Abstract
A characterization of the impact of natural disasters on the brain of survivors is critical for a better understanding of posttraumatic responses and may inform the development of more effective early interventions. Here we report alterations in white matter microstructure in survivors soon after Wenchuan earthquake in China in 2008. Within 25 days after the Wenchuan earthquake, 44 healthy survivors were recruited and scanned on a 3T MR imaging system. The survivors were divided into two groups according to their self‐rating anxiety scale (SAS) score, including the SAS(+) (SAS > 55 after correction) group and “SAS(−)” (SAS < 55 after correction) group. Thrity‐two healthy volunteers were also recruited as control group before earthquake. Individual maps of fractional anisotropy (FA) were calculated and voxel‐based analysis (VBA) was performed to allow the comparison between survivors and controls using ANCOVAs in SPM2. In addition, a correlation between SAS score and regional FA value was examined using Pearson's correlation analysis in SPSS 11.5. Compared with the healthy cohort, the whole group of 44 survivors showed significantly decreased FA values in the right prefrontal lobe, the parietal lobe, the basal ganglia, and the right parahippocampus. These effects did not appear to depend on self‐rating anxiety. For the first time we provide evidence that acute trauma altered cerebral microstructure within the limbic system; furthermore, these alterations are evident shortly after the traumatic event, highlighting the need for early evaluation and intervention for trauma survivors. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: stress, depression, PTSD, earthquake, MRI, diffusion tensor imaging
INTRODUCTION
On May 12th 2008, the epicenter of an earthquake measuring 8.0 on the Richter scale occurred in Wenchuan, in the Sichuan Province of China, resulting in enormous human and economic consequences. Sixty‐nine thousand two hundred twenty seven people were confirmed dead, 374,643 were injured and 17,923 are missing in this disaster [Lui et al., 2009]. However, besides the human and economic consequences, natural disasters such as this also pose a considerable risk to the mental health of survivors who are not physically injured [Li et al., 2009]. A significant proportion of the survivors (≈20%) are likely to develop stress‐related disorders, such as acute stress disorder (ASD) and post‐traumatic stress disorder (PTSD). A characterization of the impact of natural disasters on the brain of survivors is therefore critical for a better understanding of posttraumatic responses and may inform the development of more effective early interventions.
Diffusion tensor imaging (DTI), a magnetic resonance imaging technique sensitive to the orientation of water diffusion restricted within the neuron sheath and myelination, provides measures of white‐matter microstructure in the human brain. The orientation dependence of water diffusion—fractional anisotropy (FA) in DTI is thought to reflect anatomical features of neural fiber, such as axon caliber, fiber density, and myelination [Scholz et al., 2009]. This technique has been widely used in psychiatric research in recent years [Stam, 2007] and has been successfully applied to the detection of neuroplastic changes after short‐time training [Scholz et al., 2009].
Previous studies have shown that exposure to traumatic events, such as natural disasters [Lui et al., 2009; Sakamoto et al., 2005] or terrorist attacks [Ganzel et al., 2008], results in alterations of brain structure and function. For example, in our earlier research, the regional amplitude low frequency fluctuation (ALFF) signal changes in prefrontal cortex and basal ganglia were found to be correlated with the extent of mental distress, and furthermore the functional integration among these areas was affected [Lui et al., 2009]. This was consistent with animal models proving evidence that hippocampus and prefrontal lobe are physiologically and morphologically influenced by acute stress [Miller and McEwen, 2006]. However, the studies published so far have focused on the impact of traumatic events on gray matter and, to our knowledge, none of them has examined white matter shortly after traumatic events. It is therefore unclear whether exposure to natural disasters, such as the Wenchuan 8.0 earthquake, result in alterations of white matter microstructure. Our aim is therefore to use DTI to explore the anatomical changes among the survivors recruited from the affected areas of the Wenchuan earthquake within 25 days. Based on the findings of disrupted functional integration in patients with established postraumatic stress disorder [Lanius et al., 2003] and in healthy survivors [Lui et al., 2009], we hypothesized that the survivors of Wenchuan 8.0 earthquake would show alterations of white matter microstructure, particularly within a fronto‐limbic network involved in emotion regulation[Li and Sinha, 2008].
MATERIALS AND METHODS
Subjects
The study was approved by the local ethics committee, and all the participants signed informed consent. Forty‐four survivors (27 men and 17 women with a mean age: 37 ± 10.6 years) were recruited from the earthquake affected areas within 25 days (range 13–25 days; mean ± SD: 21 ± 3 days) after the start of the Wenchuan earthquake from the most affected regions, where seismic intensity ranged from 9 to 11 on the Mercalli intensity scale. The inclusion criteria for the survivors included: (1) physically experienced the earthquake, (2) without any personal medical injury, and (3) had personally witnessed death, serious injury, or the collapse of buildings. A structured clinical interview was used to rule out the possibility of psychiatric disorders before the earthquake based on the diagnostic and statistical manual of mental disorders‐IV (SCID IV). Thirty‐two healthy volunteers (20 males and 12 females with a mean age: 34.6 ± 11.0 years) were also recruited from local areas just a little earlier before the earthquake for normal control database. Gender, weight, height, age, and years of education were matched among the three groups (Table I). The incoming level and occupational status were also matched between the two groups.
Table I.
Demographic statistical and survivors' SAS score
| Characteristics | Controls (N = 32) | SAS(−) (N = 20) | SAS(+) (N = 24) | Survivors (N = 44) | P |
|---|---|---|---|---|---|
| Male:female | 20:12 | 12:8 | 15:9 | 27:17 | 0.998 |
| Height (cm) | 168.4 ± 4.9 | 167.7 ± 5.6 | 168.1 ± 7.9 | 167.8 ± 6.4 | 0.77 |
| Weight (Kg) | 58.4 ± 11.2 | 59.3 ± 12.1 | 57.9 ± 9.2 | 58.9 ± 10.3 | 0.685 |
| Mean age ± SD | 34.6 ± 11.0 | 35.9 ± 11.2 | 37.9 ± 10.1 | 37 ± 10.6 | 0.916 |
| Years of edu ± SD | 9.3 ± 4.2 | 9.6 ± 3.8 | 7.7 ± 4.2 | 8.6 ± 4.1 | 0.420 |
| SAS score ± SD | — | 37 ± 6.5 | 57 ± 4.8 | 48 ± 11.4 |
SAS, the self‐rating anxiety scale.
The exclusion criteria were: (1) the existence of current psychiatric disorder, (2) organic brain defects on T1 or T2 images (examined by two experienced radiologist), and (3)chronic serious illness (e.g. tumor, epilepsy, diabetes).
A recent investigation has reported that the anxiety symptoms were the survivors' dominant reaction following the earthquake [Fan et al., 2011]. Before the magnetic resonance imaging (MRI), the self‐rating anxiety scale (SAS) test was used to evaluate the stress level of each survivor [Zung, 1965]. According to their SAS scores (after correction), the survivors were divided into two groups. Those who scored over 55 with a diagnosis of anxiety status were assigned to the SAS(+) group (15 males and 9 females with a mean age: 37.9 ± 10.1 years, SAS score 57 ± 4.6) and the rest were assigned to the SAS(−) group (12 males and 8 females with a mean age: 35.9 ± 11.2 years, SAS score 38 ± 6.9) [Zung, 1965].
Data Acquisition
All DTI data were acquired on a 3T MR scanner (GE, EXCITE, Milwaukee). The DTI protocol involved using a single‐shot spin‐echo echo planar image (SE‐EPI) sequence (TR/TE = 10,000/70.8 ms; FOV 24 × 24 cm2; number of excitations (NEX) = 2; resolution matrix 128 × 128). Eddy current distortions were minimized by adding a second refocusing pulse [Reese et al., 2003]. Forty‐two contiguous slices were acquired with a 3‐mm slice thickness and no gap. The diffusion sensitizing gradients were applied along 15 noncollinear directions (b = 1,000 s/mm2) and an acquisition without diffusion weighing (b = 0). A strict quality assurance (QA) scan was acquired before each subject was examined. Foam cushions were used to reduce head translation movement and rotation. The head translation movement and rotation were within 0.5 mm and 0.5°.
Data Processing and Statistical Analysis
DTI Studio (Johns Hopkins University, Baltimore, MD; available at: http://cmrm.med.jhmi.edu) was used for data calculation. After image acquisition, the echo planar distortions induced by eddy currents were corrected using an algorithm that determines the optimum affine transformation to be applied to each diffusion‐weighted image. Individual maps of FA were calculated from the DTI data on a pixel by pixel basis according to the method proposed by Pierpaoli et al. [ 1996]. Voxel‐based analysis was performed using statistical parametric mapping (SPM2, Welcome Department of Imaging Neuroscience, Institute of Neurology, University of College London, UK; available at http://www.fil.ion.ucl.ac.uk/spm/software/) After normalizing all b0 images to standard Montreal Neurological Institute (MNI) space using the EPI template supplied by SPM2, the original voxel size of 1.875 mm ×1.875 mm ×3 mm was interpolated to a final voxel size of 2 mm ×2 mm ×2 mm. Then, these derived parameters were applied to the FA maps in order to normalize them to the MNI space. Finally, the normalized FA maps were spatially smoothed with a 8‐mm full width at half maximum (FWHM), isotropic Gaussian kernel to improve the signal‐to‐noise ratio (SNR).
Voxel‐based analysis was performed using ANCOVAs in SPM2 among the three groups to examine differences in FA. Age was used as a covariate of no interest in order to minimize its possible impact on the findings. Significant differences were defined as those which survived a statistical threshold of P < 0.05 (after family wise error correction) with a cluster size more than 10 voxels.
Pearson's correlation analysis was carried out using SPSS11.5 software to explore the correlation between the mean FA values in areas with significant differences and the SAS scores. The mean FA value of all voxels in each of these areas were extracted using a volume of interest approach in SPM2; the correlation was then performed using bivariate analysis with a statistical threshold of P < 0.05 after Bonferroni correction.
RESULTS
We first compared the survivor group as a whole against the control group. This revealed decreased FA in the right parahippocampus, bilateral parietal lobe, right prefrontal lobe, and bilateral basal ganglia of survivors (Fig. 1). We then contrasted the SAS(−) and SAS(+) groups against the control group. The SAS(−) group showed reduced FA in the right parahippocampus, bilateral parietal lobe, right prefrontal lobe, and bilateral basal ganglia; similarly, the SAS(+) group showed decreased FA in the right parahippocampus, bilateral parietal lobe, and bilateral basal ganglia (Fig. 1 and Table II). When comparing the two survivor groups directly, there was no significant difference in white matter microstructure.
Figure 1.
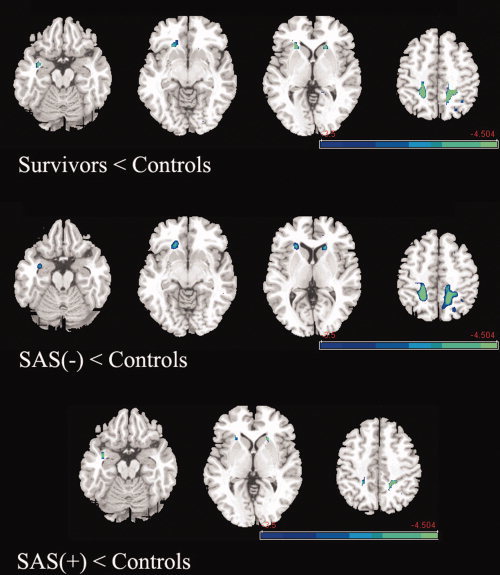
Differences of FA between the survivors and controls. Upper panel: Survivors as a whole showed decreased FA value compared with the control group in right parahippocampus, right medial prefrontal lobe, bilateral basal ganglia, and bilateral parietal lobe. Middle panel, the SAS(−) group showed decreased FA value compared with the control group in right parahippocampus, right medial prefrontal lobe, bilateral basal ganglia, and bilateral parietal lobe. Lower panel, the SAS(+) group showed decreased FA value compared with the control group in right parahippocampus, bilateral basal ganglia, and bilateral parietal lobe. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table II.
Voxel‐based analysis of global FA among three groups
| Location | Talairach (peak voxel) | Voxel size | P | ||
|---|---|---|---|---|---|
| x | y | z | |||
| NC > SAS(−) | |||||
| Right parahippo | 33 | −3 | −24 | 74 | * |
| Left parietal lobe | −21 | −54 | 57 | 680 | * |
| Right parietal lobe | 18 | −39 | 51 | 339 | * |
| Right med‐fro lobe | 30 | 21 | 24 | 69 | * |
| Left basal‐ganglia | −21 | 24 | 6 | 34 | * |
| Right basal‐ganglia | 21 | 24 | −3 | 111 | * |
| NC > SAS(+) | |||||
| Right parahippo | 33 | 0 | −18 | 34 | * |
| Left parietal lobe | −21 | −42 | 42 | 410 | * |
| Right parietal lobe | 21 | −39 | 42 | 127 | * |
| Left basal‐ganglia | −21 | 24 | 0 | 44 | * |
| Right basal‐ganglia | 24 | 24 | 0 | 37 | * |
| NC > all survivors | |||||
| Right parahippo | 33 | 0 | −21 | 88 | * |
| Left parietal lobe | −21 | −42 | 42 | 942 | * |
| Right parietal lobe | 21 | −39 | 45 | 328 | * |
| Right med‐fro lobe | 30 | 18 | 24 | 68 | * |
| Left basal‐ganglia | −21 | 24 | 3 | 59 | * |
| Right basal‐ganglia | 21 | 24 | −3 | 141 | * |
P < 0.05 (after family wise error correction).
NC: normal control, Med‐fro lobe: medial frontal lobe, parahippo: parahippocampal gyrus.
We also performed a series of Pearson's correlation analyses to examine the correlation of regional FA in the right parahippocampus, bilateral parietal lobe, right prefrontal lobe, and bilateral basal ganglia with the SAS score in the survivor's groups. Contrary to our prediction, the FA values did not correlate with the SAS scores in any of these areas.
DISCUSSION
To the best of our knowledge, this is the first study to explore the anatomical alteration in survivors shortly (within 25 days) after a natural disaster. Decreased white matter microstructure was found in the prefrontal‐limbic system including the prefrontal lobe, parahippocampus, bilateral parietal lobe, and bilateral basal ganglia. Survivors with and without high levels of anxiety showed similar patterns of FA decreases compared with the control group, and no differences were found between the two survivor groups. In addition, Pearson's correlation analysis revealed that FA values did not correlate with the SAS scores in any of these areas.
Considering that the main determinant of FA value is the architecture of the myelin, decreased FA is likely to reflect the disruption of integrity of the nerve‐sheath in white matter [Le Bihan, 2003]. This is consistent with previous studies which have reported reduced expression of glutathione in white matter in the perturbed mouse brain [Miller et al., 2009]. It is also consistent with the observation that oxidative stress is associated with neurodegeneration; in particular the antioxidant defense enzymes such as superoxide dismutase may be lower in anxiety status and lead to cytoskeletal breakdown [Ranjekar et al., 2003].
Previous animal experiments have revealed molecular alterations in response to acute stress mainly in the limbic system [Pacak et al., 1995; Yuen et al., 2009]. For example, cFos, nerve‐growth factor‐induced protein A at molecular level was found to be expressed 30 to 60 min after a stressor administration [Pacak et al., 1995]. Reports of enhanced glutamatergic transmission in prefrontal cortex [Yuen et al., 2009] under acute stress also indicate that acute stress elevates and down‐regulates neurotransmission thereby influencing the activity of cortex. However, few studies have explored the cerebral alterations which occur in human beings after exposure to highly stressful events [Ganzel et al., 2008]. Our earlier research found that alterations of cerebral function in trauma survivors are mainly localized in the limbic system [Lui et al., 2009]. The current study extends this result by revealing neuroanatomical changes within the same system as indicated by decreased FA values.
The PFC plays a key role in mood regulation [Berna et al., 2010]. More specifically, it is implicated in the “top‐down” regulation of emotional processing and plays an inhibitive role on limbic structures such as the amygdala and the hippocampus through direct anatomical connections [Tekin and Cummings, 2002]. It is therefore possible that the disruption of frontal‐subcortical circuits revealed in this study led to a disruption of the inhibition of PFC on the limbic and striatal system which could account for the increased activation of these regions during resting in these sample of survivors [Lui et al., 2009]. Increased activation of these regions may also be a biomarker of mood dysregulation as suggested by several imaging studies [Guyer et al., 2011]. In addition, the PFC has the most delayed ontogeny of all brain regions with maturation extending to the third decade; this may explain why this region expressed a high degree of plasticity in our adult sample.
Another important finding is the decreased FA in the parahippocampus in survivor groups. The parahippocampal gyrus is thought to be the interface for many neocortical efferents from and afferents to the hippocampus which is pivotal in memory conditioning [Karl and Werner, 2010]. A former experiment assessing monoamine oxidase A (MAO‐A) distribution found significantly elevated MAO‐A density in the parahippocampal gyrus in depressed patients relative to healthy volunteers [Meyer et al., 2006]; furthermore the degree of elevated MAO‐A density was associated with fear conditioning and anxiety. The reduced FA value of parahippocampus may therefore contribute to memory deficits [Yogarajah et al., 2008].
The parietal lobe is found to contribute to fear conditioning [Brewin et al., 2010] and, together with the medial prefrontal lobe and medial temporal lobe, is part of a common network of memory and image generation which is also important for mediating past and future thinking in the dual representing theory for PTSD [Brewin et al., 2010]. This theory postulates that this network integrates the information from the past and mental simulations about possible future events. A previous DTI investigation revealed lower FA bilaterally in the parietal cortex of obsessive‐compulsive disorder (OCD) patients [Szeszko et al., 2005]. Our finding of FA alteration in parietal lobe provides further anatomical evidence that this region is implicated in response to stressful or traumatic events.
The decreased FA value in the limbic lobe and striatal areas is likely to reflect focal microstructural alterations including the alteration of axon density, myelination, and possibly diameter [Finsterer and Fuglsang‐Frederiksen, 2000]. These alterations may influence the electrical conductivity of nerve fiber [Tuch et al., 2001] and eventually result in disrupted functional connectivity [Boorman et al., 2007]. Thus, widespread decreased FA in limbic and striatal areas could account for the decrease in functional connectivity within a distributed limbic network which was found in the same sample of survivors [Lui et al., 2009].
The comparison of FA between SAS(+) and SAS(−) groups did not show any difference, and furthermore the SAS scores did not correlate with the mean FA values in any areas which differed between survivors and controls. In contrast, our former work showed that the SAS score correlated with the regional ALFF signal in the same sample [Lui et al., 2009]. These results indicate that, within 25 days from the traumatic event, self‐rated anxiety is related to alterations of regional activation but not to anatomical alterations in white matter. However it is also possible that an association between SAS scores and white matter microstructure was not detected because of the relatively small sample size. Further studies would therefore benefit from greater sample size.
The present study has three limitations which require careful consideration. First, it is not possible to establish how long it took for the alterations in white matter microstructure to be expressed after the earthquake and whether they are reversible. A longitudinal study will be conducted to shed light on these questions. Second, the relatively small sample size might have limited statistical power resulting in false negative results; nevertheless, sensitivity was enhanced by the recruitment of a homogeneous sample of subjects who were exposed to the same natural disaster and had no history of substance abuse [Stam, 2007]. Finally, this is a cross sectional study and therefore potential confounds such as nutritional and socioeconomic status would might affect the results [Chiang et al., 2011]. Although we carefully matched our groups for weight, height, occupation status and incoming level, we cannot exclude the impact of other socioeconomic variables such as wealth status.
In conclusion, our study is the first to demonstrate white matter alterations in survivors of a natural disaster as early as 25 days after the traumatic event. We found these alterations to be localized in a frontal‐limbic system which is important in emotion regulation.
REFERENCES
- Berna C, Leknes S, Holmes EA, Edwards RR, Goodwin GM, Tracey I ( 2010): Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol Psychiatry 67: 1083–1090. [DOI] [PubMed] [Google Scholar]
- Boorman ED, O'Shea J, Sebastian C, Rushworth MF, Johansen‐Berg H ( 2007): Individual differences in white‐matter microstructure reflect variation in functional connectivity during choice. Curr Biol 17: 1426–1431. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Gregory JD, Lipton M, Burgess N ( 2010): Intrusive images in psychological disorders: characteristics, neural mechanisms, and treatment implications. Psychol Rev 117: 210–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, McMahon KL, de Zubicaray GI, Martin NG, Hickie I, Toga AW, Wright MJ, Thompson PM ( 2011): Genetics of white matter development: A DTI study of 705 twins and their siblings aged 12 to 29. Neuroimage 54: 2308–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Zhang Y, Yang Y, Mo L, Liu X ( 2011): Symptoms of posttraumatic stress disorder, depression, and anxiety among adolescents following the 2008 Wenchuan earthquake in China. J Trauma Stress 24: 44–53. [DOI] [PubMed] [Google Scholar]
- Finsterer J, Fuglsang‐Frederiksen A ( 2000): Concentric needle EMG versus macro EMG I. Relation in healthy subjects. Clin Neurophysiol 111: 1211–1215. [DOI] [PubMed] [Google Scholar]
- Ganzel BL, Kim P, Glover GH, Temple E ( 2008): Resilience after 9/11: multimodal neuroimaging evidence for stress‐related change in the healthy adult brain. Neuroimage 40: 788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez‐Edgar K, Fox NA, Pine DS, Ernst M ( 2011): Striatal Functional Alteration During Incentive Anticipation in Pediatric Anxiety Disorders. Am J Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl A, Werner A ( 2010): The use of proton magnetic resonance spectroscopy in PTSD research‐Meta‐analyses of findings and methodological review. Neurosci Biobehav Rev 34: 7–22. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS ( 2003): Recall of emotional states in posttraumatic stress disorder: An fMRI investigation. Biol Psychiatry 53: 204–210. [DOI] [PubMed] [Google Scholar]
- Le Bihan D ( 2003): Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci 4: 469–480. [DOI] [PubMed] [Google Scholar]
- Li CS, Sinha R ( 2008): Inhibitory control and emotional stress regulation: Neuroimaging evidence for frontal‐limbic dysfunction in psycho‐stimulant addiction. Neurosci Biobehav Rev 32: 581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Rao LL, Ren XP, Bai XW, Zheng R, Li JZ, Wang ZJ, Liu H ( 2009): Psychological typhoon eye in the 2008 Wenchuan earthquake. PLoS One 4: e4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S, Huang X, Chen L, Tang H, Zhang T, Li X, Li D, Kuang W, Chan RC, Mechelli A, Sweeney JA, Gong Q ( 2009): High‐field MRI reveals an acute impact on brain function in survivors of the magnitude 8.0 earthquake in China. Proc Natl Acad Sci USA 106: 15412–15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A, Young T, Praschak‐Rieder N, Wilson AA, Houle S ( 2006): Elevated monoamine oxidase a levels in the brain: An explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry 63: 1209–1216. [DOI] [PubMed] [Google Scholar]
- Miller MM, McEwen BS ( 2006): Establishing an agenda for translational research on PTSD. Ann NY Acad Sci 1071: 294–312. [DOI] [PubMed] [Google Scholar]
- Miller VM, Lawrence DA, Mondal TK, Seegal RF ( 2009): Reduced glutathione is highly expressed in white matter and neurons in the unperturbed mouse brain–Implications for oxidative stress associated with neurodegeneration. Brain Res 1276: 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak K, McCarty R, Palkovits M, Cizza G, Kopin IJ, Goldstein DS, Chrousos GP ( 1995): Decreased central and peripheral catecholaminergic activation in obese Zucker rats. Endocrinology 136: 4360–4367. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G ( 1996): Diffusion tensor MR imaging of the human brain. Radiology 201: 637–648. [DOI] [PubMed] [Google Scholar]
- Ranjekar PK, Hinge A, Hegde MV, Ghate M, Kale A, Sitasawad S, Wagh UV, Debsikdar VB, Mahadik SP ( 2003): Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res 121: 109–122. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ ( 2003): Reduction of eddy‐current‐induced distortion in diffusion MRI using a twice‐refocused spin echo. Magn Reson Med 49: 177–182. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Fukuda R, Okuaki T, Rogers M, Kasai K, Machida T, Shirouzu I, Yamasue H, Akiyama T, Kato N ( 2005): Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: A functional MRI study. Neuroimage 26: 813–821. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen‐Berg H ( 2009): Training induces changes in white‐matter architecture. Nat Neurosci 12: 1370–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam R ( 2007): PTSD and stress sensitisation: A tale of brain and body. Part 1: Human studies. Neurosci Biobehav Rev 31: 530–557. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Ardekani BA, Ashtari M, Malhotra AK, Robinson DG, Bilder RM, Lim KO ( 2005): White matter abnormalities in obsessive‐compulsive disorder: a diffusion tensor imaging study. Arch Gen Psychiatry 62: 782–790. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL ( 2002): Frontal‐subcortical neuronal circuits and clinical neuropsychiatry: An update. J Psychosom Res 53: 647–654. [DOI] [PubMed] [Google Scholar]
- Tuch DS, Wedeen VJ, Dale AM, George JS, Belliveau JW ( 2001): Conductivity tensor mapping of the human brain using diffusion tensor MRI. Proc Natl Acad Sci USA 98: 11697–11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogarajah M, Powell HW, Parker GJ, Alexander DC, Thompson PJ, Symms MR, Boulby P, Wheeler‐Kingshott CA, Barker GJ, Koepp MJ Duncan JS ( 2008): Tractography of the parahippocampal gyrus and material specific memory impairment in unilateral temporal lobe epilepsy. Neuroimage 40: 1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z ( 2009): Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci USA 106: 14075–14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung WW ( 1965): A self‐rating depression scale. Arch Gen Psychiatry 12: 63–70. [DOI] [PubMed] [Google Scholar]


