Abstract
Learning efficacy depends on its emotional context. The contents learned and the feedback received during training tinges this context. The objective here was to investigate the influence of content and feedback on the efficacy of implicit learning and to explore using functional imaging how these factors are processed in the brain. Twenty‐one participants completed 150 trials of a probabilistic classification task (predicting sun or rain based on combinations of playing cards). Smileys or frowneys were presented as feedback. In 10 of these subjects, the task was performed during functional magnetic resonance imaging. Card combinations predicting sun were remembered better than those predicting rain. Similarly, positive feedback fortified learning more than negative feedback. The presentation of smileys recruited bilateral nucleus accumbens, sensorimotor cortex, and posterior cingulum more than negative feedback did. The higher the predictive value of a card combination, the more activation was found in the lateral cerebellum. Both context and feedback influence implicit classification learning. Similar to motor skill acquisition, positive feedback during classification learning is processed in part within the sensorimotor cortex, potentially reflecting the activation of a dopaminergic projection to motor cortex (Hosp et al., 2011). Activation of the lateral cerebellum during learning of combinations with high predictive value may reflect the formation of an internal model. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: sensorimotor cortex, nucleus accumbens, cerebellum, reward, classification learning, implicit learning, fMRI
INTRODUCTION
Learning depends on the emotional context in which it occurs [Cahill and McGaugh, 1996]. Pleasant cues with positive valence are better remembered than unpleasant ones with negative valence [Ali and Cimino, 1997; Mneimne et al., 2010] and the opposite may be true for directed forgetting [Minnema and Knowlton, 2008]. Feedback in the form of reward or punishment provided during trial‐and‐error learning also tinges the emotional context. [Wächter et al. 2009] showed that implicit motor skill learning is more effective with positive than negative feedback. Brain networks involved in feedback processing include striatum, midbrain, amygdala, frontal, and cingulate cortices; positive and negative feedback are handled by different circuits [Liu et al., 2010].
The hypothesis here was that positive feedback, content of positive valence and high predictive value improve implicit learning and that improved learning is associated with stronger recruitment of brain networks encoding rewards. Because implicit learning mechanisms form the basis of many therapeutic interventions in rehabilitation, it is important to know the effect of these modifiable factors. We recruited subjects from middle to retirement age for a later comparison with individuals after stroke.
We tested our hypothesis using a classification learning paradigm, the weather prediction task [Knowlton et al., 1996], in which subjects had to learn associations between a certain combination of four different playing cards and a dichotomous weather outcome, sun or rain. The associations were stochastic, that is, each combination of cards predicted sun or rain with a certain probability. The subject was supposed to learn which combination predicted which weather. Feedback was given in form of smiley or frowney faces. The stochastic nature limited the subject's awareness of the association. Hence, learning this task was considered to be mainly implicit.
MATERIALS AND METHODS
Subjects and Task
Twenty‐one subjects were recruited via advertisements. Participants (14 females, 7 males) were between 43 and 85 years old (mean ± SEM: 64.6 ± 2.1). Inclusion criteria were mini mental state (MMS) ≥27 points and Beck's depression index (BDI) ≤11 points. Mean MMS was 29.5 ± 0.2, SEM, and BDI was 5.1 ± 0.8. Education quantified by the number of years spent in primary and secondary school was 11.7 ± 0.3 years. Ten of the 21 subjects qualified for (absence of claustrophobia and metal implants, six females, four males, age 60.4 ± 2.1 years, mean ± SEM) and agreed to undergo fMRI testing. The sample was recruited as a control group for a later comparison to stroke survivors. The study was approved by the Ethics Committee of the University of Tübingen, Germany. All participants provided written informed consent.
The weather prediction task (WPT) was performed as described by [Knowlton et al. 1996]. The task is a forced‐choice classification task with two alternative responses in which participants learn probabilistic associations between 14 different combinations of four playing cards (Fig. 1) and two weather outcomes, sun and rain. Each card was linked to an outcome with a prespecified probability (for sun: card 1–80%, card 2–54%, card 3–43%, card 4–20%). For each trial, either one, two, or three cards were shown composing 14 combinations that predicted the weather each with a certain combined probability. Table I shows for each combination of cards the probability and how often the combination was shown (as a fraction of 150 trials). Presentation of combinations of cards, detection of button‐press responses and feedback were computer‐controlled using Matlab (Mathworks, Natick, MA) and Psychtoolbox (http://www.psychtoolbox.org). The WPT was verbally explained and demonstrated before the experiment. Participants were instructed not to talk with the investigator during the experiment. After presentation of a card combination, the subject had to respond within 4 s or the trial was scored as “incorrect.” After 3 s, a prompt (“Please press a button”) appeared on the screen. After pressing either the “sun” or the “rain” button, feedback was shown for 2 s in form of a smiley or a frowney face. Every 50 trials, a 1‐min break was allowed. The experiment continued until 150 trials were completed.
Figure 1.

Set of playing cards. One to three cards were shown to form the card combinations described in Table I.
Table I.
Predictive value, frequency and probabilities for card combinations
| Combination | Combination class according to predictive value | Card | Percent of trials with combination | Probability for predicting sun | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| 5 | high | 0 | 0 | 1 | 1 | 13% | 0.08 |
| 1 | medium | 0 | 0 | 0 | 1 | 9.5% | 0.11 |
| 7 | medium | 0 | 1 | 1 | 1 | 9.5% | 0.11 |
| 3 | low | 0 | 1 | 0 | 1 | 6% | 0.17 |
| 11 | low | 1 | 1 | 0 | 1 | 4.5% | 0.22 |
| 6 | low | 0 | 1 | 1 | 0 | 4.5% | 0.44 |
| 2 | none | 0 | 1 | 0 | 0 | 3% | 0.5 |
| 13 | none | 1 | 0 | 1 | 1 | 3% | 0.5 |
| 4 | low | 0 | 0 | 1 | 0 | 4.5% | 0.55 |
| 9 | low | 1 | 0 | 0 | 1 | 4.5% | 0.78 |
| 12 | low | 1 | 0 | 1 | 0 | 6% | 0.83 |
| 8 | medium | 1 | 0 | 0 | 0 | 9.5% | 0.89 |
| 14 | medium | 1 | 1 | 1 | 0 | 9.5% | 0.89 |
| 10 | high | 1 | 1 | 0 | 0 | 13% | 0.92 |
Analysis of Behavioral Data
Trials were considered as “correct” when subjects chose the more probable weather (sun or rain) for the card combination presented. Trials in which subjects did not respond were scored as “incorrect.” Missed responses were few and an alternative analysis that excluded those responses yielded results similar to the ones reported below.
To investigate the time course of learning, a learning curve (performance over time) was constructed for each subject using a two‐step procedure. First, a cumulative performance curve was computed by adding 1 for each “correct” and subtracting 1 from each “incorrect” trial. This curve was then smoothed using spline interpolation (Matlab's spapi function, two knots). Second, to convert the cumulative into a performance‐over‐time curve the first derivative was computed (finder function). The resulting curve showed that performance increased in a nonlinear fashion over the course of training. Non‐linear fitting of an exponential function [Boltzmann function, p/(1 + exp(k × (a − x)))] was used to derive parameters of learning: the plateau p, the turning point a of the sigmoid Boltzmann curve and the steepness in the turning point k. The plateau values were estimated for each card combination. A general linear model was used to explore whether the variability of the plateau was explained by sun versus rain, the combinations' predictive probability and its presentation frequency. Predictive probability was classified as high, medium or low (Table I). This stratification was done because some combinations were presented less frequently than others and by grouping we obtained prediction classed of approximately equal frequency. Whether or not a subject belonged to the subgroup receiving fMRI or not was included as an additional independent dichotomous variable to test for systematic differences between the samples.
In a second analysis, we measured how well single card combinations were remembered. We counted the number of trials in which the subject responded identical to a preceding trial with the same card combination and a smiley reward. The two trials could have been subsequent or several trials apart. Trials with the same response after a rewarded (smiley) trial will be referred to as “same‐after‐smiley” trials (SAS), otherwise they will be termed “opposite‐after‐smiley” (OAS). Conversely to examine, if subjects remembered to change their response behavior after seeing a frowney, we counted “opposite‐after‐frowney” (OAF) and “same‐after‐frowney” trials. The ratio of (SAS+OAF)/all trials was then used as an index of memory. Because memory improved during training, only the last 60 trials of 150 were considered to compute this index. Using the index as a dependent variable, we tested for effects of combination (sun versus rain), predictive value (high‐medium‐low, Table I) and feedback. The independent variable feedback was defined as the number of smileys ‐ frowneys that a subject saw during the initial 30 trials of training. It was assumed that no relevant memory was formed during these initial 30 trials; hence, there was no bias towards smileys because some combinations had already been memorized. In fact frowneys were slightly but significantly more frequent during the initial 30 trials (1.9 vs. 2.8, P = 0.045) excluding a bias towards smileys. Whether or not a subject belonged to the subgroup receiving fMRI or not was included as an additional independent dichotomous variable to test for systematic differences between the samples.
JMP (version 8, SAS Institute, Cary, NC) was used for statistical calculations.
Functional Magnetic Resonance Imaging (fMRI)
Using a 3 Tesla scanner (Trio‐Tim with eight‐channel phased‐array head coil, Siemens, Erlangen, Germany) fMRI was performed in subjects without metal implants or claustrophobia who agreed to participate. Visual cues were presented via a projection system installed in the scanner room; responses were collected using an MRI‐compatible button‐box. All participants responded using their right hand.
The WPT task was performed in participants naïve to this task as described above, except that the intertrial interval was 5 s, subjects had to respond within 4 seconds and did not receive the second prompt (“Please press a button”). Additionally, a control task was performed before and after the WPT to record brain activity related to visual processing and movement similar to the WPT. In the control task one, two or three cards were shown and subjects were asked to respond with the right button when two cards were presented and the left button when one or three cards were shown. Thirty training trials of the WPT were performed outside the scanner without feedback stimuli to avoid learning before the actual experiment was started. Brain activity during WPT was measured in three blocks of 50 trials each separated by 30 s of fixation. Fifty trials of the control task were performed before the WPT.
A high‐resolution T1‐weighted scan was acquired for anatomical localization. Functional imaging used gradient‐echo planar T2*‐weighted images (EPI) with blood oxygenation level (BOLD)‐contrast (TR = 2.4 s, TE = 30 ms, flip angle = 90°). Thirty‐eight slices (slice thickness 3 mm) were acquired to cover the entire brain.
fMRI Analysis
fMRI data were processed using Brainvoyager QX (version 2.2, Brain Innovation BV, Maastricht, The Netherlands). BOLD‐weighted EPI datasets were corrected for slice acquisition timing and head motion. Motion correction parameters were used as confound predictors in first‐level GLM analyses. Datasets were registered to Talairach space in correspondence to the anatomical dataset. Images were spatially (Gaussian kernel, full‐width at half‐maximum of 8 mm) and temporally (three cycles, GLM‐Fourier‐high‐pass‐filter) smoothed.
The statistical analysis modeled each trial as two events, one before (presentation period) and one after the button press (feedback period). Five general linear models were computed:
-
1
In the first, the hemodynamic response was estimated for each of the following conditions, control trial presentation, control trial feedback, WPT trial presentation, WPT trial feedback. Random effects second‐level analysis of variance (ANOVA) was used to construct WPT versus control activation maps for the presentation and feedback periods.
-
2
The second model was constructed analogous to Model 1 except for replacing the control trials with chance trials (combination with no predictive value).
-
3
In the third model, the WPT trials were separated according to whether the card combination predicted sun or rain, and according to the predictive value of the combination (prediction class, Table I). Random effects of ANOVA were used to extract activation maps for the effects of sun/rain, predictive value and their interaction (F tests). Contrasts (t tests) were computed for high > low predictive value and sun > rain. This second model was computed for presentation and feedback periods separately.
-
4
A fourth model was computed separating trials in which a smiley and a frowney feedback was received. Random effects ANOVA was then used to extract the activation map for smiley > frowney. Only the feedback period of the trial was included in this model.
-
5
In a fifth random effects ANOVA model we investigated a potential interaction between sun/rain and smiley/frowney as independent variables. This model did not yield any results and is therefore not further mentioned.
To test for effects of age, the subject sample was split according to the median age and the age group was included as a between‐subject predictor in all models. For all random effects models the statistical threshold was set P < 0.05 corrected for multiple comparisons using a false discovery rate (FDR) method. Talairach coordinates and average p‐values were measured for each activation cluster equal or larger than 10 voxels (10 × 3 mm × 3 mm × 3 mm).
RESULTS
Learning Depends on Repetition and Predictive Value of the Combination and on the Content of the Association Being Learned (Sun versus Rain)
During the task, performance (correct responses over time) increased in a sigmoid fashion reaching a plateau between trials 70 and 80 (Fig. 2A). For card combinations predicting sun plateau performance was higher than for card combinations predicting rain (general linear model, effect of the dichotomous variable sun/rain: P = 0.039, Fig. 2B) indicating that combinations predicting sun were better learned than those predicting rain. Plateau performance was also higher for combinations occurring more frequently (general linear model, effect of frequency: P < 0.0001, r = 0.97, Fig. 2C) and for those with higher predictive value, i.e., a larger difference from chance probability to predict either sun or rain (general linear model, effect of probability: r = 0.84, P < 0.0001, Fig. 2D). The latter effect, however, did not remain significant if frequency was included in the model. Whether or not the subject was in the fMRI group had no significant effect.
Figure 2.
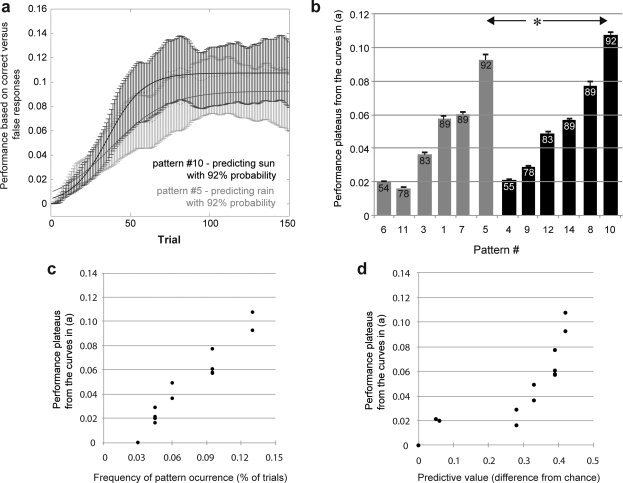
Influence on learning. (a) Learning curves constructed from correct‐versus‐incorrect responses (see Methods) and averaged for all subjects are shown for the two card combinations with highest predictive value for sun (combination no. 10, red) and rain (combination no. 5, blue). Plateau performance is reached after approximately half the trials and is lower for rain than sun card combinations. Values on the y‐axis represent the steepness, i.e., the first derivative of the cumulative performance curve, see Methods for details. (b) Plateau values estimated from the learning curves (examples in a) are shown for each card combination (bars indicate estimated plateau, error bars reflect SE, probabilities according to Table I). The plateaus are lower for card combinations predicting rain (blue) than for those predicting sun (red). This indicates that the emotional value of the learned content has an influence on learning efficacy. (c) Plateau values also depend on the frequency by which a card combination occurs and (d) on the predictive value of the card combination (difference from chance).
Learning Depends on Feedback
To evaluate learning from positive feedback, we counted how often a subject responded identical to a card combination that had occurred before and was rewarded with a smiley (“same‐after‐smiley,” SAS). Vice versa, to evaluate learning from negative feedback we counted “opposite‐after‐frowney” (OAF) trials. SAS trials were significantly more frequent than OAF trials (paired t test, P < 0.001, Fig. 3a) indicating that card combinations leading to positive feedback were remembered better than those leading to negative feedback.
Figure 3.
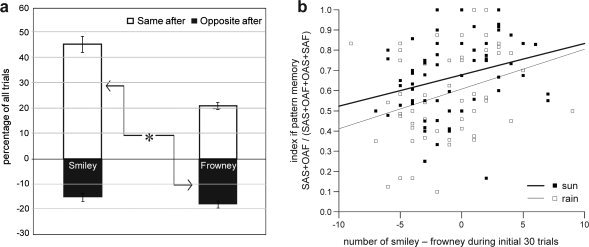
(a) Smileys are better remembered than frowneys. If a smiley was presented, the subjects were more likely to give the same answer in the subsequent trial with the same card combination (yellow bars). In contrast, frowneys did not motivate the subjects to give the opposite answer (green bars, *P < 0.001). (b) The index of memory, computed as shown in the y‐axis label, was related to how many smileys versus frowneys were received during the initial phase of WPT training and to whether sun or rain was predicted.
Using the SAS‐CAF index (see Methods) as a measure of how well card combinations were remembered, learning was better for sun than rain combinations (P = 0.036) and for combinations for which more smileys than frowneys were received during the initial 30 trials of WPT training (P = 0.001, Fig. 3b). The interaction between the two independent variables was not significant. Likewise the predictive value of the combination (high‐medium‐low) had no significant effect if the variables sun/rain and smiley‐frowneys were included in the model. Whether or not the subject was in the fMRI group had no significant effect.
Brain Activation
During the presentation period, the control task was associated with more activation in several brain areas as compared with the WPT (Table IIa). In contrast, during the feedback period, WPT lead to stronger activation in the left inferior frontal gyrus (Table IIb). In comparison with WPT card combinations without predictive value (chance trials), the combinations with a predictive value lead to stronger activation in the left posterior cingulum, Brodmann's area 31, during presentation (Table IIc). No differences between chance and predictive combinations were observed during feedback.
Table II.
Brain activation (a) during presentation period of card combinations when observing Control Task > WPT Task (b) during feedback period when observing WPT Task > Control Task (c) during presentation period when observing WPT Trials > Chance Trials
| Region of interest | Brodmann | Side | x | y | z | t | P |
|---|---|---|---|---|---|---|---|
| (a) | |||||||
| Somatosensory cortex | 3 | left | −34 | −23 | 42 | −4.93 | 0.000109 |
| Premotor cortex | 6 | left | −10 | −2 | 60 | −5.79 | 0.000017 |
| Premotor cortex | 6 | left | −28 | −2 | 45 | −4.54 | 0.000254 |
| Middle temporal gyrus | 22 | left | −55 | −35 | 6 | −9.37 | 0.000000 |
| Orbitofrontal cortex | 47 | left | −49 | 31 | −3 | −6.94 | 0.000002 |
| Thalamus | left | −1 | −11 | 12 | −5.97 | 0.000012 | |
| Putamen | left | −16 | 10 | −6 | −4.81 | 0.000141 | |
| Cerebellar hemisphere | left | −43 | −47 | −33 | −4.91 | 0.000113 | |
| Premotor cortex | 6 | right | 23 | −5 | 45 | −5.40 | 0.000040 |
| Fusiform gyrus | 20 | right | 50 | −38 | −24 | −4.35 | 0.000385 |
| Posterior cingulate | 31 | right | 11 | −32 | 33 | −5.18 | 0.000064 |
| Cerebellar hemisphere | right | 14 | −56 | −33 | −4.74 | 0.000165 | |
| (b) | |||||||
| Inferior frontal gyrus | 45 | left | −49 | 28 | 9 | 6.48 | 0.000004 |
| (c) | |||||||
| Posterior cingulum | 31 | left | −19 | −26 | 36 | 4.20 | 0.000539 |
The third random effects ANOVA model tested the effect of combinations predicting sun‐ versus rain (content) and the combinations' predictive value (combinations grouped in high—92%, medium—89%, and low—83%, 78%, 55% to account for uneven frequency of presentation, Table I) on activation. For the presentation period of the trial, no significant voxels were found related to either the interaction or to the individual effects of content (sun/rain) or predictive value. For the feedback period, estimating the effect of the within‐subject variable “predictive value” (high/medium/low) using an F test as well as the contrast (t test) high > low predictive value showed significant voxels in the right lateral cerebellum (Fig. 4, Table IIIa).
Figure 4.
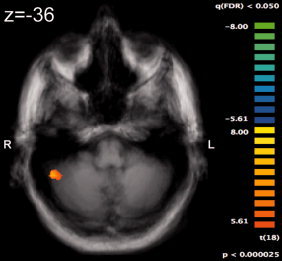
Brain activation during the feedback phase of the trial reflecting the contrast “card combinations with high (according to Table I) > low predictive value.” High value combinations recruited the lateral cerebellar hemisphere (threshold P < 0.05, FDR corrected).
Table III.
(a) Activation of cerebral regions observed in comparison of high predictive trials vs. low predictive trials in the feedback period. (b) Smiley‐related activation in the feedback period as compared to frowneys
| Region of interest | Brodmann | Side | x | Y | z | t | P |
|---|---|---|---|---|---|---|---|
| (a) | |||||||
| Cerebellar hemisphere | right | 47 | −47 | −36 | 6.97 | 0.000002 | |
| (b) | |||||||
| Primary motor cortex | 4 | left | −28 | −29 | 45 | 8.50 | 0.000014 |
| Primary motor cortex | 4 | left | −37 | −14 | 54 | 7.69 | 0.000030 |
| Posterior cingulate | 31 | left | −16 | −29 | 36 | 8.93 | 0.000009 |
| Nucleus accumbens | left | −7 | 10 | 0 | 8.37 | 0.000015 | |
| Somatosensory cortex | 3 | right | 44 | −20 | 51 | 8.68 | 0.000012 |
| Premotor cortex | 6 | right | 11 | −11 | 57 | 9.05 | 0.000008 |
| Premotor cortex | 6 | right | 8 | −26 | 57 | 6.95 | 0.000067 |
| Posterior cingulate | 23 | right | 11 | −35 | 27 | 9.70 | 0.000005 |
| Posterior cingulate | 31 | right | 23 | −20 | 33 | 7.74 | 0.000029 |
| Nucleus accumbens | right | 10 | 7 | −2 | 5.23 | 0.000585 | |
The forth random effects ANOVA model tested the effects of smiley versus frowney feedback during the feedback period of the trial. Smiley rewards were related to stronger activation in bilateral nucleus accumbens, bilateral posterior cingulum, left primary motor cortex, right postcentral gyrus, and right premotor cortex (Fig. 5, Table IIIb). No brain region was identified in which frowneys produced stronger activation than smileys.
Figure 5.
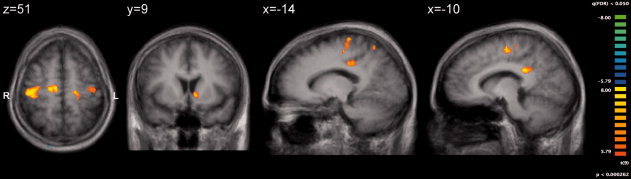
Brain activation during the feedback phase of the trial reflecting the contrast “smiley > frowney feedback.” Rewards (smileys) activated nucleus accumbens, sensorimotor and premotor cortices and cingulum more than negative (frowney) feedback (threshold P < 0.05, FDR corrected).
Including age group (dichotomous variable by median split according to age) into the smiley‐frowney model as a the between‐subject variable revealed significant voxels in left cingulate gyrus, medial frontal gyrus and putamen (Table IV) for the interaction young>old × smiley>frowney. Including age group as a between‐subject variable in the other statistical models did not reveal any significant results.
Table IV.
Activation in the brain for the interaction young>old x smiley>frowney
| Region of interest | Brodmann | Side | x | y | z | t | P |
|---|---|---|---|---|---|---|---|
| Posterior cingulate | 23 | left | −4 | −32 | 27 | 26.6 | 0.000871 |
| Anterior cingulate | left | −10 | 37 | −3 | 30.2 | 0.000580 | |
| Medial frontal gyrus | 32 | left | −16 | 13 | 42 | 37.2 | 0.000289 |
| Putamen | left | −13 | 7 | −6 | 25.7 | 0.000967 |
DISCUSSION
These results demonstrate that learning efficacy in a probabilistic classification task depends on predictive value of the cue, the content (sun/rain) to be learned and the feedback (smiley/frowney) provided. Content and feedback seem to be independent factors. Memory of card combinations was better for combinations of cards with higher predictive value, for those combinations that predicted sun instead of rain, and if smiley feedback was provided. Higher predictive values lead to stronger activation of the lateral cerebellar hemisphere. Smiley feedback was associated with stronger activation of Nucleus accumbens (NAcc), sensorimotor cortex, and cingulum.
Subjects remembered card–weather associations markedly better when positive instead of negative feedback was provided. [Wächter et al. 2009] reported a similar finding during implicit motor learning in healthy individuals. It may be that as individuals age negative feedback becomes more important as shown for avoidance learning in subjects with higher age (77 years) than our sample [Frank and Kong, 2008]. In fMRI smiley rewards were associated with stronger activation in bilateral NAcc (left>right; left dominance may be a consequence of right‐hand button presses, [Haruno et al. 2004]). NAcc activation is frequently observed during reward processing [Aron et al., 2006; Bischoff‐Grethe et al., 2009; Jensen et al., 2007; Linke et al., 2009; Poldrack et al., 2001; Seger and Cincotta, 2005; Ullsperger and von Cramon, 2003; Wachter et al., 2009]. During an over‐learned cue‐response task NAcc activation correlated with the amount of anticipated monetary reward [Knutson et al., 2001]. A metaanalysis of functional imaging studies on reward confirms bilateral activation of the NAcc and the posterior cingulum during positive versus negative feedback [Liu et al., 2010]. Also, the orbitofrontal cortex was reported to be overactive during presentation of reward versus punishment [Jensen et al., 2007; Liu et al., 2010] and reward prediction error [O'Doherty et al., 2003], but was not activated here. It is possible that this finding is related to the age of our subject sample; the frontal cortex is specifically vulnerable to age‐related metabolic dysfunction [Curiati et al., 2011] and structural atrophy [Raz et al., 2004]. In support of this interpretation frontal cortex activation was stronger in the younger half of our subjects.
We found stronger activation of sensorimotor cortex (primary motor, premotor, and somatosensory cortex) with smiley than frowney feedback which has not been reported by reward studies using explicit learning paradigms. Paradigm differences may explain this discrepancy. Most reward studies use tasks that are overlearned, involve minimal learning or are not designed to learn associations based on feedback [e.g., Elliott et al., 2003; Knutson et al., 2001]. Here, rewards were provided to learn associations between card combinations and outcomes. These associations were stochastic; hence, learning was largely implicit. Reward processing for implicit learning may involve the motor cortices like it does for motor skill learning [Wachter et al., 2009]. Whether this activation reflects a dopaminergic reward signal that is routed directly to motor cortex, remains speculative [Hosp et al., 2011; Molina‐Luna et al., 2009].
The posterior cingulate cortex (PCC) was an additional brain region activated by positive > negative feedback confirming previous reports [Liu et al., 2010]. In primates, PCC activity corresponds to decisions deviating from a standard, i.e., decision salience [Heilbronner et al., 2011]. It has been proposed that the PCC detected change relative to a standard (expected) signal in general [Pearson et al., 2011]. In our context, positive rewards may have had greater impact on changing behavior than negative ones.
As expected, learning efficacy correlated directly with the card combination's predictive value. As compared to low predictive value, high‐value trials over‐activated the right lateral cerebellum. During sensorimotor learning, the cerebellum encodes error signals. Additional activation is unrelated to error [Imamizu and Kawato, 2009] and has been suggested to reflect the generation of an internal model that serves as a cognitive framework for task‐related decisions [Ito, 2005]. One can speculate that cerebellar activation observed here for high > low predictive value trials reflects internal model formation for the classification task. High value trials are more informative than low‐value trials for the formation of such a model. If cerebellar activity would represent an error signal, activation should have been stronger for low‐value combinations, because for those subjects made more errors.
Combinations of cards predicting sun were better remembered than those predicting rain. Given everyday experience that positive events are better remembered than negative ones, this finding seems plausible in the context of explicit learning. That it transfers to implicit classification learning has—to our knowledge—not been reported before. That this finding is spurious and caused by receiving more smiley rewards for sun combinations was excluded by showing the smileys at the beginning of training were not more frequently presented for sun than rain trails. That this distribution became uneven later is expected because sun combinations were learned better and rewarded with more smileys. We also found statistically no interaction between the variables sun/rain and the number of smileys–frowney at the beginning of training in their effect on how well patterns were remembered. Nevertheless, was the behavioral difference between sun and rain trials small, which is probably why we did not observe a differential effect on brain activation.
The fMRI control task was chosen to subtract the activation related to visual presentation and motor response from performing the WPT. A comparison of the presentation phase of control and WPT trials showed stronger control‐related activation in bilateral frontal, parietal and cerebellar areas. This likely reflects the fact that more intense processing was required for counting cards than for implicitly assessing their predictive value and deciding in favor of sun or rain during WPT. Counting is known to be associated with activation of fronto‐temporal language areas, frontal and parietal cortices, and cerebellum [Ardila, 2010; Hinton et al., 2004; Kansaku et al., 2006; Sveljo et al., 2010]. During the feedback phase of a trial, findings were opposite in that the WPT was associated with stronger activation than control. This activation localized to the inferior frontal gyrus (Brodmann area 45). While this region is part of Broca's language area, it also is involved in risk assessment [d'Acremont et al., 2009]. Risk prediction error processing is likely more important during WPT than control.
A limitation of this study is that the individual valence of positive and negative stimuli was not assessed or controlled, neither for content nor feedback. Individuals may have found sun pleasant but rain rather neutral. This is a common criticism for many learning paradigms that focus on valence [Lang et al., 1990; Mneimne et al., 2010]. We used stimuli of small valence (smiley/frowney as feedback, imagined sun/rain as a response) to minimize potential differences in salience thereby hoping to reduce this confound. Although we think the effect is small, we cannot rule out a possible influence. A difference to prior fMRI studies using the WPT is that our control task was not interleaved with WPT trials. We chose this design to render the WPT data comparable to WPT training outside the scanner performed by subjects that did not qualify or opted against MR scanning. To minimize sequencing effects the control condition was performed before and after WPT training and for analysis the data of the two control periods were combined. A limitation to acknowledge is the small size of the fMRI sample. Nevertheless, random effects models that offer generalizability, yielded significant results. A limitation is also the advanced age of our subject sample. Subjects were collected as an age‐matched control group for a comparison with individuals after a stroke to be reported elsewhere. Further studies are warranted to investigate the effects of feedback, content, and predictive value in young healthy subjects.
In conclusion, our data show that pleasant content and feedback improve implicit classification learning. Positive feedback is associated with stronger activation of NAcc, sensorimotor cortex, and posterior cingulum as compared with negative feedback. Learning also depends on the predictive value of the visual cues which is in part processed within the lateral cerebellum possibly reflecting the formation of an internal model.
REFERENCES
- Ali N, Cimino CR ( 1997): Hemispheric lateralization of perception and memory for emotional verbal stimuli in normal individuals. Neuropsychology 11: 114–125. [DOI] [PubMed] [Google Scholar]
- Ardila A ( 2010): On the evolution of calculation abilities. Front Evol Neurosci 2: 7. doi: 10.3389/fnevo.2010.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Gluck MA, Poldrack RA ( 2006): Long‐term test‐retest reliability of functional MRI in a classification learning task. Neuroimage 29: 1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff‐Grethe A, Hazeltine E, Bergren L, Ivry RB, Grafton ST ( 2009): The influence of feedback valence in associative learning. Neuroimage 44: 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL ( 1996): Modulation of memory storage. Curr Opin Neurobiol 6: 237–242. [DOI] [PubMed] [Google Scholar]
- Curiati PK, Tamashiro‐Duran JH, Duran FL, Buchpiguel CA, Squarzoni P, Romano DC, Vallada H, Menezes PR, Scazufca M, Busatto GF, Alves TC ( 2011): Age‐related metabolic profiles in cognitively healthy elders: Results from a voxel‐based [18F]fluorodeoxyglucose‐positron‐emission tomography study with partial volume effects correction. AJNR Am J Neuroradiol 32: 560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Acremont M, Lu ZL, Li X, Van der Linden M, Bechara A ( 2009): Neural correlates of risk prediction error during reinforcement learning in humans. Neuroimage 47: 1929–1939. [DOI] [PubMed] [Google Scholar]
- Elliott R, Newman JL, Longe OA, Deakin JF ( 2003): Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: A parametric functional magnetic resonance imaging study. J Neurosci 23: 303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Kong L ( 2008): Learning to avoid in older age. Psychol Aging 23: 392–398. [DOI] [PubMed] [Google Scholar]
- Haruno M, Kuroda T, Doya K, Toyama K, Kimura M, Samejima K, Imamizu H, Kawato M ( 2004): A neural correlate of reward‐based behavioral learning in caudate nucleus: A functional magnetic resonance imaging study of a stochastic decision task. J Neurosci 24: 1660–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner SR, Hayden BY, Platt ML ( 2011): Decision salience signals in posterior cingulate cortex. Front Neurosci 5: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton SC, Harrington DL, Binder JR, Durgerian S, Rao SM ( 2004): Neural systems supporting timing and chronometric counting: An FMRI study. Brain Res Cogn Brain Res 21: 183–192. [DOI] [PubMed] [Google Scholar]
- Hosp JA, Pekanovic A, Rioult‐Pedotti MS, Luft AR ( 2011): Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J Neurosci 31: 2481–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamizu H, Kawato M ( 2009): Brain mechanisms for predictive control by switching internal models: Implications for higher‐order cognitive functions. Psychol Res 73: 527–544. [DOI] [PubMed] [Google Scholar]
- Ito M ( 2005): Bases and implications of learning in the cerebellum—Adaptive control and internal model mechanism. Prog Brain Res 148: 95–109. [DOI] [PubMed] [Google Scholar]
- Jensen J, Smith AJ, Willeit M, Crawley AP, Mikulis DJ, Vitcu I, Kapur S ( 2007): Separate brain regions code for salience vs. valence during reward prediction in humans. Hum Brain Mapp 28: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansaku K, Johnson A, Grillon ML, Garraux G, Sadato N, Hallett M ( 2006): Neural correlates of counting of sequential sensory and motor events in the human brain. Neuroimage 31: 649–660. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR ( 1996): A neostriatal habit learning system in humans. Science 273: 1399–1402. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D ( 2001): Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21: RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN ( 1990): Emotion, attention, and the startle reflex. Psychol Rev 97: 377–395. [PubMed] [Google Scholar]
- Linke J, Kirsch P, King AV, Gass A, Hennerici MG, Bongers A, Wessa M ( 2010): Motivational orientation modulates the neural response to reward. Neuroimage 49: 2618–2625. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J ( 2011): Common and distinct networks underlying reward valence and processing stages: A meta‐analysis of functional neuroimaging studies. Neurosci Biobehav Rev 35: 1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnema MT, Knowlton BJ ( 2008): Directed forgetting of emotional words. Emotion 8: 643–652. [DOI] [PubMed] [Google Scholar]
- Mneimne M, Powers AS, Walton KE, Kosson DS, Fonda S, Simonetti J ( 2010): Emotional valence and arousal effects on memory and hemispheric asymmetries. Brain Cogn 74: 10–17. [DOI] [PubMed] [Google Scholar]
- Molina‐Luna K, Pekanovic A, Rohrich S, Hertler B, Schubring‐Giese M, Rioult‐Pedotti MS, Luft AR ( 2009): Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS One 4: e7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ ( 2003): Temporal difference models and reward‐related learning in the human brain. Neuron 38: 329–337. [DOI] [PubMed] [Google Scholar]
- Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML ( 2011): Posterior cingulate cortex: Adapting behavior to a changing world. Trends Cogn Sci 15: 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Pare‐Blagoev EJ, Shohamy D, Creso Moyano J, Myers C, Gluck MA ( 2001): Interactive memory systems in the human brain. Nature 414: 546–550. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning‐Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD ( 2004): Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiol Aging 25: 377–396. [DOI] [PubMed] [Google Scholar]
- Seger CA, Cincotta CM ( 2005): The roles of the caudate nucleus in human classification learning. J Neurosci 25: 2941–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveljo OB, Koprivsek KM, Lucic MA, Prvulovic MB, Culic M ( 2010): Gender differences in brain areas involved in silent counting by means of fMRI. Nonlinear Biomed Phys 4( Suppl 1): S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY ( 2003): Error monitoring using external feedback: Specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J Neurosci 23: 4308–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter T, Lungu OV, Liu T, Willingham DT, Ashe J ( 2009): Differential effect of reward and punishment on procedural learning. J Neurosci 29: 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]


