Abstract
Aberrant hippocampal morphology plays an important role in the pathophysiology of aging. Volumetric analysis of the hippocampus has been performed in aging studies; however, the shape morphometry—which is potentially more informative in terms of related cognition—has yet to be examined. In this paper, we employed an advanced brain mapping technique, large deformation diffeomorphic metric mapping (LDDMM), and a dimensionality reduction approach, locally linear diffeomorphic metric embedding (LLDME), to explore age‐related changes in hippocampal shape as delineated from magnetic resonance (MR) images of 302 healthy adults aged from 18 to 94 years. Compared with the hippocampal volumes, the hippocampal shapes clearly showed the nonlinear trajectory of biological aging across the human lifespan, where the variation of hippocampal shapes by age was characterized by a cubic polynomial. By integrating of LDDMM and LLDME, we were also able to illustrate the average hippocampal shapes in each individual decade. In addition, LDDMM and LLDME facilitated the identification of 63 years as a threshold beyond which hippocampal morphological changes were accelerated. Adults over 63 years of age showed the inward‐deformation bilaterally in the head of the hippocampi and the left subiculum regardless of hippocampal volume reduction when compared to adults younger than 63. Hence, we demonstrated that the shape of anatomical structures added another dimension of structural morphological quantification beyond the volume in understanding aging. Hum Brain Mapp 34:3075–3085, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: hippocampal shape, hippocampal volume, normal aging, diffeomorphic metric mapping, locally linear embedding
INTRODUCTION
Magnetic resonance‐based volumetric assessment of the hippocampus has been widely employed in studying normal aging and various neurodegenerative diseases, including mild cognitive impairment, Alzheimer's disease (AD), and geriatric depression [Apostolova et al., 2006a, 2006b; Cardenas et al., 2003; Convit et al., 1997; Csernansky et al., 2005; Frisoni et al., 2005, 2006, 2007; Qiu et al., 2009c; Shenton et al., 2001; Wang et al., 2003, 2006; Whitwell et al., 2007]. Thus far, it has been widely accepted that the hippocampal volume declines as age increases. However, the question of whether age‐related patterns of hippocampal morphology are linear or curvilinear is debatable, even though voxel‐based morphometric analysis revealed nonlinear aging processes in the whole brain [Jernigan and Gamst, 2005; Walhovd et al., 2005]. Additionally, it is not yet known whether studies of hippocampal morphology alone can identify a critical time window when aging processes are accelerated.
Investigations of the hippocampus in normal aging have thus far been limited to examination of volumetric changes. Existing studies have yielded conflicting results on patterns of age‐related hippocampal volume reduction, where some have found the relation of the hippocampal volume with age occurs in a nonlinear fashion (e.g., cubic or quadratic polynomial) [Allen et al., 2005; Jernigan and Gamst, 2005; Kennedy et al., 2009; Raz et al., 2005; Sullivan et al., 1995; Terribilli et al., 2011; Walhovd et al., 2005], while others found a linear or no reduction of the hippocampal volume with age [Hackert et al., 2002; Jack et al., 1997; Malykhin et al., 2008; Pruessner et al., 2001; Ta et al., 2011]. These discrepancies may stem from the sample characteristic or the time window studied, or from evidence that the hippocampal volume in young adults is as variable as that in older persons [Lupien et al., 2007].
Converging data from structural and functional imaging studies suggest an anterior–posterior gradient of age‐related volume and functional reduction along the hippocampal longitudinal axis in normal aging [Chen et al., 2010; Driscoll et al., 2003; Malykhin et al., 2008; Ta et al., 2011]. The structure of the hippocampus is complex, and subtle differences in small subvolumes could well have aging implications, even if they are not large enough to affect the overall volume. This indicates that the global size of the hippocampus represented by its volume may not be a sufficiently sensitive measure for characterizing normal aging processes related to the hippocampal morphology. Regionally specific morphological changes of the hippocampus, such as the hippocampal shape characterized using brain mapping techniques, may be a key for understanding patterns within the age‐hippocampus relation in normal aging.
In the last decade, brain mapping techniques have been widely used in neuroimaging studies for identifying different patterns of hippocampal shape changes distinguishing early AD from healthy aging [Apostolova et al., 2006b; Cardenas et al., 2003; Fox et al., 1996; Ridha et al., 2006; Wang et al., 2003]. Conversely, there have been limited investigations on hippocampal shape analysis to distinguish stages of normal aging, especially regarding the concept of the evolution of hippocampal shape change across the lifespan. This may be because the hippocampal shape is characterized by high‐dimensional measures, rendering it less straightforward to intuitively visualize or study its relation with age as compared to simple hippocampal volumetric analysis.
In this study, we investigated evolution patterns of the hippocampal shapes in 302 normal adults aged from 18 to 94 years. We showed the average hippocampal shape in each individual decade and determined a critical age when the changes of the hippocampal morphology are accelerated. We hypothesized the nonlinear evolution of the hippocampal shape across the lifespan, which is similar to that of the whole brain found in previous studies [Jernigan and Gamst, 2005; Walhovd et al., 2005]. Even though this nonlinear relationship with age may not be directly observed using studies based on the hippocampal volume, this relationship is observed in studies based on the local shape changes of the hippocampus. As we will see later, this property is helpful for distinguishing different trajectories of hippocampal aging processes in young and older adults.
To test our hypothesis, we first chose an advanced brain mapping technique, large deformation diffeomorphic metric mapping (LDDMM) [Miller and Qiu, 2009], to study shape variations of individual hippocampi referenced to an atlas shape because of its mapping accuracy [Vaillant et al., 2007]. The use of LDDMM for studying hippocampal shapes allows their placement in a metric space, provides a diffeomorphic (one‐to‐one, reversible smooth) transformation characterized by vector fields (called initial momenta) and defines a metric distance that can be used to quantify the similarity between two hippocampal shapes. Because of the high dimensionality of the initial momenta that characterize individual hippocampal shapes referenced to the atlas shape, we then employed our recently developed dimensionality reduction approach, locally linear diffeomorphic metric embedding (LLDME) [Yang et al., 2011b], to seek a parsimonious representation of hippocampal shapes in an Euclidean space. This allowed us to represent the hippocampal shapes using a scalar variable such that their relation with age can be easily visualized and statistically analyzed. The integration of LDDMM and LLDME facilitated the investigation of (i) the relation between age and hippocampal shapes; (ii) average hippocampal shapes in each decade; (iii) critical time when changes of hippocampal shapes start to be accelerated.
METHODS
Subjects
This study included 302 healthy right‐handed subjects (age range: 18–94, mean age = 44.5 ± 23.8 years; gender: 114 males and 188 females). All subjects were recruited from the general Washington University community and also from the longitudinal pool of the Washington University Alzheimer Disease Research Center (ADRC). All subjects gave informed consent and participated in accordance with guidelines of the Washington University Human Studies Committee.
The screening for subject inclusion was detailed by Marcus et al. 2007. Briefly, young and middle‐aged adults were questioned by a trained technician about their medical histories and use of psychoactive drugs. Adults aged 60 and older underwent the ADRC's full clinical assessment described by Marcus et al. 2007. Subjects with a primary cause of dementia other than AD (e.g., vascular dementia, primary progressive aphasia), active neurological or psychiatric illness (e.g., major depression), serious head injury, history of clinically meaningful stroke, and use of psychoactive drugs were excluded, as were subjects with gross anatomical abnormalities evident in their MRI images (e.g., large lesions, tumors). Among all subjects aged 60 years and above, the clinical dementia rating scale (CDR) was zero and a Mini Mental Status Examination (MMSE) was greater than 25 (range, 25–30).
MRI Acquisition and Hippocampal Delineation
T1‐ weighted MP‐RAGE scans were acquired using a 1.5 T Siemens Vision scanner at Washington University at St. Louis. MP‐RAGE parameters were empirically optimized for gray‐white contrast [TR = 9.7 ms, TE = 4 ms, flip angle = 10°, inversion time (TI) = 20 ms, delay time (DT) = 200 ms, 256 × 256 (1 × 1 mm2) in‐plane resolution, one hundred twenty‐eight 1.25‐mm slices without gaps]. Head movement was minimized by cushioning and a thermoplastic facemask. All MR images used in this study are available in open access [http://www.oasis-brains.org, (Marcus et al., 2007)].
As illustrated in Figure 1, we first employed FreeSurfer to delineate the hippocampus from the intensity‐inhomogeneity corrected T1‐weighted MR images [Sled et al., 1998] using a Markov random field (MRF) model [Fischl et al., 2002]. Since the MRF model was performed in the image volume without constraints on topology of the segmented hippocampal volume, it introduced irregularities and topological errors (e.g., holes) at the hippocampal boundary (arrows in Fig. 1). This increased shape variation and thus reduced statistical power to detect group differences. To address this potential pitfall, we generated smooth hippocampal shapes of individual subjects with correct topology by injecting a hippocampal atlas shape using the large deformation diffeomorphic metric mapping (LDDMM) algorithm [Miller and Qiu, 2009; Qiu and Miller, 2008]. The hippocampal atlas shape was created from 41 manually labeled hippocampi via a large deformation diffeomorphic atlas generation algorithm in our previous study [Qiu et al., 2010]. Each hippocampal volume was approximated by the transformed atlas through the LDDMM transformation. The reader is referred to [Qiu and Miller, 2008] for the mathematical derivation of this atlas injection procedure and its evaluation as well as the segmentation accuracy on the hippocampus. This delineation approach has been successfully applied to investigate the hippocampus and other subcortical shapes in AD [Qiu et al., 2009b].
Figure 1.
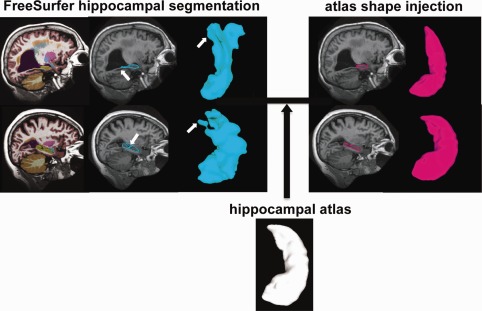
Schematic of the hippocampal segmentation. The left panel shows the volume and surface views of the hippocampus segmented using FreeSurfer, while the right panel illustrates those after injecting the atlas hippocampal shape. Arrows in the left panel point out segmentation errors from FreeSurfer.
Hippocampal Shape Analysis
Figure 2 illustrates the schematic of hippocampal shape analysis. First, we constructed triangulated surfaces using the matching cubes algorithm (left hippocampal surfaces with 1,184 vertices and 2,364 triangles; right hippocampal surfaces with 1,231 vertices and 2,458 triangles) by composing the LDDMM transformation on the atlas surface [Qiu et al., 2010]. Then, the hippocampal atlas surface (the same atlas as that was used in the hippocampal segmentation) was registered to individual subjects' hippocampal surfaces using the LDDMM‐surface mapping algorithm [Zhong et al., 2010; Zhong and Qiu, 2010]. This mapping algorithm provided a diffeomorphic transformation that is a one‐to‐one, reversible smooth transformation which aligns the atlas to the subject. It yielded initial momentum vectors defined at each vertex of the atlas surface that characterized shape variations of individual hippocampi referenced to the atlas (see an example in Fig. 2a). The initial momentum, similar to the concept in physics, uniquely determined the diffeomorphic flow deforming the atlas to individual hippocampi [Miller et al., 2006].
Figure 2.
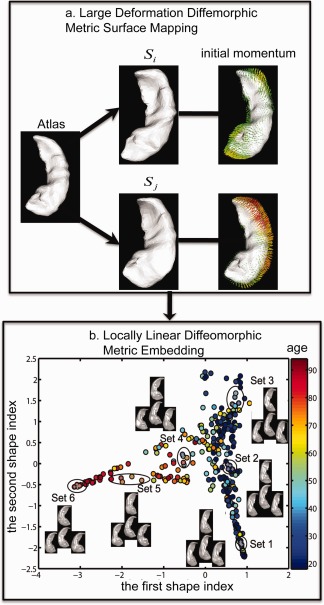
Schematic of hippocampal shape analysis. Panel (a) shows the large deformation diffeomorphic metric surface mapping (LDDMM‐surface) from a hippocampal surface atlas to subjects' surfaces, S i and S j, and obtained the initial momentum characterizing shape variations of S i and S j relative to the atlas surface. Panel (b) illustrates the representation of hippocampal shapes of 302 healthy subjects into a two‐dimensional Euclidean space that was obtained using locally linear diffeomorphic metric embedding (LLDME). Panel (b) also illustrates real hippocampal shapes at six different locations marked as set 1, 2, …, 6.
Unlike the volumetric measure of the hippocampus, whose relation with age can be easily visualized using a scatter plot, the dimensionality of the initial momentum was high (left hippocampus: 3x1184 (vertices); right hippocampus: 3x1231 (vertices)). This made it challenging to visualize the relation of the hippocampal shape with age using a scatter plot. We thus performed locally linear diffeomorphic metric embedding (LLDME) analysis [Yang et al., 2011b] that reduced the dimensionality of the initial momentum and projected the shape into a Euclidean space with a few dimensions, as illustrated in Figure 2b. In the LLDME analysis, we denoted 302 hippocampal surface shapes as S i, i = 1,2, …, 302, where S i is the hippocampal surface of subject i. (αl (i))l = 1 n are the initial momentum vectors found using LDDMM to map the atlas surface to the ith subject's surface. These momentum vectors are defined on the atlas surface with n vertices. We assumed that their age‐related variations characterized by (αl (i))l = 1 n are not random, but instead can be characterized using a small subset of unknown parameters in a Euclidean space, where the relationship of neighborhood hippocampal shapes can be preserved. The first step of LLDME was to compute pair‐wise metric distances of these hippocampal shapes using LDDMM and identify neighbors of each hippocampal shape based on its metric distances to other hippocampi [Yang et al., 2011a]. In order to reduce the number of pair‐wise LDDMM mappings among 302 subjects to 302 LDDMM mappings [Yang et al., 2011a], we estimated the metric distance between surfaces S i and S j (ρ(S i,S j)) using the first order approximation given in the form of
where x l is the coordinate of the lth point on the atlas surface and k v is the kernel associated with a Hilbert space of the initial momentum to ensure that the deformation is diffeomorphic. T denotes the transpose operation. The metric distance from this first order approximation is highly correlated with one directly computed using LDDMM to deform S i to S j (r = 0.975, P < 0.0001) [Yang et al., 2011a]. The second step of LLDME was to find a 302 × 302 matrix of weights, W = [W ij], that approximated each hippocampal shape as a linear combination of its neighbors. This was made possible by the linearity property of the initial momentum and its role of representing shape variation of any two hippocampi [Yang et al., 2011b]. We hence defined
subject to ∑j W ij = 1 and W ij = 0 when j ∉ Ni. α(i) = (αl (i))l = 1 n represents the initial momentum vectors that encode the shape variation of S i relative to the atlas, which is obtained from LDDMM. The coefficients, W = [W ij], summarize the contribution of the other subjects' anatomical shapes to that of S i and can be found via minimizing ε (W). The last step of LLDME was to find a Euclidean low‐dimensional representation of the hippocampal shapes that preserved their neighborhood relations. This was implemented by looking for a Euclidean vector, y (i) = [y 1 (i),y 2 (i), … y p (i)] with dimensionality p, representing S i such that y (i) could be approximated by a linear combination of the same neighbors when weights in W were given. This was achieved by minimizing subject to ∑i = 1 302 y i = 0, is an identity matrix. The dimensionality of the Euclidean vector (p) was determined when p gave the maximal similarity between the neighborhood relations of the hippocampal shapes characterized by using the initial momentum and y(i), that is, p was determined by maximizing R(ρ1,ρ2), where R(ρ1,ρ2) is the absolute correlation between the pair‐wise metric distance matrix computed based on the initial momentum and distance matrix computed based on y(i). In this study, the intrinsic dimension of the hippocampal shape among the 302 subjects was p = 2. These two dimensions were referred to as the first and second shape indexes and used to investigate age‐related hippocampal trajectory across the lifespan. Observe that these two shape indexes characterized local shape variations of the hippocampus beyond global increase or decrease of the hippocampal volume.
In addition to the reduction of dimensionality of the hippocampal shapes, LLDME also provided a simple scheme to compute a mean hippocampal shape among multiple subjects by first averaging their shape indexes, and then finding its neighbors and linear interpolation weights in the Euclidean space. Such neighborhood relation and weights were applied to the hippocampal shape space and used to compute the mean initial momentum that characterized the mean hippocampal shape deviation from the atlas [Yang et al., 2011b]. In our study, we employed this technique to visualize the hippocampal shape, averaged among subjects in each age decade.
Statistical Analysis
Hippocampal volume
The total hippocampal volume, a sum of the left and right hippocampal volumes, was first adjusted for the total intracranial volume (TIV, computed using FreeSurfer) and gender using linear regression, and then converted to a Z‐score reflecting the standard deviations above or below the population mean. To investigate patterns of nonlinearity in the relationship between the hippocampal volume and age, regression analysis was performed where age was a fixed factor and the dependent variable was the Z‐score of the hippocampal volume. The goodness of fit of first, second, and third order polynomial expansions was assessed and results were reported only if at least one of the regression models achieved the significance level at p < 0.05. The coefficient of determination, R 2, was used to measure how well the model is likely to fit the hippocampal volume data.
Hippocampal shape
To investigate patterns of nonlinearity in the relationship between the hippocampal shape and age, Pearson's correlation analysis was first performed to select a subset of the hippocampal shape indexes that were deemed to be significantly correlated with age. Similar to the hippocampal volume analysis, regression analysis was then performed with age as a fixed factor, the dependent variables were individual shape indexes, and gender was a covariate.
To identify a critical age when age‐related changes of the hippocampal shape are accelerated, linear discriminant analysis (LDA) was employed to optimize a classification error rate such that subjects above this critical age (older group) were best distinguished from those below this critical age (young group). Employing shape indexes with significant correlation with age as features in LDA, leave‐one‐out cross validation was applied to compute the classification error rates at each age and the critical age was determined as the age where the classification error rate was minimal. To examine the shape differences between the young and older groups regardless of the size of the hippocampus, regression was performed with age group as the fixed factor, displacement at each vertex relative to the atlas as the dependent variable, and gender and hippocampal volume as covariates. The statistical results were corrected for multiple comparisons using permutation tests to determine the overall significance of the shape difference map. In each permutation trial, the group factor was randomly assigned to each subject and the number of points with significant group shape difference (P < 0.05) was recorded. After 10,000 permutation trials, the overall significance was computed as the fraction of the time the suprathreshold area was greater in the randomized maps than in the real effect [Nichols and Holmes, 2002].
RESULTS
Age Effects on Hippocampal Volumes
Figure 3a illustrates the scatter plot of the hippocampal volume and age, showing the distribution of the hippocampal volume across the lifespan. Figure 3b illustrates a scatter plot of age and its z‐score. The age‐related decline of the hippocampal volume was best represented by the quadratic polynomial model after removing effects of TIV and gender (t = −2.16, P < 0.05) (see the red line in Fig. 3b). R 2 associated with this model was small (R 2 = 0.03). This suggested that the variation of hippocampal volume across age among this sample (Table 1) might not be well characterized by the quadratic polynomial even though this quadratic polynomial was significantly correlated with the hippocampal volume.
Figure 3.
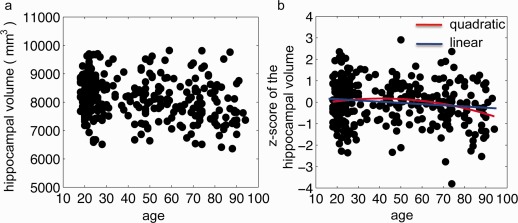
Panels (a,b) respectively show the relation of age with the hippocampal volume and its z‐score after controlling for gender and total intracranial volume. Red and blue lines on panel (b) are the quadratic and linear polynomial fittings, respectively.
Table 1.
Mean, standard deviation (SD), minimal and maximal values of the hippocampal volume (a sum of the left and right hippocampal volumes)
| Age range (years) | Subjects, N | Mean ± SD (mm3) | Min (mm3) | Max (mm3) |
|---|---|---|---|---|
| 18–30 | 139 | 8286 ± 681 | 6,512 | 9,700 |
| 31–50 | 50 | 8137 ± 754 | 6,637 | 9,517 |
| 51–65 | 39 | 8160 ± 674 | 7,059 | 9,817 |
| 66–80 | 47 | 8039 ± 759 | 6,514 | 9,810 |
| 80–94 | 30 | 7682 ± 805 | 6,360 | 9,762 |
Age Effects on Hippocampal Shapes
Figure 2b illustrates the representation of the 302 hippocampal shapes in the two‐dimensional shape index space obtained using LLDME. Intuitively, the hippocampal shapes of older adults are in the location with a larger first shape index, while the hippocampal shapes of young adults are in the location with a smaller first shape index. Several sets of the hippocampal shapes at different age ranges are also shown in Figure 2b for the intuitive illustration.
Pearson's correlation analysis revealed significant correlation of age with the first shape index (r = −0.76, P < 0.05). However, this was not the case with the second shape index (r = −0.03, P < 0.82). This suggested that the age‐related variation in hippocampal shape was represented by the first shape index only. Figure 4 illustrates the scatter plot of the first shape index and age. The plot depicts the first shape indexes among young adults (18−30 years) as restricted to a narrow range regardless of their hippocampal volumes (which was highly variable as graphed in Fig. 2). The standard deviation and the range of the first shape index are given in Table 2. This distribution suggested an increasing variation in hippocampal shapes with age. The regression model with gender as a covariate revealed a significant third order polynomial function of the first hippocampal shape index with age (t = −2.00, P < 0.05; R 2 = 0.66). This relationship remained significant (t = −1.96, P = 0.05, and R 2 = 0.68) when the hippocampal volume was considered as a covariate in the regression. The R 2 values of these two regression models with and without the hippocampal volume (with: R 2 = 0.68; without: R 2 = 0.66) as a covariate suggested that the hippocampal volume contributed little to age‐related variation of the hippocampal shapes.
Figure 4.
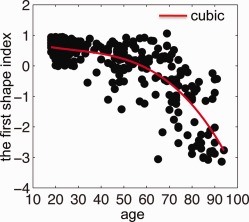
The relation between age and the hippocampal shape represented by the first shape index obtained from LLDME. The red line represents the third order polynomial fitting.
Table 2.
Mean, standard deviation (SD), minimal and maximal values of the first shape index of the hippocampal shape
| Age range (years) | Subjects, N | Mean ± SD | Min | Max |
|---|---|---|---|---|
| 18–30 | 139 | 0.57 ± 0.23 | −0.01 | 0.94 |
| 31–50 | 50 | 0.21 ± 0.53 | −1.43 | 0.89 |
| 51–65 | 39 | 0.11 ± 0.76 | −3.06 | 0.92 |
| 66–80 | 47 | −0.72 ± 0.95 | −2.89 | 1.06 |
| 80–94 | 30 | −1.99 ± 0.97 | −3.14 | −0.24 |
Figure 5 shows the hippocampal shapes averaged over subjects in each age decade and intuitively illustrates the hippocampal shapes along the nonlinear trajectory shown by the red line in Figure 4. Individual hippocampal surfaces are colored by their displacement relative to the averaged hippocampal surface among subjects in the 20 s. In particular, the head and subiculum of the bilateral hippocampi showed increasing surface inward‐deformation with increased age. At the same time, the average hippocampal shape in each age decade was slightly bent along the longitudinal axis of the hippocampus.
Figure 5.
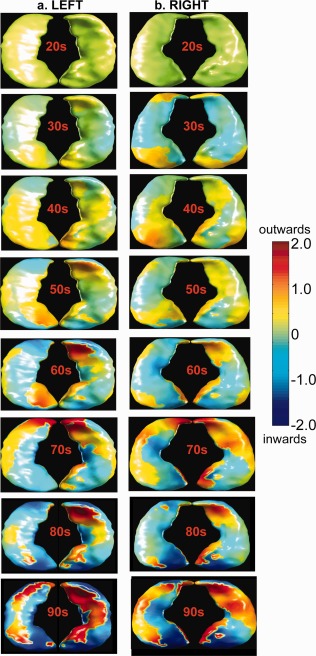
Mean hippocampal shapes among subjects in each age decade. Columns (a,b) respectively show left and right mean hippocampal shapes colored by their displacement relative to the mean hippocampal shapes among subjects aged at 20 s. The outward and inward displacements of individual mean hippocampal shapes are respectively colored in red and blue with respect to the surfaces on the first row. The inferior and superior views of the hippocampus are given on each panel.
The pattern of the local changes in hippocampal shapes due to age identified by the first shape index was also used to determine a critical age when acceleration of the hippocampal degeneration process occurs. At 63 years of age, the leave‐one‐out validation of LDA gave the minimum classification error of 9.9% for distinguishing subjects aged above and below this threshold age. When the two shape indexes were both used as features in LDA, the classification error was not improved. This observation further supported the regression results of the hippocampal shape indexes, i.e., the first shape index was the one characterizing nonlinear age‐related changes of the hippocampal shapes as shown in Figure 4.
Figure 6 shows the hippocampal shape differences between the adults with age below and above 63 after controlling for gender and the hippocampal volume. Regardless of the reduction of hippocampal volumes, adults above 63 showed the regionally specific inward deformation in the head of the bilateral hippocampi and the left subiculum field when compared with adults below 63. Permutation tests revealed that the overall significance (P‐value) of the maps in Figure 6 was less than 0.0001.
Figure 6.
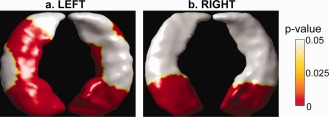
Group difference in hippocampal shapes between adults with age below and above 63 years. Panels (a,b) show the group differences in the left and right hippocampal shapes, respectively. When compared with adults younger than 63 years old, adults older than 63 years old showed statistically significant inward‐deformation in regions colored in red. The inferior and superior views of the hippocampus are given on each panel.
DISCUSSION
The present large‐scale neuroimaging study examined age‐related hippocampal shapes across a wide age range (age, 18–94 years). Traditional volumetric analysis revealed a reduction of the hippocampal volume as a quadratic function of age. However, this age‐related quadratic polynomial could only characterize limited variation of the hippocampal volumes across the lifespan. In contrast, as shown in this paper, the advanced brain mapping technique, LDDMM, combined with the data reduction approach, LLDME, clearly showed a nonlinear trajectory of the hippocampal shapes across the lifespan, which was characterized by the cubic polynomial function of age. In addition, we identified 63 years as a critical age when the hippocampal morphological changes were accelerated. Compared with young adults with age below 63, older adults with age above 63 showed the inward‐deformation in the head of the bilateral hippocampi and the subiculum of the left hippocampus regardless of the hippocampal volume reduction.
Hippocampal Volume
Previous studies that included young adults (age below 45 years) [Pruessner et al., 2001] or older adults (age above 45 years) [Chen et al., 2010; Hackert et al., 2002; Jack et al., 1997; Raz et al., 2010] suggested a linear relationship between age and hippocampal volumes. The inclusion of the middle‐aged adults in our study allowed us to reveal that age‐related nonlinear reduction exists in the hippocampal volume, which is in line with some previous studies targeting the same age range as ours (18–90 years) [Allen et al., 2005; Lupien et al., 2007; Walhovd et al., 2005]. Nevertheless, not all previous studies with the age range from 18 to 90 years found nonlinear relationships between the hippocampal volume and age. Good et al. [2001] applied voxel‐based morphometric analysis on 465 normal adults from 18 to 80 years and showed preservation of the hippocampal volume in aging. The authors discussed that their data included relatively few subjects over 65 years and these subjects could be considered to be “super normal” such that they could be missing the nonlinear trend of the aging processes in the hippocampus. Interestingly, this is not the only study reporting no change of the hippocampal volume in aging [Sullivan et al., 1995].
A few possible explanations have been raised to address the conflicting results described above. For instance, characteristics of the samples and the time window studied could have contributed to the contradictory findings. Raz et al. 2005 showed that both linear and quadratic trends in incremental age‐related shrinkage of the hippocampal volume were limited to subjects with hypertension. Fjell et al. 2010 proposed that the age range studied could dramatically influence the quadratic relation between the hippocampal volume and age. Beyond these two factors, high variability of the hippocampal volume among young and older adults could be considered as another potential complication. Our study, as well as others [Lupien et al., 2007] demonstrated that the hippocampal volume is as variable in young as in older adults even after adjusting for the total intracranial volume. In our study, the quadratic polynomial only characterized subtle variation of the hippocampal volumes in our samples, which was close to that obtained from linear fitting (quadratic: R 2 = 0.03; linear: R 2 = 0.01, Fig. 2b). As a consequence, it is difficult to conclude that the quadratic polynomial function would be the best fit for representing age‐related nonlinear trajectory of the hippocampal volume across the lifespan, although the relationship between age and hippocampal volume was found to be significant with fitting a quadratic curve. Thus, it is important to interpret existing reported data based on both the level of significance as well as the goodness of fit between age and hippocampal volume.
Hippocampal Shape
Converging evidence from postmortem, structural, and functional imaging studies have suggested heterogeneous aging effects over different regions of the hippocampus. Postmortem studies on quantifying the neuronal number suggested significant age‐associated neuronal loss in hippocampal subiculum and CA1 [Simic et al., 1997; West, 1993]. Anatomical and functional MRI studies indicated differential age‐related vulnerabilities of the anterior and posterior hippocampi, even though there are some discrepancies regarding whether the posterior hippocampus has a greater vulnerability to aging than the anterior or vice versa [Driscoll et al., 2003; Hackert et al., 2002; Jack et al., 1997; Kalpouzos et al., 2009; Malykhin et al., 2008; Raz, 2000; Ta et al., 2011]. Thus far, all of these MRI‐based anatomical and functional studies have been limited to volumetric analysis by dividing the hippocampus into the anterior and posterior segments or the head, body, and tail and then assessing the volume of each region. Hence, the precise anatomical definition of the hippocampal segregation becomes crucial, making comparison amongst different studies difficult. Moreover, the large variation of the hippocampal volume as mentioned above still exists in the volumes of its subregions. As a result, it is not surprising that the conflicting findings were reported on patterns of the hippocampal subregional volumes in aging.
Our study employed the shape analysis techniques of LDDMM and LLDME and yielded a succinct shape index, independent of the hippocampal volume, shedding light on the discrepant findings on the hippocampal volumes. Our results suggested that the local hippocampal atrophy described by its shape shows a nonlinear pattern across the lifespan regardless of the hippocampal volume. This is congruent to previous findings of a heterogeneous pattern of age‐related volume changes with region‐specific nonlinear features yielded from the whole brain studies [Terribilli et al., 2011; Walhovd et al., 2005]. We further extended the notion of nonlinear age‐associated volume changes of individual structures and/or the whole brain morphometry by showing that such patterns clearly occur within the hippocampus but based on its local shapes, and not its size. This implies that the local shape information added another dimension of structural morphological quantification beyond the volume.
Our findings further suggested that the hippocampal shape appeared to remain relatively stable until the age of 63, after which its changes were rapid. The value of this threshold age is unsurprising, given the increased prevalence of AD in individuals beyond 65; however, this is the first time that the specific timing of biological aging is identified using hippocampal shape alone. The group comparison between adults aged above and below 63 suggested that the head and subiculum of the hippocampus were crucial subregions associated with the accelerated aging process. The surface inward‐deformation in these two subregions is in conjunction with the hippocampal shape differences between normal elderly and patients with AD [Apostolova et al., 2006a; Csernansky et al., 2000; Qiu et al., 2009a]. Previous postmortem studies firmly established the association of the hippocampal neuron loss with the reduction of the hippocampal volume in both AD and normal aging, and identified neuronal loss in the subiculum and CA1 as key features in normal aging [Kril et al., 2004; West, 1993]. Taken together with our shape comparison results, these findings support the idea of the continuum trajectory of the brain anatomy from normality to the earliest signs of AD, regardless of the presence of AD neuropathology. Furthermore, these results confirm the usefulness of shape morphology as an indirect measure of neurodegeneration in normal aging.
We have thus far discussed the contribution of the first shape index for understanding age‐related hippocampal shapes. Even without association with age, the second shape index (Fig. 1b) showed a large variability of the hippocampal shapes among young adults. Nevertheless, the cause of the shape variability in the young remains uncertain. The significant interindividual variability in the hippocampal shape that we observed in young adults could arise from both genetic and experiential factors. A twin study [Sullivan et al., 2001] estimated that genetics can contribute about 40% of the variance in the hippocampal volume among older adults, while experiential factors can account for the other 60%. Buss et al. 2007 have shown significant association of birth weight and the hippocampal volume in adulthood of female subjects related to poor maternal care, suggesting that the postnatal environment modulates the neurodevelopmental consequences of prenatal risk. Animal studies have demonstrated that such environmental enrichment is a potent inducer of changes in neurogenesis and dendritic arborization in the hippocampus, which lead to changes of the hippocampal volumes in late life [Kempermann et al., 1998; Mlynarik et al., 2004]. All of these imply that understanding causes of large variation of the hippocampal shapes in the young could be very important.
Technical Strength
In the present study, LDDMM was successful in characterizing small differences of individual hippocampal shapes relative to the atlas in the scale of small subvolumes even if they are not large enough to affect the overall volume. Thus, it is a very sensitive measure of the hippocampal morphology. Additionally, it defines the shape differences of individual hippocampi as a function of the atlas coordinates such that the segregation of the hippocampus into multiple regions is not needed if hippocampal subregions are regions of interest. This avoids the anatomical ambiguity of arbitrarily defining hippocampal subregions. Integrated with LDDMM, LLDME was able to embed the hippocampal shapes (Fig. 2a) into a two‐dimensional plane (Fig. 1b) where the first dimension of the shape index is highly associated with aging. This makes the visualization and investigation of the relation of the hippocampal shapes with age as straightforward as those for the hippocampal volume. Moreover, the shape index is a convenient representation that makes it possible to directly compute the mean shape using the scalar averaging operation and to also interpolate the hippocampal shapes of missing data. The use of LDDMM and LLDME has great potential for studying anatomical morphological shapes and their association with clinical measures.
Limitations
This study was conducted based on a publicly available dataset [OASIS, (Marcus et al., 2007)]. It provides opportunities to compare anatomical findings across studies employing different brain mapping techniques. Nevertheless, the limited availability of comprehensive clinical data in this dataset restricts the ability of incorporating covariates, for instance, the education level and presence of hypertension, that do not invalidate the association of the hippocampal shapes and age but are likely to mediate this relationship. These covariates can help interpret variances of the hippocampal shapes among young and older adults that cannot be characterized by aging alone. Additionally, our segmentation approach may not be superior to recent segmentation methods [Leung et al., 2011], which needs further investigation.
CONCLUSIONS
This large‐scale neuroimaging study revealed that the evolution of the hippocampal morphology is nonlinear across the lifespan. This nonlinear age‐related association is obviously seen in local shape changes in the subiculum and the head but not the size of the hippocampus. Hence, the shape of anatomical structures added another dimension of structural morphological quantification beyond the volume in understanding aging.
REFERENCES
- Allen JS, Bruss J, Brown CK, Damasio H (2005): Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiol Aging 26:1245–1260; discussion 1279–1282. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Dinov ID, Dutton RA, Hayashi KM, Toga AW, Cummings JL, Thompson PM (2006a): 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer's disease. Brain 129( Part 11):2867–2873. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Dutton RA, Dinov ID, Hayashi KM, Toga AW, Cummings JL, Thompson PM (2006b): Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol 63:693–699. [DOI] [PubMed] [Google Scholar]
- Buss C, Lord C, Wadiwalla M, Hellhammer DH, Lupien SJ, Meaney MJ, Pruessner JC (2007): Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J Neurosci 27:2592–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Du AT, Hardin D, Ezekiel F, Weber P, Jagust WJ, Chui HC, Schuff N, Weiner MW (2003): Comparison of methods for measuring longitudinal brain change in cognitive impairment and dementia. Neurobiol Aging 24:537–544. [DOI] [PubMed] [Google Scholar]
- Chen KH, Chuah LY, Sim SK, Chee MW (2010): Hippocampal region‐specific contributions to memory performance in normal elderly. Brain Cogn 72:400–407. [DOI] [PubMed] [Google Scholar]
- Convit A, De Leon MJ, Tarshish C, De Santi S, Tsui W, Rusinek H, George A (1997): Specific hippocampal volume reductions in individuals at risk for Alzheimer's disease. Neurobiol Aging 18:131–138. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Joshi S, Miller JP, Gado M, Kido D, McKeel D, Morris JC, Miller MI (2000): Early DAT is distinguished from aging by high‐dimensional mapping of the hippocampus. Dementia of the Alzheimer type. Neurology 55:1636–1643. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Swank J, Miller JP, Gado M, McKeel D, Miller MI, Morris JC (2005): Preclinical detection of Alzheimer's disease: Hippocampal shape and volume predict dementia onset in the elderly. Neuroimage 25:783–792. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, Sutherland RJ (2003): The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex 13:1344–1351. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Kouwe A, Killiany R, Kennedy D, Klaveness S, et al. (2002): Whole brain segmentation: neurotechnique automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Westlye LT, Ostby Y, Tamnes CK, Jernigan TL, Gamst A, Dale AM (2010): When does brain aging accelerate? Dangers of quadratic fits in cross‐sectional studies. Neuroimage 50:1376–1383. [DOI] [PubMed] [Google Scholar]
- Fox NC, Warrington EK, Freeborough PA, Hartikainen P, Kennedy AM, Stevens JM, Rossor MN (1996): Presymptomatic hippocampal atrophy in Alzheimer's disease. A longitudinal MRI study. Brain 119 ( Part 6):2001–2007. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Testa C, Sabattoli F, Beltramello A, Soininen H, Laakso MP (2005): Structural correlates of early and late onset Alzheimer's disease: Voxel based morphometric study. J Neurol Neurosurg Psychiatry 76:112–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoni GB, Sabattoli F, Lee AD, Dutton RA, Toga AW, Thompson PM (2006): In vivo neuropathology of the hippocampal formation in AD: a radial mapping MR‐based study. Neuroimage 32:104–110. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Pievani M, Testa C, Sabattoli F, Bresciani L, Bonetti M, Beltramello A, Hayashi KM, Toga AW, Thompson PM (2007): The topography of grey matter involvement in early and late onset Alzheimer's disease. Brain 130( Part 3):720–730. [DOI] [PubMed] [Google Scholar]
- Hackert VH, den Heijer T, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MM (2002): Hippocampal head size associated with verbal memory performance in nondemented elderly. Neuroimage 17:1365–1372. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr, Petersen RC, Xu YC, Waring SC, O'Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E (1997): Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology 49:786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC (2005): Changes in volume with age—Consistency and interpretation of observed effects. Neurobiol Aging 26:1271–1274; discussion 1275–1278. [DOI] [PubMed] [Google Scholar]
- Kalpouzos G, Chetelat G, Baron JC, Landeau B, Mevel K, Godeau C, Barre L, Constans JM, Viader F, Eustache F, et al. (2009): Voxel‐based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging 30:112–124. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH (1998): Experience‐induced neurogenesis in the senescent dentate gyrus. J Neurosci 18:3206–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, Acker JD, Raz N (2009): Age‐related differences in regional brain volumes: A comparison of optimized voxel‐based morphometry to manual volumetry. Neurobiol Aging 30:1657–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kril JJ, Hodges J, Halliday G (2004): Relationship between hippocampal volume and CA1 neuron loss in brains of humans with and without Alzheimer's disease. Neurosci Lett 361( 1–3):9–12. [DOI] [PubMed] [Google Scholar]
- Leung KK, Barnes J, Modat M, Ridgway GR, Bartlett JW, Fox NC, Ourselin S (2011): Brain MAPS: An automated, accurate and robust brain extraction technique using a template library. Neuroimage 55:1091–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Evans A, Lord C, Miles J, Pruessner M, Pike B, Pruessner JC (2007): Hippocampal volume is as variable in young as in older adults: Implications for the notion of hippocampal atrophy in humans. Neuroimage 34:479–485. [DOI] [PubMed] [Google Scholar]
- Malykhin NV, Bouchard TP, Camicioli R, Coupland NJ (2008): Aging hippocampus and amygdala. Neuroreport 19:543–547. [DOI] [PubMed] [Google Scholar]
- Marcus DS, Wang TH, Parker J, Csernansky JG, Morris JC, Buckner RL (2007): Open Access Series of Imaging Studies (OASIS): Cross‐sectional MRI data in young, middle aged, nondemented, and demented older adults. J Cogn Neurosci 19:1498–1507. [DOI] [PubMed] [Google Scholar]
- Miller M, Trouve A, Younes L (2006): Geodesic shooting for computational anatomy. J Mathematical Imaging Vis 24:209–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Qiu A (2009): The emerging discipline of Computational Functional Anatomy. Neuroimage 45(1 Suppl):S16–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlynarik M, Johansson BB, Jezova D (2004): Enriched environment influences adrenocortical response to immune challenge and glutamate receptor gene expression in rat hippocampus. Ann NY Acad Sci 1018:273–280. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Collins DL, Pruessner M, Evans AC. (2001): Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. J Neurosci 21:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Miller MI (2008): Multi‐structure network shape analysis via normal surface momentum maps. Neuroimage 42:1430–1438. [DOI] [PubMed] [Google Scholar]
- Qiu A, Fennema‐Notestine C, Dale AM, Miller MI (2009a): Regional shape abnormalities in mild cognitive impairment and Alzheimer's disease. Neuroimage 45:656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Notestine CF, Dale AM, Miller MI (2009b): Regional subcortical shape abnormalities in mild cognitive impairment and alzheimer's disease. Neuroimage 45:656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Taylor WD, Zhao Z, MacFall JR, Miller MI, Key CR, Payne ME, Steffens DC, Krishnan KR (2009c): APOE related hippocampal shape alteration in geriatric depression. Neuroimage 44:620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Brown T, Fischl B, Ma J, Miller MI (2010): Atlas generation for subcortical and ventricular structures with its applications in shape analysis. IEEE Trans Image Process 19:1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N (2000): Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings In: Craik FIMSTA, editor.The Handbook of Aging and Cognition. Mahwah:Lawrence Erlbaum Associates; pp1–90. [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U (2010): Trajectories of brain aging in middle‐aged and older adults: Regional and individual differences. Neuroimage 51:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD (2005): Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex 15:1676–1689. [DOI] [PubMed] [Google Scholar]
- Ridha BH, Barnes J, Bartlett JW, Godbolt A, Pepple T, Rossor MN, Fox NC (2006): Tracking atrophy progression in familial Alzheimer's disease: A serial MRI study. Lancet Neurol 5:828–834. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW (2001): A review of MRI findings in schizophrenia. Schizophr Res 49( 1–2):1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simic G, Kostovic I, Winblad B, Bogdanovic N (1997): Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer's disease. J Comp Neurol 379:482–494. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC (1998): A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17:87–97. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A (1995): Age‐related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiol Aging 16:591–606. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, Swan GE, Carmelli D (2001): Heritability of hippocampal size in elderly twin men: Equivalent influence from genes and environment. Hippocampus 11:754–762. [DOI] [PubMed] [Google Scholar]
- Ta ATT, Huang S‐EH, Chiu M‐J, Hua M‐S, Tseng W‐YI, Chen S‐HA, Qiu A (2011): Age‐related vulnerabilities along the hippocampal longitudinal axis. Hum Brain Mapp. doi: 10.1002/hbm.21364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terribilli D, Schaufelberger MS, Duran FL, Zanetti MV, Curiati PK, Menezes PR, Scazufca M, Amaro E Jr, Leite CC, Busatto GF (2011): Age‐related gray matter volume changes in the brain during non‐elderly adulthood. Neurobiol Aging 32:354–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant M, Qiu A, Glaunes J, Miller MI (2007): Diffeomorphic metric surface mapping in subregion of the superior temporal gyrus. Neuroimage 34:1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B (2005): Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging 26:1261–1270; discussion 1275–1278. [DOI] [PubMed] [Google Scholar]
- Wang L, Swank JS, Glick IE, Gado MH, Miller MI, Morris JC, Csernansky JG (2003): Changes in hippocampal volume and shape across time distinguish dementia of the Alzheimer type from healthy aging. Neuroimage 20:667–682. [DOI] [PubMed] [Google Scholar]
- Wang L, Miller JP, Gado MH, McKeel DW, Rothermich M, Miller MI, Morris JC, Csernansky JG (2006): Abnormalities of hippocampal surface structure in very mild dementia of the Alzheimer type. Neuroimage 30:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ (1993): Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging 14:287–293. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr., Parisi JE, Knopman DS, Boeve BF, Petersen RC, Ferman TJ, Dickson DW, Josephs KA (2007): Rates of cerebral atrophy differ in different degenerative pathologies. Brain 130( Part 4):1148–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Goh A, Qiu A (2011a): Approximations of the Diffeomorphic Metric and their Applications in Shape Learning. Germany:IPMI. [DOI] [PubMed] [Google Scholar]
- Yang X, Goh A, Qiu A (2011b): Locally linear diffeomorphic metric embedding (LLDME) for surface‐based anatomical shape modeling. Neuroimage 56:149–161. [DOI] [PubMed] [Google Scholar]
- Zhong J, Qiu A (2010): Multi‐manifold diffeomorphic metric mapping for aligning cortical hemispheric surfaces. NeuroImage 49:355–365. [DOI] [PubMed] [Google Scholar]
- Zhong J, Phua DY, Qiu A (2010): Quantitative evaluation of LDDMM, FreeSurfer, and CARET for cortical surface mapping. Neuroimage 52:131–141. [DOI] [PubMed] [Google Scholar]


