Abstract
The dorsal frontal‐striatal circuit is implicated in executive functions, such as planning. The Tower of London task, a planning task, in combination with off‐line low‐frequency repetitive transcranial magnetic stimulation (rTMS), was used to investigate whether interfering with dorsolateral prefrontal function would modulate executive performance, mimicking dorsal frontal‐striatal dysfunction as found in neuropsychiatric disorders. Eleven healthy controls (seven females; mean age 25.5 years) were entered in a cross‐over design: two single‐session treatments of low‐frequency (1 Hz) rTMS (vs. sham rTMS) for 20 min on the left dorsolateral prefrontal cortex (DLPFC). Directly following the off‐line rTMS treatment, the Tower of London task was performed during MRI measurements. The low‐frequency rTMS treatment impaired performance, but only when the subjects had not performed the task before: we found a TMS condition‐by‐order effect, such that real TMS treatment in the first session led to significantly more errors (P = 0.032), whereas this TMS effect was not present in subjects who received real TMS in the second session. At the neural level, rTMS resulted in decreased activation during the rTMS versus sham condition in prefrontal brain regions (i.e., premotor, dorsolateral prefrontal and anterior prefrontal cortices) and visuospatial brain regions (i.e., precuneus/cuneus and inferior parietal cortex). The results show that low‐frequency off‐line rTMS on the DLPFC resulted in decreased task‐related activations in the frontal and visuospatial regions during the performance of the Tower of London task, with a behavioral effect only when task experience is limited. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: Tower of London, repetitive TMS, functional MRI
INTRODUCTION
The functional neuroanatomy of executive function primarily involves the dorsal frontal‐striatal circuit. Executive functioning implies different subdomains of higher order cognitive processes. An essential component of executive functioning is problem solving and planning, meaning the capacity to achieve a goal through a series of intermediate steps. Performance on the Tower of London task, a frequently used test to probe planning processes [Shallice,1982], strongly relies on proper functioning of dorsal frontal‐striatal and visuospatial brain regions [Owen et al.,1996; van den Heuvel et al.,2003]. Patients with altered frontal‐striatal function, such as in obsessive‐compulsive disorder [van den Heuvel et al.,2005], Parkinson's disease [Williams‐Gray et al.,2007], and Huntington's chorea [Watkins et al.,2000], commonly express executive failure. Theoretically, one might expect that enhancement of the excitability of the dorsal frontal‐striatal brain regions in these patients results in improved executive performance. A transient and noninvasive way to modulate cortical excitability is repetitive transcranial magnetic stimulation (rTMS). TMS has the potential to refine our knowledge of the neural circuits underlying neuropsychiatric disorders [Lisanby et al.,2002; Wassermann and Lisanby,2001], mainly when combined with the use of functional neuroimaging techniques [Paus,2005]. Depending on stimulation parameters, mainly pulse frequency, it is possible to either decrease or increase cortical functioning. High‐frequency rTMS induces a temporary increase in local cortical excitability, whereas low‐frequency rTMS leads to a decrease in excitability, when applied over the motor cortex [Chen,2000; Pascual‐Leone et al.,1998; Speer et al.,2000]. Extrapolating from these data, rTMS may thus be applied as a means to either stimulate under‐active cortical regions or inhibit over‐active ones in neuropsychiatric disease. Moreover, temporary inhibition of normally functioning circuits in healthy controls can simulate the decreased functioning of dorsal prefrontal regions found in patients. The feasibility of off‐line rTMS (applied directly before data collection) has been shown for motor [Chouinard et al.,2003; Rounis et al.,2005; Siebner et al.,2000; Van Der Werf and Paus,2006], emotional [Barrett et al.,2004], and cognitive [Kalbe et al.,2010; Ko et al.,2008b; Rounis et al.,2006,2007; Sack et al.,2007] processes. Using off‐line rTMS, it is possible to change cortical excitability for at least 30 min (to 1 h) poststimulation [Munchau et al.,2002; Touge et al.,2001], depending on the number of pulses delivered [Touge et al.,2001]. Although the cognitive studies using off‐line rTMS showed a clear modulating potential of rTMS on various domains of executive function, accompanied with altered neuronal activation, so far no study has investigated the ability to modulate planning performance using the combination of off‐line rTMS and functional MRI (fMRI). Earlier studies specifically investigating off‐line effects of rTMS on the performance in the Tower of London have rendered negative results; of these, one study used rTMS as treatment and did not test within a time window immediately following stimulation [Loo et al.,1999] and the other examined the Tower of London after rTMS in two sessions spaced 30 min, as part of a larger test battery lasting 2 h, thereby allowing the immediate effects of rTMS to wear off [Roth et al.,2004]. Using a different external neuromodulation technique, however, Dockery et al. [2009] showed that transcranial direct current stimulation (tDCS) on the left dorsolateral prefrontal cortex (DLPFC) is indeed able to modulate planning capacity. It is difficult to compare rTMS and tDCS as they are different stimulation modalities; yet, both cathodal (inhibitory) and anodal (excitatory) stimulation improved performance on the Tower of London task, but in different phases of the task. Since the authors did not measure brain activation during altered task performance, this does not allow to draw direct conclusions regarding the modulation of brain circuits involved.
Evidence for frontal modulation of function using off‐line rTMS is therefore inconclusive; in the present study, we aim to address the question whether off‐line low‐frequency rTMS on the left DLPFC disrupts dorsal frontal‐striatal and visuospatial function during planning performance.
MATERIALS AND METHODS
Subjects
Eleven healthy subjects (seven females, four males; mean age 25.5 years, age range 21–34 years) were recruited among university students and staff. Before scanning, subjects gave their informed consent. All procedures complied with guidelines as described in the Declaration of Helsinki and were approved by the medical ethical board of the VU university medical center.
Design
Participants were entered in a single‐blind cross‐over design. They were scanned during performance of the Tower of London task twice with an interval of on average 1 week, but never shorter than 3 days to let possible carry‐over effects wear off. Immediately prior to scanning, they received 20 min of treatment with either 1 Hz rTMS on the left DLPFC or sham rTMS (same location, stimulation coil placed at a 90° angle). The order of stimulation conditions was balanced across participants to minimize possible nonstimulation‐related order effects. The interval between stimulation and the start of the scan was kept as short as possible (interval range 12–17 min).
Tower of London Paradigm
We used an optimized version of the previously described Tower of London paradigm [van den Heuvel et al.,2003]. Briefly, participants were presented a starting and a target configuration. In both configurations, three colored beads were placed on three vertical rods, which could accommodate one, two, or three beads, respectively. One bead could be moved at a time and only when there was no other bead on top. Participants were requested to determine the minimum number of steps (ranging from one to five) needed to reach the target configuration and to choose between two possible answers. In the baseline condition, participants were instructed to count the number of blue and yellow beads. In contrast to the paradigm described previously [van den Heuvel et al.,2003], baseline trials were presented either as separate trials or as two consecutive trials, to control for possible contamination of the control trials with mental activity flowing over from the activation trial, or task switching effects. We used a pseudo‐randomized, self‐paced design with maximal response duration of 60 s for each trial, presented using E‐prime software (Psychological Software Tools, Pittsburgh, PA). Task stimuli were projected on a screen at the end of the scanner table. A mirror allowed subjects to see the projection screen. Participants' responses and response durations were registered using two magnet‐compatible button boxes. No feedback regarding the answers was provided during the task.
Before the first session, subjects received instructions and a standardized training of 10 items, two at each level of difficulty in which errors were corrected. All subjects understood the task rules and instructions.
TMS Parameters
TMS treatment was applied using a hand‐held figure‐of‐eight TMS coil (Medtronic MagOption). First, we localized the hand area of left primary motor cortex by eliciting a robust motor evoked potential. We then determined the motor threshold by gradually decreasing the amplitude of the single pulse over the primary motor cortex until the minimum amplitude of stimulation resulted in a visually detectable muscle twitch in the right hand in 5 out of 10 trials. TMS was then applied at a frequency of 1 Hz and an amplitude of 90% of the motor threshold at a distance of 5‐cm anterior to the located left primary motor cortex in the sagittal plane. Sham stimulation was performed in the same way, but with the TMS coil placed on a participant's head at a 90° angle. Participants were naïve to rTMS and blind to stimulation condition.
Data Acquisition
MR images were acquired using a Philips Intera 3T MR‐system with a sense‐8 head coil. To reduce motion artifacts, the subject's head was immobilized in the head coil using foam pads. Anatomic imaging included a sagittal 3D gradient‐echo T1‐weighted sequence (matrix 256 × 256 pixels, voxel size 1 × 1 × 1 mm, 170 sections). For fMRI, an echo planar imaging (EPI) sequence was used (TR 2.30 s, TE 30 ms, matrix 96 × 96 pixels, field of view 220 × 220 mm, flip angle 80°), creating transversal whole‐brain acquisitions (35 slices, 2.3 × 2.3 mm in‐plane resolution, 3‐mm slice thickness, no interslice gap). Slices were angled parallel to the orbital frontal surface. In total, 393–410 EPI volumes were collected per subject in ∼15 min. This ensures that the task was performed in at most 32 min after cessation of the stimulation, intended to fall within the presumed time‐window of cortical modulation of function.
Data Analyses
Imaging data were analyzed using FEAT (FMRI Expert Analysis Tool, version 5.92), a software package available within the FMRIB's Software Library (FSL, version 4.0). Prestatistic processing consisted of motion correction, removal of nonbrain structures from the EPI volumes, spatial smoothing by using a Gaussian kernel of 6 mm, and a high‐pass filter cutoff of 50 s. First‐level analyses, that is, individual planning versus baseline comparisons (“planning” contrast) and regressions across task load (“task load” contrast), were carried out using FILM (FMRIB's Improved Linear Model), a voxelwise time series analysis, based on general linear modeling. For higher‐level analyses we used FLAME (FMRIB's Local Analysis of Mixed Effects), for modeling and estimating the random‐effects component of the measured intersession mixed‐effects variance, to accurately estimate the true random‐effects variance and degrees of freedom at each voxel. In the model, event variables were defined according to task level. As the two conditions were performed in the same subjects in counterbalanced order, the design investigated within‐subject differences, thereby reducing possible variance caused by individual characteristics such as age or gender. Order of condition was entered as a separate regressor of no interest, to account for rTMS‐by‐condition effects, similar to the analysis of the behavioral data. Main effects at group‐level were considered significant at a cluster‐corrected z‐threshold of 3.1 and cluster P threshold of 0.05, and are therefore sensitive to extensive activations. BOLD signal contrasts between the real and sham TMS conditions were considered significant at a threshold of P < 0.001, uncorrected, to be sensitive to smaller but meaningful differences between the groups. To further avoid false‐positive findings, we reduced the search volume for the condition contrast analysis by masking with the appropriate main effect activation maps (i.e., task load or planning).
To test possible effects of stimulation on cortico‐striatal interactions, we conducted a follow‐up analysis of functional connectivity. From each individual functional acquisition, we extracted the mean time series of the left caudate nucleus using the Harvard‐Oxford Subcortical Structural atlas in Featquery. We then entered the mean time series into a general linear model with the filtered and motion corrected functional scans of each subject in both conditions to obtain subject/condition specific maps of connectivity between the left caudate and the rest of the brain. We subsequently computed the groupwise mean of the functional connectivity for each experimental condition using one‐sample t‐tests; and the differences between the two conditions in a within‐subject repeated measures design, with order of the conditions as a regressor of no interest in accord with the task‐related analysis, and masked for the sham groupwise mean connectivity map. Differences between conditions were considered significant at a z‐threshold of > 3.1, cluster corrected (P = 0.05).
RESULTS
Behavioral Results
Task onset was between 12 and 17 min after the end of transcranial stimulation, such that task completion was within 27 and 32 min after stimulation. All subjects completed the task successfully, with a mean error rate of less than 13% even during the highest task load (five steps). Task load showed a significant effect for task accuracy (Huynh–Feldt: F = 18.646, df = 2.730, P < 0.001) and latency (Huynh–Feldt: F = 64.704, df = 1.193, P < 0.001). There was no effect of rTMS condition on either accuracy (F = 0.145, df = 1, P = 0.712) or latency (F = 0.026, df = 1, P = 0.875). There was, however, a stimulation condition‐by‐order effect (F = 6.378, df = 1, P = 0.032), showing that those subjects who had real rTMS treatment in the first session showed significantly impaired planning compared to sham rTMS (see Fig. 1). The disruptive rTMS effect was not present in subjects who received real rTMS in the second session. This stimulation condition‐by‐order effect was not significant at the level of response latency (F = 1.234, df = 1, P = 0.293).
Figure 1.
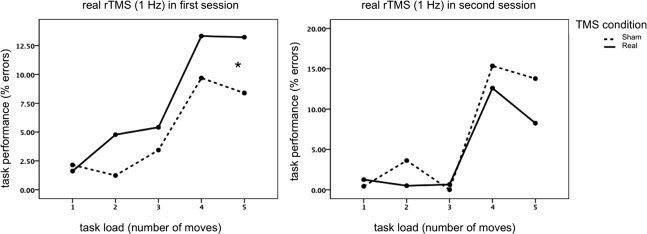
rTMS reduces cognitive performance. Task performance shows a significant TMS condition‐by‐order effect: only in those subjects who had real rTMS in the first session task performance was significantly impaired (increased % errors).
Neural Correlates
Main effects of task, both planning versus baseline and task load effects (see Table I, Fig. 2A,B), show the expected recruitment of frontal‐striatal and visuospatial brain regions for both rTMS treatment conditions. Task‐by‐rTMS condition interaction effects were most robust for the planning versus baseline analysis (see Fig. 2C), showing decreased activation after real rTMS compared with sham rTMS in prefrontal brain regions (i.e., premotor, dorsolateral prefrontal and anterior prefrontal cortices) and visuospatial brain regions (i.e., precuneus/cuneus and inferior parietal cortex). This inhibitory rTMS effect was more subtle in the task load analysis, showing decreased activation of the right superior parietal and left premotor cortices (see Table II).
Table I.
Main effects of task for both TMS conditions
| Sham rTMS | Real rTMS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI coordinates | MNI coordinates | |||||||||||
| Region | Side | BA | x | y | z | z‐score | Cluster size | x | y | z | z‐score | Cluster size |
| Planning versus baseline | ||||||||||||
| Premotor cortex | L | 6 | −20 | 2 | 66 | 5.62 | 2,396 | −22 | −8 | 64 | 5.18 | 1,123 |
| R | 6 | 28 | −2 | 66 | 4.89 | 820 | 24 | −4 | 68 | 4.82 | 505 | |
| SMA | bil. | 6 | −2 | 16 | 50 | 4.33 | 255 | |||||
| Dorsolateral PFC | R | 8 | 36 | 30 | 40 | 3.56 | 76 | 48 | 28 | 44 | 3.99 | 84 |
| L | 8 | −42 | 16 | 38 | 3.40 | 6 | ||||||
| L | 9 | −36 | 30 | 28 | 5.02 | 384 | −40 | 28 | 32 | 3.76 | 99 | |
| R | 9 | 54 | 34 | 26 | 3.42 | 25 | 52 | 36 | 26 | 4.91 | 101 | |
| L | 46 | −36 | 46 | 10 | 3.33 | 6 | ||||||
| Anterior PFC | R | 10 | 32 | 58 | −2 | 3.87 | 43 | |||||
| Ventrolateral PFC | L | 44 | −54 | 8 | 4 | 4.15 | 142 | |||||
| Insula, operculum | R | 13 | 34 | 24 | 2 | 3.79 | 76 | 32 | 26 | −6 | 4.38 | 79 |
| L | 13 | −28 | 20 | 2 | 4.39 | 610 | −30 | 22 | 0 | 4.42 | 136 | |
| Caudate nucleus | L | −16 | 16 | 2 | 3.97 | −10 | 8 | −4 | 4.14 | 150 | ||
| R | 14 | 16 | −2 | 4.29 | 336 | 10 | 14 | −2 | 4.14 | 170 | ||
| R | 10 | 2 | 10 | 3.28 | 10 | |||||||
| Precuneus | bil. | 7 | 10 | −64 | 58 | 5.42 | 2,652 | 12 | −66 | 58 | 4.76 | 2,113 |
| Sup. parietal cortex | L | 40 | −56 | −42 | 40 | 3.32 | 13 | |||||
| R | 40 | 42 | −40 | 58 | 4.27 | 444 | 40 | −42 | 54 | 4.65 | 221 | |
| L | 40 | −66 | −32 | 30 | 4.15 | 119 | −66 | −32 | 34 | 4.77 | 49 | |
| R | 40 | 62 | −28 | 38 | 4.73 | 85 | 62 | −36 | 36 | 3.65 | 20 | |
| Lat. occipital cortex | L | 19 | −44 | −78 | 20 | 4.14 | 171 | −42 | −80 | 36 | 5.34 | 170 |
| R | 19/39 | 44 | −70 | 16 | 3.84 | 101 | 44 | −52 | 34 | 4.08 | 37 | |
| R | 39 | 42 | −74 | 22 | 3.70 | 50 | ||||||
| L | 37 | −60 | −64 | −12 | 3.69 | 70 | ||||||
| L | 37 | −40 | −64 | 6 | 3.47 | 14 | ||||||
| Task load | ||||||||||||
| Dorsolateral PFC | L | 46 | −42 | 26 | 34 | 5.24 | 624 | −42 | 26 | 36 | 5.14 | 3,235 |
| Premotor cortex | L | 8 | −20 | −2 | 66 | 5.35 | 1,713 | −24 | −2 | 68 | 5.80 | |
| Dorsolateral PFC | R | 46 | 44 | 28 | 42 | 5.10 | 1,134 | 40 | 30 | 42 | 4.81 | 1,652 |
| Premotor cortex | R | 6 | 28 | 12 | 56 | 4.02 | 24 | 2 | 64 | 5.21 | ||
| SMA | L | 6 | −2 | 22 | 42 | 4.56 | 271 | |||||
| Anterior PFC | L | 10 | −42 | 46 | 6 | 3.35 | 15 | −24 | 58 | 6 | 4.09 | 79 |
| R | 11 | 24 | 58 | −12 | 3.34 | 6 | 22 | 54 | −20 | 3.49 | 13 | |
| R | 10/11 | 52 | 48 | −10 | 3.40 | 6 | 32 | 60 | 2 | 4.64 | 49 | |
| Ventrolateral PFC | L | 44 | −54 | 12 | 8 | 3.87 | 58 | −54 | 8 | 16 | 3.27 | 6 |
| L | 45 | −44 | 18 | 16 | 3.46 | 15 | ||||||
| Orbitofrontal PFC | L | 11/47 | −32 | 24 | −2 | 3.47 | 7 | −30 | 22 | −4 | 3.95 | 45 |
| L | 11/47 | −22 | 34 | −22 | 4.04 | 39 | ||||||
| Caudate nucleus | L | −14 | 6 | 16 | 3.21 | 332 | −16 | 8 | 20 | 4.11 | 644 | |
| Globus pallidum | L | −20 | 2 | 0 | 4.16 | −16 | 8 | 0 | 4.15 | |||
| Putamen | L | −16 | 12 | 2 | 3.83 | |||||||
| Caudate nucleus | R | 18 | 20 | −2 | 4.03 | 246 | 14 | 18 | 4 | 4.38 | 823 | |
| Globus pallidum | R | 14 | 4 | −4 | 3.52 | 18 | 18 | 8 | 4.49 | |||
| Thalamus | bil. | 0 | −4 | 6 | 3.69 | 53 | ||||||
| bil. | −4 | −26 | 10 | 4.14 | 284 | |||||||
| Precuneus | bil. | 7/19 | 10 | −62 | 60 | 5.29 | 2,283 | −6 | −68 | 56 | 5.05 | 3,117 |
| Sup. parietal cortex | L | 40 | −62 | −32 | 34 | 3.39 | 14 | −66 | −32 | 34 | 4.36 | 106 |
| L | 40 | −58 | −38 | 46 | 3.61 | 53 | ||||||
| L | 40 | −42 | −50 | 52 | 3.84 | 32 | ||||||
| R | 40 | 48 | −46 | 48 | 3.29 | 13 | 44 | −56 | 34 | 4.15 | 45 | |
| R | 40 | 62 | −44 | 34 | 3.85 | 45 | 64 | −32 | 36 | 3.69 | 19 | |
| R | 40 | 54 | −50 | 42 | 3.42 | 12 | ||||||
| Lat. occipital cortex | L | 19 | −38 | −62 | 38 | 3.61 | 19 | −38 | −64 | 38 | 3.93 | 34 |
| L | 19/39 | −48 | −80 | 24 | 3.54 | 14 | −44 | −80 | 36 | 5.18 | 230 | |
| R | 19/39 | 52 | −66 | 22 | 3.66 | 56 | 44 | −76 | 24 | 3.76 | 48 | |
Effects of task (both planning vs. baseline and task load effects) in both TMS stimulation conditions (sham rTMS and real rTMS), all BOLD response differences with a statistical significance of P < 0.001 uncorrected for multiple comparisons are presented.
rTMS, repetitive transcranial magnetic stimulation; BA, Brodmann area; MNI, Montreal Neurological Institute; SMA, supplementary motor area; PFC, prefrontal cortex.
Figure 2.
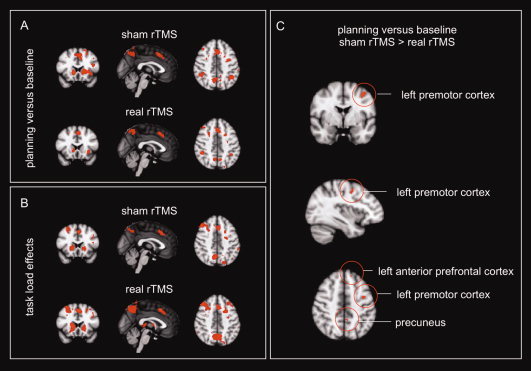
rTMS reduces task‐related activation. Neural correlates of planning performance (both planning vs. baseline and task load effects) for both rTMS conditions separately (A and B: activations significant at z > 3.1, cluster‐corrected) and the task‐by‐rTMS condition interaction effects, (C): presented at a significance threshold of P < 0.001 uncorrected for multiple comparisons.
Table II.
Task‐by‐TMS condition interaction effects
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Region | Side | BA | x | y | z | z‐score | Cluster size |
| Planning vs. baseline, sham rTMS > real rTMS | |||||||
| Premotor cortex | L | 6 | −38 | −2 | 50 | 3.76 | 41 |
| L | 9 | −46 | 2 | 32 | 3.25 | 4 | |
| Dorsolateral PFC | L | 46 | −42 | 16 | 18 | 3.19 | 2 |
| L | 46 | −54 | 16 | 30 | 3.25 | 5 | |
| Anterior PFC | R | 10 | 26 | 52 | 20 | 3.14 | 4 |
| L | 6 | −12 | 42 | 44 | 3.18 | 4 | |
| Ventrolateral PFC | R | 45 | 50 | 38 | 0 | 3.20 | 5 |
| Precuneus | bil. | 7 | −8 | −54 | 70 | 4.00 | 41 |
| bil. | 7/31 | −4 | −46 | 46 | 3.29 | 19 | |
| R | 7 | 8 | −54 | 56 | 3.31 | 4 | |
| Cuneus | R | 31 | 14 | −82 | 30 | 3.47 | 25 |
| Inferior parietal cortex | L | 40 | −48 | −60 | 40 | 3.26 | 22 |
| Planning vs. baseline, real rTMS > sham rTMS | |||||||
| No significant results | |||||||
| Task load effects, sham rTMS > real rTMS | |||||||
| Superior parietal cortex | R | 40 | 44 | −52 | 44 | 3.12 | 1 |
| Premotor cortex | L | 6 | −40 | 0 | 24 | 3.11 | 1 |
| Task load effects, real rTMS > sham rTMS | |||||||
| No significant results | |||||||
Task × rTMS condition interaction effects for planning versus baseline and task load effects; all brain regions with a statistical significance of P < 0.001 uncorrected for multiple comparisons are presented.
rTMS, repetitive transcranial magnetic stimulation; BA, Brodmann area; MNI, Montreal Neurological Institute; PFC, prefrontal cortex.
In line with the behavioral results, the neural response showed an rTMS condition‐by‐order effect for planning versus baseline (in the left motor cortex; x, y, z = −42, −2, 54; z‐score = 3.27; cluster size = 4) and for task load (in the left premotor cortex; x, y, z = −44, 0, 28; z‐score = 3.38; cluster size = 19). Posthoc analyses verified that this effect derived from the fact that those subjects who received real rTMS in the first session showed decreased activation in these regions after real rTMS compared with sham rTMS.
The follow‐up functional connectivity analysis using the left caudate as a seed region showed no differences in connectivity with the frontal cortical regions between the two conditions. Rather, a single cluster extending bilaterally in the precuneus (maximum at −10, −70, 18; cluster size 397 voxels) showed reduced connectivity with the left caudate after rTMS as compared with sham.
DISCUSSION
The present results clearly show that inhibition of left dorsal prefrontal excitability by low‐frequency repetitive transcranial magnetic modulation results in decreased planning performance and decreased recruitment of task‐related frontal and visuospatial brain regions. Behaviorally, this rTMS effect was significant only in subjects who received real rTMS in the first session, therefore in a situation of limited task experience. Exposure to a task involves learning of the stimulus‐response contingencies and task demands; we interpret the relative insensitivity to disruption of the subjects who receive verum rTMS on the second session as a “protection” against disruption due to prior training on the task, indicating that perhaps such training has led task performance to depend on subtly different brain circuits. Such a condition‐by‐order effect corresponds with the results of a recent tDCS study on planning performance, showing an effect on cathodal stimulation on performance of the Tower of London task only when the stimulation occurred during the acquisition and early consolidation phase [Dockery et al.,2009]; in their study, however, both excitatory and inhibitory stimulation improved performance; as yet the results do therefore not correspond to the rTMS findings reported here, but it is difficult to compare different types of stimulation. In addition, the study did not perform neural imaging so a comparison at the level of regional (in) activations cannot be done. In our study, real rTMS caused subjects to recruit the anterior prefrontal cortex in the planning versus baseline comparison, whereas in the placebo condition, activation of this area occurs only at higher planning loads requiring more elaborate planning [van den Heuvel et al.,2003]. One may therefore conclude that in the real rTMS condition, brain regions specifically involved in higher order cognitive processes are already recruited at lower task load and fail at higher task load, as reflected by disrupted task performance (see Fig. 1). Also, it should be noted that the observed BOLD effects are not confounded by behavioral differences, as BOLD contrasts were based on correctly answered trials only. Decreases of activation after TMS can therefore be seen as reductions of capacity, not of performance.
It is of note that the task‐related brain activation differences induced by rTMS appear not only in the area directly under the stimulating coil, that is, the left DLPFC, but also in areas distal to the coil, that is, the premotor cortex, precuneus, parietal, and middle temporal cortices. We take this to indicate that the effects of rTMS are not limited to the area stimulated, but in fact change activity in the circuit that the area is a part of, by way of its neuroanatomical connectivity, in accord with findings of distal brain activity changes upon rTMS [Cho and Strafella,2009; Chouinard et al.,2003; van der Werf et al.,2010]. The combination of areas showing changes due to rTMS would depend on the functional state of the brain, in this case involved in executive functioning; the areas affected are part of the network involved in executive task performance.
Interestingly, we observed no differences in striatal activations despite the fact that the task appears to depend heavily on striatal activation, as borne out by robust activations in both the task load and planning. To further investigate possible rTMS‐induced changes in striatal function, we conducted a follow‐up analysis of functional connectivity using the left caudate as a seed region, that is, ipsilateral to the stimulated hemisphere. This analysis revealed an increase in connectivity with the precuneus after rTMS, in a region overlapping with the medial posterior component of the so‐called default‐mode network. It appears that the cortical part of the task‐related network shows greater sensitivity to the effects of rTMS, such that caudate activity, normally associated with frontal cortical activity during executive task performance, is driven more toward nontask‐related resting state brain activity.
The combined use of rTMS and task‐related fMRI enables the study of both behavioral and neural effects of experimental modulation of brain excitability. The results show a temporary mimicking of frontal‐striatal failure in healthy controls as a result of low‐frequency rTMS. Based on these results, we may better understand the frontal‐striatal dysfunctions in patients with, for instance, obsessive‐compulsive disorder and Parkinson's disease that have been documented [Cools et al.,2010; Dagher and Nagano‐Saito,2007; Lewis et al.,2003; Owen,2004; van den Heuvel et al.,2005,2011]. One might hypothesize that high‐frequency, instead of low‐frequency rTMS on the left DLPFC of these patients normalizes executive functioning in these patients. Future studies using experimental modulation of frontal‐striatal circuits in patients may therefore contribute to discovering novel treatment options.
Based on these results, we can only speculate as to the mechanism of action of rTMS on cerebral activation. A tempting explanation is that at least part of the effect would be mediated by an rTMS‐driven mitigated release of neurotransmitters such as dopamine. Strafella and coworkers have shown that high‐frequency stimulation of the left DLPFC leads to dopamine release in both cortical and subcortical task‐relevant regions, measured during quiet wakefulness [Cho and Strafella,2009; Strafella et al.,2001]. In addition, they have shown that left, but not right, inhibitory DLPFC stimulation disrupted executive functioning and concomitant dopamine release, strengthening the idea that dopamine changes may underlie the observed performance and brain activation changes induced by rTMS [Ko et al.,2008a].
We chose to stimulate the left DLPFC as the Tower of London task appears to depend more on the left than the right frontal cortex, as borne out by lesion studies and functional activation studies [Owen et al.,1996; Shallice,1982; van den Heuvel et al.,2003]. In addition, some rTMS studies aimed at disrupting executive function have been more successful on the left than the right DLPFC [Ko et al.,2008a], whereas for other tasks this contingency may be different [Rounis et al.,2006,2007].
This study is not without methodological limitations. Coil placement for rTMS treatment was based on the assumption that the DLPFC is about 5‐cm anterior to the primary motor cortex. Considering the high intersubject variability in brain size and gyrification, this is likely an oversimplification, introducing the risk of imperfect coil localization [Sack et al.,2009]. In current and future studies, individual task‐related fMRI‐based neuronavigation will be used to obtain optimal targeting. The present method does, on the other hand, indicate that behavioral TMS‐induced changes can be achieved in an easily manageable fashion, which is interesting for potential future use in clinical settings. Another methodological issue is the use of off‐line rTMS. Because of the transient effect of rTMS, there is only a small time frame to measure the effect of stimulation. This might be solved by on‐line stimulation using MRI‐compatible TMS equipment and/or the use of stimulation paradigms with longer lasting effects, for example, θ‐burst stimulation.
CONCLUSION
As far as we know, this is the first study combining rTMS and fMRI to study the underlying neural correlates of planning performance. Here, we show a reduction of task‐related brain activity and task performance by a preceding treatment of low‐frequency rTMS. This illustrates the potential of rTMS as an experimental tool to modulate brain circuits and cognitive performance. For instance, if low‐frequency rTMS serves to lower prefrontal functioning and to impair performance, high‐frequency rTMS might conversely be used to improve impaired executive functioning in patients with frontal‐striatal disorders, like those observed in psychiatric and neurological disease.
Acknowledgements
The authors thank Maloe Hulst and Sanne Menning for contribution to data collection, Aart Nederveen for technical support, and Eus van Someren for support in equipment.
REFERENCES
- Barrett J, Della‐Maggiore V, Chouinard PA, Paus T ( 2004): Mechanisms of action underlying the effect of repetitive transcranial magnetic stimulation on mood: Behavioral and brain imaging studies. Neuropsychopharmacology 29: 1172–1189. [DOI] [PubMed] [Google Scholar]
- Chen R ( 2000): Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve 9: S26–S32. [DOI] [PubMed] [Google Scholar]
- Cho SS, Strafella AP ( 2009): rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One 4: e6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard PA, Van Der Werf YD, Leonard G, Paus T ( 2003): Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. J Neurophysiol 90: 1071–1083. [DOI] [PubMed] [Google Scholar]
- Cools R, Miyakawa A, Sheridan M, D'Esposito M ( 2010): Enhanced frontal function in Parkinson's disease. Brain 133: 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher A, Nagano‐Saito A ( 2007): Functional and anatomical magnetic resonance imaging in Parkinson's disease. Mol Imaging Biol 9: 234–242. [DOI] [PubMed] [Google Scholar]
- Dockery CA, Hueckel‐Weng R, Birbaumer N, Plewnia C ( 2009): Enhancement of planning ability by transcranial direct current stimulation. J Neurosci 29: 7271–7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbe E, Schlegel M, Sack AT, Nowak DA, Dafotakis M, Bangard C, Brand M, Shamay‐Tsoory S, Onur OA, Kessler J ( 2010): Dissociating cognitive from affective theory of mind: A TMS study. Cortex 46: 769–780. [DOI] [PubMed] [Google Scholar]
- Ko JH, Monchi O, Ptito A, Bloomfield P, Houle S, Strafella AP ( 2008a): θ burst stimulation‐induced inhibition of dorsolateral prefrontal cortex reveals hemispheric asymmetry in striatal dopamine release during a set‐shifting task: A TMS‐[(11)C]raclopride PET study. Eur J Neurosci 28: 2147–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Monchi O, Ptito A, Petrides M, Strafella AP ( 2008b): Repetitive transcranial magnetic stimulation of dorsolateral prefrontal cortex affects performance of the wisconsin card sorting task during provision of feedback. Int J Biomed Imaging 2008: 143–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM ( 2003): Cognitive impairments in early Parkinson's disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci 23: 6351–6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanby SH, Kinnunen LH, Crupain MJ ( 2002): Applications of TMS to therapy in psychiatry. J Clin Neurophysiol 19: 344–360. [DOI] [PubMed] [Google Scholar]
- Loo C, Mitchell P, Sachdev P, McDarmont B, Parker G, Gandevia S ( 1999): Double‐blind controlled investigation of transcranial magnetic stimulation for the treatment of resistant major depression. Am J Psychiatry 156: 946–948. [DOI] [PubMed] [Google Scholar]
- Munchau A, Bloem BR, Irlbacher K, Trimble MR, Rothwell JC (2002): Functional connectivity of human premotor and motor cortex explored with repetitive transcranial magnetic stimulation. J Neurosci 22:554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM ( 2004): Cognitive dysfunction in Parkinson's disease: The role of frontostriatal circuitry. Neuroscientist 10: 525–537. [DOI] [PubMed] [Google Scholar]
- Owen AM, Doyon J, Petrides M, Evans AC ( 1996): Planning and spatial working memory: A positron emission tomography study in humans. Eur J Neurosci 8: 353–364. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD ( 1998): Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol 15: 333–343. [DOI] [PubMed] [Google Scholar]
- Paus T ( 2005): Inferring causality in brain images: A perturbation approach. Philos Trans R Soc Lond B Biol Sci 360: 1109–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth HL, Nadeau SE, Triggs WJ ( 2004): Effect of repetitive transcranial magnetic stimulation on rate of memory acquisition. Neurology 63: 1530–1531. [DOI] [PubMed] [Google Scholar]
- Rounis E, Lee L, Siebner HR, Rowe JB, Friston KJ, Rothwell JC, Frackowiak RS ( 2005): Frequency specific changes in regional cerebral blood flow and motor system connectivity following rTMS to the primary motor cortex. Neuroimage 26: 164–176. [DOI] [PubMed] [Google Scholar]
- Rounis E, Stephan KE, Lee L, Siebner HR, Pesenti A, Friston KJ, Rothwell JC, Frackowiak RS ( 2006): Acute changes in frontoparietal activity after repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex in a cued reaction time task. J Neurosci 26: 9629–9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounis E, Yarrow K, Rothwell JC ( 2007): Effects of rTMS conditioning over the fronto‐parietal network on motor versus visual attention. J Cogn Neurosci 19: 513–524. [DOI] [PubMed] [Google Scholar]
- Sack AT, Kohler A, Bestmann S, Linden DE, Dechent P, Goebel R, Baudewig J ( 2007): Imaging the brain activity changes underlying impaired visuospatial judgments: Simultaneous FMRI, TMS, and behavioral studies. Cereb Cortex 17: 2841–2852. [DOI] [PubMed] [Google Scholar]
- Sack AT, Cohen Kadosh R, Schuhmann T, Moerel M, Walsh V, Goebel R ( 2009): Optimizing functional accuracy of TMS in cognitive studies: A comparison of methods. J Cogn Neurosci 21: 207–221. [DOI] [PubMed] [Google Scholar]
- Shallice T ( 1982): Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci 298: 199–209. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Peller M, Willoch F, Minoshima S, Boecker H, Auer C, Drzezga A, Conrad B, Bartenstein P ( 2000): Lasting cortical activation after repetitive TMS of the motor cortex: A glucose metabolic study. Neurology 54: 956–963. [DOI] [PubMed] [Google Scholar]
- Speer AM, Kimbrell TA, Wassermann EM, J DR, Willis MW, Herscovitch P, Post RM ( 2000): Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry 48: 1133–1141. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A ( 2001): Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci 21: RC157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touge T, Gerschlager W, Brown P, Rothwell JC (2001): Are the after‐effects of low‐frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clin Neurophysiol 112:2138–2145. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Groenewegen HJ, Barkhof F, Lazeron RH, van Dyck R, Veltman DJ ( 2003): Frontostriatal system in planning complexity: A parametric functional magnetic resonance version of Tower of London task. Neuroimage 18: 367–374. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Balkom AJ, van Hartskamp J, Barkhof F, van Dyck R ( 2005): Frontal‐striatal dysfunction during planning in obsessive‐compulsive disorder. Arch Gen Psychiatry 62: 301–309. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Mataix‐Cols D, Zwitser G, Cath DC, van der Werf YD, Groenewegen HJ, van Balkom AJ, Veltman DJ ( 2011): Common limbic and frontal‐striatal disturbances in patients with obsessive compulsive disorder, panic disorder and hypochondriasis. Psychol Med; doi:10 1017/S0033291711 000535: 1–12. [DOI] [PubMed] [Google Scholar]
- van der Werf YD, Paus T ( 2006): The neural response to transcranial magnetic stimulation of the human motor cortex. I. Intracortical and cortico‐cortical contributions. Exp Brain Res 175: 231–245. [DOI] [PubMed] [Google Scholar]
- van der Werf YD, Sanz‐Arigita EJ, Menning S, van den Heuvel OA ( 2010): Modulating spontaneous brain activity using repetitive transcranial magnetic stimulation. BMC Neurosci 11: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM, Lisanby SH ( 2001): Therapeutic application of repetitive transcranial magnetic stimulation: A review. Clin Neurophysiol 112: 1367–1377. [DOI] [PubMed] [Google Scholar]
- Watkins LH, Rogers RD, Lawrence AD, Sahakian BJ, Rosser AE, Robbins TW ( 2000): Impaired planning but intact decision making in early Huntington's disease: Implications for specific fronto‐striatal pathology. Neuropsychologia 38: 1112–1125. [DOI] [PubMed] [Google Scholar]
- Williams‐Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA ( 2007): Catechol O‐methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson's disease. J Neurosci 27: 4832–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]


