Abstract
Previous studies of brain lesions, functional activity, and gray matter structures have suggested that emotional intelligence (EI) is associated with regions involved in the network of social cognition (SCN) and in somatic marker circuitry (SMC). Our new study is the first to investigate the association between white matter (WM) integrity and EI. We examined this relationship in the brain of healthy young adult men [n = 74, mean age = 21.5 years, standard deviation (SD) = 1.6] and women (n = 44, mean age = 21.9 years, SD = 1.4). We performed a voxel‐based analysis of fractional anisotropy, which is an indicator of WM integrity, using diffusion tensor imaging and used a questionnaire (EI Scale) for measuring EI to identify the correlation of WM integrity with individual EI factor (intrapersonal, interpersonal, and situation management factors). Our results showed that (a) the intrapersonal factor of EI was positively correlated with WM integrity in the right anterior insula, and (b) the interpersonal factor of EI was associated with WM integrity in a part of the right inferior longitudinal fasciculus (ILF). The right anterior insula is one of the important nodes of the SMC, whereas the ILF connects the visual cortex and areas related to SCN, and thus, is a part of the SCN. Our findings further support the notion that the brain regions involved in the SCN and in the SMC are associated with EI. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: facial perception, emotional processing, gray matter, intrapersonal factor, interpersonal factor, situation management factor, fractional anisotropy
INTRODUCTION
Emotional intelligence (EI) is the ability to monitor one's own as well as others' emotions and to use that information to guide one's thinking and action [Salovey and Mayer, 1990]. Measurements of EI can act as valuable predictors of competence and success with regards to important lifestyle choices [Van Rooy and Viswesvaran, 2004].
According to neuroimaging studies, EI is associated with somatic marker circuitry (SMC) and neural network involved in social cognition (SCN) [Takeuchi et al., in 2011c]. The somatic marker hypothesis [Damasio et al., 1991] proposes that emotion‐based biasing signals arising from the body are integrated in higher brain regions, in particular the ventromedial prefrontal cortex (VMPFC), to regulate decision‐making in situations of complexity. Several regions, including the VMPFC, insula, and amygdala, belong to the SMC [Damasio, 1998]. On the other hand, EI is related to SCN, such as the network related to the theory of mind which involves the medial prefrontal cortex and the superior temporal sulcus (STS) and so on (for a summary of the neural circuitry involved in SCN, see Pelphrey and Carter [ 2008]). It has been illustrated that (a) the SMC's brain activity during the perception of fearful faces [Killgore and Yurgelun‐Todd, 2007], (b) lesions to the SMC [Bar‐On et al., 2003; Krueger et al., 2009], and (c) the SMC's regional gray matter density (rGMD) [Takeuchi et al., in 2011c] are associated with EI. SCN's brain activity during social reasoning [Stone et al., 2002] and its rGMD [Takeuchi et al., in 2011c] are also associated with EI.
However, despite several studies on it, the details of the relationship of EI with regard to white matter (WM) integrity remains unclear. The purpose of this study is to investigate the association between EI and WM integrity. We hypothesize that fractional anisotropy (FA), an indicator of WM integrity, in the SMC and SCN regions of the brain is associated with EI.
Understanding the underlying WM structural integrity with EI is important for gathering new insights into brain function, specifically how the SMC and SCN regions can interact and lead to decision‐making processes that are important in the achievement of a successful social life. WM integrity is possibly associated with EI in nonclinical samples for several reasons. WM integrity, found in nonclinical samples is associated with cognitive function with reference to nonsocial and nonemotional thought processes [Jung et al., 2010a; Olesen et al., 2003; Schmithorst et al., 2005; Takeuchi et al., 2010d]. Furthermore, in Autism patients, reduced WM integrity is seen in WM regions adjacent to several regions in the SCN [Barnea‐Goraly et al., 2004]. Dysfunctions of these brain region are believed to contribute to the EI‐related social deficits in autism [Frith, 2001].
We performed a voxel‐based analysis of FA using diffusion tensor imaging (DTI) [Le Bihan, 2003] to assess WM integrity. For EI assessment, we used the Japanese version of the EI Scale [Uchiyama et al., 2001], which has three factors: intrapersonal, interpersonal, and situation management factors; these factors evaluate the ability to understand and adapt emotions with regards to oneself, others, and specific situations, respectively [Uchiyama et al., 2001].
MATERIAL AND METHODS
Subjects
This study, which is a part of an ongoing project to investigate the association between brain imaging, cognitive function, and aging [Takeuchi et al., in 2011c; Takeuchi et al., 2010c; Takeuchi et al., 2010d; Takeuchi et al., 2011a; Taki et al., in press; Taki et al., 2010], included 118 healthy, right‐handed individuals (74 men and 44 women). In our previous study, data from 55 subjects were used to investigate the association between EI and rGMD [Takeuchi et al., in 2011c]. All subjects enrolled in this study also became subjects of our intervention studies [Takeuchi et al., in 2011b; Takeuchi et al., in 2011d]. In these intervention studies, psychological tests and MRI not described in this study were performed concomitantly with experiments described in this study (data from psychological tests and MRI performed before the intervention was used in this study). The mean age of the subjects was 21.6 years (standard deviation, SD = 1.57). All subjects were university students or postgraduate students, with normal vision, no history of neurological or psychiatric illness, and no recent report of usage of psychoactive or antipsychotic drugs. We provided questionnaires to all potential experimental subjects for the assessment of psychiatric illnesses and recent drug use history. In the questionnaire, subjects were asked to provide a detailed list of all drugs that they had recently used. Handedness was evaluated using the Edinburgh Handedness Inventory [Oldfield, 1971]. Written informed consent was obtained from each subject for his/her participation in the study experiment. All study procedures were approved by the Ethics Committee of Tohoku University.
Emotional Intelligence Scale
The Japanese version of the EI scale [Fukunishi et al., 2001b; Uchiyama et al., 2001] was used to assess EI as it was in our previous study [Takeuchi et al., in 2011c]. The EI Scale is a self‐reported measurement that provides an estimate of underlying emotional and social intelligence. The scale was developed and standardized for use with Japanese subjects. A detailed discussion and history of the development of the psychometric properties of this questionnaire is included in the EI Scale's technical manual [Uchiyama et al., 2001]. The EI Scale comprises of 65 items and a five‐point Likert scale with a response format ranging from “not true of me” to “very often true of me.” The subjects' responses were categorized into the following three composite scale scores (factors): (a) intrapersonal factor (comprised of self‐insight, self‐motivation, and self‐control), (b) interpersonal factor (comprised of empathy, altruism, and interpersonal control), and (c) situation management factor (comprised of insight into and control over a situation). Each composite scale score is comprised of three subscale scores.
The intrapersonal factor evaluates (1) self‐awareness, (2) the ability to sustain one's behavior, and (3) the ability to act appropriately. The interpersonal factor evaluates the ability to maintain appropriate personal relationships based on the understanding and empathy toward another person's emotions. The situation management factor evaluates (1) the ability of an individual to endure and adapt to a change, (2) provide leadership, and (3) exhibit flexibility in the control and use of their abilities in dynamic situations. Some examples of items on the EI Scale are included in our previous article [Takeuchi et al., in 2011c]. As summarized in our previous work [Takeuchi et al., in 2011c], in addition to this three‐component model of EI, a four‐component model of EI [Salovey and Mayer, 1990] and a five‐component model of EI [Bar‐On, 1997] also exist. The Bar‐On model of EI [Bar‐On, 1997] consists of two major factors, an intrapersonal and an interpersonal factor, in addition to several miscellaneous factors, such as stress coping, adaptability, and general mood. On the contrary, Otake et al. [ 2001] proposed a third major factor (situation management), which is equal to the minor factors of the Bar‐On model.
The EI Scale is an established test based on normative data with a large sample size (n = 703) [Uchiyama et al., 2001]. The scoring of each factor is based on a test manual. Confirmatory factor analyses validate the model of this test [Otake et al., 2001; Uchiyama et al., 2001]. According to the test manual [Uchiyama et al., 2001], the internal consistencies of the three factors (intrapersonal, interpersonal, and situation management factors) are 0.894, 0.915, and 0.915, respectively (Cronbach's coefficient α).
Scores on the EI Scale are associated with EI related measurements such as the Toronto Alexithymia Scale [Fukunishi et al., 2001a]. This indicates the external validity of the EI Scale.
All three factors of the EI Scale are associated with improved mental health as determined by a general health questionnaire as well as increased optimism as determined by the LOT Optimism Scale [Uchiyama et al., 2001]. Specifically, the situation management factor was strongly associated with better mental health [Uchiyama et al., 2001]. These results are consistent with the idea that higher EI leads to better mental health [Salovey et al., 2000].
Assessment of Psychometric Measures of General Intelligence
Raven's Advanced Progressive Matrix [Raven, 1998], is a measurement that is most correlated with general intelligence and is considered to be the best measurement of general intelligence [Raven, 1998]. Raven's Advanced Progressive Matrix was used to assess intelligence and adjust for the effect of general intelligence on WM structures [Schmithorst et al., 2005]. More detailed information about how this test was performed in our study is described in previous studies [Takeuchi et al., 2010c; Takeuchi et al., 2010d].
Image Acquisition
All MRI data acquisition was conducted with a 3‐T Philips Achieva scanner. As in our previous study [Takeuchi et al., 2010d], diffusion‐weighted data were acquired using a spin‐echo echo‐planar imaging (EPI) sequence (repetition time (TR) = 10293 ms, echo time (TE) = 55 ms, big delta (Δ) = 26.3 ms, little delta (δ) = 12.2 ms, field of view (FOV) = 22.4 cm, 2 × 2 × 2 mm3 voxels, slice thickness = 2 mm, 60 slices, sensitivity encoding (SENSE) reduction factor = 2, number of acquisitions = 1). Diffusion weighting was isotropically distributed along 32 directions (b value = 1,000 s/mm2). In addition, a data set with no diffusion weighting (b value = 0 s/mm2) (b = 0 image) was acquired. The total scan time was 7 min and 17 s. FA values were calculated from the collected images.
Preprocessing of Diffusion Imaging Data
In DTI, FA in each voxel was used as a measurement for the degree of diffusion anisotropy, with FA reflecting the angle (degree of directionality) of cellular structures within the fiber tracts, and therefore, reflecting the fiber structural integrity [Chua et al., 2008]. FA varies between 0, representing isotropic diffusion, and 1 representing diffusion taking place along one plane or direction.
Preprocessing and data analyses were performed using statistical parametric mapping software (SPM5; Wellcome Department of Cognitive Neurology, London, UK) and implemented in Matlab (Mathworks, Natick, MA). A normalization procedure was performed as described in our previous study [Takeuchi et al., 2010d]. Briefly, using the affine and nonlinear spatial normalization algorithm, the skull‐stripped b = 0 images of each participant were normalized to the original skull‐stripped b = 0 image template made in our previous study [for the details of this procedure, see Takeuchi et al., 2010d]. By applying the parameters derived from the normalization of the skull‐stripped b = 0 image of each participant, the FA map image of each participant was spatially normalized to give an image with 2 × 2 × 2 mm3 voxels. Finally, the normalized FA map image was spatially smoothed using a Gaussian kernel of 6‐mm full width at half‐maximum. The resulting maps representing FA were then forwarded to the group regression analysis described below.
The Statistical Analysis Using Data From Both Sexes
In the statistical analysis, we tested for a relationship between individual EI and regional FA. In the whole brain analysis, we used multiple linear regression to search for areas where FA was significantly related to the individual three EI Scale scores (intrapersonal, interpersonal, and situation management factors).
In addition to the individual three EI Scale scores, sex, age, and the Raven's Advanced Progressive Matrix score were also utilized as additional covariates, resulting in six covariates. In this analysis, we confined the search to voxels with FA > 0.20 [Albrecht et al., 2007] for each participant because FA is more susceptible to error arising from partial volumes [Pfefferbaum and Sullivan, 2003]. With this FA cutoff value, we can dissociate WM structures from other tissues [Salat et al., 2005].
The Statistical Analysis Using Data From Each Sex Separately
Covarying sex may mask any findings unique to males or females. Thus, in the whole brain analysis using only data from males or females, we performed a multiple linear regression analysis to find areas where FA was significantly related to individual scores on the three factors of the EI Scale (intrapersonal, interpersonal, and situation management factors). The analysis was performed with age and the Raven's Advanced Progressive Matrix score as additional covariates.
Statistical Threshold
Regions of significance were inferred using cluster‐level statistics [Friston et al., 1996]. Only clusters with a P < 0.05 after correcting for multiple comparisons (controlling for familywise error) at the cluster size with a voxel‐level cluster‐determining threshold of P < 0.005, uncorrected, were considered statistically significant in this analysis.
For areas with a strong a priori hypothesis, the level of statistical significance was set at 100 voxels, with an underlying voxel level of P < 0.005. Liberal thresholds on areas with a strong a priori hypothesis were previously utilized [e.g., Pochon et al., 2002]. In this study, such an approach was also the more appropriate because areas with a strong a priori hypothesis in this case did not fit the anatomical label. The standard region of interest (ROI) approach was inappropriate and we were not able to set functional ROIs in this study. Furthermore, it was difficult to perform small volume corrections using certain sized spheres in this study, because based on previous functional activation or gray matter structural studies, the centers of the spheres had to be in the gray matter areas. There were no equivalent WM studies. Any small volume corrections would consequently result in the inclusion of huge WM areas unrelated to the areas with a strong a priori hypothesis. Areas with a strong a priori hypothesis in this study were the WM regions adjacent to the VMPFC, the right anterior insula, the right STS, and the precuneus. These regions are key nodes of the SCN and the SMC. RGMD in these regions significantly correlated with individual scores on the factors of the EI Scale [Takeuchi et al., in 2011c].
RESULTS
Behavioral Data
For both sexes, the average score, the SD and range of age, the Raven's Advanced Progressive Matrix score and the scores for each factor of the EI Scale are presented in Table I. Average scores of the three EI Scale factors (intrapersonal, interpersonal, and situation management factors) for males correspond to the 72th, 68th, and 70th percentile [Uchiyama et al., 2001] among adults (Fig. 1). Average scores on the three factors for females correspond to the 71th, 77th, and 66th percentile [Uchiyama et al., 2001] among adults. However, the average EI Scale factor scores did not significantly differ between males and females (t‐test, two‐tailed, P > 0.1).
Table I.
The mean, range, and SD of scores of age, Raven's Advanced Progressive Matrix, and of each factor of EI for males and females
| Measures | Males | Females | ||||
|---|---|---|---|---|---|---|
| Mean | Range | SD | Mean | Range | SD | |
| Age | 21.5 | 18−27 | 1.6 | 21.9 | 19−27 | 1.4 |
| Raven's advanced progressive matrix | 27.9 | 18−35 | 3.5 | 27.2 | 18−35 | 4.0 |
| Intrapersonal factor | 48.7 | 23−80 | 10.5 | 47.8 | 26−78 | 11.8 |
| Interpersonal factor | 44.8 | 8−67 | 11.2 | 47.8 | 13−84 | 14.4 |
| Situation management factor | 42.7 | 14−73 | 12.5 | 41.6 | 13−73 | 14.5 |
Figure 1.
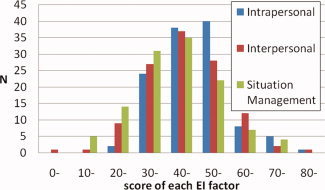
Distribution of the scores of each factor of EI in our sample.
Correlation of FA and the Intrapersonal Factor of EI in the Analysis Using Data From Both Sexes
A multiple regression analysis utilizing age, sex Raven's Advanced Progressive Matrix score as well as the three factor scores of EI as covariates revealed that intrapersonal factor scores were significantly and positively correlated with FA in a WM region in the right anterior insula (Fig. 2a,b; x, y, z = 34, 6, 18, t = 4.45, 113 voxels with an underlying voxel level of P < 0.005, uncorrected for areas with a strong a priori hypothesis. This statistical level corresponds to P = 0.106, corrected for multiple comparisons at the cluster size at the whole brain level). No regions showed significant negative correlations in this analysis.
Figure 2.
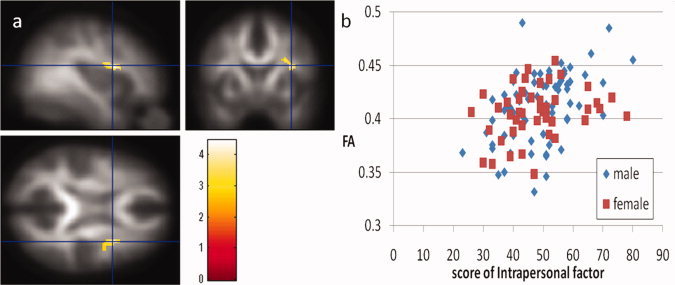
(a) Regions of significant positive correlation between FA and intrapersonal factor scores of the Emotional Intelligence Scale. Results were shown with an extent threshold of 100 voxels, with an underlying voxel level of P < 0.005, uncorrected. Regions of significant correlation are overlaid on the mean, smoothed FA images from all participants. Regions with significant positive correlation can be seen in a WM region in the right anterior insula. (b) A scatter plot of the relationship between the intrapersonal factor scores of the Emotional Intelligence Scale and mean FA values in the significant cluster of the WM region in the right anterior insula.
Correlation of FA and the Interpersonal Factor of EI in the Analysis Using Data From Both Sexes
A multiple regression analysis utilizing age, sex Raven's Advanced Progressive Matrix score as well as the three factor scores of EI as covariates revealed that interpersonal factor scores were significantly and positively correlated with FA in a WM region that extends from the right middle occipital lobe to the regions adjacent to the fusiform gyrus and the parahippocampus [Fig. 3a,b; x, y, z = 32, −60, 0, t = 3.96, P = 0.010 corrected for multiple comparisons at the cluster size at the whole brain level (194 voxels) with an underlying voxel level of P < 0.005, uncorrected]. No regions showed significant negative correlations in this analysis.
Figure 3.
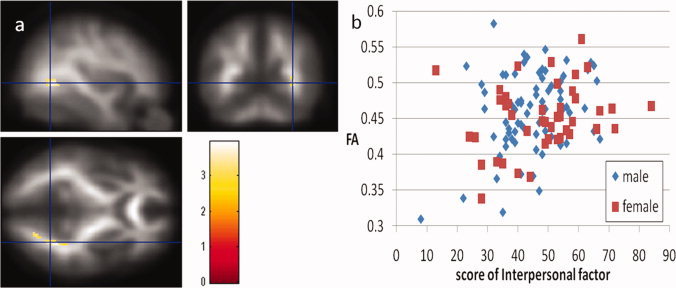
(a) Regions of significant positive correlation between FA and the interpersonal factor scores of the Emotional Intelligence Scale. Results were shown with P < 0.05 after a correction for multiple comparisons at cluster size, with a voxel‐level cluster‐determining threshold of P < 0.005, uncorrected. Regions of significant correlation are overlaid on the mean, smoothed FA images from all participants. The regions with significant positive correlation are seen in a WM region that extends from the right middle occipital lobe to the regions adjacent to the fusiform gyrus and the parahippocampus (a part of the ILF). (b) A scatter plot of the relationship between the interpersonal factor scores of the Emotional Intelligence Scale and mean FA values in the significant cluster in the ILF.
Correlation of FA and the Situation Management Factor of EI in the Analysis Using Data From Both Sexes
A multiple regression analysis utilizing age, sex Raven's Advanced Progressive Matrix score as well as the three factor scores of EI as covariates revealed that the factor score of the situation management did not significantly correlate with FA in any regions.
Correlation of FA and Each Factor of EI in the Analysis Using Data From Each Sex Separately
Data from males or females alone did not exhibit significant correlation between FA and EI factor scores in the multiple regression analysis utilizing age, Raven's Advanced Progressive Matrix score as well as the three factor scores of EI as covariates. The analysis of each sex alone did not result in a significant correlation in relationship to EI in the areas identified by the analysis of data from both sexes. This may suggest that data from both sexes show similar tendencies in these areas and that both the data from males and from females contribute to form significant correlations between these areas in the analysis using data from both sexes.
DISCUSSION
To our knowledge, this is the first study to investigate the association between EI and WM integrity using FA. The result of the analysis using data from both sexes showed that EI intrapersonal factor was positively correlated with WM integrity in the right anterior insula. This result also showed EI interpersonal factor was positively correlated with WM integrity in a WM region that extends from the right middle occipital lobe to the regions adjacent to the fusiform gyrus and the parahippocampus. The right anterior insula is an important node in the SMC [Damasio, 1998]. Furthermore, the WM region extending from the right middle occipital lobe to the regions adjacent to the fusiform gyrus and the parahippocampus is related to facial perception and the processing of emotional information (as discussed below), both of which play key roles in social interactions. The results of our study are partly consistent with our hypothesis and are in line with the notion that brain areas in the SMC and in the SCN support EI [Takeuchi et al., in 2011c].
The significant positive correlation observed between the EI intrapersonal factor and the right anterior insula is in accordance with the idea that brain regions in the SMC are related to EI. Among the regions conforming to the somatic marker hypothesis [Damasio et al., 1991], studies show that the insula, which has abundant connections to other associations and primary sensory areas, is involved in vestibular and pain perception. In addition, this region is important for monitoring ongoing somatic and visceral states and for integrating multimodal information [Dunckley et al., 2005; Nagai et al., 2007; Reiman, 1997; Reiman et al., 1997]. The structural integrity of the WM pathways adjacent to the right anterior insula may make the somatic states more accessible and increase the brain's ability to integrate information, leading to higher intrapersonal EI. Studies show the insula cortex is involved in various neuropsychiatric diseases, such as mood disorders, panic disorders, posttraumatic stress disorder (PTSD), obsessive‐compulsive disorders, eating disorders, and schizophrenia [Nagai et al., 2007]. Improved structural WM integrity adjacent to the insula may play a role in increased EI Scale scores and intrapersonal EI levels, ultimately leading to improved mental health [Schutte et al., 2007; Uchiyama et al., 2001].
Increased WM integrity in the posterior WM region, which extends from the right middle occipital lobe to the regions adjacent to the fusiform gyrus and the parahippocampus, may be associated with higher interpersonal EI through its role in facial perception and the processing of emotional information. This WM region corresponds to parts of the inferior longitudinal fasciculus (ILF) [Catani et al., 2003], which consists of fiber connections between the occipital and anterior temporal cortex. The ILF originates in the extrastriate areas of the occipital lobe and the regions around the fusiform gyrus. The ILF terminates in the lateral temporal cortex and medial temporal cortex in the region of the amygdala and parahippocampal gyrus [Catani et al., 2003]. Among these regions, the fusiform gyrus plays a key role in facial perception, [for a review, see Kanwisher and Yovel, 2006], whereas the amygdala plays a key role in fear perception and aversive conditioning [e.g., LeDoux, 2000]. Note that this region is also an important node of the SMC as was discussed in the Introduction section. The right parahippocampal gyrus also was recently proposed to have a role in the processing of paralinguistic information in social contexts [Rankin et al., 2009]. The functions of these three brain regions are essential for interpersonal communication. The fusiform gyrus processes information related to facial perception [Kanwisher and Yovel, 2006], which conveys essential information during interpersonal communication. On the other hand, the amygdala serves as a protective “brake” in social situations [Amaral, 2002]. Lesions of the amygdala cause a lack of fear and lead to a kind of “socially uninhibited” pattern of behavior, which can be seen in people without fear who cannot avoid persons bearing them potential harm. Furthermore, amygdala lesions also have deleterious consequences on primate social behavior [Amaral, 2002]. Consistent with the fact that the ILF connects the visual cortex with the fusiform gyrus and the amygdala, symptoms caused by lesions of the ILF include prosopagnosia (a disorder of facial perception), and visual hypoemotionality (a deficit of emotions evoked visually with preserved emotional responses to nonvisual stimuli) [Bauer, 1982; Benson et al., 1974; Catani et al., 2003]. In summary, the highly integrated ILF is suggested to play a key role in interpersonal EI by causing to an increase in brain function related to the processing of facial, emotional, and paralinguistic information.
The association between high interpersonal EI and increased WM integrity in the ILF may be comparable to the results of an anatomical study of autistic patients [Cheung et al., 2009]. Children with autism have deficits in facial perception [Boucher and Lewis, 1992], and they are unable to recognize paralinguistic information in a social context [e.g., Martin and McDonald, 2004]. The ILF and the regions related to the ILF play a key role in the perception of faces and in the recognition of paralinguistic information in a social context. The fact that autistic subjects have lower WM integrity in the WM regions around the ILF [Cheung et al., 2009] is consistent with these two facts. Taken together, it is a tempting speculation that decreased WM integrity in the right ILF is one of the common neural indicators of an inferior interpersonal EI and an indicator of autistic traits, both of which could be underlain by problems with facial perception and proper recognition of paralinguistic information in social contexts.
Significant findings for the intrapersonal and the interpersonal factors were identified in the right hemisphere. Although these findings were significant compared with the null hypothesis, it is unclear whether the correlations are truly right hemisphere specific or right hemisphere dominant. The lack of clarity originates from the data analysis from both sexes in which a positive, although insignificant, correlative trend between intrapersonal factor scores and FA was found in the left hemisphere area. This trend was close to the peak voxel of the significant correlations observed in the right hemisphere (x, y, z = −30, 0, 12, t = 2.78, P = 0.003, uncorrected). A similar correlative trend was also identified for interpersonal factor scores and FA in the left hemisphere area close to the peak voxel of the significant correlations observed in the right hemisphere (x, y, z = −28, −70, 2, t = 2.45, P = 0.008, uncorrected). These trends may be congruent with (1) the finding of a substantial correlation between the interpersonal factors score and rGMD in the left anterior insula, in addition to the significant correlation in the right anterior insula [see figure of Takeuchi et al., in 2011c], and (2) the finding that the ability to perceive faces, which may be relevant to interpersonal EI as described above, seems to be associated with bilateral ILF [Thomas et al., 2008]. Bilateral WM structures may be associated with EI, but whether the involvement of WM structures in the right hemisphere with EI is stronger than those in the left hemisphere remains to be investigated with more statistical power.
As was discussed in our previous studies [Takeuchi et al., 2010a], increased FA, which is presumably secondary to increased myelination [Beaulieu, 2002] or increased axonal caliber [Mori and Zhang, 2006], may be associated with greater effectiveness in neural circuit communication. This in turn may lead to enhanced EI caused by an increased conduction velocity of an action potential through (a) axon myelination [Bloom et al., 1988], and (b) thicker neuron fibers [Arbuthnott et al., 1980; Waxman, 1980]. Faster action potential conduction velocity can facilitate and increase information flow as well as allow precise temporal coding of high‐frequency bursts of neuronal activity [McDonald and Sears, 1970; Shrager, 1993; Swadlow, 1985]. Furthermore, integrity in the precise timing of sequential events in neuronal circuits can lead to a more effective cognitive performance [Peters, 2002].
No regions of the brain exhibited a significant correlation with WM integrity and the situation management factor of EI, despite the correlation between this factor and rGMD [Takeuchi et al., in 2011c]. The lack of a significant correlation could be because of a number of reasons, including a possibility that the situation management factor is related to gray matter structures but not to WM structures. A lack of statistical power, high statistical deviations, and other factors may also be responsible. Although a whole brain analysis in neuroimaging studies is apparently not suitable for proving negative findings, future studies using different samples or different methods may be needed to investigate whether the situation management factor of EI is truly not related to WM integrity.
The present findings, together with our previous studies and other published reports, conforms with the notion that EI is psychometrically distinct from general cognitive intelligence [e.g., Mayer et al., 1999; Mayer et al., 2001; Takeuchi et al., in 2011c]. Previous neuroimaging studies have also revealed the distinct neural basis of EI and general cognitive intelligence. For example, as described in the introduction, EI is associated with brain activity and gray matter structure in the regions of the SMC and the SCN; however, general intelligence, or the closely associated working memory system, is associated with neural activities and gray matter structures of regions such as the lateral prefrontal cortex and the anterior cingulate cortex [Frangou et al., 2004; Gong et al., 2005; Haier et al., 2004; Jung and Haier, 2007; Narr et al., 2007; Takeuchi et al., 2010b; Toga and Thompson, 2005; Wilke et al., 2003]. On the other hand, our present results showed WM integrity in regions related to the SMC and the SCN. Furthermore, results from previous studies showed that WM integrity in regions related to the lateral frontal and parietal cortices are associated with working memory performance [Olesen et al., 2003] and WM integrity in regions related to the lateral frontal and occipitoparietal areas is related to general intelligence [Schmithorst et al., 2005]. These regions did not show significant correlations between FA and EI in our study, even with the threshold used in our ROIs.
At least one limitation exists in this study, which was also a limitation observed with our previous studies and in other studies that use college cohorts [Jung et al., 2010b; Song et al., 2008; Takeuchi et al., 2011a; Takeuchi et al., 2010c; Takeuchi et al., 2010d]. We used young healthy subjects with high educational backgrounds. Limited sampling of the full range of intellectual abilities is a common hazard when sampling from college cohorts [Jung et al., 2010b]. In our study, the average scores of the three factors of EI were slightly higher than those of average adults. Whether our findings would also hold across the full range of population samples and a normal distribution must be determined with larger and more representative samples.
CONCLUSIONS
This is the first study to investigate the association between WM integrity and EI. Our results support previous neuroscientific studies, indicating that the SMC and the SCN are associated with EI. Our study showed that (a) intrapersonal EI was associated with WM integrity in the right anterior insula, which is one of the important nodes of the SMC, and that (b) interpersonal EI was associated with WM integrity in a part of the ILF, which connects the visual cortex and areas related to SCN, and hence, a part of the SCN, such as the amygdala, the fusiform gyrus, and the parahippocampal gyrus.
Acknowledgements
The authors respectfully thank Yuki Yamada for operating the MRI scanner and Sarah Michael for reviewing the English in this manuscript. The authors also thank study participants, the examiners of psychological tests, and all of our colleagues in Institute of Development, Aging, and Cancer and in Tohoku University for their support.
This study was performed at the Institute of Development, Aging and Cancer, Tohoku University.
REFERENCES
- Albrecht J, Dellani PR, Muller MJ, Schermuly I, Beck M, Stoeter P, Gerhard A, Fellgiebel A ( 2007): Voxel based analyses of diffusion tensor imaging in Fabry disease. Br Med J 78: 964–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG ( 2002): The primate amygdala and the neurobiology of social behavior: Implications for understanding social anxiety. Biol Psychiatry 51: 11–17. [DOI] [PubMed] [Google Scholar]
- Arbuthnott ER, Boyd IA, Kalu KU ( 1980): Ultrastructural dimensions of myelinated peripheral nerve fibres in the cat and their relation to conduction velocity. J Physiol 308: 125–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar‐On R ( 1997): Bar‐On Emotional Quotient Inventory: Technical Manual. Toronto: Multi‐Health Systems. [Google Scholar]
- Bar‐On R, Tranel D, Denburg NL, Bechara A ( 2003): Exploring the neurological substrate of emotional and social intelligence. Brain 126: 1790–1800. [DOI] [PubMed] [Google Scholar]
- Barnea‐Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL ( 2004): White matter structure in autism: Preliminary evidence from diffusion tensor imaging. Biol Psychiatry 55: 323–326. [DOI] [PubMed] [Google Scholar]
- Bauer RM ( 1982): Visual hypoemotionality as a symptom of visual‐limbic disconnection in man. Arch Neurol 39: 702–708. [DOI] [PubMed] [Google Scholar]
- Beaulieu C ( 2002): The basis of anisotropic water diffusion in the nervous system‐a technical review. NMR Biomed 15: 435–455. [DOI] [PubMed] [Google Scholar]
- Benson DF, Segarra J, Albert ML ( 1974): Visual agnosia‐prosopagnosia: A clinicopathologic correlation. Arch Neurol 30: 307–310. [DOI] [PubMed] [Google Scholar]
- Bloom FE, Lazerson A, Hofstadter L ( 1988): Brain, mind, and Behavior. San Francisco: Freeman. [Google Scholar]
- Boucher J, Lewis V ( 1992): Unfamiliar face recognition in relatively able autistic children. J Child Psychol Psychiatry 33: 843–859. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R ( 2003): Occipito‐temporal connections in the human brain. Brain 126: 2093–2107. [DOI] [PubMed] [Google Scholar]
- Cheung C, Chua SE, Cheung V, Khong PL, Tai KS, Wong TKW, Ho TP, McAlonan GM ( 2009): White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. J Child Psychol Psychiatry 50: 1102–1112. [DOI] [PubMed] [Google Scholar]
- Chua TC, Wen W, Slavin MJ, Sachdev PS ( 2008): Diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease: A review. Curr Opin Neurol 21: 83–92. [DOI] [PubMed] [Google Scholar]
- Damasio AR ( 1998): Emotion in the perspective of an integrated nervous system. Brain Res Rev 26: 83–86. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio HC ( 1991): Somatic markers and the guidance of behavior: Theory and preliminary testing In. Levin, Harvey S., Eisenberg HM, Bentaon AL. In: Levin HS, Eisenberg HM, Bentaon AL, editors. Frontal Lobe Function and Dysfunction. New York: Oxford University Press; pp 217–229. [Google Scholar]
- Dunckley P, Wise RG, Aziz Q, Painter D, Brooks J, Tracey I, Chang L ( 2005): Cortical processing of visceral and somatic stimulation: Differentiating pain intensity from unpleasantness. Neuroscience 133: 533–542. [DOI] [PubMed] [Google Scholar]
- Frangou S, Chitins X, Williams SCR ( 2004): Mapping IQ and gray matter density in healthy young people. Neuroimage 23: 800–805. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD ( 1996): Detecting activations in PET and fMRI: Levels of inference and power. Neuroimage 4: 223–235. [DOI] [PubMed] [Google Scholar]
- Frith U ( 2001): Mind blindness and the brain in autism. Neuron 32: 969–979. [DOI] [PubMed] [Google Scholar]
- Fukunishi I, Wise TN, Sheridan M, Shimai S, Otake K, Utsuki N, Uchiyama K ( 2001a): Association of emotional intelligence with alexithymic characteristics. Psychological reports 89: 651–658. [DOI] [PubMed] [Google Scholar]
- Fukunishi I, Wise TN, Sheridan M, Shimai S, Otake K, Utsuki N, Uchiyama K ( 2001b): Validity and reliability of the Japanese version of the Emotional Intelligence Scale among college students and psychiatric outpatients. Psychol Rep 89: 625–632. [DOI] [PubMed] [Google Scholar]
- Gong QY, Sluming V, Mayes A, Keller S, Barrick T, Cezayirli E, Roberts N ( 2005): Voxel‐based morphometry and stereology provide convergent evidence of the importance of medial prefrontal cortex for fluid intelligence in healthy adults. Neuroimage 25: 1175–1186. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT ( 2004): Structural brain variation and general intelligence. Neuroimage 23: 425–433. [DOI] [PubMed] [Google Scholar]
- Jung RE, Grazioplene R, Caprihan A, Chavez RS, Haier RJ ( 2010a): White matter integrity, creativity, and psychopathology: Disentangling constructs with diffusion tensor imaging. PLoS ONE 5: e9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Haier RJ ( 2007): The parieto‐frontal integration theory (P‐FIT) of intelligence: Converging neuroimaging evidence. Behav Brain Sci 30: 135–154. [DOI] [PubMed] [Google Scholar]
- Jung RE, Segall JM, Bockholt HJ, Flores RA, Smith SM, Chavez RS, Haier RJ ( 2010b): Neuroanatomy of creativity. Hum Brain Mapp 31: 398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G ( 2006): The fusiform face area: A cortical region specialized for the perception of faces. Phil Trans R Soc B: Biol Sci 361: 2109–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun‐Todd DA ( 2007): Neural correlates of emotional intelligence in adolescent children. Cogn Affect Behav Neurosci 7: 140–151. [DOI] [PubMed] [Google Scholar]
- Krueger F, Barbey AK, McCabe K, Strenziok M, Zamboni G, Solomon J, Raymont V, Grafman J ( 2009): The neural bases of key competencies of emotional intelligence. Proc Natl Acad Sci 106: 22486–22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D ( 2003): Looking into the functional architecture of the brain with diffusion MRI. Nature reviews. Neuroscience 4: 469–480. [DOI] [PubMed] [Google Scholar]
- LeDoux JE ( 2000): Emotional circuits in the brain. Annu Rev Neurosci 23: 155–184. [DOI] [PubMed] [Google Scholar]
- Martin I, McDonald S ( 2004): An exploration of causes of non‐literal language problems in individuals with Asperger syndrome. J Autism Dev Disord 34: 311–328. [DOI] [PubMed] [Google Scholar]
- Mayer JD, Caruso DR, Salovey P ( 1999): Emotional intelligence meets traditional standards for an intelligence. Intelligence 27: 267–298. [Google Scholar]
- Mayer JD, Salovey P, Caruso DR, Sitarenios G ( 2001): Emotional intelligence as a standard intelligence. Emotion 1: 232–242. [PubMed] [Google Scholar]
- McDonald WI, Sears TA ( 1970): The effects of experimental demyelination on conduction in the central nervous system. Brain 93: 583–598. [DOI] [PubMed] [Google Scholar]
- Mori S, Zhang J ( 2006): Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51: 527–539. [DOI] [PubMed] [Google Scholar]
- Nagai M, Kishi K, Kato S ( 2007): Insular cortex and neuropsychiatric disorders: A review of recent literature. Eur Psychiatry 22: 387–394. [DOI] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, Gurbani M, Toga AW, Bilder RM ( 2007): Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex 17: 2163–2171. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T ( 2003): Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto‐parietal network. Cognitive Brain Res 18: 48–57. [DOI] [PubMed] [Google Scholar]
- Otake K, Shimai S, Uchiyama K, Utsuki N ( 2001): Development of Japanese Emotional Intelligence Scale (EQS) and its validity and reliability. Job Stress Res 8. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Carter EJ ( 2008): Brain mechanisms for social perception. Anns N Y Acad Sci 1145: 283–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A ( 2002): The effects of normal aging on myelin and nerve fibers: A review. J Neurocytol 31: 581–593. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV ( 2003): Increased brain white matter diffusivity in normal adult aging: Relationship to anisotropy and partial voluming. Magn Reson Med 49: 953–961. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, Le Bihan D, Dubois B ( 2002): The neural system that bridges reward and cognition in humans: An fMRI study. Proc Natl Acad Sci USA 99: 5669–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Salazar A, Gorno‐Tempini ML, Sollberger M, Wilson SM, Pavlic D, Stanley CM, Glenn S, Weiner MW, Miller BL ( 2009): Detecting sarcasm from paralinguistic cues: Anatomic and cognitive correlates in neurodegenerative disease. NeuroImage 47: 2005–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J ( 1998): Manual for Raven's Progressive Matrices and Vocabulary Scales. Oxford: Oxford Psychologists Press. [Google Scholar]
- Reiman EM ( 1997): The application of positron emission tomography to the study of normal and pathologic emotions. J Clin Psychiatry 58: 4–12. [PubMed] [Google Scholar]
- Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, Yun LS, Chen K ( 1997): Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry 154: 918–925. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJW, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD ( 2005): Age‐related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging 26: 1215–1227. [DOI] [PubMed] [Google Scholar]
- Salovey P, Mayer JD ( 1990): Emotional intelligence. Imagin Cogn Pers 9: 185–211. [Google Scholar]
- Salovey P, Rothman AJ, Detweiler JB, Steward WT ( 2000): Emotional states and physical health. Am Psychol 55: 110–121. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK ( 2005): Cognitive functions correlate with white matter architecture in a normal pediatric population: A diffusion tensor MRI study. Hum Brain Mapp 26: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM, Thorsteinsson EB, Bhullar N, Rooke SE ( 2007): A meta‐analytic investigation of the relationship between emotional intelligence and health. Pers Indiv Differ 42: 921–933. [Google Scholar]
- Shrager P ( 1993): Axonal coding of action potentials in demyelinated nerve fibers. Brain Res 619: 278–290. [DOI] [PubMed] [Google Scholar]
- Song M, Zhou Y, Li J, Liu Y, Tian L, Yu C, Jiang T ( 2008): Brain spontaneous functional connectivity and intelligence. Neuroimage 41: 1168–1176. [DOI] [PubMed] [Google Scholar]
- Stone VE, Cosmides L, Tooby J, Kroll N, Knight RT ( 2002): Selective impairment of reasoning about social exchange in a patient with bilateral limbic system damage. Proc Natl Acad Sci 99: 11531–11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA ( 1985): Physiological properties of individual cerebral axons studied in vivo for as long as one year. J Neurophysiol 54: 1346–1362. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, Yamanouchi T, Suzuki S, Kawashima R ( 2010a): Training of working memory impacts structural connectivity. J Neurosci 30: 3297–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R ( 2011a): Failing to deactivate: The association between brain activity during a working memory task and creativity. Neuroimage 55: 681–687. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R ( 2011b): Effects of training of processing speed on neural systems. Journal of Neuroscience 31: 12139–12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R ( 2011c): Regional gray matter density associated with emotional intelligence: evidence from voxel‐based morphometry. Hum Brain Mapp 32: 1497–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R ( 2011d): Working memory training using mental calculation impacts regional gray matter of the frontal and parietal regions. PLoS ONE 6: e23175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Kawashima R ( 2010b): Effects of working memory training on cognitive functions and neural systems. Rev Neurosci 21: 427–449. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R ( 2010c): Regional gray matter volume of dopaminergic system associate with creativity: Evidence from voxel‐based morphometry Neuroimage 51: 578–585. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R ( 2010d): White matter structures associated with creativity: Evidence from diffusion tensor imaging. Neuroimage 51: 11–18. [DOI] [PubMed] [Google Scholar]
- Thomas C, Avidan G, Humphreys K, Jung K, Gao F, Behrmann M ( 2008): Reduced structural connectivity in ventral visual cortex in congenital prosopagnosia. Nat Neurosci 12: 29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM ( 2005): Genetics of brain structure and intelligence. Annual review of neuroscience 28: 1–23. [DOI] [PubMed] [Google Scholar]
- Uchiyama K, Shimai T, Utsuki N, Otake K ( 2001): EQS manual. Tokyo: Jitsumukyoiku Syuppan (Practical Education Press). [Google Scholar]
- Van Rooy DL, Viswesvaran C ( 2004): Emotional intelligence: A meta‐analytic investigation of predictive validity and nomological net. J Vocat Behav 65: 71–95. [Google Scholar]
- Waxman SG ( 1980): Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve 3: 141–150. [DOI] [PubMed] [Google Scholar]
- Wilke M, Sohn JH, Byars AW, Holland SK ( 2003): Bright spots: Correlations of gray matter volume with IQ in a normal pediatric population. Neuroimage 20: 202–215. [DOI] [PubMed] [Google Scholar]


