Abstract
Although functional imaging studies described networks associated with generalized epileptic activity, propagation patterns within these networks are not clear. In this study, electroencephalogram (EEG)‐based coherent source imaging dynamic imaging of coherent sources (DICS) was applied to different types of generalized epileptiform discharges, namely absence seizures (10 patients) and photoparoxysmal responses (PPR) (eight patients) to describe the representation and propagation of these discharges in the brain. The results of electrical source imaging were compared to EEG‐functional magnetic resonance imaging (fMRI) which had been obtained from the same data sets of simultaneous EEG and fMRI recordings. Similar networks were described by DICS and fMRI: (1) absence seizures were associated with thalamic involvement in all patients. Concordant results were also found for brain areas of the default mode network and the occipital cortex. (2) Both DICS and fMRI identified the occipital, parietal, and the frontal cortex in a network associated with PPR. (3) However, only when PPR preceded a generalized tonic‐clonic seizure, the thalamus was involved in the generation of PPR as shown by both imaging techniques. Partial directed coherence suggested that during absences, the thalamus acts as a pacemaker while PPR could be explained by a cortical propagation from the occipital cortex via the parietal cortex to the frontal cortex. In conclusion, the electrical source imaging is not only able to describe similar neuronal networks as revealed by fMRI, including deep sources of neuronal activity such as the thalamus, but also demonstrates interactions interactions within these networks and sheds light on pathogenetic mechanisms of absence seizures and PPR. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: DICS, EEG‐fMRI, absence, PPR, thalamus
INTRODUCTION
Simultaneous recordings of electroencephalogram and functional magnetic resonance imaging (EEG‐fMRI) represent a well‐established method for the investigation of hemodynamic changes in the brain associated with epileptiform discharges [Gotman,2008; Laufs and Duncan,2007]. The strength of EEG‐fMRI lies in its high spatial resolution, however, EEG‐fMRI is also characterized by a number of limitations: high susceptibility to movement artifacts [Lemieux et al.,2007], dependence of the results on statistical threshold and on the peak latency and shape of the hemodynamic response function (HRF) which can differ between subjects in a study [Jacobs et al.,2008; Lu et al.,2007], as well as its inability to differentiate brain areas of initial epileptic activity from areas of propagation [Groening et al.,2009; Vulliemoz et al.,2009; Siniatchkin et al.,2010]. In comparison to EEG‐fMRI, EEG recordings alone are less prone to movement artifacts, are less expensive, and may be performed for a longer period of time. The better temporal resolution of EEG allows better interpretation of different parts of neuronal networks [Groening et al.,2009; Siniatchkin et al.,2010; Vulliemoz et al.,2009]. However, a problem of EEG is that the signals measured on the scalp surface do not directly indicate the location of the active neurons in the brain due to the ambiguity of the underlying static electromagnetic inverse problem. Many different source configurations can generate the same distribution of potentials on the scalp and valid source reconstruction can be very difficult in some cases [Michel et al.,2004]. Particularly electrical source imaging of brain activity generated in deep brain structures, such as the thalamus, was not possible in previous studies [Holmes,2008; Holmes et al.,2004; Tucker et al.,2007]. New solutions for the inverse problem of the EEG have been developed which improve the localization power of the EEG substantially, even for deep sources. Dynamic imaging of coherent sources (DICS) is one of these solutions. DICS investigates neuronal interactions by imaging power and coherence estimates of oscillatory brain activity using a spatial filter [Gross et al.,2001]. Applied to different types of tremor and voluntary motor control, DICS was able to characterize networks including deep structures such as the diencephalon (e.g., the thalamus) and the cerebellum in magnetoencephalographic (MEG) studies [Gross et al.,2001,2002; Südmeyer et al.,2006; Schnitzler et al.,2009; Timmermann et al.,2003a,b]. In epilepsy, generalized spike and wave discharges (GSW) are an important pattern of abnormal oscillatory activity. Large amplitudes of 200–300 μV or larger and relatively regular rhythms of GSW are ideal conditions for performing DICS. In this study, we applied DICS to EEG‐fMRI data of absence seizures [Moeller et al.,2008,2010], photoparoxysmal responses (PPR) [Moeller et al.,2009a], and in PPR preceding a generalized tonic‐clonic seizure (GTCS) [Moeller et al.,2009b]. The results of the DICS analyses were compared to EEG‐fMRI results obtained from the same data sets and both techniques could be compared in every single patient. In this way, DICS results were validated by an independent method.
Absence seizures occur spontaneously and are characterized by generalized 3–4 Hz GSW. EEG‐fMRI studies have revealed positive blood oxygenation level dependent (BOLD) signal changes in the thalamus along with negative BOLD responses in brain areas of the default mode network and in the caudate nucleus during absence seizures [Aghakhani et al.,2004; Bai et al.,2010; Gotman et al.,2005; Labate et al.,2005; Laufs et al.,2006; Moeller et al.,2008,2010; Salek‐Haddadi et al.,2003]. PPR is an abnormal, highly hereditable EEG‐trait characterized by the occurrence of spike‐wave discharges in response to visual stimulation. PPR tends to generalize and represents a similar pattern of rhythmic epileptic activity as is observed in absence seizures of the majority of patients [Fisher et al.,2005]. EEG‐fMRI recordings in PPR suggest that in contrast to spontaneous GSW, PPR is a cortical phenomenon with involvement of the parietal and frontal cortex [Moeller et al.,2009a]. PPR preceding a photically induced seizure, however, revealed that the thalamus is part of the neuronal network associated with PPR [Moeller et al.,2009b]. EEG‐fMRI shows activity in areas that are involved in the generation of spike as well as areas that might be indirectly influenced by the epileptic activity [Gotman et al.,2005]. DICS of EEG recordings is only sensitive to those areas in which the rhythmic activity of the epileptic discharges is actually present. The source signals can also be used to analyze the propagation of the rhythmic epileptic discharges. In this study, we use renormalized partial directed coherence (RPDC) which describes the direction of information flow from one signal to another [Schelter et al.,2009]. The results of these analyses contribute to the understanding of pathophysiological mechanisms of absences and PPR.
SUBJECTS AND METHODS
From our database of EEG‐fMRI recordings, we selected data sets of 10 patients with absence seizures, which occurred during the recording [Moeller et al.,2008,2010], seven patients, in whom PPR was elicited during photic stimulation in the scanner [Moeller et al.,2009a], and one patient, in whom PPR preceded a photically induced seizure [Moeller et al.,2009a]. The clinical and demographic characteristics of the patients with absence seizures and PPR are summarized in Supporting Information Table 1. All patients were recruited from the Department of Neuropediatrics of the University Hospital Kiel. The study was performed according to the Declaration of Helsinki and approved by the local Ethics Committee of the University of Kiel. The patients' parents gave written informed consent before the experiment.
EEG Recording During fMRI Recording
The EEG was continuously recorded during fMRI from 30 scalp sites (10–20 system plus FC1, FC2, CP1, CP2, FC5, FC6, CP5, CP6, TP9, TP10) with a reference located between Fz and Cz. Sintered Ag/AgCl ring electrodes with built‐in 5‐k Ω resistors were attached using the “EasyCap” (Falk‐Minow Services, Herrsching‐Breitbrunn, Germany), which is part of the MR‐compatible EEG‐recording system “BrainAmp‐MR” (Brainproducts, Munich, Germany). Electrode impedance was kept below 10 k Ω. Two additional electrodes were placed on the infraorbital ridge of the left eye to record vertical electrooculogram (EOG) and on the left perivertebral part of the lower back for acquisition of the electrocardiogram to monitor heartbeat artifacts. Data were transmitted from the high‐input impedance amplifier (250 Hz low‐pass filter, 10‐s time constant, 16‐bit resolution, dynamic range of 16.38 mV) which was placed directly behind the head coil inside the scanner room and connected to a computer located outside the scanner room via a fiber optic cable. The scanner (10‐MHz sampling rate) was synchronized with the EEG amplifier (5‐kHz sampling rate). Online correction of gradient artifacts based on the averaged artifact subtraction (AAS) algorithm was performed using RecView software (Brainproducts, Munich, Germany). This enabled visual inspection of absences and PPR throughout the entire recording time. Foam pads were used to help secure the EEG leads, minimize motion and improve patient comfort.
Photic Stimulation Inside the Scanner (PPR Study)
The fMRI experiment used an epoch‐related design. The intermittent photic stimulation (IPS) consisted of flashes of light. 20‐s epochs of IPS alternated with 15‐s periods during which no IPS was applied. IPS was given in blocks of 5, 10, 15, 20, and 25 Hz in a pseudorandomized order in which every frequency was repeated five times. The light flashes were produced by a xenon‐discharge Grass PS22 stimulator (Astro‐Med) located outside the scanner suite. The flashes of light were conducted into the scanner room via two fiber optic cables (2 cm diameter each) and were presented to the patients through goggles. The patients were instructed to push an emergency button in case of discomfort or other abnormality during IPS.
EEG Data Processing
EEG recordings were processed offline using the BrainVision Analyser 1.05 software (Brainproducts, Munich, Germany). Gradient artifacts from electromagnetic distortion of the EEG due to static and dynamic magnetic field during MR data acquisition were removed using the AAS method described by Allen et al. (2000). Data were corrected relative to the onset of MR‐volume gradient artifacts, which was indicated by a trigger received from the MR system and recorded with the EEG. A moving average width of 10 MR volumes was used for gradient correction. Corrected EEG data were filtered using a high‐pass filter at 0.03 Hz and a low‐pass filter at 75 Hz. Data were then down‐sampled to 250 Hz. Ballistocardiographic artifacts were corrected using the AAS method [Allen et al.,1998]. In cases of residual artifacts after AAS correction, independent component analysis (ICA)‐based procedures were applied as described by Srivastava et al. (2005). The cleaned EEGs were free of residual artifacts. Absences and PPR were independently marked by two experienced neurophysiologists (F.M. and M.S.). Consensus was achieved by comparing and discussing the results of these independently identified absences and PPR.
Simultaneous EEG‐fMRI Recordings and fMRI Analysis
The detailed descriptions of the fMRI recordings and fMRI analysis with the general linear model and canonical hemodynamic response function are given in the Supporting Information text and in our previous publications [Moeller et al.,2008,2009a,b,2010].
EEG Analysis
Spectral analysis
The power spectra for all 30 recorded EEG channels during absences or PPR were computed using the multitaper method [Mitra et al.,1999]. The data epoch of 1s was tapered using a set of discrete prolate spheroidal sequences [Slepian et al.,1961]. The tapered data epoch was Fourier‐transformed and autospectra were computed. Finally, the spectra were averaged over tapers and data epochs, and the power spectrum was estimated. A complete description of the method is given in Muthuraman et al. (2010a). From this power spectrum, we defined the main frequency band of the EEG‐spike activity for the subsequent source analysis. For the spectral and the source analysis part of the open source package Fieldtrip was used [Oostenveld et al.,2011].
Source analysis
DICS [Gross et al.,2001] was used to find the sources of the epileptic activity in the brain. There are two major assumptions in this beamformer DICS analysis: it assumes a single dipole model which is not linearly correlated to other dipoles. This assumption is valid if the coherence is not too strong and the signal‐to‐noise ratio is sufficient [Gross et al.,2001]. By finding the spatial maximum of the power and then defining it as the seed region the coherent brain areas are found. Here the assumption is that the coherence with the reference and itself is always 1. In a next step, this area is projected out for finding further coherent areas in the brain [Schoffelen et al.,2008]. To locate the origin of specific EEG activity seen on the scalp, two problems need to be solved which are the forward and inverse problem. The forward problem is the computation of the scalp potentials for a set of neural current sources. It is solved by estimating the lead‐field matrix with specified models for the brain. In this study, the brain is modeled by a more complex, five‐concentric‐spheres model [Zhang,1995]. The models used for the forward computation are multilayer anisotropic spheres in which the innermost shell is considered to be anisotropic. The leadfields estimated due to a dipole, which lies either in the centre or in the surface can be estimated correctly without any structural bias [Zhang,1995]. The lead‐field matrix was estimated using the boundary‐element method [Fuchs et al.,2002]. The complete description of the solution for the forward problem has been described previously by Muthuraman et al. (2010b). The inverse problem is the quantitative estimation of the properties of the neural current sources underlying the EEG activity. The DICS‐method uses a spatial filter algorithm [Drongelen et al.,1996] to identify the spatial power maximum or coherence in the brain for a particular frequency band. In this study, the linear constrained minimum variance spatial filter was used which designs a bank of spatial filters that attenuates signals from other locations and allows only signals generated from a particular location in the brain [Drongelen et al.,1996]. For all analyses in this study, the same spatial filter was applied. The spatial filter algorithm uses a regularization parameter, which determines the spatial extent of source representation. For all analyses, the same regularization parameter of α = 0.001 was used. This value has been shown to yield reliable results in simulation studies [Kujala et al.,2008]. The identified brain region can subsequently be used as a reference region for cortico‐cortical coherence analysis [Gross et al.,2001]. To create tomographic maps, the spatial filter was applied to a large number of voxels covering the entire brain using a voxel size of 5 mm. The individual maps of every single coherent voxel were spatially normalized and interpolated on a standard T1 brain in SPM2. The application of the spatial filter has been described elsewhere [Muthuraman et al.,2008]. For both absences and PPR, paroxysms of 10 to 13 s were analyzed in each patient. For PPR and short absences, several paroxysms were concatenated to obtain a similar duration of the events in all patients. Despite of dynamics in GSW, we concatenated absences to obtain data with a sufficient signal‐to‐noise ratio to also detect deep sources. Since, we did not analyze temporal changes in absences a concatenation of different absences is justifiable. This is supported by the frequency band from 2 to 5 Hz used for the DICS analysis which considered both higher and lower frequencies within absences. In two patients with absences and one patient with PPR only paroxysms of 4s in total were recorded (see Supporting Information Table 1). A baseline period (at rest) of equal length as the activation period (during absence or PPR) was defined. The baseline period was chosen if it was more than 30 seconds apart from any absence or PPR and if it was free of any movement artifacts. The difference in coherence (ΔCoherence) was estimated between the baseline and the activation period: The spatial filters were estimated for both the baseline period and the activation period. The power in the frequency band of 2–5 Hz was different in the baseline and the activation period. But the cross spectrum estimation will remain the same [Schreiber and Schmitz,1996] and there is no artificial coherence developed due to changes in power in these data segments.
For both absence seizures and PPR the brain source with the strongest power in the frequency band of 2–5 Hz was identified. This frequency band was chosen, since both absences and PPR are characterized by spike and wave discharges of 2–5 Hz. The source of the strongest power in the frequency band of 2–5 Hz was defined as the reference region for further coherence analysis between brain areas. Since the coherence of a reference region with itself is always 1, the reference region was projected out of the coherence matrix, and further coherent areas were found. This analysis was performed for each patient separately, followed by a grand average across all absences or PPR events in all the patients.
Once coherent brain areas were identified, their activity was extracted from the surface EEG by the spatial filter as described in Van Veen et al. (1997). The coherence between these source signals was estimated using the Welch periodogram method [Welch et al.,1967]. The statistical significance of coherence computed with this method can be derived mathematically under the hypothesis of linear independence [Halliday et al.,1995, Timmer et al.,1998]. We calculated the 99% confidence limit, and values of coherence below this confidence limit were taken as an indication of a lack of linear dependence between the two source signals.
To investigate whether the same network would be detected when the thalamic source was used as a reference seed, the analysis was repeated taking the thalamic source as a seed instead of the source with the strongest power at the frequency band of 2–5 Hz. To justify that coherence was only present within the identified sources, a voxel in the putamen region was selected and coherence was estimated between this voxel and the other identified regions in the absence patients. The estimated ΔCoherence between the baseline and the activation period (P < 0.05) was tested for significance with a parametrical t‐test.
To investigate whether DICS artificially locates any midline subcortical sources for distributed activity in the cortex a simulation was performed: The source signal from source 1 in absences (see results section) was taken and incorporated in all cortical voxels. The “background EEG signal” was produced by incorporating independent broad band second order auto regressive processes on the other voxels to construct the EEG signals close to the real biological data. When DICS was used to find the location of the strongest source in the frequency band (2–5 Hz) only the correct distributed cortical sources and no artificial midline subcortical sources were detected.
To test whether DICS is capable of locating accurately the sources with only 32‐channel EEG data a simulation was carried out. The simulation was done with different channel configurations from 8‐ to 256‐channel EEG data to see the effect of difference in electrodes in this method [Muthuraman et al.,2011]. In this study, we showed that there is a drastic improvement from 16‐ to 32‐channel EEG data but only a moderate improvement in the case of 32 to 64‐channel.
Renormalized PDC
Coherence only reveals components that are common to two signals in the frequency domain. It does not give the direction of information flow between two signals. In this study, we applied RPDC [Schelter et al.,2009] which is a technique performed in the frequency domain to detect the direction of information flow from one signal to the other and vice versa. In this study, the open source matlab package ARFIT was used [Neumaier and Schneider,2001; Schneider and Neumaier,2001]. In this method, the multivariate modeling approach is applied which uses an autoregressive process to obtain the coefficients of the signals in the frequency band of 2–5 Hz. To obtain these coefficients, the correct model order needs to be chosen which is estimated by minimizing the Akaike Information Criterion [Akaike, 1974] and gives the optimal order for the corresponding signal [Ding et al.,2000]. After estimating the RPDC values the significance level is calculated from the applied data using a bootstrapping method [Kaminski et al.,2001].
RESULTS
Absence Seizures
Results of fMRI and DICS analyses
A more detailed description of fMRI results for absence seizures is given in the Supporting Information text. In all 10 patients, absence seizures were associated with regional changes in the BOLD signal (see Fig. 1). BOLD signal increases in the thalamus were observed in all patients. Decreases in the BOLD signal were found in default mode areas (bilaterally in the posterior parietal cortex, the precuneus and frontal cortical areas) in six patients. The results of the DICS analysis in each patient and in the group of patients are shown in Figures 1 and 2. The relative power varied from 25.1 to 33.5 (29.2 ± 2.5). The source of the strongest power at the frequency band 2–5 Hz was detected in the medial prefrontal cortex for all absence seizures (Figs. 1 and 2). The local maximum of this source varied slightly across the patients (Fig. 2). This first source was defined as the reference region for further coherence analysis between brain areas. All absences showed the same four sources coherent with the first source, and there were only small differences across the patients with respect to the local maxima of the sources (Fig. 2): The source with the strongest coherence with the reference source was found bilaterally in the lateral prefrontal cortex (second source). The second strongest coherence was detected bilaterally in eight and unilaterally in two patients in the parietal cortex (third source), while the fourth source was found bilaterally in the lateral occipital cortex. Source five was found in the medial thalamus. The grand average of all absences across all patients is displayed in Figure 1 (right panel). The coherence between each source signal is depicted in Supporting Information Figure 1: while all five sources showed coherence with each other in the frequency of 2–5 Hz, no coherence was detected between an arbitrarily chosen deep voxel in the putamen and any of the five sources associated with the absences (Supporting Information Fig. 2). When using the thalamic source as a seed, the same network as in the first analysis (seed in the frontal source with the strongest power at the frequency band 2–5 Hz) was detected (Supporting Information Fig. 3).
Figure 1.
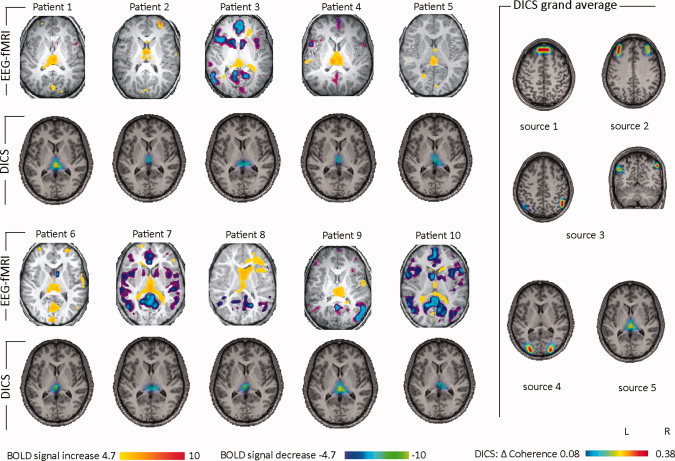
EEG‐fMRI and DICS analysis of absence seizures. EEG‐fMRI results and DICS sources (frequency band 2–5 Hz) in the thalamus for every patient are listed. Please note that all patients show thalamic involvement in both analyses. In the right of the figure, the grand averages across all patients and for all DICS sources are shown.
Figure 2.
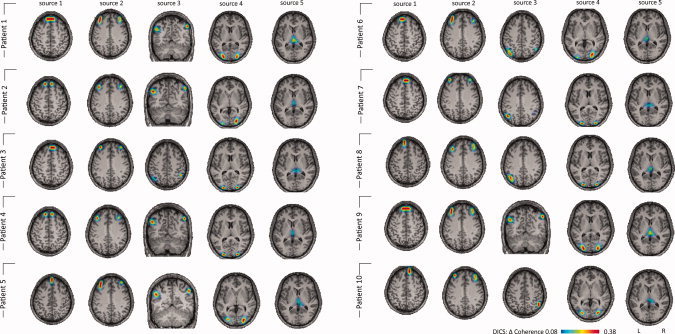
Single‐subject DICS analysis of absences. DICS sources (frequency band 2–5 Hz) for every patient are listed. Please note that all patients show the same sources, with only slight variation of the local maxima of the sources. Patients 7, 8, and 9 show a unilateral third source; in Patients 2–7, source 6 is found unilaterally.
Figure 3.
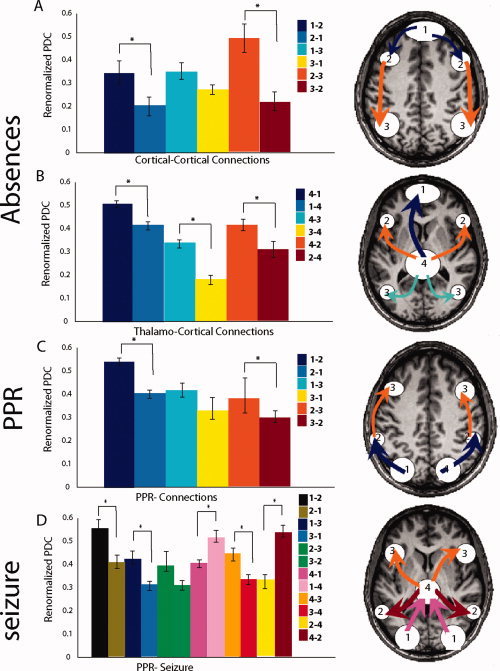
Renormalized directed partial coherence (RPDC). In the left of the figures RPDC for all directions of connections are shown in bar graphs. Directions which were significantly stronger are indicated by an asterisk. In the right RPDC connections which were significantly stronger are shown schematically for all sources overlaid on the brain (A) cortical–cortical connections of the sources in absences: information flow from the frontomedial cortex via the prefrontal cortex to the parietal cortex (B) thalamo‐cortical connections of the sources in absences: RPDC from the thalamus to all other sources are stronger. (C) cortical connections of the sources in PPR: information flow from the occipital cortex via the intraparietal slucus to the premortor cortex (D) cortical–cortical and thalamo‐cortical connections of the sources in a patient in whom PPR was followed by a generalized tonic‐clonic seizure.
Renormalized PDC
The results are depicted in Figure 3. In absences the RPDC between source 1 (medial frontal cortex) and source 2 (prefrontal cortex) was significantly stronger for the direction from source 1 to source 2. The RPDC between source 1 and source 3 (posterior parietal cortex) tended to be stronger for the direction from source 1 to source 3, but did not reach significance. Between source 2 and source 3 a significantly stronger RPDC was detected for the direction from source 2 to source 3. For the thalamus (source 4) the RPDC was significantly stronger for the direction from the thalamus to all other sources. These differences were significant (t‐test, P < 0.01).
Comparison between EEG‐fMRI and DICS analysis
Both EEG‐fMRI and DICS (source five) showed a thalamic involvement during absence seizures in all patients. Sources two and three were detected bilaterally in the parietal and frontal cortex in areas of the default mode network. While DICS showed these sources in all patients, BOLD signal changes in default mode areas were only detected in six of ten patients. BOLD signal changes in the occipital cortex related to source 4, were detected in six patients. No clear BOLD signal changes were detected in the frontopolar area of the first source.
PPR
Results of EEG‐fMRI and DICS analyses
A more detailed description of fMRI results for PPR are given in the Supporting Information text as well as in work [Moeller et al.,2009a,b]. PPR was associated with regional BOLD signal increases in the occipital, parietal and prefrontal cortical brain areas (see Fig. 4). When the PPR was followed by a tonic‐clonic seizure, a significant activation in the thalamus was observed. The results of the DICS analysis in each patient and in the group of patients are shown in Figures 4 and 5. The relative power varied from 25.1 to 33.2 (28.2 ± 2.8) and did not differ from the relative power in the absence data. The DICS analysis revealed the source of the strongest power in the frequency band of 2–5 Hz bilaterally in the occipital cortex for all PPR. This first source was defined as the reference region for further coherence analyses between brain areas. All PPR patients showed the same two coherent sources with the first source: The source with the strongest coherence with the reference source was found bilaterally adjacent to the intraparietal sulcus (second source). The second strongest coherence was detected bilaterally in the premotor cortex (third source). The local maxima of these sources varied slightly across the patients (Fig. 5). The three sources detected for each patient were also detected for the grand average across all PPR patients (Fig. 4c). No thalamic source was detected in single patient analysis or in the grand average across all PPR patients. The coherence between each source signal is depicted in Supporting Information Figure 4.
Figure 4.
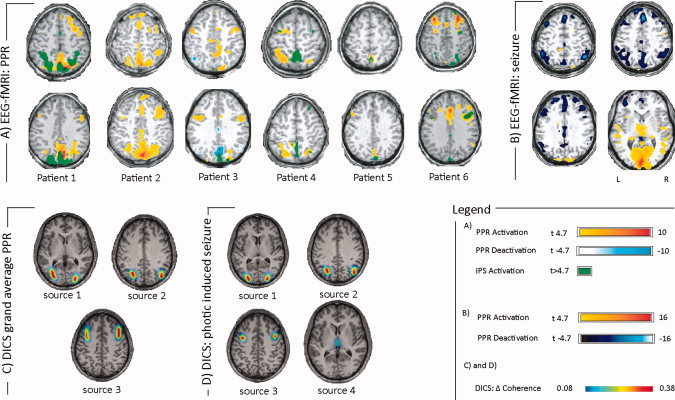
EEG‐fMRI analysis and DICS analysis of PPR. (A) EEG‐fMRI results (early regressor) for each patient are shown. Patients 1–5 show PPR‐related activation in the parietal cortex close to the intraparietal sulcus; all patients show PPR‐related activation in the frontal cortex. IPS: intermittend photic stimulation. Please not that detailed EEG‐fMRI results for each subject can be found in Moeller et al. (2009a). (B) EEG‐fMRI results of the patient in whom the blocks of PPR were followed by a generalized tonic‐clonic seizure. fMRI results show activation in the visual cortex and the thalamus. Deactivations are found bilaterally in frontoparietal areas and in the precuneus. (C) The grand averages across all patients for all DICS sources are shown. BOLD signal changes were found in areas of all sources (D) DICS results of the patient in whom the blocks of PPR were followed by a generalized tonic‐clonic seizure. A thalamic source was detected in addition to sources found in C.
Figure 5.
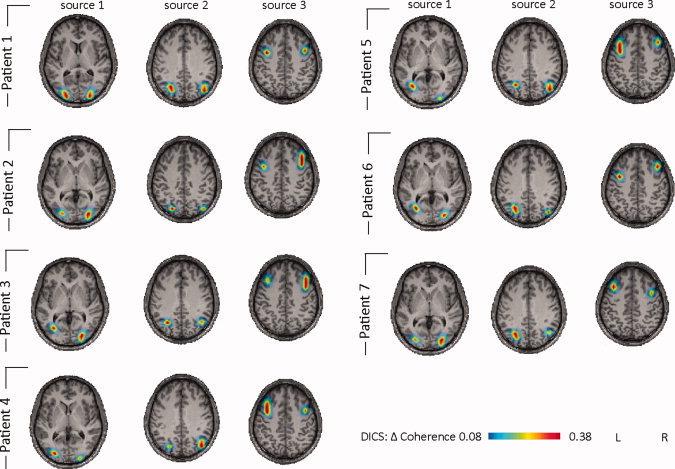
Single patient DICS analysis of PPR. DICS sources (frequency band 2–5 Hz) for each patient are listed. Please note that all patients show the same sources, with only slight variation of the local maxima of the sources. In Patient 7, the EEG‐fMRI analysis had to be discarded due to artifacts on the EPI images.
Photically induced seizure (DICS)
For the patient, in whom three blocks of PPR ended in a photically induced seizure, the same three sources were detected as in the other PPR patients. However, unlike the PPR patients, an additional source in the thalamus was detected (Fig. 4d).
Renormalized PDC
The results are depicted in Figure 3. In PPR the RPDC between source 1 (occipital cortex) and source 2 (intraparietal sulcus) was significantly stronger for the direction from source 1 to source 2. The RPDC between source 1 and source 3 (premotor cortex) tended to be stronger for the direction from source 1 to source 3, but did not reach significance. Between source 2 and source 3 a significantly stronger RPDC was detected for the direction from source 2 to source 3. For the thalamus (source 4) in the patient with a photically induced seizure the RPDC was significantly stronger for the direction from the thalamus to sources 2 and 3, while the connection between thalamus and occipital cortex was stronger from the occipital cortex to the thalamus. These differences were significant (t‐test, P < 0.001).
Comparison between EEG‐fMRI and DICS analysis
For DICS the first source was found in the lateral occipital cortex in all patients. In this area, IPS‐related BOLD signal increase was detected by EEG‐fMRI. The second source was detected bilaterally adjacent to the intraparietal sulcus, and EEG‐fMRI showed PPR‐related BOLD activation adjacent to the intraparietal sulcus in 5 of 6 patients. All patients showed PPR‐related BOLD activation in the premotor cortex, concordant with source three in the premotor cortex. However, BOLD signal changes were always more variable in space than DICS sources (Figs. 4 and 5). PPR‐related BOLD signal changes in the thalamus were only detected in one of six patients when applying a standard regressor [Moeller et al.,2009a], whereas no thalamic source was detected for any PPR patient. However, both DICS and EEG‐fMRI revealed thalamic involvement in a patient in whom PPR was followed by a photically induced seizure [Moeller et al.,2009b].
DISCUSSION
Detection of Subcortical Sources
DICS is a powerful technique of electrical source imaging, which investigates neuronal interactions by imaging power and coherence estimates of oscillatory brain activity [Gross et al.,2001]. DICS is able to characterize networks associated with different types of tremor and voluntary motor control [Gross et al.,2001,2002; Timmermann et al.,2003a,b]. It is noteworthy that not only cortical sources but also sources in deep structures such as in the diencephalon (e.g., the thalamus) and the cerebellum were detected using DICS [Gross et al.,2001,2002; Timmermann et al.,2003a,b; Südmeyer et al.,2006; Schnitzler et al.,2009]. Although such subcortical sources have been shown in these previous studies applying DICS to oscillatory signals (e.g., tremor), it remains a matter of debate whether subcortical sources can be detected in EEG signals recorded from the skull surface. It has been generally assumed that only cortical sources of at least 6.25 cm2 may be registered using the scalp EEG [Hara et al.,1999]. Intracranial recordings suggest that spikes arising from deeper structures such as the mesial temporal lobe might only be detected by the scalp EEG when averaged [Merlet et al.,1998]. Since depth electrode recordings are not used in IGE patients, there are no studies investigating whether thalamic spike and wave activity can be detected using a routine EEG recording. Studies applying dipolar and distributed modeling source analyses to GSW activity on the scalp EEG found frontal sources but did not detect thalamic sources [Holmes,2008; Holmes et al.,2004,2010; Tucker et al.,2007]. However, DICS differs from the techniques in the mentioned studies in several ways. Dipolar and distributed source modeling aim to localize sources of electric currents in the brain that give rise to potential fields at the scalp. For GSW with large sources, especially dipolar models can cluster in misleading locations [Kobayashi et al.,2005]. However, the DICS analysis does not try to explain the signal recorded on the scalp EEG by single sources, but looks for sources that are coherent to a given reference point. Since only a specific frequency range of interest is analyzed, even small oscillatory activity in this frequency range can be detected. There are different types of support showing that DICS is also sensitive to deep sources. To validate the findings presented here, we performed some additional analyses. Our simulation study demonstrated that a simulated source with a physiological signal‐to‐noise ratio placed in the diencephalon could be located correctly by DICS [Muthuraman et al.,2010b]. Since both relative power and the duration of the paroxysms did not differ between absences and PPR these factors cannot explain why a thalamic source was only detected in absences. It is known that over‐regularization may lead to “ghost sources” in the middle of the brain. However, in all our analyses we used the same moderate value for regularization which has been shown to yield adequate results [Kujala et al.,2008]. When DICS was applied to simulated distributed large sources restricted to the cortex no artificially located midline subcortical sources were detected. Furthermore, in patients with absence seizures, we took the thalamus as a seed region and were able to find the same coherent sources (medial and lateral prefrontal cortex, parietal and lateral occipital cortex and cerebellum) as in our original analysis using the source with the strongest power in the frequency band of 2–5 Hz as a seed (see Supporting Information Fig. 4). It seems likely that the network of coherent sources including the source in the thalamus is stable and independent of the seed region within this network. Additionally, an arbitrarily chosen deep source in the putamen did not demonstrate coherence with the described sources in absence patients (Supporting Information Fig. 3). This underlines the fact that the coherence is restricted to the sources revealed by DICS. The nonparametrical statistics with the period of background EEG supports the fact that the identified sources are significant and active only during the absence seizures or PPR. Moreover, the results of the DICS analysis are supported by the findings of the EEG‐fMRI which showed thalamic involvement in the same patients in whom DICS revealed thalamic sources. Thus, the results of DICS were validated by an independent method. Finally, MEG studies on different types of tremor and voluntary motor control have revealed sources in deep structures such as in the diencephalon and the cerebellum [Gross et al.,2001,2002; Timmermann et al.,2003a,b; Südmeyer et al.,2006; Schnitzler et al.,2009]. Recently, even sources of weak physiological oscillatory activity have been detected in the thalamus using a similar source analysis approach on MEG data [Cantero et al.,2009], and the general dogma that deep subcortical sources cannot be detected by surface recordings has been questioned [Kimura et al.,2008]. The fact that GSW have a high signal‐to‐noise ratio and that the electrical field of deep radial dipoles is detected better on the surface than their magnetic field [Cantero et al.,2009; Kimura et al.,2008] further supports the authenticity of the deep thalamic source of GSW activity presented in our EEG analysis.
Comparison Between DICS and EEG‐fMRI
To validate the EEG‐source analysis results we used a simultaneous application of a second technique with an undoubted spatial resolution also in the depth of the brain. We therefore applied DICS to the EEG recorded in EEG‐fMRI studies in patients with generalized epilepsy [Moeller et al.,2008,2009a,b,2010]. Thus we were able to directly compare the results of DICS to EEG‐fMRI results in the same events and patients. Oscillatory activity of GSW of large amplitudes (200–300 μV) and a well‐defined frequency range constitute ideal conditions to perform DICS. Our study showed that similar networks were detected by EEG‐fMRI and DICS in absence seizures, PPR and PPR preceding a GTCS. Interestingly, the best concordance between the two methods was found in the thalamus which was involved in all absence patients and in none of the PPR patients as revealed by both methods. However, both DICS and EEG‐fMRI showed thalamic involvement when PPR preceded a GTCS. Concordant results were also found for the default mode network and the occipital cortex in the case of absences and for the occipital cortex and the parietal and premotor regions in PPR. Thus, our study, confirms that in case of high amplitude epileptic activity DICS is able to detect networks similar to EEG‐fMRI including subcortical structures. However, the spatial resolution of both methods is different and the BOLD activation and oscillatory activity are not equivalent. This may be the reasons for few discordant results of EEG‐fMRI and DICS. While EEG‐fMRI showed clear interindividual variability, the results of the DICS analysis were very similar, only varying in the local maxima of the sources (Supporting Information Figs. 1 and 5). This limited spatial intersubject variability of electrical sources might be partly attributed to methodological factors, that is, the limited number of electrodes used in this study (n = 30), the application of the standard MRI interpolation on the MNI template without consideration of a realistic head model based on individual MRI, as well as the application of the same optimization parameters for modeling coherent sources in all subjects. The standard procedure for all participants was chosen because of our intention to perform a group analysis of coherent sources. However, the integration of algorithms for the calculation of a realistic head model is under development and will be applied in the future. Presently, the superimposition of electrical sources with the individual anatomy of the subjects was not necessary to answer the key questions of this study. Inherent differences between both methods might also lead to different results: The DICS analysis is restricted to coherent oscillatory activity in a narrow frequency band which, by definition, is more specific than looking for any spike‐related changes in blood oxygenation level which also entails methodological difficulties (see above) and may lead to more widespread and variable responses. Certain structures showed different results in EEG‐fMRI and DICS studies: For example, involvement of the caudate nucleus was found in four patients in the EEG‐fMRI analysis but not in the DICS analysis. One might argue that this area might be too small to generate a signal detectable by the scalp EEG. Moreover, the DICS revealed a source in the frontopolar area in absence seizures which did not correspond to the results of the fMRI analysis. We suggest that this source is associated with hemodynamic changes which are not closely related to the canonical HRF and thus could not be detected in the EEG‐fMRI analysis. This is supported by the observation that BOLD signal changes in the frontal and parietal cortex might precede absences not detectable by the canonical HRF [Bai et al.,2010]. Gupta and colleagues applied DICS in MEG datasets of four patients with absences. By dividing the data in segments of one second they detected an occipital source before the onset of the absence and a frontal source at the onset of the absence [Gupta et al.,2011]. This frontal source is concordant with the frontopolar source detected in or study. However, in contrast to our study, the absences were divided in segments and the detected sources were not used as a seed to detect sources coherent with the first source.
On the one hand discordant results between EEG‐fMRI and DICS challenge a one to one validation of DICS by EEG‐fMRI, on the other hand it might indicate that both methods provide complimentary information about the epileptic network.
Renormalized Partial Directed Coherence
The most important additional information derived from the coherent source analysis is the extracted EEG signal that belongs to specific sources (source signals). We used these signals to analyze the direction of interaction between the different sources of epileptic activity by renormalized partial directed coherence (PRDC). In absences RPDC demonstrated an information flow from the prefrontal sources to the parietal sources on the cortical level. However, all cortical sources were similarly influenced by the thalamic source. Animal studies in genetic models of absence epilepsy provide evidence that absences are triggered by a cortical focus [Klein et al.,2004, Meeren et al.,2002; Polack et al.,2007] and also studies in humans suggest that a cortical focus might exist in absences [Moeller et al.,2010; Westmijse et al.,2009]. However, in this study we analyzed entire absences without investigating temporal changes during the absences. Therefore our study does not answer the question how absences might be initiated, but shows that during the absence the thalamus seems to be a pacemaker of the GSW activity. In our study the thalamic source involved mainly medial regions. The centromedian portion of the thalamus mainly contains nuclei which receive input from a number of different structures and usually sends its output to more than one association area of the cerebral cortex and to the striatum whereas the regio medialis has specific connections to prefrontal cortex and basal ganglia [Herrero et al.,2002]. However, the limited spatial resolution of DICS based on only 30 EEG channels precludes an assignment of thalamic involvement to specific thalamic nuclei.
In PPR, the flow of information between the cortical sources was dominated by the opposite posterior–anterior direction, from the occipital cortex via the parietal cortex to the frontal cortex. The localization of the premotor source (source 3) is concordant with the localization of the frontal eye field [Pierrot‐Deseilligny et al.,2004]. The frontal eye field and the intraparietal cortex (source 2) are key components in the frontoparietal network mediating the generation of saccades [Pierrot‐Deseilligny et al.,2004; Schluppeck et al.,2005; Sereno et al.,2001; Silver et al.,2005]. Even if the EEG did not show clear eye movement artifact during the PPR it could be possible that subtle movements of the eyes were associated with PPR. It seems likely that PPR is based on a spreading of GSW along established pathways of physiologically linked brain areas of the occulomotor system. During the development of a photically induced seizure the occipital cortex again seems to be primarily involved, transmitting information to the thalamus which in turn seems to exert control over the frontal and parietal cortex. The fact that in both techniques a thalamic involvement was only present in absence seizures and the photically induced seizures confirms the key role of the thalamus in generalized epileptic seizures [Blumenfeld,2005]. The difference in the direction of the information flow during a photically induced seizure and absences shows that the role of the thalamus seems to depend on the type of generalized seizure. Differences in cortical representation of the networks for absences and PPR have also been reported in previous EEG‐fMRI studies [Aghakhani et al.,2004; Bai et al.,2010; Gotman et al.,2005; Labate et al.,2005; Laufs et al.,2006; Moeller et al.,2008,2009a,b,2010; Salek‐Haddadi et al.,2003]. Based on these studies, it has been hypothesized that the propagation of epileptic activity on the cortical level is different between PPR and absences [Moeller et al.,2009a,b]. Using DICS and RPDC, we now present evidence for this difference in the flow of epileptic activity within the networks.
CONCLUSION
Our study showed that EEG‐based coherent source analysis is a powerful technique to map oscillatory epileptic activity also from subcortical structures. Consistent with the EEG‐fMRI results, the source in the thalamic region only appeared in case of generalized seizures (absences or photically induced) but was absent in PPR. Further analyses of the source signals revealed propagation of cortical epileptic activity into opposite directions in PPR and absence seizures (posterior–anterior vs. anterior–posterior). The signal from the thalamic region seems to act as a pacemaker during absences whereas it seems to be recruited secondarily by occipital cortical activity in the photically induced seizure.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1
Supporting Information Figure 2
Supporting InformationFigure 3
Supporting InformationFigure 4
Table 1: Characteristics of patients with absences and patients with PPR
Acknowledgements
The authors thank the children and their parents for participating in the study.
REFERENCES
- Aghakhani Y, Bagshaw AP, Benar CG, Hawco C, Andermann F, Dubeau F, Gotman J ( 2004): fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain 27: 1127–1144. [DOI] [PubMed] [Google Scholar]
- Akaike H (1974): A new look to the stastical model identification. IEEE Transactions on automatic control 19:716–723. [Google Scholar]
- Allen PJ, Polizzi G, Krakow K, Fish DR, Lemieux L ( 1998): Identification of EEG events in the MR scanner: The problem of pulse artifact and a method for its subtraction. Neuroimage 8: 229–329. [DOI] [PubMed] [Google Scholar]
- Allen PJ, Josephs O, Turner R ( 2000): A method for removing imaging artifact from continuous EEG recorded during functional MRI. NeuroImage 12: 230–239. [DOI] [PubMed] [Google Scholar]
- Bai X, Vestal M, Berman R, Negishi M, Spann M, Vega C, Desalvo M, Novotny EJ, Constable RT, Blumenfeld H ( 2010): Dynamic time course of typical childhood absence seizures: EEG, behavior, and functional magnetic resonance imaging. J Neurosci 30: 5884–5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H ( 2005): Cellular and network mechanisms of spike‐wave seizures. Epilepsia 9: 21–33. [DOI] [PubMed] [Google Scholar]
- Cantero JL, Atienza M, Gomez‐Herrero G, Cruz‐Vadell A, Gil‐Neciga E, Rodriguez‐Romero R, Garcia‐Solis D ( 2009): Functional integrity of thalamocortical circuits differentiates normal aging from mild cognitive impairment. Hum Brain Mapp 30: 3944–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M, Bressler SL, Yang W, Liang H ( 2000): Short‐window spectral analysis of cortical event‐related potentials by adaptive multivariate autoregressive modeling: Data preprocessing, model validation, and variability assessment. Biol Cybernetics 83: 35–45. [DOI] [PubMed] [Google Scholar]
- Drongelen WV, Yuchtman M, Van Veen BD, Van Huffelen AC ( 1996): A spatial filtering technique to detect and localize multiple sources in the brain. Brain Topography 9: 39–49. [Google Scholar]
- Fisher RS, Harding G, Erba G, Barkley GL, Wilkins A ( 2005): Epilepsy Foundation of America Working Group. Photic‐ and pattern‐induced seizures: A review for the Epilepsy Foundation of America Working Group. Epilepsia 46: 1426–1441. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Kastner J, Wagner M, Hawes S, Ebersole JS ( 2002): A standardized boundary element method volume conductor model. Clin Neurophysiol 113: 702–712. [DOI] [PubMed] [Google Scholar]
- Gotman J ( 2008): Epileptic networks studied with EEG‐fMRI. Epilepsia 49: 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F ( 2005): Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci USA 102: 15236–15240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groening K, Brodbeck V, Moeller F, Wolff S, van Baalen A, Michel CM, Jansen O, Boor R, Wiegand G, Stephani U, Siniatchkin M ( 2009): Combination of EEG‐fMRI and EEG source analysis improves interpretation of spike‐associated activation networks in paediatric pharmacoresistent focal epilepsy. NeuroImage 46: 827–833. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R ( 2001): Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci USA 98: 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Timmermann L, Kujala J, Dirks M, Schmitz F, Salmelin R, Schnitzler A ( 2002): The neural basis of intermittent motor control in humans. Proc Natl Acad Sci USA 99: 2299–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D, Ossenblok P, van Luijtelaar G ( 2011): Space‐time network connectivity and cortical activations preceding spike wave discharges in human absence epilepsy: A MEG study. Med Biol Eng Comput 49: 555–565. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF ( 1995): A framework for the analysis of mixed time series/point process data—Theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol 64: 237–278. [DOI] [PubMed] [Google Scholar]
- Hara J, Musha T, Shankle WR ( 1999): Approximating dipoles from human EEG activity: The effect of dipole source configuration on dipolarity using single dipole models. IEEE Trans Biomed Eng 46: 125–129. [DOI] [PubMed] [Google Scholar]
- Herrero MT, Barcia C, Navarro JM ( 2002): Functional anatomy of thalamus and basal ganglia. [Review]. Childs Nerv Syst 18: 386–404. [DOI] [PubMed] [Google Scholar]
- Holmes MD ( 2008): Dense array EEG (2008): Methodology and new hypothesis on epilepsy syndromes. Epilepsia 49( Suppl 3): 3–14. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Quiring J, Tucker DM ( 2010): Evidence that juvenile myoclonic epilepsy is a disorder of frontotemporal corticothalamic networks. Neuroimage 49: 80–93. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Brown M, Tucker DM ( 2004): Are “generalized” seizures truly generalized? Evidence of localized mesial frontal and frontopolar discharges in absence. Epilepsia 45: 1568–1579. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Hawco C, Kobayashi E, Boor R, LeVan P, Stephani U, Siniatchkin U, Gotman J ( 2008): Variability of the hemodynamic response as a function of age and frequency of epileptic spike in children with epilepsy. NeuroImage 40: 601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski M, Ding M, Truccolo WA, Bressler SL ( 2001): Evaluating causal relations in neural systems: Granger causality, directed transfer function and statistical assessment of significance. Biol Cybernetics 85: 145–157. [DOI] [PubMed] [Google Scholar]
- Kimura T, Ozaki I, Hashimoto I ( 2008): Impulse propagation along thalamocortical fibers can be detected magnetically outside the human brain. J Neurosci 28: 12535–12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JP, Khera DS, Nersesyan H, Kimchi EY, Waxman SG, Blumenfeld H ( 2004): Dysregulation of sodium channel expression in cortical neurons in a rodent model of absence epilepsy. Brain Res 1000: 102–109. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Yoshinaga H, Ohtsuka Y, Gotman J ( 2005): Dipole modeling of epileptic spikes can be accurate or misleading. Epilepsia 46: 397–408. [DOI] [PubMed] [Google Scholar]
- Kujala J, Gross J, Salmelin R ( 2008): Localization of correlated network activity at the cortical level with MEG. Neuroimage 39: 1706–1720. [DOI] [PubMed] [Google Scholar]
- Labate A, Briellmann RS, Abbott DF, Waites AB, Jackson GD ( 2005): Typical childhood absence seizures are associated with thalamic activation. Epileptic Disord 7: 373–377. [PubMed] [Google Scholar]
- Laufs H, Lengler U, Hamandi K, Kleinschmidt A, Krakow K ( 2006): Linking generalized spike‐and‐wave discharges and resting state brain activity by using EEG/fMRI in a patient with absence seizures. Epilepsia 47: 444–448. [DOI] [PubMed] [Google Scholar]
- Laufs H, Duncan JS ( 2007): Electroencephalography/functional MRI in human epilepsy: What it currently can and cannot do. Curr Opin Neurol 20: 417–423. [DOI] [PubMed] [Google Scholar]
- Lemieux L, Salek‐Haddadi A, Lund TE, Laufs H, Carmichael D ( 2007): Modelling large motion events in fMRI studies of patients with epilepsy. Magn Res Imaging 25: 894–901. [DOI] [PubMed] [Google Scholar]
- Lu Y, Grova C, Kobayashi E, Dubeau F, Gotman J ( 2007): Using voxel‐specific hemodynamic response function in EEG‐fMRI data analysis: An estimation and detection model. NeuroImage 34: 195–203. [DOI] [PubMed] [Google Scholar]
- Meeren HK, Pijn JP, Van Luijtelaar EL, Coenen AM, Lopes da Silva FH ( 2002): Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci 22: 1480–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlet I, Garcia‐Larrea L, Ryvlin P, Isnard J, Sindou M, Mauguière F ( 1998): Topographical reliability of mesio‐temporal sources of interictal spikes in temporal lobe epilepsy. Electroencephalogr Clin Neurophysiol 107: 206–212. [DOI] [PubMed] [Google Scholar]
- Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R ( 2004): EEG source imaging. Clin Neurophysiol 115: 2195–2222. [DOI] [PubMed] [Google Scholar]
- Mitra PP, Pesaran B ( 1999): Analysis of dynamic brain imaging data. Biophys J 76: 691–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller F, Siebner H, Wolff S, Muhle H, Granert O, Jansen O, Stephani U, Siniatchkin M ( 2008): Simultaneous EEG‐fMRI in drug naïve children with newly diagnosed absence epilepsy. Epilepsia 49: 1510–1519. [DOI] [PubMed] [Google Scholar]
- Moeller F, Siebner HR, Ahlgrimm N, Wolff S, Muhle H, Granert O, Boor R, Jansen O, Gotman J, Stephani U, Siniatchkin M ( 2009a): fMRI activation during spike and wave discharges evoked by photic stimulation. Neuroimage 48: 682–695. [DOI] [PubMed] [Google Scholar]
- Moeller F, Siebner HR, Wolff S, Muhle H, Granert O, Jansen O, Stephani U, Siniatchkin M ( 2009b): Mapping brain activity on the verge of a photically induced generalized tonic‐clonic seizure. Epilepsia 50: 1632–1637. [DOI] [PubMed] [Google Scholar]
- Moeller F, Levan P, Muhle H, Stephani U, Dubeau, F , Siniatchkin M, Gotman J ( 2010): Absence seizures: Individual patterns revealed by EEG‐fMRI. Epilepsia 51: 2000–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuraman M, Raethjen J, Hellriegel H, Deuschl G, Heute U ( 2008): Imaging coherent sources of tremor related EEG activity in patients with Parkinson's disease. IEEE Eng Med Biol Soc 1: 4716–4719. [DOI] [PubMed] [Google Scholar]
- Muthuraman M, Galka A, Deuschl G, Heute U, Raethjen J ( 2010a): Dynamical correlation of non‐stationary signals in time domain—A comparative study. Biomed Sig Proc Control 5: 205–213. [Google Scholar]
- Muthuraman M, Heute U, Deuschl G, Raethjen J ( 2010b): The central oscillatory network of essential tremor. IEEE Eng Med Biol Soc 1: 154–157. [DOI] [PubMed] [Google Scholar]
- Muthuraman M, Deuschl G, Raethjen J ( 2011): Essential constraints for detecting deep sources in EEG‐application to orthostatic tremor. Conf Proc 4th IEEE Congress on Image and signal processing 2760–2763.
- Neumaier A, Schneider T ( 2001): Estimation of parameters and eigenmodes of multivariate autoregressive models. ACM Trans Math Software 27: 27–57. [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM ( 2011): FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Int Neurosci 2011: 156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot‐Deseilligny C, Milea D, Müri RM ( 2004): Eye movement control by the cerebral cortex. Curr Opin Neurol 17: 17–25. [DOI] [PubMed] [Google Scholar]
- Polack PO, Guillemain I, Hu E, Deransart C, Depaulis A, Charpier S ( 2007): Deep layer somatosensory cortical neurons initiate spike‐and‐wave discharges in a genetic model of absence seizures. J Neurosci 27: 6590–6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salek‐Haddadi A, Lemieux L, Merschhemke M, Friston KJ, Duncan JS, Fish DR ( 2003): Functional magnetic resonance imaging of human absence seizures. Ann Neurol 53: 663–667. [DOI] [PubMed] [Google Scholar]
- Schelter B, Timmer J, Eichler, M ( 2009): Assessing the strength of directed influences among neural signals using renormalized partial directed coherence. J Neurosci Methods 179: 121–130. [DOI] [PubMed] [Google Scholar]
- Schluppeck D, Glimcher P, Heeger DJ ( 2005): Topographic organization for delayed saccades in human posterior parietal cortex. J Neurophysiol 94: 1372–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Neumaier A ( 2001): Algorithm 808: ARfit—A Matlab package for the estimation of parameters and eigenmodes of multivariate autoregressive models. ACM Trans Math Software 27: 58–65. [Google Scholar]
- Schnitzler A, Münks C, Butz M, Timmermann L, Gross J ( 2009): Synchronized brain network associated with essential tremor as revealed by magnetoencephalography. Mov Disord 24: 1629–1635. [DOI] [PubMed] [Google Scholar]
- Schoffelen JM, Oostenveld R, Fries P ( 2008): Imaging the human motor system's beta‐band synchronization during isometric contraction. Neuroimage 41: 437–447. [DOI] [PubMed] [Google Scholar]
- Schreiber T, Schmitz A ( 1996): Improved surrogate data for nonlinearity tests. Phys Rev Lett 77: 635–638. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A ( 2001): Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science 294: 1350–1354. [DOI] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ ( 2005): Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol 94: 1358–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniatchkin M, Groening K, Moehring J, Moeller F, Boor R, Brodbeck V, Michel CM, Rodionov R, Lemieux L, Stephani U ( 2010): Neuronal networks in children with continuous spikes and waves during slow sleep. Brain 133: 2798–813. [DOI] [PubMed] [Google Scholar]
- Slepian D, Pollak HO ( 1961): Prolate spheroidal wavefunctions Fourier analysis and uncertainty. I Bell Syst Technol J 40: 43–63. [Google Scholar]
- Srivastava G, Grottaz‐Herbette S, Lau KM, Glover GH, Menon V ( 2005): ICA‐based procedures for removing ballistocardiogram artifacts from EEG data acquired in the MRI scanner. Neuroimage 24: 50–60. [DOI] [PubMed] [Google Scholar]
- Südmeyer M, Saleh A, Wojtecki L, Cohnen M, Gross J, Ploner M, Hefter H, Timmermann L, Schnitzler A ( 2006): Wilson's disease tremor is associated with magnetic resonance imaging lesions in basal ganglia structures. Mov Disord 21: 2134–2139. [DOI] [PubMed] [Google Scholar]
- Timmer J, Lauk M, Pfleger W, Deuschl G ( 1998): Cross‐spectral analysis of physiological tremor and muscle activity. I. Theory and application to unsynchronized electromyogram. Biol Cybern 78: 349–357. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Gross J, Butz M, Kircheis G, Häussinger D, Schnitzler A ( 2003): Mini‐asterixis in hepatic encephalopathy induced by pathologic thalamo‐motor‐cortical coupling. Neurology 61: 689–692. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Gross J, Dirks M, Volkmann J, Freund HJ, Schnitzler A ( 2003): The cerebral oscillatory network of parkinsonian resting tremor. Brain 126: 199–212. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Brown M, Luu P, Holmes MD ( 2007): Discharges in ventromedial frontal cortex during absence spells. Epilepsy Behav 11: 546–557. [DOI] [PubMed] [Google Scholar]
- Van Veen B, Drongelen WV ( 1997): Localization of brain electrical activity via lineary constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 44: 867–880. [DOI] [PubMed] [Google Scholar]
- Vulliemoz S, Thornton R, Rodionov R, Carmichael DW, Guye M, Lhatoo S, McEvoy AW, Spinelli L, Michel CM, Duncan JS, Lemieux L ( 2009): The spatio‐temporal mapping of epileptic networks: Combination of EEG‐fMRI and EEG source imaging. NeuroImage 46: 834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch PD ( 1967): The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans Audio Electron 15: 70–73. [Google Scholar]
- Westmijse I, Ossenblok P, Gunning B, van Luijtelaar G ( 2009): Onset and propagation of spike and slow wave discharges in human absence epilepsy: A MEG study. Epilepsia 50: 2538–2548. [DOI] [PubMed] [Google Scholar]
- Zhang Z ( 1995): A fast method to compute surface potentials generated by dipoles within multilayer anisotropic spheres. Phys Med Biol 40: 335–349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1
Supporting Information Figure 2
Supporting InformationFigure 3
Supporting InformationFigure 4
Table 1: Characteristics of patients with absences and patients with PPR


