Abstract
Background: Abnormalities in inhibitory control and underlying fronto‐striatal networks is common to both attention deficit hyperactivity disorder (ADHD) and obsessive‐compulsive‐disorder (OCD). The aim of this study was to investigate disorder‐specific abnormalities in neural networks mediating interference inhibition and selective attention. Method: Event‐related functional magnetic resonance imaging (fMRI) was used to compare brain activation of boys with ADHD (18), with OCD (10), and healthy boys during (20) during a Simon task that measures interference inhibition and controls for and therefore comeasures attention allocation. Results: During interference inhibition, both patient groups shared mesial frontal dysfunction compared to controls. Disorder‐specific dysfunctions were observed in OCD patients in dorsolateral prefrontal cortex during the oddball condition and in ADHD patients in inferior parietal lobe during interference inhibition and in caudate and posterior cingulate during the simpler oddball condition. The decreased activation in caudate and cingulate in ADHD was furthermore negatively correlated with ADHD symptoms and positively with OCD behavioral traits. Conclusions: The study shows that ADHD and OCD patients have shared but also disorder‐specific brain dysfunctions during interference inhibition and attention allocation. Both disorders shared dysfunction in mesial frontal cortex. Disorder‐specific dysfunctions, however, were observed in dorsolateral prefrontal cortex in OCD patients and in caudate, cingulate, and parietal brain regions in ADHD patients. The disorder‐specific dissociation of striato‐cingulate activation that was increased in OCD compared to ADHD patients, was furthermore inversely related to the symptomatology of the two disorders, and may potentially reflect differential dopamine modulation of striatal brain regions. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: obsessive‐compulsive disorder, attention deficit hyperactivity, disorder, fMRI, inhibition, attention, oddball
INTRODUCTION
Attention deficit hyperactivity disorder (ADHD) is characterized by symptoms of inattention, impulsiveness, and hyperactivity [DSM IV, American Psychiatric Association, 1994]. ADHD is associated with neuropsychological deficits in motor response inhibition and interference inhibition, the ability to protect a response from interference from a predominant, distracting information [Rubia et al., 2001b, 2007b; Sergeant et al., 2002]. Functional magnetic resonance imaging (fMRI) studies of interference inhibition in children with ADHD have been inconsistent, finding either reduced frontal‐striatal [Konrad et al., 2006], or temporal parietal activation [Rubia et al., 2009a; Vaidya et al., 2005], or no dysfunction [Booth et al., 2005; Smith et al., 2006]. Electrophysiological (EEG) and fMRI studies have further observed abnormalities in simpler selective attention tasks such as perceptual attention allocation to rare, “oddball” stimuli in frontal, temporal, and parietal areas [Barry, 2003]; [Rubia et al., 2007b]; [Rubia et al., 2009a]; [Stevens et al., 2007]; [Tamm et al., 2006].
OCD is characterized by poor inhibition over intrusive, unwanted obsessive thoughts and compulsions (DSM IV), [American Psychiatric Association, 1994]. Patients with OCD also have deficits in tasks of inhibitory control, including motor response and interference inhibition [Chamberlain et al., 2006; Menzies et al., 2008; Penades et al., 2007]. Adults and children with OCD also have structural [Huyser et al., 2009; Menzies et al., 2007, 2008] and functional abnormalities in inhibitory fronto‐striatal networks and in temporo‐parietal attention networks [Menzies et al., 2008; Woolley et al., 2008].
Therefore it has been argued that the underlying aetio‐pathophysiology for both disorders is an abnormality in fronto‐striatal inhibitory neural networks [Huyser et al., 2009; Menzies et al., 2008; Rubia et al., 1999, 2005b, 2008]. A shared pathophysiology, however, is at odds with a relatively clear symptomatic distinction of the two disorders, with compulsivity and impulsivity often considered as situated at opposite ends of a behavioral spectrum [Carlsson, 2000]. Only about 8% of ADHD children meet OCD criteria, while up to 30% of OCD children meet criteria for ADHD [Geller et al., 1996, 2000]. It remains to be clarified whether there is overlap or differences in the inhibitory networks that are affected in these two disorders. Such a difference at the neurofunctional level would provide disorder‐specific biomarkers that could assist with differential diagnosis and treatment. No functional imaging study, to our knowledge, however, has compared these two disorders during inhibition or any other functions.
The aim of this study was therefore to use fMRI to investigate the differences and commonalities in the neural substrates of interference inhibition and simple perceptual attention allocation in pediatric noncomorbid OCD compared to pediatric noncomorbid ADHD. For this purpose we used a Simon task—measuring interference inhibition and selective attention—that controls for the attentional oddball effect of low frequency appearance of incongruent trials, and thus also comeasures simple perceptual selective attention to compare healthy children with those with clinical ADHD and OCD. Based on previous evidence from fMRI studies in adolescents with ADHD, we hypothesized that ADHD children compared to controls would show reduced activation in lateral fronto‐striato‐cingulate and superior temporal and inferior parietal brain regions during interference inhibition [Konrad et al., 2006; Rubia et al., 2009a; Vaidya et al., 2005] and in inferior frontal and superior temporal regions during attention allocation [Rubia et al., 2007b, 2009a, b; Stevens et al., 2007; Tamm et al., 2006]. On the basis of our previous fMRI study in pediatric OCD, we hypothesized that adolescents with OCD would be characterized by different abnormalities compared to controls and ADHD patients in the activation of dorsolateral and orbital frontal regions during interference inhibition and in different parieto‐temporal brain regions during attention allocation [Woolley et al., 2008].
METHODS
Subjects
Patients were right‐handed, male adolescents between 9 and 16 years, 10 with a clinical diagnosis of OCD (mean age = 13 years 9 months, SD = 1 year) and 18 with a clinical diagnosis of ADHD (mean age = 14 years 3 months, SD = 2 years), recruited from clinics, parent support groups, and advertisement. Clinical diagnosis of combined hyperactive‐impulsive subtype of ADHD without the diagnosis of OCD and of OCD without clinical ADHD symptoms (DSM IV) [American Psychiatric Association, 1994] was established through interviews with a child psychiatrist using the Maudsley diagnostic interview [MDI; Goldberg and Murray, 2002]. Exclusion criteria for both patient groups were drug‐ and substance‐abuse and a history of a general or specific learning disability or comorbidity with any other major psychiatric disorder as assessed using the child behavior checklist [Achenbach and Edelbrook, 1983] and the MDI. The exception was comorbidity with CD for the ADHD group, present in one patient.
All patients with ADHD scored above threshold (over six) on the inattention/hyperactivity scale of the parent strength and difficulty questionnaire (SDQ) [Goodman, 1997] and above the 5th percentile on the Raven's standard progressive matrices intelligence questionnaire (IQ), [i.e., converted IQ estimate over 75; Raven, 1960]. All patients were medication‐naïve.
Patients with OCD were treated and in partial remission. This group was selected as we were interested in trait effects and wanted to minimize the confounding impact of concurrent anxiety, ritualizing, or the potential need to inhibit obsessions or compulsions during fMRI. A pretreatment CY‐BOCS [Children's Yale‐Brown obsessive compulsive scale; Scahill et al., 1997], (mean total score = 20.5, range: 12–33) was repeated before scanning (mean total score: 11, range: 2–21) and confirmed significant symptomatic improvement (46% for obsessions and 49% for compulsions). Some residual symptoms were present in all subjects at scanning (mean total CY‐BOCS score: 11, range: 2–21). Predominant symptom subtypes were washing and checking. To avoid potential state effects of anxiety and depression, additional exclusion criteria were scores above 15 on the Birleson depression questionnaire [Birleson, 1981] and above 19 on the Revised Children Manifest Anxiety scale [R‐CMAS; Reynolds and Richmond, 1978]. They also underwent SDQ ratings for inattention and restlessness at initial assessment. Two OCD patients scored above threshold for inattention/restlessness on the parent SDQ questionnaire but were not excluded on the basis the fact that inattention is common in patients with OCD and that patients did not meet clinical criteria for ADHD based on the clinical interview. The majority of patients (n = 8) were being treated with a selective Serotonin reuptake inhibitor (SSRI), (mean: 5 months, range: 2–12). Treated patients were selected in the OCD patient group in order to keep state effect confounds of concurrent anxiety, depression, and ritualizing to a minimum during MR scanning. Five patients had completed a course of cognitive behavioral therapy (mean, eight sessions, range: 4–10). Their IQ estimates were above 75.
Control subjects were 20 right‐handed male healthy adolescents between 10 and 17 years (mean age = 14 years, 5 months, SD = 1) with an IQ estimate of over 75 and no history of any mental or neurological disorder, or of neuro‐tropic medication or drug‐ and substance‐abuse.
Written informed consent/assent was given for all participants and the study was approved by the local Ethical Committee.
One‐way ANOVA with group as factor showed that, as expected, ADHD patients scored significantly higher than OCD patients on the SDQ ratings for inattention/hyperactivity [Mean SDQ (SD) ADHD: 9 (1); OCD: 5 (3); t = 5, df = 26, P < 0.0001]. Groups did not differ significantly in age. However, there was a significant effect for IQ estimate [Controls: IQ estimate mean (SD) = 104 (15); range: 81–125; ADHD = 93 (9) range: 75–114; OCD = 102 (20); range: 81–140; F(2, 45) = 3, P < 0.045]. Post‐hoc test (Bonferroni corrected) showed that ADHD children had a significantly lower IQ compared to healthy controls (P < 0.045). Consequently, all behavioral and fMRI analysis were conducted covarying for IQ.
fMRI Paradigm: Simon Task
Subjects practiced the Simon task once prior to scanning. The 6‐min fMRI adaptation of the Simon task involves a stimulus‐response incompatibility effect and measures interference inhibition and selective attention and controls for the attentional oddball effect [Rubia et al., 2006, 2007b, 2009a; Smith et al., 2006].
Subjects have to press a left/right button depending on whether an arrow stimulus of 300‐ms duration points either to the left or right side of the screen. The mean ITI was 1.8 s, but jittered between 1.6 and 2 s for optimal statistical efficiency of fast event‐related FMRI data analysis [Dale, 1999]. In congruent trials (160 trials), the arrow pointing left (right) appears on the left (right) side of the screen. In 12% of trials (24 trials), arrows appear on the opposite side of where they point and subjects have to inhibit responding according to the interfering, predominant spatial information while continue to respond to the iconic information (arrow direction). To control for the attentional oddball effect of the low frequency appearance of the incongruent trials, slightly slanted “oddball” but congruent stimuli appeared in another 12% of trials (24 trials), to which subjects have to respond to as to the congruent stimuli (see Fig. 1).
Figure 1.
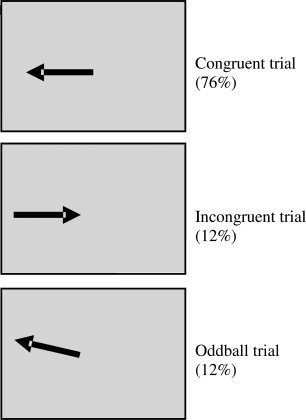
Schematic representation of the fMRI Simon task. Subjects respond to the iconic information (direction of arrow) and have to inhibit the predominant tendency to respond to the spatial information in incongruent trials (side of arrow appearance). In oddball trials the stimulus is congruent but slightly slanted.
Response times are typically slower and more erratic to incongruent compared to congruent trials, called the conflict reaction time and conflict error effects, respectively (conflict RT/error effect: MRT/error incongruent – MRT/error congruent).
The event‐related analysis compares successfully performed incongruent with successfully performed oddball trials to measure the neural correlates of interference inhibition, controlling for the attentional oddball effect (incongruent‐oddball trials). The attentional oddball effect is measured in the comparison of oddball with congruent trials (oddball‐congruent trials).
Analysis of Performance Data
Repeated measure ANCOVA with group as factor with three levels and IQ as covariate were used to compare the main performance variables of the Simon and the oddball conditions between the three groups. Post‐hoc comparisons were conducted for significant group effects using Bonferroni correction.
fMRI Image Acquisition
Gradient‐echo echoplanar MR imaging (EPI) data were acquired on a GE Signa 1.5T Horizon LX System (General; Electric, Milwaukee, WI) at the Maudsley Hospital, London. Consistent image quality was ensured by a semiautomated quality control procedure. A quadrature birdcage head coil was used for RF transmission and reception. In each of 16 noncontiguous planes parallel to the anterior–posterior commissural, 208 T2*‐weighted MR images depicting BOLD (blood oxygen level dependent) contrast covering the whole brain were acquired with TE = 40 ms, TR = 1.8 s, flip angle = 90°, in‐plane resolution = 3.1 mm, slice thickness = 7 mm, slice‐skip = 0.7 mm.
fMRI Image Analysis
We used the software package of XBAM (http://www.brainmap.co.uk) [Brammer et al., 1997] that makes no normality assumptions, which are usually violated in fMRI data, but instead uses median statistics to control for outlier effects and robust permutation rather than normal theory‐based inference. Furthermore the most common test statistic is computed by standardizing for individual difference in residual noise before embarking on second level, multi‐subject testing using robust permutation‐based methods. This allows a mixed effects approach to analysis—an approach that has recently been recommended following a detailed analysis of the validity and impact of normal theory‐based inference in fMRI in large number of subjects [Thirion et al., 2007].
Individual Analysis
fMRI data were realigned to minimize motion‐related artifacts [Bullmore et al., 1999], and smoothed using a Gaussian filter (full‐width half maximum, 7.2 mm). Time‐series analysis of individual subject activation was performed using XBAM, with a wavelet‐based resampling method previously described [Bullmore et al., 2001]. Briefly, we first convolved each experimental condition with two Poisson model functions (delays of 4 and 8 s). We then calculated the weighted sum of these two convolutions that gave the best fit (least‐squares) to the time series at each voxel. A goodness‐of‐fit statistic (the SSQ‐ratio) was then computed at each voxel consisting of the ratio of the sum of squares of deviations from the mean intensity value due to the model (fitted time series) divided by the sum of squares due to the residuals (original time series minus model time series). The appropriate null distribution for assessing significance of any given SSQ‐ratio was established using the wavelet‐based data resampling method [Bullmore et al., 2001] and applying the model‐fitting process to the resampled data. This process was repeated 20 times at each voxel and the data combined over all voxels, resulting in 20 null parametric maps of SSQ‐ratio for each subject, which were combined to give the overall null distribution of SSQ‐ratio. The same permutation strategy was applied at each voxel to preserve spatial correlation structure in the data. Activated voxels, at a <1 level of Type I error, were identified through the appropriate critical value of the SSQ‐ratio from the null distribution. For the fMRI analysis of the Simon condition, activation associated with congruent trials was subtracted from activation associated with oddball trials. For the analysis of the oddball condition, activation associated with congruent trials was subtracted from activation related to oddball trials. Individual maps were registered into standard Talairach space using rigid‐body and affine transformation.
Group Analysis
Generic group activation maps were then produced for the two task conditions. Individual SSQ‐ratio maps were transformed into standard space, first by rigid body transformation of the fMRI data into a high‐resolution inversion recovery image of the same subject, and then by affine transformation onto a Talairach template. Instead of relying on asymptotic distributions such as t or F that assume data normality, we use data‐driven, permutation‐based methods with minimal distributional assumptions that are more suitable for fMRI data analysis in this kind of sizes [Zhang et al., 2009]. A generic activation group map was produced for each experimental condition by calculating the median observed SSQ‐ratio over all subjects at each voxel in standard space and testing them against the null distribution of median SSQ‐ratios computed from the identically transformed wavelet resampled data [Brammer et al., 1997]. Then thresholding at any required level of significance proceeds exactly as for normal t or F tests where the observed statistic is tested against the appropriate critical value from a theoretical rather than a data‐derived distribution. The voxel‐level threshold was first set to 0.05 to give maximum sensitivity and to avoid Type II errors. Next, a cluster‐level threshold was computed for the resulting 3D voxel clusters such that the final expected number of Type I error clusters was <1 per whole brain. The necessary combination of voxel and cluster level thresholds was not assumed from theory but rather was determined by direct permutation for each dataset, giving excellent Type II error control [Bullmore et al., 1999]. Cluster mass rather than a cluster extent threshold was used, to minimize discrimination against possible small, strongly responding foci of activation [Bullmore et al., 1999, 2001]. Briefly, a voxel‐wide significance threshold was set (P < 0.05), and surviving voxels were assembled into 3D clusters using a contiguity criterion. The mass of each cluster was calculated by adding the statistical values of all cluster members and thresholded at P < 0.01. Less than one false positive activation locus was expected for P < 0.05 at voxel level and P < 0.01 at cluster level.
For the between‐group comparisons, one‐way ANCOVA analysis with IQ as covariate and group as factor with three levels was conducted using randomization‐based test for voxel or cluster‐wise differences as described above [Bullmore et al., 1999, 2001]. For these between‐group comparisons, less than 1 false activated cluster was expected at a P‐value of P < 0.05 for voxel and P < 0.01 for cluster comparisons. Then scalar measures of mean BOLD response for each participant were extracted in each of the significant clusters of the one way ANCOVA analysis and post‐hoc t‐tests (using Bonferroni correction) were conducted on these measures to identify comparisons between the different groups.
Correlation Between Brain Activation, Performance, and Symptoms
Scalar measure of mean BOLD response for each participant was extracted in each of the significant clusters of between‐group activation differences. Pearson correlations (two‐tailed) were then calculated between brain activation and either SDQ inattention/hyperactivity scores for ADHD patients or CY‐BOCS obsession and compulsion scores in OCD patients.
RESULTS
Task Performance
Repeated measures ANCOVA with group as factor and trial‐type as within‐subject levels (congruent, incongruent, and oddball) was conducted for reaction times and errors. There was a significant within‐subjects effect on reaction times (F = 3, df = 2, P < 0.05) which was due to a within‐subject linear effect (F = 5, df = 1, P < 0.03) where all subjects had the slowest reaction times in the Simon condition, followed by the oddball condition, with the fastest reaction times for the congruent trials. There was also a significant reaction time by group effect (F = 4, df = 3, P < 0.02). Post‐hoc group comparisons showed that this was due to a larger Simon RT effect in patients with OCD when compared to ADHD patients (P < 0.027) (see Table I).
Table I.
Performance variables of the Simon task by group
| Measure | Condition | Healthy controls (N = 20) | ADHD (N = 18) | OCD (N = 10) | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| MRT (ms) | Congruent | 451 | 102 | 494 | 120 | 445 | 86 |
| Oddball | 453 | 98 | 470 | 97 | 448 | 78 | |
| Incongruent | 544 | 111 | 570 | 129 | 598 | 112 | |
| Errors (%) | Congruent | 2 | 2 | 3 | 3 | 3 | 3 |
| Oddball | 1 | 2 | 2 | 6 | 3 | 4 | |
| Incongruent | 17 | 12 | 21 | 16 | 29 | 23 | |
MRT = mean reaction time (ms); SD = intra‐subject standard deviation.
For errors, there was a significant effect of trial type on errors within subjects (F = 13, df = 2, P < 0.0001), which was due to all subjects making more errors to incongruent compared to congruent or oddball trials (see Table I). Multivariate test, however, showed no group by error effect (F = 1, df = 4, P = n.s.).
Brain Activation
Motion.
ANOVA showed no significant between‐group differences in the extent of 3D motion during task performance.
Oddball—Congruent trials
Within‐group activations are shown in Figure 2a. Controls activated medial and dorsolateral prefrontal cortex, posterior cingulate/precuneus, superior temporal gyri, inferior parietal lobe, visual cortex, and cerebellum. ADHD children activated posterior cingulate and precuneus. OCD patients activated posterior cingulate, precuneus, and hippocampal gyrus.
Figure 2.
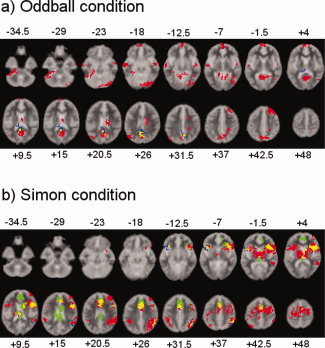
Axial slices for the group activation maps for the three groups at P < 0.05 for voxel and P < 0.01 for cluster levels for the (a) Oddball condition (oddball—congruent trials) (b) Simon condition (incongruent—oddball trials). Red = Controls; Green = ADHD; Blue = CD. Overlapping brain regions: Yellow: overlap between ADHD and Controls; Magenta: overlap between CD and Controls. Cyan: overlap between ADHD and CD. White: overlap between all groups. Talairach z‐coordinates are indicated for slice distance (in mm) from the inter‐commissural line. The right side of the picture corresponds to the right side of the brain. For the ADHD group in the oddball‐congruent contrast a cluster p value of P < 0.025 is shown as they showed no activation at P < 0.01.
ANCOVA with group as factor showed a significant group effect in the activation of right dorsolateral prefrontal cortex and of bilateral posterior cingulate (Table II, Fig. 3a). Post hoc t‐tests (peak; Bonferroni corrected) showed that OCD patients showed reduced BOLD response in right dorsolateral prefrontal cortex compared to controls (P < 0.001) and compared to ADHD patients (P < 0.001). ADHD patients did not differ from controls in this measure. ADHD patients, however, showed under‐activation in the posterior cingulate gyrus activation cluster compared to controls (P < 0.029) and compared to OCD patients (P < 0.012), both of which did not differ from each other.
Table II.
ANCOVA differences in brain activation between healthy adolescents and those with ADHD and OCD
| Subject contrast | Brain regions of activation (Brodman area; BA) | Peak Talairach coordinates (x;y;z) | N of voxels | Cluster P‐value |
|---|---|---|---|---|
| Oddball–congruent trials | ||||
| OCD, C > ADHD | R posterior cingulate/caudate (BA 31/23) | −11;−30;9 | 117 | <0.002 |
| C, ADHD > OCD | R superior/middle frontal gyrus (BA 8/9/6) | 22;41;42 | 134 | <0.003 |
| Incongruent–oddball trials | ||||
| C > ADHD, OCD | R SMA/anterior cingulate/superior parietal (6/32/7) | 29;−29;48 | 90 | <0.005 |
| C, OCD > ADHD | R inferior parietal lobe (BA 39/40) | 36;−44;26 | 63 | <0.007 |
N voxels = number of voxels. P‐value for ANCOVAs at P < 0.05 for voxel activation and P < 0.01 for cluster activation. Those P‐values were selected to yield less than one false positive cluster per brain map. SMA = supplementary motor area.
Figure 3.
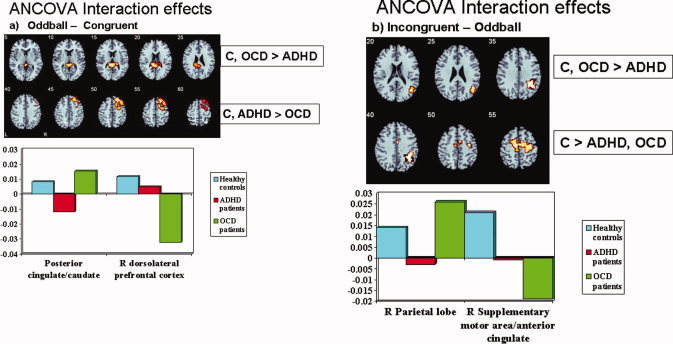
Axial sections showing the ANCOVA results for the between‐group differences in brain activation at P < 0.05 for voxel and P < 0.01 for cluster levels for the contrast of (a) Oddball condition (Oddball—congruent trials). The activation cluster in right dorsolateral prefrontal cortex was disorder‐specifically reduced in patients with OCD when compared to healthy controls and ADHD patients, while the activation cluster in posterior cingulate and caudate was disorder‐specifically reduced in ADHD compared to both OCD and controls. (b) Simon condition (Incongruent ‐ oddball trials). The cluster in SMA/anterior cingulate was reduced in both patient groups compared to healthy controls. The right inferior parietal activation, however, was specifically reduced in ADHD patients compared to both healthy controls and OCD patients. Talairach z‐coordinates are indicated for slice distance (in mm) from the inter‐commissural line. The right side of the picture corresponds to the right side of the brain.
In patients with OCD, BOLD response in the posterior cingulate activation cluster was positively correlated with the CY‐BOCS obsession scores (r = 0.7, P < 0.015) but not the compulsion score. In patients with ADHD, SDQ scores of inattention/hyperactivity were significantly negatively correlated with brain activation in this region (r = −0.5, P < 0.03). No other correlations were observed.
Incongruent—Oddball trials
Group activations for each group are shown in Figure 2b. In line with previous findings in healthy adults and children using the same interference inhibition task [Rubia et al., 2006; Smith et al., 2006], controls activated bilateral ventrolateral and dorsolateral prefrontal cortex, putamen and thalamus, middle temporal gyrus, inferior parietal gyri, and anterior cingulate and supplementary motor cortex. Patients with ADHD activated right lateral prefrontal cortex, mesial frontal cortex and anterior and posterior cingulate, caudate and putamen. OCD patients activated left inferior prefrontal cortex, and right inferior parietal/middle temporal gyri.
ANCOVA showed two significant group effect clusters in right anterior cingulate/SMA and right inferior parietal lobe. Post‐hoc comparisons showed that the cluster in parietal lobe was significantly reduced in ADHD patients compared to controls and OCD patients (P < 0.01) who did not differ from each other. The cluster in SMA was reduced in both patient groups compared to controls (P < 0.05). Patients did not differ from each other in this measure.
No correlations were observed in patients between behavioral scores and clusters of significant group effect
Discriminant Analysis to Test the Ability of Brain Activation Difference Clusters to Predict Group Membership
To explore whether the brain activation clusters that differed between groups were sensitive enough to predict group membership, we computed separate discriminant analysis from group sizes using leave‐one‐out classification using the scalar measures of mean BOLD response for each participant for each of the significant clusters of between‐group activation differences. For the parietal activation cluster that was decreased in ADHD patients compared to both controls and OCD patients (who had the highest activation) during the interference inhibition condition, the specificity was relatively moderate with 60% correct classification for controls. The sensitivity for ADHD patients, however, was very high with 89.9% correct group classification, but moderate for OCD with 60% correct classification.
For the oddball condition, two clusters differed between groups, the posterior cingulate/caudate cluster that was decreased in activation in ADHD compared to OCD and controls and a frontal lobe cluster that was disorder‐specifically reduced in OCD compared to the other two groups. Specificity as well as sensitivity for the posterior cingulate/caudate cluster was relatively moderate with 55% correct classification of controls, and 60% sensitivity to classify ADHD and OCD patients. The prefrontal lobe cluster was the least sensitive, with 50% correct classification of controls and 33% sensitivity to classify OCD patients and 22% sensitivity to classify ADHD patients.
DISCUSSION
For all subjects, the Simon condition was the most difficult condition as shown in higher error rates and slower reaction times for this condition compared to the oddball or congruent conditions. Reaction times to oddball trials were also slower for all subjects compared to the congruent condition, suggesting that attention allocation to infrequent trials led to a slowing of reaction time, presumably reflecting the attentional oddball effect. No group differences, however, were observed during performance of the simple selective attention (oddball) condition. There were significant group differences, however, for the Simon interference effect on reaction times, which was significantly higher for OCD compared to ADHD patients. The fMRI data showed that during the interference inhibition condition, boys with ADHD and with OCD shared under‐activation compared to controls in anterior cingulate, the SMA and in the dorsal superior parietal lobe. Disorder‐specific dysfunctions were observed in OCD patients in right dorsolateral prefrontal cortex during the oddball condition. ADHD patients showed disorder‐specific dysfunctions in posterior cingulate and caudate during the oddball condition and in right inferior parietal lobe during interference inhibition. Furthermore, the activation cluster in posterior cingulate and caudate showed a disorder‐specific dissociation with respect to disorder symptomatology: it correlated negatively with ADHD symptoms and positively with OCD symptoms.
Oddball Condition
During the attentional oddball condition, both disorders showed disorder‐specific under‐activations. Right dorsolateral prefrontal cortex (DLPFC) was exclusively under‐activated in OCD patients, while ADHD patients showed exclusive posterior cingulate and caudate under‐activation. Dysfunction in right DLPFC is a consistent finding in adults with OCD during higher executive selective attention and planning tasks [Menzies et al., 2008] and has previously been observed in children with OCD during inhibition failures [Woolley et al., 2008]. The findings show that patients with OCD recruit the DLPFC activation to a lesser extent not only during higher‐level executive function tasks but already during simple perceptual functions of attention allocation and, furthermore, that this is disorder‐specific when compared to ADHD.
This activation cluster in caudate and posterior cingulate was significantly decreased in ADHD compared to controls and OCD patients, who had a nonsignificantly elevated BOLD response compared to controls (see Fig. 3a). There was furthermore an interesting dissociation in the relationship between the activation cluster and disorder‐specific symptomatology. Activation in this region correlated positively with OCD symptoms, but negatively with ADHD symptoms. Underactivation of the posterior cingulate and caudate is a key finding in fMRI studies of ADHD and has been observed during several cognitive functions including the same attention allocation task [Rubia et al., 2007b, 2009a], as well as other functions of response inhibition [Durston et al., 2003; Rubia et al., 1999, 2005b, 2008; Vaidya et al., 1998], reward [Rubia et al., 2009b], and timing [Rubia et al., 1999, 2001a, 2009c]. Furthermore, in two of these studies we also observed a negative correlation between cingulate activation and ADHD symptoms [Rubia et al., 2005b, 2007b]. The posterior cingulate is connected to the limbic system and visuo‐motor pathways and is relevant for the dynamic allocation of visual‐spatial attention, in particular to visually salient events such as oddball trials [Mohanty et al., 2008; Small et al., 2003]. The caudate likewise is an important area for attention to saliency [Davidson et al., 2004; Zink et al., 2004, 2006] and is known to be mediated by dopamine that predominantly innervates the basal ganglia [Volkow et al., 2006]. The reduced activation in ADHD patients in dopaminergically innervated brain regions that are important for visual spatial attention to saliency is in line with evidence for reduced levels of dopamine in ADHD patients [Krause, 2008] which is known to modulate the attentional response to saliency [Volkow et al., 2006]. By analogy, one could argue that the increased caudate and posterior cingulate activation in OCD patients and its positive correlation with OCD symptoms could be related to enhanced dopamine levels in this patient group as indicated by reduced DAT levels and D2 receptor abnormalities [Denys et al., 2004; Hesse et al., 2005; Kim et al., 2003, 2007] that could lead to enhanced activation of brain regions that mediate salience [Volkow et al., 2006]. Several fMRI studies have found posterior cingulate (together with anterior cingulate) and caudate to be increased in adult patients with OCD during rest, symptom provocation and tasks of cognitive control and to correlate positively with OCD symptoms [Menzies et al., 2008; Page et al., 2009]. It thus appears that these two disorders that are antagonistic with respect to documented dopamine receptor and transporter binding in the basal ganglia—indicative of enhanced dopamine levels in OCD [Denys et al., 2004; Hesse et al., 2005; Kim et al., 2003, 2007] and reduced dopamine levels in ADHD [Krause, 2008]—are also antagonistic with respect to the activation of dopamine‐innervated brain regions that are involved in the processing of saliency [Volkow et al., 2006].
Interference Inhibition Condition
During the interference inhibition condition, both patient groups shared dysfunction in the SMA and anterior cingulate, typical areas for interference inhibition [Botvinick et al., 1999; Ridderinkhof et al., 2004; Rubia et al., 2006; Ullsperger and von Cramon, 2001]. In ADHD children, under‐activation of the SMA and anterior cingulate has frequently been observed during tasks of cognitive control and selective attention [Dickstein et al., 2006; Rubia et al., 2009a; Smith et al., 2006]. A study in adult OCD also found the SMA to be under‐activated during a similar interference inhibition task [Fitzgerald et al., 2005]. The under‐activation finding in anterior cingulate is in line with previous fMRI studies in OCD children and adults during similar cognitive control tasks [Page et al., 2009; Woolley et al., 2008], although there have also been reports of increased activation, in the context of error processing [Fitzgerald et al., 2005; Maltby et al., 2005; Menzies et al., 2008].
ADHD patients showed a disorder‐specific under‐activation in the inferior parietal lobes. The inferior parietal lobes have previously been found to be dysfunctional in ADHD children during similar tasks of interference inhibition [Konrad et al., 2006] and related inhibition and attention tasks [Dickstein et al., 2006; Rubia et al., 2007b, 2008, 2009c; Smith et al., 2006]. The finding of disorder‐specificity of parietal dysfunction during the higher‐level selective attention task together with the finding of disorder‐specific posterior cingulate and caudate under‐activation during the simpler, perceptive selective attention (oddball) condition, suggests that ADHD patients may have more pronounced neuro‐functional problems with the recruitment of posterior parietal visual‐spatial attention areas than patients with OCD. The activation cluster in the parietal lobes was the most sensitive to distinguish ADHD patients from OCD patients and controls, as shown in the discrimination analysis, where the BOLD response in this region was able to correctly classify ADHD patients with about 90% sensitivity. This suggests that inferior parietal activity may potentially be a diagnostic neuro‐functional biomarker for ADHD, at least when compared to controls and OCD patients.
Behaviorally, only OCD patients showed a higher interference effect in the Simon task compared to ADHD patients. The findings are in line with similar findings of performance deficits in OCD, but not ADHD patients in the same task of interference inhibition [Penades et al., 2007; Rubia et al., 2007a). Nevertheless, ADHD patients had both shared and disorder‐specific brain activation deficits with respect to OCD patients. It has consistently been shown that brain activation is more sensitive than behavioral performance and thus reduced brain activation despite comparable task performance is a common finding in ADHD patients [Rubia et al., 1999, 2005b, 2007b, 2008, 2009a, b, c; Smith et al., 2006].
A limitation of this study is the relatively small sample size of the OCD group, although the sample sizes for the other two groups were relatively large for fMRI studies. The relatively lower sample size for OCD patients compared to that of ADHD patients may have biased the positive findings towards the group with higher statistical power. Another limitation is the fact that the two disorders differed in their clinical severity and medication status. ADHD patients were fully symptomatic and medication‐naïve, while most of the patients with OCD were medicated with SSRIs and in partial remission. While this has the advantage of keeping confounding state symptoms of anxiety, depression, and ritualizing in the OCD group minimal, a comparison with fully symptomatic OCD patients may potentially elicit more severe and disorder‐specific brain dysfunctions in patients with OCD. Relatively little is known on the effects of SSRIs on functional brain activation patterns. There is evidence that SSRIs increase specific regional relative metabolic rate in healthy subjects and impulsive aggression, with differing effects depending on treatment duration [Gerdelat‐Mas et al., 2005; New et al., 2004]. Acute SSRI administration has been shown to upregulate prefrontal brain regions during cognitive control tasks [Del‐Ben et al., 2005; Vollm et al., 2005]. Tryptophan depletion that reduces brain serotonin levels by about 60% has been shown to reduce dorsolateral and inferior prefrontal brain activation during tasks of cognitive control [Rubia et al., 2005a, Evers et al., 2006]. In adult OCD, acute SSRI medication has been shown to reduce symptom related overactivation in frontal and striatal brain regions, but to increase task‐relevant brain activation during cognitive challenge [Nakao et al., 2005a, b]. Overall, the available evidence therefore implies that medication may have had a mitigating effect on brain dysfunction that might have been more pronounced in medication‐naïve adolescents with OCD.
In conclusion, this study is a first step toward delineating the underlying neuro‐functional differences between these two diagnostic disorders in relation to a commonly affected behavioral and neuropsychological phenotype that is inhibitory and attention dyscontrol. The study shows shared mesial frontal dysfunctions during higher cognitive selective attention/interference inhibition, but also disorder‐specific deficits in both patient groups. OCD patients showed disorder‐specific dysfunction in dorsolateral prefrontal activation while ADHD patients showed disorder‐specific under‐activation of parietal lobes, caudate, and posterior cingulate, the latter of which was inversely correlated with OCD and ADHD symptoms and could potentially be related to the documented inverse striatal dopamine abnormalities in the two disorders. The disorder‐specific parietal dysfunction in ADHD was the most sensitive to correctly classify ADHD patients with about 90% sensitivity. Disentangling the specificity of the underlying pathophysiology of these two disorders will ultimately help towards establishing disorder‐specific biomarkers that can provide an important aid to a more objective diagnosis and help with the development of disorder‐tailored treatment.
None of the authors has any interest to declare.
REFERENCES
- Achenbach T, Edelbrook CS ( 1983): Manual for the Child Behavior Checklist and Revised Child Behavior Profile. Vermont: University of Vermont. [Google Scholar]
- American Psychiatric Association ( 1994): Diagnostic and Statistical Manual of Mental Disorders. Washington: American Psychiatric Association. [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ ( 2003): A review of electrophysiology in attention‐deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clinical Neurophysiology 114: 171–183. [DOI] [PubMed] [Google Scholar]
- Birleson P ( 1981): The validity of depressive disorder in childhood and the development of a self‐rating scale—A research report. J Child Psychol Psychiatry Allied Disciplines 22: 73–88. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM ( 2005): Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD). J Child Psychol Psychiatry 46: 94–111. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD ( 1999): Conflict monitoring versus selection‐for‐action in anterior cingulate cortex. Nature 402: 179–181. [DOI] [PubMed] [Google Scholar]
- Brammer MJ, Bullmore ET, Simmons A, Williams SC, Grasby PM, Howard RJ, Woodruff PW, Rabe‐Hesketh S ( 1997): Generic brain activation mapping in functional magnetic resonance imaging: A nonparametric approach. Magn Reson Imaging 15: 763–770. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe‐Hesketh S, Taylor E, Brammer MJ ( 1999): Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging 18: 32–42. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Long C, Suckling J, Fadili J, Calvert G, Zelaya F, Carpenter TA, Brammer M ( 2001): Colored noise and computational inference in neurophysiological (fMRI) time series analysis: Resampling methods in time and wavelet domains. Hum Brain Mapp 12: 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson ML ( 2000): On the role of cortical glutamate in obsessive‐compulsive disorder and attention‐deficit hyperactivity disorder, two phenomenologically antithetical conditions. Acta Psychiatr Scand 102: 401–413. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Blackwell AD, Robbins TW, Sahakian BJ ( 2006): Motor inhibition and cognitive flexibility in obsessive‐compulsive disorder and trichotillomania. Am J Psychiatry 163: 1282–1284. [DOI] [PubMed] [Google Scholar]
- Dale AM ( 1999): Optimal design for event‐related fMRI. Hum Brain Mapp 8: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MC, Horvitz JC, Tottenham N, Fossella JA, Watts R, Ulug AM, Casey BJ ( 2004): Differential cingulate and caudate activation following unexpected nonrewarding stimuli. Neuroimage 23: 1039–1045. [DOI] [PubMed] [Google Scholar]
- Del‐Ben CM, Deakin JFW, McKie S, Delvai NA, Williams SR, Elliott R, Dolan M, Anderson IM ( 2005): The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: An fMRI study. Neuropsychopharmacology 30: 1724–1734. [DOI] [PubMed] [Google Scholar]
- Denys D, van der Wee N, Janssen J, De Geus F, Westenberg HGM ( 2004): Low level of dopaminergic D‐2 receptor binding in obsessive‐compulsive disorder. Biol Psychiatry 55: 1041–1045. [DOI] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP ( 2006): The neural correlates of attention deficit hyperactivity disorder: An ALE meta‐analysis. J Child Psychol Psychiatry 47: 1051–1062. [DOI] [PubMed] [Google Scholar]
- Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang YH, Ulug AM, Casey BJ ( 2003): Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry 53: 871–878. [DOI] [PubMed] [Google Scholar]
- Evers EAT, van der Veen FM, van Deursen JA, Schmitt JAJ, Deutz NEP, Jolles J ( 2006): The effect of acute tryptophan depletion on the BOLD response during performance monitoring and response inhibition in healthy male volunteers. Psychopharmacology 187: 200–208. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SF ( 2005): Error‐related hyperactivity of the anterior cingulate cortex in obsessive‐compulsive disorder. Biol Psychiatry 57: 287–294. [DOI] [PubMed] [Google Scholar]
- Geller DA, Biederman J, Griffin S, Jones J, Lefkowitz TR ( 1996): Comorbidity of juvenile obsessive‐compulsive disorder with disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry 35: 1637–1646. [DOI] [PubMed] [Google Scholar]
- Geller D, Biederman J, Faraone SV, Frazier J, Coffey BJ, Kim G, Bellordre CA ( 2000): Clinical correlates of obsessive compulsive disorder in children and adolescents referred to specialized and non‐specialized clinical settings. Depression Anxiety 11: 163–168. [DOI] [PubMed] [Google Scholar]
- Gerdelat‐Mas A, Loubinoux I, Tombari D, Rascol O, Chollet F, Simonetta‐Moreau M ( 2005): Chronic administration of selective serotonin reuptake inhibitor (SSRI) paroxetine modulates human motor cortex excitability in healthy subjects. Neuroimage 27: 314–322. [DOI] [PubMed] [Google Scholar]
- Goldberg D, Murray R, editor ( 2002): Maudsley Handbook of Practical Psychiatry. Oxford: Oxford University Press. [Google Scholar]
- Goodman R ( 1997): The strengths and difficulties questionnaire: A research note. J Child Psychol Psychiatry Allied Disciplines 38: 581–586. [DOI] [PubMed] [Google Scholar]
- Hesse S, Muller U, Lincke T, Barthel H, Villmann T, Angermeyer MC, Sabri O, Stengler‐Wenzke K ( 2005): Serotonin and dopamine transporter imaging in patients with obsessive‐compulsive disorder. Psychiatry Res Neuroimaging 140: 63–72. [DOI] [PubMed] [Google Scholar]
- Huyser C, Veltman DJ, de Haan E, Boer F ( 2009): Paediatric obsessive compulsive disorder, a neurodevelopmental disorder? Neurosci Biobehav Rev 33: 919–830. [DOI] [PubMed] [Google Scholar]
- Kim CH, Koo MS, Cheon KA, Ryu YH, Lee JD, Lee HS ( 2003): Dopamine transporter density of basal ganglia assessed with [I‐123]IPT SPET in obsessive‐compulsive disorder. Eur J Nuclear Med Mol Imaging 30: 1637–1643. [DOI] [PubMed] [Google Scholar]
- Kim CH, Cheon KA, Koo MS, Ryu YH, Lee JD, Chang JW, Lee HS ( 2007): Dopamine transporter density in the basal ganglia in obsessive‐compulsive disorder, measured with [I‐123]IPT SPECT before and after treatment with serotonin reuptake inhibitors. Neuropsychobiology 55: 156–162. [DOI] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Hanisch C, Fink GR, Herpertz‐Dahlmann B ( 2006): Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: Evidence from an event‐related functional magnetic resonance imaging study. Biol Psychiatry 59: 643–651. [DOI] [PubMed] [Google Scholar]
- Krause J ( 2008): SPECT and PET of the dopamine transporter in attention‐deficit/hyperactivity disorder. Expert Rev Neurotherap 8: 611–625. [DOI] [PubMed] [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, O'Keefe TM, Kiehl KA ( 2005): Dysfunctional action monitoring hyperactivates frontal‐striatal circuits in obsessive‐compulsive disorder: An event‐related fMRI study. Neuroimage 24: 495–503. [DOI] [PubMed] [Google Scholar]
- Menzies L, Achard S, Chamberlain SR, Fineberg N, Chen C‐H, del Campo N, Sahakian BJ, Robbins TW, Bullmore E ( 2007): Neurocognitive endophenotypes of obsessive‐compulsive disorder. Brain 130: 3223–3236. [DOI] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET ( 2008): Integrating evidence from neuroimaging and neuropsychological studies of obsessive‐compulsive disorder: The orbitofronto‐striatal model revisited. Neurosci Biobehav Rev 32: 525–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A, Gitelman DR, Small DM, Mesulam MM ( 2008): The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation‐induced attention shifts. Cereb Cortex 18: 2604–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, Kudoh A, Tada K, Yoshioka K, Kawamoto M ( 2005a): A functional MRI comparison of patients with obsessive‐compulsive disorder and normal controls during a Chinese character Stroop task. Psychiatry Res Neuroimaging 139: 101–114. [DOI] [PubMed] [Google Scholar]
- Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, Kudoh A, Tada K, Yoshioka K, Kawamoto M, Togao O, Kanba S ( 2005b): Brain activation of patients with obsessive‐compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: A functional magnetic resonance imaging study. Biol Psychiatry 57: 901–910. [DOI] [PubMed] [Google Scholar]
- New AS, Buchsbaum MS, Hazlett EA, Goodman M, Koenigsberg HW, Lo J, Iskander L, Newmark R, Brand J, Flynn K, Siever LJ ( 2004): Fluoxetine increases relative metabolic rate in prefrontal cortex in impulsive aggression. Psychopharmacology 176: 451–458. [DOI] [PubMed] [Google Scholar]
- Page LA, Rubia K, Deeley Q, Daly E, Toal F, Mataix‐Cols D, Giampietro V, Schmitz N, Murphy DGM ( 2009): A functional magnetic resonance imaging study of inhibitory control in obsessive compulsive disorder. Psychiatry Res Neuroimaging 174: 202–209. [DOI] [PubMed] [Google Scholar]
- Penades R, Catalan R, Rubia K, Andres S, Salamero M, Gasto C ( 2007): Impaired response inhibition in obsessive compulsive disorder. Eur Psychiatry 22: 404–410. [DOI] [PubMed] [Google Scholar]
- Raven J ( 1960): Guide to the Standard Progressive Matrices. London: HK Lewis. [Google Scholar]
- Reynolds CR, Richmond BO ( 1978): What I think and feel—Revised measure of children's manifest anxiety. J Abnormal Child Psychol 6: 271–280. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuiss S ( 2004): The role of the medial frontal cortex in cognitive control. Science 306: 443–447. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, Bullmore E ( 1999): Hypofrontality in attention deficit hyperactivity disorder ADHD during higher‐order motor control: A study using fMRI. Am J Psychiatry 156: 891–896. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SCR, Giampietro V, Andrew CM, et al. ( 2001a): Mapping motor inhibition: Conjunctive brain activations across different versions of go/no‐go and stop tasks. Neuroimage 13: 250–261. [DOI] [PubMed] [Google Scholar]
- Rubia K, Taylor E, Smith AB, Oksannen H, Overmeyer S, Newman S ( 2001b): Neuropsychological analyses of impulsiveness in childhood hyperactivity. Br J Psychiatry 179: 138–143. [DOI] [PubMed] [Google Scholar]
- Rubia K, Lee F, Cleare AJ, Tunstall N, Fu CHY, Brammer M, McGuire P ( 2005a): Tryptophan depletion reduces right inferior prefrontal activation during response inhibition in fast, event‐related fMRI. Psychopharmacology 179: 791–803. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer M, Toone B, Taylor E ( 2005b): Medication‐naïve adolescents with attention‐deficit hyperactivity disorder show abnormal brain activation during inhibition and error detection. Am J Psychiatry 162: 1067–1075. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer MJ ( 2006): Progressive increase of fronto‐striatal brain activation from childhood to adulthood during event related tasks of cognitive control. Hum Brain Mapp 27: 973–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer M, Taylor E ( 2007a): Performance of children with attention deficit hyperactivity disorder (ADHD) on a test battery for impulsiveness. Child Neuropsychol 13: 276–304. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E ( 2007b): Temporal lobe dysfunction in medication‐naive boys with attention‐deficit/hyperactivity disorder during attention allocation and its relation to response variability. Biol Psychiatry 62: 999–1006. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Smith AB, Mohammad M, Scott S, Giampietro V, Taylor E, Brammer ME ( 2008): Dissociated functional brain abnormalities of inhibition in boys with pure conduct disorder and in boys with pure attention‐deficit/hyperactivity disorder. Am J Psychiatry 165: 889–897. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Smith A, Mohammad M, Scott S, Brammer M ( 2009a): Shared and disorder‐specific prefrontal abnormalities in boys with pure attention‐deficit/hyperactivity disorder compared to boys with pure CD during interference inhibition and attention allocation. J Child Psychol Psychiatry 50: 669–678. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A, Halari R, Matukura F, Mohammad M, Taylor E, Brammer M ( 2009b): Disorder‐specific dissociationof orbitofrontal dysfunction in boys with pure conductdisorder during reward and ventrolateral prefrontaldysfunction in boys with pure attention‐deficit/hyperactivitydisorder during sustained attention. Am J Psychiatry 166: 83–94. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Christakou A, Taylor E ( 2009c): Impulsivenessas a disturbance of timing: Brain dysfunction in ADHD duringtemporal processes and normalisation with a dopaminergicagonist. Philos Trans R Soc (Biol Sci) 346: 1919–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L, Riddle MA, McSwigginHardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF ( 1997): Children's Yale‐Brown obsessive compulsive scale: Reliability and validity. J Am Acad Child Adolesc Psychiatry 36: 844–852. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Geurts H, Oosterlaan J ( 2002): How specific is a deficit of executive functioning for attention‐deficit/hyperactivity disorder? Behav Brain Res 130: 3–28. [DOI] [PubMed] [Google Scholar]
- Small DM, Gitelman DR, Gregory MD, Nobre AC, Parrish TB, Mesulam MM ( 2003): The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. Neuroimage 18: 633–641. [DOI] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Toone B, Rubia K ( 2006): Task‐specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication‐naive children and adolescents with attention deficit hyperactivity disorder. Am J Psychiatry 163: 1044–1051. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Kiehl KA ( 2007): An fMRI auditory oddball study of combined‐subtype attention deficit hyperactivity disorder. Am J Psychiatry 164: 1737–1749. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL ( 2006): Parietal attentional system aberrations during target detection in adolescents with attention deficit hyperactivity disorder: Event‐related fMRI evidence. Am J Psychiatry 163: 1033–1043. [DOI] [PubMed] [Google Scholar]
- Thirion B, Pinel P, Meriaux S, Roche A, Dehaene S, Poline JB ( 2007): Analysis of a large fMRI cohort: Statistical and methodological issues for group analyses. Neuroimage 35: 105–120. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY ( 2001): Subprocesses of performance monitoring: A dissociation of error processing and response competition revealed by event‐related fMRI and ERPs. Neuroimage 14: 1387–1401. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JDE ( 1998): Selective effects of methylphenidate in attention deficit hyperactivity disorder: A functional magnetic resonance study. Proc Natl Acad Sci USA 95: 14494–14499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA ( 2005): Altered neural substrates of cognitive control in childhood ADHD: Evidence from functional magnetic resonance imaging. Am J Psychiatry 162: 1605–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma YM, Fowler JS, Wong C, Jayne M, Telang F, Swanson JM ( 2006): Effects of expectation on the brain metabolic responses to methylphenidate and to its placebo in non‐drug abusing subjects. Neuroimage 32: 1782–1792. [DOI] [PubMed] [Google Scholar]
- Vollm B, Richardson P, McKie S, Elliott R, Deakin JFW, Anderson IM ( 2006): Serotonergic modulation of neuronal responses to behavioural inhibition and reinforcing stimuli: An fMRI study in healthy volunteers. Eur J Neurosci 23: 552–560. [DOI] [PubMed] [Google Scholar]
- Woolley J, Heyman I, Brammer M, Frampton I, McGuire PK, Rubia K ( 2008): Brain activation in paediatric obsessive‐compulsive disorder during tasks of inhibitory control. Br J Psychiatry 192: 25–31. [DOI] [PubMed] [Google Scholar]
- Zhang H, Nichols TE, Johnson TD ( 2009): Cluster mass inference via random field theory. Neuroimage 44: 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin‐Skurski ME, Chappelow JC, Berns GS ( 2004): Human striatal responses to monetary reward depend on saliency. Neuron 42: 509–517. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Chappelow J, Martin‐Skurski M, Berns GS ( 2006): Human striatal activation reflects degree of stimulus saliency. Neuroimage 29: 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]


