Abstract
The brain shows a high degree of activity at rest. The significance of this activity has come increasingly into focus. At present, however, the interaction between this activity and stimulus‐induced activity is not well defined. The interaction between a task‐negative (perigenual anterior cingulate cortex, pgACC) and task‐positive (supragenual anterior cingulate cortex, sgACC) region during a simple task was thus investigated using a combination of fMRI and MRS. Negative BOLD responses in the pgACC were found to show a unidirectional effective connectivity with task‐induced positive BOLD responses in the sgACC. This connectivity was shown to be related specifically with glutamate levels in the pgACC. These results demonstrate an interaction between deactivation from resting‐state and resting‐state glutamate levels in a task‐negative region (pgACC), and task‐induced activity in a task‐positive region (sgACC). This provides insight into the neuronal and biochemical mechanisms by means of which the resting state activity of the brain potentially impacts upon subsequent stimulus‐induced activity. Hum Brain Mapp, 2011. © 2011 Wiley Periodicals, Inc.
Keywords: ACC, default mode, resting state, DMN, PPI, empathy
INTRODUCTION
The significance of high resting‐state activity in the brain has been the focus of increasing interest [Carhart‐Harris and Friston,2010; Northoff et al.,2010; Raichle,2009]. Prominent in these investigations have been the set of regions, known as the default‐mode network (DMN), that show a consistent pattern of deactivation from this high resting‐state activity in response to the presentation of tasks or stimuli, as well as a high degree of functional connectivity during rest [Beckmann et al.,2005; Broyd et al.,2009; Buckner et al.,2008; Raichle and Snyder,2007; Raichle et al.,2001]. This pattern of deactivation has led to these regions being referred to as task‐negative regions, distinguishing them from task‐positive regions, in which positive BOLD responses are seen in response to specific task types.
Studies have demonstrated that resting‐state activity has an impact upon stimulus‐induced responses within both task‐negative regions [Northoff et al.,2007], and task‐positive regions [Arieli et al.,1996; Boly et al.,2007; Fox and Raichle,2007; Maandag et al.,2007; Muthukumaraswamy et al.,2009] in animals and humans. In addition, the existence of resting‐state functional interconnection between task‐positive and task‐negative regions is increasingly well supported [Hampson et al.,2010; Taylor et al.,2008]. These functional connectivity findings build on the described anti‐correlations between activity in task‐positive and task‐negative regions [Cole et al.,2010; Fox et al.,2005]. What remains unclear, however, is how resting‐state dependant activity in task‐negative regions impacts and interacts with stimulus‐induced activity in task‐positive regions. Similarly, the biochemical mechanisms underlying such interactions remain to be investigated.
The general aim of this study consisted in the investigation of the neuronal and biochemical mechanisms underlying the interaction between the deactivation from resting‐state in a task‐negative region and stimulus‐induced activity in a task‐positive region. The task‐negative region of interest, the perigenual anterior cingulate cortex (pgACC), forms part of the DMN and displays a characteristic task‐induced deactivation [Buckner et al.,2008; Grimm et al.,2009; Northoff et al.,2007]. In contrast, the task‐positive region of interest, the supragenual anterior cingulate cortex (sgACC), displays positive BOLD responses in response to a number of task types [e.g., Aron,2007; Carter and van Veen,2007; Preston and de Waal,2002]. These regions were selected due to their identification as typical task‐negative and task positive regions, respectively, that display close functional and anatomical connectivity [Ongur and Price,2000]. A simple empathy task was selected as a probe, with this task type having been consistently observed to elicit a negative BOLD response in the pgACC [Grimm et al.,2009] and a positive BOLD response in the sgACC [Prehn‐Kristensen et al.,2009; Singer and Lamm,2009].
The contrast between task‐induced positive and negative responses in the regions of interest reflects the well documented variation in anatomy and connectivity throughout the anterior cingulate cortex (ACC) [Beckmann et al.,2009; Gittins and Harrison,2004; Margulies et al.,2007; Palomero‐Gallagher et al.,2008a; Vogt et al.,1995]. Both regions studied here do, however, display a similarly rich glutamatergic innervation [Bozkurt et al.,2005; Palomero‐Gallagher et al.,2008b]. This neurotransmitter system, as the main excitatory neurotransmitter in the cortex, is thus likely to play a role in any interaction between the two regions. As such, glutamatergic function in both pgACC and sgACC was indirectly measured (as a combined value for glutamate and its precursor, glutamine) using magnetic resonance spectroscopy (MRS) to test for the biochemical mechanisms of possible rest‐stimulus interaction between both regions.
Based on the above described evidence, it was hypothesised that task‐induced negative BOLD responses in the pgACC would interact with task‐induced positive BOLD responses in the sgACC, and that this interaction would be mediated by glutamatergic communication. To ensure that the results gained were specific to both the regions and task‐type studied, rather than being examples of global effects, a control MRS region (the left anterior insula) and control tasks (a simple reward task and an emotion evaluation task) were employed.
METHODS
Subjects
Thirteen healthy subjects with no psychiatric, neurological or medical illnesses were studied (nine females, four males; average age 31.6 years, range 22–59 years; 11 right handed, 2 left handed). After a detailed explanation of the study design and potential risks, all subjects gave their written informed consent. fMRI and MRS sessions were carried out on subsequent days in a randomized order. Participants had taken no medication or caffeine prior to either scanning session. Each of the 13 subjects completed both fMRI and MRS. The study was approved by the institutional review board of the University of Magdeburg, Germany.
Experimental Paradigm
The fMRI scanning session was divided into six scanning runs. In three of these runs (2, 4, and 6) the empathy task was displayed, alternating with three runs (1, 3, and 5) in which a reward task was shown. Before entering the scanner each subject completed a number of trial presentations of the tasks in order to familiarize them fully with them. In the scanner, images were displayed using the Presentation software package (Neurobehavioural Systems, Albany, CA), and were projected onto a screen visible through a mirror mounted on the headcoil via an LCD projector.
Empathy Task
In the first component of each empathy trial, subjects were presented for 5 s with pictures from the Matsumoto and Ekman's Japanese and Caucasian Facial Expressions of Emotion [Matsumoto and Ekman,1988], with a balanced number of Caucasian and Japanese faces being displayed. Subjects were instructed to view the pictures and empathise with the person represented. Faces showed either a happy, angry, disgusted, or neutral expression, ordered randomly. All instances of these four emotion types, and both Caucasian and Japanese faces, were grouped in the analysis as a single emotional viewing condition (empathy). Also displayed were smoothed versions of these pictures to provide a control condition with no emotional content (view smoothed) that was matched for color composition and intensity, as well as for overall visual structural properties. Pictures without any emotional content were used as the comparison condition as, following a recent hypothesis, the mere perception of emotional faces, including neutral, should be sufficient for inducing an empathic response [Preston and de Waal,2002].
Following picture presentation, an evaluation condition (empathy evaluation) was displayed for 5 s. This consisted of a German text (“Ich konnte mich in die gezeigte Person hineinversetzen”) asking to what degree the subject could empathise with the person in the prior picture, along with a sliding visual analogue scale. Subjects moved the indicator to the left (not at all) or to the right (completely) to indicate how well or otherwise they could empathise using a two‐button feedback device.
An intertrial interval of 2 to 3 s occurred after each picture display and evaluation sequence was presented. These consisted of a dark cross on a light background, with participants being instructed to fixate on the cross during the ITI. In addition, five fixation‐cross periods of 6 to 8 s were located randomly in each run. These conditions were included in the design matrix as a separate condition (fixation), and used in the subsequent analysis as an operationalization of the resting state condition.
Each of the three runs contained 32 instances of emotional faces, 8 instances of smoothed faces, and 5 instances of the long fixation cross condition, giving a total number of instances of each of 96, 24, and 15, respectively (one subject aborted the final empathy task run before its completion—the data for the two empathy task runs that this subject did complete were included in the analysis).
A comparison between the results obtained using the above described paradigm and a meta‐analysis of empathy studies was carried out to ensure the paradigm's validity. The meta‐analysis was carried out using the MKDA software package [Kober et al.,2008], and included coordinates from a total of 42 studies of empathy in healthy adults (see Supporting Information for further details of the meta‐analysis method.) A close match between the regions identified in the current study from the contrast [view empathy > view control] and those identified in the meta‐analysis was observed (Supporting Information Fig. 1), providing some validation for the paradigm.
Reward Task
The reward task was a modified version of the well‐established monetary incentive delay task [Knutson et al.,2001], requiring that the subject press a button with the index finger of their right hand within a certain time of a target image (a black square in the centre of the screen) being displayed. The reward task was used only as a control task in this analysis, allowing the specificity of the particular findings discussed here for empathy to be tested. Full details of the reward task can be found in the Supporting Information.
fMRI Data Acquisition and Analysis
The fMRI component of the study was carried out on a 1.5T MR scanner (General Electric Sigma Horizon) using the standard circular polarized headcoil. Using a midsagittal scout image, a stack of 23 slices was aligned parallel to the bicomissural plane. During each functional run 320 whole brain volumes were acquired (gradient echo EPI, TR = 2 s; TE = 35 ms; flip angle = 80°; FoV = 200 × 200 mm; slice thickness = 5 mm, interslice gap = 1 mm, spatial resolution = 3.125 × 3.125 × 5 mm). Image processing and statistical analyses were carried out according to the general linear model approach [Friston et al.,1995] using the SPM8 software package (Wellcome Department of Imaging Neuroscience, London, UK) running on MATLAB 2009b (The Mathwork Inc., Natick, MA). The first five volumes were discarded due to saturation effects. All functional images were slice‐time corrected with reference to the first slice acquired, corrected for motion artefacts by realignment to the first volume (with the movement parameters obtained in this stage being included in each first‐level analysis as separate regressors), and spatially normalized to a standard T1‐weighted SPM template [Ashburner and Friston,1999]. The images were resampled to 2 × 2 × 2 mm and smoothed with an isotropic 8 mm full‐width half maximum Gaussian kernel. The time‐series fMRI data were filtered using a high pass filter and cut‐off of 128 s. A statistical model for each subject was computed by applying a canonical hemodynamic response function. Regionally specific condition effects were tested by employing linear contrasts for each subject and each condition of interest. The resulting contrast images were submitted to a second‐level random‐effects analysis by applying a one‐sample t‐test to the images acquired for all subjects in each condition. Resulting clusters of activation were only considered if they had a P value < 0.05 following correction for multiple comparison errors (FWE‐correction).
Connectivity Analysis
Effective connectivity between the pgACC and sgACC during the empathy task was tested using psychophysiological interaction (PPI) analysis [Friston et al.,1997]. Such analysis allows the identification of those regions that display an increased effective coupling with a specific seed region in response to a particular psychological factor. In this case, the NBR signal change from resting state within the pgACC in response to the viewing of emotional pictures was taken as the physiological variable of interest. By performing multiple correlation and regression analyses, a PPI analysis then searches for those signal changes in other regions that show a greater correlation with those in the seed region during the condition of interest, as compared with the contrasting psychological variable. This allows, in the case of this study, for the connectivity of a region which displays a negative BOLD response (NBR) during a task with those areas that display a positive BOLD response (PBR) to be determined. As the level of resting state activation has been shown to influence the NBR produced by stimuli [Northoff et al.,2010], this approach also allows an indirect characterization of the interaction between the resting state in the pgACC and stimulus‐induced signal changes in other regions.
Seed volumes of interest (VOI) corresponding to the pgACC MRS voxel were used to extract the eingenvariate for each subject—the physiological variable—within this region from the relevant source contrasts ([fixation > empathy]; [fixation > evaluate empathy]; [fixation > reward]) using the SPM8 “eingenvariate” function. Having defined and extracted data from these regions, three separate PPI analyses were carried out for each subject. The first of these investigated the change in functional coupling with the pgACC seed region (using the signal changes from [fixation > view empathy]) between the viewing of emotional pictures and the viewing of the control smoothed pictures. Statistical maps from the individual PPI analyses were combined into a group analysis through a one sample t‐test in SPM. This process thus identified those regions that display an effective connectivity with the pgACC during empathy. Finally, the same procedure was followed using the sgACC MRS voxel as the seed region.
In order to determine the specificity of any connectivity, two further PPI analyses were then carried out. The specificity of the connectivity between the pgACC and sgACC for empathy, as opposed to the evaluation of empathy, was tested by carrying out a PPI using the pgACC signal change from the [fixation > evaluate empathy] contrast as physiological variable and the evaluation of emotional pictures and evaluation of smoothed pictures as psychological variables. Finally, to determine the specificity of the connectivity for empathy, as opposed to other psychological functions, a PPI using the signal changes in the pgACC from the contrast [fixation > reward] as the physiological variable and the anticipation of reward and the anticipation of no outcome as psychological variables.
MRS Data Acquisition and Analysis
Single voxel 1H MR spectra were acquired during the resting state using a 3T whole body MRI system (Siemens Magnetom Trio) using an eight channel head coil (PRESS, TR = 2 s; TE = 80 ms). Voxels were prescribed on a high resolution T1‐weighted 3D data set (MPRAGE, TR = 2 s; TI = 1.1 s; TE = 4.8 ms; flip angle = 7°; FoV = 256 × 256 × 192 mm; spatial resolution = 1 × 1 × 1 mm). One voxel of 20 × 10 × 20 mm (see Supporting Information Fig. 2 for positioning of all MRS voxels) was placed in the bilateral pgACC, while a second was placed in the bilateral sgACC (20 × 10 × 20 mm). A third voxel was placed in the left anterior insula (15 × 10 × 20 mm) in order to provide a control region to establish if any potential relationship between ACC activity and global Glx levels existed. Voxels were located in relation to relevant anatomical landmarks, as identified by the researcher on a high resolution T1 anatomical image. The locations of the MRS voxels can be seen in Supporting Information Figure 2.
Spectra were eddy current corrected and analyzed using LC Model version 6.1.0 (available at: http://www.s-provencher.com/pages/lcmodel.shtml). Included in the model build were creatine, N‐acetyle aspartate, choline, glutamate plus glutamine, and (myo‐)inositol. Spectra with full‐width‐half‐maximum line widths larger than 8 Hz and quantification results with a Cramér‐Rao lower bound higher than 20% were excluded from further analysis. The measurements for one subject in each of the pgACC, sgACC and left insula were discarded for these reasons (n = 12). Metabolite concentrations are given as their ratio to the measured creatine concentration. As a slight interdependence, due to a spectral overlap in their resonances, exists between quantification results for glutamate and glutamine these were quantified together. This combined concentration ratio of glutamate/glutamine to creatine is referred to henceforth as Glx. Correlation analyses between subject age and Glx levels in each region were carried out (Spearman's rho, two tailed)—no relationship between age and Glx concentration was found in any of the MRS regions.
MRS measurements were carried out during the resting state for both methodological and conceptual reasons. The acquisition of MRS data with a suitably high resolution requires a long acquisition time—approximately 12 min in this case. Such protracted timescales, with large numbers of encoding steps, make a functional MRS approach [Mangia et al.,2007] technically extremely difficult in the context of the task type used, if not impossible. Most importantly, our hypothesis was aimed at the impact of resting state related signal changes in the pgACC on stimulus‐induced signal changes in other regions. By searching for correlations of resting state levels of glutamate in the pgACC with stimulus‐induced signal changes in the insula we were able to experimentally investigate this hypothesis.
It should be noted that the MRS technique does not allow one to distinguish between intra‐ and extracellular metabolite pools. Nor does it allow the differentiation of neuronal and non‐neuronal metabolite concentrations. As such, it is not possible to directly assess the level of transmitter within the synaptic cleft during stimulation using MRS. However, in line with previous studies [Northoff et al.,2007], our correlation results suggest that the level of Glx present at rest positively predicts the level of Glx that will be secreted upon stimulation, thus allowing an indirect discrimination between intra‐ and extracellular pools of Glx during stimulation.
Combination of fMRI and MRS Data
Individuals' measures of Glx concentration in the pgACC were included in a second‐level SPM‐based regression analysis with the statistical maps of each subject for each of the contrasts of interest. These regression analyses thus identified those brain regions in which the signal changes during the particular condition of interest are related to the level of Glx in the pgACC MRS region. Only those clusters significant after correcting for multiple comparison errors (FWE‐correction) were considered.
RESULTS
Results for each stage of the analysis are given below. Peak voxel coordinates in MNI‐space are given in parenthesis (x, y, z), along with the related cluster's P value. Unless otherwise stated, all P values given are FWE‐corrected for multiple comparison errors.
Positive Signal Changes
The empathy task evoked positive signal changes (i.e., a task‐induced activation) in the sgACC (−8 18 42, P FWE < 0.001). This cluster of activation includes the region defined as the sgACC MRS voxel (Fig. 1 and Supporting Information Table Ia). This region overlaps with the sgACC region of activity observed in the task‐validation meta‐analysis (Supporting Information Fig. 1). No activation in the sgACC was observed during the empathy evaluation task or during the reward task, demonstrating that the activation in this region is task‐specific.
Figure 1.
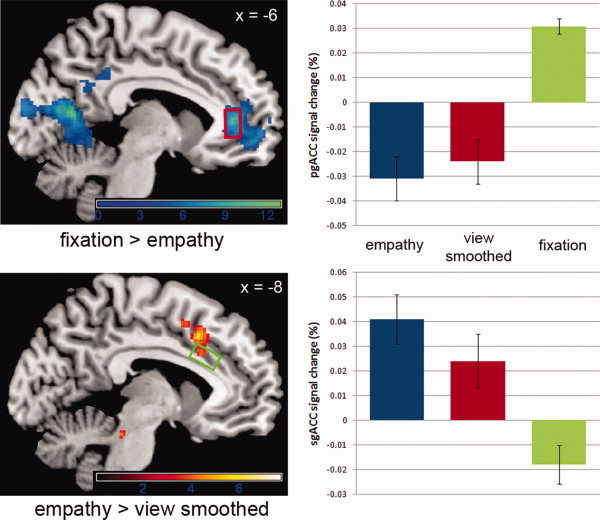
Areas of deactivation from rest in response to the empathy task are shown (contrast [fixation > empathy]), along with activations in response to the empathy task (contrast [empathy > smoothed]). Mean percent signal changes are shown for the viewing of emotional pictures, the viewing of smoothed pictures, and the fixation period in the pgACC (red box) and sgACC (green box) MRS voxels. Mean percentage signal changes were calculated using the Marsbar toolbox (available at: http://marsbar.sourceforge.net/). Error bars represent SEM. Images are shown with a threshold of P = 0.005 (unc.) for the purpose of illustration. See also Supporting Information Table Ia and Ib. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Activation in the left anterior insula was observed (−34 16 −2, P FWE = 0.005), in line with previous studies of empathy [Singer et al.,2009]. No significant activation was seen in the right anterior insula. The empathy task was also seen to induce activity in, amongst other regions, the bilateral amygdala, extending to the ventral striatum (−18 −18 −20, P FWE = 0.014; 22 −6 −16, P FWE = 0.001), again consistent with previous studies of empathy [Ruby and Decety,2004] (see Supporting Information Table Ia for full activation details).
Negative Signal Changes
As described above, the pgACC has been consistently observed to display a stimulus‐induced negative BOLD response, and so the presence of empathy‐task induced deactivation in this region was investigated. The contrast [fixation > empathy] does reveal such deactivation from rest in the pgACC as a whole, with the peak voxel of this cluster (−6 40 8, P FWE < 0.001) lying within the specific portion of this region defined as the pgACC MRS voxel (see Fig. 1 and Supporting Information Table Ib). A similar region of deactivation was observed in the pgACC in response to the empathy evaluation task, as well as the reward task. This demonstrates that the deactivation in this region is task nonspecific.
Other regions of what has been described as the default mode network [Raichle et al.,2001], which includes the pgACC, were also seen to display a task‐induced deactivation during the empathy task, specifically a cluster containing the posterior cingulate cortex (PCC) and precuneus (−10 −62 12, P FWE < 0.001) (Fig. 1 and Supporting Information Table Ib).
Interregional Connectivity
Effective connectivity between the pgACC and sgACC was tested using psychophysiological interaction (PPI) analyses. Taking the pgACC MRS voxel as the seed region, effective connectivity from this region with the sgACC was observed (0 22 32, P FWE = 0.004; Supporting Information Table IIa). The reciprocal effective connectivity, investigated using the sgACC MRS voxel as seed region, between the sgACC and pgACC was not observed (Supporting Information Table IIb). Hence, only when taking the pgACC as seed was a relationship with the signal changes in the sgACC observed, with this not being the case in the reverse direction: this suggests a unidirectional connectivity from the pgACC to the sgACC during empathy.
The pgACC also displayed effective connectivity with, amongst other regions, the right hippocampus, extending to the amygdala (22 −30 −6, P < 0.001), the bilateral posterior cingulate cortex (−12 −42 32, P FWE < 0.001), the left thalamus (−12 −14 2, P FWE = 0.001), and the right thalamus extending to the ventral striatum (10 −8 6, P FWE < 0.001) (see Supporting Information Table IIa for full details).
Effective connectivity between the sgACC and, amongst other regions, the bilateral posterior cingulate cortex (−6 0 22, P FWE < 0.001) was observed (see Supporting Information Table IIb for full details). This cluster runs posterior from the sgACC, via the midcingulate, following approximately the line of the corpus callosum.
The specificity of the effective connectivity between the pgACC and sgACC for empathy was tested by carrying out two additional PPI analyses using the pgACC MRS voxel as seed region. Neither the PPI analysis using emotional evaluation as the psychological factor, nor that using reward, showed any effective connectivity between the pgACC and the sgACC.
Resting State Glx and Regional Signal Changes
SPM‐based regression analyses of the BOLD response to the empathy task and the Glx concentration in both the pgACC and sgACC were carried out. These analyses identified in which regions of the brain the neural response to the task is related to the level of Glx in the particular MRS voxel.
A relationship between the concentration of Glx in the pgACC and the empathy‐task induced BOLD response was observed in the sgACC (−12 12 36, P FWE < 0.001), with this cluster overlapping with the sgACC MRS voxel (Fig. 2). In addition, there was a relationship between the concentration of Glx in the pgACC and the neural response in the left precuneus (−26 −66 48, P FWE = 0.001), as well as with the response in the bilateral amygdala (−26 −2 −10, P FWE < 0.001; 30 6 −14, P FWE < 0.001) (see Supporting Information Table IIIa for full details).
Figure 2.
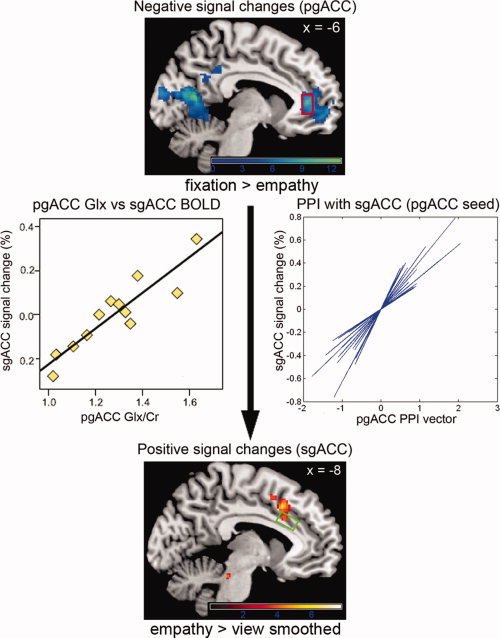
Unidirectional connectivity between negative signal changes in the pgACC MRS region (red box) and positive signal changes in the sgACC MRS region (green box) during the empathy task was demonstrated using PPI analyses. A relationship between the BOLD response during the same task in the sgACC and the level of glutamate in the pgACC was demonstrated using regression analyses. A plot of the regression between the mean sgACC BOLD response and pgACC glutamate at the peak regression voxel within the sgACC is shown. A combined plot of the PPI regression results for each individual subject obtained from their first level PPI analysis at the peak voxel within the sgACC (from the group level analysis) is also shown. Regression plots were produced by obtaining the fitted response at the peak voxel via the “fitted response” option of the “plot” function in SPM, and plotting these against the relevant explanatory variable in MATLAB (The Mathwork Inc., Natick, MA). Activation images are shown with a threshold P = 0.005 (unc.) for the purpose of illustration. See also Supporting Information Tables IIa, IIb and III. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
No relationship was observed between the concentration of Glx in the sgACC and task‐induced BOLD responses in the rest of the brain when results were corrected for multiple comparison errors. An uncorrected correlation between the sgACC Glx level and the BOLD response in the right superior frontal gyrus (24 44 26, P = 0.006 unc.), right fusiform gyrus (44 −60 −16, P = 0.01 unc.) and left mid‐insula (−40 8 −8, P = 0.032 unc.) was observed, however (Supporting Information Table IIIb).
To test the specificity of the relationship between the concentration of Glx in the pgACC and neural responses in the sgACC during empathy, regression analyses of pgACC Glx with the BOLD response during both the empathy evaluation task and the reward task were carried out. Neither regression showed any relationship between pgACC Glx and the neural response in the sgACC during the respective conditions. In addition, regression analyses of the neural response during the empathy, empathy evaluation and reward tasks with the concentration of Glx in the left anterior insula were carried out. No areas within the cingulate cortex were identified by these analyses.
DISCUSSION
The involvement of glutamate in the interaction between resting state related activity in a task‐negative region and stimulus‐induced activity in a task‐positive region is reported here. This provides insight into the neuronal and biochemical mechanisms by means of which the resting state activity of the brain potentially impacts upon subsequent stimulus‐induced activity.
The first main finding of the study concerns the relationship between signal changes in task‐negative and task‐positive regions. Positive BOLD responses (activations) in the task‐positive sgACC were reported, along with a negative BOLD response (deactivation) when compared with rest in the task‐negative pgACC. These negative BOLD responses in the pgACC were seen to display an effective connectivity with the positive BOLD responses in the sgACC. The effective connectivity between the pgACC and sgACC was shown to be unidirectional and specific to the empathy task, as compared with control empathy‐evaluation and reward tasks.
The second main finding of the study concerns apparent glutamatergic modulation of rest‐stimulus interaction between the two target regions. Glutamate concentrations in the two regions of interest within the ACC, along with a control region in the left anterior insula, were measured using MRS (as a combined measure of glutamate and glutamine). It was found that signal changes during the empathy task in the sgACC were related to the level of glutamate in the pgACC. No reciprocal relationship between the signal changes in the pgACC and the level of glutamate in the sgACC was found. Similarly, no relationship was found between signal changes in either the pgACC or sgACC and the level of glutamate in the left anterior insula, suggesting that the relationship observed with the pgACC was not due to a global effect of glutamate concentrations in the brain.
The observed interaction between the pgACC and sgACC during empathy can be interpreted as an instance of task‐induced deactivation in one region interacting with a separate area of task‐induced activation. In agreement with previous findings [e.g., Grimm et al.,2009; Northoff et al.,2007; Raichle et al.,2001; Simpson et al.,2001], the task‐induced deactivation in the pgACC was seen not to be task‐specific, with a similar extent of NBR seen in response to empathy, empathy evaluation, and reward. In contrast, the task‐induced activation in the sgACC was demonstrated to be specific to empathy, with no activation in this region in response to the two control tasks.
A relationship between task‐negative and task‐positive regions has been described in the resting state [Fox et al.,2005; Hampson et al.,2010], whilst resting connectivity within the task‐negative DMN has been shown to affect task induced BOLD responses in task‐positive regions [Mennes et al.,2010]. The current study thus extends these findings by demonstrating that negative BOLD responses in the DMN are connected to task‐specific BOLD responses in other brain regions. More specifically, a relationship between the positive and negative signal changes in the two regions of interest was demonstrated through the observed effective connectivity. This suggests that task‐induced activation in the sgACC is related to the degree of task‐induced deactivation in the pgACC. The uni‐directionality of the relationship further supports the hypothesis that the level of resting state activity in the pgACC impacts upon, and potentially modulates, stimulus‐induced activity in the sgACC. Such a situation would lend support to the concept of rest‐stimulus interaction, where resting state and stimulus‐induced activity are functionally interrelated [Northoff et al.,2010].
Both subregions of the ACC studied have rich glutamatergic innervation [Bozkurt et al.,2005; Palomero‐Gallagher et al.,2008b], and so this neurotransmitter system, the main excitatory neurotransmitter system and important in corticocortical communication, was deemed likely to play a role in communication between the two regions. This was confirmed in this study through the combination of effective connectivity analyses and the measuring of glutamate using MRS. That the relationship between glutamate concentration and interregional signal changes was found only in the case of pgACC glutamate levels further supports the unidirectional nature of the communication between the pgACC and sgACC described here.
Previous studies have demonstrated the involvement of GABA in task‐induced deactivation in both task‐positive and task‐negative regions [Muthukumaraswamy et al.,2009; Northoff et al.,2007]. These findings, however, leave open the question as to how interactions between task‐negative and task‐positive regions are mediated. The implication of glutamate in the interaction between the task‐negative pgACC and task‐positive sgACC in the current study thus provides initial evidence for a role for this neurotransmitter system in rest‐stimulus interaction.
One may also speculate as to the relationship between glutamate, an excitatory neurotransmitter, and deactivation from rest in the pgACC. The high level of resting state in this region, and by extension other task‐negative regions, may be an outcome of high ongoing glutamatergic activity in these regions. A reduction in this glutamatergic activity, or a counteraction of it by an inhibitory neurotransmitter such as GABA, may then lead to the observed task‐induced deactivations. The level of tonic glutamatergic activity would thus determine the level of resting state activity, and from this, would indirectly determine the potential for deactivation during a given task. This hypothesis remains speculative at present and requires further investigation.
In addition to their significance to our understanding of the interaction between task‐negative and task‐positive regions, this study's findings may be of some interest in the context of empathy. Both of the main findings of the study were specific to the empathy task employed, as distinct from the control empathy evaluation and reward tasks. This is in accordance with the reported central role of the sgACC in empathy [Prehn‐Kristensen et al.,2009; Singer and Lamm,2009]. The current findings extend these previous observations by showing that task‐induced activity in the sgACC during empathy interacts with task‐induced deactivation in the pgACC, providing evidence that such deactivation in the pgACC may play a similarly important role in empathy processing. Finally, it was demonstrated that the interaction between the pgACC and sgACC during empathy was related to resting‐state glutamate levels in the pgACC. This lends further support to the observed glutamatergic modulation of emotional and neural function related to the pgACC and sgACC by glutamatergic substances such as ketamine [Etkin et al.,2010; Salvadore et al.,2009,2010]. These observations in the context of empathy must, of course, be treated as speculative and need to be investigated further.
The observation of glutamatergic modulation of emotional processing as in empathy may also be relevant in psychiatric disorders. Changes in resting state activity and connectivity have been linked to a number of psychiatric disorders, including schizophrenia and major depressive disorder [Broyd et al.,2009; Buckner et al.,2008; Garrity et al.,2007; Greicius et al.,2007]. Particularly in depression, abnormally elevated resting state activity and glutamatergic abnormalities have been observed in the pgACC [Alcaro et al.,2010; Buckner et al.,2008; Walter et al.,2009]. This study's findings thus raises questions as to how this abnormally elevated resting state activity in the pgACC relates to glutamatergic function, as well as to the modulation of stimulus‐induced activity in the sgACC, a region that has been observed to show decreased activity during emotional and cognitive tasks in depression [Anand et al.,2005; Hooley et al.,2009]. Hence, it may be of interest in the future to investigate depressed patients using a similar design to that reported here.
Some limitations of the present study should be considered. Resting state activity in the pgACC is inferred indirectly via the occurrence of a negative BOLD response elicited by stimuli, while glutamate levels were measured directly in the resting state. Though previous studies pursued a similar approach [Northoff et al.,2007], future studies may wish to take a more direct approach to measuring resting state activity itself, relating this then to glutamate levels. Measurements of changes in human glutamate concentration during stimulus‐induced activity are also needed to further confirm the assumption that the direct and indirect measurements can safely be compared. In a similar vein, it should be noted that glutamate was measured here as a single value along with its precursor glutamine, as represented by the designation Glx. Replication using a more sensitive MRS analysis that allows for a distinction between glutamate and glutamine would be desirable. It should also be mentioned that the focus of the analysis was on glutamate, and as such the role of GABA was not investigated. The role of GABA in the context of rest‐stimulus interactions thus needs to be investigated in further detail in the future. The fixation cross period taken as a representative resting state condition in the study was rather short, and so can only be taken to be an approximation of a true resting state; future studies may thus wish to replicate these findings with longer resting state periods. Finally, the current sample size is not large, and so results should be treated as preliminary. Replication with a larger sample size is required.
In summary, the present study demonstrates an interaction between resting state dependant responses in the task‐negative pgACC and task‐induced activations in the task‐positive sgACC. Connectivity between the regions was further shown to be related to glutamate concentrations in the pgACC specifically. These results provide preliminary evidence that glutamate plays a central role in rest‐stimulus interaction in the human brain.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Acknowledgements
The authors thank M. de Greck for his invaluable help with the study design and analysis, D. Hayes for his useful comments, and E. Stockum and C. Ulrich for their assistance with the recruitment of subjects and conducting of scanning sessions. Thanks also to the staff at the Department of Neurology for their skilful assistance.
REFERENCES
- Alcaro A, Panksepp J, Witczak J, Hayes DJ, Northoff G ( 2010): Is subcortical‐cortical midline activity in depression mediated by glutamate and GABA? A cross‐species translational approach. Neurosci Biobehav Rev 34: 592–605. [DOI] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ ( 2005): Activity and connectivity of brain mood regulating circuit in depression: A functional magnetic resonance study. Biol Psychiatry 57: 1079–1088. [DOI] [PubMed] [Google Scholar]
- Arieli A, Sterkin A, Grinvald A, Aertsen A ( 1996): Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science 273: 1868–1871. [DOI] [PubMed] [Google Scholar]
- Aron AR ( 2007): The neural basis of inhibition in cognitive control. Neuroscientist 13: 214–228. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ ( 1999): Nonlinear spatial normalization using basis functions. Hum Brain Mapp 7: 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM ( 2005): Investigations into resting‐state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360: 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann M, Johansen‐Berg H, Rushworth MF ( 2009): Connectivity‐based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci 29: 1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S ( 2007): Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA 104: 12187–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt A, Zilles K, Schleicher A, Kamper L, Arigita ES, Uylings HB, Kotter R ( 2005): Distributions of transmitter receptors in the macaque cingulate cortex. Neuroimage 25: 219–229. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga‐Barke EJ ( 2009): Default‐mode brain dysfunction in mental disorders: A systematic review. Neurosci Biobehav Rev 33: 279–296. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL ( 2008): The brain's default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Carhart‐Harris RL, Friston KJ ( 2010): The default‐mode, ego‐functions and free‐energy: A neurobiological account of Freudian ideas. Brain 133: 1265–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, van Veen V ( 2007): Anterior cingulate cortex and conflict detection: An update of theory and data. Cogn Affect Behav Neurosci 7: 367–379. [DOI] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF ( 2010): Advances and pitfalls in the analysis and interpretation of resting‐state FMRI data. Front Syst Neurosci 4: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF ( 2010): Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry 167: 489–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME ( 2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700–711. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME ( 2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ ( 1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6: 218–229. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith C, Frackowiak RSJ ( 1995): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD ( 2007): Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry 164: 450–457. [DOI] [PubMed] [Google Scholar]
- Gittins R, Harrison PJ ( 2004): A quantitative morphometric study of the human anterior cingulate cortex. Brain Res 1013: 212–222. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover G, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. 2007. Resting state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, Ernst J, Hell D, Boeker H, Northoff G ( 2009): Altered negative BOLD responses in the default‐mode network during emotion processing in depressed subjects. Neuropsychopharmacology 34: 932–843. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen N, Roth JK, Gore JC, Constable RT ( 2010): Functional connectivity between task‐positive and task‐negative brain areas and its relation to working memory performance. Magn Reson Imaging 28: 1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley JM, Gruber SA, Parker HA, Guillaumot J, Rogowska J, Yurgelun‐Todd DA ( 2009): Cortico‐limbic response to personally challenging emotional stimuli after complete recovery from depression. Psychiatry Res 172: 83–91. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D ( 2001): Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21: RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss‐Moreau E, Lindquist K, Wager TD ( 2008): Functional grouping and cortical‐subcortical interactions in emotion: A meta‐analysis of neuroimaging studies. Neuroimage 42: 998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maandag NJ, Coman D, Sanganahalli BG, Herman P, Smith AJ, Blumenfeld H, Shulman RG, Hyder F ( 2007): Energetics of neuronal signaling and fMRI activity. Proc Natl Acad Sci USA 104: 20546–20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangia S, Tkac I, Gruetter R, Van de Moortele PF, Maraviglia B, Ugurbil K ( 2007): Sustained neuronal activation raises oxidative metabolism to a new steady‐state level: Evidence from 1H NMR spectroscopy in the human visual cortex. J Cereb Blood Flow Metab 27: 1055–1063. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP ( 2007): Mapping the functional connectivity of anterior cingulate cortex. Neuroimage 37: 579–588. [DOI] [PubMed] [Google Scholar]
- Matsumoto D, Ekman P ( 1988): Japanese and Caucasian Facial Expressions of Emotion (JACFEE). San Francisco: San Francisco State University. [Google Scholar]
- Mennes M, Kelly C, Zuo XN, Di Martino A, Biswal B, Castellanos FX, Milham MP ( 2010): Inter‐individual differences in resting state functional connectivity predict task‐induced BOLD activity. Neuroimage 50: 1690–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD ( 2009): Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci USA 106: 8356–8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Qin P, Nakao T ( 2010): Rest‐stimulus interaction in the brain: A review. Trends Neurosci 33: 277–284. [DOI] [PubMed] [Google Scholar]
- Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, Boeker H, Grimm S, Boesiger P ( 2007): GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci 10: 1515–1517. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL ( 2000): The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10: 206–219. [DOI] [PubMed] [Google Scholar]
- Palomero‐Gallagher N, Mohlberg H, Zilles K, Vogt B ( 2008a) Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol 508: 906–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero‐Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K ( 2008b) Receptor architecture of human cingulate cortex: Evaluation of the four‐region neurobiological model. Hum Brain Mapp 30: 2336–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn‐Kristensen A, Wiesner C, Bergmann TO, Wolff S, Jansen O, Mehdorn HM, Ferstl R, Pause BM ( 2009): Induction of empathy by the smell of anxiety. PLoS One 4: e5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, de Waal FB ( 2002): Empathy: Its ultimate and proximate bases. Behav Brain Sci 25: 1–20; discussion 20–71. [DOI] [PubMed] [Google Scholar]
- Raichle ME ( 2009): A paradigm shift in functional brain imaging. J Neurosci 29: 12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ ( 2007): A default mode of brain function: A brief history of an evolving idea. Neuroimage 37: 1083–1090. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J ( 2004): How would you feel versus how do you think she would feel? A neuroimaging study of perspective‐taking with social emotions. J Cogn Neurosci 16: 988–999. [DOI] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Colon‐Rosario V, Coppola R, Grillon C, Zarate CA Jr, Manji HK ( 2009): Increased anterior cingulate cortical activity in response to fearful faces: A neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry 65: 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon‐Rosario V, Carver F, Holroyd T, Diazgranados N, Machado‐Vieira R, Grillon C, Drevets WC, Zarate CA Jr ( 2010): Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology 35: 1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JR Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME ( 2001): Emotion‐induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc Natl Acad Sci USA 98: 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K ( 2009): A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci 13: 334–340. [DOI] [PubMed] [Google Scholar]
- Singer T, Lamm C ( 2009): The social neuroscience of empathy. Ann NY Acad Sci 1156: 81–96. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD ( 2008): Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp 30: 2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR ( 1995): Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol 359: 490–506. [DOI] [PubMed] [Google Scholar]
- Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U, Schnepf B, Boeker H, Boesiger P, Northoff G ( 2009): The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry 66: 478–486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


