Abstract
Although the cortical circuitry underlying saccade execution has well been specified by neurophysiological and functional imaging studies, the temporal dynamics of cortical activity predicting the occurrence of voluntary or reflexive saccades in humans are largely unknown. Here, we examined electrophysiological activity preceding the onset of correct (i.e., voluntary) or error (i.e., reflexive) saccades in an oculomotor capture task. Participants executed saccades to lateralized visual targets while attempting to inhibit reflexive glances to abruptly appearing distracters. Since the visual display was identical for both types of saccades, different electrophysiological patterns preceding correct and error saccades could not be explained by low‐level perceptual differences. Compared to correct saccades electrophysiological activity preceding error saccades showed significant differences of the scalp electric field and of voltage amplitudes at posterior electrodes. In addition, though error saccades had significantly shorter latency than correct saccades a prolonged topographic configuration of electric potentials prior to error saccades was found ∼120–140 ms following target onset. In agreement with the known asymmetry in hemispheric dominance for spatial attention, distinct electrophysiological patterns were only found for leftward saccades. While error saccades were associated with stronger activity in the right Frontal Eye Field, correct saccades were preceded by stronger activity in the inferior parietal lobule. These findings suggest that selection of the saccade target in a conflicting situation is determined by early top‐down biases originating in frontal and parietal cortical regions critical for spatial attention and saccade programming. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: saccade, visual attention, electrophysiology, frontal eye fields, inferior parietal lobe
INTRODUCTION
Visual exploration of the environment is a highly dynamic process that generates on average three gaze shifts per second [Findlay and Gilchrist, 2003; Henderson and Hollingworth, 1999]. Though saccades may be directed voluntarily to a region of the scene that is of particular behavioral significance [Antes, 1974; Yarbus, 1967], many saccades are triggered reflexively by a salient stimulus such as a moving shape or a suddenly appearing light [Itti and Koch, 2000; Parkhurst et al., 2002; Walker et al., 2000].
The cortical circuitry underlying saccade generation has well been specified. Neurophysiological, neuropsychological, and functional brain imaging studies have identified a frontoparietal network including the frontal eye fields (FEF), the supplementary eye fields (SEF), and the intraparietal sulcus (IPS) that is consistently active when participants make saccades or shift attention covertly [Corbetta et al., 1998; Everling and Fischer, 1998; Johnston and Everling, 2008; Luna et al., 1998; Müri et al., 1996; Perry and Zeki, 2000; Pierrot‐Deseilligny et al., 2002; Schall and Thompson, 1999]. Much less is known about the temporal dynamics of cortical activity associated with the generation of voluntary and reflexive saccades in humans. Brain‐imaging techniques that are based on hemodynamic measures fail to distinguish between activity related to saccade programming and saccade execution. Event‐related potential (ERP) techniques have the temporal resolution necessary for the assessment of presaccadic cortical activity [Evdokimidis et al., 1996; Evdokimidis et al., 1992; Everling et al., 1997; Everling et al., 1998; Moster and Goldberg, 1990]. However, since experimental conditions inducing voluntary and reflexive saccades often considerably differ, comparing electrophysiological dynamics of these two saccade types is problematic. For example, in the antisaccade task activity related to the voluntary saccade directed opposite a peripheral target not only reflects saccade programming, but also inhibition of a reflexive saccade toward the target and reorienting of attention [Hallett, 1978; Munoz and Everling, 2004]. Likewise, the peripheral stimulus that triggers reflexive saccades in the cued saccade task results in substantially different visual stimulation than the directional cue that is presented at fixation and elicits voluntary saccades. Consequently, differences in early ERP components may reflect differences in experimental conditions rather than distinct mechanisms involved in voluntary and reflexive processes.
The present electrical neuroimaging study employed an oculomotor capture paradigm [Theeuwes et al., 1999], which instead of inducing voluntary and reflexive saccades by different visual stimulation, sets controlled and automatic processes in opposition. In this task, though instructed to execute saccades toward a visual target, participants make a significant proportion of error saccades toward a distracter stimulus that—by virtue of its abrupt onset—has high perceptual saliency. Since the visual stimulation and task requirements are constant, differential brain activity associated with correct (voluntary) and error (reflexive) saccades can only be explained by variables related to target selection or selection of the oculomotor response. Though the global conditions leading to oculomotor capture have well been specified in behavioral studies [Maljkovic and Nakayama, 1994; McPeek et al., 1999, 2000; Theeuwes and Godijn, 2004; Theeuwes et al., 1999], the reason why on some trials gaze is captured by the distracter, but is directed to the target on other trials is still unknown. Capture of attention by irrelevant distracters has been discussed in the context of two alternative hypotheses. According to the gain amplification hypothesis a distracter captures attention when top‐down signals fail to modulate the perceptual saliency of the target [Blaser et al., 1999; Carrasco et al., 2004; Hillyard et al., 1998; Pestilli and Carrasco, 2005]. In contrast, the noise reduction hypothesis postulates that capture occurs when top‐down processes fail to reduce the saliency of external noise [Lu and Dosher, 1998; Pashler, 1998]. Thus, both hypotheses suggest that top‐down attentional factors modulate early perceptual processing in the occipital and temporal cortex. Here, we show that the selection of the target of voluntary and reflexive saccades is reflected in distinct temporal patterns of early brain activity in the right hemisphere ∼120–140 ms after target onset, preceding saccade onset by more than 100 ms.
MATERIALS AND METHODS
Participants
Sixteen healthy subjects consented to participating in this study. The data of six subjects were subsequently excluded because they produced an insufficient number of saccade errors for EEG averaging. The remaining 10 participants (four females; mean age: 28.3 ± 5 years) were all right‐handers and had normal vision. The study was approved by the ethics committee at the University Hospitals, Geneva.
Stimuli and Procedure
The experiment was a simplified version of the oculomotor capture task by Theeuwes et al. [ 1999]. Each trial started with a fixation display, which contained a central fixation cross (0.7°) and four colored dots (either red or green), arranged on an imaginary circle (radius: 7°; Fig. 1). The dots were 1.5° large and were located at positions 2, 4, 8, and 10 o'clock. The fixation display was presented for 1,200 ms and was then replaced by the target display. The target display was similar to the fixation display except that it did not contain a fixation cross, and three of the four peripheral dots (stable distracters) changed color, while the fourth dot (saccade target) retained its original color. In addition, a fifth dot (onset distracter) was presented in the target display that had the same color as the saccade target (contingent condition) or the color of the stable distracters (noncontingent). The aim of varying the color of the onset distracter was to examine whether the tendency of participants to direct their initial saccade to the distracter was contingent on visual similarity between target and distracter, i.e., whether oculomotor capture was fully stimulus‐driven or contingent on the attentional set of the observer [Folk et al., 1992; Yantis and Jonides, 1990]. In pilot experiments, participants produced only few error saccades in the noncontingent condition; this condition was included for behavioral analysis, while the EEG analysis was restricted to the contingent condition.
Figure 1.
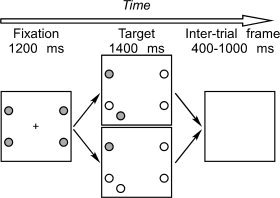
Stimulus display used in the experiment. The saccade target (in this example, the upper left dot) is revealed by a color change of the three stable distracters. Simultaneously, the onset distracter is presented. The upper panel shows a contingent display (colors of target and onset distracter are the same) while the lower panel shows a noncontingent display (colors of target and onset distracter differ). Colors: gray = red; white = green. Note that the target and distracters were shown on black background.
The onset distracter was always displayed in the same hemifield as the saccade target, but in a different quadrant. Thus, when the target was at 2 o'clock, the distracter was at 5 o'clock; a target at 4 o'clock had a distracter at 1 o'clock; a target at 8 o'clock had a distracter at 11 o'clock; and a target at 10 o'clock had a distracter at 7 o'clock. The target display was shown for 1,400 ms, and was followed by a blank screen for 400–1,000 ms, whereupon a new trial started.
Stimuli were presented on black background on a 21‐in. screen refreshed at 85 Hz and with a resolution of 1,280 × 1,024, which was placed at a distance of 65 cm. In half of the subjects the target was green and the onset distracter either green (contingent condition) or red (noncontingent condition), while the stable distracters were red; in the other half the colors were reversed. Participants were instructed to fixate the central cross while the fixation display was shown and to execute a saccade as quickly as possible toward the circle whose color remained constant. Stimuli were presented in blocks of 96 trials (7–10 blocks per participant), each containing a series of randomly intermixed target positions (left up or down and right up or down).
Acquisition and Analysis of Saccade Data
The horizontal and vertical position of the right eye was recorded with an infrared, video‐based system (HighSpeed; SMI, Germany), which has a sampling rate of 240 Hz and a spatial resolution better than 0.3°. Participants were positioned at the table with their head resting on a chin‐rest. The height of the table on which the eye‐tracker was installed, and the height of the chin‐rest were independently adjusted. The calibration procedure required participants to fixate sequentially on 13 small circles presented at different positions on the screen.
Saccades were extracted offline from the raw data using velocity (saccade onset: greater than 30°/s) and amplitude (>0.3°) criteria. Trials with saccade latency <80 ms (anticipations), > 800 ms (delayed) or blinks were excluded. Further, trials were excluded if gaze was >1.5° from the fixation cross (fixation loss), when the saccade landed in the hemifield that did not contain the target (direction error), or when saccade amplitude was <2° (significant undershot). Finally, to distinguish clearly saccades that were directed toward the target from saccades directed toward the distracter we included only saccades that landed at least 1° above or below the horizontal line separating the upper and lower hemifields. The resulting saccades belonged to one of eight conditions defined by saccade direction (left, right), distracter color (contingent, noncontingent), and saccade type (correct, error).
EEG Acquisition and Preprocessing
Continuous electroencephalogram (EEG) was acquired using the Biosemi EEG system (Biosemi V.O.F., Amsterdam, Netherlands) with 128 channels covering the entire scalp. The analysis was performed using the Cartool software (http://brainmapping.unige.ch). EEG signals were digitized at 1,024 Hz and filtered with a band‐pass between 0 and 208 Hz. As we focused our analysis on presaccadic activity, we determined the duration of epochs based on the analysis of saccade data, the criterion being that about 50% of all saccades start after the end of the epoch. This criterion reflects the compromise between considering a sufficiently long epoch to allow comparison with previous studies and limiting contamination of EEG data by potentials related to the motor execution of eye movements. For the analysis of target‐locked data we examined peri‐stimulus epochs of continuous EEG starting 100 ms prior to and ending 250 ms after onset of the saccade target. For the analysis of saccade‐locked data epochs started 350 ms prior to and ended simultaneously with the onset of the saccade.
Channels with artifacts were interpolated and artifact‐free epochs that satisfied the inclusion criteria defined by the eye movement analysis (see above) were averaged for each experimental condition to compute visual evoked potentials (VEPs). For all analysis, baseline was defined as the first 100 ms of the EEG epoch. The behavioral analysis showed that participants made too few error saccades when the color of the onset distracter was different from the target (see Results). The EEG analysis described below focused therefore on the comparison between correct and error saccades generated in response to visually identical displays in the contingent condition.
EEG Analyses and Source Estimation
In addition to prototypical event‐related potential analyses entailing area measures from selected scalp sites, VEPs associated with correct and error saccades were submitted to analysis of the electric field at the scalp. The first type of analysis was a topographic pattern (map) analysis, which attempts to identify topographic configurations of the electric field at the scalp [Murray et al., 2008]. Changes in the pattern of electrical topography across time or across conditions are indicative of differences in underlying generators [Fender, 1987]. The temporal distribution of maps (representing different functional microstates) was examined within and compared between conditions. This method is independent of the reference electrode and insensitive to pure amplitude modulations across conditions. A modified cross‐validation criterion determined the number of maps that explained the whole group‐averaged dataset [Pasqual‐Marqui et al., 1995]. The significance of the pattern of maps observed in the group‐averaged data was statistically tested by comparing each of these maps with the moment‐by‐moment scalp topography of individual VEPs. Each time point was labeled according to the map with which it best correlated spatially, yielding a measure of map presence that was in turn submitted to an ANOVA with factors condition (correct versus error) and map. This fitting procedure shows whether a given experimental condition is more often described by one map versus another, and therefore whether different generator configurations better account for particular experimental conditions. In addition to the analysis of the topographic pattern, changes in electric field strength were determined by analyzing the global field power (GFP) for each subject and each condition, a measure that reflects the spatial standard deviation of the electric field at the scalp [Lehmann and Skrandies, 1980]. Observation of a GFP modulation without accompanying topographic changes across experimental conditions is best explained by an amplitude modulation of statistically indistinguishable generators. The analysis of GFP reduces an observer bias that can follow from analysis restricted to specific selected electrodes, although we also present data from single electrodes to facilitate comparison with other studies. GFP area measures were tested with a paired t‐test against baseline (0 μV) in time periods of stable scalp topography. A modulation of the VEP was only considered significant when the t‐test exceeded the Bonferroni‐corrected 0.05 α‐criterion and when at least three electrodes exhibited differential responses at that time point. Finally, we estimated the sources in the brain underlying the VEPs that preceded correct or error saccades, using the local autoregressive average (LAURA) distributed linear inverse solution [Grave de Peralta Menendez et al., 2004]. The solution space comprised 3,005 points distributed on a spherical head model with anatomical constraints [Spinelli et al., 2001]. Source estimations were first calculated individually for each condition and each subject, and then statistically compared using a voxel‐by‐voxel t‐test.
RESULTS
Behavioral Results
Because of expected hemispheric differences in patterns of electrophysiological activity associated with saccades directed to the left versus saccades directed to the right, we analyzed behavioral responses as a function of the visual hemifield to which saccades were directed. All analyses were based on saccades that—according to the criteria defined in Methods—were classified either as correct (i.e., directed toward the target) or error saccade (i.e., directed to the onset distracter). Since the onset distracter was always presented in the same hemifield, but in the other quadrant than the target error saccades were easily distinguished from correct saccades based on their landing position. Figure 2 shows that the percentage of correct saccades was significantly higher when the distracter color was not contingent to the target color (left visual field, LVF: t(9) = 16.0, P < 0.0001; right visual field, RVF: t(9) = 10.3, P < 0.0001). In the contingent condition, approximately one third of all saccades were directed toward the distracter. Table I shows the latency and amplitude of saccades as a function of the visual hemifield and contingency (the low number of wrong saccades generated in the noncontingent condition did not permit statistical comparison). Neither latency nor amplitude differences were found between contingent and noncontingent displays. In contrast, in the contingent condition error saccades were ∼30 ms faster than correct saccades (LVF: t(9) = 5.8, P < 0.001; RVF: t(9) = 4.0, P < 0.01). Figure 3A displays a distribution of saccade latencies across the first 400 ms following target onset. The figure shows that the distribution of latencies of correct and error saccades was clearly distinct across the entire interval. Figure 3B shows the landing positions of correct and error saccades. To permit comparison between visual fields and upper/lower quadrants, data were normalized to fit into the upper left quadrant. The data shows that while correct saccades mostly landed on or close to the target (undershoot and overshoot being equally likely), error saccades mostly undershot the target. Consequently, the mean amplitude of error saccades was ∼20% smaller than the amplitude of correct saccades (Table I; LVF: t(9) = 11.9, P < 0.0001; RVF: t(9) = 10.7, P < 0.0001).
Figure 2.
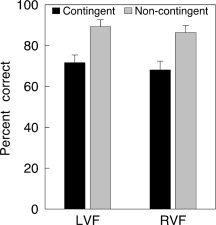
Percent correct saccades (i.e., saccades directed to the target, rather than to the onset distracter) in contingent and noncontingent conditions (LVF/RVF: left/right visual field).
Table I.
Saccade latencies and amplitudes as a function of contingency and visual hemifield
| Contingent | Noncontingent | |||||||
|---|---|---|---|---|---|---|---|---|
| Latency | Amplitude | Latency | Amplitude | |||||
| LVF | RVF | LVF | RVF | LVF | RVF | LVF | RVF | |
| Correct | 254 ± 27 | 247 ± 29 | 6.6 ± 0.2 | 6.9 ± 0.4 | 249 ± 27 | 244 ± 27 | 6.5 ± 0.2 | 6.9 ± 0.4 |
| Wrong | 221 ± 14 | 217 ± 18 | 5.4 ± 0.3 | 5.7 ± 0.6 | — | — | — | — |
LVF/RVF: left/right visual field.
Figure 3.
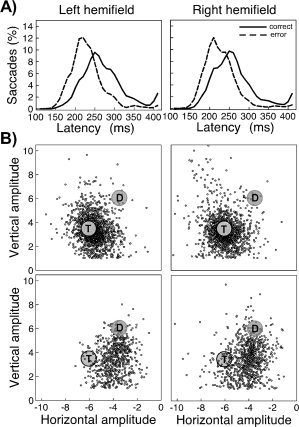
(A) Distribution of saccade latency as a function of saccade type. (B) Landing position of all correct (upper panels) and all error saccades (lower panels), collapsed over all 10 participants. The results are normalized with respect to the position of the target and onset distracter by projecting all landing positions into the upper left quadrant. T: target; D: onset distracter.
Electrophysiological Results: Target‐Locked Data
Previous studies have clearly established that modulations of electrophysiological activity preceding a saccade are stronger over the hemisphere contralateral to saccade direction [Evdokimidis et al., 1992; Moster and Goldberg, 1990]. In addition, clinical and functional brain imaging studies have revealed a hemispheric asymmetry in saccade programming [Muri and Nyffeler, 2008; Perry and Zeki, 2000], which was expected to significantly affect inverse solutions. For these reasons we performed separate analysis for saccades directed to the LVF and saccades directed to the RVF. Electrophysiological analyses were restricted to the contingent condition, which was the only condition that provided enough correct and error saccade trials.
Left Saccades
Because of the greater frequency of correct saccades the number of EEG epochs used for averaging was normalized between conditions for each participant. The average number of epochs was 73 ± 6 for correct saccades and 70 ± 20 for error saccades, which was statistically comparable (t(9) = 0.5, n.s.). To identify electrode amplitude differences in the whole electrode set between EEG activities preceding correct and error saccades, we computed point‐wise Bonferroni‐corrected t‐tests over the epoch covering 0–250 ms following target onset. These tests identified significant differences at right posterior and central electrode sites during a time‐period between 120 and 180 ms (Fig. 4A, left panel). Figure 5 shows grand average amplitude modulations at nine individual electrodes localized over anterior, central, and posterior scalp sites as a function of saccade type (correct or error saccade), together with significant differences between conditions. Significant differences involved in particular the P1 component at PO3 and POz and the N1 component at PO3, PO4, and POz.
Figure 4.
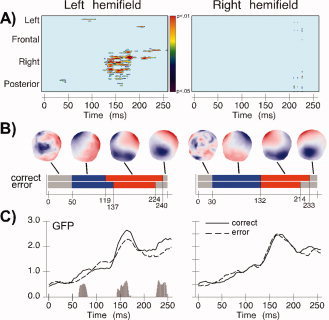
Electrophysiological results for the contrast of correct versus error saccades in the left and right hemifield. (A) Point‐wise Bonferroni‐corrected t‐tests for each electrode over the epoch covering 0–250 ms following target onset. (B) Results of the topographic pattern analysis showing the onset and end of each of the four stable topographies identified over the 250‐ms poststimulus period. (C) Global field power (GFP) as a function of time. The histogram shows time periods when GFP differed significantly between correct and error saccades. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 5.
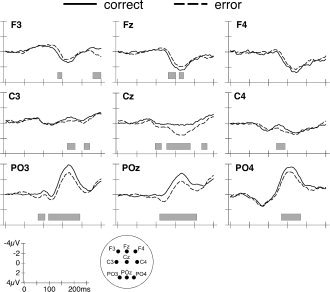
Grand average waveforms of left correct and error saccades at selected frontal, central, and posterior electrodes. The gray bars indicate intervals when significant differences between both saccade types were detected.
The topographic pattern analysis identified four stable electric field configurations that explained 92.7% of the variance (global explained variance, GEV) over 250 ms. Figure 4B (left panel) shows the temporal succession of these maps as well as the spatial distribution over the scalp. While correct and error saccades generated identical topographies over the first 50 ms (Map 1) the fitting procedure revealed a significant interaction between condition and map (F(1,9) = 10.32; P < 0.05) over the 50‐ to 224‐ms time period. However, the main effects of condition or map were not significant, suggesting that differences in map duration, but not map presence, were associated with the occurrence of correct and error saccades. Specifically, Map 2 (Fig. 4B, blue), which is partly coincident with the P1 component, ended 18 ms earlier prior to correct saccades (119 ms) compared to error saccades (137 ms). It is noteworthy that the duration modulation of Map 2 substantially preceded mean saccadic reaction time (in fact, only 1 out of 2,584 saccades had latency shorter than 137 ms) and was therefore not related to the execution of eye movements. Conversely to this first modulation of map duration, the electrical configuration of Map 3 (Fig. 4B, red), which is temporally coincident with the N1 component, ended 16 ms later prior to correct compared to error saccades. Interestingly, the end of Map 3 (224 ms) was nearly identical to the average latency of error saccades (221 ms). Its modulation might therefore have been influenced by neural activity related to the motor execution of saccades.
Analysis of the GFP, a global measure of response strength across the entire electrode set, revealed a very early difference in brain activity preceding correct and error saccades. Over the time period of 64–78 ms (corresponding to Map 2 identified in the topographic pattern analysis) there was a stronger response to correct than error saccades (t(9) = 99; P < 0.01). Further significant differences in GFP were found at the beginning (145–162 ms; t(17) = 42; P < 0.01) and end (224–240 ms; t(19) = 23.49; P < 0.01) of Map 3. These GFP modulations suggest a change in the response magnitude of the same network of brain areas that becomes active prior to correct and error saccades.
Right Saccades
The average number of epochs used for averaging was 74 ± 5 for correct saccades and 80 ± 32 for error saccades, which was statistically indistinguishable (t(9) = 0.6, n.s.). Point‐wise t‐tests computed over the 250‐ms epoch following target onset failed to identify important modulations of amplitude by correct and error saccades (Fig. 4A, right panel). Figure 6 shows data from individual electrodes that confirm the impression that amplitude differences between conditions were much less prominent than when subjects performed saccades to the left hemifield.
Figure 6.
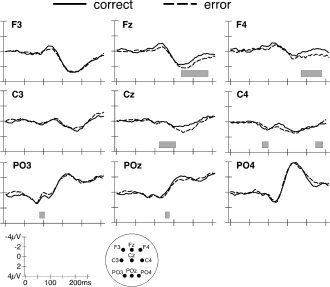
Grand average waveforms of right correct and error saccades.
The segmentation of the grand‐means identified four stable electrical configurations with 93% explained variance. Across the two experimental conditions, identical electric field topographies were observed over the interval of 0–132 ms (Fig. 4B, right panel). In contrast, the fitting procedure yielded an interaction between condition and map over the 132‐ to 250‐ms time interval (F(1,9) = 21.8; P < 0.01), indicating that Map 3 (coincident with the N1 component) ended 19 ms later prior to correct saccades than error saccades. This was, however, the only significant difference that reliably differentiated correct from error saccades. Analysis of the GFP (Fig. 4C, right panel) did not show any differences between both saccade types. In sum, these results suggest a significant prolongation without a modulation of response magnitude of a functional microstate that immediately precedes correct saccades compared to error saccades.
Electrophysiological Results: Saccade‐Locked Data
The main interest of the target‐locked analysis was to identify electrophysiological correlates of early visual or attentional processing that clearly precedes the saccadic response. However, the more time passes following onset of the target the more processes involved in saccade preparation and selection of the appropriate motor response will affect electrophysiological activity. To determine whether these processes are reflected in the EEG data we performed additional analyses with EEG epochs locked to saccade onset.
Left Saccades
Point‐wise t‐tests computed over the 250‐ms epoch preceding saccade onset failed to identify modulations of amplitude at single electrodes by correct and error saccades. The segmentation of the grand‐means identified four electrical configurations with 93% explained variance. The fitting procedure failed to identify any differences between conditions and maps, suggesting that electric field topographies were identical over the whole 250‐ms interval. The only difference between conditions was revealed in the analysis of the GFP, which showed a stronger response 50–25 ms before the onset of correct saccades.
Right Saccades
Point‐wise t‐tests did not identify modulations of amplitude by correct and error saccades at single electrodes. The segmentation of the grand‐means identified four stable electrical configurations with 91% explained variance. However, the strength and duration of these individual maps did not statistically differ, suggesting that electric field topographies were identical over the 250‐ms interval. The analysis of GFP failed to identify any significant differences in response strength.
Source Estimations
Source estimations were calculated over 119–137 ms after target presentation, which corresponds to the period during which early differences in topographies between left correct and error saccades in the target‐locked analysis were observed. VEPs for each subject and the two experimental conditions (left correct vs. left error) were averaged separately across the time period mentioned above. Source estimations were then calculated and subsequently averaged across subjects. Figure 7A shows average LAURA estimations over the 119‐ to 137‐ms period for left saccades. Based on current density measures (Fig. 7B), differences between activities associated with upcoming correct and error saccades were found at the border region between the inferior right precentral gyrus and middle frontal gyrus (maximal difference at 53, −4, 46 mm using the coordinate system of Talairach and Tournoux, [ 1988]. This region exhibited weaker sources prior to correct compared to error saccades (P = 0.003). The coordinates of this region correspond to the inferior FEF, which in functional imaging studies is active when participants shift attention or execute voluntary or reflexive saccades toward the contralateral hemifield [Grosbras and Paus, 2002; Grosbras et al., 2005; Luna et al., 1998; McDowell et al., 2008; Paus, 1996]. In contrast to the FEF, the right supramarginal gyrus (coordinates, 50, −56, 45) exhibited stronger sources when participants made correct saccades than when they made error saccades (P = 0.028).
Figure 7.
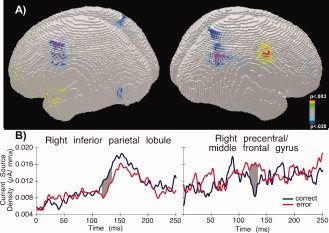
(A) LAURA inverse solution over the 119‐ to 137‐ms period following target onset depicting the mean difference of source estimations between left correct and left error saccades. Bluish colors: correct > error; reddish colors: error > correct. The right hemisphere is shown on the right side. (B) Current source density in the right inferior parietal lobule (left) and right precentral/middle frontal gyrus (right) as a function of saccade type. Grey area shows interval when significant differences were detected. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For right saccades, comparing the inverse solutions computed for correct and error saccades did not reveal any reliable differences.
DISCUSSION
This study reveals distinct patterns of electrophysiological activity associated with voluntary and reflexive saccades 120–140 ms following onset of the target, which was on average about 100 ms prior to saccade onset. In agreement with the observation that voluntary saccades have longer latency than reflexive saccades [Mort et al., 2003b; Walker et al., 2000], saccade latencies were significantly shorter for error compared to correct saccades. A second difference between correct and error saccade was that the latter had significantly smaller amplitudes. Several studies suggest that awareness of an upcoming error saccade may affect its amplitude computation, resulting in significant undershot of the target [McPeek et al., 2000; Theeuwes et al., 1999]. In addition, a recent study of oculomotor capture showed that error saccades elicit error‐related negativity, an electrophysiological marker of conscious error processing 80–120 ms after the onset of the saccade [Belopolski et al., 2008]. Together, the temporal (latency differences) and spatial (amplitude differences) characteristics of saccades in our study support our initial assumption that correct saccades reflect voluntary and error saccades reflexive processes. Previous studies of voluntary and reflexive saccades have employed paradigms such as the antisaccade task that does not allow distinguishing electrophysiological activity related to the type of upcoming saccade from initial visually evoked activity [Evdokimidis et al., 1996; Everling et al., 1997; Richards, 2003]. In contrast, in the paradigm used in the present study the visual displays evoking voluntary or reflexive saccades were identical in the critical (contingent) condition, and the type of saccade that was about to occur was therefore only determined by cognitive and physiological mechanisms. However, the term “reflexive” should not be taken as synonym to “fully stimulus‐driven,” since the degree of oculomotor capture critically depended on the visual similarity between target and distracter. Earlier studies have shown that unexpected peripheral stimuli (e.g., abrupt onsets) only capture attention of healthy participants [Folk et al., 1992; Leblanc et al., 2008; Yantis and Jonides, 1990] or patients with impaired spatial attention [Ptak and Schnider, 2006; Ptak et al., 2002] if they share task‐relevant features with the current action target. The finding that ∼30% of contingent onsets captured the gaze of participants against ∼10% of noncontingent onsets is therefore fully compatible with the hypothesis that the occurrence of reflexive saccades is contingent on the attentional set of the observer.
Our results reveal several differences between electrocortical activity associated with correct as compared to error saccades. Since these differences mainly concerned saccades directed to the left, we first discuss the latter before considering a possible explanation of hemifield differences. The first evidence of differential processing of upcoming correct and error saccades occurred already around 70 ms poststimulus in terms of a strength modulation of the electrical signal. Topographic differences followed at ∼120–140 ms and were characterized by prolonged duration of a specific electrical field configuration prior to error saccades. This temporal difference marked the onset of a strong modulation of electrode amplitude by saccade type at posterior electrodes and was followed by a significant difference in GFP. Since saccades are preceded by a cascade of cognitive processes the question arises which of them is the most likely source of the observed electrophysiological differences between voluntary and reflexive saccades. Three distinct processes are particularly relevant: motor preparation of the saccade, target selection (including successful inhibition of the distracter), and spatial attention. Several arguments suggest that electrophysiological differences observed up to ∼130 ms following target onset were not related to motor processes of saccade preparation. First, electric potential differences related to motor processes preceding the saccade should be revealed when EEG data are time‐locked to saccade onset; our analysis revealed differences when data were time‐locked to the target, but not when time‐locked to the saccade. Second, though voluntary saccades had larger amplitude than reflexive saccades the difference of ∼1.2° was too small to affect the electrophysiological correlates of saccades [Yagi, 1979]. Finally, the most salient electrophysiological topographic differences appeared substantially earlier than the first saccades and 80–100 ms prior to average saccade latency. Though there were some eye movement artifacts within the period of interest, the first of these appeared ∼20 ms after the observed differences in EEG topography.
Alternatively, it may be argued that voluntary saccades are those in which the reflexive shift toward the onset distracter has been successfully inhibited. According to this reasoning, ERPs might have been affected by the involvement of an additional inhibition component when participants performed voluntary saccades. Though our data do not provide a definite argument against this possibility, we would expect that an additional cognitive component in the voluntary condition be reflected in an additional ERP microstate, or in longer duration of a microstate that is shared with the reflexive condition. What we observed instead is a prolonged microstate in the reflexive compared to the voluntary condition, which argues for the prolongation of a shared cognitive process and against the action of an additional inhibition component.
A more straightforward explanation of the early electrophysiological differences is that they reflect modulations of early brain potentials by spatial attention. Studies examining the deployment of spatial attention and the programming of eye movements show that a shift of attention invariably precedes a saccadic eye movement [Deubel and Schneider, 1996; Hoffman and Subramaniam, 1995; Kowler et al., 1995]. Two early ERP components triggered by a visual stimulus, P1 (onset at 70–90 ms) and N1 (onset at 130–150 ms) are strongly modulated by attention [Anllo‐Vento et al., 2004; Herrmann and Knight, 2001]. For example, P1 and N1 amplitudes are larger when subjects consciously attend to a visual stimulus [Di Russo et al., 2003; Rugg et al., 1987; Yamaguchi et al., 1994], or when a peripheral stimulus suddenly captures attention [Eimer, 1994; Fu et al., 2005; Hopfinger and Ries, 2005], even if no behavioral response is required. The most interesting, though counterintuitive electrophysiological finding of our study is that the functional microstate indexed by Map 2 was ∼20 ms longer prior to error than correct saccades, though the former had ∼30 ms shorter latency. This lengthening of early processing (at ∼130 ms) was counterbalanced by the shortening of late processing (at ∼230 ms), resulting in a net speed‐up of electrophysiological processes preceding error saccades. A reasonable interpretation of these findings is that early processing indicates attentional selection of the saccade target while late processing reflects motor preparation of the upcoming saccade. However, why should attention act longer in a condition that is characterized by shorter reaction times? Attentional modulation of the P1 component has been interpreted in terms of a sensory gain control mechanism, according to which attention facilitates early sensory processing by increasing the signal‐to‐noise ratio [Hillyard et al., 1998]. The increased P1 component prior to error saccades and the prolonged functional microstate covering the period of P1 occurrence observed in our study may therefore be interpreted as the electrophysiological markers of stronger and prolonged action of a sensory gain‐control mechanism that enhances distracter saliency. Source analysis identified the FEF and the supramarginal gyrus as possible origins of top‐down effects on sensory processing. Neurophysiological studies of single neurons in the FEF have shown that—by virtue of its direct connections with visual areas and subcortical oculomotor structures—this area is strongly implicated in saccade target selection and visuomotor transformations required for eye movement programming [Bruce and Goldberg, 1985; Hanes et al., 1998; Schall and Thompson, 1999]. Recent animal and human studies indicate that the FEF exhibits very early activity in response to visual stimuli [Kirchner et al., 2009; Liu et al., 2009] and contributes to saccade target selection by sending top‐down signals that enhance or attenuate activity of visual neurons and thus directly modulate the saliency of visual stimuli [Moore and Armstrong, 2003; Treue, 2003; Walker et al., 2009]. Interestingly, activity of visual cells in the FEF discriminates between target and distracter between 120 and 150 ms after onset of the display [Thompson et al., 1996], a delay that is remarkably similar to the main electrophysiological differences observed in our study. Together, these results suggest that the FEF is the possible substrate of a gating mechanism that modulates the strength of sensory signals through amplification of target saliency or attenuation of distracter saliency—in particular when a saccade has to be planned—and thus contributes to target selection.
The second area identified by the source analysis was located in the right inferior parietal lobule (IPL), centered on the supramarginal gyrus. Damage to this region leads to spatial neglect, a severe deficit of spatial attention affecting the ability to shift attention to the contralesional space [Golay et al., 2008; Mort et al., 2003a]. The IPL and temporo‐parietal junction are strongly involved in reorienting spatial attention [Friedrich et al., 1998], and patients with damage to this region fail to inhibit reflexive glances toward irrelevant ipsilesional stimuli [Heide and Kömpf, 1998; Ptak et al., 2007, 2009]. Further, similar to the posterior parietal cortex of the monkey [Constantinidis and Steinmetz, 2001; Gottlieb et al., 1998], the human IPL is strongly involved in detecting feature singletons and salient stimuli presented within a crowded display [Husain and Rorden, 2003]. Thus, the finding that FEF and IPL were active prior to the occurrence of voluntary and reflexive saccades fits well with the role of these regions in spatial attention and saccade planning. However, what surprises is the fact that FEF activity was stronger prior to a reflexive saccade while IPL activity was stronger prior to a voluntary saccade. A possible explanation of this finding is that activity of the FEF and IPL reflects increased demands on processing resources of these regions. Thus, the higher source activity of the FEF prior to a reflexive saccade may reflect the increased requirement on top‐down enhancement of target saliency and inhibition of the highly salient distracter. Conversely, greater activation of the IPL prior to a correct saccade is evidence of the greater effort to orient attention to the target in the presence of a highly salient distracter stimulus.
The differences between electrophysiological activity preceding correct and error saccades were only found for saccades directed to the left hemifield. While robust differences in amplitude modulations of individual electrodes, GFP and topographic patterns were found between left correct and error saccades, such differences were largely absent for right saccades. Considering the similarity in behavioral performance for left and right saccades, this absence of electrophysiological differences with right saccades might seem surprising. However, neuropsychological and functional brain imaging studies strongly suggest that, although each hemisphere is specialized in programming contraversive eye movements and shifting attention contralaterally, the right hemisphere has a bilateral contribution to spatial attention [Corbetta et al., 1998; Fan et al., 2005; Mesulam, 1981; Muri and Nyffeler, 2008]. This incomplete functional asymmetry may account for the absence of electrophysiological differences between right correct and error saccades, though its precise explanation remains hypothetical. A tentative hypothesis is that the bilateral representation of spatial attention for the right hemisphere cancelled out the tiny differences between electrophysiological activity preceding voluntary and reflexive saccades. Interestingly, a recent fMRI study [Petit et al., 2009] and studies examining power modulations in EEG alpha‐band activity have observed similar asymmetries suggesting dominance of the right hemisphere in spatial processing [Brignani et al., 2007; Thut et al., 2006]. Since EEG‐power modulations may serve as an index of attentional orienting to the left or right hemifield, examining the occurrence of such modulations prior to target onset might clarify the role of spontaneous attentional fluctuations in the control of voluntary and reflexive saccades. However, such an analysis necessitates pretarget periods that are substantially longer than the intertrial intervals used in the present study.
In sum, this study reveals early temporal differences in electrophysiological processing associated with voluntary and reflexive saccades and indicates that the FEF and IPL contribute differently to the programming of these two saccade types. These findings show that the occurrence of a voluntary or reflexive saccade is predicted by the pattern of electrocortical activity more than 100 ms prior to the onset of the eye movement.
Acknowledgements
The authors thank Laetitia Golay, Estelle Robert, and Louis Nahum for technical assistance as well as Micah Murray for helpful comments on a previous version of the manuscript. The Cartool software was developed by Denis Brunet, with the support of the Center for Biomedical Imaging of Geneva and Lausanne.
REFERENCES
- Anllo‐Vento L, Schoenfeld MA, Hillyard SA ( 2004): Cortical mechanisms of visual attention In: Posner, MI, editor. Cognitive Neuroscience of Attention. New York: Guilford Press pp 180–193. [Google Scholar]
- Antes JR ( 1974): The time course of picture viewing. J Exp Psychol 103: 62–70. [DOI] [PubMed] [Google Scholar]
- Belopolsky AV, Kramer AF, Theeuwes J ( 2008): The role of awareness in processing of oculomotor capture: Evidence from event‐related potentials. J Cogn Neurosci 20: 2285–2297. [DOI] [PubMed] [Google Scholar]
- Blaser E, Sperling G, Lu Z‐L ( 1999): Measuring the amplification of attention. Proc Natl Acad Sci USA 96: 11681–11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignani D, Maioli C, Maria Rossini P, Miniussi C ( 2007): Event‐related power modulations of brain activity preceding visually guided saccades. Brain Res 1136: 122–131. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME ( 1985): Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol 53: 603–635. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Ling S, Read S ( 2004): Attention alters appearance. Nat Neurosci 7: 308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C, Steinmetz MA ( 2001): Neuronal responses in area 7a to multiple‐stimulus displays: I. Neurons encode the location of the salient stimulus. Cereb Cortex 11: 581–591. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL ( 1998): A common network of functional areas for attention and eye movements. Neuron 21: 761–773. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX ( 1996): Saccade target selection and object recognition: Evidence for a common attentional mechanism. Vis Res 36: 1827–1837. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Hillyard SA ( 2003): Source analysis of event‐related cortical activity during visuo‐spatial attention. Cereb Cortex 13: 486–499. [DOI] [PubMed] [Google Scholar]
- Eimer M ( 1994): An ERP study on visual spatial priming with peripheral onsets. Psychophysiology 31: 154–163. [DOI] [PubMed] [Google Scholar]
- Evdokimidis I, Mergner T, Lücking CH ( 1992): Dependence of presaccadic cortical potentials on the type of saccadic movement. Electroencephalogr Clin Neurophysiol 83: 179–191. [DOI] [PubMed] [Google Scholar]
- Evdokimidis I, Liakopoulos D, Constantinidis TS, Papageorgiou C ( 1996): Cortical potentials with antisaccades. Electroencephalogr Clin Neurophysiol 98: 377–384. [DOI] [PubMed] [Google Scholar]
- Everling S, Fischer B ( 1998): The antisaccade: A review of basic research and clinical studies. Neuropsychologia 36: 885–899. [DOI] [PubMed] [Google Scholar]
- Everling S, Krappmann P, Flohr H ( 1997): Cortical potentials preceding pro‐ and antisaccades in man. Electroencephalogr Clin Neurophysiol 102: 356–362. [DOI] [PubMed] [Google Scholar]
- Everling S, Spantekow A, Krappmann P, Flohr H ( 1998): Event‐related potentials associated with correct and incorrect responses in a cued antisaccade task. Exp Brain Res 118: 27–34. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI ( 2005): The activation of attentional networks. NeuroImage 26: 471–479. [DOI] [PubMed] [Google Scholar]
- Fender DH ( 1987): Methods of analysis of brain electrical and magnetic signals In: Gevins AS, Remond A, editors. Handbook of Electroencephalography and Clinical Neurophysiology, Vol. 1 Amsterdam: Elsevier; pp 355–399. [Google Scholar]
- Findlay JM, Gilchrist ID ( 2003): Active Vision. Oxford: Oxford University Press. [Google Scholar]
- Folk CL, Remington RW, Johnston JC ( 1992): Involuntary covert orienting is contingent on attentional control settings. J Exp Psychol Hum Percept Perform 18: 1030–1044. [PubMed] [Google Scholar]
- Friedrich FJ, Egly R, Rafal RD, Beck D ( 1998): Spatial attention deficits in humans: A comparison of superior parietal and temporal‐parietal junction lesions. Neuropsychology 12: 193–207. [DOI] [PubMed] [Google Scholar]
- Fu S, Greenwood PM, Parasuraman R ( 2005): Brain mechanisms of involuntary visuospatial attention: An event‐related potential study. Hum Brain Mapp 25: 378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golay L, Schnider A, Ptak R ( 2008): Cortical and subcortical anatomy of chronic spatial neglect following vascular damage. Behav Brain Funct 4: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb J, Kusunoki M, Goldberg ME. ( 1998): The representation of visual salience in monkey parietal cortex. Nature 391: 481–484. [DOI] [PubMed] [Google Scholar]
- Grave de Peralta Menendez R, Murray MM, Michel CM, Martuzzi R, Gonzalez Andino SL ( 2004): Electrical neuroimaging based on biophysical constraints. NeuroImage 21: 527–539. [DOI] [PubMed] [Google Scholar]
- Grosbras M‐H, Paus T ( 2002): Transcranial magnetic stimulation of the human frontal eye field: Effects on visual perception and attention. J Cogn Neurosci 14: 1109–1120. [DOI] [PubMed] [Google Scholar]
- Grosbras M‐H, Laird AR, Paus T ( 2005): Cortical regions involved in eye movements, shifts of attention, and gaze perception. Hum Brain Mapp 25: 140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PE ( 1978): Primary and secondary saccades to goals defined by instructions. Vis Res 18: 1279–1296. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Patterson WF, Schall JD ( 1998): Role of the frontal eye fields in countermanding saccades: Visual, movement, and fixation activity. J Neurophysiol 79: 817–834. [DOI] [PubMed] [Google Scholar]
- Heide W, Kömpf D ( 1998): Combined deficits of saccades and visuo‐spatial orientation after cortical lesions. Exp Brain Res 123: 164–171. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Hollingworth A ( 1999): High‐level scene perception. Annu Rev Psychol 50: 243–271. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Knight RT ( 2001): Mechanisms of human attention: Event‐related potentials and oscillations. Neurosci Biobehav Rev 25: 465–476. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ ( 1998): Sensory gain control (amplification) as a mechanism of selective attention: Electrophysiological and neuroimaging evidence. Philos Trans R Soc Lond B 353: 1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JE, Subramaniam B ( 1995): The role of visual attention in saccadic eye movements. Percept Psychophys 57: 787–795. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Ries AJ ( 2005): Automatic versus contingent mechanisms of sensory‐driven neural biasing and reflexive attention. J Cogn Neurosci 17: 1341–1352. [DOI] [PubMed] [Google Scholar]
- Husain M, Rorden C. ( 2003): Non‐spatially lateralized mechanisms in hemispatial neglect. Nat Rev Neurosci 4: 26–36. [DOI] [PubMed] [Google Scholar]
- Itti L, Koch C ( 2000): A saliency‐based search mechanism for overt and covert shifts of visual attention. Vis Res 40: 1489–1506. [DOI] [PubMed] [Google Scholar]
- Johnston K, Everling S ( 2008): Neurophysiology and neuroanatomy of reflexive and voluntary saccades in non‐human primates. Brain Cogn 68: 271–283. [DOI] [PubMed] [Google Scholar]
- Kirchner H, Barbeau EJ, Thorpe SJ, Regis J, Liegeois‐Chauvel C ( 2009): Ultra‐rapid sensory responses in the human frontal eye field region. J Neurosci 29: 7599–7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E ( 1995): The role of attention in the programming of saccades. Vis Res 35: 1897–1916. [DOI] [PubMed] [Google Scholar]
- Leblanc E, Prime DJ, Jolicoeur P ( 2008): Tracking the location of visuospatial attention in a contingent capture paradigm. J Cogn Neurosci 20: 657–671. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Skrandies W ( 1980): Reference‐free identification of components of checkerboard‐evoked multichannel potential fields. Electroencephalogr Clin Neurophysiol 48: 609–621. [DOI] [PubMed] [Google Scholar]
- Liu H, Agam Y, Madsen JR, Kreiman G ( 2009): Timing, timing, timing: Fast decoding of object information from intracranial field potentials in human visual cortex. Neuron 62: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZL, Dosher BA ( 1998): External noise distinguishes attention mechanisms. Vis Res 38: 1183–1198. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Strojwas MH, McCurtain BJ, Berman RA, Genovese CR, Sweeney JA ( 1998): Dorsal cortical regions subserving visually guided saccades in humans: An fMRI study. Cereb Cortex 8: 40–47. [DOI] [PubMed] [Google Scholar]
- Maljkovic V, Nakayama K ( 1994): Priming of pop‐out: I. Role of features. Mem Cogn 22: 657–672. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Dyckman KA, Austin BP, Clementz BA ( 2008): Neurophysiology and neuroanatomy of reflexive and volitional saccades: Evidence from studies of humans. Brain Cogn 68: 255–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPeek RM, Keller EL, Nakayama K ( 1999): Concurrent processing of saccades. Behav Brain Sci 22: 691–692. [Google Scholar]
- McPeek RM, Skavenski AA, Nakayama K ( 2000): Concurrent processing of saccades in visual search. Vis Res 40: 2499–2516. [DOI] [PubMed] [Google Scholar]
- Mesulam MM ( 1981): A cortical network for directed attention and unilateral neglect. Ann Neurol 10: 309–325. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. ( 2003): Selective gating of visual signals by microstimulation of frontal cortex. Nature 421: 370–373. [DOI] [PubMed] [Google Scholar]
- Mort DJ, Malhotra P, Mannan SK, Rorden C, Pambakian A, Kennard C, Husain M. ( 2003a): The anatomy of visual neglect. Brain 126: 1986–1997. [DOI] [PubMed] [Google Scholar]
- Mort DJ, Perry RJ, Mannan SK, Hodgson TL, Anderson E, Quest R, McRobbie D, McBride A, Husain M, Kennard C ( 2003b): Differential cortical activation during voluntary and reflexive saccades in man. Neuroimage 18: 231–246. [DOI] [PubMed] [Google Scholar]
- Moster ML, Goldberg G ( 1990): Topography of scalp potentials preceding self‐initiated saccades. Neurology 40: 644–648. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. ( 2004): Look away: The anti‐saccade task and the voluntary control of eye movement. Nat Rev Neurosci 5: 218–228. [DOI] [PubMed] [Google Scholar]
- Muri RM, Nyffeler T ( 2008): Neurophysiology and neuroanatomy of reflexive and volitional saccades as revealed by lesion studies with neurological patients and transcranial magnetic stimulation (TMS). Brain Cogn 68: 284–292. [DOI] [PubMed] [Google Scholar]
- Müri RM, Iba‐Zizen MT, Derosier C, Cabanis EA, Pierrot‐Deseilligny C ( 1996): Location of the human posterior eye field with functional magnetic resonance imaging. J Neurol Neurosurg Psychiatry 60: 445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MM, Brunet D, Michel CM ( 2008): Topographic ERP analyses: A step‐by‐step tutorial review. Brain Topogr 20: 249–264. [DOI] [PubMed] [Google Scholar]
- Parkhurst DJ, Law K, Niebur E ( 2002): Modeling the role of salience in the allocation of overt visual attention. Vis Res 42: 107–123. [DOI] [PubMed] [Google Scholar]
- Pashler HE ( 1998): The Psychology of Attention. Cambridge, MA: MIT Press. [Google Scholar]
- Pasqual‐Marqui RD, Michel CM, Lehmann D ( 1995): Segmentation of brain electrical activity into microstates: Model estimation and validation. IEEE Trans Biomed Eng 42: 658–665. [DOI] [PubMed] [Google Scholar]
- Paus T ( 1996): Location and function of the human frontal eye‐field: A selective review. Neuropsychologia 34: 475–483. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Zeki S ( 2000): The neurology of saccades and covert shifts in spatial attention. Brain 123: 2273–2288. [DOI] [PubMed] [Google Scholar]
- Pestilli F, Carrasco M ( 2005): Attention enhances contrast sensitivity at cued and impairs it at uncued locations. Vis Res 45: 1867–1875. [DOI] [PubMed] [Google Scholar]
- Petit L, Zago L, Vigneau M, Andersson F, Crivello F, Mazoyer B, Mellet E, Tzourio‐Mazoyer N. ( 2009): Functional asymmetries revealed in visually guided saccades: An FMRI study. J Neurophysiol 102: 2994–3003. [DOI] [PubMed] [Google Scholar]
- Pierrot‐Deseilligny C, Ploner CJ, Müri RM, Gaymard B, Rivaud‐Péchaux S ( 2002): Effects of cortical lesions on saccadic eye movements in humans. Ann N Y Acad Sci 956: 216–229. [DOI] [PubMed] [Google Scholar]
- Ptak R, Schnider A ( 2006): Reflexive orienting in spatial neglect is biased towards behaviourally salient stimuli. Cereb Cortex 16: 337–345. [DOI] [PubMed] [Google Scholar]
- Ptak R, Valenza N, Schnider A ( 2002): Expectation‐based attentional modulation of visual extinction in spatial neglect. Neuropsychologia 40: 2199–2205. [DOI] [PubMed] [Google Scholar]
- Ptak R, Schnider A, Golay L, Müri R ( 2007): A non‐spatial bias favouring fixated stimuli revealed in patients with spatial neglect. Brain 130: 3211–3222. [DOI] [PubMed] [Google Scholar]
- Ptak R, Golay L, Müri R, Schnider A ( 2009): Looking left with left neglect: The role of spatial attention when active vision selects local image features for fixation. Cortex 45: 1156–1166. [DOI] [PubMed] [Google Scholar]
- Richards JE ( 2003): Cortical sources of event‐related potentials in the prosaccade and antisaccade task. Psychophysiology 40: 878–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Milder AD, Lines CR, Phalp R. ( 1987): Modulation of visual event‐related potentials by spatial and non‐spatial visual selective attention. Neuropsychologia 25: 85–96. [DOI] [PubMed] [Google Scholar]
- Schall JD, Thompson KG ( 1999): Neural selection and control of visually guided eye movements. Annu Rev Neurosci 22: 241–259. [DOI] [PubMed] [Google Scholar]
- Spinelli L, Andino SG, Lantz G, Seeck M, Michel CM ( 2001): Electromagnetic inverse solutions in anatomically constrained spherical head models. Brain Topogr 13: 115–125. [DOI] [PubMed] [Google Scholar]
- Talairach T, Tournoux P ( 1988): Co‐planar Stereotaxic Atlas of the Human Brain: 3‐Dimensional Proportional System—An Approach to Cerebral Imaging. New York: Thieme. [Google Scholar]
- Theeuwes J, Godijn R ( 2004): Inhibition‐of‐return and oculomotor interference. Vis Res 44: 1485–1492. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Kramere AF, Hahn S, Irwin DE, Zelinsky GJ ( 1999): Influence of attentional capture on oculomotor control. J Exp Psychol Hum Percept Perform 25: 1595–1608. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Hanes DP, Bichot NP, Schall JD ( 1996): Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol 76: 4040–4055. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual‐Leone A ( 2006): Alpha‐band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci 26: 9494–9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treue S ( 2003): Visual attention: The where, what, how and why of saliency. Curr Opin Neurobiol 13: 428–432. [DOI] [PubMed] [Google Scholar]
- Walker R, Walker DG, Husain M, Kennard C ( 2000): Control of voluntary and reflexive saccades. Exp Brain Res 130: 540–544. [DOI] [PubMed] [Google Scholar]
- Walker R, Techawachirakul P, Haggard P ( 2009): Frontal eye field stimulation modulates the balance of salience between target and distractors. Brain Res 1270: 54–63. [DOI] [PubMed] [Google Scholar]
- Yagi A ( 1979): Saccade size and lambda complex in man. Physiol Psychol 7: 370–376. [Google Scholar]
- Yamaguchi S, Tsuchiya H, Kobayashi S ( 1994): Electroencephalographic activity associated with shifts of visuospatial attention. Brain 117: 553–562. [DOI] [PubMed] [Google Scholar]
- Yantis S, Jonides J ( 1990): Abrupt visual onsets and selective attention: Voluntary versus automatic allocation. J Exp Psychol Hum Percept Perform 16: 121–134. [DOI] [PubMed] [Google Scholar]
- Yarbus AL ( 1967): Eye Movements and Vision. New York: Plenum. [Google Scholar]


