Abstract
We used functional magnetic resonance imaging (fMRI) to investigate brain processes underlying control of emotional responses towards a person in distress by cognitive social distance modulation. fMRI and peripheral physiological responses (startle response and electrodermal activity) were recorded from 24 women while they watched victim–offender scenes and modulated their social distance to the victim by cognitive reappraisal. We found that emotional responses, including startle eyeblink and amygdala responses, can effectively be modulated by social distance modulation. Furthermore, our data provide evidence that activity in the dorsomedial prefrontal cortex (dmPFC) and the anterior paracingulate cortex (aPCC), two brain regions that have previously been associated with brain processes related to distant and close others, is differentially modulated by intentional social distance modulation: activity in the dmPFC increased with increasing disengagement from the victim and activity in the aPCC increased with increasing engagement with the victim. We suggest that these two regions play opposing roles in cognitive modulation of social distance and affective responses towards persons in distress that enable the adaptive and flexible social behavior observed in humans. Hum Brain Mapp 33:2464–2476, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: empathy, social cognition, emotion regulation, paracingulate cortex, medial prefrontal cortex, amygdala
INTRODUCTION
Empathy, i.e., the ability to share another person's affective state, is considered to be a mediator of prosocial behavior [Decety and Jackson, 2004; Leiberg and Anders, 2006; Preston and de Waal, 2002]. Psychological theories stress, however, that in addition to empathy, active regulatory processes are required to enable flexible and adaptive human social behavior. For example, if observers of a person in distress are overwhelmed by their own emotional response, the positive relation between empathy and helping behavior is inverted [Batson, 1991; Eisenberg and Fabes, 1998]. In that case, active down‐regulation of vicarious emotional responses might be necessary to promote prosocial behavior. Conversely, if contextual factors such as a person's physical appearance dampen empathic responses to a person in distress, active up‐regulation of these responses might facilitate helping behavior. Effective control of vicarious emotional responses might thus constitute an important prerequisite for the complex social behavior required by modern human societies.
Despite the growing interest in the neurobiology of social behavior, the neurobiological bases of control processes that enable down‐ and up‐regulation of emotional responses towards people in distress are not well investigated. Behavioral and neuroimaging studies show that cognitive reappraisal, i.e. the intentional reinterpretation of an event or situation in a way that their emotional effect is diminished or amplified [Ochsner and Gross, 2005], can effectively modulate affective peripheral physiological responses [e.g., Dillon and LaBar, 2005; Eippert et al., 2007; Jackson et al., 2000; Kalisch et al., 2005] and brain activity in affect‐related brain regions such as the amygdala [Eippert et al., 2007; Kim and Hamann, 2007; Ochsner et al., 2002, 2004b; Phan et al., 2005; Urry et al., 2006]. A number of neuroimaging studies point to a role of different prefrontal cortices in these control processes. Particularly, the dorsolateral prefrontal cortex (dlPFC) and lateral orbitofrontal cortex (lOFC) seem to play a general role in down‐ and up‐regulation of affective responses [Eippert et al., 2007; Kim and Hamann, 2007; Ochsner et al., 2004b; Urry et al., 2006]. At the same time, different subsystems of the prefrontal cortex seem to participate in affective control processes, depending on the type of reappraisal involved. For example, self‐focused reappraisal (i.e., the reinterpretation of the meaning of an emotional situation for oneself) seems to preferentially activate medial prefrontal cortex, whereas situation‐focused reappraisal (i.e., the reinterpretation of the situation per se) seems to preferentially activate lateral prefrontal cortex [Ochsner et al., 2004b]. Neuroimaging studies on affective responses towards other people, particularly towards other people in pain, point to a role of the anterior insula in empathy [Fan et al., 2011; Lamm et al., 2011]. The anterior insula has also been shown to subserve cognitive‐regulatory processes [Anderson et al., 2004; Lerner et al., 2009]. However, if and how the insula participates in control of emotional responses towards other people is currently unknown.
One important psychological dimension in control of emotional responses towards other people might be similarity [Liviatan et al., 2008], or perceived social distance [Trope and Liberman, 2010], i.e., how close or distant the observer feels to the other person. Behavioral studies provide evidence for a negative relation between perceived social distance and strength of the empathic response: the more distant an observer feels to a person in distress the weaker is his empathic response [Batson et al., 1995; Cialdini et al., 1997]. Moreover, there is evidence from neuroimaging studies that empathic responses decrease when the person in distress has previously behaved unfairly towards the observer [Singer et al., 2006]. Thus, cognitive reappraisal acting on perceived social distance might be highly relevant for the regulation of emotional responses directed towards other people.
Recent neuroimaging studies have identified brain regions that might play a specific role in such ‘interpersonal reappraisal.’ For example, there is evidence that distinct regions of the medial prefrontal cortex respond when participants are asked to think about people they perceive as either dissimilar or similar to themselves [Mitchell et al., 2005, 2006]. Specifically, thinking and making inferences about people perceived as dissimilar to oneself seems to be associated with neural activity in the posterior dorsal medial prefrontal cortex (dmPFC) (comprising the part of the superior medial frontal gyrus that corresponds to BA 8 [Mitchell et al., 2005, 2006]). In contrast, thinking and making inferences about people perceived as similar to oneself seems to be associated with neural activity in a more ventral region of the medial prefrontal cortex located at the transition between anterior cingulate cortex and the frontal poles corresponding to BA32/10 (hereafter referred to as anterior paracingulate cortex [aPCC; e.g. Frith and Frith, 2003]). Moreover, these data suggest that activity in the dmPFC is linearly related to perceived dissimilarity [Mitchell et al., 2006], while activity in the aPCC is linearly related to perceived similarity [Mitchell et al., 2006; Mobbs et al., 2009].
The current study aimed to investigate neural processes underlying the control of emotional responses towards persons in distress by cognitive modulation of social distance. Particularly, we were interested whether intentionally increasing and decreasing one's social distance to a person in distress would be linearly associated with increases and decreases of activity in the dmPFC and aPCC, and corresponding decreases and increases of affective responses. Such a finding would provide evidence that the dmPFC and aPCC are not only differentially associated with brain processes related to dissimilar and similar others, but that activity in these regions can actively be modulated by interpersonal reappraisal, which in turn might lead to down‐ and up‐regulation of emotional responses towards the other person.
Participants viewed pictures of violent victim–offender scenes and were asked to disengage from, view, or engage with the victim during functional magnetic resonance imaging (fMRI). We predicted (i) that social distance modulation would lead to down‐ and up‐regulation of affective peripheral physiological responses and activity in the amygdala and, possibly, insula, (ii) that intentional social disengagement and engagement would both activate prefrontal brain regions involved in control and regulation (i.e. dlPFC and lOFC), and (iii) that intentional social distance modulation would be associated with a linear modulation of activity in the dmPFC and the aPCC (i.e., increase of activity with increasing disengagement in the dmPFC and increase of activity with increasing engagement in the aPCC). To further test whether the dmPFC/aPCC and the amygdala and/or insula are functionally connected during social distance modulation, we also performed an inter‐individual correlation analysis of activity in these regions.
METHODS
Participants
We investigated 24 healthy right‐handed volunteers (age: 18–33 years; mean: 24.1 years) recruited through advertisements from the University of Tübingen and the surrounding community. In order to reduce between‐subject variance possibly arising from gender differences in emotional tasks [Kring and Gordon, 1998], only women were studied. A phone interview clarified that all participants were right‐handed, did not have any neurological history and no contraindication for fMRI. All participants gave written informed consent prior to participation and were paid € 30. The study was approved by the ethics committee of the University of Tübingen Medical Faculty. To ensure that participants were a representative sample of the population with regard to empathy, all participants were asked to complete the Interpersonal Reactivity Index [IRI; Davis, 1983; German version by Paulus, 2009]. Participants' self‐reported trait empathy deviated less than one standard deviation (sd) from the norm on all four subscales of the IRI (fantasy: 20.26 ± 4.33 [mean ± sd]; empathic concern: 19.26 ± 4.74; perspective taking: 18.87 ± 3.25; and personal distress: 13.65 ± 4.85). Hence the sample of the current study can be regarded as being representative of the population.
Stimuli
Stimuli consisted of negative and neutral pictures taken from the International Affective Picture System [IAPS; Lang et al., 2005] and the internet. Negative stimuli depicted war scenes or other violent scenes in which one to five offenders threatened one to three victims with a gun, knife, or fists. In all scenes, victims showed visible signs of distress in their facial expression and/or body posture. Neutral stimuli depicted everyday‐life scenes (e.g., at the workplace, in the car, doing sports) with one to five persons. In all scenes the faces of the depicted persons were clearly visible. In a pilot study, 100 pictures (78 violent victim–offender scenes and 22 neutral scenes) were rated by 48 female students with regard to the unpleasantness (valence) and arousal they elicited on scales ranging from unpleasant (1) to pleasant (9) and calm (1) to aroused (9) [Self‐Assessment Manikin (SAM); Bradley and Lang, 1994]. Based on these ratings three victim–offender sets (each consisting of 12 victim–offender scenes, mean valence: 2.42 ± 0.08 s.e.m. [standard error of mean], mean arousal: 6.05 ± 0.09 s.e.m.) and a single neutral set (consisting of 12 neutral scenes, mean valence: 5.32 ± 0.12 s.e.m., mean arousal: 2.90 ± 0.11 s.e.m.) were compiled. The victim–offender sets were balanced for valence (set 1, mean valence: 2.6; set 2, mean valence: 2.3; set 3, mean valence: 2.3), arousal (set 1, mean arousal: 5.9; set 2, mean arousal: 6.3; set 3, mean arousal: 6.1), and content (i.e., type of violence, sex of victim(s), and number of depicted persons). Different victim–offender sets were used for different types of regulation (disengagement from the victim, viewing without regulation, engagement with the victim). This assignment was counterbalanced across participants. Three pseudo‐randomized series of 16 stimuli (four stimuli from each victim–offender set and four stimuli from the neutral set) were created, with the restriction that no more than three stimuli from a set could occur in a row. The order of stimulus series was counterbalanced across participants.
Regulation Task
Participants were asked to cognitively regulate their social distance to the victim according to single word instructions—disengage, view, or engage—using the following strategies. Disengage (“Distanzieren”): participants should disengage from the victim, i.e., increase their social distance to the victim, by becoming a detached observer through thinking that the depicted person is an actor or a doll. View (“Betrachten”): participants should view the picture attentively without trying to alter their emotional reactions. Engage (“Hineinversetzen”): participants should engage with the victim, i.e., decrease their social distance to the victim, by feeling themselves into the victim's place. Instructions were chosen to match instructions used in previous studies on emotion regulation [e.g., Eippert et al., 2007; Jackson et al., 2000; Johnstone et al., 2007; Kim and Hamann, 2007], except that the focus of reappraisal in the current study was on the participant's social distance to the victim. To ensure that participants understood the strategies they were to use, and to give them practice in using these strategies, they received a training session 1–4 days before scanning with stimuli that were not used in the experiment. The training ended with completion of 16 practice trials that were similar to one experimental run.
Experimental Paradigm
The experimental paradigm (Fig. 1) was the same as that used by Eippert et al. [2007] (originally derived from Jackson et al. [2000]) except that the current study used pictures and instructions modified for investigation of social distance regulation as described above. Participants underwent three fMRI runs, each consisting of 16 trials. Pictures were projected onto a translucent screen at the rear end of the scanner which subjects could see through a tilted mirror mounted on the headcoil. At the beginning of a trial, a scene appeared in the center of the screen (pre‐regulation phase). After 2.5 s, the instruction (“disengage,” “view,” or “engage”) appeared in the center of the scene signaling the participants to view the scene or to modulate their social distance to the victim according to the practiced strategies. Neutral scenes should always be viewed attentively without trying to alter emotional responses. After 0.5 s, the instruction was replaced with a red cross‐hair for the 6 s regulation phase; during viewing and regulation participants had to fixate this cross‐hair to allow for startle recordings. Participants were told before the experiment not to close their eyes or avert them, and to view the scenes attentively the whole time, regardless of instruction type. A startle probe [a 50 ms white noise burst, loudness adjusted individually to reliably elicit startle eyeblinks and to be unpleasant but not painful; Anders et al., 2004b] was presented through headphones [HD 570, Sennheiser, Germany; modified after Baumgart et al., 1998] at 2 s into the regulation phase. After the regulation phase, the scene was replaced for 3 s by a scale on which participants rated their success in regulation according to the change of their emotional response towards the victim (1 [not successful at all] to 5 [very successful]) by pushing a button in their right hand. The starting point of the scale was “1” so that participants had to press the button once for rating “2,” twice for rating “3,” etc. No success ratings were obtained for view trials, but to hold motor activation similar across conditions, participants were asked to push the button twice in these trials (following the design of Eippert et al. [2007]). After rating, a gray rectangle appeared on the screen for 10–14 s indicating the participants to relax.
Figure 1.
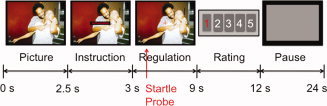
Experimental paradigm. Pictures were presented for 2.5 s, after which the instruction word (disengage, engage or view) appeared in the center of the picture for 0.5 s. From this point on participants were to regulate their social engagement with the victim for 6 s; at 2 s into the regulation phase an acoustic startle probe was delivered. After the regulation phase participants were asked to rate their success in regulation on a scale from 1 to 5 by button presses. During the inter‐trial interval a grey square was shown (10–14 s), indicating the participants to relax. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Data Acquisition
Whole‐brain fMRI was performed on a 1.5 Tesla scanner (Magnetom Sonata, Siemens, Erlangen, Germany). One hundred and one (101) functional T2*‐weighted volumes were acquired during each of the three runs (echo‐planar imaging [EPI], repetition time [TR] 4 s, 44 coronal slices, slice thickness 3 mm, 0.5 mm gap, field of view [FOV] 192 mm, matrix 64 × 64, in‐plane resolution 3 × 3 mm2, flip angle 90°, echo time [TE] 45 ms). The first five volumes of each run were discarded prior to image analysis to reduce T1 saturation effects. In addition to functional images, peripheral physiological responses were recorded during scanning. Startle data were recorded at 1000 Hz from the participants' right eye with infrared oculography (IOG)‐based fMRI compatible equipment (Eyetracker, Cambridge Research Systems, Cambridge, UK; [Anders et al., 2004b]). Electrodermal activity (EDA) was recorded at 16 Hz with fMRI compatible equipment (Varioport, Becker Meditec, Karlsruhe, Germany) with standard Ag/AgCl electrodes filled with unibase electrolyte affixed to the thenar and hypothenar of the left hand.
Analysis of Peripheral Physiological Data and Self‐Reports
Startle and EDA analysis was performed with Matlab 6.5 (The Mathworks, Natick, MA). Startle data were smoothed with a 10 ms full width at half maximum (FWHM) isotropic Gaussian kernel and visually inspected for artifacts by two of the authors (S.L. and S.A.). Eye blinks that did not show a Gaussian‐shaped time course were excluded from the analysis. Data of six participants and 12% of the eye blinks of the remaining participants were discarded due to this criterion. Startle amplitude was determined as maximum differential voltage in an interval from 21 ms to 120 ms after startle probe onset, relative to the average differential voltage during a 20 ms baseline beginning with startle probe onset. All startle amplitudes were scaled to the mean of the respective run. EDA data were smoothed with a Gaussian kernel with a FWHM of 200 ms. EDA responses were determined as the difference between the maximum in the regulation phase (4,000–10,000 ms after picture onset) and the maximum in the pre‐regulation phase (1,000–3,500 ms after picture onset). All EDA responses were log transformed (log[1+EDA[μS]]). All trials were included in the analysis, regardless of the size of the response [i.e., reported values are a measure of EDA magnitude; Dawson et al., 2000]. All analyses of peripheral physiological data followed the procedures described by Anders et al. [2004a] and Eippert et al. [2007].
To test whether victim–offender scenes elicited stronger affective peripheral physiological responses than neutral scenes, we compared peripheral physiological responses (startle and EDA) during viewing of victim–offender scenes and neutral scenes without regulation (paired T‐test). To test for effects of social distance modulation on peripheral physiological responses, we computed linear contrasts within one‐way repeated measures analyses of variance (ANOVA) with levels disengage, view and engage, analogous to the linear contrasts used to test for effects of intentional disengagement and engagement on brain activity (see below). Paired T‐tests were computed to test for differences in self‐reported regulation success between the disengage and engage condition.
fMRI Data Analysis
Image preprocessing and statistical analysis were performed with Statistical Parametric Mapping software (SPM2, Wellcome Department of Imaging Neuroscience, London, UK). Preprocessing of functional data included spatial realignment and unwarping [Andersson et al., 2001], spatial normalization to a standard template [3 × 3 × 3 mm3 voxel size, Montreal Neurological Institute; Collins et al., 1994] and spatial smoothing with an isotropic Gaussian kernel (FWHM 12 mm). In a previous study, we had ascertained that the startle probes do not introduce additional head movements [Eippert et al., 2007].
A general linear model that accounted for low‐frequency drifts (cut‐off period 128 s) and first‐order temporal autocorrelations was applied to the time series of each voxel, separately for each participant. Each model included five regressors of interest for each run: (i) pre‐regulation viewing of neutral scenes, (ii) pre‐regulation viewing of victim–offender scenes, (iii) intentional disengagement from the victim, (iv) viewing of victim–offender scenes (no regulation), and (v) intentional engagement with the victim. Please note that pre‐regulation viewing of all victim–offender scenes of a given run was modeled by the same regressor, independent of the subsequent regulation condition; thus, each of the three regulation regressors (iii–v) was only weakly correlated with the pre‐regulation regressor. In addition to the five regressors of interest, four regressors of no interest were included for each run: (vi) instruction presentation, (vii) viewing of neutral scenes, (viii) rating phase, and (ix) startle probe. Pre‐regulation viewing, regulation and the rating phase were modeled as box‐car functions (duration 2.5 s, 6 s, and 3 s, respectively) convolved with a canonical hemodynamic response function (hrf) as implemented in SPM2. The instruction and the startle probe were modeled as stick functions convolved with the hrf. The largest correlation in the filtered design matrix between a regressor of interest and any other regressor was r = 0.45.
In these first‐level models, six contrasts were computed for each participant: (1) pre‐regulation viewing of victim–offender scenes minus pre‐regulation viewing of neutral scenes (negative − neutral), (2) disengagement, (3) viewing, (4) engagement, (5) disengagement minus viewing (disengage − view), and (6) engagement minus viewing (engage − view). These contrasts were used for group analyses as described below.
First, we used a one‐sample T‐test on the negative–neutral contrast to test whether the victim–offender scenes evoked initial emotional responses towards the victim in the pre‐regulation phase in the amygdala and insula. Second, we wanted to test for brain regions that showed a common increase of brain activity during social disengagement and engagement. For this, we computed a one‐way repeated measures ANOVA with levels disengage, view, and engage. Within this ANOVA, we defined two contrasts, engagement minus viewing (engage − view) and disengagement minus viewing (disengage − view). A conjoint conjunction analysis [minimum statistic compared to the conjunction null; Nichols et al., 2005] was performed on these two contrasts, testing for common increases of brain activity during disengagement and engagement.
Third and fourth, we searched for brain activity that was linearly modulated by social distance modulation. For this, we performed two analyses. First, we searched for brain activity that was linearly related to the level of disengagement/engagement. To this end, we defined two linear contrasts in the ANOVA described above, disengage–view–engage (modeling an increase of activity from engaging over viewing to disengaging) and engage–view–disengage (modeling an increase of activity from disengaging over viewing to engaging). Please note that because these linear contrasts were modeled in the ANOVA (not in the first‐level models) they tested for linear increases/decreases across the three conditions. Second, we searched for brain activity that correlated with self‐reported success of disengagement/engagement across participants. Therefore, we computed two separate random‐effects regression analyses that used each participant's average success rating during disengagement and engagement, respectively, to predict the degree of increase/decrease of brain activity during disengagement (individual contrast disengage−view) and engagement (individual contrast engage−view).
Finally, we performed an inter‐individual analysis of functional connectivity [Atique et al., 2011; Zaki et al., 2007] between the dmPFC and aPCC and amygdala and insula during disengagement and engagement, respectively. For this, we averaged individual parameter estimates for each participant across all activated voxels in the respective region and computed two correlation analyses across participants, one between the dmPFC and the amygdala/insula (using individual disengage–view parameter estimates) and one between the aPCC and the amygdala/insula (using individual engage–view parameter estimates). For the dmPFC, all voxels were included that showed significant activity in the linear contrast disengage–view–engage. Because we did not find significant activity in the aPCC in the contrast engage–view–disengage, for the aPCC all voxels were included that showed significant activity in the regression analysis. For the amygdala and the insula, all voxels were included that showed significant activity in the negative–neutral contrast in the pre‐regulation phase.
AAL software [Automated Anatomical Labeling; Tzourio‐Mazoyer et al., 2002] was used to demarcate three regions of interest (ROIs): the amygdala, the insula, and the prefrontal cortex. For the amygdala and the insula, the bilateral regions defined in the AAL software were used. The AAL insula ROI comprises the gray matter internal to the circular sulcus, including the anterior and the posterior insula. For the prefrontal region, we combined the AAL regions for the medial superior, superior, middle and inferior frontal gyri; medial, superior and middle orbitofrontal gyri; and gyri recti bilaterally. To test for predicted effects (including the conjunction) in regions of interest, we used an uncorrected voxel‐wise height threshold of P = 0.001 (corresponding to T = 3.5) with an additional extent threshold of k = 10 contiguous voxels in the prefrontal cortex. Effects outside these regions are reported in the Results section and in the Supporting Information tables if they meet the statistical criteria used in the prefrontal cortex ROI, but are not further discussed. Coordinates are given in MNI space [Montreal Neurological Institute; Collins et al., 1994], and group activation peaks were labeled with AAL software.
RESULTS
Self‐Reported Success
Overall, participants rated their success in social distance regulation (according to changes in their emotional responses towards the victim) as 3.68 ± 0.08 s.e.m. (on a scale from 1 to 5). There was no significant difference in success ratings between disengagement (3.74 ± 0.57 s.e.m.) and engagement (3.63 ± 0.60 s.e.m.) (t[23] = 0.75, P = 0.46).
Peripheral Physiological Responses
Participants showed stronger startle responses when they viewed victim–offender scenes than when they viewed neutral scenes (N = 18, victim‐offender scenes: 101.06 ± 3.6 s.e.m., neutral scenes: 94.0 ± 4.6 s.e.m., t[17] = 1.72, P = 0.05, one‐sided) (from six participants no usable startle data were obtained, see Methods section). EDA responses did not differ significantly between victim–offender scenes and neutral scenes (N = 24, victim‐offender scenes: 0.09 ± 0.03 s.e.m., neutral scenes: 0.04 ± 0.03 s.e.m., t[23] = 0.68, P = 0.13, one‐sided).
Importantly, participants successfully modulated their startle responses by regulating their social distance to the victim: Startle amplitudes were smallest when participants disengaged from the victim (98.2 ± 5.7 s.e.m.), medium for view (101.0 ± 3.6 s.e.m.), and largest when participants engaged with the victim (114.6 ± 5.1 s.e.m.) (linear contrast, partial η2 = 0.19, t[17] = 1.99, P = 0.03, one‐tailed) (Fig. 2A). EDA responses also showed a significant linear increase from disengage (0.12 ± 0.04 s.e.m.) over view (0.09 ± 0.03 s.e.m.) to engage (0.21 ± 0.06 s.e.m.) (linear contrast, partial η2 = 0.21, t[23] = 2.45, P = 0.02, one‐tailed) (Fig. 2B).
Figure 2.
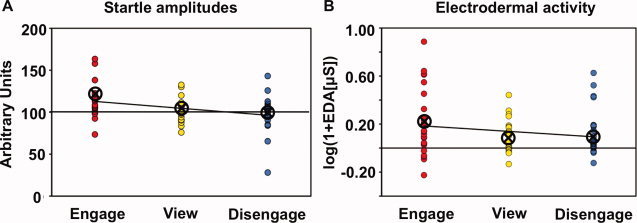
Effects of social distance modulation on peripheral physiological responses. A: Startle amplitudes. Startle amplitudes linearly decreased from engaging over viewing to disengaging. B: EDA. EDA responses linearly decreased from engaging over viewing to disengaging. Each dot depicts one participant's mean response for the specific condition. Lines represent linear fits. Circled crosses represent group means. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Brain Activity
Activity in the pre‐regulation phase
In the pre‐regulation phase, activity in the left amygdala (x = −33, y = −6, z = −12, T = 3.60, cluster size, k = 1) and in bilateral dorsal anterior insula [Kurth et al., 2010; Mesulam and Mufson, 1982] (x = −30, y = 24, z = 3, T = 7.12, k = 263; x = 39, y = 24, z = 0, T = 4.75, k = 69) was stronger when participants viewed victim–offender scenes than when they viewed neutral scenes. Outside these ROIs, the thalamus, middle temporo‐occipital regions, the precuneus, and the supplementary motor area (SMA) were more strongly activated when participants viewed victim–offender scenes than when they viewed neutral scenes (Table S1).
Common activity during social disengagement and engagement
None of the ROIs showed common activity during disengagement and engagement. Outside the predefined ROIs, the right SMA was the only region that showed an increase of activity during both disengagement and engagement (Table S2).
Modulation of activity by social distance modulation: Linear increases/decreases
As predicted, activity in the amygdala was linearly modulated by social distance modulation: activity in the left amygdala was lowest when participants disengaged from the victim and highest when participants engaged with the victim (x = −27, y = −3, z = −27, T = 3.78, k = 10, Fig. 3 and Table S3). No such effect was observed in the insula. The converse effect, a linear increase of activity with increasing disengagement was observed in the dmPFC (medial superior frontal gyrus, BA 8, x = 0, y = 30, z = 48, T = 4.34, k = 30), right dlPFC (right middle frontal gyrus, BA 46, x = 39, y = 48, z = 6, T = 3.94, k = 25), and lOFC (left middle orbitofrontal gyrus, BA 11, x = −36, y = 60, z = −12, T = 4.82, k = 27) (Fig. 3 and Table S3). Outside the predefined ROIs, occipito‐temporal regions, the cingulate cortex, posterior SMA, and the cerebellum showed a similar effect as the amygdala, i.e. a linear increase of activity with increasing engagement (Table S3).
Figure 3.
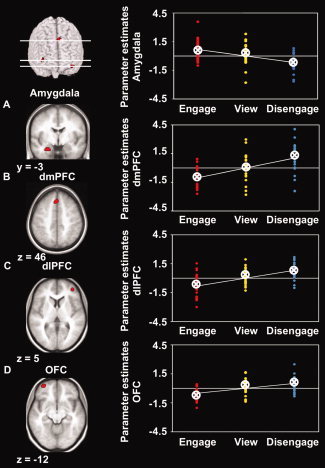
Statistical parametric maps (SPMs) (left) and individual mean parameter estimates (right) of brain activity during engagement, viewing, and disengagement in regions of interest. A: Significant linear increase of activity during engagement (contrast engage‐view‐disengage) in the left amygdala. B–D: Significant linear increase of activity during disengagement (contrast disengage–view–engage) in the dorsomedial prefrontal cortex (dmPFC) (B), dorsolateral prefrontal cortex (dlPFC) (C), and left orbitofrontal cortex (OFC) (D). SPMs are shown with a height threshold of T = 3.5, corresponding to P = 0.001, uncorrected, and an additional extent threshold of k = 10 contiguous voxels for the prefrontal cortex, and are overlaid on the participants' mean structural scan. For visualization, the SPM of amygdala activity is masked with the amygdala region‐of‐interest mask derived from the AAL toolbox. Each dot depicts one participant's mean parameter estimate for the specific condition at the peak voxel of the respective region. Lines represent linear fits. Circled crosses represent group means. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Modulation of activity by social distance modulation: Correlation with self‐reported success
Correlation analyses across participants revealed a significant increase of brain activity with increasing self‐reported success during engagement in the left aPCC (medial superior frontal gyrus, BA 10, x = −15, y = 42, z = 15, T = 5.53, k = 22) and right ventromedial PFC (vmPFC; medial orbitofrontal gyrus, BA 11, x = 15, y = 45, z = −9, T = 4.36, k = 96) (Fig. 4). No significant other correlation between brain activity and self‐reported success was observed within or outside the predefined ROIs.
Figure 4.
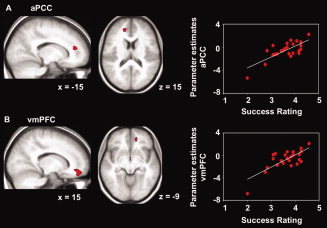
Statistical parametric maps (SPMs) (left) and scatter plots (right) of self‐reported success and brain activity during engagement in prefrontal cortex. A: Significant correlation between self‐reported success and brain activity during engagement (contrast engage‐view) in the anterior paracingulate cortex (aPCC). B: Significant correlation between self‐reported success and brain activity during engagement (contrast engage‐view) in the ventromedial prefrontal cortex (vmPFC). SPMs are shown with a height threshold of T = 3.5, corresponding to P = 0.001, uncorrected, and an additional extent threshold of k = 10 contiguous voxels, and are overlaid on the participants' mean structural scan. Scatter plots show parameter estimates of each participant at the peak voxel of the respective region plotted against self‐reported success in engaging. Lines represent linear fits. The smallest correlation detectable with the statistical threshold used in the current analysis (height threshold T = 3.5) is r = 0.60. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Functional connectivity between dmPFC/aPCC and amygdala/insula
To further investigate whether the observed increases and decreases of dmPFC and aPCC activity during social distance regulation were directly associated with modulation of amygdala and insula activity (as observed in the pre‐regulation phase), we performed an analysis of functional connectivity across participants. As predicted, increasing brain activity in the dmPFC during disengagement was significantly positively correlated with decreasing activity in the amygdala (N = 24, r[22] = 0.49, P = 0.01) (Fig. 5) and with decreasing activity in the anterior insula (right anterior insula: N = 24, r[22] = 0.47, P = 0.02; left anterior insula: N = 24, r[22] = 0.50, P = 0.01) in this condition. No correlation was observed between activity in the aPCC and amygdala or insula during engagement.
Figure 5.
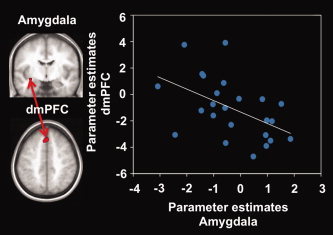
Scatter plot of brain activity during disengagement in the dorsomedial prefrontal cortex (dmPFC) and amygdala. Statistical parametric maps (SPMs) on the left indicate clusters used for parameter extraction (see text). The scatter plot shows parameter estimates of each participant during disengagement (contrast disengage‐view). The line represents the linear fit. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
The current study investigated brain processes underlying the control of emotional responses towards victims of violence by intentional social distance modulation. Participants watched violent victim–offender scenes and were asked to cognitively modulate their social distance to the victim. When viewing these scenes participants showed stronger startle responses and stronger activity in emotion‐related brain regions, including the amygdala and the dorsal anterior insula, than when viewing neutral social scenes. By intentionally increasing and decreasing their social distance to the victim participants successfully modulated their emotional responses: startle responses and activity in the left amygdala decreased when participants disengaged themselves from the victim, and increased with increasing engagement. The converse effect was observed in the dmPFC, dlPFC, and lOFC. Here, activity increased when participants disengaged themselves from the victim, and decreased with increasing engagement. Moreover, the increase of dmPFC activity during disengagement was positively correlated with decreasing amygdala and insula activity. Additionally, activity in the aPCC and the vmPFC was positively correlated with self‐reported success in engaging.
Peripheral Physiological Responses and Activity in the Amygdala and Insula
Up‐ and down‐regulation of affective peripheral physiological responses [Dillon and LaBar, 2005; Eippert et al., 2007; Jackson et al., 2000; Kalisch et al., 2005, 2006] and amygdala activity [Beauregard et al., 2001; Eippert et al., 2007; Kim and Hamann, 2007; Levesque et al., 2003; Ochsner et al., 2002, 2004b; Phan et al., 2005] by cognitive reappraisal has previously been reported in studies in which participants were asked to regulate their emotional responses to social and non‐social stimuli. In another line of research it has been shown that emotional brain responses towards other people can be modulated by several cognitive factors including cued shifts of attention and provision of contextual information [Gu and Han, 2007; Lamm et al., 2007]. The present study extends these findings by showing that affective peripheral physiological and amygdala responses towards victims of violence can intentionally be modulated by interpersonal reappraisal, i.e., by cognitively increasing or decreasing one's social distance to the other person.
Interestingly, although the dorsal anterior insula was more strongly activated by victim–offender scenes than neutral scenes in the pre‐regulation phase, we found no linear decreases and increases of insula activity during social distance modulation. The anterior insula has consistently been found to be activated when participants are asked to empathize with other people, particularly with persons in pain [for meta‐analyses see Fan et al., 2011; Lamm et al., 2011] and might thus be expected to be down‐regulated by social disengagement and up‐regulated by social engagement. However, the dorsal anterior insula has also been associated with attentional and regulatory processes, such as focusing on interoceptive processes [Critchley et al., 2004] and suppression of natural urges [Lerner et al., 2009]. Furthermore, there is evidence for overlapping activity in the insula during empathy and a wide variety of cognitive processes, including language and memory [Kurth et al., 2010]. To account for these observations, it has been suggested that the dorsal anterior insula constitutes an integration area where information about bodily states, personal motivation, and context is brought together [Craig, 2009]. In the current study, any linear modulation of insula activity might have been masked by integration‐ or regulation‐related increases of activity during both regulation conditions, or by even more general task‐related activity [see e.g. Dosenbach et al., 2006].
Activity in the dlPFC and OFC
Contrary to our hypothesis the dlPFC and the lOFC showed an increase of activity only during disengagement, but not during engagement. Both regions have consistently been seen co‐activated during non‐affective [Badre and Wagner, 2004; Harrison et al., 2005] and affective [Beauregard et al., 2001; Eippert et al., 2007; Goldin et al., 2008; Johnstone et al., 2007; Kim and Hamann, 2007; Levesque et al., 2003; Ochsner et al., 2002; Ochsner et al., 2004b; Ohira et al., 2006; Urry et al., 2006] control processes. Based on these findings, we hypothesized that the dlPFC and OFC would be commonly activated during both regulatory conditions.
Interestingly, some studies that investigated both up‐ and down‐regulation of emotion found increased dlPFC activity exclusively for either up‐ [Urry et al., 2006] or down‐regulation [Johnstone et al., 2007; Ochsner et al., 2004b], while others found dlPFC activity during both conditions (albeit at slightly different locations) [Eippert et al., 2007; Kim and Hamann, 2007]. Given these and the current findings one might speculate that the required level of control determines the strength of dlPFC‐OFC activity during regulatory processes. In the current study, intensifying a spontaneous emotional response towards the victim by social engagement might have required less effort than reversing this response by social disengagement. This assumption would also be in line with the study by Ochsner et al. [2004b] in which participants reported exerting significantly more effort during down‐ than during up‐regulation of emotional responses towards negative stimuli and where dlPFC activity was found only during down‐regulation. Alternatively, disengaging from the victim might have entailed some situation‐focused reappraisal (i.e. thinking that the depicted scene is not real), which has been suggested to preferentially activate lateral prefrontal regions (in contrast to self‐focused reappraisal, which is thought to preferentially activate medial prefrontal regions) [Ochsner et al., 2004b].
Activity in the Dorsomedial Prefrontal and Anterior Paracingulate Cortex
The focus of the current study was on the potential role of the dmPFC and aPCC in the intentional modulation of social distance. As predicted, activity in the dmPFC was linearly related to the level of disengagement from the victim. DmPFC activity was highest during disengagement and decreased over viewing to engagement. The dmPFC has been shown to be activated when participants think about a person they perceive as dissimilar to themselves, and its activity has been shown to be linearly related to perceived dissimilarity [Mitchell et al., 2006]. The current study provides evidence that dmPFC activity can actively be modulated by social distance regulation. Moreover, we found a significant positive correlation between the increase of dmPFC activity during disengagement and decreasing amygdala and insula activity during that condition. The correlation of dmPFC activity and amygdala activity during social disengagement in the present study is consistent with the idea that the dmPFC might be a mediator of the inverse relation between social distance and empathic responses observed at the behavioral level [Batson et al., 1995; Cialdini et al., 1997]. Interestingly, another study, in which participants were asked to judge the emotion of a person in a video, found that participants who scored higher on trait personal distress showed weaker dmPFC activity [Lawrence et al., 2006].
In addition to the predicted linear modulation of dmPFC activity during social distance modulation, we found a positive correlation between self‐reported success during engagement and activity in the aPPC. APCC activity has been observed during both self‐ and other‐related processing [Ames et al., 2008; David et al., 2006; Jenkins et al., 2008; Kelley et al., 2002; Lane et al., 1997; Mitchell et al., 2005; Ochsner et al., 2004a], and it has been suggested that this functional overlap occurs because people employ similar brain regions for other‐related processes and self‐related processes if they perceive the other person as sufficiently similar to themselves. In line with this, it has been shown that aPCC activity during other‐related reasoning is linearly related to perceived similarity [Mitchell et al., 2006; Mobbs et al., 2009]. In the current study, neural activity in the aPCC increased when participants successfully reduced their social distance to the victim. Thus, the current study provides evidence that not only dmPFC activity, but also aPCC activity can actively be modulated by intentional social engagement with people in distress. Taken together, these findings provide evidence that both the dmPFC and the aPCC are important nodes in social distance regulation and that the two regions might play opposing roles in the intentional control of empathic responses.
Activity in the vmPFC
Finally, a cluster located more ventrally in the vmPFC showed a similar response pattern as the aPCC. In contrast to the aPCC, the vmPFC (here defined as medial prefrontal cortex below z = 0 [Ochsner et al., 2004a]) has been associated with representation of emotional states and concomitant physiological changes [Anders et al., 2004a; Damasio, 1996]. The vmPFC also seems to play a role in emotional perspective taking [Eslinger, 1998; Hynes et al., 2006; Shamay‐Tsoory et al., 2003]. Particularly, participants showed stronger vmPFC activity when they reappraised emotional events in an emotional‐schematic, “hot,” way than when they reappraised the same events in an unemotional‐conceptual, “cold,” way [Schaefer et al., 2003]. These findings are in line with the observed increase of vmPFC activity during intentional engagement with, but not disengagement from, people in distress in the current study.
Open Questions and Future Directions
The current study investigated brain processes underlying the control of emotional responses towards victims of violence by intentional social distance modulation through cognitive reappraisal. While our findings provide evidence that (i) peripheral physiological responses and amygdala activity can be down‐ and up‐regulated by social distance modulation and that (ii) activity in the dmPFC and aPCC is differentially associated with social disengagement and engagement, they also leave some open questions.
First, it is not entirely clear why activity in the dmPFC significantly increased from engagement to disengagement, but showed no correlation with self‐reported success during disengagement, while the increase of aPCC activity during engagement was only evident when participants' self‐reported success was used to model inter‐individual differences in activity. This is particularly puzzling as level and variance of self‐reported success did not differ between the two conditions. It has to be noted, though, that both findings are robust and persist when the statistical threshold is corrected for the number of tested contrasts. One possibility is that engagement‐related activity in the aPCC is more variable across participants than dmPFC activity. Additionally, participants might have been less accurate in reporting their success during disengagement.
Second, interpersonal reappraisal, like other forms of cognitive reappraisal, likely depends on a number of different subprocesses [e.g., Ochsner and Gross, 2005, 2008], and further studies are needed to disentangle these subprocesses. Particularly, the (unpredicted) finding of significantly stronger activity in the lOFC and dlPFC during disengagement than during engagement needs further investigation. Including additional conditions in which participants are asked to modulate their social distance to the offender (rather than the victim) might help to disentangle required effort and direction of social distance modulation. This would also help to address the intriguing question whether there is a “default response” of the human brain to empathize with people in distress (but not with offenders) that increases if activity in the OFC/dlPFC is down‐regulated.
A third question pertains to the matter of how specifically the dmPFC and aPCC are involved in intentional social distance modulation, and whether other types of cognitive reappraisal also draw upon these regions. A literature search showed that previous studies that investigated emotional reappraisal in diverse social and non‐social contexts have often found an increase of dlPFC activity, while significant increases and decreases of dmPFC and aPCC activity have been observed much less frequently (Table S4). Interestingly, the only study in which participants were explicitly asked to increase their interpersonal distance to moral offenders [Harenski and Hamann, 2006] found activity in the dmPFC very close to the cluster of dmPFC activity observed in the current study (Table S4). However, at the moment these data are too limited to permit conclusion at a meta‐analysis level and call for studies that directly compare social distance regulation and emotion regulation in non‐social contexts.
Finally, the current study did not directly investigate the consequences of social distance modulation and associated brain activity on subjective empathic experience or social behavior. First evidence for a role of the dmPFC in social behavior comes from a study by Lotze et al. [2007] in which increased dmPFC activity was associated with the selection of a painful revenge stimulus in a competitive game. It will remain a challenging task for future studies to investigate the interplay between the aPCC and dmPFC in social behavior, and to establish whether there is a balance of neural activity in these regions (and perhaps other regions of the prefrontal cortex) that promotes adequate prosocial behavior. Experimental paths that promise to be fruitful in this endeavor are the use of economic games [de Quervain et al., 2004; Rilling et al., 2002] and virtual realities [King et al., 2006; Mathiak and Weber, 2006].
CONCLUSION
The present study examined neural processes underlying the modulation of emotional responses towards victims of violence by intentional social distance modulation through cognitive reappraisal. Our findings provide evidence that (i) affective responses, including peripheral physiological responses and amygdala activity, can effectively be modulated by intentional disengagement from and engagement with people in distress, and that (ii) neural activity in two medial prefrontal brain regions that have previously been associated with brain processes related to dissimilar and similar others, the dmPFC and aPCC, can intentionally be modulated by cognitive reappraisal acting on social distance. These findings are in line with the assumption that the dmPFC and the aPCC play important roles in the maintenance of a balance between social disengagement and engagement that promotes the flexible and complex human social behavior required by modern human societies. A disruption of this balance might be one cause for the maladaptive social behavior observed in many psychiatric illnesses.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Acknowledgements
Scanning was performed at the Section for Experimental MR of the CNS, Department of Neuroradiology, University of Tübingen, Germany.
Contributor Information
Susanne Leiberg, Email: susanne.leiberg@econ.uzh.ch.
Silke Anders, Email: silke.anders@neuro.uni-luebeck.de.
REFERENCES
- Ames DL, Jenkins AC, Banaji MR, Mitchell JP ( 2008): Taking another person's perspective increases self‐referential neural processing. Psychol Sci 19: 642–644. [DOI] [PubMed] [Google Scholar]
- Anders S, Lotze M, Erb M, Grodd W, Birbaumer N ( 2004a): Brain activity underlying emotional valence and arousal: A response‐related fMRI study. Hum Brain Mapp 23: 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Weiskopf N, Lule D, Birbaumer N ( 2004b) Infrared oculography‐validation of a new method to monitor startle eyeblink amplitudes during fMRI. Neuroimage 22: 767–770. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Glover GH, Gabrieli JD ( 2004): Neural systems underlying the suppression of unwanted memories. Science 303: 232–235. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Hutton C, Ashburner J, Turner R, Friston K ( 2001): Modeling geometric deformations in EPI time series. Neuroimage 13: 903–919. [DOI] [PubMed] [Google Scholar]
- Atique B, Erb M, Gharabaghi A, Grodd W, Anders S ( 2011): Task‐specific activity and connectivity within the mentalizing network during emotion and intention mentalizing. Neuroimage 55: 1899–1911. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD ( 2004): Selection, integration, and conflict monitoring; assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron 41: 473–487. [DOI] [PubMed] [Google Scholar]
- Batson CD ( 1991): The Altruism Question: Towards a Social‐Psychological Answer. Mahwah, NJ: Erlbaum. [Google Scholar]
- Batson CD, Turk CL, Shaw LL, Klein TR ( 1995): Information function of empathic emotion: Learning that we value the other's welfare. J Pers Soc Psychol 68: 300–313. [Google Scholar]
- Baumgart T, Kaulisch T, Tempelmann C, Gaschler‐Markefski B, Tegeler C, Schindler F, Stiller D, Scheich H ( 1998): Electrodynamic headphones and woofers for application in magnetic resonance imaging scanners. Med Phys 25: 2068–2070. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P ( 2001): Neural correlates of conscious self‐regulation of emotion. J Neurosci 21: RC165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ ( 1994): Measuring emotion: The self‐assessment Manikin and the semantic differential. J Behav Ther Exp Psychiatry 25: 49–59. [DOI] [PubMed] [Google Scholar]
- Cialdini RB, Brown SL, Lewis BP, Luce C, Neuberg SL ( 1997): Reinterpreting the empathy‐altruism relationship: When one into one equals oneness. J Pers Soc Psychol 73: 481–494. [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC ( 1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18: 192–205. [PubMed] [Google Scholar]
- Craig AD ( 2009): How do you feel‐now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59–70. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ ( 2004): Neural systems supporting interoceptive awareness. Nat Neurosci 7: 189–195. [DOI] [PubMed] [Google Scholar]
- Damasio AR ( 1996): The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci 351: 1413–1420. [DOI] [PubMed] [Google Scholar]
- David N, Bewernick BH, Cohen MX, Newen A, Lux S, Fink GR, Shah NJ, Vogeley K ( 2006): Neural representations of self versus other: Visual‐spatial perspective taking and agency in a virtual ball‐tossing game. J Cogn Neurosci 18: 898–910. [DOI] [PubMed] [Google Scholar]
- Davis MH ( 1983): Measuring individual differences in empathy: Evidence for a multidimensional approach. J Pers Soc Psychol 44: 113–126. [Google Scholar]
- Dawson ME, Schell AE, Filion DL ( 2000): The electrodermal sytem In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. New York: Cambridge University Press; pp. 200–223. [Google Scholar]
- Decety J, Jackson PL ( 2004): The functional architecture of human empathy. Behav Cogn Neurosci Rev 3: 71–100. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Fischbacher U, Treyer V, Schellhammer M, Schnyder U, Buck A, Fehr E ( 2004): The neural basis of altruistic punishment. Science 305: 1254–1258. [DOI] [PubMed] [Google Scholar]
- Dillon DG, LaBar KS ( 2005): Startle modulation during conscious emotion regulation is arousal‐dependent. Behav Neurosci 119: 1118–1124. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE ( 2006): A core system for the implementation of task sets. Neuron 50: 799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S ( 2007): Regulation of emotional responses elicited by threat‐related stimuli. Hum Brain Mapp 28: 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA ( 1998): Prosocial development In: Damon W, editor. Handbook of Child Psychology, Vol. 3: Social, Emotional, and Personality Development. New York: Wiley; 701–778. [Google Scholar]
- Eslinger PJ ( 1998): Neurological and neuropsychological bases of empathy. Eur Neurol 39: 193–199. [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de GM, Northoff G ( 2011): Is there a core neural network in empathy? An fMRI based quantitative meta‐analysis. Neurosci Biobehav Rev 35: 903–911. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD ( 2003): Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci 358: 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Han S ( 2007): Attention and reality constraints on the neural processes of empathy for pain. Neuroimage 36: 256–267. [DOI] [PubMed] [Google Scholar]
- Harenski CL, Hamann S ( 2006): Neural correlates of regulating negative emotions related to moral violations. Neuroimage 30: 313–324. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Shaw M, Yucel M, Purcell R, Brewer WJ, Strother SC, Egan GF, Olver JS, Nathan PJ, Pantelis C ( 2005): Functional connectivity during Stroop task performance. Neuroimage 24: 181–191. [DOI] [PubMed] [Google Scholar]
- Hynes CA, Baird AA, Grafton ST ( 2006): Differential role of the orbital frontal lobe in emotional versus cognitive perspective‐taking. Neuropsychologia 44: 374–383. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ ( 2000): Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology 37: 515–522. [PubMed] [Google Scholar]
- Jenkins AC, Macrae CN, Mitchell JP ( 2008): Repetition suppression of ventromedial prefrontal activity during judgments of self and others. Proc Natl Acad Sci USA 105: 4507–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ ( 2007): Failure to regulate: Counterproductive recruitment of top‐down prefrontal‐subcortical circuitry in major depression. J Neurosci 27: 8877–8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Critchley HD, Seymour B, O'Doherty JP, Oakley DA, Allen P, Dolan RJ ( 2005): Anxiety reduction through detachment: Subjective, physiological, and neural effects. J Cogn Neurosci 17: 874–883. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Herrmann K, Dolan RJ ( 2006): Neural correlates of self‐distraction from anxiety and a process model of cognitive emotion regulation. J Cogn Neurosci 18: 1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF ( 2002): Finding the self? An event‐related fMRI study. J Cogn Neurosci 14: 785–794. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S ( 2007): Neural correlates of positive and negative emotion regulation. J Cogn Neurosci 19: 776–798. [DOI] [PubMed] [Google Scholar]
- King JA, Blair RJ, Mitchell DG, Dolan RJ, Burgess N ( 2006): Doing the right thing: A common neural circuit for appropriate violent or compassionate behavior. Neuroimage 30: 1069–1076. [DOI] [PubMed] [Google Scholar]
- Kring AM, Gordon AH ( 1998): Sex differences in emotion: Expression, experience, and physiology. J Pers Soc Psychol 74: 686–703. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB ( 2010): A link between the systems: Functional differentiation and integration within the human insula revealed by meta‐analysis. Brain Struct Funct 214: 519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J ( 2007): The neural substrate of human empathy: Effects of perspective‐taking and cognitive appraisal. J Cogn Neurosci 19: 42–58. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T ( 2011): Meta‐analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54: 2492–2502. [DOI] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM, Dolan RJ ( 1997): Neural activation during selective attention to subjective emotional responses. Neuroreport 8: 3969–3972. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN ( 2005): International affective picture system (IAPS): Digitized photographs, instruction manual and affective ratings Technical Report A‐6. Gainsville, FL. [Google Scholar]
- Lawrence EJ, Shaw P, Giampietro VP, Surguladze S, Brammer MJ, David AS ( 2006): The role of ‘shared representations’ in social perception and empathy: An fMRI study. Neuroimage 29: 1173–1184. [DOI] [PubMed] [Google Scholar]
- Leiberg S, Anders S ( 2006): The multiple facets of empathy: A survey of theory and evidence. Prog Brain Res 156: 419–440. [DOI] [PubMed] [Google Scholar]
- Lerner A, Bagic A, Hanakawa T, Boudreau EA, Pagan F, Mari Z, Bara‐Jimenez W, Aksu M, Sato S, Murphy DL, Hallett M ( 2009): Involvement of insula and cingulate cortices in control and suppression of natural urges. Cereb Cortex 19: 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M ( 2003): Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry 53: 502–510. [DOI] [PubMed] [Google Scholar]
- Liviatan I, Trope Y, Liberman N ( 2008): Interpersonal similarity as a social distance dimension: Implications for perception of other's actions. J Exp Soc Psychol 44: 1256–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Veit R, Anders S, Birbaumer N ( 2007): Evidence for a different role of the ventral and dorsal medial prefrontal cortex for social reactive aggression: An interactive fMRI study. Neuroimage 34: 470–478. [DOI] [PubMed] [Google Scholar]
- Mathiak K, Weber R ( 2006): Toward brain correlates of natural behavior: fMRI during violent video games. Hum Brain Mapp 27: 948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mudson EJ ( 1982): Insula of the old world monkey. I. Architectonics in the insulo‐orbito‐temporal component of the paralimbic brain. J Comp Neurol 212: 1–22. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN ( 2005): The link between social cognition and self‐referential thought in the medial prefrontal cortex. J Cogn Neurosci 17: 1306–1315. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR ( 2006): Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron 50: 655–663. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Meyer M, Passamonti L, Seymour B, Calder AJ, Schweizer S, Frith CD, Dalgleish T ( 2009): A key role for similarity in vicarious reward. Science 324: 900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB ( 2005): Valid conjunction inference with the minimum statistic. Neuroimage 25: 653–660. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ ( 2005): The cognitive control of emotion. Trends Cogn Sci 9: 242–249. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD ( 2002): Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14: 1215–1229. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC ( 2004a): Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci 16: 1746–1772. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ ( 2004b): For better or for worse: Neural systems supporting the cognitive down‐ and up‐regulation of negative emotion. Neuroimage 23: 483–499. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ ( 2008): Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Curr Dir Psychol Sci 17: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira H, Nomura M, Ichikawa N, Isowa T, Iidaka T, Sato A, Fukuyama S, Nakajima T, Yamada J ( 2006): Association of neural and physiological responses during voluntary emotion suppression. Neuroimage 29: 721–733. [DOI] [PubMed] [Google Scholar]
- Paulus C ( 2009): Der Saarbrücker Persönlichkeitsfragebogen SPF(IRI) zur Messung von Empathie: Psychometrische Evaluation der deutschen Version des Interpersonal Reactivity Index. http://psydok.sulb.uni-saarland.de/volltexte/2009/2363. Accessed June 14th 2011.
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME ( 2005): Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry 57: 210–219. [DOI] [PubMed] [Google Scholar]
- Preston SD, de Waal FB ( 2002): Empathy: Its ultimate and proximate bases. Behav Brain Sci 25: 1–20. [DOI] [PubMed] [Google Scholar]
- Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C ( 2002): A neural basis for social cooperation. Neuron 35: 395–405. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Collette F, Philippot P, van der LM, Laureys S, Delfiore G, Degueldre C, Maquet P, Luxen A, Salmon E ( 2003): Neural correlates of “hot” and “cold” emotional processing: A multilevel approach to the functional anatomy of emotion. Neuroimage 18: 938–949. [DOI] [PubMed] [Google Scholar]
- Shamay‐Tsoory SG, Tomer R, Berger BD, Aharon‐Peretz J ( 2003): Characterization of empathy deficits following prefrontal brain damage: The role of the right ventromedial prefrontal cortex. J Cogn Neurosci 15: 324–337. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD ( 2006): Empathic neural responses are modulated by the perceived fairness of others. Nature 439: 466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trope Y, Liberman N ( 2010): Construal‐level theory of psychological distance. Psychol Rev 117: 440–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ ( 2006): Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci 26: 4415–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Ochsner KN, Hanelin J, Wager TD, Mackey SC ( 2007): Different circuits for different pain: Patterns of functional connectivity reveal distinct networks for processing pain in self and others. Soc Neurosci 2: 276–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


