14 | 3.6af | ||||
| 40 | −86 | −6 | 3.5ag | |||||
| Fusiform gyrus | −42 | −70 | −16 | 5.9a, be | ||||
| −40 | −66 | −18 | 5.2a, be | |||||
| Lingual gyrus | −18 | −86 | −12 | 4.1ae | ||||
| Cerebellum | 34 | −74 | −18 | 4.5a, bf | ||||
| 34 | −68 | −20 | 3.8af | |||||
| C. Conjunction of the activations of pseudoword reading and motor sequence learning tasks | ||||||||
| Inf. frontal gyrus, pars orbitalis | 58 | 22 | 26 | 3.1p | ||||
| 42 | 26 | −14 | 3.8aj | |||||
| Inf. frontal gyrus, pars opercularis | −58 | 10 | 20 | 4.7a, bh | 60 | 20 | 22 | 3.3ap |
| −60 | 12 | 26 | 4.7a, bh | |||||
| Precentral gyrus | −56 | 6 | 18 | 4.6a, bh | ||||
| −56 | 6 | 28 | 4.4ah | |||||
| SMA | −6 | 16 | 48 | 3.9al | ||||
| −2 | 8 | 56 | 3.3al | |||||
| Insula | −34 | 22 | −6 | 4.8a, bh | ||||
| Inf. parietal gyrus | −52 | −44 | 54 | 4.8a, bi | ||||
| Sup. temporal pole | −50 | 12 | −10 | 5.6a, bh | 46 | 24 | −16 | 4.0aj |
| −56 | 10 | −6 | 5.5a, bh | 54 | 22 | −10 | 3.4aj | |
| Mid. temporal pole | 52 | 16 | −22 | 3.4aj | ||||
| Cerebellum | −38 | −58 | −30 | 5.1a, bn | 34 | −70 | −28 | 3.9am |
| 0 | −36 | −6 | 4.0ak | 32 | −66 | −28 | 3.8am | |
FDR corrected.
FWE corrected.
Areas of overlap between the data of the conjunction analysis and the hypoactivations reported by Paulesu et al. (2001 in dyslexics during single words and pseudowords reading.
Cluster‐size: (A) a = 917 voxels; b = 672 voxels; c = 111 voxels; d = 52 voxels; (B) e = 735 voxels; f = 57 voxels; g = 43 voxels; (C) h = 737 voxels; i = 175 voxels; j = 143 voxels; k = 78 voxels; l = 44 voxels; m = 41 voxels; n = 20 voxels; p = 10 voxels.
Figure 2.
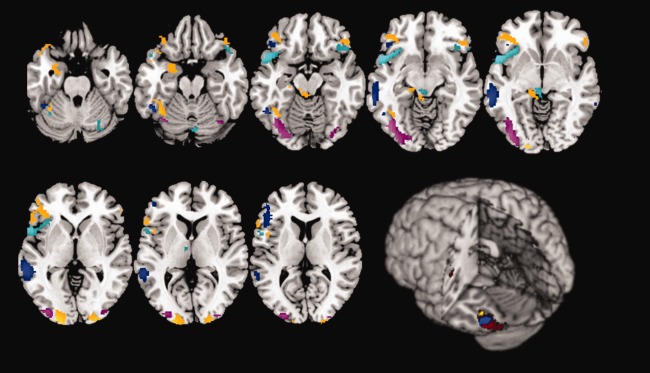
Intersections between reading and the three systems. This figure shows the overlap between brain areas normally activated during pseudoword reading and those involved in auditory phonological discrimination (blue areas), in visual motion perception (purple areas) and in the motor sequence learning task (cyan areas). Areas that were specifically involved in reading per‐se (orange areas) are also reported. Finally, the three‐dimensional rendering shows the overlap between the hypoactivated areas (in red) observed in dyslexics during word and pseudoword reading tasks as reported by Paulesu et al. 2001 and the intersection areas observed in our analyses
Intersections between pseudoword reading and visual motion perception
Areas of shared activations were located in the posterior occipitotemporal regions, bilaterally (see Table 3 and Fig. 2). It is worthy of note that these areas of overlap did not extend towards that between reading and rhyming, nor to the so‐called Visual Word Form Area (Cohen, et al. 2002). A further area of intersection was found in the right cerebellum.
Intersections between pseudoword reading and motor sequence learning
Areas of shared activation were found in the inferior frontal gyri, in the left precentral gyrus, in the left supplementary motor area (SMA), in the left inferior parietal lobule, in the superior temporal poles, in the right middle temporal lobe and in the cerebellum bilaterally (see Table 3 and Fig. 2).
Second‐ and third‐order intersections
The patterns of the three tasks (reading, visual motion perception and motor learning) overlapped in the cerebellar hemispheres bilaterally and in the left dorsal premotor (see Table 4).
Table 4.
Brain areas normally involved in pseudoword reading: conjunctions of three and four tasks
| Brain region | Left hemisphere | Right hemisphere | ||||||
|---|---|---|---|---|---|---|---|---|
| MNI coordinates | ||||||||
| x | y | z | Z score | x | y | z | Z score | |
| A. Conjunction of the activations of pseudoword reading, visual motion perception and motor sequence learning tasks | ||||||||
| Precentral gyrus | −54 | 0 | 44 | 3.9d | ||||
| −42 | −4 | 56 | 3.8ac | |||||
| Cerebellum | −40 | −68 | −26 | 5.3a, ba | 38 | −74 | −24 | 4.3ab |
| −32 | −70 | −24 | 4.8a, ba | 36 | −64 | −26 | 3.4b | |
| B. Conjunction of the activations of pseudoword reading, auditory rhyming and motor sequence learning tasks | ||||||||
| Inf. frontal gyrus, pars opercularis | −52 | 12 | 24 | 3.9e | ||||
| Precentral gyrus | −46 | 6 | 34 | 3.4e | ||||
| −50 | 10 | 32 | 3.3e | |||||
| C. Conjunction of the activations of pseudoword reading, auditory rhyming and visual motion perception tasks | ||||||||
| — | ||||||||
| D. Conjunction of the activations of pseudoword reading, auditory rhyming, visual motion perception and motor sequence learning tasks | ||||||||
| — | ||||||||
FDR corrected.
FWE corrected.
Cluster‐size: (A) a = 179 voxels; b = 58 voxels; c = 25 voxels; d = 11 voxels; (B) e = 89 voxels.
Further, the neural network associated with reading, phonological discrimination and motor learning patterns overlapped in the Broca's area (see Table 4).
However, no overlap of the reading, phonological discrimination and visual motion perception patterns was identified in any brain region.
Furthermore, no cerebral areas showed an overlap with all four activation patterns.
Reading per‐se
Once even the weakest trends of activation for the phonological, magnocellular, and cerebellar tasks were masked out, a number of regions were found which specifically activated only when reading, i.e., part of the left and of the right frontal cortices, the left insula, the left postcentral gyrus, the left superior temporal pole, the left superior temporal gyrus, the left middle occipital gyrus, the left fusiform gyrus and the left cerebellum. Subcortical activation was also found in the left amygdala (see Table 5 and Fig. 2). Notably, the occipitotemporal regions activated exclusively by reading, included a region in stereotactic coordinates compatible with the location of the VWFA [Cohen et al., 2002].
Table 5.
Brain areas involved in reading per‐se
| Brain region | Left hemisphere | Right hemisphere | ||||||
|---|---|---|---|---|---|---|---|---|
| MNI coordinates | ||||||||
| x | y | z | Z score | x | y | z | Z score | |
| Mid. frontal gyrus | −44 | 14 | 48 | 3.9ai | ||||
| −44 | 12 | 52 | 3.7ai | |||||
| Inf. frontal gyrus, pars orbitalis | −36 | 30 | −4 | 5.8a, ba | 48 | 30 | −18 | 4.8a, bd |
| −48 | 34 | −2 | 5.4a, ba | 54 | 40 | −4 | 3.7ad | |
| Inf. frontal gyrus, pars triangularis | −52 | 20 | 2 | 5.1a, ba | 58 | 30 | 22 | 4.1ag |
| −54 | 20 | 6 | 5.1a, ba | 60 | 26 | 20 | 3.9ag | |
| Inf. frontal gyrus, pars opercularis | −58 | 16 | 12 | 4.6a, ba | 62 | 22 | 20 | 3.6ag |
| Rolandic opercular gyrus | −46 | 6 | 14 | 4.8a, ba | ||||
| −46 | 2 | 18 | 3.9aa | |||||
| Precentral gyrus | −48 | −2 | 36 | 5.4a, ba | ||||
| −48 | 0 | 30 | 5.2a, ba | |||||
| Insula | −44 | 16 | 4 | 3.9aa | ||||
| Postcentral gyrus | −52 | −8 | 48 | 4.0aa | ||||
| Sup. temporal pole | −44 | 22 | −22 | 4.7a, ba | ||||
| −50 | 10 | −22 | 4.1aa | |||||
| Sup. temporal gyrus | −58 | −44 | 16 | 3.6ah | ||||
| Amygdala | −24 | −2 | −24 | 4.6a, be | ||||
| Mid. occipital gyrus | −22 | −100 | −4 | Inf.a, bb | ||||
| Inf. occipital gyrus | 26 | −100 | 0 | Inf.a, bf | ||||
| Fusiform gyrus (VWFA)c | −40 | −56 | −16 | 6.8a, bc | ||||
| −40 | −58 | −12 | 6.2a, bc | |||||
| Cerebellum | −38 | −54 | −22 | 7.6a, bc | ||||
| −36 | −52 | −26 | 7.0a, bc | |||||
FDR corrected.
FWE corrected.
Areas of overlap between the brain areas activated in reading per se and the hypoactivations reported by Paulesu et al. (2001) in dyslexics during reading. Cluster‐size: a = 778 voxels; b = 249 voxels; c = 201 voxels; d = 156 voxels; e = 143 voxels; f = 137 voxels; g = 76 voxels; h = 30 voxels; i = 22 voxels.
Current fMRI Results Compared With Previous Findings on Dyslexia
The comparison of the above patterns of shared or exclusive activations emerging from the present fMRI data and those of reduced activation reported by Paulesu et al. 2001 revealed an anatomical overlap in the more anterior ventral occipitotemporal region that was activated by both the reading and rhyming tasks; a more medial region of overlap was found in the ventral occipitotemporal area, identified by the present reading‐per‐se analysis (Fig. 2) and corresponding with the VWFA.
The 2001 dyslexia data, on the contrary, did not overlap within the more posterior occipitotemporal regions, in which the present fMRI data identified a convergence for the reading and the moving Gabor patch tasks, nor in the regions activated during the motor learning task.
DISCUSSION
The purpose of this study was to better characterize the functional properties of the cortices involved in reading. The same goal can be pursued in several ways such as, for example, by designing subtle paradigms and manipulating the psycholinguistic or perceptual properties of the reading stimuli accordingly [Dehaene et al., 2004; Duncan et al., 2010; Koyama et al., 2011; Kronbichler et al., 2004, 2009]. However, as our long term aim is to reach a better understanding of dyslexia, we preferred to approach the issue by determining the functional properties of the brain areas typically involved in single word and pseudoword reading and the topography of their intersections with the neurocognitive systems frequently called into play by theories on dyslexia, specifically the auditory phonological, the visuo‐magnocellular and the motor/cerebellar systems (henceforth called the three systems).
The logic behind our experiment was straightforward: given the vast literature supporting the involvement of the three systems in dyslexia [Frith, 1999; Nicolson et al., 2001; Pernet et al., 2009; Reid et al., 2007; Snowling, 2001; Stein, 2001], and because reading remains the primary task on which the diagnosis of dyslexia is made, it would be expected that a certain degree of functional anatomical intersection between the three systems and the one for reading would be observed in normal subjects.
Before proceeding with the discussion, certain potential “limitations” of our study should be borne in mind.
In the first place, we evaluated mature adult brains of healthy readers, and assessed the anatomical convergence of different systems once the reading process had been fully mastered. As a consequence, the resulting data may be limited in their relevance for children, and it would be interesting to make a comparison of similar data in children, while they are still learning to read.
In the second place, we assessed the neurofunctional contiguity of the three systems and the reading system using the simple‐minded test of spatial congruity of the patterns of activation. In principle, the three systems and the reading system may cooperate by processes of functional connectivity of anatomically separated brain areas through long‐range connections rather than through the convergence on higher‐order multimodal brain regions [Twomey et al., 2011; van der Mark et al., 2011]. In fact, the reading system may not even exist as a separate entity, and it may manifest itself as the emergent property of distant (but connected) systems during the task of reading [Twomey et al., 2011]. The importance of connectivity processes through functional connectivity cannot be excluded: these may play a crucial role when learning to read [Beaulieu et al., 2005; Qiu et al., 2008], or, put negatively, their absence may be one of the causes behind the fact that the left inferior occipitotemporal cortex does not commit itself to reading in dyslexia [see Silani et al., 2005 for further discussion]. However, the idea that some form of convergence on higher‐order areas of intersection may appear through the maturation of the reading skills remains viable [Price and Devlin, 2011], at least in the light of data of acquired and developmental dyslexia: these syndromes are definitively associated with focal damage in their acquired form [Dejerine, 1891; Dejerine, 1892; Price et al., 2003], and, even in the developmental variant, reading difficulties are associated with localized brain dysfunction [Paulesu et al., 2001; Shaywitz et al., 1998].
In the third place, the congruity of the three systems and the reading system has been considered for single word and pseudoword reading only rather than in a more ecologically valid task such as sentence reading. The latter creates higher functional demands on oculomotor control and visual processes that might be relevant in reading such as saccadic suppression [see discussion below and Rayner, 1998].
However, it should also be considered that subjects with dyslexia usually have decoding problems even with single words and pseudoword reading, tasks similar to those adopted in our fMRI experiment. Hence any congruity between the three systems and that activated by our minimalistic reading task should be even more relevant, showing the existence of a hard‐wired basis for the congruity.
Finally, it must be pointed out that our study was based only on the highly regular Italian orthography, which may constitute a limitation. Indeed the transparency of the Italian writing system may pose less of a stress on the neural system devoted to reading, so reducing the possibility of detecting intersections between the different systems and the reading system [Paulesu, 2006]. However, previous studies have shown that the reading systems for alphabetic orthographies of different complexities (e.g., Italian and English) largely overlap, with the functional differences being a point of emphasis on the different subcomponents by readers of different cultures, whereas the sub‐components are available to both groups of readers [Paulesu et al., 2000]. We therefore deduce that our results will be of sufficient general importance; indeed, it could even be assumed that any congruity of the three systems with the reading system for the simple Italian orthography would be of greater significance.
While keeping these potential limitations in mind, the main trust of our results remains straightforward: the seemingly distant brain systems considered showed considerable degrees of anatomical intersection with that involved in reading.
These intersections are not complete in every region; as reading is a multicomponent skill, we observed a gradient from the early extrastriate visual cortex to more anterior occipitotemporal cortices of the left hemisphere and to the left temporal and frontal areas. Moreover the reading system also included a brain region that in our experiment was uniquely activated by the reading task,8 in stereotactic coordinates compatible with Cohen et al.'s VWFA 2002.
In the following section, we will focus on the intersections in the left occipitotemporal cortices, i.e., on brain regions that play a crucial role in reading [Cohen and Dehaene, 2004; Cohen et al., 2002; Fiez and Petersen, 1998] and whose malfunction has been associated with developmental dyslexia by many previous studies in different cultural contexts [Kronbichler et al., 2006; Paulesu et al., 2001; Shaywitz et al., 1998] including Chinese [Siok et al., 2004], at least for a lexical decision task.9
A Rostro‐Caudal Functional Gradient in the Left Occipitotemporal Cortex for Reading
The conjunction analyses revealed the existence of a rostro‐caudal gradient in the left inferior occipitotemporal cortex: (i) the most anterior conjunction effect was located in the posterior part of the inferior temporal gyrus (local maximum: −50, −54, −18) and corresponded to the overlap between the reading and auditory phonological awareness patterns, whereas (ii) the most posterior conjunction effect, corresponding to the overlap between the magnocellular system and the reading system, was located in the posterior part of the fusiform gyrus (local maximum: −42, −70, −16).
Based on these results, the left inferior occipitotemporal cortex appears to be an intersection between the different functional systems.
The most anterior inferior occipitotemporal conjunction effect, in particular, may correspond to a brain region labeled LIMA (Lateral Inferior‐temporal Multimodal Area) by Cohen et al. 2004, which was centered, in line with Cohen's findings, at −52, −56, −18. The LIMA has recently been described as an “interface area”10 [Devlin et al., 2006] between orthographic, phonological and semantic information. It is assumed to be sensitive to task demands [Starrfelt and Gerlach, 2007], to be involved in both auditory and visual word processing [Booth et al., 2002] and to have a role in the explicit manipulation of sublexical units [Booth et al., 2002; Burton et al., 2000; Cohen and Dehaene, 2004]. The identification of this brain region as an area of intersection for both the reading and auditory phonological awareness implies that the integration of orthographic representations with their phonological counterparts may take advantage of short‐range connectivity within the same brain region, which most likely receives input from the peri‐sylvian auditory‐phonological regions on the one hand and, on the other, from the ventral extrastriate visual cortices devoted to visual orthographic decoding.
The new data, which emerge from this study, show that most probably this region does not receive direct magnocellular input, nor it is involved in the motor learning task.
A first‐order anatomical intersection was also found between the reading system and the activations for the visual motion perception task in the more posterior areas, i.e., in part of the left fusiform gyrus, of the left inferior occipital cortex and of the left lingual gyrus, once all trends of activation for tasks tackling the other two systems had been excluded. A visual motion perception task for low‐frequency Gabor patches was used in this study as it is recognized as being able to powerfully activate the visual magnocellular system [Chen et al., 2008], including the dorso‐parietal stream. Given the nature of this stimulation, it can be inferred that the regions associated with it should normally receive signals, regardless of how directly, from the magnocellular pathway [Zeki, 1974]. As anticipated, the area of the intersection with reading did not involve the V5/MT area, a region strongly activated in the visual motion perception task, for which a malfunction in dyslexia is controversial [Eden et al., 1996; Stein, 2003; Vanni et al., 1997]. Indeed, this area has little if anything to do with single word or pseudoword reading, as demonstrated by the fact that these tasks are not associated with its activation, which in itself lessens the relevance of the reported abnormality of its activation in some dyslexics.
However, it may be argued that the functionality of the V5/MT area should not be used as the only benchmark in evaluating the importance of the magnocellular system in reading and dyslexia. Indeed, the magnocellular visual pathways are not merely involved in visual motion perception, but also in a range of behavior and phenomena that were, admittedly, not tested in our experiments such as the control of eye movements [Thiele et al., 2002], which is crucial for sentence reading and its typical alternation of fixations and saccades [Stein and Walsh, 1999]. The magnocellular system should also be functionally suppressed to inhibit the sensation of motion during eye movements [Ross et al., 2001]; as with every eye movement, the image of the visual world abruptly moves over the retina making the peripheral visual analysis less effective. Contrast sensitivity [Legge, 1993], letter position encoding [Cornelissen et al., 1998b], visual attention [Vidyasagar, 2004] and binocular control [Stein and Fowler, 1993] are other putative magnocellular processes that could influence reading abilities and, as a consequence, could play a role in determining reading difficulties in dyslexia [Skottun, 2000].
Our study does not allow us to determine the role of the magnocellular system in normal reading or in dyslexia, but our data do show the existence of a considerable overlap of the reading system and the brain regions driven by a strong magnocellular stimulus in the ventral extrastriate cortices, outside the V5/MT area. These areas may be candidate targets of the malfunction of an interaction between the reading (decoding) system and the magnocellular system. Further experiments are needed in this area to clarify the interaction of the spatial attentional networks with the oculomotor control networks and the reading system to depict their role in normal reading and dyslexia.
Furthermore, our data showed the existence of a significant overlap between the reading and the motor/cerebellar systems. In the cerebellum, these areas of overlap were located bilaterally in the sixth lobule and in the crus, in the middle part of the third and seventh portion of the vermis.11 These portions of the cerebellum have been associated with the ability to monitor the discrepancy between the actual and intended phonological rehearsal [Ben‐Yehudah and Fiez, 2008; Desmond et al., 1997]. Moreover, they would support the phonological loop functioning through a feedback connection to the frontal lobes, which would keep the phonological information stored in the loop updated [Desmond et al., 1997]. This interpretation would also be consistent with Nicolson and Fawcett's results 2001, which suggested that the reading ability would be strictly associated with cerebellar functional activity [Laycock et al., 2008].
Interestingly, no higher order intersections were observed in the left occipitotemporal stream.
Second‐Order Intersections
There were two second‐order intersections based on three tasks.
The first such intersection was between the neural network associated with reading, visual motion perception and motor learning; it involved the cerebellum and the left dorsal premotor cortex. This finding can be attributed to the premotor aspects embedded in these tasks. While it is hardly surprising that the premotor cortex is involved in a motor learning task [Lacourse et al., 2005], it is also most likely involved in aspects of the articulatory planning for reading [Watkins et al., 2008]; finally, its activation during the moving Gabor patch task may be the result of the fixation and inhibition of saccades imposed by the task [Dieterich et al., 2009]. Just a few years ago, the question as to why the cerebellum was active also in our motion perception task might have been a matter of mere speculation. However, recent anatomical findings showed the existence of strong cerebellar projections into the dorsal parietal cortex [Dum and Strick, 2003; Middleton and Strick, 2000], which is part of the dorsal visual stream. More importantly, it is now well documented that patients with cerebellar lesions have problems with coherent motion discrimination tasks [Handel et al., 2009]. Hence, while we cannot give a detailed account of the contribution of the cerebellum in the motion perception task, we can say that its activation is in line with this recent evidence. However the real challenge may be to provide an explanation of why shared cerebellar activation was observed for these three tasks. We can only make an educated guess here, particularly if a basic and shared physiological mechanism has to be identified. It is well known that the same brain structure, outside the primary cortices, may participate in more than one process, just as it is recognized that a single lesion, particularly when placed in higher order cortices or in subcortical structures, may cause more than one behavioral deficit. As discussed above, the cerebellum has been seen to play a role also in visual motion perception. While cerebellar activation is to be expected in a motor learning task, it is not difficult to imagine the cerebellum playing a role in pseudoword reading, particularly as this is a task that may be more demanding in terms of eye movement control, given the greater ambiguity of pseudowords, or inner articulation. Future studies on dyslexics showing reduced activation of these cerebellar sites together with specific behavioral patterns, may contribute to understanding the intimate meaning of these shared activations in normal subjects.
A further second‐order intersection for reading, phonological awareness and motor learning, involved Broca's area and the ventral premotor cortex, brain regions well‐known for their involvement in motor planning, in speech and hand motor control [for a review, see Fadiga et al., 2009].
Finally, it is of great interest to see that no four‐task intersection was found. As the three systems do not fully converge on any of the brain regions involved in reading, their independent malfunctioning may account for different aspects of the complex behavior associated with dyslexic reading.
Reading Per‐Se Areas?
A pattern of activation specific for reading was found after excluding all the voxels showing even the smallest trend of activation (P < 0.05 uncorrected) in any of the other three tasks from the statistical tests. Among others, activation peaks occurred in a region compatible with the so‐called visual word form area [Cohen et al., 2002]. In the last decade, numerous studies have investigated the role of the left ventral fusiform gyrus in reading tasks [Cohen et al., 2002; Dehaene et al., 2004; Kronbichler et al., 2009; McCandliss et al., 2003; Woodhead et al., 2011], supporting the idea that this region would be an unimodal area associated with written word recognition (i.e., with orthographic processing). The role of this area in reading processes was supported by several studies investigating the acquisition of reading skills in children [Ben‐Shachar et al., 2011; Dehaene et al., 2010; Houde et al., 2010; Turkeltaub et al., 2003], and its hypoactivation, together with that of surrounding left occipitotemporal cortices, is often reported as one of the typical neurofunctional markers of developmental dyslexia [Paulesu et al., 2001; Richlan et al., 2011; Shaywitz et al., 1998].
Whereas some authors have suggested that the VWFA has a specific role in supporting orthographic processing [Cohen and Dehaene, 2004; Cohen et al., 2002], other groups have suggested that this brain region may represent an interface between general visual input and verbal processing [Hillis et al., 2005; Price and Devlin, 2003; Price and Devlin, 2011]. Therefore, the VWFA would be activated during other cognitive tasks such as picture naming [McCrory et al., 2005a; Price, 2000; Shinkareva et al., 2011]. Based on these considerations, the specific role of the VWFA during the reading task in our study could be consistent both with the unimodal hypothesis and with the interface hypothesis. Further studies are needed to gain a deeper understanding of the specific contribution of the VWFA region.
The Hypoactivation of the Occipitotemporal Cortex in Developmental Dyslexia
A comparison between the present data and the data on dyslexia reported by Paulesu et al. 2001 showed that the reading‐related hypo‐activations observed in their sample of 36 developmental dyslexics do overlap, in part, with the reading per‐se system described in this article, and in part with the area activated during both the phonological awareness task and the reading task; in line with Cohen et al. 2004, we believe that this LIMA area may well operate as an interface between phonology and orthography. Paulesu et al.'s data 2001, based on patients from different cultural environments, were consistent with previous results on North American dyslexics found by Shaywitz et al. 1998 and have since been replicated by several subsequent studies to represent a well‐established pattern of dysfunction for single word reading in dyslexia in alphabetic orthographies [for a review see also Richlan et al.'s meta‐analysis, 2011], but also in nonalphabetic orthographies such as the Chinese one [Wei Hu et al., 2010]. The present data show that in normal subjects this area is heterogeneous because it contains both a subregion activated for both auditory phonological awareness and for reading, and an area which is more specifically activated by the visual orthographic stimuli.12 In addition, this area does not seem to receive magnocellular input nor does it belong to the network that cooperates with the cerebellum in a motor learning task.
Does this evidence support any of the theories of dyslexia? The observation of the above mentioned heterogeneity does not permit us to embrace fully any of the theories previously described. Clearly, neither the visual magnocellular theory nor the cerebellar theory are supported by our data because no overlap was found either in the specific tasks used here or in the hypoactivations described in the PET experiment on dyslexia taken as an initial test‐bed of the present data.
Of course, the data from the PET experiment were derived from a combination of tasks (implicit and explicit reading) and materials (word and nonword reading combined), none of which were explicitly magnocellular or cerebellar in nature. In principle, it is possible that dyslexia data resulting specifically from the visually more demanding pseudoword reading task or from a sentence‐reading task that inevitably imposes more stress on oculomotor control, may also reveal a topographical overlap with regions activated by the visual magnocellular task or by the motor learning task, but it remains to be demonstrated. Thus, the present findings are relevant only as far as the pattern of single word reading is concerned and the novel mapping of the composition of the ventral occipitotemporal cortex in the region, which was found to be hypoactivated in the 2001 dyslexia study. Furthermore, the present findings do not exclude the relevance of the magnocellular or cerebellar hypotheses for the malfunctioning of other cortical or subcortical systems other than that identified for single word reading in ventral occipitotemporal cortex.
On the other hand, the present data also indicate that a pure phonological explanation cannot be accepted because the area of malfunctioning in dyslexia contained a region, which possibly corresponds to the VWFA [Cohen et al., 2002], that was activated only by the reading task while not showing even the weakest activation trend in the phonological awareness task.
Of course, as Paulesu et al. 2001 dyslexia findings were based on PET studies, we are not in the position right now to assess them beyond a group‐level meta‐analytic comparison, when referring to the present fMRI data. Clearly, the patterns observed in individual dyslexics, as seen with fMRI, could shed new light on the different scenarios that one may hypothesize. Individual dyslexics may present reduced activation of the VWFA, or of the LIMA, in isolation or in combination. In addition, the lack of activation of either of these regions, or of both together, could be highly task dependent: for example, the LIMA may not show a commitment to the integration of orthography and phonology when reading, because of the lack of a development of the neural expertise of the VWFA region; however in dyslexics this same region, which operates as a LIMA in normal subjects, may be activated during auditory phonological tasks. These different patterns may of course have interesting implications for the understanding of the role of these cortices in reading and in dyslexia. A complete interpretation of each of these possible scenarios clearly requires single subject fMRI data complemented by the observation of specific behavioral patterns.
However, although further studies are needed to address these interesting issues, our initial observations suggest that the current theories on dyslexia fail to capture the complexity of the syndrome and its anatomo‐dysfunctional patterns.
ACKNOWLEDGMENTS
The authors are grateful to Uta Frith, Cathy Price, and Jean‐Francois Démonet for allowing them to use the PET results described in Paulesu et al. (2001).
Footnotes
A further theory, not specifically discussed in this article, postulates that dyslexia may be due more specifically to a faulty visuospatial attentional system [Facoetti et al., 2000, 2006; Peyrin et al., 2011]. We regard to this theory as a variant of the magnocellular theory, which is a more general one as it tries to accommodate a wider range of phenomena.
There is a further functional and anatomical hypothesis of dyslexia not explicitly considered in this experiment, the disconnection hypothesis: this suggests that a loose connectivity within a distributed system might be at the root of the disorder. Initially proposed by Paulesu et al. 1996 on the basis of a qualitative assessment of the PET activation patterns in a small sample of dyslexics challenged with visual phonological tasks, the hypothesis has been further supported by DTI data [Klingberg et al., 2000], morphometric VBM MRI data [Silani et al., 2005], DTI and VBM data combined [Steinbrink et al., 2008], fMRI data [van der Mark et al., 2011], and even anatomical data from animal models that mimic the pathology of dyslexia [Rosen et al., 2000]. One well established finding is, for example, that regarding the left arcuate fasciculus; this was found to be abnormal in dyslexic subjects in at least three studies [Klingberg et al., 2000; Silani et al., 2005; Steinbrink et al., 2008]: the left arcuate fasciculus spans from the ventral occipitotemporal cortex up to the temporoparietal junction and forward to Broca's region [Catani et al., 2005], offering connections within the language system and possibly to regions interfacing between vision and language [Steinbrink et al., 2008]. In the same vein, Van der Mark et al. 2011 used fMRI to study the functional connectivity of the inferior occipitotemporal regions in children with normal reading skills: they proposed the existence of three separated routes, one of which connects the middle part of the OTC (including the VWFA proper) to the inferior parietal lobule and to the inferior frontal gyrus. According to this study, dyslexics show a reduction of the functional connectivity of this particular route, a finding consistent with the observations about the arcuate fasciculus. The same general disconnection hypothesis could also be used to explain more complex deficits in dyslexia (e.g., those which emerge when reading also involves extensive eye movements) as the result of a faulty interaction between multiple systems. Admittedly, this connectionist perspective is not considered in our article, as we preferred to characterize the properties of the cortices involved in reading by assessing the level of convergence of the three systems called into play by the three dyslexia theories discussed in the Introduction. However, we imply that the discovery of convergence of the three systems on cortical reading regions could be taken as initial evidence of a hardwired localized opportunity for interaction between the phonological, the magnocellular and the motor/cerebellar systems when reading.
In the following sections, the motor learning task is labeled as cerebellar both for convenience and for historical reasons. This category of tasks has been repeatedly associated with the activation of the cerebellum in normal subjects while a lack of cerebellar activation was seen in subjects with dyslexia during motor learning. The same evidence was used in support of the cerebellar theory of dyslexia [Nicolson et al., 1999]. However, we maintain both the well‐documented notion that motor learning tasks are by no means the only kind that challenges the function of the cerebellum as well as the notion that motor learning tasks depend on a distributed network exceeding the cerebellum.
For regular orthographies such as Italian, errors in reading pseudowords are obviously phonemic paralexias, given the deterministic nature of the relationship between print and sound at the grapheme to phoneme correspondence level.
There are subtle differences between the present motor learning task and that of Jenkins et al. 1994 and Nicolson et al. 1999, particularly as far as the time scale of the experiment is concerned: the earlier experiments had more learning trials. As a consequence, our task captured the motor learning process more than the complete consolidation and rehearsal of the entire motor sequence. Yet, as shown in the results section, our participants learned a substantial part of the sequence in spite of the shorter learning process.
Exclusive masking in the SPM implies a statistical assessment of all voxels, except those contained in the mask.
It is worth mentioning here that the PET study effectively sampled the entire brain and the cerebellum down to −56 mm from the bicommissural plane. The original raw data of the PET had been smoothed with a 16 × 16 × 16 mm3 Gaussian filter. The PET dyslexia data results were derived from a random‐effect analysis corrected for multiple comparisons [Paulesu et al., 2001].
The fact that a brain region was identified in the reading per‐se analysis does not imply that this region would not be activated for tasks not considered in our experiment, such as, for example, picture naming [Price and Devlin, 2003].
In Siok et al.'s 2004 study of Chinese dyslexic subjects, the authors tested homophone judgements and lexical decision making rather than reading per‐se; a reduced activation of the left inferior occipitotemporal cortex was found in the lexical decision task.
The concept of interface areas in the brain was suggested over 20 years ago by Damasio in a theorethical paper in which he postulated the existence of different “convergence zones” that would have a role in feature and semantic binding and in supporting the interaction between different neurofunctional systems [Damasio, 1989].
The anatomical labels for the subportion of the cerebellum have been taken from the cerebellum atlas by Schmahmann et al. 1999.
As already mentioned, the fact that the reading pe‐se area was not activated by other stimuli used in this study does not imply that it is purely devoted to orthography; forms of picture naming, for example, may also activate the same region [McCrory et al., 2005a; Price, 2000; Shinkareva et al., 2011]. However, the present paper suggests that this region does not receive magnocellular input nor is it directly involved in auditory phonological processing or motor learning.
REFERENCES
- Aghababian V, Nazir TA (2000): Developing normal reading skills: Aspects of the visual processes underlying word recognition. J Exp Child Psychol 76:123–150. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Plewes C, Paulson LA, Roy D, Snook L, Concha L, Phillips L (2005): Imaging brain connectivity in children with diverse reading ability. Neuroimage 25:1266–1271. [DOI] [PubMed] [Google Scholar]
- Ben‐Shachar M, Dougherty RF, Deutsch GK, Wandell BA (2011): The development of cortical sensitivity to visual word forms. J Cogn Neurosci 23:2387–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Yehudah G, Fiez JA (2008): Impact of cerebellar lesions on reading and phonological processing. Ann N Y Acad Sci 1145:260–274. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E (2005): Some neurophysiological constraints on models of word naming. Neuroimage 27:677–693. [DOI] [PubMed] [Google Scholar]
- Boden C, Giaschi D (2007): M‐stream deficits and reading‐related visual processes in developmental dyslexia. Psychol Bull 133:346–366. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM (2002): Modality independence of word comprehension. Hum Brain Mapp 16:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitmeyer B (1993): The roles of sustained (P) and transient (M) channels in reading and reading disability In: Wright SF, Groner R, editors. Facets of Dyslexia and its Remediation. London: Elsevier; pp 13–31. [Google Scholar]
- Burton MW, Small SL, Blumstein SE (2000): The role of segmentation in phonological processing: An fMRI investigation. J Cogn Neurosci 12:679–690. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH (2005): Perisylvian language networks of the human brain. Ann Neurol 57:8–16. [DOI] [PubMed] [Google Scholar]
- Chen Y, Grossman ED, Bidwell LC, Yurgelun‐Todd D, Gruber SA, Levy DL, Nakayama K, Holzman PS (2008): Differential activation patterns of occipital and prefrontal cortices during motion processing: Evidence from normal and schizophrenic brains. Cogn Affect Behav Neurosci 8:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Dehaene S (2004): Specialization within the ventral stream: The case for the visual word form area. Neuroimage 22:466–476. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S (2002): Language‐specific tuning of visual cortex? Functional properties of the visual word form area. Brain 125( Part 5):1054–1069. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Langdon R, Haller M (1997): Computational cognitive neuropsychology and reading In: Dodd B, Worrall L, Campbell R, editors. Evaluating Theories of Language. London: WHURR Publishers; p. 9–36. [Google Scholar]
- Cornelissen P, Richardson A, Mason A, Fowler S, Stein J (1995): Contrast sensitivity and coherent motion detection measured at photopic luminance levels in dyslexics and controls. Vis Res 35:1483–1494. [DOI] [PubMed] [Google Scholar]
- Cornelissen P, Hansen PC, Gilchrist I, Cormack F, Essex J, Frankish C (1998a): Coherent motion detection and letter position encoding. Vis Res 38:2181–2191. [DOI] [PubMed] [Google Scholar]
- Cornelissen PL, Hansen PC, Gilchrist I, Cormack F, Essex J, Frankish C (1998b): Coherent motion detection and letter position encoding. Vis Res 38:2181–2191. [DOI] [PubMed] [Google Scholar]
- Cutting LE, Clements AM, Courtney S, Rimrodt SL, Schafer JG, Bisesi J, Pekar JJ, Pugh KR (2006): Differential components of sentence comprehension: Beyond single word reading and memory. Neuroimage 29:429–438. [DOI] [PubMed] [Google Scholar]
- Damasio A (1989): The brain binds entities and events by multiregional activation from convergence zones. Neural Comput 1:123–132. [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline JB, Le Bihan D, Cohen L (2004): Letter binding and invariant recognition of masked words: Behavioral and neuroimaging evidence. Psychol Sci 15:307–313. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Pegado F, Braga LW, Ventura P, Nunes Filho G, Jobert A, Dehaene‐Lambertz G, Kolinsky R, Morais J, Cohen L (2010): How learning to read changes the cortical networks for vision and language. Science 330:1359–1364. [DOI] [PubMed] [Google Scholar]
- Dejerine J (1891): Sur un cas de cécité verbale avec agraphie, suivi d'autopsie. Mémoires de la Société de Biologie 3:197–201. [Google Scholar]
- Dejerine J (1892): Contribution a l'étude anatomo‐pathologique et clinique des differentes variétés de cécité verbale. Mémoires de la Société de Biologie 4:61–90. [Google Scholar]
- Demb JB, Boynton GM, Heeger DJ (1998): Functional magnetic resonance imaging of early visual pathways in dyslexia. J Neurosci 18:6939–6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH (1997): Lobular patterns of cerebellar activation in verbal working‐memory and finger‐tapping tasks as revealed by functional MRI. J Neurosci 17:9675–9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Jamison HL, Gonnerman LM, Matthews PM (2006): The role of the posterior fusiform gyrus in reading. J Cogn Neurosci 18:911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich M, Muller‐Schunk S, Stephan T, Bense S, Seelos K, Yousry TA (2009): Functional magnetic resonance imaging activations of cortical eye fields during saccades, smooth pursuit, and optokinetic nystagmus. Ann N Y Acad Sci 1164: 282–292. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL (2003): An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol 89:634–639. [DOI] [PubMed] [Google Scholar]
- Duncan KJ, Pattamadilok C, Devlin JT (2010): Investigating occipito‐temporal contributions to reading with TMS. J Cogn Neurosci 22:739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden GF, VanMeter JW, Rumsey JM, Zeffiro TA (1996): The visual deficit theory of developmental dyslexia. Neuroimage 4( 3, Part 3):S108–S117. [DOI] [PubMed] [Google Scholar]
- Facoetti A, Paganoni P, Turatto M, Marzola V, Mascetti GG (2000): Visual‐spatial attention in developmental dyslexia. Cortex 36:109–123. [DOI] [PubMed] [Google Scholar]
- Facoetti A, Zorzi M, Cestnick L, Lorusso ML, Molteni M, Paganoni P, Umilta C, Mascetti GG (2006): The relationship between visuo‐spatial attention and nonword reading in developmental dyslexia. Cogn Neuropsychol 23:841–855. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Craighero L, D'Ausilio A (2009): Broca's area in language, action, and music. Ann N Y Acad Sci 1169:448–458. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE (1998): Neuroimaging studies of word reading. Proc Natl Acad Sci USA 95:914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Frith CD, Liddle PF, Frackowiak RSJ (1991): The cerebellum in skill learning J Cerebr Blood Flow Metab 11:S440. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Turner R, Frackowiak RS (1995): Characterizing evoked hemodynamics with fMRI. Neuroimage 2:157–165. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ (1999): Multisubject fMRI studies and conjunction analyses. Neuroimage 10:385–396. [DOI] [PubMed] [Google Scholar]
- Frith U (1999): Paradoxes in the definition of dyslexia. Dyslexia 5:192–214. [Google Scholar]
- Galaburda AM (1993): Neurology of developmental dyslexia. Curr Opin Neurobiol 3:237–242. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Menard MT, Rosen GD (1994): Evidence for aberrant auditory anatomy in developmental dyslexia. Proc Natl Acad Sci USA 91:8010–8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves WW, Desai R, Humphries C, Seidenberg MS, Binder JR (2010): Neural systems for reading aloud: A multiparametric approach. Cereb Cortex 20:1799–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel B, Thier P, Haarmeier T (2009): Visual motion perception deficits due to cerebellar lesions are paralleled by specific changes in cerebro‐cortical activity. J Neurosci 29:15126–15133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Renvall H (2001): Impaired processing of rapid stimulus sequences in dyslexia. Trends Cogn Sci 5:525–532. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker P, Herskovits E, Degaonkar M (2005): The roles of the “visual word form area” in reading. Neuroimage 24:548–559. [DOI] [PubMed] [Google Scholar]
- Holmes A, Friston K (1998): Generalisability, random effects and population inference. NeuroImage 7:S754. [Google Scholar]
- Houde O, Rossi S, Lubin A, Joliot M (2010): Mapping numerical processing, reading, and executive functions in the developing brain: An fMRI meta‐analysis of 52 studies including 842 children. Dev Sci 13:876–885. [DOI] [PubMed] [Google Scholar]
- Hu W, Lee HL, Zhang Q, Liu T, Geng LB, Seghier ML, Shakeshaft C, Twomey T, Green DW, Yang YM, Price CJ (2010): Developmental dyslexia in Chinese and English populations: Dissociating the effect of dyslexia from language differences. Brain 133( Part 6):1694–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE (1994): Motor sequence learning: A study with positron emission tomography. J Neurosci 14:3775–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio‐Mazoyer N (2003): Evaluation of the dual route theory of reading: A metanalysis of 35 neuroimaging studies. Neuroimage 20:693–712. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA (2000): Microstructure of temporo‐parietal white matter as a basis for reading ability: Evidence from diffusion tensor magnetic resonance imaging. Neuron 25:493–500. [DOI] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Zuo XN, Kelly C, Mennes M, Jutagir DR, Castellanos FX, Milham MP (2011): Resting‐state functional connectivity indexes reading competence in children and adults. J Neurosci 31:8617–8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H, Mair A, Staffen W, Ladurner G (2004): The visual word form area and the frequency with which words are encountered: Evidence from a parametric fMRI study. Neuroimage 21:946–953. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H (2006): Evidence for a dysfunction of left posterior reading areas in German dyslexic readers. Neuropsychologia 44:1822–1832. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Klackl J, Richlan F, Schurz M, Staffen W, Ladurner G, Wimmer H (2009): On the functional neuroanatomy of visual word processing: Effects of case and letter deviance. J Cogn Neurosci 21:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacourse MG, Orr EL, Cramer SC, Cohen MJ (2005): Brain activation during execution and motor imagery of novel and skilled sequential hand movements. Neuroimage 27:505–519. [DOI] [PubMed] [Google Scholar]
- Laycock SK, Wilkinson ID, Wallis LI, Darwent G, Wonders SH, Fawcett AJ, Griffiths PD, Nicolson RI (2008): Cerebellar volume and cerebellar metabolic characteristics in adults with dyslexia. Ann N Y Acad Sci 1145:222–236. [DOI] [PubMed] [Google Scholar]
- Legge GE (1993): The role of contrast in reading: Normal and low vision In: Shapley RM, Lam DMK, editors. Contrast Sensitivity. Cambridge, MA: MIT Press; pp269–287. [Google Scholar]
- Levy J, Pernet C, Treserras S, Boulanouar K, Aubry F, Demonet JF, Celsis P (2009): Testing for the dual‐route cascade reading model in the brain: An fMRI effective connectivity account of an efficient reading style. PLoS One 4:e6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy T, Walsh V, Lavidor M (2010): Dorsal stream modulation of visual word recognition in skilled readers. Vis Res 50: 883–888. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S (2003): The visual word form area: Expertise for reading in the fusiform gyrus. Trends Cogn Sci 7:293–299. [DOI] [PubMed] [Google Scholar]
- McCrory E, Mechelli A, Frith U, Price CJ (2005a): More than words: A common neural basis for reading and naming deficits in developmental dyslexia? Brain 128( Part 2):261–267. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (2000): Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Res Brain Res Rev 31:236–250. [DOI] [PubMed] [Google Scholar]
- Nichols T, Hayasaka S (2003): Controlling the familywise error rate in functional neuroimaging: A comparative review. Stat Methods Med Res 12:419–446. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ (1990): Automaticity: A new framework for dyslexia research? Cognition 35:159–182. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Dean P (1995): Time estimation deficits in developmental dyslexia: Evidence of cerebellar involvement. Proc Biol Sci 259:43–47. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Berry EL, Jenkins IH, Dean P, Brooks DJ (1999): Association of abnormal cerebellar activation with motor learning difficulties in dyslexic adults. Lancet 353: 1662–1667. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Dean P (2001): Developmental dyslexia: The cerebellar deficit hypothesis. Trends Neurosci 24:508–511. [DOI] [PubMed] [Google Scholar]
- Paulesu E (2006): On the advantage of ‘shallow’ orthographies: Number and grain size of the orthographic units or consistency per se? Dev Sci 9:443–444; discussion 451–453. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RS, Frith CD (1996): Is developmental dyslexia a disconnection syndrome? Evidence from PET scanning. Brain 119( Part 1):143–157. [DOI] [PubMed] [Google Scholar]
- Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, Cotelli M, Cossu G, Corte F, Lorusso M, Pesenti S, Gallagher A, Perani D, Price C, Frith CD, Frith U. (2000): A cultural effect on brain function. Nat Neurosci 3:91–96. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Demonet JF, Fazio F, McCrory E, Chanoine V, Brunswick N, Cappa SF, Cossu G, Habib M, Frith CD, Frith U. (2001): Dyslexia: Cultural diversity and biological unity. Science 291:2165–2167. [DOI] [PubMed] [Google Scholar]
- Penny W, Holmes A (2004): Random‐effects analysis In: Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Price CJ, Zeki S, Ashburner J, Penny W, editors. Human Brain Function. Elsevier: San Diego: pp 843–850. [Google Scholar]
- Pernet C, Andersson J, Paulesu E, Demonet JF (2009): When all hypotheses are right: A multifocal account of dyslexia. Hum Brain Mapp 30:2278–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrin C, Demonet JF, N'Guyen‐Morel MA, Le Bas JF, Valdois S (2011): Superior parietal lobule dysfunction in a homogeneous group of dyslexic children with a visual attention span disorder. Brain Lang 118:128–138. [DOI] [PubMed] [Google Scholar]
- Plaut DC, McClelland JL, Seidenberg MS, Patterson K (1996): Understanding normal and impaired word reading: Computational principles in quasi‐regular domains. Psychol Rev 103:56–115. [DOI] [PubMed] [Google Scholar]
- Price CJ (2000): The anatomy of language: Contributions from functional neuroimaging. J Anat 197( Part 3):335–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT (2003): The myth of the visual word form area. Neuroimage 19:473–481. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT (2011): The interactive account of ventral occipitotemporal contributions to reading. Trends Cogn Sci 15: 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Gorno‐Tempini ML, Graham KS, Biggio N, Mechelli A, Patterson K, Noppeney U (2003): Normal and pathological reading: Converging data from lesion and imaging studies. Neuroimage 20(Suppl 1):S30–S41. [DOI] [PubMed] [Google Scholar]
- Qiu D, Tan LH, Zhou K, Khong PL (2008): Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: Voxel‐wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage 41:223–232. [DOI] [PubMed] [Google Scholar]
- Ramus F, Rosen S, Dakin SC, Day BL, Castellote JM, White S, Frith U (2003): Theories of developmental dyslexia: Insights from a multiple case study of dyslexic adults. Brain 126( Part 4):841–865. [DOI] [PubMed] [Google Scholar]
- Rayner K (1986): Eye movements and the perceptual span in beginning and skilled readers. J Exp Child Psychol 41:211–236. [DOI] [PubMed] [Google Scholar]
- Rayner K (1998): Eye movements in reading and information processing: 20 years of research. Psychol Bull 124:372–422. [DOI] [PubMed] [Google Scholar]
- Reid AA, Szczerbinski M, Iskierka‐Kasperek E, Hansen P (2007): Cognitive profiles of adult developmental dyslexics: Theoretical implications. Dyslexia 13:1–24. [DOI] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H (2011): Meta‐analyzing brain dysfunctions in dyslexic children and adults. Neuroimage 56:1735–1742. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M (2000): Stereotaxic display of brain lesions. Behav Neurol 12:191–200. [DOI] [PubMed] [Google Scholar]
- Rosen GD, Burstein D, Galaburda AM (2000): Changes in efferent and afferent connectivity in rats with induced cerebrocortical microgyria. J Comp Neurol 418:423–440. [PubMed] [Google Scholar]
- Ross J, Morrone MC, Goldberg ME, Burr DC (2001): Changes in visual perception at the time of saccades. Trends Neurosci 24:113–121. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Andreason P, Zametkin AJ, Aquino T, King AC, Hamburger SD, Pikus A, Rapoport JL, Cohen RM (1992): Failure to activate the left temporoparietal cortex in dyslexia. An oxygen 15 positron emission tomographic study. Arch Neurol 49:527–534. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Service E, Kiesila P, Uutela K, Salonen O (1996): Impaired visual word processing in dyslexia revealed with magnetoencephalography. Ann Neurol 40:157–162. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, Kabani N, Toga A, Evans A, Petrides M (1999): Three‐dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage 10( 3, Part 1): 233–260. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, et al. (2002): Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry 52:101–110. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA (2008): Paying attention to reading: The neurobiology of reading and dyslexia. Dev Psychopathol 20:1329–1349. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, Shankweiler DP, Liberman AM, Skudlarski P, Fletcher JM, et al. (1998): Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci USA 95:2636–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkareva SV, Malave VL, Mason RA, Mitchell TM, Just MA (2011): Commonality of neural representations of words and pictures. Neuroimage 54:2418–2425. [DOI] [PubMed] [Google Scholar]
- Silani G, Frith U, Demonet JF, Fazio F, Perani D, Price C, Frith CD, Paulesu E (2005): Brain abnormalities underlying altered activation in dyslexia: A voxel based morphometry study. Brain 128( Part 10):2453–2461. [DOI] [PubMed] [Google Scholar]
- Siok WT, Perfetti CA, Jin Z, Tan LH (2004): Biological abnormality of impaired reading is constrained by culture. Nature 431:71–76. [DOI] [PubMed] [Google Scholar]
- Skottun BC (2000): The magnocellular deficit theory of dyslexia: The evidence from contrast sensitivity. Vis Res 40:111–127. [DOI] [PubMed] [Google Scholar]
- Snowling MJ (2001): From language to reading and dyslexia. Dyslexia 7:37–46. [DOI] [PubMed] [Google Scholar]
- Starrfelt R, Gerlach C (2007): The visual what for area: Words and pictures in the left fusiform gyrus. Neuroimage 35:334–342. [DOI] [PubMed] [Google Scholar]
- Stein J (2001): The magnocellular theory of developmental dyslexia. Dyslexia 7:12–36. [DOI] [PubMed] [Google Scholar]
- Stein J (2003): Visual motion sensitivity and reading. Neuropsychologia 41:1785–1793. [DOI] [PubMed] [Google Scholar]
- Stein J, Fowler MS (1993): Unstable binocular control in dyslexic children. J Res Reading 16:30–45. [Google Scholar]
- Stein J, Walsh V (1997): To see but not to read: The magnocellular theory of dyslexia. Trends Neurosci 20:147–152. [DOI] [PubMed] [Google Scholar]
- Steinbrink C, Vogt K, Kastrup A, Muller HP, Juengling FD, Kassubek J, Riecker A (2008): The contribution of white and gray matter differences to developmental dyslexia: Insights from DTI and VBM at 3.0 T. Neuropsychologia 46:3170–3178. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Fawcett AJ, Nicolson RI, Stein JF (2005): Impaired balancing ability in dyslexic children. Exp Brain Res 167:370–380. [DOI] [PubMed] [Google Scholar]
- Tallal P (1980): Auditory temporal perception, phonics, and reading disabilities in children. Brain Lang 9:182–198. [DOI] [PubMed] [Google Scholar]
- Thiele A, Henning P, Kubischik M, Hoffmann KP (2002): Neural mechanisms of saccadic suppression. Science 295:2460–2462. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF (2003): Development of neural mechanisms for reading. Nat Neurosci 6:767–773. [DOI] [PubMed] [Google Scholar]
- Twomey T, Kawabata Duncan KJ, Price CJ, Devlin JT (2011): Top‐down modulation of ventral occipito‐temporal responses during visual word recognition. Neuroimage 55:1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Mark S, Klaver P, Bucher K, Maurer U, Schulz E, Brem S, Martin E, Brandeis D (2011): The left occipitotemporal system in reading: Disruption of focal fMRI connectivity to left inferior frontal and inferior parietal language areas in children with dyslexia. Neuroimage 54:2426–2436. [DOI] [PubMed] [Google Scholar]
- Vanni S, Uusitalo MA, Kiesila P, Hari R (1997): Visual motion activates V5 in dyslexics. Neuroreport 8:1939–1942. [DOI] [PubMed] [Google Scholar]
- Vidyasagar TR (2004): Neural underpinnings of dyslexia as a disorder of visuo‐spatial attention. Clin Exp Optom 87:4–10. [DOI] [PubMed] [Google Scholar]
- Wandell BA (2011): The neurobiological basis of seeing words. Ann N Y Acad Sci 1224:63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Smith SM, Davis S, Howell P (2008): Structural and functional abnormalities of the motor system in developmental stuttering. Brain 131( Part 1):50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1981): The Wechsler Adult Intelligence Scale‐Revised. New York: Psychological Corporation. [Google Scholar]
- Weger UW, Inhoff AW (2006): Attention and eye movements in reading: Inhibition of return predicts the size of regressive saccades. Psychol Sci 17:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhead ZV, Brownsett SL, Dhanjal NS, Beckmann C, Wise RJ (2011): The visual word form system in context. J Neurosci 31:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K, Friston K (1995): Analysis of fMRI time‐series revisited—Again. NeuroImage 2:173–181. [DOI] [PubMed] [Google Scholar]
- Worsley K, Friston K (2000): A test for a conjunction. Stat Probability Lett 47:135–140. [Google Scholar]
- Worsley K, Marrett S, Neelin P, Vandal A, Friston K, Evans A (1996): A unified statistical approach for determining significant voxels in images of cerebral activation. Hum Brain Mapp 4:58–73. [DOI] [PubMed] [Google Scholar]
- Zeki SM (1974): Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. J Physiol 236:549–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzi M, Hougthon G, Butterworth B (1998): Two routes or one in reading aloud? A connectionist “dual‐process” model. J Exp Psychol Hum Percept Perform 24:1131–1161. [Google Scholar]


