Abstract
Coherent behavior depends on attentional control that detects and resolves conflict between opposing actions. The current functional magnetic resonance imaging study tested the hypothesis that emotion triggers attentional control to speed up conflict processing in particularly salient situations. Therefore, we presented emotionally negative and neutral words in a version of the flanker task. In response to conflict, we found activation of the dorsal anterior cingulate cortex (ACC) and of the amygdala for emotional stimuli. When emotion and conflict coincided, a region in the ventral ACC was activated, which resulted in faster conflict processing in reaction times. Emotion also increased functional connectivity between the ventral ACC and activation of the dorsal ACC and the amygdala in conflict trials. These data suggest that the ventral ACC integrates emotion and conflict and prioritizes the processing of conflict in emotional trials. This adaptive mechanism ensures rapid detection and resolution of conflict in potentially threatening situations signaled by emotional stimuli. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: affect, anterior cingulate cortex, control, flanker task, fMRI
INTRODUCTION
The hypothesis that emotion triggers executive attentional control was put forward more than two decades ago. Nevertheless, it has not been directly tested yet. For example, Norman and Shallice [1986] argued that dangerous situations trigger a supervisory attentional system. Similarly, Scherer [1984,1994] described emotions as relevance detectors that support behavioral control (see also Tomkins [1962] and Gray [2004]). In this study, we introduce a novel paradigm that allows to directly test the question whether emotion triggers attentional control and to assess the neural basis of this influence with functional magnetic resonance imaging (fMRI).
Executive control of attention has been defined as detection and resolution of conflict [Posner et al.,2007]. Experimentally, it has been studied with paradigms, such as the Stroop [Stroop,1935], flanker [Eriksen and Eriksen,1974], and Simon tasks [Simon and Small,1969]. These tasks allow comparing a congruent condition, in which a single response tendency is present, with an incongruent condition, in which two conflicting response tendencies are elicited by a stimulus. Typically, incongruent conditions yield prolonged reaction times (RTs) and increase activation in the anterior cingulate cortex (ACC; for a study testing three different conflict tasks see Fan et al. [2003]). As has been previously suggested, highly salient situations may require particularly efficient executive control of attention [Norman and Shallice,1986]. Often such situations are indicated by emotional stimuli such as the presence of a threatening animal that may trigger rapid fight or flight behavior. Stimuli and associated action tendencies that are in conflict with a particular goal can then be ignored more easily. It may thus be that attentional control is modulated in reactions to emotional (compared to neutral) stimuli. However, in contrast to the robust evidence on the influence of emotion on orienting of spatial attention [Stormark et al.,1995] or the attentional blink [Keil and Ihssen,2004], there is little evidence on the influence of emotion on executive control of attention. We therefore developed a task that directly tests the influence of emotion on attentional control and investigate the neural underpinnings of this potential influence with fMRI.
A version of the flanker task was used, in which participants determined the ink color of a centrally presented word, with the flanker words in the same (congruent) or in a different (incongruent) color (see Fig. 1). Word meaning was manipulated by incorporating emotionally negative and neutral words. This design allows assessing conflict processing (incongruent–congruent) as a function of target stimulus quality (emotional–neutral). To our knowledge, this is the first study that independently manipulates conflict and emotion in task‐relevant stimuli.
Figure 1.
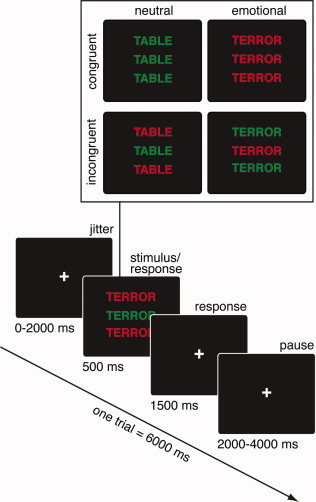
Color flanker task used in this study. The participant's task was to name the print color of the center word, ignoring that of the flanker words (the actual colors were red and green). Words were either emotional or neutral yielding a fully crossed design of conflict and emotion. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In a few previous studies, emotional stimuli were presented before a conflict task (e.g. a Stroop or flanker task). These experiments aimed at the potential influence of transiently induced emotions on subsequent conflict processing, but did not directly test attentional control in reaction to emotional stimuli. Also, the results of these studies appear largely incoherent [Dennis and Chen,2007a,b; Dennis et al.,2008; Kuhl and Kazén,1999]. Following suggestions by Scherer [1984,1994], we manipulated the emotionality of target stimuli, which increased the saliency of the behaviorally relevant stimuli and allowed to directly test the effect of emotion on executive attentional control. Furthermore, the present experiment investigates a mechanism that significantly differs from those targeted with the emotional Stroop [e.g. Williams,1996], the emotional conflict task [e.g. Etkin et al.,2006], or the Wall of Faces task [Simmons et al.,2006]. The emotional Stroop engages participants in a particular task (e.g. counting stimuli or identifying a stimulus color; Mohanty et al., [2007]; Whalen et al., [1998]) with emotional target stimuli. There are no conflict‐eliciting distractors present nor is one of the stimulus dimensions incongruent with the response [Algom et al.,2004]. The emotional Stroop allows the assessment of the “implementation of […] emotion regulation” [Mohanty et al.,2007], but does not allow conclusions as to whether emotion modulates conflict processing. In contrast, the emotional conflict task [Etkin et al.,2006] elicits conflict between different emotional responses by presenting emotional words superimposed on emotional faces (e.g. an angry face with the word “happy” superimposed). Although this task allows the testing of emotional conflict, it does not test the influence of emotion on non‐emotional conflict processing. Similarly, the Wall of Faces task [Simmons et al.,2006] creates emotionally ambiguous situations by presenting equal numbers of angry and happy faces in one display, whereas the participants' task is to decide which category outnumbers the other. Again, a form of conflict is created, but the influence of emotion on conflict processing cannot be studied as all stimuli are emotional.
Even though the emotional Stroop and emotional conflict tasks aim at different processes than this study, they may still foster hypotheses regarding the neural basis of an influence of emotion on executive attention. In contrast to the cognitive conflict tasks (including flanker, Stroop, and Simon tasks), which mainly activate the dorsal portion of the ACC [Banich et al.,2001; Bush et al.,1998; Carter et al.,1995,2000; Derbyshire et al.,1998; Egner and Hirsch,2005; Fan et al.,2003,2005,2007,2008; George et al.,1994, 1997; Luks et al.,2007; MacDonald et al.,2000; Mohanty et al.,2007; Pardo et al.,1990; Peterson et al.,2002; Taylor et al.,1997; van Veen and Carter,2005; van Veen et al.,2001; Wagner et al.,2006], emotional Stroop and emotional conflict tasks have yielded activation of the ventral ACC [Bishop et al.,2004; Engels et al.,2007; Etkin et al.,2006; George et al.,1994; Haas et al.,2006,2007; Mohanty et al.,2007; Whalen et al.,1998]. Two studies directly compared cognitive and emotional conflict or ambiguity. Simmons et al. [2006] report a clear distinction between the ventral and the dorsal ACC for emotional and cognitive ambiguity, respectively. Egner et al. [2008] also found ventral ACC activity for emotional conflict, whereas the dorsal ACC was sensitive to cognitive and emotional conflict. In conclusion, while the dorsal portion of the ACC is engaged in conflict processing irrespective of emotion, the ventral ACC seems to be sensitive to conflict when stimuli are emotional or when emotional distractors are inhibited. This raises the possibility that the ventral ACC is involved in the modulation of cognitive conflict processing in an emotional context in addition to the dorsal portion of the ACC.
Neuroanatomically, the ventral and dorsal portions of the ACC are interconnected [Stein et al.,2007]. The ventral ACC is also strongly connected to the amygdala (for a review, see Bush et al. [2000]), a structure in the temporal lobe that rapidly responds to emotion in stimuli (for a recent meta‐analysis, see Sergerie et al. [2008] and Sabatinelli et al. [2007]). Thus, we closely examined activation in the amygdala to emotional words [Zald,2003]. Information from the dorsal ACC regarding conflict and emotional significance signaled by the amygdala may be integrated in the ventral ACC, which then modulates processing of conflict. Therefore, we compared functional connectivity between the putative ventral ACC activity and activity in the dorsal ACC and amygdala for conflict processing in neutral and in emotional stimuli.
In summary, this study aims to investigate the influence of emotion on executive attentional control. Emotional stimuli are signals of relevance in a situation that potentially requires more efficient attentional control to deal with distracting information [Gray,2004; Norman and Shallice,1986; Scherer,1994]. We therefore addressed the following questions: (1) Is RT conflict reduced in emotional trials? (2) Are the dorsal and the ventral subdivisions of the ACC differently involved in conflict processing in neutral and emotional trials? (3) Is the amygdala activated by negative emotional words? and (4) Are measures of conflict‐related functional connectivity modulated by emotion?
MATERIALS AND METHODS
Rating and Stimuli
One‐thousand selected German nouns were rated by 32 participants (16 females) for emotional valence (negative–neutral–positive), arousal (low arousing–high arousing), and concreteness (concrete–abstract) on a nine‐point scale. We also evaluated concreteness as this factor has been shown to interact with the emotionality of words [Kanske and Kotz,2007]. Based on these ratings, 30 negative and 30 neutral words were selected. The word groups differed significantly in valence and arousal, but were controlled for concreteness, word frequency according to the Wortschatz Lexikon of the University of Leipzig (http://wortschatz.uni-leipzig.de/), and word length in number of letters and syllables (see Table I).
Table I.
Word stimuli
| Word type | Example word | Rated valence | Rated concreteness | Rated arousal | Word frequency | Number of letters | Number of syllables |
|---|---|---|---|---|---|---|---|
| Negative | Tod [death] | 2.35 (0.41) | 6.27 (1.35) | 6.88 (0.48) | 11.03 (2.09) | 5.50 (1.07) | 1.67 (0.48) |
| Neutral | Phase [phase] | 5.02 (0.09) | 6.01 (1.29) | 2.29 (0.31) | 11.10 (1.94) | 5.87 (1.04) | 1.83 (0.38) |
Valence, arousal, and concreteness ratings for the selected word groups. The material was also controlled for word frequency, and number of letters and syllables.
Participants
Twenty volunteers (10 females) participated in the experiment. None had taken part in similar studies before. The mean age was 24.3 (SD 2.5). All participants were native speakers of German and right‐handed according to the Edinburgh Handedness Inventory [Oldfield,1971] with a mean LQ of 94.6 (SD 9.4) and reported normal or corrected‐to‐normal vision. The study was approved by the Ethics Committee of the University of Leipzig. All participants gave written informed consent before participation.
Task and Procedure
Participants performed a color flanker task (see Fig. 1). The goal was to determine the print color of a target word presented at fixation (red or green) and to press a corresponding button (left or right). Response function was counterbalanced across participants. Two identical flanker words in either the same (congruent) or a different color (incongruent) were presented above and below the target word. Half of the words were of neutral, and the other half were of negative valence. Each of the 30 positive and negative words was presented with congruent and incongruent flankers, yielding a total of 120 trials. The colors were pseudorandomly assigned to the trials such that emotional and neutral words were presented equally often in green or red. All trials were presented in pseudorandomized order. A new randomization was created. Twenty null events were added and randomly interspersed in the experimental trials to allow for baseline contrasts. Null events were trials without word stimuli; a fixation cross was presented for 6,000 ms. All stimuli were presented in upper case. The target array extended maximally 2.3° of visual angle horizontally and 1.2° vertically from fixation. Presentation time was 500 ms, and maximal response time was 2,000 ms. Each trial lasted for 6,000 ms. Stimulus onset was jittered (0–2,000 ms) to avoid temporal orienting and to allow for measurements at multiple time points along the BOLD signal curve. Stimuli were presented with the Experimental Run Time System (Beringer). After instructions, participants completed a practice block of 16 trials.
fMRI Recording
MRI data were collected at 3.0 T using a Bruker 30/100 Medspec system (Bruker Medizintechnik GmbH, Ettlingen, Germany). A standard bird cage head coil was used. The experiment consisted of two separate, but consecutive sessions. In the first session, high‐resolution whole‐head 3D MDEFT brain scans (128 sagittal slices, 1.5‐mm thickness, FOV 25.0‐25.0‐19.2 cm, data matrix of 256‐256 voxels) were acquired to improve localization [Ugurbil et al.,1993]. The second session started with the collection of scout spin echo sagittal scans to define the anterior and posterior commissures on a midline sagittal section. For each participant, structural and functional (echo‐planar) images were obtained from 22 axial slices parallel to the plane intersecting the anterior and posterior commissures (AC–PC plane). The whole range of slices covered almost the entire brain. For functional imaging, a gradient‐echo EPI sequence was used with a TE of 30 ms, a flip angle of 90°, a TR of 2,000 ms, and an acquisition bandwidth of 100 kHz. The matrix acquired was 64 × 64 with a FOV of 19.2 cm, resulting in an in‐plane resolution of 3 × 3 mm. The slice thickness was 4 mm with an interslice gap of 1 mm. To align the functional EPI images to 3D‐MDEFT images, conventional T1‐weighted, MDEFT, and T1‐weighted EPI images were obtained in‐plane with the T2* echo‐planar images as reference.
Data Analysis
The data processing was performed using the software package LIPSIA [Lohmann et al.,2001]. This software package contains tools for preprocessing, coregistration, statistical evaluation, and visualization of fMRI data. Functional data were corrected for motion using a matching metric based on linear correlation. To correct for the temporal offset between the slices acquired in one scan, a cubic‐spline‐interpolation was applied. A temporal high‐pass filter with a cutoff frequency of 1/104 Hz was used for baseline correction of the signal as well as a spatial gaussian filter with 4.24‐mm FWHM. To align the functional data slices with a 3D stereotactic coordinate reference system, a rigid linear registration with six degrees of freedom (three rotational and three translational) was performed. The rotational and translational parameters were acquired on the basis of the MDEFT [Norris,2000; Ugurbil et al.,1993] and EPI‐T1 slices to achieve an optimal match between these slices and the individual 3D reference data set. This 3D reference data set was acquired for each participant during a previous scanning session. The MDEFT volume data set with 160 slices and 1‐mm slice thickness was standardized to the Talairach stereotactic space [Talairach and Tournoux,1988]. The rotational and translational parameters were subsequently transformed by linear scaling to a standard size. The resulting parameters were then used to transform the functional slices using trilinear interpolation, so that the resulting functional slices were aligned with the stereotactic coordinate system. This linear normalization process was improved by a subsequent processing step that performed an additional nonlinear normalization.
The statistical evaluation was based on a least‐square estimation using the general linear model for serially autocorrelated observations [Friston,1994; Friston et al.,1995a,b; Worsley and Friston,1995]. The design matrix was generated with a synthetic hemodynamic response function [Friston et al.,1998; Josephs et al.,1997] and its first derivative. The model equation, including the observation data, the design matrix, and the error term, was convolved with a Gaussian kernel of dispersion of 4‐s FWHM to deal with the temporal autocorrelation [Worsley and Friston,1995]. In the following, contrast‐images (i.e. estimates of the raw‐score differences between specified conditions) were generated for each participant for incongruent versus congruent and negative versus neutral trials (for additional contrasts, see Supporting Information). As noted previously, each individual functional dataset was aligned with the standard stereotactic reference space, so that a group analysis based on the contrast‐images could be performed. The contrast between the different conditions was calculated using the t‐statistic. Subsequently, t‐values were transformed into Z‐scores. A multiple comparisons correction was done using Monte–Carlo simulations. This procedure generates voxels at a rate equal to the significance criterion specified, proportional to the total number of voxels in the dataset, and calculates a cluster size that corresponds to the true false‐positive rate for these conditions. Using 1,000 iterations, a false positive cluster probability of P < 0.05 was achieved with a minimum cluster size of 216 mm3 at a threshold of P < 0.001 (uncorrected) for individual voxels. In the next step, this synthetically determined statistical threshold was applied to all voxels in the real data. The advantages of combining a voxel‐based threshold with a minimum cluster size have been described elsewhere [Forman et al.,1995]. As we had strong hypotheses regarding the amygdala, we performed a region of interest analysis based on observed activations of the negative–neutral words contrast with a critical Z‐score of greater than 2.33 (P < 0.01) and a volume threshold of greater than 108 mm3 (four measured voxels). For similar approaches, see, for example, Nomura et al. [2004], Etkin et al. [2006], and Dougal et al. [2007].
To assess functional connectivity, a correlational analysis was applied (see Obleser et al.2007]). Time‐courses for each trial were extracted for all voxels in the clusters in the amygdala, the ventral ACC, and the dorsal ACC, and subsequently averaged across participants. This resulted in 30 sampling points (one per trial) in each condition. Pearson's correlation coefficients were then analyzed between the brain regions for incongruent emotional and neutral conditions.
RESULTS
Behavioral Data
Overall accuracy was 95.6% (SD 4.2). There were no significant differences between conditions. Mean RTs were 680 ms (SD 85). An ANOVA with the factors conflict (congruent and incongruent) and emotion (negative and neutral) yielded a significant main effect of conflict [F(1,19) = 16.1, P < 0.001]. Congruent trials were responded to faster than incongruent trials (see Table II). There was an interaction of conflict and emotion [F(1,19) = 4.6, P < 0.05]. Follow‐up analyses revealed a reduced conflict effect for emotionally negative compared to neutral trials [F(1,19) = 7.9, P < 0.05, ω2 = 0.15 vs. F(1,19) = 18.2, P < 0.001, ω2 = 0.30]. The effect was driven by reduced RTs in the emotional incongruent condition compared to the neutral incongruent trials [F(1,19) = 4.93, P < 0.05)], whereas the difference between negative and neutral was not significant in the congruent condition.
Table II.
Reaction times
| Word type | Congruent | Incongruent | Conflict |
|---|---|---|---|
| Negative | 665 (79) | 688 (87) | 23 |
| Neutral | 663 (80) | 703 (97) | 40 |
Mean RTs and SD (in parentheses). The conflict effects (incongruent–congruent) are provided in the right hand column.
fMRI Data
Table III shows the areas that were more activated for incongruent than congruent trials. These include the left and right ventral portion of the ACC (see Fig. 2). We also report activation of the left and right dorsal ACC, which extended into more dorsal and posterior medial frontal cortex (see Supporting Information 1 for additional pictures of the activation clusters). For these activations, a time‐line statistics was conducted averaging BOLD signal change (in %) from 5 to 9 s poststimulus for all voxels in the cluster. For the dorsal regions of the ACC, there was a main effect of conflict [right: F(1,19) = 30.4, P < 0.0001; left: F(1,19) = 20.4, P < 0.001], but no effect of emotion nor an interaction. For the ventral part of the ACC, however, we found a main effect of conflict [right: F(1,19) = 16.4, P < 0.001; left: F(1,19) = 20.9, P < 0.001] as well as an interaction of emotion and conflict [right: F(1,19) = 4.4, P < 0.05; left: F(1,19) = 8.8, P < 0.01], showing that the ventral ACC was only significantly activated in negative incongruent trials [right: F(1,19) = 18.7, P < 0.001; left: F(1,19) = 19.7, P < 0.001], not in neutral incongruent trials when compared with the corresponding congruent condition (see Fig. 2). The interaction effect in the time‐line statistics was corroborated by a whole‐brain voxel‐wise analysis (see Supporting Information 2).
Table III.
Peak activations
| Incongruent–Congruent | H | x | y | z | C s | Z max | BA |
|---|---|---|---|---|---|---|---|
| Dorsal ACC/medial frontal lobe | L | −8 | −5 | 51 | 756 | 3.46 | 24/6 |
| L | −11 | 7 | 44 | 513 | 3.36 | 24/6 | |
| R | 6 | 17 | 44 | 1296 | 3.38 | 32/6 | |
| Ventral ACC | L | −9 | 16 | −9 | 459 | 3.41 | 32 |
| R | 7 | 19 | −9 | 1026 | 3.57 | 32 | |
| Postcentral gyrus | L | −15 | −36 | 60 | 702 | 3.64 | 3 |
| R | 36 | −24 | 45 | 513 | 3.27 | 3 | |
| Precentral gyrus | L | −42 | −3 | 36 | 378 | 3.30 | 6 |
| L | −42 | −15 | 27 | 297 | 3.32 | 6 | |
| Cuneus | R | 30 | −84 | 30 | 2646 | 3.38 | 19 |
| Middle occipital gyrus | L | −33 | −87 | 21 | 864 | 3.39 | 19 |
| Inferior occipital | L | −36 | −75 | −3 | 459 | 3.32 | 19 |
| Fusiform gyrus | R | 36 | −66 | −9 | 324 | 3.39 | 19 |
| Superior temporal gyrus | R | 60 | −18 | 0 | 513 | 3.57 | 22 |
Incongruent versus congruent BOLD response. H, hemisphere; Cs, cluster size in mm3; BA, Brodmann area.
Figure 2.
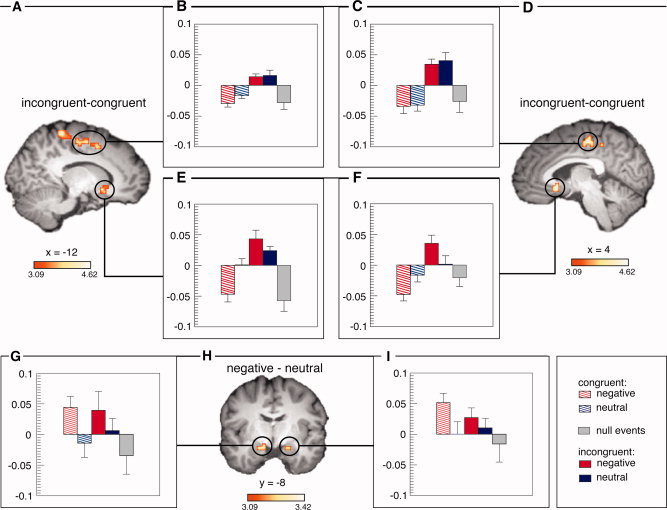
Incongruent–congruent contrast images for the left (A) and right (D) hemisphere. Activation of the left (E) and right (F) ventral ACC, and the left (B) and right (C) dorsal ACC in percent signal change. Negative–neutral contrast image (I) and activation of the left (E) and right (G) amygdala in percent signal change. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
We also report bilateral amygdala activation for negative when compared with neutral words (see Fig. 2; right: x = 19, y = −8, z = −12; C s = 108; Z = 2.61; left: x = −16, y = −8, z = −12; C s = 135; Z = 2.61). The time‐line statistics (4–8 s poststimulus, as the BOLD response peaked earlier than in the ACC regions) yielded a significant effect of emotion [right: F(1,19) = 5.8, P < 0.05; left: F(1,19) = 5.9, P < 0.05], but no effect of conflict or an interaction.
As described earlier, the dorsal ACC showed a main effect of conflict, whereas the amygdala showed a main effect of emotion. The ventral ACC was also active in the contrast of incongruent versus congruent trials. However, the time‐line statistics yielded an interaction of emotion and conflict in this region. We, therefore, compared functional connectivity between the ventral ACC and the amygdala and dorsal ACC in incongruent trials that were emotional and neutral. Interestingly, functional connectivity between these regions was modulated by emotion, such that the activation in the ventral ACC was significantly correlated with amygdala and dorsal ACC activation in incongruent emotional, but not in incongruent neutral trials (see Table IV and Fig. 3).
Table IV.
Correlations for emotional and neutral incongruent conditions
| l vACC | r vACC | |||
|---|---|---|---|---|
| Neutral | l amygdala | −0.13 n.s. | r amygdala | 0.05 n.s. |
| l dACC | 0.01 n.s. | r dACC | 0.16 n.s. | |
| Emotional | l amygdala | 0.67** | r amygdala | 0.47** |
| l dACC | 0.37* | r dACC | 0.45* | |
Correlations between left and right ventral ACC and hemisphere‐corresponding amygdala and dorsal ACC.
n.s. P > 0.05, * P < 0.05, ** P < 0.01.
Figure 3.
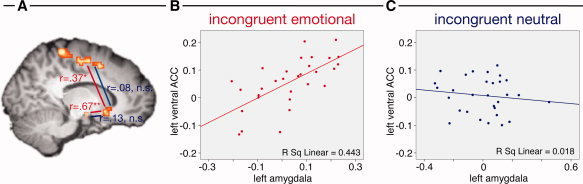
Display of correlation coefficients between left hemisphere brain regions for incongruent emotional (red) and neutral (blue) conditions (A). Exemplary scatterplots for the correlations of the left amygdala and ventral ACC are also displayed (B, C). For details, see Table III. *P < 0.05; **P < 0.01; n.s. P > 0.10. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
To investigate the influence of emotion on executive control of attention, we examined the flanker conflict effect (incongruent–congruent) in emotional and neutral word stimuli. RT conflict was reduced in emotional trials, suggesting that executive attention is enhanced in reactions to emotional stimuli. This is also evidenced by fMRI data. The dorsal ACC showed a main effect of conflict, whereas the ventral ACC was activated for conflict only in emotional stimuli. The ventral ACC seems to integrate conflict and emotional information, possibly via input from the amygdala, an area that was activated by emotional stimuli only. In line with these results, we report increased functional connectivity between the ventral ACC and activity in the dorsal ACC and the amygdala in emotional compared to neutral incongruent trials.
The present data allow addressing a long‐standing open question: Does emotion trigger executive attentional control [Norman and Shallice,1986]? We replicated a behavioral main effect of conflict with longer RTs to incongruent versus congruent stimuli [Eriksen and Eriksen,1974] and showed that this effect was reduced in emotional trials. Thus, the processing of conflict was faster when target and flanker stimuli are emotional. This mechanism is highly adaptive as emotional stimuli signal the particular relevance of a situation that may require especially efficient attentional control [Scherer,1984,1994]. For example, stimuli that constitute a threat to the organism and elicit fear make it necessary to rapidly detect and resolve conflicting action tendencies to reduce the time that the organism is incapable of responding.
We found that this mechanism is subserved by a neural network consisting of the dorsal and ventral ACC and the amygdala. As previously reported, the dorsal ACC was more responsive to incongruent than congruent stimuli [Banich et al.,2001; Bush et al.,1998; Carter et al.,1995,2000; Derbyshire et al.,1998; Egner and Hirsch,2005; Fan et al.,2003,2005,2007,2008; George et al.,1994, 1997; Luks et al.,2007; MacDonald et al.,2000; Mohanty et al.,2007; Pardo et al.,1990; Peterson et al.,2002; Taylor et al.,1997; van Veen and Carter,2005; van Veen et al.,2001; Wagner et al.,2006]. The activation in the dorsal ACC was independent of the emotional quality of words, which fits nicely with the results of Egner et al. [2008]. In their study, the dorsal ACC responded to cognitive conflict and conflict between stimuli of different emotional valence. Similarly, Simmons et al. [2006] reported dorsal ACC activation when contrasting emotional and non‐emotional ambiguity with unambiguous stimuli. Therefore, our results provide further evidence that the dorsal ACC is a vital part of the attentional control network and that it is sensitive to conflict irrespective of emotion. In contrast, the ventral ACC seems to be selectively activated in response to emotional conflict or ambiguity [Egner et al.,2008; Simmons et al.,2006]. Thus, our results extend the functional role of the ventral ACC. We report that the ventral ACC responds to a form of cognitive conflict (stimuli of different colors), however, only when the stimuli are emotional (words of negative valence).
What exactly are the implications of this finding for ventral ACC function? The amygdala, a structure that responds to emotional stimulus quality early on, is strongly connected to the ventral ACC [Devinsky et al.,1995; Vogt et al.,1992]. Furthermore, the dorsal and the ventral portions of the ACC are interconnected [Stein et al.,2007]. Interestingly, we found that functional connectivity between the ventral ACC and activity in dorsal ACC and the amygdala was increased for incongruent stimuli that were emotional compared to neutral ones. Thus, the ventral ACC seems to integrate emotional and conflict information, showing activation and increased communication with the amygdala and the dorsal ACC only when conflict and emotion coincide in a given stimulus. Behaviorally, this goes along with reduced RT conflict, which suggests that the ventral ACC not only integrates emotion and conflict, but prioritizes these situations yielding facilitated processing of conflict. As communication between the areas could be bidirectional, the ventral ACC may prioritize conflict processing in the dorsal portion of the ACC and protect the system from emotional overreactivity by downregulating amygdala activity. Although these interpretations need to be validated in future experiments (e.g. via dynamic causal modeling), they fit nicely with previous studies showing top‐down regulation of amygdala activation by the ventral ACC in emotional conflict [Etkin et al.,2006] and common activation of the dorsal ACC for emotional and cognitive conflict [Egner et al.,2008; Simmons et al.,2006].
Several further issues need to be considered. We observed amygdala activation for negative compared to neutral words, suggesting that the amygdala responds to emotionally negative words even when word meaning is task irrelevant [Sergerie et al.,2008; Zald,2003]. Amygdala activation was not modulated by conflict. We are not aware of any reports showing that the amygdala is sensitive to conflict per se. Only emotional conflict has been shown to elicit amygdala activation. Etkin et al. [2006], for example, presented emotionally ambiguous stimuli (e.g. a fearful face with the word “happy” superimposed) and report increased amygdala activation compared to emotionally congruent stimuli (e.g. a fearful face with the word “fear” superimposed). In this study, however, all stimuli were emotionally congruent, that is, only emotionally negative or neutral stimuli were presented in a given trial. Therefore, conflict in the current experiment did not arise from the emotional quality of the stimuli, but from the presentation of differently colored target and flanker stimuli. Thus, the observed amygdala activation for emotional stimuli, independent of the conflict condition, is the most plausible result.
One further issue is whether emotion modulates the detection or resolution of conflict. The reduced RT conflict effect along with the additional activation in the ventral ACC makes an effect of emotion on conflict resolution more likely than an effect on conflict monitoring. This should be directly tested in future studies that could analyze sequence effects, which are believed to show activation in “control areas” that resolve conflict when incongruent stimuli that were preceded by incongruent stimuli are contrasted with incongruent stimuli that were preceded by congruent stimuli [e.g. Egner et al., 2007]. Sequence effects were not analyzed in this study as this would have required a multiple of the number of presented trials.
A possible alternative explanation for the observed behavioral advantage of emotional stimuli in the incongruent condition may be that attention is automatically focused by emotional target words, thereby reducing flanker interference. If this were the case, executive attentional control would not be influenced by emotion. Rather, we would see a side effect of emotion on attentional orienting. Two arguments can be put forward to refute this possibility. First, in emotional trials, target as well as flanker words were emotional. Consequently, flankers should also have attracted attention, thereby increasing flanker interference. Second, emotional stimuli did not induce activation of the classical frontoparietal attention orienting network [Fan et al.,2005].
One caveat, regarding the design of the study, may arise from investigations of the “emotional Stroop” phenomenon stating that color naming latencies of emotional words are prolonged as emotional stimuli create some conflict‐like interference [Ray,1979; Williams,1996]. This should have affected RTs in the emotional trials. However, we did not observe such a main effect of emotionality. Also, the emotional Stroop task has been criticized as (1) the interference effect is only present in blocked designs [Chajut et al.,2005], (2) effects have been mainly restricted to clinical populations and are not present in healthy controls [Williams et al.,1996] or habituate rapidly [Compton et al.,2003], and (3) Larsen et al. [2006] showed that response slowing in the emotional Stroop task may be due to the fact that emotional words are of lower frequency of usage and are longer and have smaller orthographic neighborhoods. It is therefore unlikely that the emotional Stroop phenomenon confounded results in the present experiment.
What should be done in the future is to test the generalizability of the present results to other conflict tasks and to emotional stimuli of different valence. We predict that the effects are not specific to the present task as other conflict tasks such as the Simon and Stroop task also yield activation of the dorsal ACC [Fan et al.,2003]. Also, positive emotional stimuli may activate the amygdala (for a recent meta‐analysis, see Sergerie et al. [2008]). As the critical main effects of conflict and emotion generalize, so may their interaction. Future studies should target these possibilities by testing different tasks and stimulus qualities. Furthermore, this study could not disentangle the effects of emotional valence and arousal on executive attentional control as only negative emotional stimuli were presented. The inclusion of positive emotional stimuli in future investigations should help to identify whether emotional valence or arousal push the influence on executive attentional control.
To conclude, the current results clearly demonstrate an influence of emotion on executive control of attention. Emotional information is detected in the amygdala, whereas the dorsal ACC is activated by conflict. The ventral portion of the ACC integrates this information. Thus, it shows activity when emotion and conflict coincide in a stimulus and prioritizes these situations as evidenced in reduced RT conflict effects. This adaptive mechanism enables rapid processing of conflict in highly salient situations signaled by emotional stimuli. It thereby reduces the time that an organism is “caught in conflict” and incapable of responding. Thus, emotion does act as a trigger for attentional control processes.
Supporting information
Additional supporting information may be found in the online version of this article.
Supplement 1 Coronal and saggital slices showing the activation in the dorsal ACC. As can be seen, the clusters extend beyond the dorsal ACC into more dorsal medial frontal gyrus, SMA/pre‐SMA.
Supplement 2 A supplementary whole‐brain voxelwise analysis of the interaction effect was computed. The specific interaction hypothesis was to detect areas that show a conflict effect (incongruent ‐ congruent) in emotional, but not in neutral trials. Therefore, the incongruent emotional condition was contrasted with all other conditions, yielding activation bilaterally in the ventral ACC, but not the dorsal ACC. The results corroborate the interaction effect of the BOLD signal change analysis in the ventral ACC.
Acknowledgements
We thank Erich Schröger for constructive discussions and Kerstin Flake for graphical support. Also, we thank five anonymous reviewers for very helpful comments on an earlier version of this manuscript.
REFERENCES
- Algom D, Chajut E, Lev S ( 2004): A rational look at the emotional stroop phenomenon: A generic slowdown, not a stroop effect. J Exp Psychol Gen 133: 323–338. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Jacobson BL, Webb A, Wszalek T, Cohen NJ, Kramer AF ( 2001): Attentional selection and the processing of task‐irrelevant information: Insights from fMRI examinations of the Stroop task. Prog Brain Res 134: 459–470. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD ( 2004): State anxiety modulation of the amygdala response to unattended threat‐related stimuli. J Neurosci 24: 10364–10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL ( 1998): The counting Stroop: An interference task specialized for functional neuroimaging—Validation study with functional MRI. Hum Brain Mapp 6: 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI ( 2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Cohen JD ( 1995): Interference and facilitation effects during selective attention: An H215O PET study of Stroop task performance. Neuroimage 2: 264–272. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD ( 2000): Parsing executive processes: Strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA 97: 1944–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chajut E, Lev S, Algom D ( 2005): Vicissitudes of a misnomer: Reply to Dalgleish (2005): J Exp Psychol Gen 134: 592–595. [Google Scholar]
- Compton RJ, Banich MT, Mohanty A, Milham MP, Herrington J, Miller GA, Scalf PE, Webb A, Heller W ( 2003): Paying attention to emotion: An fMRI investigation of cognitive and emotional stroop tasks. Cogn Affect Behav Neurosci 3: 81–96. [DOI] [PubMed] [Google Scholar]
- Dennis TA, Chen CC ( 2007a) Emotional face processing and attention performance in three domains: Neurophysiological mechanisms and moderating effects of trait anxiety. Int J Psychophysiol 65: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, Chen CC ( 2007b) Neurophysiological mechanisms in the emotional modulation of attention: The interplay between threat sensitivity and attentional control. Biol Psychol 76: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, Chen CC, McCandliss BD ( 2008): Threat‐related attentional biases: An analysis of three attention systems. Depress Anxiety 25: E1–E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire SW, Vogt BA, Jones AK ( 1998): Pain and Stroop interference tasks activate separate processing modules in anterior cingulate cortex. Exp Brain Res 118: 52–60. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA ( 1995): Contributions of anterior cingulate cortex to behaviour. Brain 118 ( Pt 1): 279–306. [DOI] [PubMed] [Google Scholar]
- Dougal S, Phelps EA, Davachi L ( 2007): The role of medial temporal lobe in item recognition and source recollection of emotional stimuli. Cogn Affect Behav Neurosci 7: 233–242. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J ( 2005): The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage 24: 539–547. [DOI] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J ( 2008): Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cerebral Cortex 18: 1475–1484. [DOI] [PubMed] [Google Scholar]
- Engels AS, Heller W, Mohanty A, Herrington JD, Banich MT, Webb AG, Miller GA. ( 2007): Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology 44: 352–363. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW ( 1974): Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys 16: 143–149. [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J ( 2006): Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51: 871–882. [DOI] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI ( 2003): Cognitive and brain consequences of conflict. Neuroimage 18: 42–57. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI ( 2005): The activation of attentional networks. Neuroimage 26: 471–479. [DOI] [PubMed] [Google Scholar]
- Fan J, Kolster R, Ghajar J, Suh M, Knight RT, Sarkar R, McCandliss BD ( 2007): Response anticipation and response conflict: An event‐related potential and functional magnetic resonance imaging study. J Neurosci 27: 2272–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Hof PR, Guise KG, Fossella JA, Posner MI ( 2008): The functional integration of the anterior cingulate cortex during conflict processing. Cereb Cortex 18: 796–805. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC ( 1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Friston KJ. 1994. Statistical parametric mapping In: Thatcher RW, Hallet M, Zeffiro T, John ER, Huerta M, editors. Functional Neuroimaging: Technical Foundations. San Diego: Academic Press; pp 61–64. [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R ( 1995a) Analysis of fMRI time‐series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ ( 1995b) Statistical parametric maps in functional imaging: A genereal linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R ( 1998): Event‐related fMRI: Characterizing differential responses. Neuroimage 7: 30–40. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Rosinsky N, Ring H, Casey BJ, Trimble MR, Horwitz B, Herscovitch P, Post RM ( 1994): Regional brain activity when selecting a response despite interference: A H2O15 PET study of the Stroop and emotional Stroop. Hum Brain Mapp 1: 194–209. [DOI] [PubMed] [Google Scholar]
- Gray JR ( 2004): Integration of emotion and cognitive control. Curr Dir Psychol Sci 13: 46–48. [Google Scholar]
- George MS, Ketter TA, Parekh PI, Rosinsky N, Ring HA, Pazzaglia PJ, Marangell LB, Callahan AM, Post RM ( 1997): Blunted left cingulate activation in mood disorder subjects during a response interference task (the Stroop). J Neuropsychiatry Clin Neurosci, 9: 55–63. [DOI] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, Canli T ( 2006): Interference produced by emotional conflict associated with anterior cingulate activation. Cogn Affect Behav Neurosci 6: 152–156. [DOI] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, Canli T ( 2007): Emotional conflict and neuroticism: Personality‐dependent activation in the amygdala and subgenual anterior cingulate. Behav Neurosci 121: 249–256. [DOI] [PubMed] [Google Scholar]
- Josephs O, Turner R, Friston K ( 1997): Event‐related fMRI. Hum Brain Mapp 5: 243–248. [DOI] [PubMed] [Google Scholar]
- Kanske P, Kotz SA ( 2007): Concreteness in emotional words: ERP evidence from a hemifield study. Brain Res 1148: 138–148. [DOI] [PubMed] [Google Scholar]
- Keil A, Ihssen N ( 2004): Identification facilitation for emotionally arousing verbs during the attentional blink. Emotion 4: 23–35. [DOI] [PubMed] [Google Scholar]
- Kuhl J, Kazén M ( 1999): Volitional facilitation of difficult intentions: Joint activation of intention memory and positive affect removes Stroop interference. J Exp Psychol Gen 128: 382–399. [Google Scholar]
- Larsen RJ, Mercer KA, Balota DA ( 2006): Lexical characteristics of words used in emotional Stroop experiments. Emotion 6: 62–72. [DOI] [PubMed] [Google Scholar]
- Lohmann G, Müller K, Bosch V, Mentzel H, Hessler S, Chen L, Zysset S, von Cramon DY ( 2001): LIPSIA—A new software system for the evaluation of functional magnetic resonance images of the human brain. Comput Med Imaging Graph 25: 449–457. [DOI] [PubMed] [Google Scholar]
- Luks TL, Simpson GV, Dale CL, Hough MG ( 2007): Preparatory allocation of attention and adjustments in conflict processing. Neuroimage 35: 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW III, Cohen JD, Stenger VA, Carter CS ( 2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Engels AS, Herrington JD, Heller W, Ho MH, Banich MT, Webb AG, Warren SL, Miller GA ( 2007): Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology 44: 343–351. [DOI] [PubMed] [Google Scholar]
- Norman DA, Shallice T 1986. Attention to action: Willed and automatic control of behavior In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and Self‐Regulation. New York: Plenum Press; pp 1–18. [Google Scholar]
- Nomura M, Ohira H, Haneda K, Iidaka T, Sadato N, Okada T, Yonekura Y ( 2004): Functional association of the amygdala and ventral prefrontal cortex during cognitive evaluation of facial expressions primed by masked angry faces: An event‐related fMRI study. Neuroimage 21: 352–363. [DOI] [PubMed] [Google Scholar]
- Norris DG ( 2000): Reduced power multislice MDEFT imaging. J Magn Reson Imaging 11: 445–451. [DOI] [PubMed] [Google Scholar]
- Obleser J, Wise RJ, Alex Dresner M, Scott SK ( 2007): Functional integration across brain regions improves speech perception under adverse listening conditions. J Neurosci 27: 2283–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME ( 1990): The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci USA 87: 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung HC, May J, Gore JC ( 2002): An event‐related functional MRI study comparing interference effects in the Simon and Stroop tasks. Brain Res Cogn Brain Res 13: 427–440. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rueda MR, Kanske P ( 2007): Probing the mechanisms of attention In: Cacioppo JT, Tassinary JG, Berntson GG, editors. Handbook of Psychophysiology. Cambridge: Cambridge University Press; pp 410–432. [Google Scholar]
- Ray C ( 1979): Examination stress and performance on a color‐word interference test. Percept Mot Skills 49: 400–402. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Lang PJ, Costa VD, Versace F ( 2007): Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. J Neurophysiol 98: 1374–1379. [DOI] [PubMed] [Google Scholar]
- Scherer KR ( 1984): On the nature and function of emotion: A component process approach In: Scherer KR, Ekman P, editors. Approaches to Emotion. Hillsdale: Lawerence Erlbaum Associates; pp 293–317. [Google Scholar]
- Scherer KR. 1994. Emotion serves to decouple stimulus and response In: Ekman P, Davidson RJ, editors. The nature of Emotion: Fundamental Questions. New York: Oxford University Press; pp 127–130. [Google Scholar]
- Sergerie K, Chochol C, Armony JL ( 2008): The role of the amygdala in emotional processing: A quantitative meta‐analysis of functional neuroimaging studies. Neurosci Biobehav Rev 32: 811–830. [DOI] [PubMed] [Google Scholar]
- Simmons A, Stein MB, Matthews SC, Feinstein JS, Paulus MP ( 2006): Affective ambiguity for a group recruits ventromedial prefrontal cortex. Neuroimage 29: 655–661. [DOI] [PubMed] [Google Scholar]
- Simon JR, Small AM Jr. ( 1969): Processing auditory information: Interference from an irrelevant cue. J Appl Psychol 53: 433–435. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer‐Lindenberg A ( 2007): A validated network of effective amygdala connectivity. Neuroimage 36: 736–745. [DOI] [PubMed] [Google Scholar]
- Stormark KM, Nordby H, Hugdahl K ( 1995): Attentional shifts to emotionally charged cues: Behavioral and ERP data. Cogn Emot 9: 507–523. [Google Scholar]
- Stroop JR ( 1935): Studies of interference in serial verbal reactions. J Exp Psychol 18: 643–662. [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐Planar Stereotactic Atlas of the Human Brain. New York: Thieme. [Google Scholar]
- Taylor SF, Kornblum S, Lauber EJ, Minoshima S, Koeppe RA ( 1997): Isolation of specific interference processing in the Stroop task: PET activation studies. Neuroimage 6: 81–92. [DOI] [PubMed] [Google Scholar]
- Tomkins SS ( 1962): Affect, Imagery, Consciousness. New York: Springer. [Google Scholar]
- Ugurbil K, Garwood M, Ellermann J, Hendrich K, Hinke R, Hu X, Kim SG, Menon R, Merkle H, Ogawa S, Salmi R ( 1993): Imaging at high magnetic fields: Initial experiences at 4 T. Magn Reson Q 9: 259–277. [PubMed] [Google Scholar]
- van Veen V, Carter CS ( 2005): Separating semantic conflict and response conflict in the Stroop task: A functional MRI study. Neuroimage 27: 497–504. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS ( 2001): Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage 14: 1302–1308. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR ( 1992): Functional heterogeneity in cingulate cortex: The anterior executive and posterior evaluative regions. Cereb Cortex 2: 435–443. [DOI] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Kohler S, Marinou V, Mentzel HJ, Sauer H, Schlosser RG ( 2006): Cortical inefficiency in patients with unipolar depression: An event‐related FMRI study with the Stroop task. Biol Psychiatry 59: 958–965. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL ( 1998): The emotional counting Stroop paradigm: A functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry 44: 1219–1228. [DOI] [PubMed] [Google Scholar]
- Williams JM, Mathews A, MacLeod C ( 1996): The emotional Stroop task and psychopathology. Psychol Bull 120: 3–24. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ ( 1995): Analysis of fMRI time‐series revisited—Again. Neuroimage 2: 173–181. [DOI] [PubMed] [Google Scholar]
- Zald DH ( 2003): The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev 41: 88–123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Supplement 1 Coronal and saggital slices showing the activation in the dorsal ACC. As can be seen, the clusters extend beyond the dorsal ACC into more dorsal medial frontal gyrus, SMA/pre‐SMA.
Supplement 2 A supplementary whole‐brain voxelwise analysis of the interaction effect was computed. The specific interaction hypothesis was to detect areas that show a conflict effect (incongruent ‐ congruent) in emotional, but not in neutral trials. Therefore, the incongruent emotional condition was contrasted with all other conditions, yielding activation bilaterally in the ventral ACC, but not the dorsal ACC. The results corroborate the interaction effect of the BOLD signal change analysis in the ventral ACC.


