Abstract
Cigarette smoking, a major, yet avoidable, cause of disability and premature death, is the most prevalent form of nicotine addiction. An emerging theme in the neurobiology of nicotine addiction is the integrity of the amygdala. Using functional MRI, amygdala responses during a face perception task were compared between 28 chronic smokers [14 females, 14 males; age, 26.3 (2.8) years; age at onset of smoking, 15.8 (2.6) years; years smoked, 9.1 (2.1); cigarettes per day, 17.1 (3.7); Fagerström test for nicotine dependence score, 4.1 (1.9); exhaled carbon‐monoxide level, 17.8 (9.5) ppm] and 28 age‐ and education‐matched nonsmokers [14 females, 14 males; age, 26.9 (2.4) years]. Subjects underwent imaging on two separate occasions 1 week apart: smoking satiety versus overnight smoking deprivation, in a randomized counterbalanced order. Our results show no difference in amygdala responses to faces between nonsmokers and satiated smokers. However, overnight deprivation from smoking was associated with a significantly lowered amygdala response to fear, an effect that was probabilistically mapped to the basolateral amygdala. We suggest that aberrant amygdala reactivity in overnight‐deprived smokers may reflect a pre‐existing vulnerability to smoking and/or increase the risk of smoking relapse after a cessation attempt. Hum Brain Mapp, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: addiction, amygdala, dependence, faces, fear, fMRI, nicotine, smoking
INTRODUCTION
Drug addiction is a chronically relapsing disorder characterized by compulsive drug use and loss of control over drug intake. The addiction to smoking differs from other drug addictions such as opioid, cocaine, or alcohol addiction in that it has much less severe binge/intoxication and somatic withdrawal/negative affect stages [Koob,2009]. In terms of its clinical significance, however, it is even more dramatic: worldwide, an estimated 1.2 billion people are current cigarette smokers, and the risk that this population will decease prematurely from a medical complication of smoking is estimated up to 50% [Benowitz,2010]. A key obstacle to the prevention of these premature deaths is that cigarettes contain nicotine, the major, if not sole, addictive compound in tobacco [Changeux,2010]. Of those who try to quit smoking each year, only 3–5% sustain nicotine cravings without the use of nicotine replacement therapies, and no more than 30% are successful with them [Dome et al.,2010; Stead et al.,2008]. Contingent on the presence or absence of smoking‐related cues, two forms of nicotine cravings have been differentiated: Cue‐elicited (phasic) nicotine cravings are thought to result from a behavioral conditioning process in which stimuli associated with cigarette smoking initiate drug‐seeking behavior [Benowitz,2010; Robinson and Berridge,2001]. In contrast, unprovoked abstinence‐induced (tonic) cravings quickly develop after smoking cessation, in the absence of smoking‐related cues [Jarvik et al.,2000]. Despite their significant predictive value for relapse [Killen and Fortmann,1997; Shiffman et al.,2004], there are remarkable deficits in our neurobiological understanding of the impact of unprovoked abstinence‐induced nicotine cravings on human brain function, including differential effects on emotional neural circuitry while craving nicotine versus while being satiated. Nicotine activates α4β2 nicotinic acetylcholine receptors (nAChRs) in the ventral tegmental area (VTA) of the midbrain, resulting in dopamine release in the shell of the nucleus accumbens, the prefrontal cortex, and the amygdala [Benowitz,2010]. Consistent with the topographic profile of α4β2 nAChRs, unprovoked abstinence‐induced cravings are associated with elevated resting state blood flow in widely distributed brain regions, including the dopaminergic projection areas of the VTA [Wang et al.,2007]. The question remains open, though, whether or not unprovoked abstinence‐induced nicotine cravings critically alter brain emotional functions. The brain region most commonly affiliated with emotion is the amygdala, predominantly due to its widely studied role in threat perception and fear [LeDoux,2007]. Across species, selective bilateral inactivation or lesion of the amygdala disrupts fear‐motivated avoidance of threats [Adolphs,2010; Hurlemann et al.,2009]. This raises the hypothesis that continued cigarette smoking, notwithstanding the medical risks and dangers attached to it, could be related, at least in part, to interference of abstinence‐induced nicotine cravings with threat perception. One of the most intriguing findings from human functional MRI (fMRI) studies is the large sensitivity of the amygdala to threat‐related signals such as fearful faces [Phan et al.,2002]. Consequently, 28 chronic smokers (and 28 nonsmoking controls) in the present fMRI study were scanned, using a face perception task including fearful faces, on two separate occasions: smoking satiety versus overnight deprivation, in a randomized counterbalanced order. Amygdala response changes associated with overnight deprivation were further examined for correlations with the severity of nicotine addiction. Moreover, we applied voxel‐based morphometry (VBM) to control for potential gray matter deficits in smokers [Gallinat et al.,2006], which could, in principle, underlie altered fMRI activation patterns. We predicted that unprovoked abstinence‐induced nicotine cravings would critically interfere with amygdala function, evident in reduced responses to fearful faces.
MATERIALS AND METHODS
Participants
Fifty‐six adults (28 females, 28 males; recruited by advertisement) volunteered after giving written, informed consent. The study had full ethics approval and was accomplished in compliance with the latest revision of the Declaration of Helsinki. Twenty‐eight subjects were chronic smokers (14 females, 14 males; mean age: 26.32; SD, 2.79 years) and 28 subjects were nonsmokers (14 females, 14 males; mean age: 26.88; SD, 2.44 years). Smokers started smoking at least 5 years prior to the start of the study in January 2008 and smoked more than 15 cigarettes per day. Nonsmokers declared that they have smoked less than 20 cigarettes in lifetime. Before study enrollment, subjects were screened for MRI compatibility and determined to be free of current or past medical, neurological or psychiatric illness, with particular focus on the exclusion of comorbid substance/alcohol abuse. Psychiatric screening included the beck depression inventory (BDI) [Beck et al.,1996] and the Mini International Neuropsychiatric Interview (M.I.N.I.) [Sheehan et al.,1998].
Neuropsychology
Neuropsychological screening (Table I) included the Verbaler Lern‐ und Merkfähigkeitstest [Helmstaedter et al.,2001], a German version of the Rey auditory verbal learning test (RAVLT) [Rey,1941], to assess verbal learning skills, the digit‐span task (DST) derived from the Wechsler adult intelligence scale, revised [Wechsler,1997] to test working memory, and the facial expressions of emotions: stimuli and test (FEEST) [Young et al.,2002] to evaluate facial emotion recognition skills. Smoking habits were assessed by the Fagerström‐test for nicotine dependence (FTND) [Bleich et al.,2002; Heatherton et al.,1991] and by the SERG (Self‐efficacy smoking) [Batra,2000]. Because of reported correlations between specific personality traits and a predisposition to smoking [Sabol et al.,1999; see also Iwahashi and Aoki,2009], neuropsychological assessment also included the tridimensional personality questionnaire [Cloninger,1987; Weyers et al.,1995] and the NEO‐five factor inventory (NEO‐FFI) [Borkenau and Ostendorf,1993; Costa and McCrae,1985]. Handedness was determined by the Edinburgh handedness inventory (EHI) [Oldfield,1971].
Table I.
Between‐group comparison: demographics and neuropsychological characteristics
| Variable | Mean (SD) | P value | |
|---|---|---|---|
| Smokers (n = 28) | Nonsmokers (n = 28) | ||
| Age (years) | 26.32 (2.79) | 26.88 (2.44) | 0.428 |
| Education (years) | 16.11 (1.89) | 15.74 (1.99) | 0.487 |
| RAVLT | |||
| Trials 1–5a | 55.11 (7.21) | 60.25 (7.17) | 0.010 |
| Trial 7 delayed recallb | 11.71 (2.64) | 12.71 (1.94) | 0.112 |
| DST | 19 (3.44) | 19.75 (3.19) | 0.402 |
| FEEST total | 17.64 (3.22) | 18.37 (1.90) | 0.315 |
| TPQ | |||
| Novelty seeking | 18.61 (5.91) | 16.82 (3.33) | 0.174 |
| Harm avoidance | 12.29 (5.60) | 10.30 (4.51) | 0.154 |
| Reward dependence | 17.07 (3.98) | 18.26 (3.80) | 0.263 |
| NEO‐FFI | |||
| Neuroticism | 1.55 (0.65) | 1.41 (0.46) | 0.367 |
| Extraversion | 2.58 (0.38) | 2.70 (0.46) | 0.285 |
| Openness | 2.42 (0.60) | 2.59 (0.51) | 0.280 |
| Agreeableness | 2.40 (0.63) | 2.76 (0.33) | 0.011 |
| Conscientiousness | 2.52 (0.61) | 2.54 (0.46) | 0.847 |
| EHI | 66.17 (53.16) | 83.82 (29.53) | 0.138 |
Maximum possible score, 75.
Maximum possible score, 15.
RAVLT, Rey auditory verbal learning test (16); DST, digit‐span task derived from the WAIS‐R (Wechsler adult intelligence scale, revised) (17); FEEST, facial expressions of emotions: stimuli and test (18); TQP, tridimensional personality questionnaire (24, 25); NEO‐FFI, NEO‐five factor inventory (26, 27); EHI, Edinburgh handedness inventory (28).
Experimental Procedures
The rationale of the present study was to compare amygdala responses between satiated smokers and nonsmokers (between‐group analysis), and, among the smokers, between satiated and abstinent (after overnight deprivation) states (within‐group comparison) (Fig. 1). Venous blood samples were taken to determine plasma levels of cotinine (nicotine is metabolized to cotinine primarily by the liver enzyme CYP2A6) [Benowitz,2010] and to confirm smoking habits (smokers: mean, 296.52; SD, 137.97 ng/ml; nonsmokers: < 10 ng/ml). In addition, breath carbon‐monoxide (CO) levels were measured (Smokerlyzer EC50 Micro IV, Bedfont Scientific, Kent, UK). Both smokers and nonsmokers were asked to participate in two experimental fMRI sessions at least 1 week apart. For one session, smokers were asked to abstain from smoking for 12 h prior to the measurement (overnight deprivation) (exhaled CO: mean, 8.67; SD, 7.31 ppm). For the other session, they were instructed to smoke the last cigarette until 1 h prior to the measurement (satiated state) (exhaled CO: mean, 17.82, SD, 9.54 ppm). The order of satiated/deprived states was thoroughly counterbalanced across subjects. Before undergoing scanning, subjects indicated acute nicotine cravings on a visual analog scale (VAS). Moreover, nicotine cravings were assessed by the questionnaire of smoking urges (QSU) [Mueller et al.,2001] and by criteria defined in the ICD‐10 (10th Revision of the International Classification of Diseases and Related Health Problems). Furthermore, the POMS (Psychometric analyses of the revised Profile of Mood States, accessing acute emotional state) [McNair et al.,1971] and the BDI served to measure potential mood changes. An overview of smoking habits and psychometric scores is given in Tables II and III.
Figure 1.
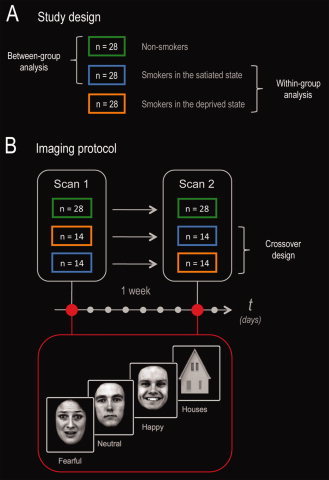
Study design and imaging protocol. (A) Smokers (and nonsmokers) were scanned on two separate occasions 1 week apart: smoking satiety versus overnight deprivation, in a randomized counterbalanced order. This enabled us to perform both between‐group (nonsmokers vs. satiated smokers) and within‐group (satiated state vs. deprived state) analyses. (B) To specifically assess amygdala function, smokers and nonsmokers were scanned on a face perception task including fearful, happy, and neutral facial expressions (plus houses as nonfacial control stimuli).
Table II.
Between‐group comparison: smoking habits
| Variable | Mean (SD) | P value | |
|---|---|---|---|
| Smokers (n = 28) | Nonsmokers (n = 28) | ||
| Years of smoking | 9.12 (2.11) | 0 | — |
| Cigarettes per day | 17.14 (3.67) | 0 | — |
| Pack years | 7.90 (2.73) | 0 | — |
| Onset of smoking (years) | 15.79 (2.60) | — | — |
| Exhaled CO (ppm) | 17.82 (9.54) | 1.86 (1.53) | < 0.0001 |
| Serum cotinine (mg/ml) | 296.52 (137.97) | < 10 | — |
| FTND | 4.07 (1.85) | 0 | — |
| SERG total | 44.78 (8.72) | 99.15 (3.80) | < 0.0001 |
| Positive affect | 11.44 (3.30) | 29.70 (1.34) | < 0.0001 |
| Negative affect | 12.82 (3.86) | 29.70 (1.34) | < 0.0001 |
| Habitual seduction | 13.70 (2.81) | 24.85 (0.67) | < 0.0001 |
FTND, Fagerström‐test for nicotine dependence (19, 20); SERG, self‐efficacy smoking (21).
Table III.
Within‐group comparison: smoking state‐dependent effects
| Variable | Mean (SD) | P value | |
|---|---|---|---|
| Satiated state (n = 28) | Deprived state (n = 28) | ||
| Exhaled CO (ppm) | 17.82 (9.54) | 8.67 (7.31) | < 0.0001 |
| Serum cotinine (mg/ml) | 296.52 (137.97) | 250.48 (147.91) | 0.005 |
| BDI | 3.07 (2.83) | 3.29 (3.20) | 0.659 |
| POMS | |||
| Fatigue | 10.46 (7.34) | 9.64 (7.14) | 0.453 |
| Vigor | 12.00 (10.43) | 11.54 (11.75) | 0.902 |
| Depression | 14.54 (11.80) | 13.18 (10.45) | 0.711 |
| Anger | 2.89 (4.48) | 3.18 (4.43) | 0.669 |
| Withdrawal ICD‐10 | 12.96 (2.91) | 15.25 (3.25) | 0.004 |
| QSU total | 101.33 (32.30) | 141.56 (36.60) | < 0.0001 |
| QSU 1 | 46.70 (14.95) | 61.70 (14.58) | < 0.0001 |
| QSU 2 | 18.70 (8.41) | 30.78 (12.84) | < 0.0001 |
| VAS (pre‐experiment) | 26.11 (23.28) | 62.25 (24.73) | < 0.0001 |
| VAS (post‐experiment) | 50.07 (24.53) | 65.39 (25.98) | 0.003 |
BDI, Beck depression inventory (13); POMS, psychometric analyses of the revised profile of mood states (30); ICD‐10, 10th revision of the International Statistical Classification of Diseases and Related Health Problems; QSU, questionnaire of smoking urges (29); VAS, visual analog scale of acute craving intensity.
FMRI Paradigm
To stimulate the amygdala, we adopted a modified version of an established face perception task [Goossens et al.,2009]. Briefly, stimuli consisted of photographs depicting 40 individuals showing fearful, neutral, and happy facial expressions. The faces were selected from “The Karolinska Directed Emotional Faces” database [Lundqvist et al.,1998]. Given their spatial resemblance to complex facial features, photographs of house facades [Reinders et al.,2005] were implemented as nonfacial control stimuli [Vuilleumier et al.,2001; Wojciulik et al.,1998; Yovel and Kanwisher,2004]. All stimuli were gray‐scaled and equated for size and luminance (Fig. 1). Using Presentation 12 (Neurobehavioral Systems, Albany, CA), stimuli were presented blockwise, via liquid crystal display video goggles. Each block included four exemplars of the same stimulus category (e.g., a sequence of four different neutral faces). Each stimulus occurred for 2,625 ms on‐screen, and the interstimulus interval (IS) varied between 250 ms and 1,500 ms, resulting in a mean block length of 14.5 s. In total, there were 10 blocks for each stimulus category (further called “condition”). The sequence of blocks was randomized, and blocks were separated from each other by a low‐level baseline period of 14.5‐s duration, where a fixation cross was depicted in the center of the screen. To assure attentive stimulus processing, subjects were instructed to press a keypad button in response to each and every stimulus occurring on‐screen. In total, the face perception task lasted ∼20 min.
Data Acquisition
A Siemens Avanto MRI system (Siemens, Erlangen, Germany) operating at 1.5T was used to obtain T2*‐weighted echoplanar (EPI) images with blood‐oxygen‐level‐dependent contrast (TR = 2.90 s, TE = 50 ms, matrix size: 64 × 64, pixel size: 3.3 × 3.3 mm2, slice thickness = 3.0 mm, distance factor = 10%, FoV = 210 mm, flip angle = 90°, 39 axial slices). Based on our a priori anatomical focus, slices were oriented centrally to the amygdala. Four hundred and five volumes were acquired; the first five volumes were discarded to allow for T1 equilibration effects. In addition, high‐resolution anatomical MRI images were acquired (T1‐weighted 3D MPRAGE).
Analysis of Brain Functional Data
The image preprocessing was performed using Matlab7 (The MathWorks, Natick, MA) and SPM5 (Wellcome Trust Centre for Neuroimaging, London, United Kingdom; http://www.fil.ion.ucl.ac.uk/spm). A standardized and automated procedure was applied for the assessment of data quality and the detection of artifacts by inspecting temporal variations [Stoecker et al.,2005]. The EPI images were corrected for head movement between scans by an affine registration [Ashburner and Friston,2003]. For realignment, a two‐pass procedure was used, by which images were initially realigned to the first image of the time‐series and subsequently re‐realigned to the mean of all images. Then, the mean EPI image for each subject was computed and spatially normalized to the Montreal Neurological Institute (MNI) template [Collins et al.,1994; Evans et al.,1992; Holmes et al.,1998] using the “unified segmentation” function in SPM5. This algorithm is based on a probabilistic framework that enables the combination of image registration, tissue classification, and bias correction within the same generative model. The resulting parameters of a discrete cosine transform, which define the deformation field necessary to move the subjects' data into the space of the MNI tissue probability maps [Evans et al.,1994], were then combined with the deformation field transforming between the latter and the MNI single subject template. The ensuing deformation was subsequently applied to the individual EPI volumes. All images were hereby transformed into standard stereotaxic space and resampled at 2 × 2 × 2 mm3 voxel size. The normalized images were spatially smoothed using an 8‐mm full‐width half‐maximum (FWHM) Gaussian kernel, according to studies showing no advantage of smaller smoothing kernels, even at higher field strengths [Hurlemann et al.,2008]. The four conditions were modeled by a boxcar function convolved with a hemodynamic response function [Friston et al.,1995]. A design matrix comprising contrasts of alternating intervals of the different blocks, the time derivative, and movement parameters was created. Specific effects were assessed by applying appropriate linear contrasts to the parameter estimates of the experimental trials resulting in t‐statistics for each voxel. These formed Statistical Parametric Maps (SPM{T}) of differences between the conditions. SPM{T}‐statistics were interpreted in light of the theory of probabilistic behavior of Gaussian random fields. Between‐group (satiated smokers vs. nonsmokers) and within‐group (satiated vs. deprived smoking state) differences associated with the conditions were assessed by two separate second‐level analyses constituting a random effects model. For each simple effect of any of the two fMRI sessions, individual contrast images of each subject or session were entered into a second‐level analysis based on an analysis of variance. To determine between‐group differences, a between‐subjects analysis with the factors “group” (satiated smokers, nonsmokers) and “condition” was performed, whereas within‐group differences were analyzed applying a within‐subjects analysis with the factors “smoking state” (satiety, overnight deprivation) and “condition”. To assess potential correlations with nicotine addiction severity indexed by the FTND, the individual contrast images were entered into another second‐level analysis using a multiple regression model with the “FTND score” as covariate. For a hypothesis‐driven analysis, the left and right amygdala—including their basolateral, centromedial, and superficial subdivisions—were defined as regions‐of‐interests (ROIs) based on cytoarchitectonic maximum probability maps derived from histological analysis of 10 human postmortem brains [Amunts et al.,2005; Eickhoff et al.,2005]. The feasibility of a subdivision‐level functional parcellation of the amygdala has been shown in our previous fMRI studies [Goossens et al.,2009; Hurlemann et al.,2008,2010; Onur et al.,2009]. The a priori research focus on the amygdala served to prevent false‐positive findings.
Analysis of Brain Structural Data
To control for structural differences between smokers and nonsmokers as a potential source of functional discrepancies between groups [Gallinat et al.,2006], a VBM analysis was performed according to predefined protocols (http://dbm.neuro.uni-jena.de/vbm). Briefly, anatomical images were segmented into gray and white matter and normalized to standard MNI space using a unified model. Voxel values were then modulated by the Jacobian determinants derived from the spatial normalization. Modulation of the gray matter images preserved the quantity of tissue that was deformed in the normalization process. The final voxel resolution after normalization was 2 × 2 × 2 mm3. After smoothing (FWHM isotropic Gaussian kernel = 12 mm), the resulting gray matter images from each participant were subjected to statistical analysis.
RESULTS
Between‐Group Comparisons: Satiated Smokers Versus Nonsmokers
Demographics and neuropsychology
The groups of smokers and nonsmokers were well matched for age, education, and handedness (Table I). Moreover, the neuropsychological screening showed no between‐group differences in facial emotion recognition (FEEST), working memory (DST), and delayed verbal memory recall (RAVLT). Furthermore, personality traits (determined by the TPQ and NEO‐FFI) did not differ between groups, with exception of the factor “agreeableness” (P = 0.011; Table I). As expected, smokers and nonsmokers differed in FTND and SERG scores, age at onset of smoking, years of smoking, cigarettes per day, pack years, exhaled CO levels, and serum cotinine levels (Table II).
Brain structure
A VBM‐based assessment of potential brain structural between‐group differences showed that neither whole‐brain volume nor total gray matter volume differed between smokers and nonsmokers. In addition, familywise error (FWE)‐corrected VBM whole‐brain analysis yielded no regionally discrete gray‐matter deficits in smokers.
Brain function
FWE‐corrected whole‐brain analysis for the contrast “(all faces > houses) nonsmokers > (all faces > houses) satiated smokers” showed no differences between nonsmokers and satiated smokers, suggesting that there is no global deficit in face‐selective processing in smokers. Figure 2 illustrates the pattern of face‐selective activations (collapsed across groups) obtained with our fMRI paradigm. Importantly, an amygdala‐specific FWE‐corrected ROI analysis for the contrasts “(fearful faces > neutral faces) nonsmokers > (fearful faces > neutral faces) satiated smokers” and “(fearful faces > neutral faces) nonsmokers < (fearful faces > neutral faces) satiated smokers” showed no difference in the amygdala response to fear between nonsmokers and satiated smokers.
Figure 2.

Displayed are face‐selective activations across satiated smokers and nonsmokers. k, cluster size; L, left hemisphere; R, right hemisphere; T, t‐value.
Within‐Group Comparisons: Deprived Smokers Versus Satiated Smokers
Behavioral and physiological indices of nicotine cravings
Nicotine cravings (as indexed by the VAS, QSU, and ICD‐10 criteria) were more severe after overnight smoking deprivation than in the satiated state (all P‐values < 0.005). This behavioral effect was confirmed by a decline in exhaled CO (P < 0.0001) and serum cotinine (P = 0.005) levels (Table III). In contrast, there were no mood changes (as assessed by the BDI and POMS) (Table III).
Brain function
As the within‐group analysis was restricted to a comparison between satiated and deprived smoking states, activity changes in this comparison are thought to reflect state‐dependent effects, irrespective of potential regionally discrete differences in gray matter volumes between smokers and nonsmokers. FWE‐corrected whole‐brain analysis for the contrast “(all faces > houses) satiated state > (all faces > houses) deprived state” and“(all faces > houses) satiated state < (all faces > houses) deprived state” showed no variation in face‐selective processing as a function of smoking state. To address the central question whether overnight smoking deprivation specifically interfered with the amygdala response to fear, we performed an amygdala‐focused ROI analysis for the contrast “(fearful faces > neutral faces) satiated state > (fearful faces > neutral faces) abstinent state”. This analysis revealed a significantly lowered activation of the right amygdala in response to fear in deprived smokers, an effect that probabilistically mapped to the basolateral amygdala (BLA) (MNI coordinates, xyz = +29, −6, −25; cluster size = 2 voxels; FWE‐corrected, P < 0.05) (Fig. 3A). The reversed contrast “(fearful faces > neutral faces) satiated state < (fearful faces > neutral faces) abstinent state” yielded no significant activation.
Figure 3.
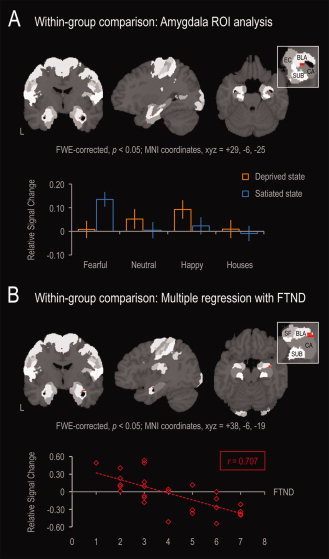
Results of the within‐group ROI analysis of the amygdala. The amygdala ROI was defined based on cytoarchitectonic maximum probability maps [Amunts et al.,2005; Eickhoff et al.,2005]. Bars represent relative signal change from low‐level baseline. (A) Compared to nonsmokers, satiated smokers exhibited normal amygdala activations to face stimuli, with fearful faces evoking the largest response. The within‐group analysis revealed that smoking deprivation was associated with a selective decline in amygdala activation to fearful faces. (B) Fear responses were lower, the higher smokers scored in the Fagerstöm test for nicotine dependence (FTND), an effect which was probabilistically mapped to the basolateral subregion of the amygdala (BLA). BLA, basolateral amygdala; CA, cornu ammonis; EC, entorhinal cortex; SF, superficial amygdala; SUB, subiculum.
Brain‐behavior relationships
A regression analysis demonstrated variation of decreases in the BLA response to fear as a function of nicotine addiction severity indexed by the FTND, that is, the higher the smokers scored in the FTND, the lower was their BLA response to fear in the nicotine‐deprived state (r = 0.707) (MNI coordinates, xyz = +38, −6, −19; cluster size = 5 voxels; FWE‐corrected, P < 0.05) (Fig. 3B). No other correlations were observed.
DISCUSSION
This is, to our knowledge, the first fMRI study specifically designed to assess amygdala reactivity in nicotine addiction. We show that unprovoked abstinence‐induced nicotine cravings are associated with a lowered amygdala response to fear, an effect that was probabilistically mapped to the BLA. This effect was in direct correlation with nicotine addiction severity, that is, the more addicted the smokers were, the more blunted their amygdala response to fear. Given the absence of a significant smoking state‐dependent difference in amygdala responses to appetitive and neutral stimuli, the observed effect is unlikely to result from increased resting state perfusion [Wang et al.,2007] and rather implies a fear‐selective malfunction of the amygdala while craving nicotine versus while being satiated. Our findings may reflect the consequences of nicotine addiction or a pre‐existing vulnerability. These two factors may function independently or interact.
Abnormal Amygdala Reactivity in the Nicotine‐Deprived State
Our finding of a specific decrease in BLA responses to fear in the nicotine‐deprived state links unprovoked abstinence‐induced cravings to functional abnormalities of the amygdala, which may be compensated by elevated brain nicotine levels in the satiated state. The detection and validation of fear signals has been the function most associated with the BLA in rodent models, and ample evidence links fear‐motivated avoidance learning to synaptic plasticity in the BLA [LeDoux,2007]. In humans, numerous imaging studies have confirmed the central role of the amygdala in orchestrating the brain's alarm response to fear signals such as fearful faces [LaBar et al.,1998; Whalen et al.,2001]. In a review of 55 imaging studies of the functional neuroanatomy of emotion, 25 studies reported amygdala activation to fear signals [Phan et al.,2002]. To complement the imaging work, it has been demonstrated that patients with temporal lobectomy and resulting amygdala loss express impaired fear‐conditioned startle responses [Funayama et al.,2001]. Furthermore, patients with selective bilateral amygdala calcification damage as a result of Urbach‐Wiethe disease show impaired fear recognition from facial expressions, reduced fearfulness in social contexts, and a failure to acquire conditioned fear responses [Adolphs et al.,1994,1998,2005; see also Hurlemann et al.,2009]. The present study suggests impaired fear perception in the nicotine‐deprived state, where a satiety‐related compensation of a pre‐existing deficit and/or nicotine addiction‐related dysfunction of the amygdala may be expired.
Our data appear to conflict with reports on elevated irritability, restlessness, anhedonia, and anxiety in smokers, who were abstinent over several days [Hughes and Hatsukami,1986]. However, intra‐amygdala infusions of nicotine have been shown to produce anxiogenic effects [Zarrindast et al.,2008], suggesting that a decline in amygdala nicotine levels following overnight smoking deprivation may reduce amygdala reactivity, an effect that may most likely become functionally evident in the fear domain. Nicotine addiction critically differs from other drug addictions such as opioid, cocaine, or alcohol addiction by the absence of severe somatic withdrawal/negative affect symptoms [Changeux,2010]. Therefore, current concepts of amygdala overactivation and overexpression of conditioned fear responses [Quirk and Gehlert,2003] may not necessarily apply to nicotine withdrawal. Support for this interpretation comes from reports on sustained heavy smoking in cancer patients with tracheostoma or in patients suffering from Buerger's Disease (thromboangiitis obliterans), where peripheral ischemic tissue damage is often complicated by fatal ulcerations and gangrene, resulting in the need for surgical amputation of extremities [Malecki et al.,2009]. Sustained heavy smoking, despite its deleterious physical consequences, implies reduced fear perception and underexpression of fear‐motivated avoidance of threats in nicotine cravings.
Behavioral Implications
Severity of unprovoked abstinence‐induced nicotine cravings is a reliable predictor of relapse after a cessation attempt [Killen and Fortmann,1997; Shiffman et al.,1997]. The present study demonstrates an association of unprovoked abstinence‐induced nicotine cravings with reduced perception of fear signals, an effect that was positively correlated with nicotine addiction severity, that is, the more severely addicted the smokers were, the more blunted the BLA response to these signals. Our results may thus point to a neural mechanism, which is not inherently addictive per se, but might support addictive behavior by compromising threat perception and self‐preservation abilities. Furthermore, threat perception deficits during unprovoked abstinence‐induced nicotine cravings could undermine public health awareness campaigns based on fear appeals (e.g., warning labels on cigarette packaging, alarming advertising depicting the fatal consequences of cigarette smoking in many people) from having their intended effect to promote smoking avoidance behavior. Diminished fear perception could thus decrease the effectiveness of negative reinforcement for smoking cessation and increase the risk of relapse into smoking behavior. Moreover, our results imply a compensation of BLA malfunction by increased brain nicotine levels in the satiated state. This illustrates that cigarette smoking may additionally attain a positive reinforcement value due to its well‐known psychoactive and procognitive effects [Hong et al.,2009]. These factors could act synergistically to lower quit intentions and/or predispose to smoking relapse.
Strengths and Limitations
The rationale of the present study was to investigate amygdala reactivity in nicotine addiction, with a priori focus on possibly altered fear responses during unprovoked abstinence‐induced cravings. This experimental strategy bears two significant limitations. First, our findings of aberrant amygdala reactivity in overnight deprived smokers cannot be interpreted causally. They may reflect the sequelae of nicotine addiction, a pre‐existing vulnerability, or an interaction of both. Prospective longitudinal studies would be needed to disentangle the contributions of dispositional factors and the consequences of nicotine addiction to the altered amygdala reactivity in overnight‐deprived smokers demonstrated here. Second, based on our study design, it cannot be specified whether amygdala responsivity to fear is exclusively altered in nicotine addiction or extends to other forms of drug addiction. We propose, based on our findings, a neural mechanism, which may not be inherently addictive per se, but might contribute to prevent nicotine‐addicted individuals from quitting smoking. The prevailing concepts of nicotine addiction range from pharmacological models emphasizing the mood‐enhancing, prohedonic, and cognition‐augmenting effects of nicotine [Benowitz,2010] over the biopsychological model of incentive salience sensitization [Robinson and Berridge,2001] to systems models of a nicotine‐induced facilitation of cortico‐basal ganglia‐thalamic interplay by direct stimulation of nAChRs and/or indirect stimulation via increased dopaminergic input [Brody et al.,2006]. The present study complements these concepts by implicating aberrant amygdala reactivity in deprived smokers.
Acknowledgements
The authors thank A. Kirsch, A.K. Rehme, and M. Staudinger for research assistance.
REFERENCES
- Adolphs R ( 2010): What does the amygdala contribute to social cognition? Ann N Y Acad Sci 1191: 42–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A ( 1994): Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372: 669–672. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio A ( 1998): The human amygdala in social judgment. Nature 393: 470–474. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR ( 2005): A mechanism of impaired fear recognition after amygdala damage. Nature 433: 68–72. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Scheider F, Zilles K ( 2005): Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Emryol 210: 343–352. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ ( 2003): Rigid body registration In: Frackowiak RS, Friston KJ, Frith CD, Dolan RJ, Price CJ, Ashburner J, Penny WD, Zeki S, editors. Human Brain Function, 2nd ed. London, UK: Academic Press; pp 635–655. [Google Scholar]
- Batra A ( 2000): Tobacco use and smoking cessation in the psychiatric patient. Fortschr Neurol Psychiatr 68: 80–92. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Rainieri W ( 1996): Comparison of beck depression inventories ‐IA and ‐II in psychiatric outpatients. J Pers Assess 67: 588–597. [DOI] [PubMed] [Google Scholar]
- Benowitz NL ( 2010): Nicotine addiction. N Engl J Med 362: 2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleich S, Havemann‐Reinecke U, Kornhuber J ( 2002): Der Fagerström‐Test für Nikotinabhängigkeit (FTNA). Göttingen: Hogrefe. [Google Scholar]
- Borkenau P, Ostendorf F ( 1993): NEO‐Fünf‐Faktoren‐Inventar (NEO‐FFI) nach Costa und McCrae. Göttingen: Hogrefe. [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, Koren AO, Mukhin AG ( 2006): Cigarette smoking saturates brain α4 β2 nicotinic acetylcholine receptors. Arch Gen Psychiatry 63: 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP ( 2010): Nicotine addiction and nicotinic receptors: Lessons from genetically modified mice. Nat Rev Neurosci 11: 389–401. [DOI] [PubMed] [Google Scholar]
- Cloninger CR ( 1987): A systematic method for clinical description and classification of personality variants: A proposal. Arch Gen Psychiatry 44: 573–588. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC ( 1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18: 192–205. [PubMed] [Google Scholar]
- Costa PT Jr, McCrae RR ( 1985): The NEO Personality Inventory Manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Dome P, Lazary J, Kalapos MP, Rihmer Z ( 2010): Smoking, nicotine and neuropsychiatric disorders. Neurosci Biobehav Rev 34: 295–342. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K ( 2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Evans AC, Marrett S, Neelin P, Collins L, Worsley K, Dai W, Milot S, Meyer E, Bub D ( 1992): Anatomical mapping of functional activation in stereotactic coordinate space. Neuroimage 1: 43–53. [DOI] [PubMed] [Google Scholar]
- Evans AC, Kamber M, Collins DL, MacDonald D ( 1994): An MRI based probabilistic atlas of neuroanatomy In: Shorvon S, Fish D, Andermann F, Bydder GM, editors. Magnetic Resonance Scanning and Epilepsy. New York: Plenum; 263–274. [Google Scholar]
- Friston KJ, Holmes A, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ ( 1995): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Funayama ES, Grillon C, Davis M, Phelps EA ( 2001): A double dissociation in the affective modulation of startle in humans: Effects of unilateral temporal lobectomy. J Cogn Neurosci 13: 721–729. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedtgen M ( 2006): Smoking and structural brain deficits: A volumetric MR investigation. Eur J Neurosci 24: 1744–1750. [DOI] [PubMed] [Google Scholar]
- Goossens L, Kukolja J, Fink GR, Maier W, Griez EJ, Schruers K, Hurlemann R ( 2009): Selective processing of social stimuli in the superficial amygdala. Hum Brain Mapp 30: 3332–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO ( 1991): The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addict 86: 1119–1127. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Lendt M, Lux S ( 2001): VLMT—Verbaler Lern und Merkfähigkeitstest. Göttingen: Beltz. [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC ( 1998): Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 22: 324–333. [DOI] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA ( 2009): Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry 66: 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D ( 1986): Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry 43: 289–294. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Rehme AK, Diessel M, Kukolja J, Maier W, Walter H, Cohen MX ( 2008): Segregating intra‐amygdalar responses to dynamic facial emotion with cytoarchitectonic maximum probability maps. J Neurosci Methods 172: 13–20. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Schlaepfer TE, Matusch A, Reich H, Shah NJ, Zilles K, Maier W, Bauer A ( 2009): Reduced 5‐HT(2A) receptor signaling following selective bilateral amygdala damage. Soc Cogn Affect Neurosci 4: 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Walter H, Rehme AK, Kukolja J, Santoro SC, Schmidt C, Schnell K, Musshoff F, Keysers C, Maier W, Kendrick KM, Onur OA ( 2010): Human amygdala reactivity is diminished by the beta‐noradrenergic antagonist propranolol. Psychol Med 27: 1–10. [DOI] [PubMed] [Google Scholar]
- Iwahashi K, Aoki J ( 2009): A review of smoking behavior and smokers evidence (chemical modification, inducing nicotine metabolism, and individual variations by genotype: Dopaminergic function and personality traits). Drug Chem Toxicol 32: 301–306. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Madsen DC, Olmstead RE, Iwamoto‐Schaap PN, Elins JL, Benowitz NL ( 2000): Nicotine blood levels and subjective craving for cigarettes. Pharmacol Biochem Behav 66: 553–558. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP ( 1997): Craving is associated with smoking relapse: Findings from three prospective studies. Exp Clin Psychopharmacol 5: 137–142. [DOI] [PubMed] [Google Scholar]
- Koob GF ( 2009): Brain stress systems in the amygdala and addiction. Brain Res 1293: 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA ( 1998): Human amygdala activation during conditioned fear acquisition and extinction: A mixed‐trial fMRI study. Neuron 20: 937–945. [DOI] [PubMed] [Google Scholar]
- LeDoux J ( 2007): The amygdala. Curr Biol 17: R868–R874. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Ohman A ( 1998): The Karolinska Directed Emotional Faces‐KDEF [CD‐ROM]. Stockholm, Sweden: Department of Clinical Neuroscience, Psychology section, Karolinska Institutet. [Google Scholar]
- Malecki R, Zdrojowy K, Adamiec R ( 2009): Thromboangiitis obliterans in the 21st century—A new face of disease. Atherosclerosis 206: 328–334. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Doppleman LF ( 1971): EITS Manual for Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service. [Google Scholar]
- Mueller V, Mucha RF, Ackermann K, Pauli P ( 2001): The assessment of craving in smokers with a German version of the “Questionnaire on Smoking Urges” (QSU‐G). Zeitschrift fur Klinische Psychologie 30: 164–171. [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Onur OA, Walter H, Schlaepfer TE, Rehme AK, Schmidt C, Keysers C, Maier W, Hurlemann R ( 2009): Noradrenergic enhancement of amygdala responses to fear. Soc Cogn Affect Neurosci 4: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I ( 2002): Functional neuroanatomy of emotion: A meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Gehlert DR ( 2003): Inhibition of the amygdala: Key to pathological states? Ann N Y Acad Sci 985: 263–272. [DOI] [PubMed] [Google Scholar]
- Reinders AA, den Boer JA, Buchel C ( 2005): The robustness of perception. Eur J Neurosci 22: 524–530. [DOI] [PubMed] [Google Scholar]
- Rey LB ( 1941): L'examen psychologique dans les cas d'encephalopathie traumatique. Arch Psychol 28: 286–340. [Google Scholar]
- Robinson TA, Berridge KC ( 2001): Incentive‐sensitization and addiction. Addiction 96: 103–114. [DOI] [PubMed] [Google Scholar]
- Sabol SZ, Nelson ML, Fisher C, Gunzerath L, Brody CL, Hu S, Sirota LA, Marcus SE, Greenberg BD, Lucas FR IV, Benjamin J, Murphy DL, Hamer DH ( 1999): A genetic association for cigarette smoking behavior. Health Psychol 18: 7–13. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC ( 1998): The Mini‐International Neuropsychiatric Interview (M.I.N.I.) the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 59 ( Suppl 20): 22–33. [PubMed] [Google Scholar]
- Shiffman S, West R, Gilbert D; SRNT Work Group on the Assessment of Craving and Withdrawal in Clinical Trials ( 1997): Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine Tob Res 6: 599–614. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gwaltney CJ, Dang Q ( 2004): Immediate antecedents of cigarette smoking: An analysis of unrestricted smoking patterns. J Abnorm Psychol 113: 166–171. [DOI] [PubMed] [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T ( 2008): Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 1: CD000146. [DOI] [PubMed] [Google Scholar]
- Stoecker T, Schneider F, Klein M, Habel U, Kellermann T, Zilles K, Shah NJ ( 2005): Automated quality assurance routines for fMRI data applied to a multicenter study. Hum Brain Mapp 25: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ ( 2001): Effects of attention and emotion on face processing in the human brain: An event‐related fMRI study. Neuron 30: 829–841. [DOI] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, Detre JA, Lerman C ( 2007): Neural substrates of abstinence‐induced cigarette cravings in chronic smokers. J Neurosci 27: 14035–14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D ( 1997): Wechsler Adult Intelligence Scale. Administration and Scoring Manual, 3rd ed. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Weyers P, Krebs H, Janke W ( 1995): Reliability and construct validity of the German version of Cloninger's Tridimensional Personality Questionnaire. Pers Individ Dif 19: 853–861. [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL ( 2001): A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion 1: 70–83. [DOI] [PubMed] [Google Scholar]
- Wojciulik E, Kanwisher N, Driver J ( 1998): Covert visual attention modulates face‐specific activity in the human fusiform gyrus: fMRI study. J Neurophysiol 79: 1574–1578. [DOI] [PubMed] [Google Scholar]
- Young AW, Perrett DI, Calder AJ, Sprengelmeyer R, Ekman P ( 2002): Facial Expressions of Emotion: Stimuli and Tests (FEEST). Bury St. Edmunds: Thames Valley Test Company. [Google Scholar]
- Yovel G, Kanwisher N ( 2004): Face perception: Domain specific, not process specific. Neuron 44: 889–898. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Solati J, Oryan S, Parivar K ( 2008): Effect of intra‐amygdala injection of nicotine and GABA receptor agents on anxiety‐like behaviour in rats. Pharmacology 82: 276–284. [DOI] [PubMed] [Google Scholar]


