Abstract
Sustained responsiveness to external stimulation is fundamental to many time‐critical interactions with the outside world. We used functional magnetic resonance imaging during speeded stimulus detection to identify convergent and divergent neural correlates of maintaining the readiness to respond to auditory, tactile, and visual stimuli. In addition, using a multimodal condition, we investigated the effect of making stimulus modality unpredictable. Relative to sensorimotor control tasks, all three unimodal detection tasks elicited stronger activity in the right temporo‐parietal junction, inferior frontal cortex, anterior insula, dorsal premotor cortex, and anterior cingulate cortex as well as bilateral mid‐cingulum, midbrain, brainstem, and medial cerebellum. The multimodal detection condition additionally activated left dorsal premotor cortex and bilateral precuneus. Modality‐specific modulations were confined to respective sensory areas: we found activity increases in relevant, and decreases in irrelevant sensory cortices. Our findings corroborate the modality independence of a predominantly right‐lateralized core network for maintaining an alert (i.e., highly responsive) state and extend previous results to the somatosensory modality. Monitoring multiple sensory channels appears to induce additional processing, possibly related to stimulus‐driven shifts of intermodal attention. The results further suggest that directing attention to a given sensory modality selectively enhances and suppresses sensory processing—even in simple detection tasks, which do not require inter‐ or intra‐modal selection. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: fMRI, response readiness, sustained attention, multimodal stimulation, simple reaction time, intrinsic alertness
INTRODUCTION
Many every‐day behaviors depend on our ability to stay responsive to signals from our environment. Failure to do so, for example, due to mental fatigue [Langner et al.,2010b ], can result in minor lapses but can also have catastrophic consequences, for instance for car drivers hitting the brake too late after noticing an obstacle. In keeping with several taxonomies of attentional functions, we refer to this responsiveness to external stimulation as “alertness” [e.g., Posner and Boies,1971; Raz and Buhle,2006; Sturm and Willmes,2001]. Short‐term (“phasic”) increases in alertness after warning cues have been differentiated from “intrinsic” alerting, which refers to the voluntary (endogenous) control of response readiness over seconds to minutes without the help of cues [Sturm et al.,1999; Sturm and Willmes,2001]. As such, intrinsic alertness is the most basic form of sustaining attention.
Alertness differs from the related concept of vigilance [Davies and Parasuraman,1982; Mackworth,1948] in two important ways: first, unlike vigilance, alertness does not predominantly focus on perceptual sensitivity but is equally concerned with motor readiness. As such, it is a broader concept than vigilance, comprising modulations of the entire stimulus–response chain. Second, on its perceptual side, alertness is only concerned with the most basic form of input receptivity, namely the speed of stimulus detection (without requiring stimulus identification), whereas vigilance is typically concerned with (the time‐related decline of) perceptual sensitivity for stimulus discrimination [requiring stimulus identification; See et al.,1995]. Alertness is also different from (generalized) arousal [Humphreys and Revelle,1984; Pfaff,2006], which has no direction in any way but modulates the intensity of alertness.
In neuropsychological assessment, alertness is usually measured with stimulus‐detection tasks that require an invariable, speeded response to stimuli that occur at unpredictable times. Such simple reaction‐time (RT) tasks have been chosen as operationalization of the “general responsiveness to external events,” since they constitute the most basic form of translating external sensory input into speeded motor output. This makes those tasks less susceptible to practice and strategy effects, which, in turn, ensures their sensitivity and validity for capturing fluctuations in attentiveness [Henderson and Dittrich,1998]. For this reason, speeded stimulus‐detection tasks are also frequently employed in chronobiological or sleep‐deprivation research [Blatter and Cajochen,2007; Lim and Dinges,2008]. However, despite the apparent simplicity and homogeneity of the concept of alertness, its typical operationalization (i.e., simple‐RT performance) is subserved by an interplay of multiple processes. By means of careful task analysis, we aimed to identify the neural correlates of several key processes involved in alert responding.
In simple‐RT tasks, all perceptual and motor processes can be prepared before stimulus/response onset, since there is no uncertainty about the stimuli and the response they require [Jennings and van der Molen,2005; Requin et al.,1991]. For sustained readiness, maintaining (or repeatedly reactivating) this task‐specific preparatory set over time is essential. This includes maintaining the relevant stimulus–response mapping, sustained sensory anticipation, and maintaining a balance between motor preparation and inhibition to avoid premature responding. Second, response speed in simple‐RT tasks with variable interstimulus intervals benefits from building implicit temporal expectations based on the temporal structure of previous stimuli [Coull and Nobre,2008; Langner et al.,2010a]. Finally, preparation for speeded action is assumed to include the regulation of arousal, which determines the general responsiveness of the brain and “energizes” cognitive processes [Pfaff,2006; Sturm et al.,1999; Stuss,2006]. The optimal interplay of these processes should result in maximal response readiness (i.e., high alertness).
Studies in patients suffering from brain damage revealed a dominant role of the right hemisphere (RH) in the control of alertness. For instance, Howes and Boller [1975], Ladavas [1987], and Posner et al. [1987] reported a substantial increase in simple visual and auditory RT following RH lesions. However, when testing phasic alerting with forewarned simple‐RT tasks, RH patients showed much smaller performance deficits [Posner et al.,1987; Tartaglione et al.,1986]. This suggests that RH lesions mainly impair the control of intrinsic, and not phasic, alertness.
The number of brain imaging studies on alertness is rather limited, but their results point to a comparable differentiation between brain mechanisms underlying warning‐cue‐induced (phasic) versus self‐regulated (intrinsic) alertness. For instance, Sturm and colleagues [Sturm et al.,1999,2004] found in two studies with positron emission tomography (PET) a predominantly RH network subserving intrinsic alertness. The network included right anterior cingulate cortex (ACC), right dorsolateral prefrontal cortex, right inferior parietal lobule as well as thalamic and brainstem structures (possibly including the locus coeruleus) for both visual and auditory intrinsic alertness tasks. A recent functional magnetic resonance imaging (fMRI) study largely replicated these findings [Périn et al.,2010]: a visual alertness task activated, relative to a sensorimotor control task, right prefrontal, parietal, and anterior cingulate cortices as well as right thalamus, putamen, and brainstem.
In contrast, and consistent with the aforementioned findings in patients, studies on phasic alerting revealed (additional) left‐hemisphere activity. Using fMRI, Sturm and Willmes [2001] reported right and left frontal and parietal activations in forewarned auditory detection tasks. Bilateral activity was also reported in other fMRI studies on phasic alerting [Fan et al.,2005; Konrad et al.,2005; Thiel et al.,2004; Thiel and Fink,2007]. Mainly left‐sided activity related to phasic alerting was found in anterior insula, dorsal premotor cortex, and superior and inferior parietal cortex in a study by Coull et al. [2001]. The differences in brain activity between phasic and intrinsic alerting, which may be attributed to several task factors (see Discussion for details), complicate generalizations.
The question as to the modality independence of brain activity related to maintaining response readiness has been discussed previously, since some modality‐related differences had been found [Sturm and Willmes,2001]. To our knowledge, there is only one study [Kinomura et al.,1996] that tested intrinsic alerting with stimuli of different sensory modalities (visual and tactile) in the same participants. In this pioneering study, however, alertness and sensorimotor‐control tasks were performed by two different and rather small samples (n = 9 each). More importantly, the study only focused on subcortical areas; no cortical activity was reported. Apart from that, there appears to exist only one other imaging study that investigated the modality independence of alertness‐related brain activity [Thiel and Fink,2007]. Focusing on phasic alertness, the authors contrasted cued with uncued trials of simple‐RT tasks with visual or auditory stimuli and found the only modality‐independent activity in the posterior part of the right superior temporal gyrus.
Furthermore, it is plausible to assume that to maintain high responsiveness to a predefined stimulus, participants are likely to focus their attention on the given stimulus modality. Although previous intrinsic‐alertness studies [Périn et al.,2010; Sturm et al.,1999,2004] did not report any alertness‐related activity in sensory cortices, relative to sensorimotor control tasks, modulations of brain activity by modality‐specific top–down attention have been shown in studies using bi‐ or tri‐modal stimulation. There, participants were required to direct their attention to one out of two or three modalities to perform a discrimination task within a given modality while ignoring simultaneously presented stimuli of another modality [Kawashima et al.,1999; Macaluso et al.,2002,2003; Roland,1982; Shomstein and Yantis,2004]. That is, these studies did not only require the mere focusing on a given modality (as in alertness tasks) but required intermodal selection (selection of one modality vs. another in the presence of distraction) as well as intramodal selection (within‐modality discrimination). Some of these studies additionally included demands on spatial attention. Furthermore, cross‐modal attention‐related deactivations have been observed in sensory cortices that subserve the processing of irrelevant sensory input [Johnson and Zatorre,2006; Mozolic et al.,2008].
These differences between intrinsic‐alertness and selective‐attention studies might imply that significant modality‐specific attentional modulations depend on the joint impact of several simultaneous attentional demands. However, the null findings in previous intrinsic‐alertness studies might also be related to methodological differences: alertness conditions, which contained relatively few stimuli, were compared with sensorimotor control tasks, in which sensory stimulation was provided at a much higher rate. This difference in presentation rate may have produced increased activity in sensory cortices, preventing the detection of alertness‐related modulations of sensory processing in a subtraction analysis [Rinne et al.,2005]. In contrast, in the aforementioned studies on selective attention, modality‐specific effects were assessed by comparing the different modality conditions with each other. Therefore, we investigated whether or not modality‐specific modulations in sensory cortices are also produced by directing attention to a given modality in the absence of any selection demands.
In summary, our study investigated modality‐independent and modality‐specific modulations of brain activity by intrinsic alertness using auditory, tactile, and visual stimuli in simple, uncued RT tasks performed by the same participants. We expected to find a common right‐lateralized network across modalities as well as modality‐specific activations and deactivations in relevant and irrelevant sensory cortices, respectively. In addition, we were interested in the effects of increased monitoring demands, which were manipulated by introducing a condition in which the modality of the upcoming stimulus was unpredictable. To the best of our knowledge, this is the first investigation of brain activity related to the simultaneous monitoring for stimuli from three different modalities unpredictably presented one at a time and without any selection demands.
METHODS
Participants
Twenty (nine female) healthy, right‐handed university students (mean age = 24.0 years, SD = 3.5) were recruited via advertisements on campus and were paid for their participation in the experiment. The study complied with the ethical standards laid down in the Declaration of Helsinki and was approved by the Ethics Committee of the RWTH Aachen University Hospital. All participants gave written informed consent before entering the study.
Tasks and Procedure
The experiment was run on a standard personal computer using the software Presentation 10.0 (Neurobehavioral Systems, http://www.neurobs.com). The experiment comprised four experimental (auditory, tactile, visual, and mixed) and three sensorimotor control (auditory, tactile, and visual) conditions. In all experimental conditions, the task was to respond as fast as possible to the imperative stimulus by a button press with the right‐hand index finger. In the auditory condition, the imperative stimulus was a 1,000‐Hz sine tone (70 dB), presented binaurally via noise‐shielded, MRI‐compatible headphones. The tactile imperative stimulus was a vibration generated by the repeated 1‐mm extension of eight blunt plastic rods (each generating a force of about 5 mN) from two MRI‐compatible Braille stimulators strapped to the inner side of each ring finger's upper phalanx. The visual imperative stimulus consisted of two white squares (5.7° visual angle each) to the left and right of a central fixation cross, presented via MRI‐compatible goggles. All imperative stimuli were bilaterally presented for 500 ms with a frequency of 10 Hz (five 50‐ms‐on/50‐ms‐off cycles each). This was done to maximize comparability between modalities, since by their nature, vibrotactile stimuli can only be presented in a cyclic on/off fashion. In the mixed condition, stimuli of all three modalities were presented in an unpredictably mixed way. The interstimulus intervals in all experimental conditions varied randomly between 1,350 and 5,100 ms (Mean = 2,785 ms), sampled from an exponential distribution.
In the three sensorimotor control conditions (auditory, tactile, and visual), the same stimuli were used but were presented continuously throughout the block. Participants were instructed to perceive the stimulation without paying attention to it and to press the response button with their right index finger approximately every 2 s in a self‐paced manner, independently of the sensory input [Sturm et al.,2004]. These control conditions were supposed to capture the purely sensory and motor aspects of the experimental conditions. The continuous high‐rate stimulation was necessary to prevent participants from synchronizing their button presses with the stimuli, which would have turned the control conditions into another alertness task [Sturm et al.,1999]. The button‐press rate of about 0.5 Hz was chosen to approximately match the response rate during the experimental tasks and to induce routinization, thereby minimizing alertness demand. Thus, the control tasks required neither maintaining responsiveness (i.e., increased perceptual receptivity and motor readiness) nor responding (i.e., stimulus–response translation) to external events, which enabled us to isolate subprocesses specific to maintaining efficient performance in simple‐RT tasks.
From an information‐theoretic perspective, the highly repetitive stream of irrelevant sensory input during the control tasks is devoid of information and surprise. This contrasts with the RT tasks, which are, due to the temporally unpredictable presentation of relevant signals, relatively information‐rich. According to recent theorizing on brain arousal [Pfaff,2006], absence of information renders situations de‐arousing. RT‐task performance, therefore, should be associated with higher levels of nonspecific arousal than performing the information‐poor sensorimotor control tasks. Finally, any task features that might be specific to the control conditions (e.g., the need to self‐initiate the finger movement or the more frequent sensory input) were encountered by restricting the analysis of differences to those voxels that showed “genuine” task activations relative to resting baseline.
All conditions were presented in separate sessions, each consisting of six 20‐s task blocks and seven 20‐s resting‐baseline blocks. Each experimental task block contained seven imperative stimuli. Throughout all task blocks, a central fixation cross was presented; its disappearance indicated the beginning of a baseline block. Toward the end of each baseline block (between 1,500 and 3,500 ms before task‐block onset), an auditory 2,000‐Hz sine tone was presented as a warning signal indicating the imminent beginning of the next task block. This was done to help participants relax their alertness during the resting blocks. Before each session, the type of condition was announced by the experimenter. Condition order was chosen at random for each participant. Before the experiment, participants were given 10 practice trials of each experimental condition.
fMRI Data Acquisition
Brain imaging data were obtained with a 3T MRI scanner (Philips Achieva, Philips Medical Systems, Best, The Netherlands) with a SENSE head coil. Participants lay supine in the scanner, and their heads were immobilized with cushions to minimize movements. A T1‐weighted structural image was used for anatomical reference (TE = 2.3 ms, TR = 506 ms, flip angle = 80°, matrix size = 240 × 240, 32 sagittal slices, voxel size = 0.94 × 1.17 × 4 mm3, 1 mm gap between slices). Blood oxygenation level‐dependent (BOLD) signals were acquired using echo‐planar imaging (EPI) covering the whole brain in 28 transverse slices parallel to the AC/PC line (TE = 32 ms, TR = 2.0 s, flip angle = 80°, SENSE factor = 1.3, matrix size = 64 × 74, field of view = 192 × 228 mm2, voxel size = 3 × 3 × 3.6 mm3, 0.8 mm gap between slices, interleaved slice acquisition). During each run, 133 volumes were acquired, preceded by seven dummy scans.
fMRI Data Analysis
Data were analyzed with the SPM5 software package (Wellcome Department of Imaging Neuroscience, London) implemented in Matlab 7.2 (The MathWorks, Sherborn, MA). After discarding the dummy scans, EPI images were corrected for head movement by affine registration using a two‐pass procedure by which images were initially realigned to the first image and subsequently to the mean of the realigned images. Spatial normalization into standard stereotaxic MNI (Montreal Neurological Institute) space was achieved by applying the “unified segmentation” procedure of SPM5 to the mean EPI of all runs using the segmented structural MNI single‐subject template as tissue probability map and then applying the normalization parameters to all EPI images (resampled voxel size: 2 × 2 × 2 mm3). The normalized EPI data were smoothed with an isotropic Gaussian filter of 8 mm to accommodate assumptions of random‐field theory as well as remaining interindividual variation in brain anatomy.
The statistical analysis of the data was done according to a block‐design approach: The expected hemodynamic response for each block was modeled by a canonical hemodynamic response function [HRF; Friston et al.,1998]. This function was convolved with block onsets (defined as the onset of the alerting warning stimulus that indicated the end of the resting block) and block durations to create predictors in a general linear model. We also modeled the parametric modulation of task‐related hemodynamic activity by mean‐centered reaction time (mean RT of each experimental block). Low‐frequency signal drifts were filtered using a cutoff period of 128 s. To reduce residual variance in the time series (induced, e.g., by head movements), a dispersion measure of each volume from the respective mean of the time series was entered as covariate of no interest [see Stöcker et al.,2005, for details]. After correction of the time series for dependent observations according to an autoregressive first‐order correlation structure, parameter estimates of the HRF regressors were calculated from the least‐mean‐squares fit of the model to the time series. Group analysis was done by entering parameter estimates of all 11 regressors of interest (four experimental task conditions and their parametric modulations plus three sensorimotor‐control conditions) into a random‐effects repeated‐measurement analysis of variance (ANOVA). Alertness‐related activity was analyzed by contrasting activity in experimental task conditions with activity in sensorimotor control conditions. Modality‐specific modulations of brain activity were tested by contrasting alertness‐related activity associated with a given stimulus modality against alertness‐related activity associated with the other two modalities. Generally, activity differences were considered significant when surviving a single‐voxel threshold of P < 0.001 and a cluster‐level threshold of P < 0.05, family‐wise error (FWE) corrected for multiple comparisons across the whole brain.
RESULTS
Behavioral Data
Mean reaction time (standard deviation) was 272 (49) ms in the auditory condition, 240 (46) ms in the tactile condition, 274 (45) ms in the visual condition, and 313 (52) ms in the mixed condition (averaged across modalities). Separate averaging for each modality within the mixed condition yielded 351 (60) ms for auditory targets, 267 (57) ms for tactile targets, and 321 (51) ms for visual targets, largely repeating the pattern of the blocked presentation at a higher RT level. A repeated‐measures ANOVA yielded a significant effect of modality condition (auditory, tactile, visual, and mixed) on mean RT [F (3, 57) = 30.6, P < 0.001]. Simple contrasts revealed that this effect was driven by both significantly faster responses to tactile stimuli than to auditory ones [F (1, 19) = 19.6, P < 0.001] and significantly slower responses in the mixed condition than in the slowest unimodal (i.e., visual) one [F (1, 19) = 18.8, P < 0.001]. The RT values lie in the typical range of simple‐RT tasks, confirming that our participants responded as instructed. Also, the significantly longer RT in the mixed condition attests to the higher processing load imposed by monitoring three sensory channels simultaneously, reproducing the well‐known behavioral effect of mixed stimulus presentation [Los,1996].
Imaging Data
Modality‐independent effects
To identify supramodal activity related to maintaining responsiveness to external events, the main effect across all three unimodal RT‐task conditions was contrasted against the main effect across all three sensorimotor control conditions, applying a mask to only retain voxels whose activation during each single RT task was stronger than during both baseline and their respective sensorimotor control task. Results are presented in Table I and Figure 1; contrast estimates for peak voxels are shown in Figure 2. The analysis revealed significant right‐sided activity in the supplementary motor area (SMA), pre‐SMA, dorsal premotor cortex (dPMC), anterior cingulate cortex (ACC), inferior frontal cortex (pars opercularis and pars triangularis), anterior insula, and temporo‐parietal junction. Further significant activity was found in bilateral rostral motor cingulate areas and left medial cerebellum. The cerebellar cluster extended into the brainstem (pontine reticular formation). Finally, a subcortical cluster comprised activity in bilateral rostrodorsal pons (possibly locus coeruleus) and midbrain (vicinity of nucleus ruber, ventral tegmental area, and substantia nigra).
Table I.
Supramodal alertness‐related brain activity
| Cluster/Area | x, y, z | z score |
|---|---|---|
| Cerebellum/pons (k = 772, P < 0.001) | ||
| L cerebellar vermis | −6, −48, −24 | 5.84 |
| R dorsal pons (reticular formation) | 14, −34, −32 | 4.34 |
| L cerebellar intermediate hemisphere | −18, −50, −24 | 4.21 |
| R medial pons (reticular formation) | 6, −22, −34 | 3.86 |
| Medial frontal/cingulate cortex (k = 706, P < 0.001) | ||
| R supplementary motor area (BA 6) | 8, 18, 50 | 4.60 |
| R anterior cingulate cortex | 4, 38, 24 | 4.01 |
| L middle cingulate cortex | −2, 6, 34 | 3.84 |
| R pre‐supplementary motor area (BA 8) | 10, 32, 46 | 3.82 |
| R middle cingulate cortex | 6, −6, 34 | 3.62 |
| L middle cingulate cortex | −6, 16, 32 | 3.44 |
| L anterior cingulate cortex | −1, 24, 30 | 3.40 |
| Temporo‐parietal junction (k = 593, P < 0.001) | ||
| R inferior parietal cortex | 50, −50, 22 | 4.11 |
| R superior temporal gyrus | 50, −46, 14 | 3.73 |
| R superior temporal gyrus | 56, −42, 12 | 3.43 |
| Precentral gyrus (k = 204, P = 0.024) | ||
| R dorsal premotor cortex (BA 6) | 36, −4, 46 | 4.45 |
| Inferior frontal/insular cortex (k = 189, P = 0.031) | ||
| R inferior frontal gyrus (pars triangularis) | 42, 22, 4 | 4.15 |
| R anterior insula | 39, 24, 0 | 3.70 |
| Midbrain/brainstem (k = 179, P = 0.038) | ||
| R substantia nigra | 16, −14, −10 | 3.85 |
| R nucleus ruber | 10, −18, −12 | 3.74 |
| R rostrodorsal pons (locus coeruleus) | 2, −28, −18 | 3.71 |
| L rostrodorsal pons (locus coeruleus) | −4, −26, −18 | 3.46 |
| L nucleus ruber | −6, −20, −12 | 3.37 |
| L ventral tegmental area | −4, −16, −14 | 3.30 |
Coordinates x, y, and z refer to MNI space; k = number of voxels in cluster; P value represents cluster‐level error probability FWE‐corrected for multiple comparisons.
L, left; R, right; BA, Brodmann area.
Figure 1.
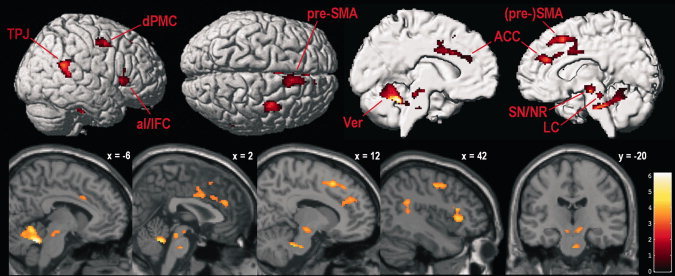
Supramodal alertness‐related brain activity (averaged unimodal alertness conditions vs. averaged control conditions, masked to only include voxels that showed stronger activity during each unimodal alertness condition than during resting baseline and the respective control condition). TPJ, temporo‐parietal junction; dPMC, dorsal premotor cortex; aI/IFC, anterior insula/inferior frontal cortex; pre‐SMA, pre‐supplementary motor area; Ver, cerebellar vermis; ACC, anterior cingulate cortex; SN/NR, substantia nigra/nucleus ruber; LC, locus coeruleus. Parasagittal slices show activity overlaid over the SPM5 single‐subject template brain; coordinates refer to MNI space; color codes t values; voxel‐wise P < 0.001 and FWE‐corrected cluster‐level P < 0.05.
Figure 2.

Activation level in peak voxels of eight key regions of the supramodal alertness network during uni‐ and multi‐modal intrinsic alerting as well as sensorimotor control conditions. Error bars show the standard error of the mean. A., alertness; C., sensorimotor control; Aud., auditory; Tact., tactile; Vis., visual; Mult., multimodal (mixed). For a list of abbreviations of the anatomical labels, see Figure 1. Coordinates refer to MNI space.
Modality‐specific effects
Selective increases in brain activity during each unimodal RT‐task condition were analyzed by calculating balanced contrasts between one unimodal task condition and the other two conditions. Again, each contrast was masked to only include voxels that showed stronger activity during the RT task than during both baseline and its respective sensorimotor control condition. The results of these analyses are reported in Table II and Figure 3. Generally, modality‐specific activity was only found in areas specialized in processing signals of a given sensory modality: Contrasting auditory with somatosensory and visual alertness revealed stronger bilateral activity in posterior aspects of the superior and middle temporal gyri [Brodmann areas (BAs) 41, 42, and 22; see Fig. 3A]. These areas correspond to auditory core and belt regions. Comparing somatosensory with auditory and visual alertness revealed stronger bilateral activity in the postcentral gyrus (BAs 1 and 2), parietal operculum, and supramarginal gyrus, with activity being more pronounced in the right hemisphere (see Fig. 3B). These regions correspond to primary (S1), secondary (S2), and higher‐order somatosensory cortices. Finally, contrasting visual with auditory and somatosensory alertness revealed stronger bilateral activity in superior, middle, and inferior occipital gyri, fusiform gyrus, caudal superior and inferior parietal lobules, and inferior temporal gyrus (see Fig. 3C). In addition, activity was found in right cuneus (BAs 18 and 19) and lingual gyrus. These regions correspond to secondary (V2) and higher‐order visual association areas of both the dorsal and ventral visual‐processing streams.
Table II.
Modality‐specific activations during unimodal intrinsic alerting
| Cluster/Area | x, y, z | z score |
|---|---|---|
| Auditory alertness | ||
| Cluster 1 (k = 306, P = 0.004) | ||
| L posterior superior temporal gyrus | −52, −30, 8 | 7.73 |
| L posterior middle temporal gyrus | −66, −28, 6 | 7.03 |
| L posterior superior temporal sulcus | −54, −30, 4 | 6.38 |
| Cluster 2 (k = 169, P = 0.047) | ||
| R posterior superior temporal gyrus | 62, −26, 4 | 6.50 |
| R posterior middle temporal gyrus | 64, −30, −2 | 4.68 |
| R posterior superior temporal sulcus | 50, −30, 0 | 3.57 |
| Tactile alertness | ||
| Cluster 1 (k = 1,043, P < 0.001) | ||
| R Rolandic operculum (S2) | 46, −20, 16 | 6.18 |
| R postcentral sulcus (BA 2/5) | 44, −36, 56 | 4.72 |
| R supramarginal gyrus | 54, −34, 48 | 4.21 |
| R postcentral sulcus (BA 2/40) | 44, −26, 40 | 4.09 |
| R postcentral gyrus (BA 1; S1) | 32, −42, 66 | 3.86 |
| Cluster 2 (k = 405, P = 0.001) | ||
| L Rolandic operculum (S2) | −54, −18, 18 | 4.22 |
| L postcentral gyrus (BA 2/7) | −40, −30, 46 | 3.95 |
| L supramarginal gyrus | −56, −28, 22 | 3.85 |
| L postcentral sulcus (BA 2/5) | −44, −34, 52 | 3.75 |
| L postcentral sulcus (BA 2/40) | −36, −32, 40 | 3.62 |
| Visual alertness | ||
| Cluster 1 (k = 1,174, P < 0.001) | ||
| R middle occipital gyrus | 30, −80, 24 | 7.58 |
| R lateral middle occipital gyrus | 42, −68, 10 | 6.80 |
| R superior parietal lobule (BA 7A) | 26, −60, 52 | 4.35 |
| R superior parietal lobule (BA 7P) | 26, −74, 48 | 3.98 |
| R cuneus (BA 19) (V3) | 12, −86, 28 | 3.95 |
| R cuneus (BA 18) (V2) | 10, −90, 24 | 3.80 |
| Cluster 2 (k = 995, P < 0.001) | ||
| L fusiform gyrus | −46, −62, −16 | 6.48 |
| L middle occipital gyrus | −38, −80, 2 | 5.64 |
| L inferior occipital gyrus (V4) | −30, −86, −12 | 3.68 |
| L inferior temporal gyrus | −56, −52, −16 | 3.18 |
| Cluster 3 (k = 578, P < 0.001) | ||
| L middle occipital gyrus | −30, −78, 20 | Inf. |
| L lateral middle occipital gyrus | −44, −72, 10 | 5.22 |
| Cluster 4 (k = 372, P = 0.001) | ||
| R inferior occipital gyrus | 38, −66, −8 | 5.80 |
| R inferior temporal gyrus | 42, −52, −14 | 4.49 |
| R fusiform gyrus | 32, −42, −20 | 3.32 |
| Cluster 5 (k = 292, P = 0.005) | ||
| L superior parietal lobule (BA 7A) | −26, −64, 56 | 5.38 |
| Cluster 6 (k = 262, P = 0.008) | ||
| R inferior occipital gyrus | 34, −76, −4 | 5.74 |
| R middle occipital gyrus | 40, −80, 2 | 5.60 |
| R lingual gyrus | 24, −84, −4 | 5.23 |
Coordinates x, y, and z refer to MNI space; k = number of voxels in cluster; P value represents cluster‐level error probability FWE‐corrected for multiple comparisons.
L, left; R, right; BA, Brodmann area.
Figure 3.
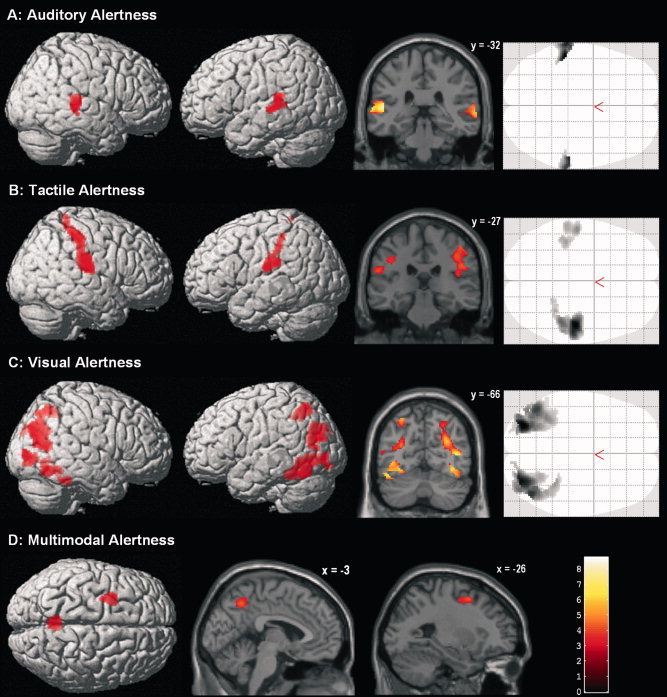
A–C: Modality‐specific activations during unimodal intrinsic alerting (one unimodal alertness condition vs. the other two unimodal alertness conditions, respectively; masked to only include voxels that showed stronger activity during each unimodal alertness condition than during resting baseline and the respective control condition). D: Alertness‐related brain activity specific to the unpredictably mixed presentation of stimuli of different modalities (multimodal condition vs. all three unimodal alertness conditions, masked to only include voxels that showed stronger activity during the multimodal condition than during resting baseline and each single unimodal condition). Coronal and parasagittal slices show activity overlaid over the SPM5 single‐subject template brain; coordinates refer to MNI space; color codes t values; voxel‐wise P < 0.001 and FWE‐corrected cluster‐level P < 0.05.
Modality‐specific cross‐modal deactivations were analyzed by pair‐wise contrasts between unimodal RT‐task conditions to find voxels that were less activated in one modality condition than in another, only including voxels that were truly deactivated below baseline during the RT task of interest (i.e., the subtrahend). The deactivation effects were much smaller than the activation ones; none of them survived our strict cluster‐level threshold. However, since these analyses were driven by specific hypotheses about modality‐selective effects in sensory cortices, we tested the results without applying a cluster‐level correction for multiple comparisons; minimum cluster size was set to k = 20 voxels. Foci of modality‐specific deactivations are listed in Table III, and composite renderings are presented in Figure 4. The analyses revealed cross‐modal deactivations during auditory alerting in right somatosensory cortex and bilateral visual areas (see Fig. 4A). During tactile alerting, deactivations below resting baseline levels were found in bilateral auditory and visual cortices (see Fig. 4B). Finally, during visual alerting, cross‐modal deactivations were found in right somatosensory and auditory cortices (see Fig. 4C).
Table III.
Modality‐specific deactivations during unimodal intrinsic alerting
| Cluster/Area | x, y, z | z score |
|---|---|---|
| Auditory alertness | ||
| Cluster 1 (k = 243) | ||
| R postcentral gyrus (BA 2) | 42, −36, 56 | 4.34 |
| R postcentral gyrus (BA 1) | 32, −42, 66 | 3.93 |
| R postcentral gyrus (BA 5/7) | 48, −26, 40 | 3.92 |
| Cluster 2 (k = 739) | ||
| R lingual gyrus (BA 18) | 16, −82, −10 | 7.56 |
| R fusiform gyrus | 30, −62, −10 | 5.64 |
| R fusiform gyrus | 30, −42, −20 | 3.79 |
| Cluster 3 (k = 1,368) | ||
| L middle occipital gyrus | −30, −78, 20 | 7.23 |
| L cuneus (BA 17/18) | −8, −94, 14 | 4.56 |
| L superior occipital gyrus | −24, −80, 42 | 3.44 |
| Cluster 4 (k = 1,220) | ||
| R middle occipital gyrus | 30, −80, 22 | 6.95 |
| R superior occipital gyrus | 26, −82, 28 | 6.68 |
| R cuneus (BA 18) | 18, −94, 18 | 6.22 |
| Cluster 5 (k = 614) | ||
| L fusiform gyrus | −42, −66, −14 | 5.99 |
| L lingual gyrus (BA 18) | −12, −82, −10 | 4.86 |
| L fusiform gyrus | −26, −52, −12 | 3.41 |
| Cluster 6 (k = 96) | ||
| L superior parietal lobule (BA 7) | −28, −62, 52 | 4.66 |
| Cluster 7 (k = 56) | ||
| R superior parietal lobule (BA 7) | 24, −58, 52 | 3.66 |
| Tactile alertness | ||
| Cluster 1 (k = 39) | ||
| L middle temporal gyrus | −54, −20, −2 | Inf. |
| Cluster 2 (k = 27) | ||
| R middle temporal gyrus | 58, −10, −6 | 6.01 |
| Cluster 3 (k = 4,981) | ||
| R lingual gyrus (BA 18) | 18, −82, −10 | 7.41 |
| R middle occipital gyrus | 30, −82, 20 | 6.98 |
| L middle occipital gyrus | −30, −78, 20 | 6.95 |
| R superior occipital gyrus | 28, −78, 32 | 6.23 |
| R cuneus (BA 17/18) | 10, −82, 2 | 6.17 |
| L lingual gyrus (BA 18) | −10, −86, −6 | 5.93 |
| L superior occipital gyrus | −16, −94, 14 | 5.78 |
| L cuneus (BA 17/18) | −8, −84, 2 | 4.78 |
| L fusiform gyrus | −34, −48, −18 | 4.52 |
| R fusiform gyrus | 32, −48, −18 | 3.94 |
| Cluster 4 (k = 118) | ||
| L superior parietal lobule (BA 7) | −26, −64, 56 | 5.02 |
| Cluster 5 (k = 27) | ||
| R superior parietal lobule (BA 7) | 24, −56, 50 | 4.04 |
| Cluster 6 (k = 54) | ||
| R precentral gyrus (BA 4) | 34, −76, −4 | 5.74 |
| Visual alertness | ||
| Cluster 1 (k = 170) | ||
| R superior temporal gyrus | 50, −18, 2 | Inf. |
| R superior temporal gyrus | 58, −10, −4 | 6.26 |
| Cluster 2 (k = 20) | ||
| R posterior insula | 50, −26, 30 | 4.49 |
| Cluster 3 (k = 40) | ||
| R parietal operculum | 38, −14, 12 | 4.31 |
| Cluster 4 (k = 30) | ||
| R superior temporal gyrus (BA 41/42) | 48, −18, 8 | 4.29 |
| Cluster 5 (k = 26) | ||
| R postcentral gyrus (BA 2) | 46, −34, 56 | 4.14 |
For each modality, deactivations from two separate unimodal contrasts are combined (see text for details). Coordinates x, y, and z refer to MNI space; k = number of voxels in cluster.
L, left; R, right; BA, Brodmann area.
Figure 4.
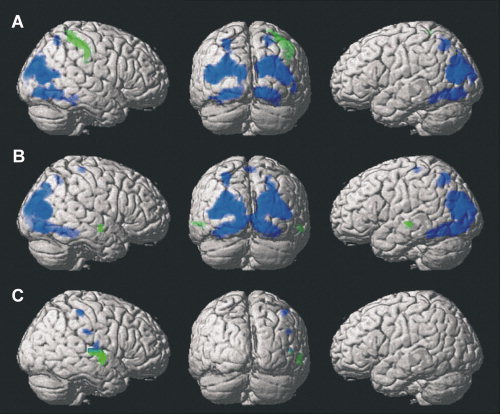
Modality‐specific deactivations during unimodal intrinsic alerting. Contrasts are masked to only include voxels that showed a deactivation below resting baseline. Voxel‐wise P < 0.001, k = 20. A: Auditory alertness (green: auditory < tactile alertness; blue: auditory < visual alertness). B: Tactile alertness (green: tactile < auditory alertness; blue: tactile < visual alertness). C: Visual alertness (green: visual < auditory alertness; blue: visual < tactile alertness).
Effects of multimodal response readiness
Activity related to the multimodal monitoring for external response signals was examined via a balanced contrast between the mixed‐modalities condition and all three sensorimotor control conditions, using a mask to only include voxels with more activity during multimodal alertness than during baseline. In general, the pattern of results was similar to the supramodal network described above but included additional foci of significant activity (see Table IV). The analysis revealed bilateral activity in dPMC, precuneus, and posterior aspects of middle and superior temporal gyri, which, at the right side, extended into inferior parietal cortex. Right‐sided activity was found in inferior frontal cortex (pars opercularis and pars triangularis), inferior parietal lobule and angular gyrus. Activity in right pre‐SMA was found to be slightly above our cluster threshold (corrected cluster‐level P = 0.085). Other areas with significant supramodal alertness‐related activity reported above (right SMA, anterior insula, and ACC; left medial cerebellum; bilateral mid‐cingulum, midbrain, and brainstem) were only active at a lower level (uncorrected voxel‐wise P < 0.01).
Table IV.
Effects of multimodal alertness
| Cluster/Area | x, y, z | z score |
|---|---|---|
| Cluster 1 (k = 338, P = 0.002) | ||
| L posterior STS (TPJ) | −58, −46, 10 | 5.53 |
| Cluster 2 (k = 435, P < 0.001) | ||
| R precentral gyrus (dPMC) | 36, 0, 48 | 5.07 |
| R middle frontal gyrus | 32, 10, 48 | 4.56 |
| Cluster 3 (k = 623, P < 0.001) | ||
| L middle frontal gyrus | −30, 2, 52 | 4.90 |
| L precentral gyrus (dPMC) | −28, −4, 50 | 4.86 |
| L superior frontal gyrus | −20, 2, 56 | 4.43 |
| Cluster 4 (k = 800, P < 0.001) | ||
| R middle precuneus | 0, −58, 48 | 4.83 |
| L anterior precuneus | −8, −48, 56 | 3.98 |
| L middle precuneus | −12, −70, 44 | 3.65 |
| L anterior precuneus | −8, −46, 48 | 3.60 |
| R middle precuneus | 6, −68, 42 | 3.21 |
| Cluster 5 (k = 363, P = 0.002) | ||
| R posterior STG (TPJ) | 52, −46, 14 | 4.47 |
| R posterior STS (TPJ) | 46, −44, 14 | 4.33 |
| Cluster 6 (k = 286, P = 0.005) | ||
| R inferior frontal gyrus (pars triangularis) | 40, 20, 18 | 4.42 |
| R inferior frontal gyrus (pars triangularis) | 40, 14, 24 | 4.01 |
| R inferior frontal gyrus (pars opercularis) | 36, 6, 24 | 3.42 |
| Cluster 7* (k = 140, P = 0.085) | ||
| R pre‐SMA (BA 8) | 6, 30, 46 | 4.17 |
| R pre‐SMA (BA 6) | 8, 22, 50 | 4.16 |
| Cluster 8 (k = 232, P = 0.014) | ||
| R angular gyrus | 34, −62, 40 | 3.86 |
| R angular gyrus | 36, −58, 46 | 3.84 |
Coordinates x, y, and z refer to MNI space; k = number of voxels in cluster; P value represents cluster‐level error probability corrected for multiple comparisons.
Significant at corrected cluster‐level P < 0.1.
L, left; R, right; BA, Brodmann area; TPJ, temporo‐parietal junction; dPMC, dorsal premotor cortex; pre‐SMA, pre‐supplementary motor area.
To directly test for differences between the network identified in the mixed condition and the supramodal network identified across unimodal conditions, we computed a balanced contrast between both, again applying a composite filter to only retain voxels with more activity during the mixed condition than during baseline and each of the three unimodal conditions. Only two areas survived this direct test: bilateral precuneus (BA 7; MNI coordinates: 4/−56/46; z = 4.15) and left dPMC (BA 6; −28/6/52, z = 4.40; −28/−4/50, z = 3.80; see Fig. 3D). Conversely, contrasting all three unimodal against the mixed condition yielded a single significant difference in the bilateral anterior medial cerebellum (−8/−50/−6, z = 4.31; 10/−48/−8, z = 3.99).
Modulatory effects of response speed
When analyzing modulatory effects of the parametric RT regressors, it turned out that blockwise averaged response speed was not significantly associated with brain activity in any of the four experimental task conditions. Thus, mean RT per block appears not to explain any additional variance in alertness‐related brain activity beyond the task regressors themselves.
DISCUSSION
We examined the influence of stimulus modality on the brain network activated when maintaining a high level of responsiveness to temporally unpredictable external stimuli. First, we examined the supramodal (i.e., modality‐independent) nodes of this network by analyzing the common activity in response to auditory, tactile, and visual stimuli. Second, we tested whether monitoring for external response signals produces sustained modality‐specific modulations of activity in sensory cortices. Finally, we explored the effect of increasing monitoring demands by making stimulus modality unpredictable. Our results provide evidence for a mainly right‐lateralized supramodal core network controlling sustained response readiness (i.e., intrinsic alertness). This network, identified across all three sensory modalities (alertness conditions contrasted against sensorimotor control conditions), consisted of right dPMC, SMA, pre‐SMA, ACC, inferior frontal cortex, anterior insula, and temporo‐parietal junction as well as left medial cerebellum, bilateral motor cingulate areas, midbrain, and brainstem areas. These findings are in good agreement with previous studies on intrinsic alertness using only one sensory modality [auditory or visual; Périn et al.,2010; Sturm et al.,1999,2004; Sturm and Willmes,2001].
The marked right‐hemisphere dominance suggests computational differences between intrinsic and phasic alerting, since during the latter, the activation pattern is usually much less lateralized. One difference may be the increased task difficulty of phasic‐alertness tasks, leading to the recruitment of additional (left‐hemisphere) resources [for related discussions, see Helton et al.,2010; Nebel et al.,2005]: Phasic alerting requires participants to distinguish warning stimuli from imperative ones [Sturm and Willmes,2001], which may be especially demanding when cued and uncued trials are presented in a randomly mixed fashion [see, e.g., Fan et al.,2005; Thiel and Fink,2007; but see Sturm and Willmes,2001, for a non‐mixed approach]. In fact, Jaffard and coworkers [Jaffard et al.,2007,2008] showed that this mixing leads to sustained proactive inhibition to prevent erroneous responding to cues. They argued that relative RT improvements in cued trials, which are usually attributed to performance gains from alerting, should rather be attributed to inhibition‐related performance losses in uncued trials. Moreover, the warning stimulus in phasic‐alertness tasks is likely to be used as a temporal cue inducing endogenous temporal orienting, as compared to more exogenous temporal preparation processes in intrinsic‐alertness tasks [Coull and Nobre,2008; see also below]. It remains for future studies to answer the question of commonalities and differences between intrinsic and cue‐induced phasic alerting by using time‐resolved imaging techniques or analysis approaches that allow the distinct characterization of pre‐ and post‐warning‐cue activity in carefully designed comparisons between cued and uncued simple‐RT tasks presented in mixed and non‐mixed blocks of trials. In the following, we will discuss possible contributions of the observed network's parts to endogenously maintaining response readiness, before turning to modality‐specific modulations and the effects of not knowing the stimulus modality in advance.
Maintaining the Preparatory Set: Stimulus–Response Mapping, Motor Preparation, and the Prevention of Premature Responses
Lesion studies in non‐human primates [Halsband and Passingham,1985; Petrides,1985] and human patients [Petrides,1997] as well as electrophysiological recordings in monkeys [Hoshi and Tanji,2006] showed that dPMC is essential for learning and using arbitrary stimulus–response associations (i.e., sensorimotor mapping). Dorsal PMC also processes spatial cues to direct movements, regardless of the cue's sensory modality [Weinrich and Wise,1982]. Further, there is electrophysiological evidence for sensory functions of dPMC [Wise et al.,1997], and a recent fMRI study [Kansaku et al.,2004] reported bilateral (more pronounced on the right side) dPMC activity when participants attended to auditory, tactile, or visual stimuli without any response requirement. This was assumed to reflect a role of dPMC in facilitating cue detection. This non‐motor, input‐related function of dPMC is also supported by fMRI and PET studies on the maintenance of spatial and non‐spatial attention [Kelley et al.,2008; Pardo et al.,1991; Simon et al.,2002].
At the same time, dPMC has been found to be directly involved in motor control [Geyer et al.,2000; Graziano et al.,2002], via direct connections to the primary motor area (M1) and the spinal cord [Barbas and Pandya,1987; Dum and Strick,1991]. Thus, it can be argued that bilateral activation of dPMC in our task versus baseline contrast reflects both (i) maintaining the mapping between stimuli and instructed motor response (including the facilitation of stimulus detection) and (ii) sending motor‐execution signals further down the motor hierarchy. Both functional aspects have been proposed to be differentially localized in more rostral versus caudal subdivisions of dPMC, respectively [reviewed in Abe and Hanakawa,2009; see also Chouinard and Paus,2006; Picard and Strick,2001; Simon et al.,2002]. In fact, comparing alertness conditions against resting baseline yielded a somewhat more rostral focus of dPMC activity at the right compared to the left side (data not shown). This may explain why only right dPMC survived the comparison with the sensorimotor control tasks: Both experimental and control tasks required the repeated execution of button presses, to which caudal dPMC may contribute equally in both tasks, resulting in elimination by subtraction. Sustained stimulus–response mapping and signal detection, however, were only required in the experimental tasks. This differential processing demand might be preferably subserved by the contralateral rostral dPMC.
A similar logic may apply to alertness‐induced right‐sided SMA activations. Unilateral movements usually activate bilateral SMA [e.g., Naito et al.,2000], which is thought to be involved in initiating and executing movements [Cunnington et al.,2003]. This notion is supported by recent effective‐connectivity studies [Eickhoff et al.,2008; Grefkes et al.,2008a,b], which revealed strong context‐specific influences of SMA on M1. SMA and, to a greater extent, pre‐SMA are also involved in the preparation of movements [Cunnington et al.,2005; Hülsmann et al.,2003]. Picard and Strick [1996] suggested that SMA proper is more active in simple, automatic tasks and pre‐SMA is more active in complex, cognitively controlled ones [Jakobs et al.,2009]. Recently it has been proposed that functional differences between SMA proper and pre‐SMA follow a caudal–rostral continuum rather than a discrete parcellation [Nachev et al.,2008]. We argue that maintaining a preparatory set for a given movement is subserved by rostral SMA and its anterior continuation, pre‐SMA. In contrast, more basic processes related to response initiation/execution may be localized more caudally. Since the latter are shared by our experimental and control tasks, caudal SMA activity is eliminated by the subtraction of activity during sensorimotor control tasks.
The ACC has been found involved in a wealth of motor, cognitive, and affective processes [reviewed in Devinsky et al.,1995]. Motor functions have been associated with different cingulate motor zones, among them caudal BA 24, which is active during simple movements [Picard and Strick,1996]. The stronger activation of caudal ACC during intrinsic alerting, relative to our repetitive control tasks, might reflect a preparatory attentional modulation that facilitates the generation of motor output. This notion is supported by studies showing that ACC activity is closely related to the generation of the contingent negative variation (CNV), an electrocortical potential indicating top‐down preparatory activity following a warning signal that announces an impending imperative stimulus [Fan et al.,2007; Lütcke et al.,2009; Nagai et al.,2004]. The CNV is a slow cortical potential that reflects an increase in neural excitability (i.e., responsiveness to input) during expectancy states [Birbaumer et al.,1990; Elbert,1993]. Thus, the increased caudal ACC activity during sustained response readiness may subserve an expectancy‐induced modulation of higher‐frequency neural oscillations, facilitating the stimulus‐contingent elicitation of the motor output cascade [see He and Raichle,2009, for a discussion on the relation between slow potentials and BOLD activity].
Maintaining a preparatory set for a given movement involves motor preparation as well as motor inhibition, largely implemented in parallel [Davranche et al.,2007; Duque and Ivry,2009; Hasbroucq et al.,1999; Jennings and van der Molen,2005]. Motor preparation results in increased readiness to respond in a predefined way upon occurrence of a signal. The resulting “urge to move,” however, must be held in check to prevent premature responding. Also, competing action tendencies need be suppressed while preparing for a given motor response [Jennings et al.,2009]. Such inhibitory processes are presumably subserved by pre‐SMA and right inferior frontal gyrus (BA 44). Picard and Strick [1996] argued for an important role of pre‐SMA in higher motor control including motor inhibition, which is corroborated by a patient study showing that lesions in pre‐SMA and SMA were selectively associated with deficits in response inhibition [Picton et al.,2007]. Furthermore, the deactivation of pre‐SMA by means of TMS was shown to disrupt the ability to suppress responses after stop signals [Chen et al.,2009]. Since our temporally unpredictable experimental conditions required a rapid stimulus‐contingent implementation of motor plans, alertness‐related pre‐SMA activity may reflect increased demands for preparatory motor control to hold motor plans on line for rapid use as well as to mediate constraints on excitatory activity to prevent the premature release of motor plans.
There is evidence that these constraining signals may originate from inferior prefrontal cortex (BA 44), which has direct anatomical connections to pre‐SMA [Aron et al.,2007; Johansen‐Berg et al.,2004]. Functional MRI studies revealed common activity during response inhibition [Coxon et al.,2009; Xue et al.,2008] and greater effective connectivity when inhibition was successful [Duann et al.,2009]. Further, TMS over right BA 44 [Chambers et al.,2006] and lesions in this area [Aron et al.,2003] impaired the ability to stop a motor act. On the basis of these and other findings, Jakobs et al. [2009] suggested that BA 44 acts as a hold‐and‐release switch in situations of temporal uncertainty about the moment of movement execution. Our results agree with this interpretation, and we suggest that BA 44 modulates pre‐SMA activity to bias the balance (or permanent conflict) between “going” and “withholding” according to current task demands.
The Timing of Preparation
It has been postulated that the brain constantly predicts future events based on previous experiences to minimize computational load and to disambiguate incoming information—a feature that has been labeled “predictive coding” [Friston,2005; Rao and Ballard,1999]. Situations with uncertainty result in frequent prediction errors and constant updating of beliefs to improve the accuracy of future predictions [Behrens et al.,2007; Kilner et al.,2007]. Under such circumstances, computational activity should increase in areas that subserve predictive coding. With respect to uncertainty about action parameters (type and onset of responses), the right temporo‐parietal junction (TPJ) has recently been implicated to play a central role in predictive motor coding, including the updating of action expectations and/or the comparison of prepared motor programs with current requirements [Eickhoff et al.,2010; Jakobs et al.,2009].
In RT tasks involving temporal uncertainty such as our experimental conditions, participants develop temporal expectations about the onset of the next stimulus and its associated response [Coull and Nobre,2008; Langner et al.,2010a]. We propose that the activity observed in right TPJ reflects the updating of such temporal predictions. This is consistent with recent theories of trial‐to‐trial conditioning processes governing temporal preparation in RT tasks under temporal uncertainty [Los et al.,2001; Steinborn et al.,2008]. Importantly, our sensorimotor control conditions also entailed monitoring the passage of time to properly self‐pace motor output, which suggests that the increased TPJ activity in simple‐RT tasks is related to changing temporal expectancies rather than simply encoding elapsed time. In fact, a recent fMRI study [Cui et al.,2009] also found activity in right superior temporal gyrus (although more anterior than our cluster) to encode expectation of cue arrival times. A modality‐independent role of the right TPJ in establishing and updating implicit probabilistic models of the temporal structure of sensory input under time uncertainty agrees with Kansaku et al.'s [2004] study, in which right TPJ activity was observed during both monitoring of, and speeded responding to, temporally unpredictable stimuli of different modalities. This shows the independence of this process from motor output [see also Cui et al.,2009].
Our assumption that TPJ plays a role in facilitating the speeded detection of stimuli by predictive coding is also in line with previous studies on the stimulus‐driven orienting of non‐temporal attention [Corbetta and Shulman,2002; Downar et al.,2000; see also Corbetta et al.,2008], which reported increased activity in the TPJ region during disruptions in expectation about incoming visual stimuli (i.e., increasing prediction error) or detection of sensory changes in the environment (i.e., updating the present prediction). Taken together, this argues for a general role of the right TPJ in integrating bottom‐up information with predictions to facilitate target detection under uncertainty [see also Eickhoff et al.,2010; Shulman et al.,2010]. Considering its presumed role in stimulus‐driven attentional orienting, the observed TPJ activity agrees well with the notion that a state of optimal responsiveness to external events facilitates stimulus‐driven orienting to those events by giving more weight to bottom‐up sensory input (i.e., prediction errors), relative to top–down expectations.
Another brain region associated with temporal preparation under time uncertainty is the cerebellum [Courchesne and Allen,1997], which showed modality‐independent activity related to response readiness in the anterior vermis and left intermediate hemisphere. This finding agrees well with other reports of cerebellar activity during alertness, sustained attention, and simple‐RT performance [Kansaku et al.,2004; Lawrence et al.,2003; Sturm and Willmes,2001; Sturm et al.,2004]. Our results are also consistent with a recent study showing that the absence of the cerebellar vermis because of a congenital dysplasia is related to deficits in endogenously maintaining responsiveness to visual signals [Michael et al.,2009]. It should be noted, though, that the chosen field of view of our fMRI measurement allowed us to only image the superior cerebellum, precluding inferences on its lower parts.
Cerebellar involvement in precise motor timing has long been known from lesion studies [Ivry,1996; Sailer et al.,2005]. Furthermore, increased activation of the cerebellum in random compared to fixed timing was observed in a task‐switching experiment [Dreher and Grafman,2002], and Martin et al. [2006] showed with magnetoencephalography (MEG) that cerebellar activity predicted response speed in temporally unpredictable simple‐RT tasks. Another recent MEG study [Pollok et al.,2008] suggested, based on functional connectivity analyses in a cerebello‐diencephalic‐parietal network, that the cerebellum is involved in both anticipatory motor control and mismatch‐contingent updating of an internal model, which is in line with the predictive‐coding framework outlined above [see also Nixon and Passingham,2001; Trillenberg et al.,2004]. This notion is also supported by the finding that patients with cerebellar damage show impaired anticipatory timing of postural adjustments [Diedrichsen et al.,2005]. Given that our control tasks also involved monitoring/estimating the passage of time, our results suggest, in line with the aforementioned findings, that the medial cerebellum may be involved in synchronizing motor preparation with the predicted temporal structure of target events.
Arousal Regulation and the Implementation of Task Sets
Apart from the cingulate motor zones, controversy surrounds the function of the ACC. Paus [2001] argued that the ACC is the place where motor control, executive attention, and arousal regulation interface. This is in line with our data and previous studies on intrinsic alertness or vigilance, which consistently found ACC activity related to maintaining responsiveness [Paus et al.,1997; Périn et al.,2010; Sturm et al.,1999,2004; Sturm and Willmes,2001]. One role of the ACC in simple‐RT tasks might be the regulation of midbrain and brainstem arousal systems in order to maintain optimal efficiency of information processing [Aston‐Jones and Cohen,2005; Critchley et al.,2003; Fischer et al.,2008; Sturm et al.,1999]. This notion is corroborated by a PET study [Paus et al.,1997], in which the decline of arousal during a 60‐min vigilance task covaried with activity in ACC and midbrain structures. Further support comes from an effective‐connectivity analysis of brain activity during a simple‐RT task [Mottaghy et al.,2006], which revealed top–down influences of the ACC on thalamus and brainstem structures associated with arousal regulation. Notably, in a sensorimotor control condition similar to the one we used here, this top‐down connectivity was absent. Also, ACC activity has been repeatedly shown to increase with preparatory attention [Luks et al.,2002; Murtha et al.,1996] and attentional effort [Mulert et al.,2005; Walton et al.,2003]. Along these lines, we suggest that in tasks that demand intense attentiveness, the ACC is important for initiating and maintaining efficient goal‐directed behavior by “energizing” cognition [see also Stuss,2006].
This energizing is thought to be mediated via input from midbrain and brainstem arousal systems [for reviews, see Jones,2003; Pfaff,2006]. These systems employ various neurotransmitters, such as noradrenalin, dopamine, acetylcholine, or serotonin, which innervate large parts of the cortex. In their target zones, they modulate computational processes and thus control the efficiency of information processing [Grefkes et al.,2010; Hasselmo,1995]. These neuromodulatory systems originate in subcortical regions, some of which correspond to activity foci of our study, such as the ventral tegmental area and substantia nigra (dopamine) or the locus coeruleus (noradrenalin). Furthermore, midbrain structures like the substantia nigra as well as the pons are implicated in the control of sympathetic autonomic arousal [Critchley et al.,2002]. We are aware that fMRI as used here cannot localize these structures with certainty, since its spatial resolution is too coarse. Nevertheless, our data provide further evidence that distinct midbrain and brainstem structures involved in neuromodulation contribute to voluntarily maintaining an alert state.
The network of ACC and midbrain/brainstem regions, mediating cerebral and bodily arousal, is also tightly connected with another area of the supramodal alertness network: the anterior insula, an area that has been found involved in representing bodily states [Craig,2002; Critchley et al.,2004]. There are strong anatomical connections between anterior insula and ACC [Augustine,1996; Mesulam and Mufson,1982], and a recent fMRI study provided evidence from resting‐state connectivity for a functional anterior‐insula–cingulate system [Taylor et al.,2009]. Similar to ACC, anterior insula activity has not only been found in response to stimulus events but also in preparation for expected ones. For instance, a meta‐analysis revealed that anterior insula activation increases prior to both expected monetary losses and gains and correlates with self‐reported negative and positive arousal [Knutson and Greer,2008].
Thus, during intrinsic alerting, right anterior insula might feed integrated information on bodily states to ACC, which monitors and, if necessary, adjusts arousal to maintain optimal readiness in body and brain. This assumption is supported by the finding that activity in right anterior insula, right ACC, cerebellum and brainstem covaries with peripheral cardiovascular arousal (i.e., sympathetic autonomic activity) induced by mental or physical stressors [Critchley et al.,2000; see also Pollatos et al.,2007]. Our view is also in line with the recent finding of significant functional connectivity between right anterior insula and ACC across different attention‐demanding tasks, leading to the conclusion that the right anterior insula may be especially critical for modulating cognitive control systems under challenging conditions [Eckert et al.,2009]. Apart from cognitive challenges, this might also, or even particularly, apply to very simple tasks like ours, which are challenging, because they are repetitive and ultimately boring but still require the continuous, effortful maintenance of attention [Fischer et al.,2008; Langner et al., 2010b; Walker et al.,2009].
Recently, joint ACC–insula activity has been discussed in a somewhat different framework, which we think can be integrated with the arousal‐regulation account: Dosenbach et al. [2006] found across ten different tasks and 183 subjects that dorsal ACC and bilateral anterior insula showed reliable start‐cue and sustained activations across tasks. This was taken as evidence that these regions form a core system for implementing and maintaining task sets [see also Dosenbach et al.,2007]. Obviously, adhering to a given task set is a prerequisite for efficient performance in any attention‐demanding task and, as alluded to above, might be especially challenged during simple, repetitive tasks like ours [Smallwood and Schooler,2006]. It appears, though, as if this kind of challenge in computationally simple tasks is predominantly met by right anterior insula.
How might task sets be implemented, and how could this process be integrated with arousal regulation? Apart from modulating activity in other cortical regions via direct cortico‐cortical interactions (e.g., connections between ACC and mid‐cingulate motor zones), ACC and insula might indirectly send modulatory signals to cortical areas via subcortical loops that involve brainstem arousal centers [Corbetta et al.,2008]. The LC, a major brainstem nucleus involved in arousal regulation [Jones,2003], receives input from ACC and insula [Aston‐Jones and Cohen,2005; Ongür et al.,2003], which might top–down modulate LC firing activity [Mottaghy et al.,2006]. This top–down control may drive changes in tonic LC firing, thereby producing transitions between behavioral states characterized by different arousal levels (e.g., sleep or alert wakefulness; Aston‐Jones and Cohen,2005). Apart from that, the LC system might also contribute to implementing task sets via its phasic burst activity. Phasic LC firing has been interpreted as a “network reset” signal [Bouret and Sara,2005], driving the (re)configuration of task‐relevant networks after a target is detected and also, maybe, when a target is expected. Thus, the observed readiness‐related activity in the vicinity of the LC suggests that “staying on the job” in simple‐RT tasks might depend on repeated, LC‐mediated resets of the task‐relevant brain network, which are possibly timed via top–down signals from ACC and/or anterior insula.
This conceptualization of ACC control over subcortical arousal structures is also consistent with inferences drawn from behavioral deficits in patients with lesions in the ACC and medial frontal cortex: Stuss and colleagues [Picton et al.,2006; Shallice et al.,2008; Stuss et al.,1995,2005; Stuss,2006] proposed that these regions subserve the “energization” of cognition, that is, they activate specific task schemata or task sets. Taking these and our findings together, we conjecture that the allocation of attentional resources (i.e., the implementation of a task set) might correspond to a network reset [Bouret and Sara,2005], and the dependence of the phasic LC mode on the underlying tonic mode [Aston‐Jones and Cohen,2005] might constitute one of the neural underpinnings of the influence of arousal on the availability of such attentional resources, as suggested by Kahneman [1973] or Humphreys and Revelle [1984]. The ACC then might influence both LC firing modes, adaptively adjusting LC activity according to task demands as well as behavioral goals. This view of ACC function is also consistent with studies that argue for a general role of the ACC in self‐regulation [Posner and Rothbart,1998; Posner et al.,2007], which agrees with our proposal of a hierarchical model of depletable self‐regulatory power underlying performance decrements in tasks that require sustained attention [Langner et al.,2010b].
Modality‐Specific Modulations of Brain Activity by Intrinsic Alertness
Maintaining alertness involves sustained (preparatory) attention to sensory input, which facilitates subsequent processing. Our findings corroborate the hypothesis that modality‐specific attentional modulations should be found in areas that process sensory information of the given modality. Indeed, the only modality‐selective increases in brain activity during auditory, tactile, or visual alertness were found in auditory core and belt regions, primary and higher‐order somatosensory cortex, and visual association cortex, respectively. The absence of selective modulations in primary visual cortex during visual alertness is most likely due to the processing of some visual input (fixation cross) in all three unimodal alertness conditions. Importantly, the unimodal activity is not due to simple stimulus processing, since the outcome only included voxels that showed higher activity during intrinsic alerting than during the sensorimotor control tasks, which actually provided much more sensory stimulation.
In general, the modality‐specific activations show remarkable overlap with findings from a study that had its participants attend to auditory, somatosensory, and visual stimulation, each of which randomly alternated between two modes [Downar et al.,2000]. This suggests that change detection is subserved by cortical modules that are also the target of attentional modulation in simple tasks like ours, which merely require detecting the presence of stimuli of a given modality. Our results are also consistent with previous studies on intermodal selective attention [Kawashima et al.,1999; Macaluso et al.,2000,2002,2003; Shomstein and Yantis,2004]. Unlike these studies, however, our task did not include additional requirements for spatial or within‐modality selective attention. This supports the notion that it is not the increased task complexity or interactions with other kinds of attention that produce attentional modulations: directing attention to modality appears to suffice.
Our findings are based on direct comparisons of alertness‐related brain activity between different modalities, which strengthens the assumption that previous failures to find modality‐specific modulations by intrinsic alerting may have been a consequence of using comparisons between unimodal simple‐RT tasks and sensorimotor control conditions with a non‐matched stimulus presentation rate (see Introduction). This interpretation is also in line with studies on phasic alertness that found modality‐specific modulations of sensory processing when comparing trials with and without alerting pre‐cue [Daumann et al.,2009; Thiel and Fink,2007]. Thus, we conclude that modality‐specific modulations by intrinsic alerting are sufficient to produce sustained activity increases in sensory cortices. Our blocked design, however, did not allow distinguishing preparatory modulations [i.e., baseline increases; Kastner et al.,1999] from modulations of sensory processing.
In addition, we found sustained activity decreases in irrelevant sensory cortices. These modality‐specific cross‐modal deactivations are consistent with previous findings during selective intermodal attention [Johnson and Zatorre,2006; Mozolic et al.,2008] and further attest to a two‐way strategy during modality‐specific attention, which comprises the enhancement of relevant, and the suppression of irrelevant, sensory processing. Finally, the absence of any other modality‐specific modulations underlines that other subprocesses during intrinsic alerting, for example, related to motor preparation, timing, or arousal regulation, are independent of stimulus modality.
Effects of Unpredictable Stimulus Modality
In the mixed condition, in which stimulus modality was unpredictable, the pattern of activity largely overlapped with the network shared by all three unimodal conditions (Fig. 2). The direct comparison between the three unimodal conditions and the mixed condition did not yield stronger activity in sensory cortices during unimodal alerting. This argues for similar modality‐specific modulations during multimodal monitoring for external response signals, which is consistent with the view that the deployment of attentional resources across modalities is less limited than dividing attention between stimuli within a modality [Talsma et al.,2006]. Conversely, two additional foci of activity emerged for the mixed condition when it was directly compared against the unimodal conditions: left dPMC and bilateral precuneus. We suggest that the additional left‐sided dPMC activity reflects (i) increased demands on signal detection because of the larger number of input channels to be monitored and (ii) the associated more complex stimulus–response mapping to be maintained. This interpretation is in line with findings from studies requiring attention to be divided between simultaneously presented stimuli of different sensory modalities [Saito et al.,2005; Vohn et al.,2007].
Precuneus activation has been previously shown during voluntary attentional shifts between spatial [Krumbholz et al.,2009] and non‐spatial [Le et al.,1998] features of auditory and visual stimuli as well as between auditory and visual stimulus modalities during bimodal stimulation [Shomstein and Yantis,2004; Townsend et al.,2006]. Although modality unpredictability in our mixed condition discouraged voluntary shifts of attention between modalities, it will all the more have induced involuntary, stimulus‐driven shifts. Thus, the precuneus might be involved in both voluntary and involuntary shifts of attention to targets. This view is supported by studies showing precuneus activity related to attentional set shifts between object features implicitly triggered by performance feedback [Nagahama et al.,1998,1999]. Further support on a more general level comes from an experiment on task switching that found precuneus activity related to increased attentional demands imposed by the cued preparation of task shifts as well as the execution‐related shifting per se during the response period [Barber and Carter,2005] and from a study comparing the networks involved in alertness for central versus spatially distributed stimuli [Sturm et al.,2006], with the precuneus only active under the distributed condition. Finally, our finding is also consistent with a recent investigation that showed precuneus activity to be associated with better performance in a non‐spatial auditory detection task [Sadaghiani et al.,2009]. In summary, those findings and ours argue for a model of multimodal intrinsic alertness that achieves sensory facilitation (and, thus, increased responsiveness) by biasing the system to allocate attentional resources in a stimulus‐driven manner. That is, the input channels are kept “open” by facilitating attentional capture by relevant stimuli, which is consistent with the functions ascribed to the ventral attention system [Corbetta and Shulman,2002; see also Serences et al.,2005].
CONCLUSIONS
We performed one of the first fMRI studies on the brain network that supramodally controls the readiness to respond to external stimuli under conditions of time uncertainty (i.e., intrinsic alertness). To induce a high degree of sustained responsiveness, we used non‐cued simple‐RT tasks with variable interstimulus intervals and three different stimulus modalities. Regardless of sensory modality, maintaining response readiness was subserved by a mainly right‐hemisphere network, which confirms findings of earlier unimodal studies and extends them to the somatosensory modality. We identified multimodal brain regions which have been associated with different subprocesses of maintaining optimal performance in simple‐RT tasks, such as sustained stimulus–response mapping, balancing motor preparation and inhibition, building temporal expectations, regulating arousal, and maintaining the task set. In this supramodal network, the ACC might be the central coordinating structure for readying body and brain for action. Modality‐specific modulations of brain activity were confined to sensory cortices. When monitoring demands were increased by making stimulus modality unpredictable, additional left frontal and medial parietal areas were recruited, possibly to meet the demands for shifting attention between modalities.
A future challenge is to further specify the functional significance of each identified brain region by delineating the neurocomputational operations subserved by these regions and their interplay during simple‐RT performance. Conversely, different paradigms and analytical approaches should be used to obtain results that converge on an even more generalizable neurobiological core substrate of alertness. This might range from using different effectors for responding or using purely perceptual tasks [Kansaku et al.,2004] to examining neural correlates of sophisticated and validated behavioral and physiological markers of alertness [Schmidt et al.,2009]. The endeavor has direct relevance for the treatment of impaired alertness regulation in various neurological and psychiatric patient groups, since understanding the neural basis of specific and general subprocesses offers the chance for more focused diagnostic and therapeutic approaches with substantially improved outcome.
REFERENCES
- Abe M, Hanakawa T ( 2009): Functional coupling underlying motor and cognitive functions of the dorsal premotor cortex. Behav Brain Res 198: 13–23. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW ( 2003): Stop‐signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci 6: 115–116. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA ( 2007): Triangulating a cognitive control network using diffusion‐weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci 27: 3743–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston‐Jones G, Cohen JD ( 2005): An integrative theory of locus coeruleus‐norepinephrine function: Adaptive gain and optimal performance. Annu Rev Neurosci 28: 403–450. [DOI] [PubMed] [Google Scholar]
- Augustine JR ( 1996): Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev 22: 229–244. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN ( 1987): Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol 256: 211–228. [DOI] [PubMed] [Google Scholar]
- Barber AD, Carter CS ( 2005): Cognitive control involved in overcoming prepotent response tendencies and switching between tasks. Cereb Cortex 15: 899–912. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Walton ME, Rushworth MF ( 2007): Learning the value of information in an uncertain world. Nat Neurosci 10: 1214–1221. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Elbert T, Canavan AGM, Rockstroh B ( 1990): Slow potentials of the cerebral cortex and behavior. Physiol Rev 70: 1–41. [DOI] [PubMed] [Google Scholar]
- Blatter K, Cajochen C ( 2007): Circadian rhythms in cognitive performance: Methodological constraints, protocols, theoretical underpinnings. Physiol Behav 90: 196–208. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ ( 2005): Network reset: A simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci 28: 574–582. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH, Morris AP, Mattingley JB ( 2006): Executive "brake failure" following deactivation of human frontal lobe. J Cogn Neurosci 18: 444–455. [DOI] [PubMed] [Google Scholar]
- Chen CY, Muggleton NG, Tzeng OJ, Hung DL, Juan CH ( 2009): Control of prepotent responses by the superior medial frontal cortex. NeuroImage 44: 537–545. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Paus T ( 2006): The primary motor and premotor areas of the human cerebral cortex. Neuroscientist 12: 143–152. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL ( 2008): The reorienting system of the human brain: From environment to theory of mind. Neuron 58: 306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull J, Nobre A ( 2008): Dissociating explicit timing from temporal expectation with fMRI. Curr Opin Neurobiol 18: 137–144. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC, Frith CD ( 2001): The noradrenergic alpha2 agonist clonidine modulates behavioral and neuroanatomical correlates of human attentional orienting and alerting. Cereb Cortex 11: 73–84. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Allen G ( 1997): Prediction and preparation, fundamental functions of the cerebellum. Learn Mem 4: 1–35. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD ( 2009): Stop and go: The neural basis of selective movement prevention. J Cogn Neurosci 21: 1193–1203. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2002): How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ ( 2000): Cerebral correlates of autonomic cardiovascular arousal: A functional neuroimaging investigation in humans. J Physiol 523 ( Part 1): 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ ( 2002): Volitional control of autonomic arousal: A functional magnetic resonance study. NeuroImage 16: 909–919. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ ( 2003): Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain 126: 2139–2152. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ ( 2004): Neural systems supporting interoceptive awareness. Nat Neurosci 7: 189–195. [DOI] [PubMed] [Google Scholar]
- Cui X, Stetson C, Montague PR, Eagleman DM ( 2009): Ready…go: Amplitude of the fMRI signal encodes expectation of cue arrival time. PLoS Biol 7: e1000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E ( 2003): The preparation and readiness for voluntary movement: A high‐field event‐related fMRI study of the Bereitschafts‐BOLD response. NeuroImage 20: 404–412. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Moser E ( 2005): Premovement activity of the pre‐supplementary motor area and the readiness for action: Studies of time‐resolved event‐related functional MRI. Hum Mov Sci 24: 644–656. [DOI] [PubMed] [Google Scholar]
- Daumann J, Wagner D, Heekeren K, Neukirch A, Thiel C, Gouzoulis‐Mayfrank E ( 2009): Neuronal correlates of visual and auditory alertness in the DMT and ketamine model of psychosis. J Psychopharmacol. doi: 10.1177/0269881109103227. [DOI] [PubMed] [Google Scholar]
- Davies DR, Parasuraman R ( 1982): The Psychology of Vigilance. San Diego, CA: Academic Press; 288 p. [Google Scholar]
- Davranche K, Tandonnet C, Burle B, Meynier C, Vidal F, Hasbroucq T ( 2007): The dual nature of time preparation: Neural activation and suppression revealed by transcranial magnetic stimulation of the motor cortex. Eur J Neurosci 25: 3766–3774. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA ( 1995): Contributions of anterior cingulate cortex to behavior. Brain 118 ( Part 1): 279–306. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Verstynen T, Lehman SL, Ivry RB ( 2005): Cerebellar involvement in anticipating the consequences of self‐produced actions during bimanual movements. J Neurophysiol 93: 801–812. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE ( 2006): A core system for the implementation of task sets. Neuron 50: 799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME ( 2007): Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 104: 11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD ( 2000): A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci 3: 277–283. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Grafman J ( 2002): The roles of the cerebellum and basal ganglia in timing and error prediction. Eur J Neurosci 16: 1609–1619. [DOI] [PubMed] [Google Scholar]
- Duann JR, Ide JS, Luo X, Li CS ( 2009): Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci 29: 10171–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL ( 1991): The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci 11: 667–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Ivry RB ( 2009): Role of corticospinal suppression during motor preparation. Cereb Cortex 19: 2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR ( 2009): At the heart of the ventral attention system: The right anterior insula. Hum Brain Mapp 30: 2530–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Dafotakis M, Grefkes C, Shah NJ, Zilles K, Piza‐Katzer H ( 2008): Central adaptation following heterotopic hand replantation probed by fMRI and effective connectivity analysis. Exp Neurol 212: 132–144. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Pomjanski W, Jakobs O, Zilles K, Langner R ( 2010): Neural correlates of developing and adapting behavioral biases in speeded choice reactions: An fMRI study on predictive motor coding. Cereb Cortex, doi: 10.1093/cercor/bhq188. [DOI] [PubMed] [Google Scholar]
- Elbert T ( 1993): Slow cortical potentials reflect the regulation of cortical excitability In: McCallum WC, Curry SH, editors. Slow Potential Changes in the Human Brain. New York: Plenum Press; pp 235–251. [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI ( 2005): The activation of attentional networks. NeuroImage 26: 471–479. [DOI] [PubMed] [Google Scholar]
- Fan J, Kolster R, Ghajar J, Suh M, Knight RT, Sarkar R, McCandliss BD ( 2007): Response anticipation and response conflict: An event‐related potential and functional magnetic resonance imaging study. J Neurosci 27: 2272–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer T, Langner R, Birbaumer N, Brocke B ( 2008): Arousal and attention: Self‐chosen stimulation optimizes cortical excitability and minimizes compensatory effort. J Cogn Neurosci 20: 1443–1453. [DOI] [PubMed] [Google Scholar]
- Friston K ( 2005): A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci 360: 815–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Fletcher PC, Josephs O, Holmes A, Rugg MD, Turner R ( 1998): Event‐related fMRI: Characterizing differential responses. NeuroImage 7: 30–40. [DOI] [PubMed] [Google Scholar]
- Geyer S, Matelli M, Luppino G, Zilles K ( 2000): Functional neuroanatomy of the primate isocortical motor system. Anat Embryol (Berl) 202: 443–474. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Taylor CS, Moore T ( 2002): Complex movements evoked by microstimulation of precentral cortex. Neuron 34: 841–851. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Eickhoff SB, Nowak DA, Dafotakis M, Fink GR ( 2008a): Dynamic intra‐ and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. NeuroImage 41: 1382–1394. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, Eickhoff SB, Dafotakis M, Kust J, Karbe H, Fink GR ( 2008b): Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol 63: 236–246. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Wang LE, Eickhoff SB, Fink GR ( 2010): Noradrenergic modulation of cortical networks engaged in visuomotor processing. Cereb Cortex 20: 783–797. [DOI] [PubMed] [Google Scholar]
- Halsband U, Passingham RE ( 1985): Premotor cortex and the conditions for movement in monkeys (Macaca fascicularis). Behav Brain Res 18: 269–277. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Osman A, Possamai CA, Burle B, Carron S, Depy D, Latour S, Mouret I ( 1999): Cortico‐spinal inhibition reflects time but not event preparation: Neural mechanisms of preparation dissociated by transcranial magnetic stimulation. Acta Psychol (Amst) 101: 243–266. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME ( 1995): Neuromodulation and cortical function: Modeling the physiological basis of behavior. Behav Brain Res 67: 1–27. [DOI] [PubMed] [Google Scholar]
- He BJ, Raichle ME ( 2009): The fMRI signal, slow cortical potential and consciousness. Trends Cogn Sci 13: 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton WS, Warm JS, Tripp LD, Matthews G, Parasuraman R, Hancock PA ( 2010): Cerebral lateralization of vigilance: A function of task difficulty. Neuropsychologia 48: 1683–1688. [DOI] [PubMed] [Google Scholar]
- Henderson L, Dittrich WH ( 1998): Preparing to react in the absence of uncertainty: I. New perspectives on simple reaction time. Br J Psychol 89 ( Part 4): 531–554. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J ( 2006): Differential involvement of neurons in the dorsal and ventral premotor cortex during processing of visual signals for action planning. J Neurophysiol 95: 3596–3616. [DOI] [PubMed] [Google Scholar]
- Howes D, Boller F ( 1975): Simple reaction time: Evidence for focal impairment from lesions of the right hemisphere. Brain 98: 317–332. [DOI] [PubMed] [Google Scholar]
- Hülsmann E, Erb M, Grodd W ( 2003): From will to action: Sequential cerebellar contributions to voluntary movement. NeuroImage 20: 1485–1492. [DOI] [PubMed] [Google Scholar]
- Humphreys MS, Revelle W ( 1984): Personality, motivation, and performance: A theory of the relationship between individual differences and information processing. Psychol Rev 91: 153–184. [PubMed] [Google Scholar]
- Ivry RB ( 1996): The representation of temporal information in perception and motor control. Curr Opin Neurobiol 6: 851–857. [DOI] [PubMed] [Google Scholar]
- Jaffard M, Benraiss A, Longcamp M, Velay JL, Boulinguez P ( 2007): Cueing method biases in visual detection studies. Brain Res 1179: 106–118. [DOI] [PubMed] [Google Scholar]
- Jaffard M, Longcamp M, Velay JL, Anton JL, Roth M, Nazarian B, Boulinguez P ( 2008): Proactive inhibitory control of movement assessed by event‐related fMRI. NeuroImage 42: 1196–1206. [DOI] [PubMed] [Google Scholar]
- Jakobs O, Wang LE, Dafotakis M, Grefkes C, Zilles K, Eickhoff SB ( 2009): Effects of timing and movement uncertainty implicate the temporo‐parietal junction in the prediction of forthcoming motor actions. NeuroImage 47: 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, van der Molen MW ( 2005): Preparation for speeded action as a psychophysiological concept. Psychol Bull 131: 434–459. [DOI] [PubMed] [Google Scholar]
- Jennings JR, van der Molen MW, Tanase C ( 2009): Preparing hearts and minds: Cardiac slowing and a cortical inhibitory network. Psychophysiology 46: 1170–1178. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, Smith SM, Higham DJ, Matthews PM ( 2004): Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci USA 101: 13335–13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JA, Zatorre RJ ( 2006): Neural substrates for dividing and focusing attention between simultaneous auditory and visual events. NeuroImage 31: 1673–1681. [DOI] [PubMed] [Google Scholar]
- Jones BE ( 2003): Arousal systems. Front Biosci 8: s438–S451. [DOI] [PubMed] [Google Scholar]
- Kahneman D ( 1973): Attention and Effort. Englewood Cliffs, NJ: Prentice‐Hall; 246 p. [Google Scholar]
- Kansaku K, Hanakawa T, Wu T, Hallett M ( 2004): A shared neural network for simple reaction time. NeuroImage 22: 904–911. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG ( 1999): Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22: 751–761. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Imaizumi S, Mori K, Okada K, Goto R, Kiritani S, Ogawa A, Fukuda H ( 1999): Selective visual and auditory attention toward utterances: A PET study. NeuroImage 10: 209–215. [DOI] [PubMed] [Google Scholar]
- Kelley TA, Serences JT, Giesbrecht B, Yantis S ( 2008): Cortical mechanisms for shifting and holding visuospatial attention. Cereb Cortex 18: 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Friston KJ, Frith CD ( 2007): Predictive coding: An account of the mirror neuron system. Cogn Process 8: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinomura S, Larsson J, Gulyas B, Roland PE ( 1996): Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science 271: 512–515. [DOI] [PubMed] [Google Scholar]
- Knutson B, Greer SM ( 2008): Anticipatory affect: Neural correlates and consequences for choice. Philos Trans R Soc Lond B Biol Sci 363: 3771–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Thiel CM, Specht K, Hanisch C, Fan J, Herpertz‐Dahlmann B, Fink GR ( 2005): Development of attentional networks: An fMRI study with children and adults. NeuroImage 28: 429–439. [DOI] [PubMed] [Google Scholar]
- Krumbholz K, Nobis EA, Weatheritt RJ, Fink GR ( 2009): Executive control of spatial attention shifts in the auditory compared to the visual modality. Hum Brain Mapp 30: 1457–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladavas E ( 1987): Is the hemispatial deficit produced by right parietal lobe damage associated with retinal or gravitational coordinates? Brain 110 ( Part 1): 167–180. [DOI] [PubMed] [Google Scholar]
- Langner R, Steinborn MB, Chatterjee A, Sturm W, Willmes K ( 2010a): Mental fatigue and temporal preparation in simple reaction‐time performance. Acta Psychol (Amst) 133: 64–72. [DOI] [PubMed] [Google Scholar]
- Langner R, Willmes K, Chatterjee A, Eickhoff SB, Sturm W ( 2010b): Energetic effects of stimulus intensity on prolonged simple reaction‐time performance. Psychol Res 74: 499–512. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA ( 2003): Multiple neuronal networks mediate sustained attention. J Cogn Neurosci 15: 1028–1038. [DOI] [PubMed] [Google Scholar]
- Le TH, Pardo JV, Hu X ( 1998): 4 T‐fMRI study of nonspatial shifting of selective attention: cerebellar and parietal contributions. J Neurophysiol 79: 1535–1548. [DOI] [PubMed] [Google Scholar]
- Lim J, Dinges DF ( 2008): Sleep deprivation and vigilant attention. Ann N Y Acad Sci 1129: 305–322. [DOI] [PubMed] [Google Scholar]
- Los SA ( 1996): On the origin of mixing costs: Exploring information processing in pure and mixed blocks of trials. Acta Psychol (Amst) 94: 145–188. [Google Scholar]
- Los SA, Knol DL, Boers RM ( 2001): The foreperiod effect revisited: Conditioning as a basis for nonspecific preparation. Acta Psychol (Amst) 106: 121–145. [DOI] [PubMed] [Google Scholar]
- Luks TL, Simpson GV, Feiwell RJ, Miller WL ( 2002): Evidence for anterior cingulate cortex involvement in monitoring preparatory attentional set. NeuroImage 17: 792–802. [PubMed] [Google Scholar]
- Lütcke H, Gevensleben H, Albrecht B, Frahm J ( 2009): Brain networks involved in early versus late response anticipation and their relation to conflict processing. J Cogn Neurosci 21: 2172–2184. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Frith C, Driver J ( 2000): Selective spatial attention in vision and touch: Unimodal and multimodal mechanisms revealed by PET. J Neurophysiol 83: 3062–3075. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Frith CD, Driver J ( 2002): Directing attention to locations and to sensory modalities: Multiple levels of selective processing revealed with PET. Cereb Cortex 12: 357–368. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Eimer M, Frith CD, Driver J ( 2003): Preparatory states in crossmodal spatial attention: Spatial specificity and possible control mechanisms. Exp Brain Res 149: 62–74. [DOI] [PubMed] [Google Scholar]
- Mackworth NH ( 1948): The breakdown of vigilance during prolonged visual search. Q J Exp Psychol 1: 6–21. [Google Scholar]
- Martin T, Houck JM, Bish JP, Kicic D, Woodruff CC, Moses SN, Lee DC, Tesche CD ( 2006): MEG reveals different contributions of somatomotor cortex and cerebellum to simple reaction time after temporally structured cues. Hum Brain Mapp 27: 552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ ( 1982): Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol 212: 38–52. [DOI] [PubMed] [Google Scholar]
- Michael GA, Garcia S, Bussy G, Lion‐Francois L, Guibaud L ( 2009): Reactivity to visual signals and the cerebellar vermis: Evidence from a rare case with rhombencephalosynapsis. Behav Neurosci 123: 86–96. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM, Willmes K, Horwitz B, Muller HW, Krause BJ, Sturm W ( 2006): Systems level modeling of a neuronal network subserving intrinsic alertness. NeuroImage 29: 225–233. [DOI] [PubMed] [Google Scholar]
- Mozolic JL, Joyner D, Hugenschmidt CE, Peiffer AM, Kraft RA, Maldjian JA, Laurienti PJ ( 2008): Cross‐modal deactivations during modality‐specific selective attention. BMC Neurol 8: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulert C, Menzinger E, Leicht G, Pogarell O, Hegerl U ( 2005): Evidence for a close relationship between conscious effort and anterior cingulate cortex activity. Int J Psychophysiol 56: 65–80. [DOI] [PubMed] [Google Scholar]
- Murtha S, Chertkow H, Beauregard M, Dixon R, Evans A ( 1996): Anticipation causes increased blood flow to the anterior cingulate cortex. Hum Brain Mapp 4: 103–112. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M ( 2008): Functional role of the supplementary and pre‐supplementary motor areas. Nat Rev Neurosci 9: 856–869. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Sadato N, Yamauchi H, Katsumi Y, Hayashi T, Fukuyama H, Kimura J, Shibasaki H, Yonekura Y ( 1998): Neural activity during attention shifts between object features. Neuroreport 9: 2633–2638. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Sawamoto N, Toma K, Nakamura K, Hanakawa T, Konishi J ( 1999): Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. NeuroImage 10: 193–199. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Critchley HD, Featherstone E, Fenwick PB, Trimble MR, Dolan RJ ( 2004): Brain activity relating to the contingent negative variation: An fMRI investigation. NeuroImage 21: 1232–1241. [DOI] [PubMed] [Google Scholar]
- Naito E, Kinomura S, Geyer S, Kawashima R, Roland PE, Zilles K ( 2000): Fast reaction to different sensory modalities activates common fields in the motor areas, but the anterior cingulate cortex is involved in the speed of reaction. J Neurophysiol 83: 1701–1709. [DOI] [PubMed] [Google Scholar]
- Nebel K, Wiese H, Stude P, de Greiff A, Diener HC, Keidel M ( 2005): On the neural basis of focused and divided attention. Cogn Brain Res 25: 760–776. [DOI] [PubMed] [Google Scholar]
- Nixon PD, Passingham RE ( 2001): Predicting sensory events: The role of the cerebellum in motor learning. Exp Brain Res 138: 251–257. [DOI] [PubMed] [Google Scholar]
- Ongür D, Ferry AT, Price JL ( 2003): Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol 460: 425–449. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME ( 1991): Localization of a human system for sustained attention by positron emission tomography. Nature 349: 61–64. [DOI] [PubMed] [Google Scholar]
- Paus T, Zatorre RJ, Hofle N, Caramanos Z, Gotman J, Petrides M, Evans AC ( 1997): Time‐related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. J Cogn Neurosci 9: 392–408. [DOI] [PubMed] [Google Scholar]
- Paus T ( 2001): Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat Rev Neurosci 2: 417–424. [DOI] [PubMed] [Google Scholar]
- Périn B, Godefroy O, Fall S, de Marco G ( 2010): Alertness in young healthy subjects: An fMRI study of brain region interactivity enhanced by a warning signal. Brain Cogn 72: 271–281. [DOI] [PubMed] [Google Scholar]
- Petrides M ( 1985): Deficits in non‐spatial conditional associative learning after periarcuate lesions in the monkey. Behav Brain Res 16: 95–101. [DOI] [PubMed] [Google Scholar]
- Petrides M ( 1997): Visuo‐motor conditional associative learning after frontal and temporal lesions in the human brain. Neuropsychologia 35: 989–997. [DOI] [PubMed] [Google Scholar]
- Pfaff DW ( 2006): Brain Arousal and Information Theory: Neural and Genetic Mechanisms. Cambridge, MA: Harvard University Press; 205 p. [Google Scholar]
- Picard N, Strick PL ( 1996): Motor areas of the medial wall: A review of their location and functional activation. Cereb Cortex 6: 342–353. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL ( 2001): Imaging the premotor areas. Curr Opin Neurobiol 11: 663–672. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Shallice T, Alexander MP, Gillingham S ( 2006): Keeping time: Effects of focal frontal lesions. Neuropsychologia 44: 1195–1209. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S ( 2007): Effects of focal frontal lesions on response inhibition. Cereb Cortex 17: 826–838. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Schandry R, Auer DP, Kaufmann C ( 2007): Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res 1141: 178–187. [DOI] [PubMed] [Google Scholar]
- Pollok B, Gross J, Kamp D, Schnitzler A ( 2008): Evidence for anticipatory motor control within a cerebello‐diencephalic‐parietal network. J Cogn Neurosci 20: 828–840. [DOI] [PubMed] [Google Scholar]
- Posner MI, Boies SJ ( 1971): Components of attention. Psychol Rev 78: 391–408. [Google Scholar]
- Posner MI, Inhoff AW, Friedrich FJ, Cohen A ( 1987): Isolating attentional systems: A cognitive‐anatomical analysis. Psychobiology 15: 107–121. [Google Scholar]
- Posner MI, Rothbart MK ( 1998): Attention, self‐regulation and consciousness. Philos Trans R Soc Lond B Biol Sci 353: 1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Tang Y ( 2007): The anterior cingulate gyrus and the mechanism of self‐regulation. Cogn Affect Behav Neurosci 7: 391–395. [DOI] [PubMed] [Google Scholar]
- Rao RP, Ballard DH ( 1999): Predictive coding in the visual cortex: A functional interpretation of some extra‐classical receptive‐field effects. Nat Neurosci 2: 79–87. [DOI] [PubMed] [Google Scholar]
- Raz A, Buhle J ( 2006): Typologies of attentional networks. Nat Rev Neurosci 7: 367–379. [DOI] [PubMed] [Google Scholar]
- Requin J, Brener J, Ring C ( 1991): Preparation for action In: Jennings JR, Coles MGH, editors. Handbook of Cognitive Psychophysiology: Central and Autonomic Nervous System Approaches. Oxford, UK: John Wiley & Sons; pp 357–448. [Google Scholar]
- Rinne T, Pekkola J, Degerman A, Autti T, Jaaskelainen IP, Sams M, Alho K ( 2005): Modulation of auditory cortex activation by sound presentation rate and attention. Hum Brain Mapp 26: 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland PE ( 1982): Cortical regulation of selective attention in man. A regional cerebral blood flow study. J Neurophysiol 48: 1059–1078. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, Hesselmann G, Kleinschmidt A ( 2009): Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. J Neurosci 29: 13410–13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer U, Eggert T, Straube A ( 2005): Impaired temporal prediction and eye‐hand coordination in patients with cerebellar lesions. Behav Brain Res 160: 72–87. [DOI] [PubMed] [Google Scholar]
- Saito DN, Yoshimura K, Kochiyama T, Okada T, Honda M, Sadato N ( 2005): Cross‐modal binding and activated attentional networks during audio‐visual speech integration: A functional MRI study. Cereb Cortex 15: 1750–1760. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Collette F, Leclercq Y, Sterpenich V, Vandewalle G, Berthomier P, Berthomier C, Phillips C, Tinguely G, Darsaud A et al. ( 2009): Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area. Science 324: 516–519. [DOI] [PubMed] [Google Scholar]
- See JE, Howe SR, Warm JS, Dember WN ( 1995): Meta‐analysis of the sensitivity decrement in vigilance. Psychol Bull 117: 230–249. [Google Scholar]
- Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, Yantis S ( 2005): Coordination of voluntary and stimulus‐driven attentional control in human cortex. Psychol Sci 16: 114–122. [DOI] [PubMed] [Google Scholar]
- Shallice T, Stuss DT, Alexander MP, Picton TW, Derkzen D ( 2008): The multiple dimensions of sustained attention. Cortex 44: 794–805. [DOI] [PubMed] [Google Scholar]
- Shomstein S, Yantis S ( 2004): Control of attention shifts between vision and audition in human cortex. J Neurosci 24: 10702–10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Pope DL, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M ( 2010): Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci 30: 3640–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SR, Meunier M, Piettre L, Berardi AM, Segebarth CM, Boussaoud D ( 2002): Spatial attention and memory versus motor preparation: Premotor cortex involvement as revealed by fMRI. J Neurophysiol 88: 2047–2057. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW ( 2006): The restless mind. Psychol Bull 132: 946–958. [DOI] [PubMed] [Google Scholar]
- Steinborn MB, Rolke B, Bratzke D, Ulrich R ( 2008): Sequential effects within a short foreperiod context: Evidence for the conditioning account of temporal preparation. Acta Psychol (Amst) 129: 297–307. [DOI] [PubMed] [Google Scholar]
- Stöcker T, Schneider F, Klein M, Habel U, Kellermann T, Zilles K, Shah NJ ( 2005): Automated quality assurance routines for fMRI data applied to a multicenter study. Hum Brain Mapp 25: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm W, de Simone A, Krause BJ, Specht K, Hesselmann V, Radermacher I, Herzog H, Tellmann L, Müller‐Gärtner HW, Willmes K ( 1999): Functional anatomy of intrinsic alertness: Evidence for a fronto‐parietal‐thalamic‐brainstem network in the right hemisphere. Neuropsychologia 37: 797–805. [DOI] [PubMed] [Google Scholar]
- Sturm W, Willmes K ( 2001): On the functional neuroanatomy of intrinsic and phasic alertness. NeuroImage 14: S76–S84. [DOI] [PubMed] [Google Scholar]
- Sturm W, Longoni F, Fimm B, Dietrich T, Weis S, Kemna S, Herzog H, Willmes K ( 2004): Network for auditory intrinsic alertness: A PET study. Neuropsychologia 42: 563–568. [DOI] [PubMed] [Google Scholar]
- Sturm W, Schmenk B, Fimm B, Specht K, Weis S, Thron A, Willmes K ( 2006): Spatial attention: More than intrinsic alerting? Exp Brain Res 171: 16–25. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Shallice T, Alexander MP, Picton TW ( 1995): A multidisciplinary approach to anterior attentional functions. Ann N Y Acad Sci 769: 191–211. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Shallice T, Picton TW, Binns MA, Macdonald R, Borowiec A, Katz DI ( 2005): Multiple frontal systems controlling response speed. Neuropsychologia 43: 396–417. [DOI] [PubMed] [Google Scholar]
- Stuss DT ( 2006): Frontal lobes and attention: Processes and networks, fractionation and integration. J Int Neuropsychol Soc 12: 261–271. [DOI] [PubMed] [Google Scholar]
- Talsma D, Doty TJ, Strowd R, Woldorff MG ( 2006): Attentional capacity for processing concurrent stimuli is larger across sensory modalities than within a modality. Psychophysiology 43: 541–549. [DOI] [PubMed] [Google Scholar]
- Tartaglione A, Bino G, Manzino M, Spadavecchia L, Favale E ( 1986): Simple reaction‐time changes in patients with unilateral brain damage. Neuropsychologia 24: 649–658. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD ( 2009): Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp 30: 2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR ( 2004): Cerebral correlates of alerting, orienting and reorienting of visuospatial attention: an event‐related fMRI study. NeuroImage 21: 318–328. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Fink GR ( 2007): Visual and auditory alertness: Modality‐specific and supramodal neural mechanisms and their modulation by nicotine. J Neurophysiol 97: 2758–2768. [DOI] [PubMed] [Google Scholar]
- Townsend J, Adamo M, Haist F ( 2006): Changing channels: An fMRI study of aging and cross‐modal attention shifts. NeuroImage 31: 1682–1692. [DOI] [PubMed] [Google Scholar]
- Trillenberg P, Verleger R, Teetzmann A, Wascher E, Wessel K ( 2004): On the role of the cerebellum in exploiting temporal contingencies: Evidence from response times and preparatory EEG potentials in patients with cerebellar atrophy. Neuropsychologia 42: 754–763. [DOI] [PubMed] [Google Scholar]
- Vohn R, Fimm B, Weber J, Schnitker R, Thron A, Spijkers W, Willmes K, Sturm W ( 2007): Management of attentional resources in within‐modal and cross‐modal divided attention tasks: An fMRI study. Hum Brain Mapp 28: 1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AD, Muth ER, Odle‐Dusseau HN, Moore D, Pilcher JJ ( 2009): The effects of 28 hours of sleep deprivation on respiratory sinus arrhythmia during tasks with low and high controlled attention demands. Psychophysiology 46: 217–224. [DOI] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Alterescu K, Rushworth MF ( 2003): Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort‐related decisions. J Neurosci 23: 6475–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrich M, Wise SP ( 1982): The premotor cortex of the monkey. J Neurosci 2: 1329–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R ( 1997): Premotor and parietal cortex: Corticocortical connectivity and combinatorial computations. Annu Rev Neurosci 20: 25–42. [DOI] [PubMed] [Google Scholar]
- Xue G, Aron AR, Poldrack RA ( 2008): Common neural substrates for inhibition of spoken and manual responses. Cereb Cortex 18: 1923–1932. [DOI] [PubMed] [Google Scholar]


