Abstract
We compared cortical anatomy, using 3D brain MRI scans, between three groups of university students: proficient readers (skilled at phonological decoding and text comprehension), poor readers (impaired at phonological decoding and text comprehension), and resilient readers (impaired at phonological decoding but skilled in text comprehension). This latter group provides a unique opportunity to investigate associations between cortical morphology and phonological decoding deficits in individuals without attendant reading comprehension deficits. We predicted widespread reductions in gray matter thickness and brain size in temporal and frontal regions in poor readers, and more focal differences in brain morphology in resilient readers. Typical asymmetry of gray matter thickness in the temporo‐parietal region was reduced in both poor and resilient readers. Poor readers also exhibited smaller brain sizes in the right inferior frontal region than both proficient and resilient readers. Altered asymmetry in the temporo‐parietal region may therefore be associated with poor phonological decoding and impaired text comprehension may be associated with altered frontal morphology. Resilient readers show relatively focal behavioral differences from typical readers, so it is interesting that they show reliable differences in brain morphology. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: MRI, temporal lobe, frontal lobe, dyslexia, cerebral cortex
INTRODUCTION
Relationships between neuroanatomy and reading skill have long been sought. Although there is a wide range of reading abilities among nondyslexic readers, most prior studies have compared individuals with dyslexia to those with typical reading abilities. In the current study, we investigate brain morphology in a sample of university students with different reading profiles, including a group that showed a dissociation between phonological decoding and text comprehension abilities. The existence of such individuals challenges the widely held belief that strong phonological skills are necessary for skilled reading comprehension and allows us to investigate differences in cortical anatomy between individuals with specific deficits in phonological processing. Such an approach allows us to investigate morphological differences between groups of readers with more focal behavioral differences than prior studies have considered. A demonstration that cortical morphology differs between individuals with relatively minor and focal reading deficits may suggest that relationships exist between reading ability and brain anatomy even among those with highly skilled reading comprehension. To gain insight into potential neuroanatomical characteristics of this novel group, prior studies of developmental changes in brain morphology and function that may relate to typical reading development and studies comparing reading impaired individuals to typical readers should be considered.
Studies investigating associations between neurodevelopmental changes and changes in reading skill have suggested that frontal and temporal lobe maturation may relate to reading acquisition. In typically developing children, gray matter thickening in the inferior frontal cortex was associated with improving phonological abilities [Lu et al., 2007]. Reading ability also correlated with fractional anisotropy, a measure of white matter integrity derived from diffusion‐weighted imaging, in the left temporal lobe in individuals between the age of 8 and 18 [Nagy et al., 2004]. Between the ages of 6 and 22, individuals with better reading skills showed more activity in left inferior frontal and middle temporal cortex, and less activity in right infero‐temporal regions [Turkeltaub et al., 2003].
These developmental studies largely agree with studies comparing cortical anatomy between impaired and normal readers. Across studies, perhaps the most consistent finding is altered morphology in the left temporal lobe. Decreased tissue volume throughout the left temporal lobe was observed in dyslexic men [Brown et al., 2001; Eliez et al., 2000]. In other samples, more focal group differences in temporal lobe anatomy have been reported in the left supramarginal gyrus [Eckert et al., 2005], the posterior superior temporal gyrus and temporo‐parieto‐occipital junction [Brown et al., 2001], and in the left superior temporal gyrus [Brambati et al., 2004]. Some have found that dyslexics have altered morphology in more ventral cortical regions [Brambati et al., 2004; Eckert et al., 2005; Kronbichler et al., 2008; Vinckenbosch et al., 2005]. These reductions in gray matter are frequently more extensive in the left hemisphere, resulting in a reduction of the typical leftward asymmetry.
Reduced asymmetry of temporal regions has long been proposed to be associated with dyslexia [Galaburda, 1989; Galaburda et al., 1985]. Such reductions in asymmetry in language relevant regions have also been observed in functional imaging studies [Shaywitz et al., 2002], suggesting that the left‐lateralized regions that support normal reading have not developed properly in dyslexic individuals. Some more recent studies have found reductions in temporal asymmetry in dyslexics, while others have found no reading group differences [reviewed in Beaton, 1997; Eckert, 2004]. Others have suggested that reading disability may be associated with increased leftward asymmetry. It has been proposed that extreme asymmetry of the planum may characterize readers with intact comprehension and impaired phonological processing [reviewed in Leonard and Eckert, 2008]. In a case study, a compensated dyslexic adult showed extreme leftward asymmetry of the planum temporale [Chiarello et al., 2006a]. Dyslexic engineering students show more leftward asymmetry of the parietal operculum than controls [Robichon et al., 2000].
Inferior frontal morphology also may differ between reading groups, given its role in language processing. Dyslexic men showed reduced gray matter volume in the left orbital and frontal pole and bilaterally in the inferior and superior frontal gyri [Brown et al., 2001]. Relatives of dyslexics who themselves showed either clinically evident or compensated dyslexia had reduced gray matter volume in left superior and inferior frontal gyri [Brambati et al., 2004]. It has also been reported that dyslexic children had smaller pars triangularis volumes bilaterally [Eckert et al., 2003].
These previous studies of brain morphology in reading disabled individuals have been somewhat inconsistent in the extent and localization of morphological differences. These inconsistencies may result from different subject selection criteria, as readers varied greatly in age and severity of reading disability across studies. In the present sample, we had the opportunity to investigate whether different neural substrates relate to phonological decoding and reading comprehension. Such a finding might explain apparent inconsistencies between prior studies in which reading skills were not considered independently. Additionally, prior studies have employed a variety of image processing techniques. The present study used sophisticated cortical matching algorithms to investigate differences in brain morphology. Compared to voxel‐based morphometry (VBM), cortical pattern matching controls for variation in gyral anatomy across subjects, increasing our ability to identify group difference in some anatomical regions. We examined gray matter thickness, radial expansion (a measure of local brain size), and left‐right asymmetries of gray matter thickness across the entire cortical surface of the brain at thousands of anatomical points matched across subjects. We identified proficient readers (skilled in both phonological decoding and reading comprehension), poor readers (impaired at both phonological decoding and reading comprehension), and resilient readers (impaired at phonological decoding, but skilled at reading comprehension) from a large sample of college students. Resilient readers have phonological and orthographic skills similar to those of poor readers, and show greater semantic priming than both poor and proficient readers [Welcome et al., submitted for publication]. Additional behavioral characteristics of resilient readers have been described previously [Jackson and Doellinger, 2002; Welcome et al., 2009]. Because resilient and poor readers were identified by shared deficits in phonological decoding, morphological alterations common to these two groups might shed light onto the neural substrates of phonological decoding. Poor readers were distinguished from resilient readers by reading comprehension; therefore, morphological differences between these groups may reflect, at least in part, neural substrates of reading comprehension level.
Functional imaging studies using tasks that place differing demands on various reading processes have identified brain regions that are active during reading, and we used these findings to inform our predictions regarding brain morphology. A bilateral ventral frontal region, including the inferior frontal gyrus, has been implicated in semantic processing [reviewed in Fiez, 1997]. The left inferior frontal region has also been shown to be active during phonological processing [reviewed in Pugh et al., 1996]. A left lateral temporal region, centered on the middle temporal gyrus, has shown activation during semantic processing tasks [Vandenberghe et al., 1996; Hoenig and Scheef, 2005]. A left ventral region, including ventral extrastriate cortex and the fusiform gyrus, has been proposed to support pre‐lexical orthographic analysis [Cohen et al., 2000]. A left temporo‐parietal region, including the posterior superior temporal, supramarginal and angular gyri, showed activation during the conversion of orthography to phonology [Booth et al., 2002; Joubert et al., 2004].
In our sample, relative to proficient readers, poor readers showed deficits in both phonological decoding and reading comprehension. Therefore, poor readers were predicted to show structural brain abnormalities throughout the frontal and temporal regions thought to support phonological processing and meaning access. Specifically, we hypothesized that poor readers would show (1) bilateral reductions in gray matter thickness and radial expansion in the lateral ventral frontal region relative to proficient readers; (2) less leftward asymmetry of the temporo‐parietal region than proficient readers; and (3) reduced size and gray matter thickness in the left temporal lobe compared to both proficient and resilient readers. Resilient readers show a more focal behavioral deficit, with primary impairments in phonological processing. Therefore, they were predicted to show alterations in brain structure confined to regions thought to support phonological processing. Resilient readers were predicted to show (1) reduced radial expansion and gray matter thickness in the posterior inferior frontal region relative to proficient readers; and (2) alterations in leftward asymmetry of the temporo‐parietal region relative to proficient readers.
METHODS
Participants
A total of 55 university students participated in the study. Fifty‐two of these individuals were selected on the basis of their reading profile from a pool of 200 university students who participated in the Biological Substrates for Language Project [Chiarello et al., 2006b]. To increase the number of poor readers in the study, an additional three participants were recruited from the Riverside community through ads targeting individuals with a history of reading disability. All participants were native speakers of English with normal or corrected‐to‐normal vision and ranged in age between 18 and 34 years of age (mean age = 21.1, SD = 3.4 years). Participants with a history of brain injury or disease or conditions incompatible with MRI were excluded. A neuroradiologist confirmed the absence of visible neuropathology.
This sample included 22 proficient readers, 21 resilient readers, and 12 poor readers identified on the basis of their performance on the Word Attack and Passage Comprehension subtests of the Woodcock Reading Mastery Test Revised/Normative Update (WRMT‐R/NU) [Woodcock, 1998]. All proficient readers had scaled scores above 98 on both subtests, indicating that their reading performance was at least typical for their age. All poor readers had scaled scores below 95 on both subtests, indicating that their performance was at least a third of a standard deviation below the age‐based norm. As the poor readers' scores were in the lowest 10% of 200 university students, they represent the lower extreme of reading ability in our sample. Resilient readers had scaled scores below 95 on the Word Attack subtest and above 98 on the Passage Comprehension subtest, with a discrepancy of at least 10 scaled score units between an individual's Word Attack score and Passage Comprehension score. Mean reading scores are presented in Table I. Overall, the sample consisted of 51% males, 89% right‐handers, and had a mean age of 21.1 years. Groups did not significantly differ in proportion of males, age, handedness, or level of parental education [Welcome et al., 2009; Welcome et al., submitted for publication].
Table I.
Mean scaled scores (standard deviations) on the WRMT‐R/NU
| Proficient readers (N = 22) | Resilient readers (N = 21) | Poor readers (N = 12) | |
|---|---|---|---|
| Word attack | 104.9 (5.8) | 87.2 (3.2) | 86.4 (8.0) |
| Passage comprehension | 108.5 (6.8) | 107.0 (7.2) | 91.7 (2.3) |
Procedures
In a 2‐h preliminary session, participants completed measures of handedness, questionnaires regarding language and family background, and standardized measures of reading skill and intelligence. The Word Attack and Passage Comprehension subtests of the WRMT‐R/NU [Woodcock, 1998] were administered to assess participants' ability to read pseudowords and to supply contextually appropriate completions to increasingly complex stimuli. Age norms were used to calculate scaled scores and percentile ranks. Performance of the reading groups on other standardized measures and experimental tasks is described in a previous manuscript [Welcome et al., 2009; Welcome et al., submitted for publication].
Imaging Acquisition
Each participant received a volumetric MRI scan (3‐D SPGR, 1.2‐mm thick slices) in a GE Signa 1.5 Tesla Scanner at the Computerized Diagnostic Imaging Center in Riverside. The imaging parameters used were as follows: TR 11 ms; TE 2.2 ms; flip angle 25°; field of view 24 cm; acquisition matrix 256 × 256; acquisition time, 4.36 min.
Image Processing
The image analysis procedures have been described in detail previously [Sowell et al., 2001, 2004]. Briefly, MRI scans from each individual were processed through a series of manual and automated procedures including: (1) nonbrain tissue (i.e., scalp, orbits) was removed from a brain mask [Dogdas et al., 2005]; (2) the hemispheres were separated and the cerebellum was removed; (3) magnet inhomogeneity was corrected using a Bias Field Correcting algorithm; (4) a tissue classification algorithm categorized each voxel in the image as white matter, gray matter, or CSF [Shattuck et al., 2001]; (5) the processed image was temporarily converted to a standard space using a completely automated 12‐parameter transformation which employs scaling [Woods et al., 1993]; (6) signal intensity midway between the average value for gray matter and the average value for CSF was used to guide an automatic extraction of the cortical surface [MacDonald et al., 1994]. This step results in a spherical mesh of 131,072 triangulated elements spanning 65,536 surface points that follows the contour of the brain surface; (7) 17 sulci in each hemisphere were manually traced on the lateral surface (Sylvian fissure, central sulcus, precentral sulcus, postcentral sulcus, superior temporal sulcus (STS) main body, STS ascending branch, STS posterior branch, primary intermediate sulcus, secondary intermediate sulcus, inferior temporal sulcus, superior frontal sulcus, inferior frontal sulcus, intraparietal sulcus, transverse occipital sulcus, olfactory sulcus, occipito‐temporal sulus, and collateral sulcus). An additional set of 12 sulci was outlined on the medial surface of each hemisphere (callosal sulcus, inferior callosal outline, superior rostral sulcus, inferior rostral sulcus, paracentral sulcus, anterior, and posterior segments of the cingulate sulcus, outer segment double parallel cingulate sulcus, parieto‐occipital sulcus, anterior and posterior segments of the calcarine sulcus, and the subparietal sulcus). A set of six curves was outlined to establish gyral limits at midline. Protocols, including criteria for start and stop points, have been developed and reliability has been previously reported [Sowell et al., 2002, 2004]; (8) the 12‐parameter transformation that was used to scale each participant's brain was inverted and the surface, lines, and images were transformed back into reoriented space; (9) the first and last points of each sulcal line were used to compute a rigid‐body transformation that put subjects into a common orientation while retaining the native size and shape of the brain; (10) averaged sulcal landmarks were used as anchors to drive the cortical surface mesh models from each subject into correspondence [Thompson et al., 2004]. In this way, points were matched across individuals relative to specific sulci so that comparisons of gray matter and surface location were based on homologous surface points, as far as possible.
The measure of radial expansion has been developed primarily to quantify group differences in local brain shape. Radial expansion was calculated by measuring the distance, in mm, from the center of mass of the hemisphere to each of the 65,536 brain surface points [Sowell et al., 2001]. This measure presumably reflects expansion of the cortical surface due to both underlying gray and white matter. Gray matter thickness was calculated using the Eikonal Fire Equation [Sapiro, 2001; Thompson et al., 2004]. The 3D Eikonal equation was applied only to voxels that segmented as gray matter. The image data were supersampled to create voxel dimensions of 0.33 mm3. Cortical thickness (in millimeters) was averaged within a 15‐mm sphere centered on each surface point. This approach allowed us to calculate cortical thickness with an effective resolution finer than the original voxel size given that the error associated with localizing anatomy on the inner and outer cortical surfaces was averaged with the unbiased error of all other voxels within the smoothing kernel (i.e., ∼42,000 0.33‐mm3 voxels). This approach has been shown to be both valid and reliable [Sowell et al., 2004].
To investigate group differences in asymmetry of gray matter thickness, asymmetry maps for each measure were created for each individual. Each subject's right hemisphere was reflected in the midsagittal plane, to have the appearance of a left hemisphere, and sulcal matching was performed on the full set of left and “flipped” right hemispheres. In this way, sulcal anatomy was matched not only across individuals but also between hemispheres. We then used these flipped sulcally matched brain surface representations to assess ratios of gray matter thickness at analogous surface points. Gray matter thickness asymmetry was calculated as the ratio of gray matter thickness on the left to gray matter thickness on the right. This ratio is greater than 1 if gray matter is thicker on the left and less than 1 if gray matter is thicker in the right hemisphere.
Statistical Analyses
To visualize group differences in local brain size and gray matter thickness, uncorrected statistical maps were created comparing groups of proficient, resilient, and poor readers. In these maps, the correlation (Pearson's r) between group membership and thickness or brain size was calculated. Proficient readers were compared with both poor and resilient readers separately, and resilient and poor readers were compared. A surface‐point significance threshold of P = 0.05 (uncorrected) was considered significant.
These maps allow for easy visualization of the spatial patterns of relationships between brain morphology and performance or group membership. To confirm localization of effects and to correct for multiple comparisons, permutation methods [Bullmore et al., 1999] were used. To localize effects at a lobar level, eight coarse regions of interest (ROIs) were modified from a probabilistic atlas [Evans et al., 1994]. On the lateral surface, parietal, temporal, and occipital ROIs were used. The lateral frontal lobe was separated into ventral and dorsal regions by dividing it in half along the dorsal/ventral axis. A temporo‐parietal ROI was created by centering a sphere 20 mm in diameter at the posterior extent of the Sylvian fissure on an average brain image. Medial ROIs encompassed the 11 slices closest to midline. The medial surface was divided in frontal and posterior portions. The ROIs in standard space were transformed into each individual's native space, and then averaged across individuals to create regional masks. An example showing these ROIs displayed over a representative brain surface is displayed in Figure 1. The area encompassing the corpus callosum, ventricular wall, and brain stem was not analyzed, as no gray matter is present in those regions.
Figure 1.
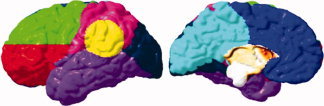
ROIs used in permutation analyses. Regions are coded as follows: Lateral dorsal frontal, green; lateral ventral frontal, red; lateral parietal, pink; temporo‐parietal, yellow; lateral occipital, dark blue; temporal, purple; medial frontal, blue; medial posterior, teal.
In the permutation analyses, group membership was randomly reassigned 10,000 times and new correlation analyses were conducted for each reassignment. This resulted in a null distribution of results that occurred by chance that was compared to the results from the real assignment. For each permutation, the number of points that reached significance within each ROI are counted and compared to the number of points that reach significance with the actual subject assignments. A statistical threshold of 0.01 was used to identify voxels in which significant group differences were observed. If fewer than 5% of the random reassignments resulted in more points below the 0.01 threshold than the real assignment, the effect within an ROI was considered significant.
RESULTS
Poor Readers Compared With Proficient Readers
Comparisons between poor (N = 12) and proficient (N = 22) readers were performed to explore differences in brain anatomy between individuals who decode and comprehend text well and those who do not. Group differences in radial expansion, gray matter thickness, and gray matter thickness asymmetry are shown in Figure 2 and statistical values are presented in Table II. Compared with proficient readers, poor readers show significantly less radial expansion in the right lateral inferior frontal, right lateral superior frontal, and right lateral parietal regions (Fig. 2A). The magnitude of difference in radial expansion between poor and proficient readers is illustrated in Figure 2B. In regions of maximal difference, brain surface extent is reduced up to 5 mm in poor readers. Proficient and poor readers show no reliable differences in gray matter thickness (Fig. 2C,D). Compared with proficient readers, poor readers show a decrease in leftward asymmetry in the temporo‐parietal region (Fig. 2E). The magnitude of this alteration in asymmetry is shown in Figure 2F. In the temporo‐parietal region, gray matter asymmetry is reduced by a maximum of 20% in poor readers. Proficient readers show a leftward asymmetry in this region (with ∼10% more gray matter in the left hemisphere in areas of maximal asymmetry), but poor readers show a rightward asymmetry (with ∼10% less gray matter in the left hemisphere in areas of maximal asymmetry).
Figure 2.
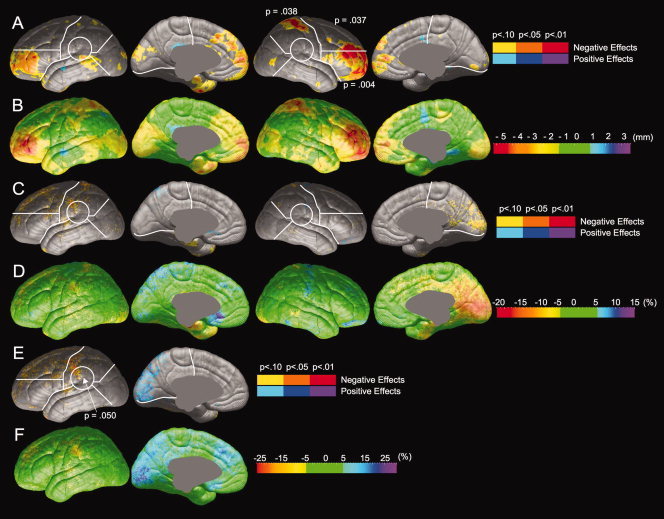
Comparison of brain morphology between proficient and poor readers. Probability values, represented by color, are mapped onto an average cortical surface model. Regions shown in hot colors (yellow, orange, and red) are areas in which poor readers have less radial expansion, thickness, or leftward thickness asymmetry than proficient readers; regions in cool colors (blue and purple) are areas in which poor readers have greater radial expansion, thickness, or leftward thickness asymmetry than proficient readers. Significance values from permutation tests are shown for each ROI in which group differences were significant by permutation. (A) Statistical comparison of radial expansion between proficient and poor readers. (B) Magnitude of differences in radial expansion between proficient and poor readers. (C) Statistical comparison of gray matter thickness between proficient and poor readers. (D) Magnitude of differences in gray matter thickness between proficient and poor readers. (E) Statistical comparison of gray matter thickness asymmetry between proficient and poor readers. (F) Magnitude of differences in gray matter thickness asymmetry.
Table II.
Significance of permutation values from each ROI at a threshold of 0.01
| Region | Poor versus proficient readers | Proficient versus resilient readers | Resilient versus poor readers | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Radial exp. | Thickness | Asymmetry | Radial exp. | Thickness | Asymmetry | Radial exp. | Thickness | Asymmetry | |
| Left inf. frontal lat. | 0.08 | NS | NS | NS | NS | NS | NS | NS | NS |
| Left sup. frontal lat. | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Left parietal lat. | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Left occipital lat. | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Left temporal | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Left tempro‐parietal | NS | 0.10 | 0.05 | NS | 0.08 | 0.04 | NS | NS | NS |
| Left frontal med. | NS | NS | NS | NS | NS | NS | 0.06 | NS | NS |
| Left posterior med. | NS | NS | NS | NS | NS | NS | NS | 0.05 | NS |
| Right inf. frontal lat. | 0.00 | NS | NS | NS | 0.01 | NS | |||
| Right sup. frontal lat. | 0.04 | NS | NS | NS | 0.07 | NS | |||
| Right parietal lat. | 0.04 | NS | NS | NS | 0.04 | NS | |||
| Right occipital lat. | NS | NS | NS | NS | NS | NS | |||
| Right temporal | NS | NS | NS | 0.10 | NS | NS | |||
| Right tempro‐parietal | NS | NS | NS | NS | NS | NS | |||
| Right frontal med. | NS | NS | NS | NS | NS | NS | |||
| Right posterior med. | NS | NS | NS | NS | NS | NS | |||
Resilient Readers Versus Proficient Readers
Comparisons of resilient (N = 21) and proficient (N = 22) readers were performed to investigate differences in brain anatomy between individuals who are impaired at phonological decoding and those who are not in samples matched for reading comprehension scores. Group differences in radial expansion, gray matter thickness, and gray matter thickness asymmetry are shown in Figure 3 and Table II. Resilient and proficient readers did not reliably differ in radial expansion (Fig. 3A) and maximal differences in radial expansion did not exceed 3 mm (Fig. 3B). Resilient and proficient readers did not significantly differ in gray matter thickness (Fig. 3C,D). Resilient readers show significantly less leftward asymmetry in the temporo‐parietal region (Fig. 3E). The magnitude of differences of asymmetry between resilient and proficient readers (Fig. 3F) was similar to the magnitude of difference between poor and proficient readers (Fig. 2F).
Figure 3.
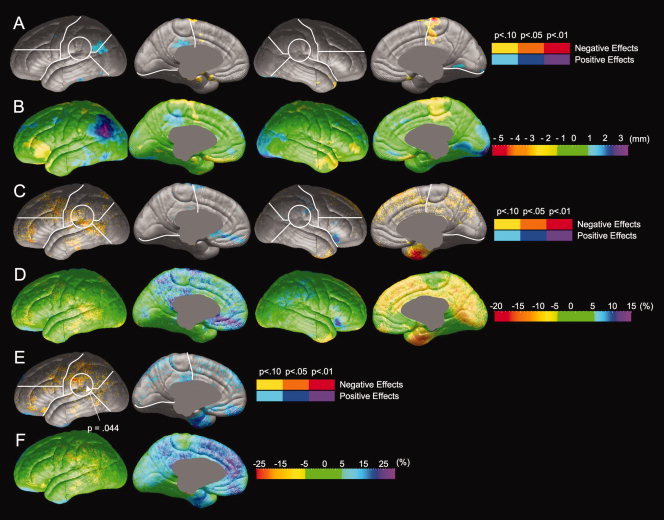
Comparison of brain morphology between proficient and resilient readers. Negative effects (Proficient > Resilient) are shown in hot colors, and positive effects (Resilient > Proficient) are shown in cool colors. Significance values from permutation tests are shown for each ROI in which group differences were significant by permutation. (A) Statistical comparison of radial expansion between proficient and resilient readers. (B) Magnitude of differences in radial expansion between proficient and resilient readers. (C) Statistical comparison of gray matter thickness between proficient and resilient readers. (D) Magnitude of differences in gray matter thickness between proficient and resilient readers. (E) Statistical comparison of gray matter thickness asymmetry between proficient and resilient readers. (F) Magnitude of differences in gray matter thickness asymmetry.
Poor Readers Versus Resilient Readers
Comparisons of resilient (N = 21) versus poor readers (N = 12) were performed to investigate differences in brain anatomy between individuals who are skilled at Passage Comprehension and those who are not in samples equally impaired in phonological decoding. Group differences in radial expansion, gray matter thickness, and gray matter thickness asymmetry are shown in Figure 4 and Table II. Compared to poor readers, resilient readers show a significant increase in radial expansion in the right lateral inferior frontal and right lateral parietal regions (Fig. 4A). The magnitude of the reduction in radial expansion in poor readers (shown in Fig. 4B) is similar to the reduction shown in poor readers relative to proficient readers (Fig. 2B). Resilient and poor readers do not show reliable differences in gray matter thickness (Fig. 4C,D). Resilient and poor readers did not reliably differ in the asymmetry of gray matter thickness (Fig. 4E,F).
Figure 4.
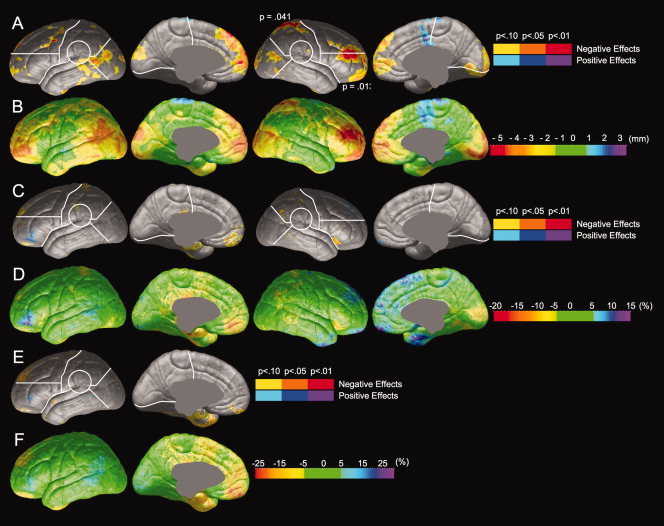
Comparison of brain morphology between resilient and poor readers. Negative effects (Resilient > Poor) are shown in hot colors, and positive effects (Poor > Resilient) are shown in cool colors. Significance values from permutation tests are shown for each ROI in which group differences were significant by permutation. (A) Statistical comparison of radial expansion between resilient and poor readers. (B) Magnitude of differences in radial expansion between resilient and poor readers. (C) Statistical comparison of gray matter thickness between resilient and poor readers. (D) Magnitude of differences in gray matter thickness between resilient and poor readers. (E) Statistical comparison of gray matter thickness asymmetry between resilient and poor readers. (F) Magnitude of differences in gray matter thickness asymmetry.
DISCUSSION
Several studies have examined neural correlates of reading ability, but this study was the first to examine brain morphology in adults with impaired phonological processing and intact reading comprehension. Such individuals are of theoretical interest because they suggest that skilled reading comprehension can develop in individuals with relatively poor phonological skills. Because we classified readers according to performance on subtests that represent two different dimensions of reading ability, we were able to detect differences in brain structure between groups that differ in specific reading subskills. This may allow us to observe more precise anatomical‐functional associations than generalized comparisons between normal and dyslexic readers. Proficient readers were compared with resilient readers (poor phonological decoders with good passage comprehension) and poor readers (poor phonological decoding and passage comprehension). We predicted that resilient readers would show altered morphology in the posterior inferior frontal and temporo‐parietal regions thought to support skilled phonological decoding. These predictions were partially supported by the finding that resilient readers, compared to proficient readers, showed less leftward asymmetry of gray matter thickness in the temporo‐parietal region. We predicted that poor readers would share this reduced asymmetry with resilient readers, and show additional anatomical differences in the lateral ventral frontal region, and inferior temporal lobe. These predictions were partially supported, as we found that poor readers showed less leftward asymmetry of the temporo‐parietal region and an additional reduction in radial expansion in the frontal lobes that was not seen in resilient readers.
Our finding of less leftward asymmetry in the temporal‐parietal region of both poor and resilient readers agrees with prior findings that reading disabled participants show reduced gray matter in the left supramarginal gyrus [Eckert et al., 2005] and the posterior superior temporal gyrus and temporo‐parieto‐occipital juction [Brambati et al., 2004; Brown et al., 2001]. This observed reduction in asymmetry in both groups with poor phonological skills is consistent with the findings of Galaburda et al. [ 1985]. Our findings extend these prior findings to suggest that less leftward gray matter asymmetry in this region may characterize individuals with poor phonological skills, regardless of reading comprehension abilities. Using cortical matching techniques, we found no evidence for exaggerated asymmetry in the temporo‐parietal region in resilient readers similar to that observed in a compensated dyslexic [Chiarello et al., 2006b] and dyslexic engineering students [Robichon et al., 2000]. It should be noted that the temporo‐parietal asymmetry measured in this study differs considerably from the sulcal length measures employed by these prior studies. As poor and resilient readers show similarly decreased performance on pseudoword reading tasks and an auditory phoneme deletion task [Welcome et al., 2009; Welcome et al., submitted for publication], it is suggestive that the two groups show a similar pattern of reduced asymmetry in this region. Functional imaging studies suggest that the left temporo‐parietal region is active during the conversion of orthography to phonology [Booth et al., 2002] and tasks that require assembled phonology [Chen et al., 2002].
Alternately, it is possible that altered morphology in the temporo‐parietal region relates to text comprehension. Reading disabled children show overactivation in the left superior temporal region in response to sentence comprehension tasks [Rimrodt et al., 2008]. Poor readers exposed to remedial instruction show increases in activation in the left angular gyrus and left superior parietal lobule during sentence comprehension [Meyler et al., 2008]. Our behavioral findings with these participants suggest that resilient readers rely more on semantic relationships between words than either of the other groups [Welcome et al., 2009; Welcome et al., submitted for publication]. Given functional studies that suggest a role for this region in text comprehension, it is possible that morphological differences in this region relate to group differences in semantic processing.
The reduced radial expansion in right frontal regions shown by poor, but not resilient, readers is partially consistent with prior findings that dyslexics show altered morphology in bilateral frontal regions [Brown et al., 2001; Eckert et al., 2003]. Our findings suggest that this morphological feature characterizes individuals with less skill at reading comprehension. This interpretation is supported by functional imaging studies that suggest a role for a bilateral ventral frontal region in semantic processing [reviewed in Fiez, 1997]. Poor readers show reduced radial expansion of the ventral frontal region that is reliable in the right hemisphere, but not the left. These findings suggest that right ventral frontal morphology, as well as left, may relate to text comprehension ability. Consistent with this finding, bilateral activation in the inferior frontal region was seen during concrete/abstract decisions [Chee et al., 1999], semantic category generation [Shaywitz et al., 1994], comprehension of complex sentences [Just et al., 1996], and discourse processing [St. George et al., 1999] suggesting that both left and right inferior frontal regions may be involved in tasks that involve meaning access.
Neither poor nor resilient readers in our sample showed the predicted reductions in gray matter thickness and brain size in the left posterior inferior frontal region, although this region is typically active during phonological processing [reviewed in Pugh et al., 1996]. There were also no reductions in the inferior temporal regions that are active during orthographic processing [Cohen et al., 2000]. It is possible that we simply lacked the power to uncover morphological differences in these regions. Alternately, it is possible that reading impaired groups that have shown these alterations in morphology [e.g., Eckert et al., 2005; Kronbichler et al., 2008] have more extensive reading impairments or more atypical developmental trajectories than the college students in the current study. While both resilient and poor readers showed decreased performance on an orthographic choice task [Welcome et al., 2009; Welcome et al., submitted for publication], the presence of these individuals in a university setting suggests that their reading skills may not be as poor as typical dyslexics, and perhaps that their exposure to print is more similar to that of typical readers. Another possibility is that the sulcal pattern matching employed in the current study yielded different results from VBM methods used in previous work. Prior work has demonstrated that manual measures and VBM have identified differences between dyslexics and controls in different portions of the reading network [Eckert et al., 2005], suggesting that such differences are sensitive to measurement technique. The cortical pattern matching technique employed in the present study control for variability in sulcal patterns across individuals in a way that current VBM techniques cannot, and it is possible that this methodological difference underlies differences between our findings and those of some prior studies.
In the temporo‐parietal region, both poor and resilient readers show less leftward asymmetry of gray matter thickness. This leftward asymmetry appeared to result from a slight, nonreliable, reduction of gray matter thickness in the right temporo‐parietal cortex relative to proficient readers. The cellular correlates of gray matter thickness observed with MRI are not fully understood, but this measure may reflect soma size [Wellman and Sengelaub, 1991], neuron number [Wegner et al., 2006], and arborization of dendritic processes [O'Kusky et al., 1996]. Alternately, thinner gray matter may reflect greater myelination of fibers, as unmyelinated axons may be classified as gray matter on the basis of their signal value on MRI [see Sowell et al., 2004], although the absence of a parallel increase in radial expansion argues against this interpretation.
Poor readers show reduced radial expansion in the ventral frontal regions without reduced gray matter thickness in the same region. The cellular determinants of reduced radial expansion without reduced gray matter thickness are again unknown, but this morphological alteration may have implications for processing differences between dyslexics and controls. Similar to our findings, a previous study found that total cerebral volume was reduced in dyslexics, while cortical thickness was not [Casanova et al., 2004]. These alterations were related to reduced gyrification, which the authors suggest could result in more cortical integration and slower processing [Casanova et al., 2004]. Alternately, the reduced radial expansion in our study may indicate reductions in underlying white matter in this region. Reduced connectivity from the inferior frontal region in dyslexics has been demonstrated with diffusion tensor imaging [Richards et al., 2008] and functional connectivity studies [Quaglino et al., 2008]. Thus, reduced radial expansion in the poor readers in the present sample may reflect alterations in underlying white matter, perhaps indicating differences in the connections made between the inferior frontal cortex and other brain regions.
It is interesting to speculate as to whether these anatomical differences are the cause or consequence of the behavioral differences between groups. It is possible that morphology is altered in such a way that sufficiently accurate phonological representations cannot be supported, leading to reading deficits. However, recent studies suggest that brain anatomy can be altered by learning experiences [Draganski et al., 2006] and activation profiles normalize following successful reading remediation [Temple et al., 2003], indicating that brain anatomy may be sensitive to environmental factors. Without detailed knowledge of the educational experiences of our participants, it is not possible to know whether differences in reading instruction might contribute to differences in brain morphology between our reading groups.
Our study had some limitations. Our sample sizes were small, especially for the group of poor readers, and therefore the study may have lacked the power to uncover subtle anatomical differences. Prior studies using this methodology have used similar sample sizes to produce findings that have replicated across independent samples [Sowell et al., 2002]. As the participants in this study were all adults, some aspects of their educational background, including objective measures of their childhood reading abilities, were not available. Longitudinal studies identifying and tracking resilient and poor readers from early in development might provide additional insight into the relationships between brain anatomy and reading ability. An additional limitation of the present study was that multiple comparisons were necessary to examine group differences in brain morphology. Within a statistical map, permutation analyses were used to correct for the number of comparisons for each region of interest evaluated. However, the relatively large number of statistical maps, and ROIs examined within each map, means that many comparisons were made. Nonetheless, the approach of investigating relationships between morphology and specific components of reading was novel and provided unique information about relationships between cortical anatomy and reading abilities, and we feel confident interpreting those results that are consistent with our a priori predictions.
Additionally, it should be noted that even the poor readers in this study were attending college and had higher scores than reading impaired participants of previous studies. The inclusion of less reading impaired individuals may have minimized anatomical differences between groups, and we run the risk of underestimating the extent of anatomical differences related to reading ability. However, the finding that groups with relatively minor reading impairments show reliable differences in brain morphology extends these prior findings and suggests that relationships between reading ability and brain anatomy exist across the entire spectrum of reading skill. Further, the use of resilient readers allowed us to demonstrate that some of these anatomical differences likely relate to phonological decoding impairments, while others may index other reading skills.
Together, our findings suggest that brain morphology differs between college students with different reading profiles. Resilient readers were shown to represent a separate population of readers, distinguishable from poor and proficient readers in neuroanatomy. Resilient readers show relatively subtle behavioral differences from typical readers, so it is intriguing that they show consistent differences in brain morphology. These differences were focal, and restricted to the temporo‐parietal region as predicted. In those students with more extensive reading difficulties, morphological differences were more extensive, present in inferior frontal and temporo‐parietal areas thought to support both print‐to‐sound conversion and meaning access. Future studies may build on these results to provide a more comprehensive picture of the relationships between brain anatomy, brain function, and reading behavior.
Acknowledgements
The authors thank Laura K. Halderman, Janelle Julagay, and Eric Kan for assistance with data collection and processing.
REFERENCES
- Beaton AA ( 1997): The relation of the planum temporale asymmetry and morphology of the corpus callosum to handedness, gender, and dyslexia: A review of the evidence. Brain Lang 60: 255–322. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM ( 2002): Functional anatomy of intra‐ and cross‐modal lexical tasks. Neuroimage 16: 7–22. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Termine C, Ruffino M, Stella G, Fazio F, Cappa SF, et al. ( 2004): Regional reductions of gray matter volume in familial dyslexia. Neurology 63: 742–745. [DOI] [PubMed] [Google Scholar]
- Brown WE, Eliez S, Menon V, Rumsey JM, White CD, Reiss AL ( 2001): Preliminary evidence of widespread morphological variations of the brain in dyslexia. Neurology 56: 781–783. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe‐Hesketh S, Taylor E, Brammer MJ ( 1999): Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging 18: 32–42. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Araque J, Giedd J, Rumsey JM ( 2004): Reduced brain size and gyrification in the brains of dyslexic patients. J Child Neurol 19: 275–281. [DOI] [PubMed] [Google Scholar]
- Chee MW, O'Craven KM, Bergida R, Rosen BR, Savoy RL ( 1999): Auditory and visual word processing studied with fMRI. Hum Brain Mapp 7: 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Fu S, Iversen SD, Smith SM, Matthews PM ( 2002): Testing for dual brain processing routes in reading: A direct contrast of chinese character and pinyin reading using FMRI. J Cogn Neurosci 14: 1088–1098. [DOI] [PubMed] [Google Scholar]
- Chiarello C, Lombardino LJ, Kacinik NA, Otto R, Leonard CM ( 2006a): Neuroanatomical and behavioral asymmetry in an adult compensated dyslexic. Brain Lang 98: 169–181. [DOI] [PubMed] [Google Scholar]
- Chiarello C, Welcome SE, Halderman LK, Julagay J, Otto R, Leonard CM ( 2006b): Individual differences in lexical processing and cerebral asymmetries. Paper presented at the meeting of the Psychonomic Society, Houston, TX.
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene‐Lambertz G, Henaff MA, Michel F ( 2000): The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split‐brain patients. Brain 123: 291–307. [DOI] [PubMed] [Google Scholar]
- Dogdas B, Shattuck DW, Leahy RM ( 2005): Segmentation of skull and scalp in 3‐D human MRI using mathematical morphology. Hum Brain Mapp 26: 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, May A ( 2006): Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci 26: 6314–6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert M ( 2004): Neuroanatomical markers for dyslexia: A review of dyslexia structural imaging studies. Neuroscientist 10: 362–371. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Richards TL, Aylward EH, Thomson J, Berninger VW ( 2003): Anatomical correlates of dyslexia: Frontal and cerebellar findings. Brain 126: 482–494. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Wilke M, Eckert M, Richards T, Richards A, Berninger V ( 2005): Anatomical signatures of dyslexia in children: Unique information from manual and voxel based morphometry brain measures. Cortex 41: 304–315. [DOI] [PubMed] [Google Scholar]
- Eliez S, Rumsey JM, Giedd JN, Schmitt JE, Patwardhan AJ, Reiss AL ( 2000): Morphological alteration of temporal lobe gray matter in dyslexia: An MRI study. J Child Psychol Psychiatry 41: 637–644. [DOI] [PubMed] [Google Scholar]
- Evans AC, Kamber M, Collins DL, MacDonald D ( 1994): An MRI‐based probabilistic atlas of neuroanatomy In: Shorvon MD, editor. Magnetic Resonance Scanning and Epilepsy. New York: Plenum; pp 263–274. [Google Scholar]
- Fiez JA ( 1997): Phonology, semantics, and the role of the left inferior prefrontal cortex. Hum Brain Mapp 5: 79–83. [PubMed] [Google Scholar]
- Galaburda AM ( 1989): Ordinary and extraordinary brain development: Anatomical variation in developmental dyslexia. Ann Dyslexia 39: 67–79. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Sherman GF, Rosen GD, Aboitiz F, Geschwind N ( 1985): Developmental dyslexia: Four consecutive cases with cortical anomalies. Ann Neurol 18: 222–233. [DOI] [PubMed] [Google Scholar]
- Hoenig K, Scheef L ( 2005): Mediotemporal contributions to semantic processing: fMRI evidence from ambiguity processing during semantic context verification. Hippocampus 15: 597–609. [DOI] [PubMed] [Google Scholar]
- Jackson NE, Doellinger HL ( 2002): Resilient readers? University studentss who are poor recoders but sometimes good text comprehenders. J Educ Psychol 94: 64–78. [Google Scholar]
- Joubert S, Beauregard M, Walter N, Bourgouin P, Beaudoin G, Leroux JM, Karama S, Lecours AR ( 2004): Neural correlates of lexical and sublexical processes in reading. Brain Lang 89: 9–20. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR ( 1996): Brain activation modulated by sentence comprehension. Science 4: 114–116. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Wimmer H, Staffen W, Hutzler F, Mair A, Ladurner G ( 2008): Developmental dyslexia: Gray matter abnormalities in the occipitotemporal cortex. Hum Brain Mapp 29: 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CM, Eckert MA ( 2008): Asymmetry and dyslexia. Dev Neuropsychol 33: 663–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Leonard C, Thompson P, Kan E, Jolley J, Welcome S, Toga A, Sowell E ( 2007): Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: A longitudinal MRI analysis. Cereb Cortex 17: 1092–1099. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Avis D, Evans A ( 1994): Multiple surface identification and matching in magnetic resonance images. Proc SPIE Visual Biomed Comput 2359: 160–169. [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Gabrielli JDE, Just MA ( 2008): Modifying the brain activation of poor readers during sentence comprehension with extended remedial instruction: A longitudinal study of neuroplasticity. Neuropsychologia 46: 2580–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T ( 2004): Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci 16: 1227–1233. [DOI] [PubMed] [Google Scholar]
- O'Kusky JR, Akers MA, Vinters HV ( 1996): Synaptogenesis in hemimegalencephaly: The numerical density of asymmetric and symmetric synapses in the cerebral cortex. Acta Neuropathol 92: 156–163. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Shankweiler DP, Katz L, Fletcher JM, Gore JC ( 1996): Cerebral organization of component processes in reading. Brain 119: 1221–1238. [DOI] [PubMed] [Google Scholar]
- Quaglino V, Bourdin B, Czternasty G, Vrignaud P, Fall S, Meyer ME, Berquin P, Devauchelle B, de Marco G ( 2008): Differences in effective connectivity between dyslexic children and normal readers during a pseudoword reading task: An fMRI study. Clin Neurophysicol 38: 73–82. [DOI] [PubMed] [Google Scholar]
- Richards T, Stevenson J, Crouch J, Johnson LC, Maravilla K, Stock P, et al. ( 2008): Tract‐based spatial statistics of diffusion tensor imaging in adults with dyslexia. Am J Neuroradiol 29: 1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimrodt SL, Clements‐Stephens AM, Pugh KR, Courtney SM, Gaur P, Pekar JJ, Cutting LE ( 2008): Functional MRI of sentence comprehension in children with dyslexia: Beyond word recognition. Cereb Cortex 19: 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichon F, Levrier O, Farnarier P, Habib M ( 2000): Developmental dyslexia: Atypical cortical asymmetries and functional significance. Eur J Neurol 7: 35–46. [DOI] [PubMed] [Google Scholar]
- Sapiro G ( 2001): Geometric Partial Differential Equations and Image Analysis. New York: Cambridge University Press. [Google Scholar]
- Shattuck DW, Sandor‐Leahy SR, Schaper KA, Rottenberg DA, Leahy RM ( 2001): Magnetic resonance image tissue classification using a partial volume model. NeuroImage 13: 856–876. [DOI] [PubMed] [Google Scholar]
- Shaywitz DA, Pugh KR, Constable RT, Shaywitz SE, Bronen RA, Fulbright RK, et al. ( 1994): Localization of semantic processing using functional magnetic resonance imaging. Hum Brain Mapp 2: 149–158. [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, Gore JC ( 2002): Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry 52: 101–110. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW ( 2001): Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci 21: 8819–8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Rex D, Komsand D, Tessner KD, Jernigan TL, Toga AW ( 2002): Mapping sulcal pattern asymmetry and local cortical surface gray matter distribution in vivo: Maturation in perisylvian cortices. Cereb Cortex 12: 17–26. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW ( 2004): Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci 24: 8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. George M, Kutas M, Martinez A, Sereno MI ( 1999): Semantic integration in reading: Engagement of the right hemisphere during discourse processing. Brain 122: 1317–1325. [DOI] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, Gabrieli JDE ( 2003): Neural deficits in children with dyslexia ameliorated by behavioral remediation: Evidence from functional MRI. Proc Natl Acad Sci USA 4: 2860–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Sowell ER, Gogtay N, Giedd JN, Rapoport JL, de Zubicaray GI, Janke AL, Rose SE, Semple J, Doddrell DM, Wang Y, van Erp TGM, Cannon TD, Toga AW ( 2004): Mapping cortical change in Alzheimer's disease, brain development, and schizophrenia. NeuroImage 23: S2–S18. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF ( 2003): Development of neural mechanisms for reading. Nat Neurosci 6: 767–773. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RS ( 1996): Functional anatomy of a common semantic system for words and pictures. Nature 383: 254–256. [DOI] [PubMed] [Google Scholar]
- Vinckenbosch E, Robichon F, Eliez S ( 2005): Gray matter alteration in dyslexia: Converging evidence from volumetric and voxel‐by‐voxel MRI analyses. Neuropsychologia 43: 324–331. [DOI] [PubMed] [Google Scholar]
- Wegner C, Esiri MM, Chance SA, Palace J, Matthews PM ( 2006): Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology 67: 960–967. [DOI] [PubMed] [Google Scholar]
- Welcome SE, Chiarello C, Halderman LK, Leonard CM ( 2009): Lexical processing skill in college‐age resilient readers. Read Writing Interdiscipl J 22: 353–371. [Google Scholar]
- Wellman CL, Sengelaub DR ( 1991): Cortical neuroanatomical correlates of behavioral deficits produced by lesion of the basal forebrain in rats. Behav Neural Biol 56: 1–24. [DOI] [PubMed] [Google Scholar]
- Woodcock RW ( 1998): Woodcock Reading Mastery Test‐Revised Normative Update. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR ( 1993): MRI‐PET registration with automated algorithm. J Comput Assist Tomogr 17: 536–546. [DOI] [PubMed] [Google Scholar]


