Abstract
The technique of diffusion tensor imaging (DTI) has been used to investigate alterations in white matter architecture following long‐term training and expertise. Professional simultaneous interpreters (SI) provide an ideal model for the investigation of training‐induced plasticity due to the high demands placed on sound to motor mapping mechanisms, which are vital for executing fast interpretations. In line with our hypothesis, we found clusters with decreased fractional anisotropy (FA) in the SI group in brain regions previously shown to support sensory‐motor coupling mechanisms and speech articulation (cluster extent family‐wise error corrected, P < 0.01). Furthermore, we found an altered white matter architecture indicated by lower FA values in the SI group in the most anterior and posterior parts of the corpus callosum. Our results suggest that language expertise is accompanied by plastic adaptations in regions strongly involved in motor aspects of speech and in interhemispheric information transfer. These results have implications for our understanding of language expertise in relation to white matter adaptations. Hum Brain Mapp, 2011. © 2010 Wiley Periodicals, Inc.
Keywords: diffusion tensor imaging, plasticity, multilingualism, insula, corpus callosum, simultaneous interpreters
INTRODUCTION
Several recent longitudinal [Draganski et al.,2004; Hyde et al.,2009] and cross‐sectional [Maguire et al.,2000] studies have described the exceptional potential of the human brain to alter its function and morphology following short‐ and long‐term training or exposure to specific environments. As many as 10 studies investigating plastic adaptations in professional musicians have clearly demonstrated white matter reorganization after intensive instrumental training that required the integration of sensory and motor information [Bengtsson et al.,2005; Imfeld et al.,2009]. Similar adaptations in the white matter architecture have also been observed in other domains, for instance, in individuals undergoing intensive motor training that places high demands on sensory‐motor coordination [Jäncke et al.,2009; Hänggi et al.,2009; Baumann et al.,2007; Jäncke2009; Jäncke et al.,2000; Münte et al.,2002].
An established dual stream model [Hickok and Poeppel,2007] suggests that anatomically and functionally segregated pathways are involved in different aspects of language processing. This model postulates that a bilateral ventral processing stream originating from posterior perisylvian sites and stretching to anterior temporal regions supports a variety of language comprehension functions, implying that this ventral stream is strongly involved in mapping sound to meaning. On the other hand, a left dorsal stream that extends from posterior perisylvian regions to the frontal operculum is proposed to be involved in auditory‐to‐motor transformations and in articulation processes, and thus important for mapping sounds to articulation. A previous study [Saur et al.,2008] investigated the neuronal substrate of the dual stream model by combining functional magnetic resonance imaging (fMRI) during performance of two prototypical language tasks with diffusion tensor imaging‐based (DTI) tractography techniques. These two tasks, sublexical repetition and higher‐level language comprehension, were designed to differentially recruit the dorsal and ventral processing streams. The results of this study corroborated the integrity of the model proposed by Hickok and Poeppel [2007] and pointed to a functional and anatomical segregation of the two processing streams.
In speech production, the left inferior frontal gyrus and particularly Broca's area were long thought to be critical for the motor act of speech [Ruff and Arbit,1981; Schiff et al.,1983]. However, lesion‐based analyses conducted with large samples [Bates et al.,2003; Dronkers,1996] have shown that the integrity of the left anterior insula and not necessarily Broca's area is fundamental for accurate articulation. The functional relevance of the left insula for speech articulation is further supported by a positron emission tomography (PET) study conducted by Wise et al. [1999]. These authors showed that the repetition of single words did not activate just Broca's area but a more comprehensive motor‐related network including the left anterior insula, the lateral premotor cortex, and the basal ganglia. Wise and colleagues were further able to establish that the left anterior insula is activated during both hearing and articulation, thus supporting the notion that this region plays a pivotal role in sensory‐motor coupling mechanisms in language. Such a sensory‐motor interplay supported by the anterior insula was previously been shown to be restricted to overt task performances [Riecker et al.,2000].
Several neuroimaging studies of overt speech production [Eickhoff et al.,2009] and observations in patients with specific language impairments such as verbal apraxia [Dronkers,1996] have revealed a broad network that contributes to the control of speech motor output. A previous meta‐analysis [Eickhoff et al.,2009] of neuroimaging data acquired in healthy subjects during verbal fluency tasks identified the involvement of a core motor network in overt speech production. This network is composed by the pars opercularis, left anterior insula, basal ganglia, cerebellum, premotor cortex, and primary motor cortex. Further evidence for structures mediating speech production has been derived from the analysis of patients with specific clinical syndromes [Nestor et al.,2003] and developmental language disorders [Jüncke et al.,2007]. Nestor et al. used the PET technique to investigate 10 patients affected by progressive nonfluent aphasia, a syndrome in which the patient loses the ability to communicate fluently [2003]. Compared with controls and with a group of Alzheimer's disease patients without nonfluent aphasic features, the nonfluent aphasia group showed hypometabolic activation patterns most notably in the left anterior insula/frontal opercular region. This clinical finding underscores the crucial role of this structure in motor aspects of speech.
The functional relevance of the white matter fiber bundles in speech production has been investigated in individuals diagnosed with specific speech disorders such as persistent developmental stuttering. A previous voxel‐based morphometry (VBM) study [Jäncke et al.,2004] found enhanced stuttering‐related white matter volumes in regions supporting speech production functions, including the white matter underlying the pars triangularis and the precentral gyrus in the vicinity of the face and mouth representation. Another VBM study [Beal et al.,2007] delivered further evidence in favor of the notion that stuttering is related to differential white matter architecture in regions involved in speech production. That study reported lower white matter densities in affected individuals in a region enclosing the left anterior insula.
The DTI technique can be especially useful for understanding the role of brain connectivity in human behavior, since the white matter bundles support the crosstalk between grey‐matter areas that enables them to function in concert. In the domain of speech processing, SI represent an ideal experimental group for studying the architecture of white matter fiber bundles in conjunction with language expertise and plastic adaptations [Elmer et al.,2010]. Professional simultaneous interpreting places high demands on sensory‐motor coupling mechanisms; since linguistic input needs to be almost simultaneously translated into an adequate output format. Furthermore, the intensive training of SI requires fine‐tuned sensory‐motor adjustments in order to achieve an excellent pronunciation in the foreign languages.
In this study, we used a voxel‐wise approach to compare fractional anisotropy (FA) in SI and control subjects. We focussed on the white matter architecture in brain regions previously shown to be involved in mapping sounds to articulation and in the motor control of speech. We expected that the fast sound‐to‐motor mapping typical for simultaneous interpreting must be accompanied by plastic adaptations in white‐matter pathways situated along the dorsal stream, in keeping with the model proposed by Hickok and Poeppel [2007]. In particular, we expected to find FA differences between the two groups in white‐matter fibers that encompass the left anterior insula, the Broca's area, the basal ganglia, the left ventral prefrontal region, and the motor/premotor areas.
MATERIALS AND METHODS
Participants
We investigated 24 healthy right‐handed subjects divided into two groups of 12 subjects each. The experimental group (SI, eight women and four men, mean/standard deviation: 37.9 ± 5.8 years) was compared with a control group (eight women and four men, 28.4 ± 2.8 years). The SI group consisted of specifically trained and certified graduated professional SI. Since all participants had a comparable level of education (i.e., university degree or advanced university students), we exclude that different IQ values between the two groups may influence the results in some directions. Additionally, the regions that we observed to differ between groups have not yet been attributed to IQ [Chiang et al.,2009; Jung and Haier,2007; Li et al.,2009]. The subjects reported no past or current neurological, psychiatric, or neuropsychological problems and denied to take drugs or illegal medication. Subjects were paid for participation. The local ethics committee (Zürich, Switzerland) approved the study and written informed consent was obtained from all participants.
Imaging Data Acquisition
Magnetic resonance imaging (MRI) scans were acquired on a 3.0 T Philips Achieva whole body scanner (Philips Medical Systems, Best, The Netherlands) equipped with a transmit‐receive body coil and a commercial eight‐element sensitivity encoding (SENSE) head coil array.
A diffusion‐weighted spin echo, echo‐planar imaging (EPI) sequence was used to obtain diffusion‐weighted scans with a measured spatial resolution of 2.08 × 2.08 × 2.0 mm (acquisition matrix 96 × 96 pixels, 50 slices) and a reconstructed resolution of 1.56 × 1.56 × 2.0 mm (reconstructed matrix 128 × 128 pixels, 50 slices). Further imaging parameters were as follows: Field of view, FOV = 200 × 200 mm; echo time, TE = 50 ms; repetition time, TR = 10,166 ms; flip angle, FA = 90°; sensitivity encoding (SENSE) factor, R = 2.1; and b‐value = 1,000 s/mm2. Diffusion was measured in 15 noncollinear directions followed by a nondiffusion‐weighted volume (reference volume). We used the Philips standard diffusion gradient mode (“medium”) that is comprised by 16 diffusion directions (15 noncollinear diffusion directions and one nondiffusion weighted volume). We did not acquire a b0‐field map for correcting EPI‐distortions because we used the sensitivity encoding (SENSE) technique that minimizes these EPI‐distortions typically occurring at the base of the brain and in medial prefrontal regions. All these areas were not expected to be altered in SI. Total acquisition time was about 15 min.
Analysis of Fractional Anisotropy and Axial Versus Radial Diffusivity
To analyze interconnectivity, measured by means of fractional anisotropy (FA), we preprocessed the diffusion‐weighted images with the scripts of tract‐based spatial statistics (TBSS) [Behrens et al.,2003] and the diffusion toolbox (FDT). This toolbox is part of the FSL [Smith et al.,2006] software implemented in the functional magnetic resonance imaging of the brain (FMRIB) software library (http://www.fmrib.ox.ac.uk/fsl/) and was used to create FA as well as axial (AD) and radial diffusivity (RD) maps. The following steps were realized: (1) Head movement and eddy current correction was applied using EDDY_CORRECT of FDT. (2) A brain mask of the reference volume (no diffusion) was created using the brain extraction tool (BET). (3) Tensors were fitted to the data using DTIFIT to generated FA, AD, and RD maps. (4) Nonlinear registration of all FA, AD, and RD maps into standard space was applied. (5) FA maps were smoothed with a Gaussian kernel of full width at half maximum (FWHM) of 9 mm. (6) All voxels with FA values smaller than 10% of the mean FA values were excluded from the statistical analyses because we were only interested in the diffusion characteristics of white matter tissue. We additionally regressed the age of the participants against local FA values in order to control for age differences between the interpreters and the control subjects. AD and RD was computed only for the clusters that showed a significant difference in FA.
Statistical Analysis
The statistical group comparisons of local FA as well as the regression with age were performed by applying the general linear model implemented in the statistical parametric mapping (SPM5) software (http://www.fil.ion.ucl.ac.uk/spm/). Global mean FA was modeled as a nuisance variable in the analysis of covariance of local FA as well as in the regressions applied for controlling the variable age. Although strong a‐priori hypotheses were postulated, the statistical extent threshold was corrected for multiple comparisons combined with a nonstationary smoothness correction [Hayasaka and Nichols,2004; Hayasaka et al.,2004]. We used cluster extent family‐wise error (FWE) correction with P = 0.01 and a height threshold of P = 0.001 (uncorrected) for FA group comparisons and the regression with age. Note that the number of voxels that were found to be different in the whole brain FA analysis and those used to compute AD and RD slightly differs. This is due to the nonstationary smoothness correction applied in the FA analysis. This correction cannot be applied to the region‐of‐interest approach used to compute AD and RD.
RESULTS
Fractional Anisotropy and Axial Versus Radial Diffusivity
In this study, fractional anisotropy (FA) maps in SI (n = 12) and control subjects (n = 12) were compared using a voxel‐wise approach to examine the directedness and integrity of the white matter architecture in brain regions previously shown to be involved in the mapping sounds to articulation, in the motor control of speech, as well as in the interhemispheric transfer.
In line with our hypothesis, subjecting the contrast controls versus SI to the voxel‐wise analysis of FA values, we revealed strong between‐group differences (Table 1 and Figure 1) in fiber tracts encompassing the left anterior insula (peak in Montreal Neurological Institute coordinates: x = −26, y = 23, z = 12; t = 6.49), the upper part of the corticospinal tract (peak: x = −22, y = −3, z = 38; t = 6.05), the right inferior parietal lobe (peak: x = 45, y = −76, z = 29; t = 6.08 and peak: x = 51, y = −57, z = 35; t = 4.38), and the dorsal part of the right caudate nucleus (peak: x = 11, y = 7, z = 19; t = 5.92).
Table I.
Results of the FA analysis (controls > interpreters)
| Fractional anisotropy | Hemisphere | MNI coordinates | Cluster size | t‐value | ||
|---|---|---|---|---|---|---|
| Controls > Interpreters | x | y | z | k = 1,359 voxels nonstationarity corrected | P < 0.01 (FWE) cluster extent corrected | |
| Orbitofrontal cortex | Right | 18 | 30 | −15 | 1,635 | 6.82 |
| Genu of corpus callosum | Right | 21 | 30 | −3 | 3.91 | |
| Insula | Left | −26 | 23 | 12 | 1,546 | 6.49 |
| Splenium of corpus callosum | Left | −5 | −44 | 20 | 3,226 | 6.46 |
| Splenium of corpus callosum | Left | −21 | −57 | 18 | 5.04 | |
| Body of corpus callosum | Left | −13 | −39 | 19 | 5.82 | |
| Cingulum | Left | −3 | −24 | 30 | 2,228 | 6.11 |
| Corticospinal tract | Left | −22 | −3 | 38 | 6.05 | |
| Cingulum | Left | −10 | −27 | 38 | 5.07 | |
| Inferior parietal lobe | Right | 45 | −76 | 29 | 1,372 | 6.08 |
| Inferior parietal lobe | Right | 51 | −57 | 35 | 4.38 | |
| Forceps minor | Left | −17 | 48 | 2 | 2,219 | 5.94 |
| Forceps minor | Left | −14 | 57 | 9 | 5.38 | |
| Frontal pole | Left | −21 | 56 | 1 | 4.15 | |
| Nucleus caudatus | Right | 11 | 7 | 19 | 4,968 | 5.92 |
| Forceps minor corpus callosum | Right | 1 | 31 | 9 | 5.18 | |
| Genu of corpus callosum | Left | −5 | 25 | 5 | 5.13 | |
Figure 1.

FA, controls versus SI. Statistical parametric maps of the voxel‐based fractional anisotropy analysis overlaid on the MNI‐152 template and thresholded with P < 0.01, FWE‐corrected at the cluster extent level. Shown are regions with increased fractional anisotropy in the control subjects compared with simultaneous interpreters in the left insula (A, left image), in the genu (B, left and right image), and in the body and splenium (C) of the corpus callosum (right image).The color bar represents the t‐values.
As a second main result, we found lower FA values in the SI group in the genu (peak: x = 21, y = 30, z = −3; t = 3.91 and peak: x = −5, y = 25, z = 5; t = 5.13) and splenium (peak: x = −5, y = −44, z = 20; t = 6.46 and peak: x = −21, y = −57, z = 18; t = 5.04) of the corpus callosum. Furthermore, we revealed lower FA values in the SI group in the body of the corpus callosum (peak: x = −13, y = −39, z = 19; t = 5.82). No brain area was found to have higher FA values for the opposite contrast (SI vs. controls). A comprehensive list of all results is depicted in Table I.
To better comprehend the decrease in FA values for the highly trained subjects, we calculated the average axial and radial diffusivity (mm2/s) for each significant cluster and group. As visible in Figure 2, our DTI data show that the lower FA values in the interpreters group are associated with reduced axial and enhanced radial diffusivity.
Figure 2.
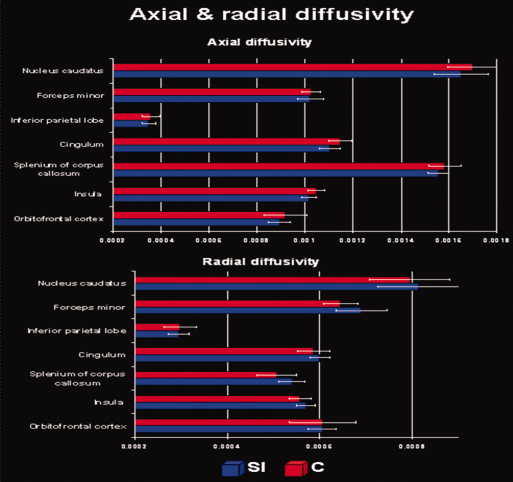
Average mean diffusivity (mm2/s). The figure depicts average axial and radial diffusivity for each significant cluster and group.
Regression With Age
Since the two groups significantly differed in age (t‐test for independent samples, two‐tailed, controls vs. SI, t(df = 22) = −4.71, P < 0.001), we computed a regression of FA values with age. This analysis yielded a positive correlation between FA and age in the white matter residing in the orbitofrontal cortex (peak: x = 7, y = 48, z = −15; t = 6.81 and peak: x = 4, y = 45, z = −25; t = 5.34 and peak: x = −3, y = 37, z = −23; t = 4.21).
DISCUSSION
General Discussion
The motivation of this study was based on the hypothesis that the fast sound‐to‐motor transformations necessary for simultaneous interpreting must be accompanied by plastic adaptations in white matter pathways stretching along the left dorsal language stream according to the model proposed by Hickok and Poeppel [2007] and supported by previous studies concerning overt speech production [Eickhoff et al.,2009] or speech disorders [Dronkers,1996]. With this background in mind, we tested for white matter differences between SI and matched controls by comparing FA values derived from the DTI data and report two principal findings (Fig. 1 and Table I). The first main result was that we found substantial between‐group differences in white matter architecture as indicated by reduced FA in the SI group in regions involved in the control of speech articulation and sensory‐motor coupling mechanisms. These areas are the left anterior insula, the basal ganglia, the inferior parietal lobe, and the upper part of the corticospinal tract. The second main result was the finding of reduced FA values in the SI group in the anterior (genu), middle (body), and posterior (splenium) part of the corpus callosum, a large fiber bundle in the human brain that predominantly interconnects homologous cortical areas of both hemispheres [Nolte,1999]. Our results thus suggest differential white matter architecture in regions supporting speech articulation, sensory‐motor coupling mechanisms, and interhemispheric callosal transfer. We propose that these expertise‐related morphological adaptations are due to specific language‐related demands associated with simultaneous interpreting.
Fractional Anisotropy and White Matter Plasticity
Our results are comparable with previous work showing that intensive musical training [Imfeld et al.,2009] or fine tuned motor praxis [Hänggi et al.,2009; Jäncke et al.,2009] abets plastic adaptations associated with decreased FA values. Partly in contrast to our findings, a former neuroanatomical study investigating professional pianists using the DTI technique revealed increased FA values with increasing amount of practice hours in this group [Bengtsson et al.,2005]. Although this correlation suggests that increased practice might lead to increased FA, the mean FA values were mostly smaller in pianists compared with nonpianists (see Table I in Bengtsson et al.,2005]. Thus, overall the experts (here the pianists) demonstrate generally lower FA values in many fiber tracts compared with nonexperts.
To date, it results difficult to fundamentally explain the findings arising from FA and diffusivity measurements and associate them with the knowledge deriving from cellular neurophysiology. On the basis of a previous work conducted by Mori and Zhang [2006], we know that there are at least three possibilities that lead to lower FA: (1) an increase in radial diffusivity (along the shorter axes); (2) a decrease in axial diffusivity (along the longest axis); (3) the combination of the two. In addition, crossing fibers lead to decreased FA values at the intersections. On the other hand, a meticulous technical review [Beaulieu,2002] of the DTI technique evidences that the interpretation of water molecules diffusion is much more complex as previously supposed. Beaulieu provides empirical evidence for the fact that the available data do not permit the dissection of the individual contributions of myelin and axonal membranes to the degree of anisotropy. However, empirical evidence suggests that axonal membranes play the primary role and that myelination, although not necessary for significant anisotropy, can modulate the degree of anisotropy. This implicates further reasoning to explain how training‐induced neuronal adaptation can lead to lower FA values.
As visible in Figure 2 our DTI data militate in favor of the view that the lower FA values in the interpreters' group are associated with reduced axial and enhanced radial diffusivity. For example, it might be that the specific stimulation and usage of the language and sensory‐motor control systems might have resulted in a more complex architecture of the fiber bundles in the form of less parallel devolution. A further possibility might be that increased myelin sheaths may reduce the volume of the extracellular space and simultaneously increase the intracellular radial diffusivity relative to its extracellular counterpart; this leading to lower FA values. To further clarify which of these aforementioned mechanisms (or even others) are responsible for the changes in FA reported here, it is necessary to conduct further methodological research.
Sensory‐Motor Coupling and Speech Articulation
The insula has a varying cytoarchitectonic arrangement throughout its length and subserves a plethora of different functions: visceral, motor, vestibular, somatosensory, and auditory functions [Flynn et al.,1999]. However, the anterior part of the insula, in the proximity of the extreme capsule, has been shown to have connections to the brain stem and to the pontine nuclei [Ozaki et al.,1986]. The latter serve as a relay station for corticopontine fibers that carry information from the primary motor cortex and have projections to the cerebellum via the middle cerebellar peduncle. The direct and indirect anatomical connections of the anterior insula with several motor structures suggest that this region is strongly involved in fine tuned motor adjustments and modification of actions. In addition, the anterior insula is known to have connections to the supratemporal plane [Flynn et al.,1999] and to the caudate nucleus [Turner et al.,2009]. These direct or indirect crosslinks between the anterior insula with several motor structures and its connections to the supratemporal plane support the notion that this region subserves speech articulation and sounds to motor mapping functions. In fact, previous neuroimaging [Ackermann and Riecker,2004; Riecker et al.,2000; Wise et al.,1999] and lesion studies [Bates et al.,2003; Dronkers,1996] have demonstrated that the left anterior insula is generally involved in the coordination of motor aspects of speech [Riecker et al.,2000; Wise et al.,1999], of muscles engaged in articulation and phonation [Ackermann and Riecker,2004] and involved in the motor planning of speech, as shown in patients with apraxia of speech [Dronkers,1996]. In view of the differential white matter architecture we found between the two groups in the left anterior insula, the caudate nucleus, and in the upper part of the corticospinal tract, our results suggest an expertise‐related structural adaptation in fiber bundles supporting speech articulation and sensory‐motor coupling mechanisms. In line with our hypothesis, we suggest that this expertise‐related adaptation facilitates a more efficient neural processing during sound‐to‐motor mapping, which is a fundamental prerequisite for the simultaneous translation from an input language to a target language.
The participation of the left anterior insula in sounds‐motor associations was previously corroborated in music‐naïve individuals undergoing a short‐term musical training [Mutschler et al.,2007]. Mutschler et al. investigated subjects who passively listened to simple piano melodies that had been either actively learned beforehand or simply listened to passively and found increased fMRI responses to actively compared with passively learned melodies in a region similar to that we found in this study. These previous results show that the left anterior insula becomes involved in sensory‐motor coupling processes already after a short period of learning and may therefore play a role in learning and supporting sensory‐motor associations. These results further suggest that the left anterior insula is involved in auditory‐motor integration such as when sounds become meaningful to the motor system, probably reflecting a general auditory‐motor interface.
In a meta‐analysis of previously published neuroimaging data reporting insula effects, Mutschler et al. [2009] found language tasks and perception of vocalization to preferentially activate an area in the dorsal part of the left anterior insular cortex, thus suggesting that this subregion may be involved in general language functions. While we are fully aware of the anterior insula's involvement in a variety of heterogeneous functions, we are concerned here only with its role in language processing. The contribution of the left anterior insula to language processing is further supported by the results of a previous published voxel‐based‐morphometry (VBM) study [Golestani and Pallier,2007]. This particular study revealed that individuals who more accurately learned to pronounce a Persian consonant that does not exist in French but which could easily be distinguished from French speech sounds had higher white matter densities in the white matter enclosing left anterior insula. Interestingly, the differential cluster we found in the anterior insula (x = −26, y = 23, z = 12) has almost the same anatomical location as reported by Golestani and Pallier (x = −29, y = 29, z = 10) in fast foreign speech learners. This is not surprising, since SI can be considered as individuals able to learning foreign languages with high proficiency. Foreign speech learning places a high demand on sensory‐motor coupling mechanisms, since the pronounced sound continuously undergoes fine tuned sensory‐motor adjustments in order to achieve an excellent pronunciation in the foreign language. We thus propose that the ability to rapidly regulate sound‐to‐motor transformations may be a basic prerequisite for enrolment in simultaneous interpreting college. However, an additional intensive training in this specific domain is necessary to become a certified and graduated professional SI. This hypothesis could be the starting point for developing a sensitive test for separating suitable training candidates, since selection during education is known to be time consuming and costly.
Meanwhile, it is established that the left anterior insular region is phylogenetically ancient and belongs to the archicortex. Even if this region evolved relatively early in evolution, there is evidence in favor of the view that this brain region plays an important role in audio‐motor integration [Mutschler et al.,2009] and in particular in the coordination of the up to 100 muscles engaged in articulation and phonation [Ackermann and Riecker,2004,2010]. Conceivably, the functional involvement of the left anterior insular region in speech production might have evolved within the framework of phylogenetically older connections between the insula and limbic structures. Otherwise, it is imaginable that the contribution of the left anterior insula to speech production was established through connections between this structure and nonspeech functions of the upper midline musculature in association with swallowing [Ackermann and Riecker,2004].
Callosal Transfer
Genu of the corpus callosum
The anatomy of the corpus callosum has been proposed to be a potential marker for functional lateralization because its size is considered proportional to the number of fibers connecting the two hemispheres [Josse et al.,2008]. In line with this, a previous morphometric study [Schlaug et al.,1995] showed that professional musicians who began musical training before the age of 7 revealed a larger mid‐sagittal area in the anterior part of the corpus callosum than did control subjects, indicating a training‐induced difference in interhemispheric communication between sensory‐motor areas.
The anterior part of the corpus callosum contains fiber tracts connecting frontal and orbitofrontal areas of both hemispheres [Huang et al.,2005]. Many of these fibers are involved in the control of motor and somatosensory functions. However, other fibers support the interhemispheric communication between both insulae and prefrontal areas [Delacoste et al.,1985; Dimond et al.,1977]. It is now established that prefrontal regions play an essential role in the integration of information and the management of multiple tasks [Reynolds et al.,2006] and are thus crucial in subserving higher cognitive functions such as memory, attention, and inhibition. Indeed, all these cognitive functions are strongly involved in simultaneous interpreting. In line with this, the differential architecture we revealed in the genu of the corpus callosum suggests that professional interpreting is accompanied by plastic adaptations in a structure that facilitates executive control mechanisms. In particular, the morphological differences we found in the genu of the corpus callosum suggest that simultaneous interpreting may have an influence on the transfer time of information across multiple frontal areas for enhancing executive control.
One particular PET study [Rinne et al.,2000] measured brain activation in professional interpreters during simultaneous interpreting versus repetition of auditorily presented sentences, reporting clear evidence of the high demands placed on executive control during interpreting. The relevance of prefrontal regions for language translation is further supported by another PET study [Klein et al.,1995] designed to investigate translation compared with the repetition of single auditorily presented words in English‐French bilinguals. Similar prefrontal activations were also found in a language‐switching fMRI study [Hernandez et al.,2000], which investigated single‐ and dual‐language picture naming in a group of Spanish‐English bilinguals. Interestingly, a comparable involvement of prefrontal areas was also reported in several switching tasks that were not principally related to language processing [Yeung et al.,2006]. All these findings are congruent with the view that language switching, a fundamental prerequisite for translation, is part of a general executive attentional system residing in the prefrontal cortex [Hernandez et al.,2000]. Hence, we suggest that the differential architecture in the genu of the corpus callosum may support efficient control of switching‐related mechanisms by keeping one language from interfering with the other during speech. Our results are also consistent with a previous morphometric study [Coggins et al.,2004] that evaluated the area of five subregions of the mid‐sagittal corpus callosum in bilingual compared with monolingual individuals. The authors proposed that the measured adaptation in the anterior mid‐body occurred in tracts connecting prefrontal regions, mainly to accommodate multiple language capacities and increase processing speed between the frontal lobes; this being advantageous in compensating for the processing load of maintaining multiple languages.
Splenium of the corpus callosum
The fibers connecting the temporal and parietal language‐related regions of the two hemispheres cross the caudal part of the corpus callosum [Delacoste et al.,1985; Waddington,1984]. Although language processing is the result of the coordination of activity between both hemispheres via the cerebral commissures [Coggins et al.,2004], it is known that the processing of speech sounds relies on important computational differences between the two hemispheres [Hickok and Poeppel,2007]. The left auditory‐related cortex is more proficient at processing stimuli requiring high‐temporal resolution (segmental information), whereas the right counterpart is more responsive to processing spectral information (suprasegmental information) [Zatorre and Belin,2001].
Although speech processing relies on fast changing signals more strongly involving the left auditory‐related cortex [Zaehle et al.,2004], the prosody of speech is preferentially processed in the right auditory‐related cortex [Meyer et al.,2002]. Professional interpreters rely on both segmental and suprasegmental information for performing an adequate translation, especially taking into account the huge variability of the speakers, their cultural background, the numerous languages spoken, and the informational relevance mediated by speech prosody. In this context, a previous EEG study [Friederici et al.,2007] has shown the functional relevance of the posterior third of the corpus callosum for the dynamic interplay of right and left auditory‐related areas in patients with posterior callosal lesions. These results in combination with what is known about the functional anatomy of the corpus callosum lead to the suggestion that the FA differences we found in the splenium are the result of training‐induced adaptations in interhemispheric connectivity between temporal and temporo‐parietal auditory‐related brain regions and that this is of relevance for a fine‐tuned interplay between perisylvian regions.
A previous study on language expertise [Golestani et al.,2002] did show a relationship between white matter architecture and non‐native speech sound learning. This study demonstrated that the speed of sound learning is correlated with FA in parieto‐occipital regions and parts of the splenium of the corpus callosum. These anatomical differences are thought to be related to the more efficient neuronal processing in faster non‐native speech sounds learners, this possibly due to greater interhemispheric connectivity in temporal and temporo‐parietal auditory‐related brain regions in fast phonetic learners. In a similar way, we suggest that the reduced FA we uncovered in the posterior part of the corpus callosum may be related to a higher or smaller degree of functional asymmetry in speech‐related functions of temporo‐parietal regions. Since several neuroimaging studies have repeatedly shown leftward asymmetries during tasks requiring phonetic processing [Demonet et al.,1992; Zatorre et al.,1992] and verbal working memory functions [Paulesu et al.,1993], we propose that the reduced FA we revealed in the splenium could be related to a more asymmetrical/symmetrical representation of phonetic perception or verbal working memory functions, these being essential for interpreting.
Differential White Matter Architecture in the Right Hemisphere
Our findings of differential white matter architecture deserve a particular consideration in the right hemisphere. In particular, we discuss the lower FA values we found in the SI group in the dorsal part of the right caudate nucleus and the right inferior parietal lobe in turn.
Caudate Nucleus
Meanwhile, there is mounting evidence supporting the notion that not only the left but also the right caudate nucleus is strongly involved in speech production [Abutalebi et al.,2008; Liu et al.,2010]. Furthermore, the participation of the right caudate nucleus in overt speech processing was previously found in bilingual subjects during both language switching [Abutalebi et al.,2008; Price et al.,1999] and translation tasks [Abutalebi et al.,2007; Price et al.,1999]. In the context of bilingualism, previous researches [Abutalebi and Green,2007; Abutalebi et al.,2008] have also associated the functional contribution of this anatomical structure with inhibition mechanisms, because during speech production one language has to be inhibited in order to avoid interferences between the two languages. Taken together, these previous observations speak in favor of the view that the right caudate nucleus may not only be related to articulo‐motor processing but also to general brain mechanisms such as switching and inhibition mechanisms. Even though we are fully aware that bilingual subjects cannot be directly contrasted with the exceptional faculties observed in professional SI due to the intensive training demands, simultaneous interpreting strongly involves switching and inhibition. However, in the context of the findings of this work, it results difficult to allocate a univocal functional contribution on this anatomical structure, since this study only placed emphasis on anatomy without integration of behavioral aspects.
Inferior Parietal Lobe
Our present data agree with several recent neuroimaging and morphometric studies that sketched a bilateral cerebral implementation of language processing. In fact, right hemisphere structures were previously shown to play a prominent role in both speech production [Abutalebi et al.,2008; Golestani and Pallier,2007; Golestani et al.,2002; Liu et al.,2010] and speech perception [Meyer et al.,2004; Schmidt et al.,2008]. Furthermore, previous findings argue in favor of the view that the right inferior parietal lobe is implicated in the storage of phonological information in verbal short‐term memory [Jonides et al.,1998; Paulesu et al.,1993].
Meanwhile, there is willingness to consider the functional and morphologic characteristics of the right inferior parietal lobe as a likely neuroanatomical substrate subserving different aspects of language processing in association with training and expertise [Golestani and Pallier,2007; Price et al.,1999]. Along this vein, the view of a right inferior parietal contribution to language expertise is consistent with a previous research conducted with German‐English bilinguals who were scanned whilst either translating or reading visually presented words in German (L1), English (L2), or switching between L1 and L2 [Price et al.,1999]. The fact that switching between L1 and L2 (in both directions) was associated with increased bilateral activation of the inferior parietal lobe may indicate that switching modulates word processing at a phonological stage. Furthermore, a right inferior parietal contribution to phonemic processing is supported by a previous study [Zatorre et al.,1996], which showed that this region was stronger involved in phonemic detection than in nonverbal pitch detection. On the basis of this previous evidence for phonological processing in inferior parietal regions, we propose that professional interpreting may favor brain connectivity in regions associated with the storage of phonological information in verbal short‐term memory. Otherwise, right hemispheric language processing was previously associated with the perception of auditory suprasegmental cues [Geiser et al.,2008; Meyer et al.,2002] that may help facilitate professional interpreting. These previous observations in compliance with our present data partially agree with the notion that language skills and expertise are associated with a right inferior parietal implementation of language processing.
CONCLUSIONS
In the present DTI study, we compared FA between SI and control subjects and expected to find differential white matter properties in regions supporting sensory‐motor coupling mechanisms and speech articulation. In line with our hypothesis, we found reduced FA in SI in a motor network including the left anterior insula, the basal ganglia, and the upper part of the corticospinal tract. As a second main result, we found significantly reduced FA in SI in the anterior and posterior part of the corpus callosum, suggesting that intensive language training as experienced in simultaneous interpreting influences interhemispheric transfer functions. Our results might have implications for the understanding of language expertise in relation to plastic changes in the white matter architecture.
Author contributions
SE conceived the study, designed the experimental paradigm, and drafted the manuscript. JH contributed to the hypothesis, to the manuscript, and performed the data analysis. MM contributed to the hypothesis, design, results discussion, and preparation of the manuscript. LJ contributed to the hypothesis, design, results, discussion, and preparation of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Marcus Cheetham for helpful comments on a previous version of the manuscript and Lucas Marrama for assisting in data acquisition.
REFERENCES
- Abutalebi J, Annoni JM, Zimine I, Pegna AJ, Seghier ML, Lee‐Jahnke H, Lazeyras F, Cappa SF, Khateb A ( 2008): Language control and lexical competition in bilinguals: An event‐related fMRI study. Cereb Cortex 18: 1496–1505. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Brambati SM, Annoni JM, Moro A, Cappa SF, Perani D ( 2007): The neural cost of the auditory perception of language switches: An event‐related functional magnetic resonance imaging study in bilinguals. J Neurosci 27: 13762–13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abutalebi J, Green D ( 2007): Bilingual language production: The neurocognition of language representation and control. J Neurolinguistics 20: 242–275. [Google Scholar]
- Ackermann H, Riecker A ( 2004): The contribution of the insula to motor aspects of speech production: A review and a hypothesis. Brain Lang 89: 320–328. [DOI] [PubMed] [Google Scholar]
- Ackermann H, Riecker A ( 2010): The contribution(s) of the insula to speech production: A review of the clinical and functional imaging literature. Brain Struct Funct 214: 419–433. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF ( 2003): Voxel‐based lesion‐symptom mapping. Nat Neurosci 6: 448–450. [DOI] [PubMed] [Google Scholar]
- Baumann S, Koeneke S, Schmidt CF, Meyer M, Lutz K, Jüncke L ( 2007): A network for audio‐motor coordination in skilled pianists and non‐musicians. Brain Res 1161: 65–78. [DOI] [PubMed] [Google Scholar]
- Beal DS, Gracco VL, Lafaille SJ, De Nil LF ( 2007): Voxel‐based morphometry of auditory and speech‐related cortex in stutterers. Neuroreport 18: 1257–1260. [DOI] [PubMed] [Google Scholar]
- Beaulieu C ( 2002): The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed 15: 435–455. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen‐Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM ( 2003): Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magn Reson Med 50: 1077‐1088. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F ( 2005): Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci 8: 1148–1150. [DOI] [PubMed] [Google Scholar]
- Chiang MC, Barysheva M, Schattuk DW, Lee AD, Madsen SK, Avedissian C, Klunder AD, Toga AW, McMahon KL, de Zubicaray GI, Wright MJ, Srivastava A, Balov N, Thompson PM ( 2009): Genetics of brain fiber architecture and intellectual performance. J Neurosci 29: 2212–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggins PE, Kennedy TJ, Armstrong TA ( 2004): Bilingual corpus callosum variability. Brain Lang 89: 69–75. [DOI] [PubMed] [Google Scholar]
- Delacoste MC, Kirkpatrick JB, Ross ED ( 1985): Topography of the human corpus‐callosum. J Neuropathol Exp Neurol 44: 578–591. [DOI] [PubMed] [Google Scholar]
- Demonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R ( 1992): The anatomy of phonological and semantic processing in normal subjects. Brain 115: 1753–1768. [DOI] [PubMed] [Google Scholar]
- Dimond SJ, Scammell RE, Brouwers EYM, Weeks R ( 1977): Functions of center section (trunk) of corpus‐callosum in man. Brain 100: 543–562. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A ( 2004): Neuroplasticity: Changes in grey matter induced by training—Newly honed juggling skills show up as a transient feature on a brain‐imaging scan. Nature 427: 311–312. [DOI] [PubMed] [Google Scholar]
- Dronkers NF ( 1996): A new brain region for coordinating speech articulation. Nature 384: 159–161. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K ( 2009): A systems perspective on the effective connectivity of overt speech production. Philos Transact A Math Phys Eng Sci 1896: 2399–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer S, Meyer M, Jüncke L ( 2010): Simultaneous interpreters as a model for neuronal adaptation in the domain of language processing. Brain Res 1317: 147–156. [DOI] [PubMed] [Google Scholar]
- Flynn FG, Benson DF, Ardila A ( 1999): Anatomy of the insula—Functional and clinical correlates. Aphasiology 13: 55–78. [Google Scholar]
- Friederici AD, von Cramon DY, Kotz SA ( 2007): Role of the corpus callosum in speech comprehension: Interfacing syntax and prosody. Neuron 53: 135–145. [DOI] [PubMed] [Google Scholar]
- Geiser E, Zaehle T, Jüncke L, Meyer M ( 2008): The neural correlate of speech rhythm as evidenced by metrical speech processing. J Cogn Neurosci 20: 541–552. [DOI] [PubMed] [Google Scholar]
- Golestani N, Pallier C ( 2007): Anatomical correlates of foreign speech sound production. Cereb Cortex 17: 929–934. [DOI] [PubMed] [Google Scholar]
- Golestani N, Paus T, Zatorre RJ ( 2002): Anatomical correlates of learning novel speech sounds. Neuron 35: 997–1010. [DOI] [PubMed] [Google Scholar]
- Hänggi J, Koeneke S, Bezzola L, Jäncke L ( 2009): Structural neuroplasticity in the sensorimotor network of professional female ballet dancers. Hum Brain Mapp 31: 1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE ( 2004): Combining voxel intensity and cluster extent with permutation test framework. Neuroimage 23: 54–63. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE ( 2004): Nonstationary cluster‐size inference with random field and permutation methods. Neuroimage 22: 676–687. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Martinez A, Kohnert K ( 2000): In search of the language switch: An fMRI study of picture naming in Spanish‐English bilinguals. Brain Lang 73: 421–431. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D ( 2007): Opinion—The cortical organization of speech processing. Nat Rev Neurosci 8: 393–402. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang JY, Jiang HY, Wakana S, Poetscher L, Miller MI, van Zijl PC, Hillis AE, Wytik R, Mori S ( 2005): DTI tractography based parcellation of white matter: Application to the mid‐sagittal morphology of corpus callosum. Neuroimage 26: 195–205. [DOI] [PubMed] [Google Scholar]
- Hyde KL, Lerch J, Norton A, Forgeard M, Winner E, Evans AC, Schlaug G ( 2009): Musical training shapes structural brain development. J Neurosci 29: 3019–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imfeld A, Oechslin MS, Meyer M, Loenneker T, Jüncke L ( 2009): White matter plasticity in the corticospinal tract of musicians: A diffusion tensor imaging study. Neuroimage 46: 600–607. [DOI] [PubMed] [Google Scholar]
- Jüncke L ( 2009): The plastic human brain. Restor Neurol Neurosci 27: 521–538. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Hänggi J, Steinmetz H ( 2004): Morphological brain differences between adult stutterers and non‐stutterers. BMC Neurol 4: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäncke L, Koeneke S, Hoppe A, Rominger C, Hänggi J ( 2009): The architecture of the golfer's brain. PLoS One 4: e4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jüncke L, Shah NJ, Peters M ( 2000): Cortical activations in primary and secondary motor areas for complex bimanual movements in professional pianists. Cogn Brain Res 10: 177–183. [DOI] [PubMed] [Google Scholar]
- Jüncke L, Siegenthaler T, Preis S, Steinmetz H ( 2007): Decreased white‐matter density in a left‐sided fronto‐temporal network in children with developmental language disorder: Evidence for anatomical anomalies in a motor‐language network. Brain Lang 102: 91–98. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter‐Lorenz PA, Marshuetz C, Willis CR ( 1998): The role of parietal cortex in verbal working memory. J Neurosci 18: 5026–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse G, Seghier ML, Kherif F, Price CJ ( 2008): Explaining function with anatomy: Language lateralization and corpus callosum size. J Neurosci 28: 14132–14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Haier RJ ( 2007): The parieto‐frontal integration theory (P‐FIT) of intelligence: Converging neuroimaging evidence. Behav Brain Sci 30: 135–187. [DOI] [PubMed] [Google Scholar]
- Klein D, Milner B, Zatorre RJ, Meyer E, Evans AC ( 1995): The neural substrates underlying word generation—A bilingual functional‐imaging study. Proc Natl Acad Sci USA 92: 2899–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HY, Hu ZG, Guo TM, Peng DL ( 2010): Speaking words in two languages with one brain: Neural overlap and dissociation. Brain Res 1316: 75–82. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T ( 2009): Brain anatomical network and intelligence. PLoS Comput Biol 5: e1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD ( 2000): Navigation‐related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA 97: 4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Alter K, Friederici AD, Lohmann G, von Cramon DY ( 2002): FMRI reveals brain regions mediating slow prosodic modulations in spoken sentences. Hum Brain Mapp 17: 73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Steinhauer K, Alter K, Friederici AD, von Cramon DY ( 2004): Brain activity varies with modulation of dynamic pitch variance in sentence melody. Brain Lang 89: 277–289. [DOI] [PubMed] [Google Scholar]
- Mori S, Zhang JY ( 2006): Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51: 527–539. [DOI] [PubMed] [Google Scholar]
- Münte TF, Altenmuller E, Jüncke L ( 2002): The musician's brain as a model of neuroplasticity. Nat Rev Neurosci 3: 473–478. [DOI] [PubMed] [Google Scholar]
- Mutschler I, Schulze‐Bonhage A, Glauche V, Demandt E, Speck O, Ball T ( 2007): A rapid sound‐action association effect in human insular cortex. PLoS One 2: e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler I, Wieckhorst B, Kowalevski S, Derix J, Wentlandt J, Schulze‐Bonhage A, Ball T ( 2009): Functional organization of the human anterior insular cortex. Neurosci Lett 457: 66–70. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR ( 2003): Progressive non‐fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain 126: 2406–2418. [DOI] [PubMed] [Google Scholar]
- Nolte J ( 1999): The Human Brain: An Introduction to its Functional Anatomy. St. Louis: Mosby. [Google Scholar]
- Ozaki I, Baba M, Narita S, Matsunaga M, Takebe K ( 1986): Pure dysarthria due to anterior internal capsule and‐or corona radiata infarction—A report of 5 cases. J Neurol Neurosurg Psychiatr 49: 1435–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RSJ ( 1993): The neural correlates of the verbal component of working memory. Nature 362: 342–345. [DOI] [PubMed] [Google Scholar]
- Price CJ, Green DW, von Studnitz R ( 1999): A functional imaging study of translation and language switching. Brain 122: 2221–2235. [DOI] [PubMed] [Google Scholar]
- Reynolds JR, McDermott KB, Braver TS ( 2006): A direct comparison of anterior prefrontal cortex involvement in episodic retrieval and integration. Cereb Cortex 16: 519–528. [DOI] [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, Dogil G, Grodd W ( 2000): Opposite hemispheric lateralization effects during speaking and singing at motor cortex, insula and cerebellum. Neuroreport 11: 1997–2000. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Tommola J, Laine M, Krause BJ, Schmidt D, Kaasinen V, Teräs M, Sipilä H, Sunnari M ( 2000): The translating brain: Cerebral activation patterns during simultaneous interpreting. Neurosci Lett 294: 85–88. [DOI] [PubMed] [Google Scholar]
- Ruff RL, Arbit E ( 1981): Aphemia resulting from a left frontal hematoma. Neurology 31: 353–356. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, Umarova R, Musso M, Glauche V, Abel S, Huber W, Rijntjes M, Hennig J, Weiller C ( 2008): Ventral and dorsal pathways for language. Proc Natl Acad Sci USA 105: 18035–18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff HB, Alexander MP, Naeser MA, Galaburda AM ( 1983): Aphemia. Clinical‐anatomic correlations. Arch Neurol 40: 720–727. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Jüncke L, Huang YX, Staiger JF, Steinmetz H ( 1995): Increased corpus‐callosum size in musicians. Neuropsychologia 33: 1047–1055. [DOI] [PubMed] [Google Scholar]
- Schmidt CF, Zaehle T, Meyer M, Geiser E, Boesiger P, Jüncke L ( 2008): Silent and continuous fMRI scanning differentially modulate activation in an auditory language comprehension task. Hum Brain Mapp 29: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ ( 2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. NeuroImage 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- Turner BH, Mishkin M, Knapp M ( 2009): Organization of the amygdalopedal projections from modality‐specic cortical association areas in the monkey. J Comp Neurol 191: 515–543. [DOI] [PubMed] [Google Scholar]
- Waddington MM ( 1984): Atlas of the Human Intracranial Anatomy. Vermont: Rutland. [Google Scholar]
- Wise RJS, Greene J, Buchel C, Scott SK ( 1999): Brain regions involved in articulation. Lancet 353: 1057–1061. [DOI] [PubMed] [Google Scholar]
- Yeung N, Nystrom LE, Aronson JA, Cohen JD ( 2006): Between‐task competition and cognitive control in task switching. J Neurosci 26: 1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaehle T, Wustenberg T, Meyer M, Jüncke L ( 2004): Evidence for rapid auditory perception as the foundation of speech processing: A sparse temporal sampling fMRI study. Eur J Neurosci 20: 2447–2456. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P ( 2001): Spectral and temporal processing in human auditory cortex. Cereb Cortex 11: 946–953. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E, Gjedde A ( 1992): Lateralization of phonetic and pitch discrimination in speech processing. Science 256: 846–849. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Meyer E, Gjedde A, Evans, AC ( 1996): PET studies of phonetic processing of speech: Review, replication, and reanalysis. Cereb Cortex 6: 21–30. [DOI] [PubMed] [Google Scholar]


