Abstract
Histological studies have shown a relatively high iron concentration in the subthalamic nucleus (STN). T2‐ and T2*‐weighted sequences have previously been used to visualize the STN in vivo. The phase information of gradient‐echo images reflects the magnetic tissue properties more directly, e.g., iron is more paramagnetic than water. Unfortunately, phase images suffer from non‐local effects and orientation dependency. The goal of this study is to delineate the STN more precisely using susceptibility maps, calculated from phase images, which directly index magnetic tissue properties while removing the non‐local effects and orientation dependency. Use of 7T MRI enables high spatial resolution with good signal to noise ratio (SNR). Eight healthy subjects were scanned at 7T using a high‐resolution 3D gradient‐echo sequence. Susceptibility maps were calculated from phase data using a thresholding Fourier approach and a regularization approach using spatial priors. The susceptibility maps clearly distinguish the STN from the adjacent substantia nigra (SN). Their susceptibilities are quantitatively different (0.06 and 0.1 ppm for the STN and SN, respectively). These maps allowed the STN, SN, and the red nucleus to be manually segmented, thus providing 3D visualization of their boundaries. In sum, the STN can be more clearly distinguished from adjacent structures in susceptibility maps than in T2*‐weighted images or phase images. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: STN, subthalamic nucleus, SWI, iron, susceptibility, phase
INTRODUCTION
The subthalamic nucleus (STN) is an almond‐shaped structure within the mesencephalon. It is a small nucleus with its dimensions of ∼ 3 × 5 × 12 mm3. This nucleus is one of the key structures in motor control [Temel et al., 2005] and target for electrical stimulation to treat Parkinson's disease (PD) [Wichmann and DeLong, 2006]. Direct visualization of the STN is difficult because this structure is relatively small and hard to distinguish from the pars reticularis of the adjacent substantia nigra (SN) [Schaltenbrand and Wahren, 1977]. Most MRI methods for visualizing the STN rely on its high iron concentration. It appears strikingly dark in T2 and T2* weighted images [Dormont et al., 2004], especially at the high field strength of 7T. The size and position of the STN vary considerably in different brain atlases [Schaltenbrand and Wahren, 1977; Talairach and Tournoux, 1988] based on one or two brain samples. An MRI study [Richter et al., 2004] using fast‐spin echo acquisition gave size ranges for the STN of 3.4–9.7 mm (anteroposterior), 2.5–5.3 mm (mediolateral), and 2.9–9.4 mm (dorsoventral) measured in 18 Parkinson's disease patients. This demonstrates the high inter‐subject variability. It is thus crucial to display the STN individually for each subject rather than co‐registering MR images to brain atlases.
A recent study used the high contrast of phase images to visualize the STN [Vertinsky et al., 2009]. The presence of iron yields a T2* shortening and consequent signal reduction in gradient‐echo, magnitude images [Dormont et al., 2004; Elolf et al., 2007; Volz et al., 2009], but this approach only indirectly measures iron content. Phase images show the effect of spatial variations in resonance frequency, and because brain iron is paramagnetic, regions with high iron concentration have a higher resonance frequency than their surroundings [Manova et al., 2009; Ogg et al., 1999; Rauscher et al., 2005; Schäfer et al., 2009; Vertinsky et al., 2009]. Furthermore, in certain brain areas phase images have higher contrast‐to‐noise ratio (CNR) than magnitude images [Duyn et al., 2007]. Unfortunately, however, phase images suffer from misleading non‐local effects and care must be taken in their interpretation [Schäfer et al., 2009]. Furthermore, previous studies have invariably been based upon images of rather thick slices. This can lead to partial volume effects, especially for small brain structures like the STN. The smallest value of slice thickness used in previous studies was 1.5 mm [Vertinsky et al., 2009].
In the present study, high‐resolution phase images with nearly isotropic voxels of size 0.5 × 0.5 × 0.6 mm3 were acquired at 7 Tesla (T), enabling a much more precise characterization of the STN. These images were then used to calculate susceptibility maps which are free from non‐local effects. Using these maps we could manually segment the STN, red nucleus (RN), and SN and create 3D models of these regions for each subject.
METHODS
Phase images show the effects of a field perturbation B
dz (r) due to a susceptibility distribution χ(r) in the presence of a field
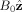 . The susceptibility distribution can be simply calculated in the Fourier domain using
. The susceptibility distribution can be simply calculated in the Fourier domain using
| (1) |
where “∼ ” denotes the 3D Fourier transform (FT) and C(k) is the FT of the deconvolution kernel, expressed as C(k) = 3k /|k|2 − 1, that links the susceptibility and the field [Marques and Bowtell, 2005; Salomir et al., 2003]. Unfortunately, on the magic‐angle cone the computed susceptibility tends to infinity, because the kernel here passes through zero. It has been shown that measuring phase maps at multiple orientations to the main magnetic field and averaging the deconvolution kernel can overcome this problem [Liu et al., 2009; Wharton et al., 2010]. However, rotating the head to get different orientations to B 0 is difficult if one wants to apply this approach to Parkinson's disease patients, which is the main target group for STN visualization. If only the zero values of the convolution kernel at the magic angle cone are omitted, the susceptibility maps show streaking artifacts [Wharton et al., 2010]. However, a simple appropriate thresholding of the convolution kernel has been shown to allow solution of the inversion problem [Shmueli et al., 2009; Wharton et al., 2010]. This will be referred to threshold method (TM). Another method is based on a regularization approach using the spatial priors of brain structures from the magnitude data [de Rochefort et al., 2010; Wharton and Bowtell, 2010]. In this regularization method (RM) the inverse problem can be transferred to a minimization of
| (2) |
where W and W 0 are weighting matrices, and α and β are regularization parameters. G denotes the gradient operator in three directions.
Eight young healthy subjects (22–28 years, three female), who gave informed consent were examined on a whole‐body 7T scanner (MAGNETOM, Siemens Medical Solutions, Erlangen, Germany) using a 24 channel phased array coil (Nova Medical). All eight volunteers were scanned twice with a time interval of 4 months. The study was approved by the local ethics committee. For imaging, a 3D, spoiled, gradient multi‐echo sequence (TR = 40 ms; TE = 11.22/21.41/31.59 ms; bw = 150 Hz/pixel; voxel = 0.5 × 0.5 × 0.6 mm3) was used. About 56 coronal slices were acquired in 14:20 min. covering the STN, SN, and RN.
The T2* maps were calculated by using a voxel‐wise least square fit of the three echo times. The phase data from the first echo were unwrapped using ϕUN [Witoszynskyj et al., 2009]. For one subject the unwrapped phase of the entire 3D data set was high‐pass filtered using a dipole filter approach to remove the unwanted background field [Wharton et al., 2010]. For all other subjects the region around the STN and SN was masked with a binary mask of an ellipsoidal shape since we were only interested in the STN. In previous work, it was shown that restricting the region of interest to a similar extent to that used here has little effect (less than 1%) on the calculated susceptibility values [Wharton et al., 2010]. To remove the unwanted bias field in the masked region, a 2nd‐order polynomial fit to the unwrapped data was subtracted from the unwrapped data to yield high‐pass filtered phase data using the masked data. The filtered phase data were divided by γB 0TE × 10−6 to convert the field‐shift to units of ppm and resampled to 0.5 mm isotropic resolution. The susceptibility map was then calculated using Eq. (1). Voxels with C(k) < |0.25| were set to zero before the inverse Fourier transforms so as to maximize the CNR [Wharton et al., 2010]. Because of the arbitrary setting of the resonant frequency and the use of high‐pass filtering to eliminate the unwanted bias field, the calculated susceptibility values should not be regarded as absolute, but the differences of two regions are expected to be correct. To avoid errors due to variability across subjects in the high‐pass filtering, the susceptibility values given in this study are expressed as a difference from a control region, e.g., χSTN − χcontrol, where the control region is located just above the STN (dotted ellipsoid in Fig. 1a).
Figure 1.

(a) One slice drawn from the 3D gradient echo magnitude image data from Subject 8 is shown. The ellipsoidal mask used for post‐processing is marked with a line. The dotted ellipsoid displays the control region for normalizing the susceptibility. For comparison, one similar slice of a whole brain T2* map (b), phase image (c), and susceptibility map (d) for the same data set as shown in (a).
Based on the susceptibility maps the SN, RN, and, STN were manually segmented and Matlab (MathWorks, MA) was then used to create a surface plot showing the three compartments.
To quantify the differentiation of the STN from the SN, the contrast‐to‐noise ratio (CNR) was calculated for all susceptibility maps and the magnitude images derived from the three different echoes. This CNR is defined as (M STN ‐ M SN)/stdSTN, where M denotes the mean value of the structure and std denotes the standard deviation within the structure. This may underestimates the CNR if the magnitude signal, phase values or susceptibility values are inhomogeneous within the structure. The region of interests were drawn on the magnitude images of the second echo time to calculate the CNR for the magnitude images and the T2* maps and on the susceptibility maps to calculate the CNR for the susceptibility maps. To address the inter‐rater reliability two different persons segmented the STN and SN. Inter‐rater reliability for the manual segmentation was assessed using the intra‐class correlation coefficient (ICC) for brain volume [Shrout and Fleiss, 1979] and Cohen's kappa [Cohen, 1960], a statistical measure of inter‐rater agreement for qualitative items. While ICC provides a measure for agreement between the raters across all segmentations of the same structure, Cohen's kappa is used to assess inter‐rater agreement for every segmentation individually.
To address the qualitative local iron distribution within the STN, the susceptibility map of the region around the SN and STN was compared with a histological study using Perl's staining by Dormont et al. [ 2004].
RESULTS
Figure 1 shows one coronal slice displaying the Substantia Nigra and the Subthalamic Nucleus. Together with the magnitude of the T2* weighted image (Fig. 1a) and the calculated T2* map (Fig. 1b), the high‐pass filtered phase (Fig. 1c) and the calculated susceptibility map (Fig. 1d) is shown. For further post‐processing only the ellipsoidal masked regions shown in Figure 1a were used, since we are only interested in the region around the STN and SN. To remove the unwanted BIAS field effects in phase data, arising from B 0 inhomogeneity, a polynomial fit was applied to the unwrapped phase data. To address the order of the polynomial fit to the phase data and susceptibility maps, polynomial fits of with orders from one to four were applied to the phase data. In Figure 2 the influence of the different orders to the phase and susceptibility maps for Subject 7 are displayed. As shown a second order polynomial fit is sufficient for a small region of interest as used here to remove the field arising from inhomogeneity in the main magnetic field. The calculated susceptibility difference between the SN and STN yield 0.095, 0.065, 0.064, and 0.064 ppm using a first, second, third, and fourth order polynomial, respectively. Figure 3 shows the magnitude image, filtered phase image, and the calculated susceptibility maps using the thresholding (TM) and the regularized (RM) approach for Subject 6 for the masked region, containing the SN and the STN. The phase images clearly show the dipolar field pattern reflecting the non‐local fields which appear as brighter areas (higher frequencies) around the STN, especially in the coronal and sagittal views. The susceptibility maps show none of these non‐local effects and clearly distinguish between the SN and the STN, especially in the coronal and sagittal views. Interestingly, the susceptibility using the thresholding approach of these regions is quantitatively different (0.06 ± 0.01 ppm and 0.1 ± 0.02 ppm for the STN and SN, respectively), and a dividing septum can typically be discerned. The susceptibility values calculated by the regularized approach yield a slightly higher difference (10%) and is thus within the range of the standard deviation of the mean. To address the inter‐rater reliability for the manual segmentation by two different persons, Cohen's kappa varied between 0.776 and 0.844 with mean 0.817 (SD = 0.0172) across subjects and hemispheres. ICC for absolute agreement between raters with respect to the volume of the segmented structure was 0.98. A profile analysis for Subject 5 through the SN and STN for the magnitude data, phase data and susceptibility map is shown in Figure 4, separately for the left and right side. For better comparison the gray values were normalized by their maximum value for each modality. The boundary between the SN and STN is more obvious in the profile for the susceptibility map than in the magnitude data. The local maximum at this point in the profile formed from the magnitude image is less striking than the local minimum in the profile derived from the susceptibility map. An overview for all subjects is displayed in Figure 5, showing the magnitude, phase an susceptibility images together with the profile plots for the left and right side. Additionally, all slices where the STN is visible in Subject 3 are shown in Figure 6. Table I displays all values of the volume‐averaged signal for the magnitude data, T2* values and averaged volume susceptibilities for the STN and SN for all subjects of the first measurement. The averaged CNR based on the SN and STN signal difference is highest for the susceptibility maps. For six subjects the CNR of the SN/STN were highest based on the susceptibility maps. The CNR for Subject 2 were highest using magnitude image of the first echo time and for Subject 4 for the magnitude image of the first and second echo time. The results from the second measurement after 4 months are displayed in Table II. These measurements yield very similar results compared to the first measurements.
Figure 2.

The influence of the order of the polynomial fit used for high‐pass filtering (Subject 7). For a small region as used in this study a second order polynomial is sufficient to remove unwanted BIAS field.
Figure 3.

Magnitude, filtered phase, and susceptibility images (from left to right) of Subject 4. Two susceptibility maps are shown, which were calculated by the thresholding (TM) and the regularization (RM) approach. Displayed are the axial (top row), coronal (middle row) and sagittal (bottom row) view. Note the difference in susceptibility values in the STN and SN.
Figure 4.

(Top) A coronal magnitude image (left), phase image (middle) and susceptibility map (right) from one slice of Subject 6 are shown and the position of the profile through the SN and STN is marked with a line. (Bottom) Normalized gray values along the profile for the magnitude image, phase image and susceptibility map for the left and right side of the brain. I, II, III, and IV denote the positions of the profiles in the images.
Figure 5.

Coronal magnitude, phase images and susceptibility maps for all subjects displaying the STN and SN along with the profiles thru the STN and SN for both the left and right side of the brain. The profiles used to plot the values are shown as a line in the MR images.
Figure 6.

All coronal slices of the magnitude, phase images and susceptibility maps of Subject 3, where the STN was visible.
Table I.
Mean signal in the magnitude images, susceptibility values, and T2* values for the subthalamic nuclei, substantia nigra, and the calculated contrast‐to‐noise ratio for all subjects for the first measurement
| TE1 | TE2 | TE3 | χ [ppm] | T2* [ms] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| STN | SN | CNR | STN | SN | CNR | STN | SN | CNR | STN | SN | CNR | STN | SN | CNR | |
| #1 | 57.61 | 57.42 | 0.03 | 30.37 | 28.42 | 0.35 | 14.89 | 13.38 | 0.25 | 0.06 | 0.08 | 1.34 | 15.77 | 14.72 | 0.34 |
| #2 | 60.66 | 65.14 | 0.52 | 32.11 | 35.27 | 0.37 | 17.14 | 19.47 | 0.33 | 0.06 | 0.07 | 0.37 | 16.52 | 16.79 | 0.07 |
| #3 | 71.89 | 66.51 | 0.75 | 38.96 | 32.35 | 0.79 | 19.88 | 16.14 | 0.54 | 0.06 | 0.1 | 2.35 | 16.51 | 14.55 | 0.58 |
| #4 | 62.45 | 53.83 | 1.11 | 33.74 | 24.63 | 0.99 | 17.33 | 12.11 | 0.6 | 0.09 | 0.11 | 0.65 | 15.93 | 13.73 | 0.59 |
| #5 | 76.04 | 74.49 | 0.22 | 43.53 | 39.07 | 0.56 | 23.53 | 20.19 | 0.44 | 0.07 | 0.1 | 1.46 | 17.98 | 16 | 0.55 |
| #6 | 70.89 | 62.98 | 1.21 | 40.51 | 30.32 | 1.32 | 23.39 | 16.36 | 1.06 | 0.05 | 0.1 | 1.59 | 18.78 | 14.97 | 0.82 |
| #7 | 75.77 | 59.56 | 2.31 | 41.9 | 24.55 | 2.2 | 20.94 | 11.13 | 1.48 | 0.05 | 0.13 | 3.29 | 16.59 | 11.97 | 1.41 |
| #8 | 70.61 | 61.86 | 1.09 | 38.11 | 25.39 | 1.46 | 20.07 | 12.36 | 1 | 0.04 | 0.12 | 3.6 | 16.64 | 12.15 | 1.16 |
| Mean | 68.24 | 62.72 | 0.9 | 37.4 | 30 | 1 | 19.65 | 15.14 | 0.71 | 0.06 | 0.1 | 1.8 | 16.84 | 14.36 | 0.69 |
| stdev | 7.05 | 6.29 | 0.71 | 4.8 | 5.32 | 0.63 | 3.06 | 3.44 | 0.43 | 0.01 | 0.02 | 1.18 | 1.02 | 1.69 | 0.53 |
Table II.
Mean signal in the magnitude images, susceptibility values, and T2* values for the subthalamic nuclei, substantia nigra, and the calculated contrast‐to‐noise ratio for the second measurement
| TE1 | TE2 | TE3 | χ [ppm] | T2* [ms] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| STN | SN | CNR | STN | SN | CNR | STN | SN | CNR | STN | SN | CNR | STN | SN | CNR | |
| #1 | 59.57 | 57.31 | 0.37 | 29.72 | 26.15 | 0.57 | 14.47 | 12.52 | 0.4 | 0.04 | 0.07 | 2.15 | 14.79 | 13.48 | 0.37 |
| #2 | 59.36 | 62.14 | 0.31 | 30.68 | 32.81 | 0.21 | 15.52 | 17.07 | 0.19 | 0.07 | 0.08 | 0.14 | 15.76 | 16.42 | 0.13 |
| #3 | 71.27 | 63.22 | 1.13 | 38.23 | 29.32 | 0.99 | 19.43 | 13.92 | 0.8 | 0.05 | 0.09 | 2.54 | 16.29 | 13.62 | 0.85 |
| #4 | 62.97 | 50.74 | 1.59 | 33.55 | 21.3 | 1.27 | 17.21 | 10.49 | 0.82 | 0.09 | 0.1 | 0.49 | 16.44 | 12.78 | 0.91 |
| #5 | 76.35 | 71.82 | 0.51 | 43.86 | 36.34 | 0.74 | 24.56 | 19.39 | 0.57 | 0.05 | 0.1 | 1.95 | 18.44 | 15.7 | 0.85 |
| #6 | 72.9 | 63.04 | 1.61 | 38.47 | 29.32 | 1.29 | 21.97 | 15.01 | 1.22 | 0.05 | 0.09 | 2.47 | 16.84 | 14.17 | 0.78 |
| #7 | 73.01 | 61.56 | 1.97 | 39.7 | 26.4 | 2.21 | 23.17 | 12.16 | 1.82 | 0.06 | 0.1 | 2.35 | 17.67 | 12.5 | 1.56 |
| #8 | 76.52 | 65.06 | 1.55 | 42.37 | 27.54 | 1.88 | 22.25 | 12.46 | 1.37 | 0.04 | 0.11 | 3.24 | 17.1 | 12.24 | 1.42 |
| Mean | 68.99 | 61.86 | 1.13 | 37.07 | 28.65 | 1.15 | 19.82 | 14.13 | 0.9 | 0.06 | 0.09 | 1.92 | 16.66 | 13.86 | 0.86 |
| stdev | 7.22 | 6.06 | 0.65 | 5.23 | 4.54 | 0.66 | 3.75 | 2.92 | 0.54 | 0.02 | 0.01 | 1.06 | 1.13 | 1.5 | 0.48 |
The excellent discrimination of the STN from the adjacent SN in the susceptibility maps allowed the STN and SN to be manually segmented and easily rendered in 3D together with the nearby red nuclei. One view of this 3D segmentation is shown in Figure 7. To address the inter‐subject variability of the size of the STN, the number of voxels of the manually segmented STN based on the susceptibility map was counted and multiplied by the voxel size (0.5 mm)3. The mean volume was found to be 48 ± 13 mm3, averaged over the eight subjects that were studied. The minimum and maximum volumes for the STN in either hemisphere are 31 and 72 mm3, respectively. There is thus a substantial variation in the STN volume of more than 100% across subjects. This agrees well with the existing literature values [Richter et al., 2004]. If one approximates the STN as an ellipsoid and takes the mean dimensions as given in [Richter et al., 2004], the volume of the STN is calculated to be 60 mm3.
Figure 7.

A 3D surface plot of one subject based on a manually segmented RN (red), SN (green), and STN (blue). The units of the axis are pixels (voxelsize = 0.5 × 0.5 × 0.5 mm3).
To compare the in vivo susceptibility distribution with iron concentration ex vivo within the STN a Perls staining image of the STN were taken from the literature [Dormont et al., 2004]. For a better visualization, the gray values of the in vivo susceptibility map were converted into a color map using a look‐up‐table. Figure 8 shows the in vivo magnitude data and susceptibility map for one subject in conjunction with the figure by Dormont et al. displaying iron distribution using a Perl's reaction (ex vivo) [Dormont et al., 2004].
Figure 8.

A comparison of local iron distribution in and ex vivo within the STN. Displayed are the in vivo magnitude image (left), susceptibility map (middle), and the ex vivo histology result using Perl's reaction taken from [Dormont et al., 2004] (with permissions). Note the similar pattern of gray values in the susceptibility map and Perl's staining, probably reflecting the iron distribution within the STN.
DISCUSSION
It is well known that iron yields hypointensities in the STN and SN in T2‐ and T2*‐weighted images [Dormont et al., 2004], because iron is paramagnetic and decreases the transverse relaxation times. Several studies have used T2‐ or T2*‐weighted imaging to visualize the STN in vivo. A recent study has developed a fast gray matter acquisition T1 inversion recovery sequence (FGATIR) to visualize subcortical structure for DBS targeting [Sudhyadhom et al., 2009]. Because of the proximity of the STN to the SN it is sometimes hard to distinguish between the two structures, especially in axial images. It has been shown that coronal images allow easier differentiation of the STN and SN [Coenen et al., 2004]. This is corroborated by our data. Using 3D acquisition it is possible to display views perpendicular to the acquired slice orientation without having “stripes” between the acquired slices. As depicted in Figures 1 and 3, the magnitude images produced using a gradient‐echo sequence give the clearest depiction of the STN when a coronal slice orientation is employed.
Phase images show differences in magnetic properties more directly and provide a higher contrast‐to‐noise ratio than the magnitude images in certain areas of the brain [Duyn et al., 2007]. A recent study investigated the use of phase information from gradient‐echo sequences for visualizing the STN in a qualitative manner [Vertinsky et al., 2009]. The mean scores of visualization and delineation of the STN were higher for phase images than for magnitude and susceptibility weighted images (SWI), the latter involving combination of magnitude and phase images [Haacke et al., 2004; Reichenbach et al., 1997]. Furthermore Manova et al. show excellent contrast in phase images for the substantia nigra [Manova et al., 2009]. The phase images of relatively thick slices (2 mm) in the study by Manova were acquired axial and high‐pass filtered with a homodyne filter, which is a disadvantage if one aims to calculate susceptibility maps [Schweser et al., 2011]. Unfortunately, phase images suffer from non‐local dipolar effects which mean that the images do not necessarily reflect the underlying anatomy [Schäfer et al., 2009]. These dipolar patterns can easily be calculated for simple structures such as spheres or cylinders, but are quite complicated for arbitrary shapes such as the STN or SN. Thus, care must be taken in relating structures in phase images to anatomical sources. The phase images in Figure 3 show dipolar patterns around the STN which do not directly reflect any underlying anatomical structures. Furthermore, the dipolar pattern changes with orientation to the main magnetic field. Thus, phase images even for the same subject may depend on the angling of the head inside the scanner. The ability to acquire images with a contrast that reflects differences in magnetic tissues properties more directly than T2‐ or T2*‐weighted images and that is independent of the orientation of the object to the B 0 field would be extremely valuable. Susceptibility maps provide this possibility since they potentially enable the direct measurement of concentration of para‐ and diamagnetic tissue properties while removing the orientation dependency. Thus the susceptibility mapping approach is appealing for visualization and investigation of brain areas with high iron concentrations such as the SN and STN. Figure 3 clearly shows that the non‐local effects evident in the phase images are removed in the susceptibility maps. In the sagittal view of the magnitude image (see Fig. 3), no sharp boundary between the SN and STN can be seen. In contrast, the susceptibility map of the same view allows clear discrimination of these structures. This is likely to be due to the different iron concentration in these areas as reflected in their fractional susceptibility difference [(χSTN − χSN)/χSN] of −0.4. This difference is remarkable compared to the fractional contrast of −0.06 and −0.2 that has previously been achieved using fast spin‐echo (FSE) and short inversion time inversion recovery (STIR) imaging, respectively [Kitajima et al., 2008]. Furthermore, the inter‐subject variability of the CNR of the STN and SN is larger for the susceptibility compared to the magnitude data. The susceptibility maps probably reflect differences in iron concentration more directly than the magnitude data [Haacke et al., 2005, 2010; Hopp et al., 2010; McAuley et al., 2010; Yao et al., 2009]. Note that these differences are most likely driven by inter‐individual differences in the iron content present in STN and SN, rather than artifacts arising from image acquisition and processing. This is corroborated by our repeated measurements which yield reproducible susceptibility values (Tables I and II).
Recently it has been shown that there are a number of potential sources of frequency differences in phase images besides iron concentration. These include the effects of exchange with macromolecules [Luo et al., 2009; Zhong et al., 2008], myelin content [Duyn et al., 2007], deoxyhemoglobin [Lee et al., 2010a; Marques et al., 2009; Petridou et al., 2010], tissue microstructure and anisotropic magnetic susceptibility [Lee et al., 2010b; Liu, 2010].
In particular, different frequency offsets can be detected in white matter structures depending on the orientation of the fibre bundles to the main magnetic field [He and Yablonskiy, 2009]. This has been attributed to the effect of anisotropic MR invisible inclusions [He and Yablonskiy, 2009] and more recently to an intrinsic anisotropy in the susceptibility of myelin [Lee et al., 2010b] in highly orientated structures like white matter. In gray matter regions, especially the SN and STN, no such strongly oriented microstructures present and previous susceptibility mapping indicates that the main frequency difference arises from differences in iron concentration in grey matter nuclei [Shmueli et al., 2009]. Assuming that susceptibility differences result mainly from elevated iron content in the STN and SN and that most of the iron detected is in Fe3+ form bound to ferritin [Duyn et al., 2007; Massey and Yousry, 2010; Zecca et al., 2001, 2004], our susceptibility measurements imply a ferritin content that is lower by 0.03 mg gtissue −1 in the STN compared to the SN. This agrees well with the recent study by Schweser et al. Assuming that myelin content is not a significant contribution to the contrast in the SN and STN like in gray/white matter contrast, the difference in putative iron content between the SN and STN can be calculated using Eq. (17) in [Schweser et al., 2011]. Our calculated susceptibility difference of 0.04 ppm yields a difference in putative iron of 3.1 mg/100 gtissue.
Based on the excellent discrimination of the STN and SN, the compartments were manually segmented in three dimensions and rendered in 3D as shown in Figure 7. As demonstrated in Figure 8 not all parts of the STN have high iron content. To further improve the contrast it would be possible to combine the susceptibility map and the magnitude image, similar to the SWI processing, where the filtered phase image and the magnitude image are combined. This would avoid errors due to the non‐local effects of the phase images. Future work will concentrate on automatic segmentation of the STN, saving the time needed for manually drawing the masks, and also avoiding subjectivity in defining the structures. The clear boundaries of the SN and STN and their strikingly different susceptibilities should make this an easy task.
It is well known that the iron within the STN is not homogenously distributed. Dormont et al. showed in a histological study that the iron concentration is higher in the inferior part compared to the superior part of the STN [Dormont et al., 2004]. As shown in Figure 8, the in vivo susceptibility map of the STN reveals a very similar pattern compared to iron distribution using Perl's staining probably reflecting local iron heterogeneity within the STN. In future work, we aim to validate this iron distribution using the same post mortem brain for susceptibility mapping and histological Perl's staining. The in vivo susceptibility map in Figure 8 show significantly decreased susceptibility around the SN and STN. By comparing the ex vivo Perls staining image in Figure 8, it is obvious that the decreased susceptibility can not be attributed to a decreased iron content. The susceptibility maps are calculated by using the phase images (displaying the frequency‐shift). It has been shown that white matter microstructure [He and Yablonskiy, 2009], myelin content [Schweser et al., 2011], or even susceptibility anisotropy of WM [Lee et al., 2010b; Liu, 2010] can contribute to the frequency‐shift measured in phase images. The decreased susceptibility of the white matter around the SN and STN may be explained by these different sources of white matter to the phase contrast and thus the susceptibility maps. However, we are not aware of any strong orientation or myelin content within the STN and thus, the susceptibility values should reflect almost true iron content.
Acknowledgements
The authors thank Prof. Dormont and the American Journal of Neuroradiology for the permission using the Figure 2 in [Dormont et al., 2004].
REFERENCES
- Coenen VA, Prescher A, Schmidt T, Picozzi P, Gielen FLH ( 2004): What is dorso‐lateral in the subthalamic Nucleus (STN)? A topographic and anatomical consideration on the ambiguous description of today's primary target for deep brain stimulation (DBS) surgery. Acta Neurochirurg 150: 1163–1165. [DOI] [PubMed] [Google Scholar]
- Cohen J ( 1960): A coefficient of agreement for nominal scales. Educ Psychol Meas 20: 37–46. [Google Scholar]
- de Rochefort L, Liu T, Kressler B, Liu J, Spincemaille P, Lebon V, Wu J, Wang Y ( 2010): Quantitative susceptibility map reconstruction from MR phase data using bayesian regularization: Validation and application to brain imaging. Magn Reson Med 63: 194–206. [DOI] [PubMed] [Google Scholar]
- Dormont D, Ricciardi KG, Tande D, Parain K, Menuel C, Galanaud D, Navarro S, Cornu P, Agid Y, Yelnik J ( 2004): Is the subthalamic nucleus hypointense on T2‐weighted images? A correlation study using MR imaging and stereotactic atlas data. AJNR Am J Neuroradiol 25: 1516–1523. [PMC free article] [PubMed] [Google Scholar]
- Duyn JH, van Gelderen P, Li TQ, de Zwart JA, Koretsky AP, Fukunaga M ( 2007): High‐field MRI of brain cortical substructure based on signal phase. Proc Natl Acad Sci USA 104: 11796–11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elolf E, Bockermann V, Gringel T, Knauth M, Dechent P, Helms G ( 2007): Improved visibility of the subthalamic nucleus on high‐resolution stereotactic MR imaging by added susceptibility (T2*) contrast using multiple gradient echoes. AJNR Am J Neuroradiol 28: 1093–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke EM, Xu YB, Cheng YCN, Reichenbach JR ( 2004): Susceptibility weighted imaging (SWI). Magnet Reson Med 52: 612–618. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Cheng NY, House MJ, Liu Q, Neelavalli J, Ogg RJ, Khan A, Ayaz M, Kirsch W, Obenaus A ( 2005): Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 23: 1–25. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Garbern J, Miao Y, Habib C, Liu M ( 2010): Iron stores and cerebral veins in MS studied by susceptibility weighted imaging. Int Angiol 29: 149–157. [PubMed] [Google Scholar]
- He X, Yablonskiy DA ( 2009): Biophysical mechanisms of phase contrast in gradient echo MRI. Proc Natl Acad Sci USA 106: 13558–13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp K, Popescu BF, McCrea RP, Harder SL, Robinson CA, Haacke ME, Rajput AH, Rajput A, Nichol H ( 2010): Brain iron detected by SWI high pass filtered phase calibrated with synchrotron X‐ray fluorescence. J Magn Reson Imaging 31: 1346–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M, Korogi Y, Kakeda S, Moriya J, Ohnari N, Sato T, Hayashida Y, Hirai T, Okuda T, Yamashita Y ( 2008): Human subthalamic nucleus: Evaluation with high‐resolution MR imaging at 3.0 T. Neuroradiology 50: 675–681. [DOI] [PubMed] [Google Scholar]
- Lee J, Hirano Y, Fukunaga M, Silva AC, Duyn JH ( 2010a): On the contribution of deoxy‐hemoglobin to MRI gray‐white matter phase contrast at high field. NeuroImage 49: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Shmueli K, Fukunaga M, van Gelderen P, Merkle H, Silva AC, Duyn JH ( 2010b): Sensitivity of MRI resonance frequency to the orientation of brain tissue microstructure. Proc Natl Acad Sci USA 107: 5130–5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C ( 2010): Susceptibility tensor imaging. Magnet Reson Med 63: 1471–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Spincemaille P, de Rochefort L, Kressler B, Wang Y ( 2009): Calculation of susceptibility through multiple orientation sampling (COSMOS): A method for conditioning the inverse problem from measured magnetic field map to susceptibility source image in MRI. Magn Reson Med 61: 196–204. [DOI] [PubMed] [Google Scholar]
- Luo J, He X, d'Avignon DA, Ackerman JJ, Yablonskiy DA ( 2009): Protein‐induced water (1)H MR frequency shifts: Contributions from magnetic susceptibility and exchange effects. J Magn Reson 202: 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manova ES, Habib CA, Boikov AS, Ayaz M, Khan A, Kirsch WM, Kido DK, Haacke EM ( 2009): Characterizing the mesencephalon using susceptibility‐weighted imaging. AJNR Am J Neuroradiol 30: 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques JP, Bowtell R ( 2005): Application of a Fourier‐based method for rapid calculation of field inhomogeneity due to spatial variation of magnetic susceptibility. Concept Magn Reson B 25B: 65–78. [Google Scholar]
- Marques JP, Maddage R, Mlynarik V, Gruetter R ( 2009): On the origin of the MR image phase contrast: An in vivo MR microscopy study of the rat brain at 14.1 T. NeuroImage 46: 345–352. [DOI] [PubMed] [Google Scholar]
- Massey LA, Yousry TA ( 2010): Anatomy of the substantia nigra and subthalamic nucleus on MR imaging. Neuroimaging Clin N Am 20: 7–27. [DOI] [PubMed] [Google Scholar]
- McAuley G, Schrag M, Sipos P, Sun SW, Obenaus A, Neelavalli J, Haacke EM, Holshouser B, Madacsi R, Kirsch W ( 2010): Quantification of punctate iron sources using magnetic resonance phase. Magn Reson Med 63: 106–115. [DOI] [PubMed] [Google Scholar]
- Ogg RJ, Langston JW, Haacke EM, Steen RG, Taylor JS ( 1999): The correlation between phase shifts in gradient‐echo MR images and regional brain iron concentration. Magn Reson Imaging 17: 1141–1148. [DOI] [PubMed] [Google Scholar]
- Petridou N, Wharton SJ, Lotfipour A, Gowland P, Bowtell R ( 2010): Investigating the effect of blood susceptibility on phase contrast in the human brain. NeuroImage 50: 491–498. [DOI] [PubMed] [Google Scholar]
- Rauscher A, Sedlacik J, Barth M, Mentzel HJ, Reichenbach JR ( 2005): Magnetic susceptibility‐weighted MR phase imaging of the human brain. AJNR Am J Neuroradiol 26: 736–742. [PMC free article] [PubMed] [Google Scholar]
- Reichenbach JR, Venkatesan R, Schillinger DJ, Kido DK, Haacke EM ( 1997): Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology 204: 272–277. [DOI] [PubMed] [Google Scholar]
- Richter EO, Hoque T, Halliday W, Lozano AM, Saint‐Cyr JA ( 2004): Determining the position and size of the subthalamic nucleus based on magnetic resonance imaging results in patients with advanced Parkinson disease. J Neurosurg 100: 541–546. [DOI] [PubMed] [Google Scholar]
- Salomir R, De Senneville BD, Moonen CTW ( 2003): A fast calculation method for magnetic field inhomogeneity due to an arbitrary distribution of bulk susceptibility. Concept Magn Reson B 19B: 26–34. [Google Scholar]
- Schäfer A, Wharton S, Gowland P, Bowtell R ( 2009): Using magnetic field simulation to study susceptibility‐related phase contrast in gradient echo MRI. NeuroImage 48: 126–137. [DOI] [PubMed] [Google Scholar]
- Schaltenbrand G, Wahren W ( 1977): Atlas for Stereotaxy of the Human Brain. Stuttgart: Thieme. [Google Scholar]
- Schweser F, Deistung A, Lehr BW, Reichenbach JR ( 2011): Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: An approach to in vivo brain iron metabolism? NeuroImage 54: 2789–2807. [DOI] [PubMed] [Google Scholar]
- Shmueli K, de Zwart JA, van Gelderen P, Li TQ, Dodd SJ, Duyn JH ( 2009): Magnetic susceptibility mapping of brain tissue in vivo using MRI phase data. Magn Reson Med 62: 1510–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL ( 1979): Intraclass correlations: Uses in assessing rater reliability. Psychol Bull 86: 420–428. [DOI] [PubMed] [Google Scholar]
- Sudhyadhom A, Haq IU, Foote KD, Okun MS, Bova FJ ( 2009): A high resolution and high contrast MRI for differentiation of subcortical structures for DBS targeting: The fast gray matter acquisition T1 inversion recovery (FGATIR). NeuroImage 47( Suppl 2): T44–T52. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐Planar Stereotactic Atlas of the Human Brain. Stuttgart: Thieme. [Google Scholar]
- Temel Y, Blokland A, Steinbusch HW, Visser‐Vandewalle V ( 2005): The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog Neurobiol 76: 393–413. [DOI] [PubMed] [Google Scholar]
- Vertinsky AT, Coenen VA, Lang DJ, Kolind S, Honey CR, Li D, Rauscher A ( 2009): Localization of the subthalamic nucleus: Optimization with susceptibility‐weighted phase MR imaging. AJNR Am J Neuroradiol 30: 1717–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz S, Hattingen E, Preibisch C, Gasser T, Deichmann R ( 2009): Reduction of susceptibility‐induced signal losses in multi‐gradient‐echo images: Application to improved visualization of the subthalamic nucleus. NeuroImage 45: 1135–1143. [DOI] [PubMed] [Google Scholar]
- Wharton S, Bowtell R ( 2010): Whole‐brain susceptibility mapping at high field: A comparison of multiple‐ and single‐orientation methods. NeuroImage 53: 515–525. [DOI] [PubMed] [Google Scholar]
- Wharton SJ, Schäfer A, Bowtell RW ( 2010): Susceptibility mapping in the human brain using threshold‐based k‐space division. Magn Reson Med 63: 1292–1304. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR ( 2006): Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron 52: 197–204. [DOI] [PubMed] [Google Scholar]
- Witoszynskyj S, Rauscher A, Reichenbach JR, Barth M ( 2009): Phase unwrapping of MR images using Phi UN‐a fast and robust region growing algorithm. Med Image Anal 13: 257–268. [DOI] [PubMed] [Google Scholar]
- Yao B, Li TQ, Gelderen P, Shmueli K, de Zwart JA, Duyn JH ( 2009): Susceptibility contrast in high field MRI of human brain as a function of tissue iron content. NeuroImage 44: 1259–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR ( 2004): Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci 5: 863–873. [DOI] [PubMed] [Google Scholar]
- Zecca L, Gallorini M, Schunemann V, Trautwein AX, Gerlach M, Riederer P, Vezzoni P, Tampellini D ( 2001): Iron, neuromelanin and ferritin content in the substantia nigra of normal subjects at different ages: Consequences for iron storage and neurodegenerative processes. J Neurochem 76: 1766–1773. [DOI] [PubMed] [Google Scholar]
- Zhong K, Leupold J, von Elverfeldt D, Speck O ( 2008): The molecular basis for gray and white matter contrast in phase imaging. NeuroImage 40: 1561–1566. [DOI] [PubMed] [Google Scholar]


