Abstract
A subgroup of patients with breast cancer suffers from mild cognitive impairment after chemotherapy. To uncover the neural substrate of these mental complaints, we examined cerebral white matter (WM) integrity after chemotherapy using magnetic resonance diffusion tensor imaging (DTI) in combination with detailed cognitive assessment. Postchemotherapy breast cancer patients (n = 17) and matched healthy controls (n = 18) were recruited for DTI and neuropsychological testing, including the self‐report cognitive failure questionnaire (CFQ). Differences in DTI WM integrity parameters [fractional anisotropy (FA) and mean diffusivity (MD)] between patients and healthy controls were assessed using a voxel‐based two‐sample‐t‐test. In comparison with healthy controls, the patient group demonstrated decreased FA in frontal and temporal WM tracts and increased MD in frontal WM. These differences were also confirmed when comparing this patient group with an additional control group of nonchemotherapy‐treated breast cancer patients (n = 10). To address the heterogeneity observed in cognitive function after chemotherapy, we performed a voxel‐based correlation analysis between FA values and individual neuropsychological test scores. Significant correlations of FA with neuropsychological tests covering the domain of attention and processing/psychomotor speed were found in temporal and parietal WM tracts. Furthermore, CFQ scores correlated negatively in frontal and parietal WM. These studies show that chemotherapy seems to affect WM integrity and that parameters derived from DTI have the required sensitivity to quantify neural changes related to chemotherapy‐induced mild cognitive impairment. Hum Brain Mapp 32:480–493, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: diffusion tensor imaging, voxel‐based analysis, white matter, brain, chemotherapy, cognition, executive functioning
INTRODUCTION
The survival rate of breast cancer patients has increased significantly with earlier diagnosis and improved cancer treatments. However, 10–40% breast cancer survivors experience cognitive defects when returning to their lives and this can seriously impact quality of life [Matsuda et al., 2005]. With the increase in survivorship, adjuvant systemic chemotherapy in breast cancer patients has been recognized to have a potential adverse effect on cognition in domains mainly involving memory, attention, psychomotor speed, executive functioning, and multitasking [Ahles et al., 2002, 2008; Castellon et al., 2004; Jenkins et al., 2006; Quesnel et al., 2009; Schagen et al., 2006; Wefel et al., 2004]. However, the pathophysiology of this impaired cognitive functioning is still unclear. Different candidate mechanisms have been suggested including direct neurotoxic effects, induced‐hormonal changes, immune dysregulation, microemboli, and genetic polymorphisms that may render individuals more susceptible to these effects [Ahles and Saykin, 2007]. Predominantly, the effects of adjuvant therapy on cognitive functioning have been evaluated with neuropsychological tests and self‐rated subjective questionnaires [Tchen et al., 2003; Vardy et al., 2007]. Additionally, animal experiments have revealed learning deficits after the administration of chemotherapeutic agents [Foley et al., 2008; Macleod et al., 2007]. Only a limited number of studies however, have used in vivo human brain imaging techniques to investigate potential physical changes induced by the therapy [Ferguson et al., 2007b; Inagaki et al., 2007; Silverman et al., 2007; Yoshikawa et al., 2005]. These studies have suggested both functional and structural changes in the brain. A functional imaging study using positron emission tomography (PET) [Silverman et al., 2007] reported altered metabolic activity in the prefrontal cortex during memory tasks. A functional magnetic resonance imaging study (fMRI) [Ferguson et al., 2007b] compared a pair of monozygotic twins, of whom one received cytotoxic treatment. Both completed a self‐reported cognitive questionnaire and an fMRI memory task. More brain areas were recruited to attain a comparable completion of the fMRI task for the chemo‐treated patient, which could explain the higher self‐reported cognitive complaints. Structural MRI studies have described reductions in white and gray matter volume for brain areas important for executive functioning [Inagaki et al., 2007; Yoshikawa et al., 2005]. A potential mechanism by which chemotherapy could impair cognitive functioning is direct neurotoxicity, causing toxic injury to brain parenchyma, producing demyelination and/or altered water content, resulting in alterations of the white matter (WM) integrity of the brain [Beaulieu, 2002]. There is some evidence that a frequently used chemotherapeutic agent, 5‐fluorouracil (5‐FU), crosses the blood–brain barrier by simple diffusion [Bourke et al., 1973; Kerr et al., 1984]. Systemic treatment with 5‐FU in mice has been shown to cause damage to myelinated WM tracts of the central nervous system [Han et al., 2008]. Also Mustafa et al. [ 2008] reported a reduction in the proliferation of cells in neurogenic brains of rat after the administration of 5FU.
The successful execution of complex cognitive tasks depends on coordinated activity in distributed brain networks and the integrity of brain WM pathways to pass the necessary information (neural signals) in a timely way [Engel et al., 2001]. The speed with which neural signals are conducted across long myelinated axons is related to their thickness and degree of myelination [Gutierrez et al., 1995]. Diffusion tensor imaging (DTI) is a technique that enables visualization and characterization of the integrity of WM fibers in the brain based on the measurement of the diffusion of water [Basser et al., 1994; Lebihan et al., 2001; Pierpaoli et al., 1996]. In intact WM tissue the diffusion is highly directional because axonal membranes and myelin hinder random diffusion. Damage to WM structures will result in changes in quantitative DTI parameters, including fractional anisotropy (FA) which characterizes the directionality of diffusion and mean diffusivity (MD) which describes the average amount of diffusion [Pierpaoli and Basser, 1996]. DTI parameters are sensitive to the detection of subtle changes in the microstructure of WM fiber tracts, as various DTI studies have linked neurodegenerative diseases and age‐related cognitive impairments with impaired WM integrity reflected by decreased FA and increased MD values [Bai et al., 2009; Schiavone et al., 2009a]. As such, DTI is a promising technique to assess whether therapy‐induced subtle WM changes could explain the cognitive complaints in women after chemotherapy. To our knowledge, only one pilot DTI study [Abraham et al., 2008] investigated chemotherapy‐induced WM changes. This study was limited to the analysis of WM in the corpus callosum. They found affected, albeit normal‐appearing WM in the genu of the corpus callosum which could be related to cognitive deficits. The purpose of this DTI study is to analyze whole brain WM to detect possible changes in WM integrity in a group of chemotherapy‐treated breast cancer patients compared to age‐matched healthy controls. As the diagnosis of cancer itself might also have an impact on cognition, an additional control group of nonchemotherapy‐treated breast cancer patients was added to validate our findings. Furthermore, we obtained a comprehensive battery of cognitive tests and self‐reported cognitive scores which we used in voxel‐based correlation analysis between WM integrity (as assessed by the DTI measures) and possible cognitive impairment (as assessed by the test scores). We hypothesize that FA would be lower and MD higher in important WM tracts of the chemo‐exposed brain and that such changes would be associated with cognitive impairment.
MATERIALS AND METHODS
Subjects
The study was approved by the local Ethical Commission and conducted in accordance with the declaration of Helsinki. After signing the informed consent, 17 postchemotherapy (C+) (80–160 days after completion), early‐stage breast cancer patients with no metastasis (age 45.4 ± 4.2 years), 18 healthy controls (age 45.2 ± 3.9 years), and 10 nonchemotherapy‐treated breast cancer patients (C‐) (age 42.9 ± 4 years) participated in the study. Healthy controls were free from cognitive complaints and were recruited via advertisement on local websites. All patients underwent breast cancer surgery, were premenopauzal at the start of the study, and had received standard education (minimally until 18 years). All subjects were assessed with the Mini International Neuropsychiatric Interview [Sheehan et al., 1998] to exclude potential psychiatric disorders including depression and anxiety disorders. Other exclusion criteria included previous history of cancer, history of any neurological condition, traumatic brain injury, mental retardation, and use of psychotropic medication.
MRI Imaging
All subjects were imaged on a 3T scanner (Intera, Philips, Best, the Netherlands) with an eight channel phased‐array head coil. A whole brain DTI SE‐EPI (diffusion‐weighted single shot spin‐echo echoplanar imaging) was acquired with the following scanning parameters: 68 contiguous sagittal slices, 112 × 109 matrix size, 220 × 220 mm2 FOV, 4,956 ms TR, 55 ms TE, 2.5 parallel imaging factor, 2.2‐mm slice thickness, 1.96 × 1.96 × 2.2 mm3 voxel size. Diffusion gradients were applied along 45 noncollinear directions with a b‐value of 800 s mm−2. Additionally one nondiffusion‐weighted (b = 0) set of images was acquired resulting in a total scan time of 10.34 min. A T1‐weighted whole brain 3D‐TFE (182 contiguous coronal slices; 250 × 250 mm2 FOV; 4.6 ms TE; 9.7 ms TR; 1.2‐mm slice thickness; 256 × 256 matrix; 0.98 × 0.98 × 1.2 mm3 voxel size), a T2‐weighted TSE (28 transversal slices; 230 × 184 mm2 FOV; 4‐mm slice thickness; 3,000 ms TR; 80 ms TE), and a FLAIR (28 transverse slices; 230 × 183 mm2 FOV; 125 ms TE; 11,000 ms TR; 2,800 ms IR delay; 4‐mm slice thickness; 256 × 256 matrix; 0.65 × 0.87 × 4 mm3 voxel size) were also acquired to search for primary brain pathology as an exclusion criterion.
DTI Data Processing
DTI images were visually checked for possible artifacts. DTI post‐processing was performed as in [Sage et al., 2009]: First, eddy‐current and motion‐induced artifacts were corrected using CATNAP with the required reorientation of the b‐matrix [Landman et al., 2007; Leemans and Jones, 2009]. Second, the diffusion tensor and derived DTI parameters such as FA, MD, radial diffusivity (RD, the diffusivity perpendicular to the principal diffusion directory which was calculated by averaging the second and third eigenvalues) and parallel diffusivity (PD, the diffusivity parallel to the principal diffusion directory, which is represented by the first eigenvalue) were calculated with the diffusion MR toolbox ExploreDTI [Leemans et al., 2009]. Third, the individual datasets were nonrigidly normalized to an in‐house population‐based full‐tensor‐information variant of the ICBM81‐DTI‐template [Mori et al., 2008; Verhoeven et al., 2010] in MNI (Montreal Neurological Institute) space, using an affine and, subsequently, a nonrigid DTI‐based coregistration algorithm [Leemans et al, 2005; Van Hecke et al., 2007]. The spatially normalized FA and MD maps were then smoothed with a 3D Gaussian kernel of FWHM 6 mm and processed in a voxel‐based analysis (VBA) using SPM5 [Ashburner and Friston, 2005, http://www.fil.ion.ucl.ac.uk/spm/software/spm5/]. The choice of registration algorithm and smoothing kernel were based on a previous study, in which it was demonstrated that these choices yield reliable VBA results that were similar to those obtained with a hybrid technique such as tract‐based spatial statistics (TBSS) [Sage et al., 2009].
Neuropsychological Assessment
All chemotherapy‐treated patients and healthy controls were evaluated using a neuropsychological test battery, covering several domains: (1) Attention and concentration (Bourdon‐Wiersma Dot cancellation test [Grewelf, 1953], Test of every day attention: auditory elevator (TEA) [Robertson et al., 1996], WAIS Digit span (Wechsler, 1981], CORSI block span [Corsi, 1972], WAIS substitution [Wechsler, 1981]); (2) Memory (Auditory verbal learning test [Corsi, 1972; Rey, 1958; Van der Elst et al., 2005], Rey visual design learning test [Rey, 1941]); (3) Executive functioning (Stroop color word test [Stroop, 1935], controlled oral word association test [Benton and Hamsher, 1978]); (4) Cognitive/Psychomotor processing speed (WAIS digit symbol [Wechsler, 1981], nine‐hole pegboard (9HPT), trail making test [TMT; Army Individual Test Battery, 1944; Klove, 1963]). Self‐reported cognitive functioning was assessed using the cognitive failure questionnaire (CFQ) [Broadbent et al., 1982; Muris and Merckelbach, 1995], providing subscales on distraction, distraction in social situations, names and word finding, orientation, and a total summary score. All subjects, including the C‐ patients, completed the Spielberger State‐Trait‐Anxiety Inventory (STAI) [Spielberger and Gorsuch, 1985] and the Beck depression inventory (BDI) [Bosscher et al., 1986] to assess possible anxiety and depressive symptoms. Verbal IQ was measured using the Dutch adult reading test NLV [Schmand et al., 1991]. Neuropsychological evaluation and MR scans took place on the same day. One patient did not complete the cognitive failure questionnaire.
Statistical Analysis
Possible differences in neuropsychological test scores and demographics between chemotherapy‐treated patients and healthy controls were assessed with two‐tailed two‐sample Student‐t‐tests and Mann‐Whitney U‐tests for normally and nonnormally distributed parameters, respectively. Threshold for statistical significance was set at P < 0.05. Tests showing significant difference between groups were subsequently selected for voxel‐based correlation analysis. To be sufficiently sensitive in selecting the tests for the explorative correlation analysis, we did not apply a correction for multiple comparisons. Although we cannot exclude false positives by doing so, this procedure was used only as a data reduction technique and not to make any claims on differences in neuropsychological test results between chemotherapy‐treated patients and healthy controls.
Two‐sample‐t‐tests were performed to assess differences in FA and MD maps between chemotherapy‐treated patients and healthy controls on a voxel‐by‐voxel basis in SPM5. We added verbal IQ and the depression score BDI as covariates‐of‐no‐interest in the analysis. A WM mask, obtained by segmentation of the mean FA map into white and grey matter and cerebrospinal fluid (CSF), was applied to limit the analysis to WM voxels only.
The resulting statistical parametric maps were thresholded at P < 0.001 uncorrected for multiple comparisons. Clusters significant at the P < 0.05 level corrected for multiple comparisons were retained. These thresholds have been used for all reported analysis. Thresholded t‐maps were overlaid over the mean FA image from the whole group for visualization. Anatomical labeling of the significant clusters was performed using the ICBM81 labels [Mori et al., 2008].
From the significant clusters, the average FA, MD, RD, and PD values were calculated for the chemotherapy‐treated patients, the healthy control group, and the nonchemotherapy‐treated control group and compared between the groups.
To study the effect of degree of cognitive impairment on WM integrity, we carried out two separate analysis. First, we divided the patients in an impaired and an unimpaired group. Cognitive impairment was defined as having at least two neuropsychological test scores outside normal variance (>2 standard deviations (SD) difference from the mean of healthy controls) [Vardy et al., 2007]. We repeated the voxel‐based two‐sample t‐test to compare FA of both unimpaired and impaired patients with the FA of the healthy controls. Second, we conducted a voxel‐based correlation analysis in SPM5 with the subjects' FA as dependent variable, the selected neuropsychological test scores as regressors and BDI and verbal IQ as covariates‐of‐no‐interest. We choose FA for the correlation analysis as it has already been shown that FA and cognition correlate well [Turken et al., 2008] and more strongly than other WM integrity parameters like MD [Schiavone et al., 2009b].
Finally, to examine whether self‐reported cognitive impairment is associated with WM changes, we correlated FA values of patients with the self‐reported CFQ scores (total and distraction) using the same procedure as described above. Healthy controls were not included in this part of the analysis as they did not have any cognitive complaints. Age was incorporated as a covariate‐of‐no‐interest for the CFQ scores as a significant correlation between age and CFQ scores was found.
RESULTS
Structural MR Image and Data Quality Assessment
T1, T2, and FLAIR images were used to search for primary brain pathology as an exclusion criterion. This resulted in the exclusion of three subjects: one C+ patient and one healthy volunteer were diagnosed with MS and another healthy volunteer with intracranial vessel atherosclerosis leading to vascular periventricular leucomalacia. Three additional subjects (two C+ patients and one healthy control) were excluded from the image analysis due to excessive head motion, resulting in image intensity differences between odd and even slices of more than 10%. In summary, from the initial 18 healthy controls, 17 C+ and 10 C‐ patients, DTI images from 15 healthy controls, 14 C+ and 10 C‐ patients were included in the imaging analysis.
Subject Demographics
Background and medical information of included patients and controls are summarized in Table I. All subjects were active women between 38 and 51 years old. At the time of testing, all C+ patients had completed chemotherapy and local radiation‐therapy. Twelve C+ and nine C‐ patients had started additional hormonal treatment with tamoxifen 1 month before the testing.
Table I.
Background and clinical characteristics of included patients and controls
| Patients (C+; n = 14) | Healthy controls (HC; n = 15) | Difference C+ vs. HC (t test) | Patient controls group (C−; n = 10) | Difference C+ vs. C− (t test) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean or count | SD | Mean | SD | P value | Mean or count | SD | P value | |
| Age (years) | 45.4 | 4.5 | 45.1 | 4.0 | 0.83 | 42.9 | 6.15 | 0.23 |
| Breast cancer stage | ||||||||
| Stage I | 5 | NA | NA | NA | NA | 8 | NA | NA |
| Stage II | 4 | NA | NA | NA | NA | 2 | NA | NA |
| Stage III | 5 | NA | NA | NA | NA | 0 | NA | NA |
| Hormonal therapy (Nolvadex) | 12 | NA | NA | NA | NA | 9 | NA | NA |
| Protocol of adjuvant chemotherapy | ||||||||
| FEC (six cycles) | 3 | NA | NA | NA | NA | NA | NA | NA |
| FEC (three cycles) + Taxol (three cycles) | 11 | NA | NA | NA | NA | NA | NA | NA |
| Radiotherapy | 12 | NA | NA | NA | NA | 9 | NA | NA |
| Time of investigation after completion of chemotherapy (days) | 129 | 20 | NA | NA | NA | NA | NA | NA |
| Depression BDI | 6.4 | 4.6 | 3.7 | 3.3 | 0.06 | 3.9 | 3.7 | 0.15 |
| Anxiety STAI | 34.7 | 7.9 | 32.6 | 6 | 0.38 | 37 | 8.5 | 0.50 |
| Years of education (years) | 14.2 | 2.1 | 14.5 | 1.8 | 0.83 | 14.9 | 1.7 | 0.26 |
| Verbal IQ | 112 | 4.3 | 115 | 7.4 | 0.09 | 114 | 7.1 | 0.27 |
All units are arbitrary, except specified otherwise. C+: chemotherapy‐treated patients; C−: patients not treated with chemotherapy; HC: healthy controls; BDI: beck depression inventory; STAI: Spielberger state‐trait anxiety; FEC: fiuorouracil, epirubicin, cyclophosphamide.
No significant differences (P < 0.05) were found between C+ patients and the two control groups (healthy controls and C‐ patients) in terms of verbal IQ, depression BDI, and anxiety STAI scores.
Neuropsychological Assessment
Two‐tailed t‐tests comparing neuropsychological test scores for C+ patients and healthy controls revealed that patients performed significantly worse on attention tests (Bourdon‐Wiersma avg time (P = 0.02) and Every day Attention, TEA_ST5 (P = 0.01)) and cognitive processing/psychomotor speed (9HPT hole (P = 0.004), TMT A (P = 0.02) and WAIS digit symbol (P = 0.05)) (Table II). These tests were subsequently selected for voxel‐based correlation analysis with FA. Seven C+ patients were classified as impaired according to the definition described above. A detailed summary of all neuropsychological test results can be found in the Supporting Information.
Table II.
Summary of neuropsychological assessment
| Domain test | Patients (C+; n = 14) | Healthy controls (HC; n = 15) | Impaired patients (IC+; n = 7) | Difference C+ vs. HC (t test or M‐U) | Difference IC+ vs. HC (t test or M‐U) | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | P value | P value | |
| Attention and concentration | ||||||||
| Attention Bourdon‐Wiersma avg/row (s) | 12.56 | 3.09 | 10.61 | 1.32 | 13.7 | 3.56 | 0.023 | 0.0018 |
| Every day attention—auditory elevator TEA ST5 | 5.44 | 3.58 | 7.86 | 2.21 | 3.2 | 2.91 | 0.015 | 0.0002 |
| Cognitive/psychomotor speed | ||||||||
| Trail making test A (s) | 28.09 | 9.52 | 22.23 | 3.66 | 32.8 | 9.67 | 0.025 | 0.0006 |
| Nine‐hole pegboard, Nondominant hand (s) | 20.26 | 2.73 | 17.94 | 1.31 | 21.1 | 2.65 | 0.004 | 0.0005 |
| WAIS digit symbol | 58.56 | 14.17 | 67.41 | 11.01 | 49.9 | 10.95 | 0.053 | 0.0004 |
| Self reported cognitive complaints: CQF | ||||||||
| Distraction | 11.2 | 4.9 | 9.1 | 2.1 | 12.8 | 4.5 | 0.06 | 0.02 |
| Distraction in social situations | 6.1 | 2.2 | 6.4 | 1.8 | 7.3 | 1.9 | 0.35 | 0.25 |
| Names and words | 4.8 | 2.2 | 5.8 | 2.1 | 4.8 | 2.6 | 0.11 | 0.18 |
| Orientation | 6.2 | 1.5 | 5.7 | 1.5 | 6.8 | 1.9 | 0.22 | 0.06 |
| Total score | 36.7 | 13.6 | 35.2 | 7.5 | 42.0 | 12.3 | 0.35 | 0.09 |
All units are arbitrary, except specified otherwise. C+: chemotherapy‐treated patients; HC: healthy controls; IC: impaired patients, defined as C+ patients having more than two cognitive tests outside normal variance (>2 standard deviation (SD) difference from mean of controls).
Note: Higher scores on TEA‐ST5 and WAIS digit symbol and lower scores on Bourdon‐Wiersma, Nine‐hole Peg and Trail Making Test indicate better performance.
No significant differences were found between the C+ patients and the healthy controls in terms of self‐reported complaints. For the impaired patient group however, significant differences were found for the CQF distraction subscale (P = 0.02).
Assessment of DTI Parameter Differences Between Patients and Controls (FA, MD, RD, and PD)
Compared to healthy controls, the C+ patient group demonstrated significantly decreased FA in frontal [superior fronto‐occipital fasciculus (SFOF), anterior limb of internal capsula (ALIC), superior corona radiata] and temporal [inferior longitudinal (ILF) and fronto‐occipital fasciculus (IFOF)] WM tracts. A significant increase of MD in patients compared to controls was demonstrated in frontal WM [superior longitudinal fasciculus (SLF), superior corona radiata, corpus callosum, and cingulum]. Additionally, RD values for the above reported regions were significantly higher in C+ patients than in controls. No significant differences were found in PD values between the groups. Compared to C‐ patients, the C+ patient group had significant lower FA, higher MD, and higher RD in the above reported regions (Table III, Fig. 1a,b).
Table III.
FA and MD voxel‐wise comparison
| Region | Cluster P corr | Cluster size | MNI coord [X Y Z] | Anatomical extent of cluster | T value | Mean FA | Mean MD (× 10−3 mm2 s−1) | Mean RD (×10−3 mm2 s−1) | Mean PD (102 mm2 s−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PA C+ | PA C− | HC | PA C+ | PA C− | HC | PA C+ | PA C− | HC | PA C+ | PA C‐ | HC | ||||||
| FA voxel‐wise comparison | |||||||||||||||||
| Frontal | <0.0001 | 599 | [21 12 24] | Cluster covering parts of : superior fronto‐occipital fasciculus, anterior limb of internal capsula, superior corona radiata | 5.36 | 0.39a , b | 0.43 | 0.43 | 0.75a , b | 0.70 | 0.70 | 0.58a , b | 0.52 | 0.52 | 0.11 | 0.10 | 0.11 |
| Temporal | 0.01 | 227 | [36 −45 −6] | Sagittal stratum (includes inferior longitudinal and inferior fronto‐occipital fasciculus) | 5.61 | 0.42a , b | 0.46 | 0.47 | 0.93a , b | 0.88 | 0.89 | 0.71a , b | 0.64 | 0.65 | 0.14 | 0.14 | 0.14 |
| MD voxel‐wise comparison | |||||||||||||||||
| Frontal | <0.0001 | 1164 | [30 14 26] | Cluster covering parts of : superior longitudinal fasciculus, superior corona radiata, corpus callosum and cingulum | 5.84 | 0.38a , b | 0.42 | 0.42 | 0.76a , b | 0.69 | 0.71 | 0.60a , b | 0.52 | 0.54 | 0.11 | 0.10 | 0.10 |
FA voxel‐wise comparison: clusters where FA value is significantly lower in chemo‐therapy treated patients than healthy controls (P < 0.05, corrected for multiple comparisons). Mean FA, MD, RD, and PD values are calculated and reported for those identified clusters.
MD voxel‐wise comparison: clusters where MD value is significantly higher in chemo‐therapy treated patients than healthy controls (P < 0.05, corrected for multiple comparisons). Mean FA, MD, RD, and PD values are calculated and reported for those identified clusters.
PA C+: patients who received chemotherapy; PA C−: patients who did not receive chemotherapy; HC: healthy controls; FA: fractional anisotropy; MD: mean diffusivity; RD: radial diffusivity; PD: parallel diffusivity.
Mean chemotherapy‐treated patient group significantly different (P < 0.05) from mean healthy control group.
Mean chemotherapy‐treated patient group significantly different (P < 0.05) from mean non‐chemotherapy‐treated patient control group.
Note: no significant differences were found between mean C− patient control group and mean healthy control group.
Figure 1.
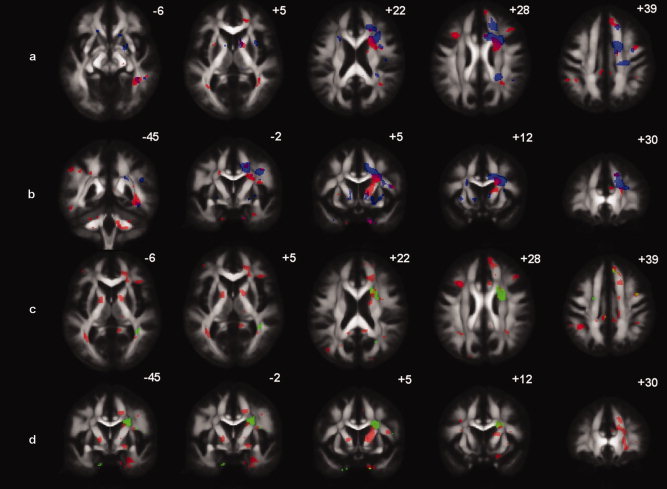
Axial (a) and coronal (b) slices with significantly decreased FA (red) and significantly increased MD (blue) in patients that received chemotherapy versus healthy controls (P < 0.05 corrected for multiple comparisons). Regions where both effects are present are colored purple/brown. Axial (c) and coronal (d) slices with significantly decreased FA in impaired patients (red) and in unimpaired patients (green) versus healthy controls (P < 0.05 corrected for multiple comparisons). Regions where both effects are present are colored yellow/light green. Numbers (mm) define the location relative to z (a–c) and y (b–d) in MNI coordinates.
No WM regions were identified where C+ patients had higher FA or lower MD when compared to the healthy control group.
Assessment of FA Between Impaired/Unimpaired Patients and Controls
When compared to healthy controls, the impaired patient group (n = 7) had much more extensive WM degeneration (significant lower FA value) than the unimpaired patient group, which is shown in Figure 1c,d.
Correlation Analysis of Neuropsychological Test Scores With FA Values
Significant correlations between FA values and neuropsychological test scores were found when including all subjects in the voxel‐based correlation analysis in frontal, parietal, and temporal WM regions (Table IV and Fig. 2a–d). FA correlated significantly with performance on attention tests in parietal parts of the superior longitudinal fasciculus (SLF), in which a strong negative correlation with the Bourdon‐Wiersma average time/row and a positive correlation for TEA_ST5 were demonstrated. FA was negatively correlated with 9HPT in parietal (parts of SLF, cingulum, corpus callosum, and superior corona radiata) and parieto‐temporal (parts of posterior thalamic radiation and SLF) WM, with reduced FA being associated with longer time required to complete the 9HPT test using the nondominant hand. Additionally, the WAIS digit symbol score correlated positively with FA in temporal WM regions covering parts of the SLF and inferior longitudinal fasciculus (ILF). Significant negative correlations of FA with TMTA in the cingulum were observed. Note that there is an indication that cognitively impaired patients have lower FA values in these identified regions, indicating more extensive WM injury as shown in Figure 2a–d.
Table IV.
Brain regions showing significant correlations (P corr < 0.05) between FA and cognitive scores
| Domain test | Region | Cluster P corr | Cluster size | MNI coord (X Y Z mm) | Anatomical extent of cluster | T value |
|---|---|---|---|---|---|---|
| FA and Neuropsychological test scores in C+ patients and healthy controls: | ||||||
| Attention | ||||||
| BW‐avg | Parietal | <0.0001 | 769 | [12 −26 34] | Cluster covering parts of corpus callosum, cingulum and superior longitudinal fasciculus (SLF) | 5.49(n) |
| TEA‐ST5 | Parietal | 0.039 | 163 | [−39 −42 2]] | Superior longitudinal fasciculus (SLF) | 6.07(p) |
| Psychomotor/processing speed | ||||||
| 9HPT | Parietal | <0.0001 | 495 | [10 −26 36] | Cluster covering parts of corpus callosum, cingulum and superior corona radiata | 5.46(n) |
| Parieto‐temporal | 0.004 | 265 | [−33 −38 14] | Cluster covering parts of posterior thalamic radiation and superior longitudinal fasciculus | 4.23(n) | |
| Parietal | 0.017 | 204 | [39 −44 18] | Superior longitudinal fasciculus (SLF) | 4.61(n) | |
| WAIS‐digit symbol | Temporal | 0.039 | 172 | [−45 −27 −8] | Cluster covering superior longitudinal fasciculus (SLF) and inferior longitudinal fasciculus (ILF) | 5.71(p) |
| TMTA | Parietal | 0.005 | 263 | [14 −32 38] | Cingulum | 5.53(n) |
| Self‐rated cognitive complaint (FA and Self‐rated cognitive impairment scores in C+ patients) | ||||||
| CFQ‐Distraction score | Frontal | 0.004 | 137 | [−33 −12 21] | Cluster covering parts of : superior longitudinal fasciculus (SLF), superior corona radiata, external capsula | 6.8(n) |
| CFQ‐Total score | Parietal | 0.007 | 128 | [18 −51 56] | Posterior corona radiata | 8.33(n) |
| Frontal | 0.002 | 159 | [−32 −15 22] | Cluster covering parts of : superior longitudinal fasciculus (SLF), superior corona radiata, external capsula | 7.43(n) | |
C+: chemotherapy‐treated patients; P corr: p‐value corrected for multiple comparisons at cluster level.
(p): indicates a positive correlation; (n): indicates a negative correlation.
CFQ: cognitive failure questionnaire; BW: Bourdon‐Wiersma Dot cancellation test; TEA‐ST5: test of every day attention—auditory elevator; 9HPT: nine‐hole pegboard test; TMTA: trail making test, version A.
Figure 2.
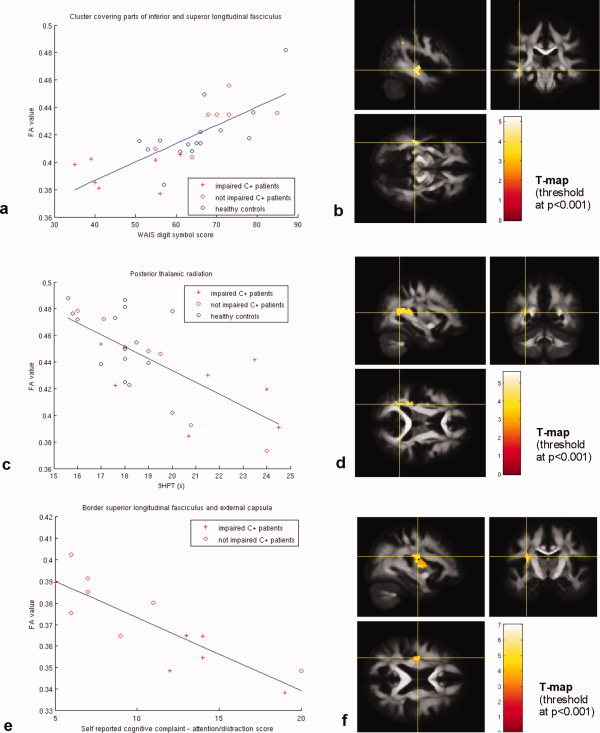
(a) Scatter plot of WAIS digit symbol scores and mean FA values in a cluster covering part of the superior and inferior longitudinal fasciculus. (b) Thresholded T‐map showing areas with significant positive correlation between WAIS digit symbol test score and FA values, overlaid on the mean FA image. (c) Scatter plot of nine hole pegboard test (9HPT) results and mean FA values in a cluster covering the posterior thalamic radiation. (d) Thresholded T‐map showing areas with significant negative correlation between 9HPT score and FA values, overlaid on the mean FA image. (e) Scatter plot of cognitive failure questionnaire (CQF)—distraction scores and mean FA values in resulting a cluster at the border of superior longitudinal fasciculus and external capsula. (f) Thresholded T‐map showing areas with significant negative correlation between CQF‐distraction score and FA value, overlaid on the mean FA image.
Correlation Analysis of the “Self‐Reported Cognitive Function” Measure With FA Values in Patients
Voxel‐based correlation analysis of FA maps and subjective self‐reported cognitive function scores revealed significant correlations in frontal and parietal regions. CFQ total and distraction scores correlated negatively with FA in frontal cerebral WM regions covering parts of SLF and superior corona radiata. The CFQ total score additionally correlated with FA in parietal corona radiata (Table IV and Fig. 2e,f). There was an inversely proportional relationship between self‐reported cognitive complaints (higher) and FA values in the identified regions (lower).
DISCUSSION
This study shows that WM integrity, as determined by quantitative DTI measures, of important WM tracts involved in cognition is significantly lower in chemotherapy‐treated breast cancer patients when compared with healthy controls or with nonchemotherapy‐treated patients. Moreover, the affected WM areas were much more extensive in the impaired patient group, in comparison with the unimpaired group. Additionally, performance on neuropsychological tests of attention and processing speed correlated significantly with the integrity of important temporal and parietal WM tracts, including the SLF and ILF. In these regions the impaired patient group had the lowest FA values. Interestingly, also subjective self‐reported cognitive complaints in patients correlated well with FA in frontal and parietal WM regions. All together, these results suggest that microstructural WM abnormalities in chemotherapy‐exposed brain may underlie cognitive dysfunctions.
The decreased WM integrity in chemotherapy‐treated patients could not be explained by differences in verbal IQ, age, anxiety, and depression scores. Major depression disorder has been associated with decreased FA in the ALIC and the inferior part of the SLF by Zou et al. [ 2008]. However, we included the BDI depression score and verbal IQ as covariates‐of‐no‐interest, and still observed significant differences in WM integrity. The same was true when adding age or anxiety scores as covariates of no interest.
The execution of complex cognitive tasks requires fast transfer of information between different brain regions [Mesulam, 1998, 2000] and damage to any part of the WM connections between these regions could in principle lead to changes in performance [Morris et al., 1994]. Various studies have linked neurodegenerative diseases and age‐related cognitive impairments with impaired WM integrity as reflected by decreased FA and increased MD values [Grieve et al., 2007; Huang et al., 2007; Lipton et al., 2008; Medina et al., 2006; Shim et al., 2008]. Several WM tracts reported in this study (e.g., SLF, ILF, IFOF, and SFOF) are long association tracts subserving functional integration among frontal, parietal, and temporal association cortices, needed for the execution of complex cognitive tasks [Schmahmann et al., 2008]. These tracts have been implicated in both normal cognitive functioning in healthy subjects and impairment of cognitive functioning in diseased subjects. For instance, a recent study in healthy subjects, reported a positive correlation between FA values and cognitive processing speed (WAIS digit‐Symbol test) in SLF, ILF, IFOF, SFOF, and corona radiata [Turken et al., 2008]. Cho et al. [ 2008], described altered FA and MD values in SLF, ILF, and internal capsula (IC) in mild cognitive impairment (MCI). Furthermore, Parente et al. [ 2008] reported significantly lower FA values in SLF in MCI patients. In temporal lobe epilepsy, FA decrease in IFOF was related to poorer verbal memory and word naming [McDonald et al., 2008]. In mild traumatic brain injury, microstructural WM lesions in ILF and corona radiata were associated with persistent cognitive deficits [Niogi et al., 2008].
Although this study does not provide a definite answer on what the etiology is of treatment‐induced cognitive impairment, it provides for the first time a quantitative measure that reflects cognitive complaints due to structural WM differences in patients. Direct neurotoxicity, causing toxic injury to brain parenchyma and producing WM demyelination, could be one of the factors explaining such cognitive impairment. To gain a better understanding of the observed changes in WM integrity, we also studied radial and parallel diffusivity (RD, PD). We revealed significantly higher values of RD in the WM of chemotherapy‐treated patients compared with controls, whereas no significant differences were found in PD. This observed pattern may be related to the demyelination of WM axons [Pierpaoli et al., 2001; Song et al., 2002].
We do not conclude that cytotoxic treatment would affect only one or two specific WM tracts, but rather find the evidence which suggests that there is a diffuse pattern of microstructural WM damage, resulting in subtle cognitive impairment in a subgroup of patients. We hypothesize that those patients not only have more affected WM, as demonstrated in this study, but that they also have less redundancy in the WM circuitry to compensate for the damaged WM when compared with the unimpaired patient group. Therefore these patients are more vulnerable to chemotherapy‐induced cognitive deficits.
Self‐reported cognitive assessment could not always be linked to objective performance on neuropsychological tests in previous studies [Castellon et al., 2004; Ferguson et al., 2007b]. In this study, we found correlations of subjective self‐reported cognitive scores with frontal and parietal WM integrity, suggesting that patients with more self‐reported cognitive dysfunction have more frontal and parietal WM damage. Because of the presence of extensive WM impairment in these patients, more effort may be required for comparable cognitive performance. It should be noted however, that our patients were scanned quite shortly after completion of the chemotherapy (129 days on average). Therefore we cannot determine whether these effects are reversible. Ahles et al. [ 2002], reported long term cognitive impairment for a subset of patients, while others [Inagaki et al., 2007; Wefel et al., 2004] described recovery for some patients. This heterogeneity in the longitudinal course of cognitive impairment may be related to the degree of WM changes.
Finally, some limitations of this study should be mentioned. These limitations mainly relate to the fact that we cannot completely rule out that the difference in cognitive performance between patients and controls can only be attributed to the chemotherapy treatment. First, no baseline data of the patients were acquired before the treatment. One study showed that cognitive functioning could already be impaired at baseline in women diagnosed with breast cancer [Ahles et al., 2008]. In this exploratory study, we have tried to match the patient and control group as well as possible, and we added an additional control group of breast cancer patients not treated with chemotherapy to address the potential impact of cancer diagnosis. In a future longitudinal study, we will also acquire pretreatment data to assess this possible baseline difference. Second, the explorative DTI analysis assessing WM integrity of impaired and unimpaired patients contains only small sample sizes in both groups and needs further confirmation in future analysis. Third, the major part of our C+ patient group (12 out of 14) additionally started hormonal treatment 1 month before the time of assessment. We cannot completely rule out the potential effect of antiestrogen agents on cognition. There is some evidence that tamoxifen may impair cognition [Castellon et al., 2004; Jenkins et al., 2007], although other studies reported no significant differences in cognitive performance between patients with and without antiestrogen treatment [Hermelink et al., 2008; Mar Fan et al., 2005]. This study seems to support the latter findings. As a major part of the additional control group of nonchemotherapy‐treated patients (9 out of 10) also just started hormonal treatment and we did not find any significant difference in WM integrity parameters between the C‐ patients and the healthy controls, our observations suggest that the subtle cognitive impairment that some cancer patients experience might be because of chemotherapy.
In summary, this study demonstrates that DTI provides WM integrity parameters that are sufficiently sensitive to investigate neural changes related to cognitive complaints of patients who underwent chemotherapy. Additionally, it could be a clinically useful tool to identify groups of patients who might benefit from cognitive behavioral training [Ferguson et al., 2007a] or the selection of appropriate pharmacological treatment [Rozans et al., 2002].
We have shown WM impairment in important WM tracts involved in cognition in chemotherapy‐treated patients compared with healthy controls and nonchemotherapy‐treated patients suggesting that there is a link between WM integrity and chemotherapy‐induced impaired cognition.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supplementary Material: Results neuropsychological tests
REFERENCES
- Abraham J, Haut MW, Moran MT, Filburn S, Lemiuex S, Kuwabara H ( 2008): Adjuvant chemotherapy for breast cancer: Effects on cerebral white matter seen in diffusion tensor imaging. Clin Breast Cancer 8: 88–91. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ ( 2007): Candidate mechanisms for chemotherapy‐induced cognitive changes. Nat Rev Cancer 7: 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, Whedon MB, Bivens S, Mitchell T, Greenberg ER, Silberfarb PM ( 2002): Neuropsychologic impact of standard‐dose systemic chemotherapy in long‐term survivors of breast cancer and lymphoma. J Clin Oncol 20: 485–493. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA ( 2008): Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat 110: 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Army Individual Test Battery ( 1944): Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General's Office. [Google Scholar]
- Bai F, Zhang Z, Watson DR, Yu H, Shi Y, Yuan Y, Qian Y, Jia J ( 2009): Abnormal integrity of association fiber tracts in amnestic mild cognitive impairment. J Neurol Sci 278: 102–106. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, Lebihan D ( 1994): Mr diffusion tenser spectroscopy and imaging. Biophys J 66: 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C ( 2002): The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed 15: 435–455. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher KS ( 1978): Multilingual Aphasia Examination. North Florida: Psychological Assessment Resources. [Google Scholar]
- Bosscher RJ, Koning H, Van MR ( 1986): Reliability and validity of the Beck depression inventory in a Dutch college population. Psychol Rep 58: 696–698. [DOI] [PubMed] [Google Scholar]
- Bourke RS, West CR, Chheda G, Tower DB ( 1973): Kinetics of entry and distribution of 5‐fluorouracil in cerebrospinal fluid and brain following intravenous injection in a primate. Cancer Res 33: 1735–1746. [PubMed] [Google Scholar]
- Broadbent DE, Cooper PF, FitzGerald P, Parkes KR ( 1982): The cognitive failures questionnaire (CFQ) and its correlates. Br J Clin Psychol 21 ( Part 1): 1–16. [DOI] [PubMed] [Google Scholar]
- Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA ( 2004): Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol 26: 955–969. [DOI] [PubMed] [Google Scholar]
- Cho H, Yang DW, Shon YM, Kim BS, Kim YI, Choi YB, Lee KS, Shim YS, Yoon B, Kim W, Ahn KJ ( 2008): Abnormal integrity of corticocortical tracts in mild cognitive impairment: A diffusion tensor imaging study. J Korean Med Sci 23: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi PM ( 1972): Human Memory and the Medial Temporal Region of the Brain, 34 ed University Microfilms, Mc GIII university: (No.AAI05‐77717). pp 891B. [Google Scholar]
- Engel AK, Fries P, Singer W ( 2001): Dynamic predictions: Oscillations and synchrony in top‐down processing. Nat Rev Neurosci 2: 704–716. [DOI] [PubMed] [Google Scholar]
- Ferguson RJ, Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Mott LA ( 2007a): Cognitive‐behavioral management of chemotherapy‐related cognitive change. Psychooncology 16: 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA ( 2007b): Brain structure and function differences in monozygotic twins: Possible effects of breast cancer chemotherapy. J Clin Oncol 25: 3866–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JJ, Raffa RB, Walker EA ( 2008): Effects of chemotherapeutic agents 5‐fluorouracil and methotrexate alone and combined in a mouse model of learning and memory. Psychopharmacology (Berl) 199: 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewelf F ( 1953): The Bourdon‐Wiersma test. Folia Psychiatr Neurol Psychiatr 56: 694–703. [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E ( 2007): Cognitive aging, executive function, and fractional anisotropy: A diffusion tensor MR imaging study. AJNR Am J Neuroradiol 28: 226–235. [PMC free article] [PubMed] [Google Scholar]
- Gutierrez R, Boison D, Heinemann U, Stoffel W ( 1995): Decompaction of CNS myelin leads to a reduction of the conduction velocity of action potentials in optic nerve. Neurosci Lett 195: 93–96. [DOI] [PubMed] [Google Scholar]
- Han R, Yang YM, Dietrich J, Luebke A, Mayer‐Proschel M, Noble M ( 2008): Systemic 5‐fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J Biol 7: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermelink K, Henschel V, Untch M, Bauerfeind I, Lux MP, Munzel K ( 2008): Short‐term effects of treatment‐induced hormonal changes on cognitive function in breast cancer patients: Results of a multicenter, prospective, longitudinal study. Cancer 113: 2431–2439. [DOI] [PubMed] [Google Scholar]
- Huang J, Friedland RP, Auchus AP ( 2007): Diffusion tensor imaging of normal‐appearing white matter in mild cognitive impairment and early Alzheimer disease: Preliminary evidence of axonal degeneration in the temporal lobe. AJNR Am J Neuroradiol 28: 1943–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, Akechi T, Wada N, Imoto S, Murakami K, Uchitomi Y ( 2007): Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer 109: 146–156. [DOI] [PubMed] [Google Scholar]
- Jenkins V, Shilling V, Deutsch G, Bloomfield D, Morris R, Allan S, Bishop H, Hodson N, Mitra S, Sadler G, Shah E, Stein R, Whitehead S, Winstanley J ( 2006): A 3‐year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer 94: 828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins V, Atkins L, Fallowfield L ( 2007): Does endocrine therapy for the treatment and prevention of breast cancer affect memory and cognition? Eur J Cancer 43: 1342–1347. [DOI] [PubMed] [Google Scholar]
- Kerr IG, Zimm S, Collins JM, O'Neill D, Poplack DG ( 1984): Effect of intravenous dose and schedule on cerebrospinal fluid pharmacokinetics of 5‐fluorouracil in the monkey. Cancer Res 44: 4929–4932. [PubMed] [Google Scholar]
- Klove H ( 1963): Clinical neuropsychology. Med Clin North Am 47: 1647–1658. [PubMed] [Google Scholar]
- Landman BA, Farrell JA, Jones CK, Smith SA, Prince JL, Mori S ( 2007): Effects of diffusion weighting schemes on the reproducibility of DTI‐derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5T. Neuroimage 36: 1123–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H ( 2001): Diffusion tensor imaging: Concepts and applications. J Magn Reson Imaging 13: 534–546. [DOI] [PubMed] [Google Scholar]
- Leemans A, Jones DK ( 2009): The B‐matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 61: 1336–1349. [DOI] [PubMed] [Google Scholar]
- Leemans A, Jeurissen B, Sijbers J, Jones DK ( 2009): ExploreDTI: A Graphical Toolbox for Processing, Analyzing, and Visualizing Diffusion MR Data. In: 17th Annual Meeting of Intl Soc Mag Reson Med, Hawaii, USA. pp 3537.
- Leemans A, Sijbers J, De Backer S, Vandervliet E, Parizel PM ( 2005): Affine coregistration of diffusion tensor magnetic resonance images using mutual information. Lecture Notes in Computer Science 3708: 523–530. [Google Scholar]
- Lipton ML, Gellella E, Lo C, Gold T, Ardekani BA, Shifteh K, Bello JA, Branch CA ( 2008): Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: A voxel‐wise analysis of diffusion tensor imaging. J Neurotrauma 25: 1335–1342. [DOI] [PubMed] [Google Scholar]
- Macleod JE, DeLeo JA, Hickey WF, Ahles TA, Saykin AJ, Bucci DJ ( 2007): Cancer chemotherapy impairs contextual but not cue‐specific fear memory. Behav Brain Res 181: 168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar Fan HG, Houede‐Tchen N, Yi QL, Chemerynsky I, Downie FP, Sabate K, Tannock IF ( 2005): Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1‐ and 2‐year follow‐up of a prospective controlled study. J Clin Oncol 23: 8025–8032. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Takayama T, Tashiro M, Nakamura Y, Ohashi Y, Shimozuma K ( 2005): Mild cognitive impairment after adjuvant chemotherapy in breast cancer patients—Evaluation of appropriate research design and methodology to measure symptoms. Breast Cancer 12: 279–287. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, Dale AM, Halgren E ( 2008): Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology 71: 1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina D, DeToledo‐Morrell L, Urresta F, Gabrieli JD, Moseley M, Fleischman D, Bennett DA, Leurgans S, Turner DA, Stebbins GT ( 2006): White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol Aging 27: 663–672. [DOI] [PubMed] [Google Scholar]
- Mesulam MM ( 1998): From sensation to cognition. Brain 121 ( Part 6): 1013–1052. [DOI] [PubMed] [Google Scholar]
- Mesulam MM ( 2000): Brain, mind, and the evolution of connectivity. Brain Cogn 42: 4–6. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van ZP, Mazziotta J ( 2008): Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40: 570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JG, Grattan‐Smith P, Panegyres PK, O'Neill P, Soo YS, Langlands AO ( 1994): Delayed cerebral radiation necrosis. Q J Med 87: 119–129. [PubMed] [Google Scholar]
- Muris P, Merckelbach H ( 1995): De Cognitive failures questionnaire (CFQ). Gedragstherapie 28: 123–128. [Google Scholar]
- Mustafa S, Walker A, Bennett G, Wigmore PM ( 2008): 5‐Fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur J Neurosci 28: 323–330. [DOI] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, Ghajar J, Johnson C, Kolster RA, Sarkar R, Lee H, Meeker M, Zimmerman RD, Manley GT, McCandliss BD ( 2008): Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: A 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol 29: 967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente DB, Gasparetto EL, da CL Jr, Domingues RC, Baptista AC, Carvalho AC, Domingues RC ( 2008): Potential role of diffusion tensor MRI in the differential diagnosis of mild cognitive impairment and Alzheimer's disease. AJR Am J Roentgenol 190: 1369–1374. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ ( 1996): Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 36: 893–906. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, DiChiro G ( 1996): Diffusion tensor MR imaging of the human brain. Radiology 201: 637–648. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix L, Virta A, Basser P ( 2001): Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage 13: 1174–1185. [DOI] [PubMed] [Google Scholar]
- Quesnel C, Savard J, Ivers H ( 2009): Cognitive impairments associated with breast cancer treatments: Results from a longitudinal study. Breast Cancer Res Treat 116: 129–130. [DOI] [PubMed] [Google Scholar]
- Rey A ( 1941): L'examen psychologie dans les cas d'encéphalopathie traumatique (les problèmes). Arch Psychol 28: 286–340. [Google Scholar]
- Rey A ( 1958): L'examin Clinique en Psychologie. Paris: Presses Universitaires de France. [Google Scholar]
- Robertson IH, Ward T, Ridgeway V, Nimmo‐Smith I ( 1996): The structure of normal human attention: The test of everyday attention. J Int Neuropsychol Soc 2: 525–534. [DOI] [PubMed] [Google Scholar]
- Rozans M, Dreisbach A, Lertora JJ, Kahn MJ ( 2002): Palliative uses of methylphenidate in patients with cancer: A review. J Clin Oncol 20: 335–339. [DOI] [PubMed] [Google Scholar]
- Sage CA, Van Hecke W, Peeters R, Sijbers J, Robberecht W, Parizel P, Marchal G, Leemans A, Sunaert S ( 2009): Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis: Revisited. Hum Brain Mapp 30: 3657–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagen SB, Muller MJ, Boogerd W, Mellenbergh GJ, van Dam FS ( 2006): Change in cognitive function after chemotherapy: A prospective longitudinal study in breast cancer patients. J Natl Cancer Inst 98: 1742–1745. [DOI] [PubMed] [Google Scholar]
- Schiavone F, Charlton RA, Barrick TR, Morris RG, Markus HS ( 2009a): Imaging age‐related cognitive decline: A comparison of diffusion tensor and magnetization transfer MRI. J Magn Reson Imaging 29: 23–30. [DOI] [PubMed] [Google Scholar]
- Schiavone F, Charlton RA, Barrick TR, Morris RG, Markus HS ( 2009b): Imaging age‐related cognitive decline: A comparison of diffusion tensor and magnetization transfer MRI. J Magn Reson Imaging 29: 23–30. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Smith EE, Eichler FS, Filley CM ( 2008): Cerebral white matter: Neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci 1142: 266–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmand B, Bakker D, Saan R, Louman J ( 1991): The dutch reading test for adults: A measure of premorbid intelligence level. Tijdschr Gerontol Geriatr 22: 15–19. [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC ( 1998): The mini‐international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 59 ( Suppl 20): 22–33. [PubMed] [Google Scholar]
- Shim YS, Yoon B, Shon YM, Ahn KJ, Yang DW ( 2008): Difference of the hippocampal and white matter microalterations in MCI patients according to the severity of subcortical vascular changes: Neuropsychological correlates of diffusion tensor imaging. Clin Neurol Neurosurg 110: 552–561. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, Waddell K, Petersen L, Phelps ME, Ganz PA ( 2007): Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant‐treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat 103: 303–311. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH ( 2002): Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17: 1429–1436. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL ( 1985): Assessment of state and trait anxiety: Conceptual and methodological issues. Southern Psychol 2: 6–16. [Google Scholar]
- Stroop J ( 1935): Studies of interference in serial verbal reactions. J Exp Psychol 18: 643–662. [Google Scholar]
- Tchen N, Juffs HG, Downie FP, Yi QL, Hu H, Chemerynsky I, Clemons M, Crump M, Goss PE, Warr D, Tweedale ME, Tannock IF ( 2003): Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. J Clin Oncol 21: 4175–4183. [DOI] [PubMed] [Google Scholar]
- Turken A, Whitfield‐Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD ( 2008): Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. Neuroimage 42: 1032–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Elst W, van Boxtel MP, van Breukelen GJ, Jolles J ( 2005): Rey's verbal learning test: Normative data for 1855 healthy participants aged 24–81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsychol Soc 11: 290–302. [DOI] [PubMed] [Google Scholar]
- Van Hecke W, Leemans A, D'Agostino E, De Backer S, Vandervliet E, Parizel PM, Sijbers J ( 2007): Nonrigid coregistration of diffusion tensor images using a viscous fluid model and mutual information. IEEE Trans Med Imaging 26: 1598–1612. [DOI] [PubMed] [Google Scholar]
- Vardy J, Rourke S, Tannock IF ( 2007): Evaluation of cognitive function associated with chemotherapy: A review of published studies and recommendations for future research. J Clin Oncol 25: 2455–2463. [DOI] [PubMed] [Google Scholar]
- Verhoeven J, Sage CA, Leemans A, Van Hecke W, Callaert D, Peeters R, De Cock P, Lagae L, Sunaert S ( 2010): Construction of a stereotaxic DTI atlas with full diffusion tensor information for studying white matter maturation from childhood to adolescence using tractography‐based segmentations. Hum Brain Mapp 31: 470–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D ( 1981): Wechsler Adult Intelligence Scale‐Revised. San Antonio: The Psychological Corporation. [Google Scholar]
- Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA ( 2004): The cognitive sequelae of standard‐dose adjuvant chemotherapy in women with breast carcinoma: Results of a prospective, randomized, longitudinal trial. Cancer 100: 2292–2299. [DOI] [PubMed] [Google Scholar]
- Yoshikawa E, Matsuoka Y, Inagaki M, Nakano T, Akechi T, Kobayakawa M, Fujimori M, Nakaya N, Akizuki N, Imoto S, Murakami K, Uchitomi Y ( 2005): No adverse effects of adjuvant chemotherapy on hippocampal volume in Japanese breast cancer survivors. Breast Cancer Res Treat 92: 81–84. [DOI] [PubMed] [Google Scholar]
- Zou K, Huang X, Li T, Gong Q, Li Z, Ou‐yang L, Deng W, Chen Q, Li C, Ding Y, Sun X ( 2008): Alterations of white matter integrity in adults with major depressive disorder: A magnetic resonance imaging study. J Psychiatry Neurosci 33: 525–530. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supplementary Material: Results neuropsychological tests


