Abstract
Practice of tasks in an interleaved order generally induces superior learning compared with practicing in a repetitive order, a phenomenon known as the contextual‐interference (CI) effect. Increased neural activity during interleaved over repetitive practice has been associated with the beneficial effects of CI. Here, we used psychophysiological interaction (PPI) analysis to investigate whether the neural connectivity of the dorsal premotor (PM) and the dorsolateral prefrontal (DLPFC) cortices changes when motor sequences are acquired through interleaved practice. Sixteen adults practiced a serial reaction time task where a set of three 4‐element sequences were arranged in a repetitive or in an interleaved order on 2 successive days. On Day 5, participants were tested with practiced sequences to evaluate retention. A within‐subjects design was used so that participants practiced sequences in the other condition (repetitive or interleaved) 2–4 weeks later. Functional magnetic resonance images were acquired during practice and retention. On Day 2 of practice, there was greater inter‐regional functional connectivity in the interleaved compared with the repetitive condition for both PM‐seeded and DLPFC‐seeded connectivity. The increased functional connectivity between both seeded regions and sensorimotor cortical areas correlated with the benefit of interleaved practice during later retention. During retention, a significant PPI effect was found in DLPFC‐seeded connectivity, with increased DLPFC‐supplementary motor area connectivity correlated with the benefits of interleaved practice. These data suggest that interleaved practice benefits learning by enhancing coordination of sensorimotor cortical regions, and superior performance of sequences learned under CI is characterized by increased functional connectivity in frontal cortex. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: contextual interference, serial reaction time task, psychophysiological interaction, functional connectivity, fMRI, practice schedule, TMS
INTRODUCTION
Existing literature has shown that introducing manipulations that make performance more difficult during practice may nonetheless improve long‐term retention [Schmidt and Bjork, 1992]. Bjork proposed the notion of “desirable difficulties” that challenges for learners such as context shifts and retrieval during study result in better learning and should be introduced into skill practice [Christina and Bjork, 1991]. An example of desirable difficulty is the contextual interference (CI) effect, where practice context is manipulated by presenting multiple tasks in either a repetitive (blocked) order or an interleaved (random) order [Shea and Morgan, 1979]. Practicing tasks in an interleaved order generally results in inferior practice performance but induces superior retention compared with practicing in a repetitive order [Brady, 2008]. This differential effect of practice condition during practice and retention phases is an example of the distinction between performance and learning. Although CI is detrimental to performance during the practice phase, it benefits the learning of skills.
The CI effect was first demonstrated in the verbal learning literature [Battig and Berry 1966] and was subsequently studied in motor learning [Shea and Morgan, 1979]. In a classic study of the CI effect by Shea and Morgan, participants learned three arm‐movement tasks presented in a repetitive (less difficult) or an interleaved (more difficult) order. Participants were retested after a 10‐min or 10‐day delay with both repetitive and interleaved presentation of the practiced tasks. Subsequent transfer to a task of either the same or greater complexity than the originally learned tasks was also investigated. During the practice phase, there was an advantage for the participants who practiced under the repetitive condition, especially in the early phases of practice, but continuing until the end of practice. After the 10‐min retention interval, performance was better for those participants who had trained in the interleaved condition even when they were tested in a repetitive condition. After the 10‐day retention interval, though participants were always faster when tested with repetitive condition compared with interleaved condition, those subjects who had been trained in the interleaved condition performed better in both testing conditions than those who were trained using a repetitive order. Shea and Morgan's results support Bjork's conceptualization of desirable difficulties in cognitive learning that during practice, a more‐difficult condition (interleaved practice) led to worse performance compared with a less‐difficult condition (repetitive practice). In contrast, retention and transfer were superior after interleaved practice than after repetitive practice. The poorer performance during interleaved practice implies that there is greater effort disbursed in a more‐difficult condition, and this increased effort during practice results in better long‐term retention [Schmidt and Bjork, 1992; Shea and Zimny, 1983].
Despite the robust benefits of CI on learning, little is currently known about the neural basis of how CI leads to better retention. Previous work used functional imaging to demonstrate increased frontoparietal hemodynamic responses when motor sequences were practiced in an interleaved compared with a repetitive condition [Cross et al., 2007; Lin et al., 2011]. It is likely, however, that CI does not merely increase activity during learning it rather enhances the interaction between regions during practice. These previous studies did not examine how inter‐regional brain connectivity evolves as a function of learning under different practice conditions. In this study, we investigate whether practice of motor sequences in an interleaved compared with a repetitive condition would result in different learning‐related changes in inter‐regional functional connectivity of the cerebral motor learning network using psychophysiological interaction (PPI) analysis of functional magnetic resonance imaging (fMRI) data.
In the last decade, a great effort has been devoted to investigate inter‐regional connectivity in different tasks in order to provide a fuller picture of underlying brain mechanisms [Liao et al., 2010; Mesquita et al., 2010; Mulder et al., 2010). Inter‐regional connectivity was often determined by performing temporal correlation between spatially remote neurophysiological events (e.g., correlating blood‐oxygen‐level‐dependent (BOLD) signals between the prefrontal and cingulate gyrus on a Stroop test). The correlation approach, however, is limited in drawing causal inference about the direction of influences that the elements of one neural system exert on another. Friston et al., therefore, developed PPI analysis which is a bilinear model of how a psychological context changes the influence of one brain region on another, and corresponds to differences in regression slopes for different psychological contexts [Friston et al., 1997]. The PPI method has been extensively used in neuroscientific studies, and is commonly accepted as a powerful way to characterize neural interactions among brain regions during behavior paradigms [Hattori et al., 2009; Liao et al., 2010; Mulder et al., 2010; Neufang et al., 2008; Wu et al., 2008].
The purpose of this study was to identify condition‐dependent changes in inter‐regional functional connectivity during motor sequence learning using PPI analyses. We chose the dorsal premotor cortex (PM) and the dorsal lateral prefrontal cortex (DLPFC) as a priori seed regions to start with the PPI analysis. The PM and DLPFC were chosen based on previous work demonstrating increased activity in these regions during interleaved compared with repetitive practice of motor skills [Cross et al., 2007; Lin et al., 2011]. Both of these seed regions are key components of the motor learning network. PM has been shown to be involved in planning and organizing sequential movements [Abe et al., 2007; Bortoletto and Cunnington, 2010; Schwarb and Schumacher, 2009], and has been linked to both parietal modules for sensorimotor transformation during action preparation [Gail et al., 2009; Kroliczak and Frey, 2009], cerebellar‐motor pathways for action coordination [Debaere et al., 2004; Matsumura et al., 2004], and the primary motor cortex (M1) for the process of movement selection and execution [Ortu et al., 2009; Rickert et al., 2009]. DLPFC is part of prefrontal‐parietal and prefrontal‐striatal networks, and together with the supplementary motor area (SMA), these networks are essential for executive function, task switching, and sequencing [Honda et al., 1998; Mansouri et al., 2009; Neufang et al., 2008; Schwarb and Schumacher, 2009; Yoshida et al., 2010]. It is likely that all of these processes are engaged during interleaved practice. As such, we hypothesized that interleaved practice may be associated with enhanced connectivity between the PM and regions within parietal cortex, cerebellum, and M1 while DLPFC connectivity with the SMA, parietal cortex, and striatum would be enhanced as well.
The CI benefit to skill learning has been explained in terms of greater information processing during the interleaved practice condition, including repeated task‐switching and retrieval of action plans [Lee and Magill, 1983; Shea and Zimny, 1983]. Our choice of DLPFC and PM as seeds was theoretically motivated based on the role of these regions in these cognitive functions. To accomplish multiple tasks in a nonrepetitive order, learners must switch their attention and task sets (engaging the DLPFC, medial frontal network [Rossi et al., 2009]), and to program an action plan for a different upcoming trial (engaging the premotor network [Gail et al., 2009]). It is conceivable that greater changes in DLPFC and PM networks during interleaved compared with repetitive practice may support such elaborative information processing and retrieval practice, leading to enhanced learning.
We aimed to address three research questions. First, would functional connectivity of the cortical motor learning network be altered when motor sequences were acquired under different practice conditions? Second, would any increased functional connectivity of sensorimotor areas during practice be associated with greater behavior gain during the retention phase? Third, during the retention phase, would successful retrieval be associated with increased inter‐regional connectivity between specific regions? We hypothesized that, compared with a repetitive practice condition, interleaved practice of sequences would lead to greater functional connectivity in a cerebral network involved in motor planning and executive function. We also hypothesized that the increased connectivity during practice will be functionally relevant in that it will be associated with better retention of the motor sequences. To our knowledge, this study is the first to apply PPI analysis to model inter‐regional connectivity differences as a function of practice schedule. Our results suggest that enhanced functional connectivity during and after interleaved practice contribute to the well‐known benefits of CI to skill learning.
METHODS
Participants
Sixteen right‐handed young adults were enrolled in the study (9 men and 7 women, ages 19–29). Participants were recruited from the University and adjacent community. All participants gave informed consent using an institutionally approved consent form. Participants were excluded if they had any contraindications to MRI, including significant medical, neurological, or psychiatric history, metal implants, uncorrected vision loss, or scored less than 28 on the Mini‐Mental State Exam (MMSE; [Folstein, et al. 1975]).
Study Design
We applied a within‐subject cross‐over design. The participants practiced the serial reaction time task (Fig. 1A) on two consecutive training days (Days 1 and 2, Fig. 1B). To measure the effects of practice on learning, we tested delayed retention performance on Day 5 (Fig. 1B) [Cahill et al., 2001; Perez et al., 2005; Shea and Morgan, 1979; Wright et al., 2005]. Behavioral and fMRI data were acquired concurrently on each testing day within an MRI scanner (Fig. 1B).
Figure 1.
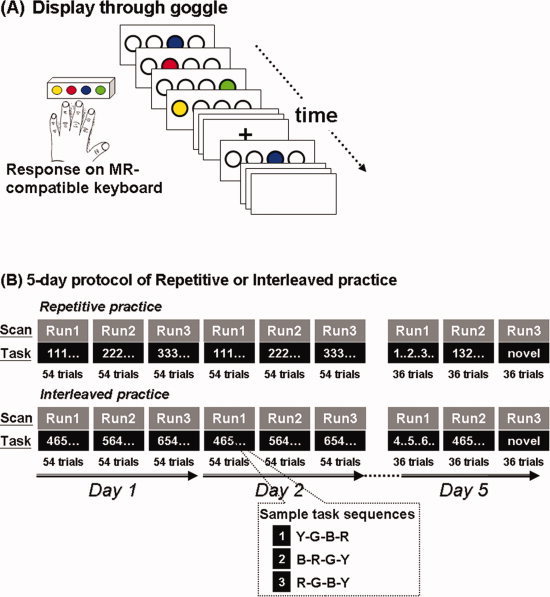
Participants practiced serial reaction time sequence learning tasks (A) in both a repetitive and an interleaved order (B). Participants practiced tasks for two consecutive days with the retention tests of trained and untrained sequences taking place on Day 5. Imaging data were acquired concurrently while the participants practiced the tasks. During repetitive practice, participants performed the same sequence repeatedly for the same scan run. 2 weeks after practice and retention for one training order, participants returned to practice a different set of three sequences in the other condition (e.g., repetitive practice → interleaved practice).
Each participant practiced a variation of the serial response task (SRT) for 2 consecutive days and then was tested on the 5th day. The SRT task consisted of three different four‐element sequences, presented in either a repetitive or interleaved order (Fig. 1B, also see “behavioral task” below for details). In this within‐subject cross‐over design, each participant started in the first week with either the repetitive practice (RP) or the interleaved practice (IP) condition; 2 weeks later, each participant performed in the other practice condition (i.e., repetitive → interleaved, or vice versa). The order of the practice conditions and the SRT sequences was counter‐balanced across participants.
Functional images were acquired concurrently, while the SRT was performed inside the MR scanner. For Days 1 and 2, there were three functional imaging runs on each day. Each run consisted of 54 movement trials, where the participants practiced one test sequence in each trial (Fig. 1B). For repetitive practice (RP), each of the three test sequences was practiced for 54 consecutive trials (i.e., one fMRI run) before the next sequence appeared, leading to 162 trials (54 trials × 3 test sequences) for each day (Fig. 1B top). The order of the three sequences was counter‐balanced across the participants. For interleaved practice (IP), the three tested sequences were arranged in a nonrepetitive manner within each 54‐trial fMRI run (Fig. 1B bottom), and the same arrangement of the test sequences was applied to all the participants. In other words, when the participants performed IP, they practiced the same order of test sequences.
On the retention day (Day 5), the participants underwent 3 fMRI runs, with 36 trials per run (Fig. 1B, Day 5). In the first two runs they were tested with the three sequences they had practiced in the previous 2 days. In one run, the practiced sequences were presented in a repetitive order (denoted by repetitive testing condition, or RC), and in the other run they were presented in an interleaved order (denoted by interleaved testing condition, or IC). This was to ensure practice‐test compatibility was equal for either practice condition [Lee, 1988; Lee and Magill, 1983; Shea and Morgan, 1979]. The order of these runs was counterbalanced across subjects. Each sequence was presented for 24 trials across these two runs on Day 5 (in contrast, during practice on Days 1 and 2, each sequence was presented for 54 trials on each day). The reduced number of trials was used in order to limit further learning processing for the sequences [Cross et al., 2007]. To assess whether learning was specific to the training sequences, on the third fMRI run the participants were tested with three novel, or unpracticed, test sequences (Fig. 1B).
Behavioral Task
The SRT task during fMRI scanning was executed as follows (Fig. 1A). Participants positioned the four fingers of the left hand (all except the thumb) on the four light‐sensitive response keys of a magnet‐compatible button box (Current Designs). The left hand was used to increase the overall difficulty of the task (analogous to the approach in Cross et al., 2007). Subjects watched colored circles (yellow, red, blue, and green) through magnet‐compatible goggles. Only one colored circle appeared at a time while the other circles were transparent (Fig. 1A). Each color was always displayed at the same circle location, thus providing a consistent spatial and color cue for the motor response on the spatially corresponding response key. Participants were instructed to “respond as quickly as possible.” Within each sequence, one circle would be colored at a time with the next colored circle appearing as soon as the previous response was made.
Participants pressed four consecutive keys (four elements, equivalent to one sequence) to complete one task trial. To ensure that each subject practiced an equal number of trials, each sequence (four key presses) was presented for a fixed duration of 3 s. If the participant completed the four key presses before 3 s, 4 transparent circles would appear on the screen, thus controlling visual stimulation (Fig. 1A). Since we applied a blocked design for functional imaging acquisition with 18‐second task blocks interleaved with 18‐second rest blocks, behavioral trials were presented as six sequences per task block. During the rest block, the circles would be replaced by a fixation cross in the center of the screen. Participants were instructed to remain relaxed but gaze at the fixation cross. A custom‐designed computer software program written with presentation (Neurobehavioral Systems) controlled the appearance of the colored circles and recorded the participants' responses. Response times (RT) were recorded for each key press.
Functional Magnetic Resonance Imaging
Images were acquired using a Siemens Trio 3.0 T MRI scanner. Two sets of high‐resolution anatomical images were acquired for image registration. We acquired an MP‐RAGE structural volume (TR = 1,900, TE = 2.26, flip angle = 8°) with 176 sagittal slices, each 1 mm thick with 0.5 mm gap and 1.33 × 1.33 mm2 in‐plane resolution. We also acquired a T2‐weighted coplanar volume (TR = 5,000, TE = 34, flip angle = 90°) with 34 transverse slices covering the whole brain, each 4 mm thick with 1 mm gap, a 128 × 128 matrix and an in‐plane resolution of 1.5 × 1.5 mm2.
Functional images were acquired while the participants performed the sequence learning task. There were three functional runs which corresponded to the three practice sessions on each day (Fig. 1B). On Days 1 and 2, each functional run lasted for 5 min and 48 s, and consisted of 153 EPI volumes (gradient‐echo, TR = 2,000, TE = 30, flip angle = 90°), each with 34 transverse slices, 4 mm thick, 1 mm gap, and a 64 × 64 matrix yielding an in‐plane resolution of 3 × 3 mm2. The first four volumes of each functional run were removed from analysis to allow for magnetization to approach equilibrium. On Day 5, the participants underwent three functional runs (Fig. 1B, two runs for sequence specific tests and one run for the novel sequence test). Each run lasted for 3 minutes and 48 s, and involved the acquisition of 99 EPI volumes.
Data Processing and Statistical Analysis
Behavioral data
The behavioral data have been reported in our previous study [Lin et al., 2011]. Response time (RT) was defined as the time between stimulus onset to key press. The total RT for each 4‐element sequence trial was calculated by adding up the RT of each of the four key presses. For both practice and retention, we calculated the median total RT for every six sequence trials for use in all analyses (Supporting Information Fig. 1).
To compare the effects of training conditions (repetitive versus interleaved) during practice, RT across 2 days of practice was averaged before determining the main condition effect. Participants' performance during the retention test on Day 5 was also evaluated. The condition effect on day 5 was evaluated by comparing the averaged RT of the practiced sequences. Averaged RT was computed separately for the repetitive testing condition (RC) and the interleaved testing condition (IC) on day 5. We also calculated the averaged RT of the novel sequences to investigate whether the superior performance on retention for interleaved practice may simply reflect general improvement in key‐pressing speed. Full model analysis, including the two practice conditions, was conducted for practice and retention phases separately. Paired sample t‐tests were applied to contrast averaged RT between the repetitive and interleaved practice conditions on the baseline performance, the end of practice, and performance on the trained and novel sequences on Day 5.
Imaging preprocessing and first level analyses
Functional images were processed using the Statistical Parametric Mapping software (SPM8, Wellcome Department of Cognitive Neurology, London, UK). To correct for motion artifacts, functional image data were realigned to the first volume in each functional run and then resliced with 4th‐degree B‐Spline interpolation [Friston et al., 1995]. None of the subjects had scans with head motions greater than 2 mm. After realignment, the resulting mean images of each subject were normalized to the standard Montreal Neurological Institute (MNI) EPI template [Evans et al., 1993]. The normalization parameters were then applied to all the functional images of that subject. The normalized images were then resampled to 3 × 3 × 3 mm3 per voxel, and subsequently spatially smoothed with an isotropic Gaussian filter with full width at half maximum (FWHM) = 8 mm. Statistical analysis was first carried out separately for each participant using the general linear model (GLM) [Friston et al., 1995]. The fMRI data were modeled using a boxcar function that included an explicit baseline model convolved with the hemodynamic response function (HRF). An additional parametric regressor with the mean response time for each task block was applied to ensure that any differences in brain activities during practice and retention was due to the influences of the practice conditions but not to differences in the response time.
Second level and BOLD contrast analyses
The fMRI data were first examined to determine brain regions that are involved in sequence learning. Group comparisons under the two practice conditions were performed using a second‐level random effects analysis on the contrast images (task versus rest) derived from the above model fitting [Handwerker et al., 2004; Worsley et al., 2002]. Since the nature of sequence practice in an interleaved condition involves planning and executive function [Brady, 2008], the dorsal premotor (PM) and dorsal lateral prefrontal cortices (DLPFC) were selected as seed regions for the connectivity analyses. The coordinates of the peak activation voxel of the right PM and DLPFC from the group analyses was used as the center of each seed region for the subsequent PPI connectivity analysis.
Psychophysiological interaction (PPI) analysis (Question 1)
To assess the hypothesis that the interleaved practice led to greater prefrontal‐connectivity and premotor‐connectivity with the sensorimotor network, we estimated the functional connectivity for seed regions DLPFC and PM in separate PPI analyses. For each participant, after statistical analysis that was first carried out separately using the general linear model (GLM) [Friston et al., 1995], the BOLD signal time course in the right DLPFC and right PM were separately entered into the PPI analysis [Friston et al., 1997; Gitelman et al., 2003; Murata et al., 2000]. The PPI analysis was modeled to explain how neural activity in one brain area interacts differently with another brain region when there are changes in a cognitive/perceptual process [Neufang et al., 2008]. In this study, we focus on PM and DLPFC connectivity with sensorimotor regions of the brain, and the experimental manipulation examined is the interleaved or repetitive practice conditions. In our PPI analysis, the design matrix includes three regressors: (i) the “psychological variable,” representing the cognitive/perceptual process of interest (here interleaved practice versus repetitive practice), (ii) the “physiological variable,” representing the BOLD activity timecourse in a given brain region (here we implemented two seed regions: the right DLPFC and the right PM), and (iii) the interaction term of (i) and (ii).
The psychological variable used was a vector specified for the task condition (1 for interleaved practice, −1 for repetitive practice) convolved with the HRF. For PPI, fMRI time‐series data deconvolved with the HRF for the right DLPFC or the right PM was extracted from each participant's normalized data, based on a sphere of radius 6 mm around the peak activation voxel detected by the second‐level group analysis. These time series were mean‐corrected and high‐pass filtered to remove low‐frequency drifts. The product of this activation time‐series data and the psychological vector of interest (interleaved practice minus repetitive practice) resulted in the PPI interaction term. New statistical parametric mappings with the physiological variable (PM or DLPFC activity), psychological variable, and their interaction as regressors were computed for each subject for each practice day (Day 1 and Day 2) and retention day (Day 5). These subject level PPI‐statistical parametric mappings were then entered into a random‐effects group analysis using a one‐sample t‐test within the rest of the brain where practice condition differentially altered the BOLD signal.
For both BOLD and PPI analyses, overall significance were thresholded and corrected for multiple comparisons using the topological false discovery rate (FDR) method [Chumbley et al., 2010] at P < 0.05 and a cluster size of greater than six contiguous voxels. This indicated that on average less than 5% of the significant voxels were false positive.
Correlations between inter‐regional connectivity and learning (Questions 2 and 3)
We next sought to determine if any differences in functional connectivity between the two practice conditions from our primary PPI analyses were correlated with relative differences in the learning benefits of interleaved vs. repetitive practice. All correlations were performed using a contrast between practice conditions (either repetitive‐minus‐interleaved, denoted by R‐I, or interleaved‐minus‐repetitive, denoted by I‐R, depending on hypothesized direction of effect). Specifically, as a measure of relative behavioral benefits of learning between practice conditions, mean response time (RT) during retention was computed on practice sequences on Day 5 with a positive R‐I contrast indicating faster RT (behavioral improvement) for interleaved practice. Similarly, for PPI contrasts during practice and retention, a positive I‐R contrast indicates brain regions with greater premotor and/or DLPFC connectivity for interleaved practice.
To index the relative PPI contrasts between practice conditions, we computed an interleaved‐repetitive contrast in regression slopes between time series of seed regions (PM or DLPFC) and candidate brain regions. The candidate brain regions are those which showed significant connectivity with the seed regions through each primary PPI analysis as well as being among those which were previously identified as being key regions to networks related to each seed (e.g., parietal cortex, cerebellum, and M1 for PM seeded networks; SMA, basal ganglia for DLPFC seeded networks). Since the group‐level time series of each candidate brain region is known to show a significant correlation with the time series of the corresponding seed region, for an individual subject, each PPI analysis can then be visualized as a pair of linear regression lines relating the time series of each other (the seed and the candidate brain region) with each line corresponding to one practice condition (e.g., Figs. 3, 4, 5). As such, we computed our index of PPI contrasts as a difference in regression slopes to represent relative differences in inter‐regional connectivity between practice conditions. For PPI contrasts during practice and retention, a positive I‐R contrast indicates brain regions with greater PM and/or DLPFC connectivity (i.e., greater regression slope) for interleaved practice.
Figure 3.
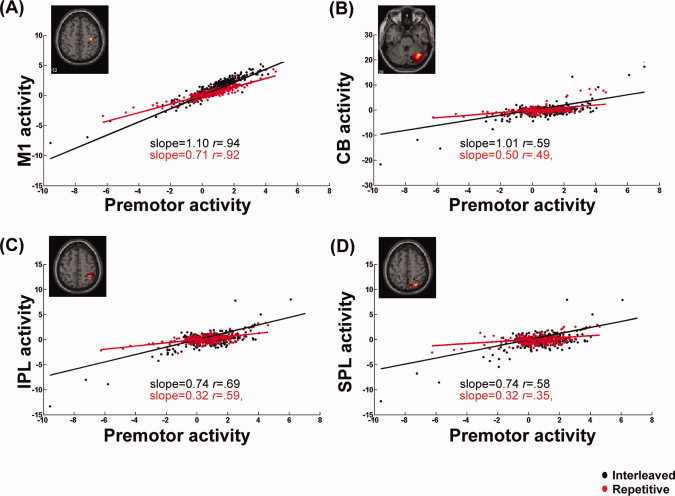
Interaction effect of practice conditions on functional connectivity between the right premotor area and other brain regions. BOLD activities, corrected for head movements, in the primary motor area (M1, A), cerebellum [CB, (B)], the inferior parietal [IPL, (C)], and superior parietal areas [SPL,(D)], were plotted against the right premotor activities, respectively under the interleaved (black dots) and repetitive practice conditions (red dots) on Day 2 for a typical participant. Regression slopes with respect to these four ROIs were all significantly greater under the interleaved than repetitive practice condition. These findings indicate that on the second day of practice, interleaved tasks evoked stronger premotor modulation on brain regions involved in sensorimotor integration and error corrections.
Figure 4.
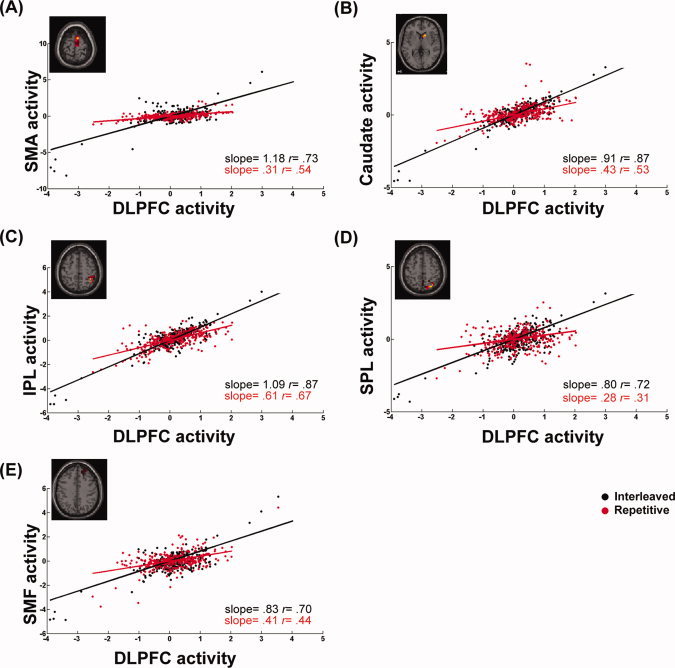
Differences in the connectivity of the right DLPFC circuit for a typical participant practicing in the interleaved (black dots) and repetitive (red dots) conditions on Day 2. The Regression slope of BOLD activities between the right DLPFC and the supplementary motor area [SMA, (A)], the caudate nucleus (B), the inferior parietal [IPL, (C)] and the superior parietal areas [SPL, (D)], and the superior medial frontal area [SMF, (E)], is higher in the interleaved practice, showing a stronger functional connectivity in the interleaved than the repetitive condition. These findings indicate that on Day 2, interleaved practice induced more DLPFC modulation on prefrontal, parietal, and subcortical regions, suggesting the stronger demand for attention, executive function, and task switching during interleaved practice.
Figure 5.
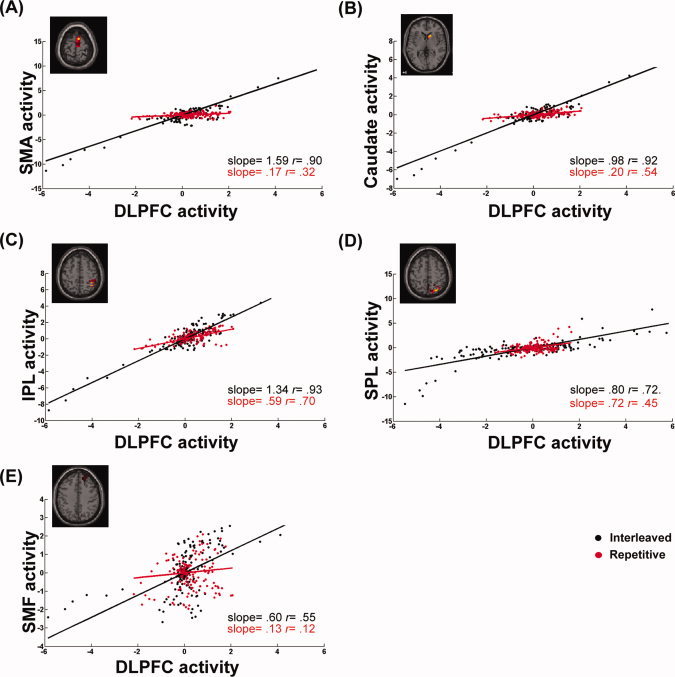
Differences in the connectivity of the right DLPFC circuit between the interleaved (black dots) and repetitive practices (red dots) on Day 5. The participant selected for demonstration and the experimental settings are the same as Fig. 4, except that the BOLD responses were measured on Day 5. Similar to Day 2 (as shown in Fig. 4), regression slopes with respect to retention testing were higher following the interleaved practice, measured between the BOLD responses for the right DLPFC and the supplementary motor area [SMA, (A)], the caudate nucleus (B), the inferior parietal area [IPL, (C)], and the superior medial frontal area [SMF, (E)]. However, the interaction effect of the interleaved practice almost disappeared for connectivity between the DLPFC and the superior parietal regression [SPL, (D)]. These findings indicate that successful retrieval of the practiced sequences, especially the performance following interleaved practice, requires more DLPFC modulation on prefrontal areas of the brain.
Since the most robust learning benefits of interleaved practice were identified in the interleaved testing condition, only relative RT of the interleaved testing condition was applied for subsequent PPI‐learning correlation analyses. Pearson's correlation tests were applied to identify how the relative inter‐regional functional connectivity was related to the learning benefits of interleaved practice. The correlation analyses, based on different between‐condition contrasts on response time and inter‐regional PPI, were performed to test specific hypotheses. First, was increased PM‐ and/or DLPFC‐connectivity during practice predictive of enhanced learning? Relative PM‐, and DLPFC‐regression slopes (slope differences, I‐R) during practice were correlated with the amount of benefit of interleaved practice on subsequent retention performance (RT, R‐I). Since significant maps were only identified for DLPFC‐seeded modulation on Day 5 retention, we also tested whether the increase in DLPFC‐modulation during the Day 5 retention test after interleaved practice was associated with enhanced retention performance. For all statistical tests of behavioral data, significance level was set at P < 0.05. SPSS 13.0 (SPSS, Chicago, IL) software was used for the statistical analyses.
RESULTS
Behavior Results
The participants' behavior, as described in our previous study [Lin et al., 2011], followed the expected pattern of the CI effect. Performance in both conditions improved after 2 days of practice, and the overall response time (RT) during the practice phase was faster in the repetitive than in the interleaved condition (mean RT, repetitive = 880.8 ± 69.4, interleaved = 1121.6 ± 64.7, P = 0.006). However, this pattern was reversed on the retention test on Day 5, where the RT of the trained sequences was faster for the sequences practiced in the interleaved than in the repetitive condition (mean RT, repetitive = 1114.9 ± 74.4, interleaved = 897.4 ± 37.7, P < .001), especially when the participants were tested using interleaved sequences [Supporting Information Fig. 1‐(1)]. We also investigated whether the benefit of the interleaved practice on retention was sequence‐specific, or simply reflected general improvement in key‐pressing speed, which we denoted by nonspecific learning. Supporting Information Fig. 1‐(2)A compares sequence‐specific learning between the two training conditions. Interleaved practice significantly facilitated sequence‐specific learning compared with the repetitive practice (P = .003, Supporting Information Fig. 1‐(2)A). By contrast, the difference in nonspecific learning between the interleaved and repetitive practice was not significant (P = .741, Supporting Information Fig. 1‐(2)B).
These results replicate the findings of many other studies in the CI literature using different paradigms [Lee and Magill, 1983; Lee et al., 1992; Lin et al., 2008; Maslovat et al., 2004; Perez et al., 2005; Wright et al., 2005].
Functional MRI Results
Practice of SRT evoked increased BOLD activity in sensorimotor regions of the brain during interleaved practice compared with repetitive practice, a pattern that we have demonstrated previously [Lin et al., 2011]. To detect the difference in inter‐regional functional connectivity between repetitive and interleaved practice in sequence learning, functional BOLD time series data at every voxel of the brain were regressed against that of the right dorsal premotor cortex (PM) or the right dorsal lateral prefrontal cortex (DPLFC), with the interleaved or repetitive condition as the interaction term, and between‐participant variability as the random‐effects variable (PPI analysis [Friston et al., 1997]). The PPI analyses were performed separately for each practice day (Day 1 and Day 2) and retention (Day 5).
Seed I: Right Dorsal Premotor Cortex
We identified common cerebral substrates across participants where task‐related PM connectivity was different between the two practice conditions. Figures 2A–C show brain regions where PM seeded connectivity was significantly different between the repetitive and interleaved conditions, separately for the 2 practice days and the retention phase on Day 5. Regions where the PPI effects were significant are listed in Table I. Unless otherwise noted, all results were significant at the topological FDR < 0.05. On Day 1, no brain regions showed significant differences between practice conditions in functional connectivity with right PM (Fig. 2A). The PPI effects of the interleaved condition were strongest and widespread across the brain on the second day of practice (Fig. 2B). This is also demonstrated by the scatter plots in Fig. 3, where the regression slopes of the BOLD signal between PM and M1, the cerebellum, and the inferior and the superior parietal areas were higher in the interleaved condition. These areas are classically associated with movement execution and sensorimotor transformation [Cross et al., 2007; Karni et al., 1998]. These findings complement previous studies in which different levels of neural activity were detected when sequential finger pressing tasks were practiced in different conditions [Karni et al., 1995; Lin et al., 2011], and indicate the unique contributions of the PM in coordinating circuits involved in skill encoding. Interestingly, the above condition‐specific PPI effects on PM connectivity disappeared when subjects performed the practiced sequences on Day 5 (Fig. 2C). This may indicate that modulation of PM connectivity occurs as subjects achieve asymptotic performance when practicing interleaved tasks, not when well‐learned sequences are retrieved.4
Figure 2.
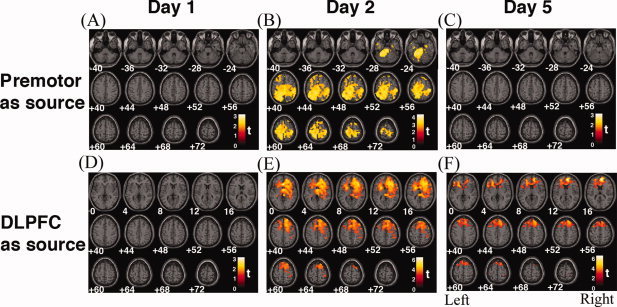
Mapping the modulatory effects of learning on cerebral functional networks. To detect the difference in functional connectivity of the brain between repetitive and interleaved practices in sequence learning, at every voxel of the brain, the functional BOLD time series was regressed against that of the right premotor cortex (upper row) or the right dorsal lateral prefrontal cortex (DLPFC, lower row), with the interleaved or repetitive condition as the interaction term, and between‐participant variability as the random‐effects variable. The resulting significance maps are displayed in neurological orientation, with the MNI coordinate (in mm) at the bottom. Colored regions, where voxel t‐values are positive, indicate that the interaction effects of interleaved practice had significantly greater interaction effects than repetitive practice on the functional relationship between that region and the premotor (upper row) or DLPFC (lower row) cortex, corrected for multiple comparisons using the topological false discovery rate (FDR) method. On Day 1 (A and D), modulatory effects of practice conditions were not significant, evaluated using either the premotor cortex or DLPFC as the seed region. On Day 2, interleaved practice led to higher correlations between the premotor cortex with sensorimotor planning regions, including the cerebellum, the medial frontal, and posterior parietal areas (B). Higher correlations between DLPFC and the caudate, the precentral and postcentral gyri, and the posterior parietal areas (E), were also associated with interleaved practice. On Day 5, modulatory effects of practice on the premotor cortex disappeared (C), but still remained with connectivity between DLPFC and other frontal regions, although the significance was attenuated (F). These results suggest that more brain regions with strong functional linkage need to be recruited during the acquisition phase of motor learning in the interleaved than repetitive practice conditions.
Table I.
Brain areas showing positive change in coupling with the right premotor (upper section) and right DLPFC (lower section) while sequences were practiced in interleaved versus repetitive conditions on Day 2 (thresholded at P < 0.05, topological FDR corrected)
| Regions | x | y | z | T | P |
|---|---|---|---|---|---|
| Coupling with Rt PM | |||||
| Rt inferior frontal gyrus | 57 | 30 | 12 | 3.24 | 0.01 |
| 18 | 36 | −12 | 3.07 | 0.01 | |
| Rt superior frontal gyrus | 6 | 33 | 45 | 3.05 | 0.01 |
| 15 | 0 | 75 | 3.05 | 0.01 | |
| Rt insula gyrus | 48 | 6 | −6 | 3.23 | 0.01 |
| Rt postcentral gyrus | 51 | −27 | 54 | 4.59 | 0.01 |
| Lt postcentral gyrus | −63 | −15 | 36 | 3.22 | 0.01 |
| Lt precentral gyrus | −60 | 3 | 27 | 3.48 | 0.01 |
| Lt supramarginal gyrus | −66 | −24 | 30 | 4.13 | 0.01 |
| Rt medial temporal lobule | 57 | −66 | −3 | 3.28 | 0.01 |
| Lt medial temporal lobule | −54 | −66 | 18 | 4.55 | 0.01 |
| Lt superior temporal lobule | −45 | −6 | −12 | 3.15 | 0.01 |
| Rt cerebellum | 42 | −69 | −24 | 3.12 | 0.01 |
| Rt cuneus | 18 | −90 | 39 | 3.7 | 0.01 |
| Rt inferior occipital lobule | 27 | −96 | −9 | 3.76 | 0.01 |
| Rt middle occipital lobule | 42 | −90 | 0 | 3.48 | 0.01 |
| Lt rolandic operculum | −57 | −3 | 3 | 3.06 | 0.01 |
| Coupling with Rt DLPFC | |||||
| Lt ant cingulum | −2 | 27 | −3 | 6.56 | 0.011 |
| Lt cingular gyrus | −15 | 3 | 27 | 4.51 | 0.011 |
| Rt medial frontal | 48 | 9 | 54 | 4.17 | 0.011 |
| 40 | 40 | 48 | 4.12 | 0.011 | |
| Rt superior frontal gyrus | 18 | 27 | 63 | 4.21 | 0.011 |
| Lt superior medial frontal gyrus | 2 | 38 | 50 | 5.28 | 0.011 |
| Rt insular gyrus | 42 | −9 | −3 | 4.13 | 0.011 |
| Lt supplementary motor cortex | −3 | 18 | 48 | 4.23 | 0.011 |
| Rt precentral gyrus | 36 | 0 | 39 | 4 | 0.011 |
| Rt rolandic operculum | 63 | 9 | 12 | 4.53 | 0.011 |
| Rt caudate | 3 | 9 | 18 | 5.5 | 0.011 |
| Rt medial temporal gyrus | 51 | 6 | −30 | 4.05 | 0.011 |
Coordinates are in MNI space [Evans et al., 1993].
Rt PM, right dorsal premotor cortex; Rt DLPFC, right dorsal lateral prefrontal cortex; Rt, right; Lt, left.
Seed II: Right Dorsal Lateral Prefrontal Cortex
Figures 2D–E show maps of regions where DLPFC‐seeded modulations were significantly different between the repetitive and interleaved conditions, separately for the 2 practice days and the retention phase on Day 5. Regions where the PPI effects were significant are listed in Table I. Similar to the above findings with the right PM, the PPI effects of the interleaved vs. the repetitive conditions on DLPFC connectivity were not detected on Day 1 (Fig. 2D), but became highly significant on Day 2, especially in the frontal lobe and basal ganglia (Fig. 2E). Moreover, scatter plots in Fig. 5 show that the right DLPFC seeded connectivity increased during the interleaved practice more than the repetitive practice on brain networks that are functionally associated with executive function, sequence learning, and sensorimotor transformation, including the superior medial frontal regions, the supplementary motor area, caudate nucleus, and the inferior and superior parietal areas [Schwarb and Schumacher, 2009; Sun et al., 2007; Tamas Kincses et al., 2008]. These results suggest that the connectivity between the motor and the higher order cognitive networks is enhanced during interleaved sequence practice. Unlike the PM connectivity described in the previous section, the effects of practice condition on the DLPFC connectivity were sustained during the retention test on Day 5, showing that successful retrieval of the practiced sequences requires the integration of DLPFC activity and brain regions known for memory and executive control (the superior medial frontal area), sequencing (the supplementary motor area), retrieval (the prefrontal areas), and automation (the caudate nucleus) (Figs. 2E and 5). On the other hand, however, the DLPFC connectivity with the superior parietal region was only significant during Day 2 of practice, which may indicate that the requirements of sensorimotor integration are less critical when the participants retrieved the practiced sequences on Day 5, compared with when they were still acquiring motor sequences on Day 2.
Increased PM‐ and DLPFC‐Connectivity on Day 2 is Associated with Enhanced Learning
Figure 6A and B demonstrated that the PPI effects of the interleaved vs. repetitive condition on functional connectivity significantly correlated with enhanced learning on Day 5. Greater interleaved‐repetitive differences in regression slopes (Day 2) between two pairs of brain regions, PM‐M1 and DLPFC‐superior parietal (SPL), were associated with better learning following the interleaved practice (differences in regression slopes correlated with differences in response time; PM‐M1: r = .51, P = .04, Fig. 6A; DLPFC‐SPL: r = .603, P = .013; Fig. 6B). These findings demonstrate that the CI effects on brain functional connectivity are associated with the CI enhancement of skill learning. Stronger functional connectivity between the PM and M1, and DLPFC and parietal regions during interleaved practice is associated with behavioral gain (shorter RT) during a later retention test.
Figure 6.
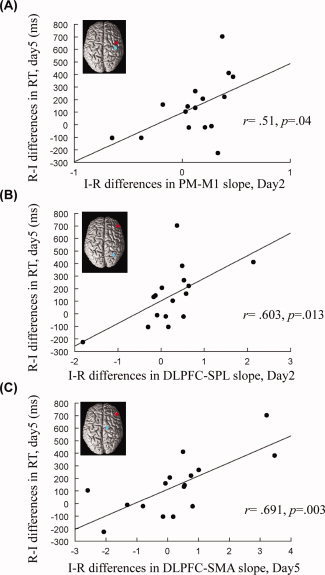
Greater interaction effects of practice conditions are associated with greater learning benefit. The x‐axis indicates the difference in regression slopes of the interleaved minus the repetitive practice condition, for BOLD activities between the ROI pair. Higher x‐values mean a greater interaction effect of the interleaved practice. Learning benefit is represented by the difference in the response time (RT) between the interleaved and the repetitive practices, measured on the retention testing on Day 5 and displayed on the y‐axis. Here, we used the difference in RT of the repetitive minus the interleaved practice (note that this is opposed to the “interleaved minus repetitive” difference in the x‐axis), as we already knew that the interleaved practice led to better learning performance (Lin et al., 2011), and preferred the same direction for data in the x‐ and y‐axes. Our results show that greater differences in regression slopes for BOLD activities between the interleaved and repetitive practices, no matter measured during practice (Day 2, A and B) or retention testing (Day 5, C), correlate to greater learning benefit. This also indicates that when one practices sequences in an interleaved order, stronger connectivity within the cortical motor network is required for successful sequence retrieval. PM, dorsal premotor area; M1, primary motor area; DLPFC, dorsolateral prefrontal cortex; SPL, superior parietal area; SMA, supplementary motor area.
Increased DLPFC‐SMA Connectivity may Indicate Efficient Retrieval
We next demonstrated that enhanced learning following interleaved practice appears to be associated with a more efficient cerebral network for retrieval than following repetitive practice. Figure 6C shows a significant positive correlation between the differences in DLPFC‐SMA regression slopes on Day 5 (interleaved minus repetitive, I‐R) and the learning benefits, quantified by the shorter response time during the retention test, following interleaved practice (r = .691, P = .003). This finding indicates that the increased connectivity between DLPFC and SMA supports more efficient retrieval of motor memory following the interleaved practice.
DISCUSSION
Our purpose was to investigate whether learning skills in an interleaved manner evokes different levels of inter‐regional connectivity in the human brain compared with when those skills are learned in a repetitive manner. Inter‐regional connectivity was determined using PPI analysis [Friston et al., 1997], separately for early and late phases of practice (Day 1 and Day 2, respectively) and when participants performed the trained sequences during a retention test (Day 5). Dorsal premotor cortex (PM) and dorsal lateral prefrontal cortex (DLPFC) were selected as a priori seed regions because their functional roles in movement planning and executive function. There are four main findings. First, the differences in inter‐regional connectivity for the interleaved vs. repetitive conditions did not emerge until the second day of practice, and this was consistent in both PM‐seeded and DLPFC‐seeded connectivity. Second, during the retention phase on Day 5, DLPFC‐seeded connectivity was significantly greater in the interleaved than the repetitive practice conditions, suggesting that interleaved practice enhanced DLPFC modulations during sequence retrieval. Third, increased functional connectivity in sensorimotor areas of the brain on Day 2 significantly correlated with the behavioral benefits of interleaved condition measured during retention on Day 5. In addition, increased DLPFC‐supplementary motor area connectivity during retention was associated with the behavioral benefits of the interleaved condition.
The present results suggest that different levels of contextual interference, namely practicing sequences in an interleaved vs. a repetitive condition, evoke different levels of functional connectivity in the cerebral network of motor learning. This distinct effect of practice condition was not significant until the later phase of practice (Day 2) where compared with the repetitive condition, practicing in the interleaved condition led to increased functional connectivity between the PM or DLPFC and the sensorimotor learning network. The significant interleaved‐repetitive contrast in functional connectivity on Day 2 suggests that interleaved practice facilitates inter‐regional coupling. To confirm that the significant PPI effect on Day 2 was due to increased functional connectivity as a result of interleaved practice, we further computed the contrast in the PPI effect between practice Day 1 and Day 2 separately for the interleaved and repetitive conditions. We found that for both PM and DLPFC, functional connectivity on Day 2 was significantly greater than Day 1 for the interleaved condition but not for the repetitive condition (Supporting Information Fig. 2). This increased connectivity during interleaved practice was accompanied by trends in decreased BOLD activity (Supporting Information Fig. 3). However, the functional imaging approach used in the current study was based on a blocked‐design, and thus we were unable to differentiate neural processing during the different phases of each trial, including planning and execution of specific events. A study separating the phase of task processing may be able to pinpoint more precisely the cognitive level of the beneficial effect of CI on skill learning. Our interpretation is also limited by the small sample size and limited number of a priori seed regions, which makes it difficult to generalize our findings to other cerebral areas.
Our findings corroborate previous work by Büchel et al. who demonstrated that learning that engages multiple cortical regions is accompanied by adaptation of evoked cortical responses and changes in inter‐regional coupling between these regions over time [Büchel et al., 1999]. Their data suggest that as activation within a brain region decreases with learning, connectivity between cortical areas increases. Using two distinct practice conditions, we further demonstrated that manipulation of practice condition alters the development of functional connectivity during skill learning. Consistent with the trends shown by Büchel et al.'s study, we found that the interleaved‐repetitive contrast in functional connectivity was not significant until Day 2. This significant PPI is accompanied by decreased hemodynamic response and increased functional connectivity preferentially for the interleaved condition (Supporting Information Figs. 1 and 2). This pattern of results suggest that the interleaved condition may facilitate more efficient neural encoding during the later phase of skill acquisition, and such efficiency (evident by decreased BOLD and increased functional connectivity) during the encoding phase may facilitate subsequent consolidation and retrieval.
An important finding of this study was that increases in functional connectivity during interleaved practice may explain the learning benefits under this practice schedule. We found that increased functional connectivity on Day 2 between two pairs of brain regions: the PM‐M1 and the DLPFC‐superior parietal areas, significantly correlated with the learning benefits in the interleaved condition on the Day 5 retention test. Enhanced functional connectivity in corticomotor and executive control networks may reflect more proficient neural encoding of motor sequences that benefit memory consolidation and subsequent retrieval. Interleaved practice involves greater retrieval demands compared with repetitive practice in which a single sequence can be held in working memory. By one view, the increased effort devoted to the retrieval of sequences during interleaved practice is the source of the benefit of this practice schedule. Retrieval practice has been shown to have a powerful effect on the long‐term retention of memories [Roediger and Butler, 2011], and the increased connectivity in the PM‐M1 and DLPFC‐superior parietal circuits during interleaved practice may reflect this increased retrieval effort.
Another novel finding is that the enhanced learning, reflected by shorter response time when the participants performed the practiced sequences on Day 5, is accompanied by increased functional connectivity between the DLPFC and SMA. There is evidence demonstrating that SMA activity is related to decreased motor response time [Honda et al., 1998], and the SMA is selectively active when motor sequences are retrieved from memory [Mushiake et al., 1991]. Neurophysiology and imaging studies have identified DLPFC as an important substrate for attention, task switching, and executive function [Yoshida et al., 2010]. Since successful retrieval of practiced sequences requires appropriate allocation of attention (presumably operated by DLPFC) to select the neural representations of sequences (presumably stored in SMA), it is conceivable that when the coupling between these two regions are strengthened, retrieval would be enhanced. The positive correlation between increased DLPFC‐SMA modulation and the performance benefits following interleaved practice supports this interpretation.
Looking at the pattern of results across the experiment, there was a large increase in functional connectivity on the second day of interleaved practice emanating from both the PM and DLPFC, with the DLPFC‐seeded PPI effect becoming sustained and more focal during Day 5 (Fig. 2C). This pattern suggests that increasing premotor modulation after skills have been consolidated is no longer necessary. The interleaved condition appears to demand stronger premotor modulation during the encoding phase when the demands of movement planning, parameterization, and error correction are high. Once the interleaved motor sequences were well‐learned and the consolidation of learning took place, the prefrontal areas continued to be important for coordinating the retrieval of appropriate representations during the retention phase. Our results agree with existing imaging studies that have associated frontal activity with explicit motor learning [Goghari and MacDonald, 2009; Schwarb and Schumacher, 2009; Sun et al., 2007]. In the context of prefrontal contributions to motor memory retrieval, our results suggest that interleaved practice results in long‐term changes in connectivity between the strategic network and the sensorimotor network that facilitates successful retrieval of the trained sequences.
We observed increased functional connectivity between the dorsal premotor with M1, superior and inferior parietal cortex, and the cerebellum in the interleaved compared with the repetitive condition. This suggests that these regions would be more engaged in the interleaved condition. PM is known for its role in movement planning and scaling in studies using neurophysiology (e.g., [Pesaran et al., 2006]) and neuroimaging (e.g., [Bortoletto and Cunnington, 2010] methods. It is also involved in sequence learning [Schwarb and Schumacher, 2009] and has been shown to increase activation during interleaved compared with repetitive practice of motor sequences [Cross et al., 2007; Lin et al., 2011]. M1 has been implicated in the storage of motor sequence information [Ben‐Shaul et al., 2004; Lu and Ashe, 2005]. The posterior parietal cortex, including the superior and inferior regions, plays an important role in integrating and scaling planned movements. It processes the transformation between reference frames in which sensory stimuli are encoded, which often differ from those of motor effectors [Cohen and Andersen, 2002]. The cerebellum, in contrast, is important for the learning of skilled movements [Doyon et al., 1998; Hikosaka et al., 1998; Jueptner and Krukenberg, 2001; Molinari et al., 1997], and is critical for both error correction and execution of automatic movements [Doyon et al., 1997; Wu et al., 2008]. The cerebellum‐premotor coupling may help synchronize the learned motor elements to generate a well‐executed motor sequence [Laforce and Doyon, 2002].
In addition, we observed increased DLPFC modulation over the SMA, superior and inferior parietal, superior prefrontal areas, and the caudate nucleus when sequences were acquired in an interleaved condition. Rowe et al., (2000) suggested a role of DLPFC for the selection of upcoming action representations [Rowe et al., 2005]. There is also evidence suggesting that the DLPFC might be involved in action selection and could play a unique part in top–down regulation of neural activity in regions processing task‐relevant representations [Egner and Hirsch, 2005]. SMA activation has been demonstrated during sequence learning in humans using a neuroimaging paradigm [Hikosaka et al., 1996; Sakai et al., 1999]. Evidence from neurophysiology studies suggest that different types of neurons in the SMA help to encode not only where in a sequence the monkey is but also the conditional links between the previous response and the upcoming response [Rushworth et al., 2004]. In the interleaved practice condition, more conditional links are active than when the subject is repetitively practicing a single sequence.
Previous studies regarding the role of superior prefrontal cortex in executive control are also consistent with the idea that this region plays an important role in the interleaved practice. Increased superior prefrontal activation has been identified when human subjects perform a task switching paradigm [Sohn et al., 2000], and when inhibiting a mental set is required to initiate an appropriate response [Konishi et al., 2003]. Such inhibitory function is required in set‐shifting paradigms and is specifically needed in an interleaved practice condition.
The increased functional connectivity between the caudate nucleus and DLPFC during interleaved practice is consistent with the idea that motor learning is associated with increased functional connectivity in cortico‐subcortical circuits [Toni et al., 2002; Wu et al., 2008]. The caudate nucleus projects to corticomotor areas including M1 and premotor areas [Alexander and Crutcher 1990], and these caudate‐corticomotor connections are thought to be important to the acquisition and coordination of motor sequences [Nakano, 2000]. In addition, significant increase in prefrontal‐caudate modulation was observed during retention on Day 5. This stronger prefrontal‐caudate modulation supports previous findings that some post‐learning processes may be mediated by subcortical structures such as the basal ganglia [Toni et al., 2002; Wu et al., 2008]. The basal ganglia may participate in developing a motor repertoire that can be initiated in response to appropriate environmental cues [Laforce and Doyon, 2001]. We speculate that the interleaved condition facilitates prefrontal‐basal ganglia connectivity during the encoding phase and this connectivity was maintained after memory consolidation. The basal ganglia and prefrontal cortex may function jointly to optimize retrieval processing during retention by transforming a series of motor elements (e.g., the motor elements that compose a motor sequence) as one motor representation, making the retrieval performance more automatic.
Learning in the SRT task was shown to include both sequence specific and nonspecific components. Nonspecific learning was shown by the faster RT after practice when tested with novel sequences, while sequence specific learning was assessed by the further reduction in RT for practiced sequences compared with novel sequences. Although interleaved practice improved averaged RT during retention compared with repetitive practice, it is unclear whether the changes in functional connectivity that we observed supported enhanced sequence‐specific representations, or whether the enhancement was general for SRT task performance. Further studies where sequence specific and nonspecific learning can be assessed separately are needed to determine how changes in functional connectivity support these different types of learning.
To our knowledge, this is the first study to demonstrate that changes in neural couplings contribute to the beneficial effects of CI on learning. Introducing the desirable difficulty of CI during practice increased premotor‐ and DLPFC‐modulations of the cerebral motor learning network that appear to support more efficient long‐term retrieval. Future directions for this work include the implementation of neural modulation techniques, such as repetitive transcranial magnetic stimulation, to validate the role of dorsal premotor and DLPFC and their distinct contributions to the CI effect. Another important direction is to examine the effect of neurological disorders on the development of inter‐regional connectivity with skill practice, which may lead to better design of rehabilitation protocols.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Figure 3.
Acknowledgements
For generous support the authors also wish to thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson‐Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones‐Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family and Northstar Fund.
REFERENCES
- Abe M, Hanakawa T, Takayama Y, Kuroki C, Ogawa S, Fukuyama H ( 2007): Functional coupling of human prefrontal and premotor areas during cognitive manipulation. J Neurosci 27: 3429–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD ( 1990): Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends Neurosci 13: 266–271. [DOI] [PubMed] [Google Scholar]
- Battig WF, Berry JK ( 1966): Effects of number and similarity of pretraining alternatives on paired‐associate performance on pretrained and new items under correction and noncorrection procedures. J Exp Psychol 72: 722–730. [DOI] [PubMed] [Google Scholar]
- Ben‐Shaul Y, Drori R, Asher I, Stark E, Nadasdy Z, Abeles M ( 2004): Neuronal activity in motor cortical areas reflects the sequential context of movement. J Neurophysiol 91: 1748–1762. [DOI] [PubMed] [Google Scholar]
- Bortoletto M, Cunnington R ( 2010): Motor timing and motor sequencing contribute differently to the preparation for voluntary movement. Neuroimage 49: 3338–3348. [DOI] [PubMed] [Google Scholar]
- Brady F ( 2008): The contextual interference effect and sport skills. Perceptual Motor Skills 106: 461–472. [DOI] [PubMed] [Google Scholar]
- Büchel C, Coull JT, Friston KJ ( 1999): The predictive value of changes in effective connectivity for human learning. Science 283: 1538–1541. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL, Weinberger NM ( 2001): The neurobiology of learning and memory: Some reminders to remember. Trends in Neuroscience 24: 578–581. [DOI] [PubMed] [Google Scholar]
- Christina RW, Bjork RA ( 1991): Optimizing long‐term retention and transfer In: Druckman D, Bjork RA, editors. In the Mind's Eye: Enhancing Human performance. Washington DC: National Academy Press; pp 23–56. [Google Scholar]
- Chumbley J, Worsley K, Flandin G, Friston K ( 2010): Topological FDR for neuroimaging. Neuroimage 49: 3057–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen YE, Andersen RA ( 2002): A common reference frame for movement plans in the posterior parietal cortex. Nat Rev Neurosci 3: 553–562. [DOI] [PubMed] [Google Scholar]
- Cross ES, Schmitt PJ, Grafton ST ( 2007): Neural substrates of contextual interference during motor learning support a model of active preparation. J Cogn Neurosci 19: 1854–1871. [DOI] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP ( 2004): Cerebellar and premotor function in bimanual coordination: Parametric neural responses to spatiotemporal complexity and cycling frequency. Neuroimage 21: 1416–1427. [DOI] [PubMed] [Google Scholar]
- Doyon J, Gaudreau D, Laforce R Jr., Castonguay M, Bedard PJ, Bedard F, Bouchard JP ( 1997): Role of the striatum, cerebellum, and frontal lobes in the learning of a visuomotor sequence. Brain Cogn 34: 218–245. [DOI] [PubMed] [Google Scholar]
- Doyon J, Laforce R, Jr. , Bouchard G, Gaudreau D, Roy J, Poirier M, Bedard PJ, Bedard F, Bouchard JP ( 1998): Role of the striatum, cerebellum and frontal lobes in the automatization of a repeated visuomotor sequence of movements. Neuropsychologia 36: 625–641. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J ( 2005): Cognitive control mechanisms resolve conflict through cortical amplification of task‐relevant information. Nat Neurosci 8: 1784–1790. [DOI] [PubMed] [Google Scholar]
- Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM ( 1993): 3D Statistical Neuroanatomical Models from 305 MRI Volumes Nuclear Science Symposium and Medical Imaging Conference, 1993 IEEE Conference Record, Vol. 3 pp 1813–1817. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR ( 1975): “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ ( 1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6: 218–229. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R ( 1995): Analysis of fMRI time‐series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Gail A, Klaes C, Westendorff S ( 2009): Implementation of spatial transformation rules for goal‐directed reaching via gain modulation in monkey parietal and premotor cortex. J Neurosci 29: 9490–9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ ( 2003): Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage 19: 200–207. [DOI] [PubMed] [Google Scholar]
- Goghari VM, MacDonald AW,3rd ( 2009): The neural basis of cognitive control: response selection and inhibition. Brain Cogn 71: 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker DA, Ollinger JM, D'Esposito M ( 2004): Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage 21: 1639–1651. [DOI] [PubMed] [Google Scholar]
- Hattori N, Shibasaki H, Wheaton L, Wu T, Matsuhashi M, Hallett M ( 2009): Discrete parieto‐frontal functional connectivity related to grasping. J Neurophysiol 101: 1267–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Miyashita K, Miyachi S, Sakai K, Lu X ( 1998): Differential roles of the frontal cortex, basal ganglia, and cerebellum in visuomotor sequence learning. Neurobiol Learn Mem 70: 137–149. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakai K, Miyauchi S, Takino R, Sasaki Y, Putz B ( 1996): Activation of human presupplementary motor area in learning of sequential procedures: a functional MRI study. J Neurophysiol 76: 617–621. [DOI] [PubMed] [Google Scholar]
- Honda M, Deiber MP, Ibanez V, Pascual‐Leone A, Zhuang P, Hallett M ( 1998): Dynamic cortical involvement in implicit and explicit motor sequence learning. A PET study. Brain 121( Part 11): 2159–2173. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Krukenberg M ( 2001): Motor system: Cortex, basal ganglia, and cerebellum. Neuroimaging Clin N Am 11: 203–219, viii. [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG ( 1995): Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377: 155–158. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey‐Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG ( 1998): The acquisition of skilled motor performance: Fast and slow experience‐driven changes in primary motor cortex. Proc Natl Acad Sci USA 95: 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Jimura K, Asari T, Miyashita Y ( 2003): Transient activation of superior prefrontal cortex during inhibition of cognitive set. J Neurosci 23: 7776–7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroliczak G, Frey SH ( 2009): A common network in the left cerebral hemisphere represents planning of tool use pantomimes and familiar intransitive gestures at the hand‐independent level. Cereb Cortex 19: 2396–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforce R Jr., Doyon J ( 2001): Distinct contribution of the striatum and cerebellum to motor learning. Brain Cogn 45: 189–211. [DOI] [PubMed] [Google Scholar]
- Laforce R Jr., Doyon J ( 2002): Differential role for the striatum and cerebellum in response to novel movements using a motor learning paradigm. Neuropsychologia 40: 512–517. [DOI] [PubMed] [Google Scholar]
- Lee TD ( 1988): Transfer‐appropriate processing: A framework for conceptualizing practice effects in motor learning In: Meijer OG, Roth K, editors. Complex Movement Behaviour: The motor‐action controversy. North‐Holland: Elsevier Science Publishers B.V; pp 201–215. [Google Scholar]
- Lee TD, Magill RA ( 1983): The locus of contextual interference in motor‐skill acquisition. J Exp Psychol: Hum Learn Memor 9: 730–746. [Google Scholar]
- Lee TD, Wulf G, Schmidt RA ( 1992): Contextual interference in motor learning: dissociated effects due to the nature of task variations. Quart J Exp Psychol 44A: 627–644. [Google Scholar]
- Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, Luo C, Wang Z, Tan Q, Lu G, Chen H ( 2010): Default mode network abnormalities in mesial temporal lobe epilepsy: A study combining fMRI and DTI. Hum Brain Mapp 32: 883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Fisher BE, Winstein CJ, Wu AD, Gordon J ( 2008): Contextual interference effect: elaborative processing or forgetting‐reconstruction? A post hoc analysis of transcranial magnetic stimulation‐induced effects on motor learning. J Motor Behav 40: 578–586. [DOI] [PubMed] [Google Scholar]
- Lin CH, Knowlton BJ, Chiang MC, Iacoboni M, Udompholkul P, Wu AD ( 2011): Brain‐behavior correlates of optimizing learning through interleaved practice. Neuroimage 56: 1758–1772. [DOI] [PubMed] [Google Scholar]
- Lu X, Ashe J ( 2005): Anticipatory activity in primary motor cortex codes memorized movement sequences. Neuron 45: 967–973. [DOI] [PubMed] [Google Scholar]
- Mansouri FA, Tanaka K, Buckley MJ ( 2009): Conflict‐induced behavioural adjustment: A clue to the executive functions of the prefrontal cortex. Nat Rev Neurosci 10: 141–152. [DOI] [PubMed] [Google Scholar]
- Maslovat D, Chus R, Lee TD, Franks IM ( 2004): Contextual interference: single task versus multi‐task learning. Motor Control 8: 213–233. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Sadato N, Kochiyama T, Nakamura S, Naito E, Matsunami K, Kawashima R, Fukuda H, Yonekura Y ( 2004): Role of the cerebellum in implicit motor skill learning: a PET study. Brain Res Bull 63: 471–483. [DOI] [PubMed] [Google Scholar]
- Mesquita RC, Franceschini MA, Boas DA ( 2010): Resting state functional connectivity of the whole head with near‐infrared spectroscopy. Biomed Opt Exp 1: 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M, Leggio MG, Solida A, Ciorra R, Misciagna S, Silveri MC, Petrosini L ( 1997): Cerebellum and procedural learning: Evidence from focal cerebellar lesions. Brain 120( Part 10): 1753–1762. [DOI] [PubMed] [Google Scholar]
- Mulder MJ, van Belle J, van Engeland H, Durston S ( 2010): Functional connectivity between cognitive control regions is sensitive to familial risk for ADHD. Hum Brain Mapp 32: 1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata A, Gallese V, Luppino G, Kaseda M, Sakata H ( 2000): Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol 83: 2580–2601. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Inase M, Tanji J ( 1991): Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J Neurophysiol 66: 705–718. [DOI] [PubMed] [Google Scholar]
- Nakano K ( 2000): Neural circuits and topographic organization of the basal ganglia and related regions. Brain Dev 22( Suppl 1): S5–S16. [DOI] [PubMed] [Google Scholar]
- Neufang S, Fink GR, Herpertz‐Dahlmann B, Willmes K, Konrad K ( 2008): Developmental changes in neural activation and psychophysiological interaction patterns of brain regions associated with interference control and time perception. Neuroimage 43: 399–409. [DOI] [PubMed] [Google Scholar]
- Ortu E, Ruge D, Deriu F, Rothwell JC ( 2009): Theta Burst Stimulation over the human primary motor cortex modulates neural processes involved in movement preparation. Clin Neurophysiol 120: 1195–203. [DOI] [PubMed] [Google Scholar]
- Perez CR, Meira CM Jr., Tani G ( 2005): Does the contextual interference effect last over extended transfer trials? Perceptual Motor Skills 100: 58–60. [DOI] [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA ( 2006): Dorsal premotor neurons encode the relative position of the hand, eye, and goal during reach planning. Neuron 51: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert J, Riehle A, Aertsen A, Rotter S, Nawrot MP ( 2009): Dynamic encoding of movement direction in motor cortical neurons. J Neurosci 29: 13870–13882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger HL 3rd, Butler AC ( 2011): The critical role of retrieval practice in long‐term retention. Trends Cogn Sci 15: 20–27. [DOI] [PubMed] [Google Scholar]
- Rossi AF, Pessoa L, Desimone R, Ungerleider LG ( 2009): The prefrontal cortex and the executive control of attention. Exp Brain Res 192: 489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Stephan KE, Friston K, Frackowiak RS, Passingham RE ( 2005): The prefrontal cortex shows context‐specific changes in effective connectivity to motor or visual cortex during the selection of action or colour. Cereb Cortex 15: 85–95. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM ( 2004): Action sets and decisions in the medial frontal cortex. Trends Cogn Sci 8: 410–417. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Sasaki Y, Fujimaki N, Putz B ( 1999): Presupplementary motor area activation during sequence learning reflects visuo‐motor association. J Neurosci 19: RC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RA, Bjork RA ( 1992): New conceptualizations of practice: Common principles in three paradigms suggest new concepts for training. Psychol Sci 3: 207–217. [Google Scholar]
- Schwarb H, Schumacher EH ( 2009): Neural evidence of a role for spatial response selection in the learning of spatial sequences. Brain Res 1247: 114–125. [DOI] [PubMed] [Google Scholar]
- Shea JB, Morgan R ( 1979): Contextual interference effects on the acquisition, retention, and transfer of a motor skill. J Exp Psychol: Hum Learn Mem 5: 179–187. [Google Scholar]
- Shea JB, Zimny ST ( 1983). Contextual effects in memory and learning movement information In: Magill RA, editor. Memory and Control of Action. Amsterdam: Elsevier; pp 345–366. [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS ( 2000): The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci USA 97: 13448–13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FT, Miller LM, Rao AA, D'Esposito M ( 2007): Functional connectivity of cortical networks involved in bimanual motor sequence learning. Cereb Cortex 17: 1227–1234. [DOI] [PubMed] [Google Scholar]
- Tamas Kincses Z, Johansen‐Berg H, Tomassini V, Bosnell R, Matthews PM, Beckmann CF ( 2008): Model‐free characterization of brain functional networks for motor sequence learning using fMRI. Neuroimage 39: 1950–1958. [DOI] [PubMed] [Google Scholar]
- Toni I, Rowe J, Stephan KE, Passingham RE ( 2002): Changes of cortico‐striatal effective connectivity during visuomotor learning. Cereb Cortex 12: 1040–1047. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC ( 2002): A general statistical analysis for fMRI data. Neuroimage 15: 1–15. [DOI] [PubMed] [Google Scholar]
- Wright DL, Magnuson CE, Black CB ( 2005): Programming and reprogramming sequence timing following high and low contextual interference practice. Res Quart Exe Sports 76: 258–266. [DOI] [PubMed] [Google Scholar]
- Wu T, Chan P, Hallett M ( 2008): Modifications of the interactions in the motor networks when a movement becomes automatic. J Physiol 586( Part 17): 4295–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida W, Funakoshi H, Ishii S ( 2010): Hierarchical rule switching in prefrontal cortex. Neuroimage 50: 314–322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Figure 3.


