Abstract
Training one hand on a motor task results in performance improvements in the other hand, also when stimuli are randomly presented (nonspecific transfer). Corpus callosum (CC) is the main structure involved in interhemispheric information transfer; CC pathology occurs in patients with multiple sclerosis (PwMS) and is related to altered performance of tasks requiring interhemispheric transfer of sensorimotor information. To investigate the role of CC in nonspecific transfer during a pure motor reaction‐time task, we combined motor behavior with diffusion tensor imaging analysis in PwMS. Twenty‐two PwMS and 10 controls, all right‐handed, were asked to respond to random stimuli with appropriate finger opposition movements with the right (learning) and then the left (transfer) hand. PwMS were able to improve motor performance reducing response times with practice with a trend similar to controls and preserved the ability to transfer the acquired motor information from the learning to the transfer hand. A higher variability in the transfer process, indicated by a significantly larger standard deviation of mean nonspecific transfer, was found in the PwMS group with respect to the control group, suggesting the presence of subtle impairments in interhemispheric communication in some patients. Then, we correlated the amount of nonspecific transfer with mean fractional anisotropy (FA) values, indicative of microstructural damage, obtained in five CC subregions identified on PwMS's FA maps. A significant correlation was found only in the subregion including posterior midbody (Pearson's r = 0.74, P = 0.003), which thus seems to be essential for the interhemispheric transfer of information related to pure sensorimotor tasks. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: corpus callosum, diffusion tensor MRI, finger opposition movement, intermanual transfer, multiple sclerosis, reaction time
INTRODUCTION
The corpus callosum (CC) is the major white matter fiber bundle connecting brain areas of the two hemispheres. Several studies investigated its role in different experimental paradigms, demonstrating that CC is crucial for interhemispheric information transfer [Glickstein and Berlucchi, 2008; Wahl and Ziemann, 2008].
In serial reaction‐time tasks, subjects are asked to respond to visual stimuli by key‐presses on a keyboard, and performance improvements with practice are measured as decrease in response time (RT) [Nissen and Bullemer, 1987]. The procedural knowledge acquired with these tasks in one hand can be transferred to the other hand by a process called intermanual transfer, resulting in a significant motor performance improvement in the transfer hand [Japikse et al., 2003; Parlow and Kinsbourne, 1989]. Intermanual transfer can be related to both sequence‐specific (when stimuli are presented in a predetermined repeating sequence) and nonspecific learning (when stimuli are randomly presented) [Willingham, 1999].
Functional connectivity between the primary motor cortex (M1) hand areas of the two hemispheres can be measured by transcallosal interhemispheric inhibition (IHI) [Di Lazzaro et al., 1999; Ferbert et al., 1992; Gerloff et al., 1998]. IHI from the learning to the transfer M1 has been found to correlate with nonspecific but not with sequence‐specific performance improvement in the transfer hand, suggesting that transcallosal interactions between M1 areas may contribute to nonspecific transfer, optimizing the timing of visuomotor processing [Perez et al., 2007]. Also, IHI between the M1 hand areas was correlated with fractional anisotropy (FA) of the hand callosal motor fibers, showing that functional connectivity is strictly related to microstructural integrity in CC [Wahl et al., 2007].
In patients with multiple sclerosis (PwMS), the duration of transcallosal inhibition was negatively correlated with activation in the ipsilateral M1 during hand movement [Lenzi et al., 2007]. Further, it has been widely demonstrated that PwMS show microstructural damage in CC also in the normal‐appearing white matter outside plaques as revealed by nonconventional magnetic resonance imaging (MRI) techniques [Bonzano et al., 2008; Coombs et al., 2004; Hasan et al., 2005].
In a previous work on PwMS, we found that an impairment in bimanual coordination correlated with low FA in anterior CC, while we did not find a significant involvement of the body of CC [Bonzano et al., 2008], suggesting that complex bimanual motor tasks require the contribution of prefrontal cortices which are involved in higher‐order processing of motor control and planning.
Here, we hypothesized that an interhemispheric transfer of a pure motor task, as a nonspecific motor learning task, could reveal a major role of CC body. To this aim, we implemented a motor reaction‐time (mRT) task based on thumb‐to‐finger opposition movements in response to random visual stimuli, and explored motor performance in PwMS with minimal disability. We asked whether PwMS could improve performance with the dominant hand and, if so, whether they showed nonspecific transfer. Then, we investigated the contribution of CC in intermanual transfer by correlating the amount of nonspecific transfer with the degree of damage measured by FA in different areas of CC.
MATERIALS AND METHODS
Subjects
Twenty‐two patients affected by relapsing remitting multiple sclerosis (MS) were included in this study (8 males and 14 females; mean age: 39.8 ± 4.5 years; mean disease duration: 8.9 ± 4.5 years; mean EDSS [Kurtzke, 1983]: 1.1 ± 0.5). They showed MRI distribution patterns typical for MS with multiple, asymmetrical, white matter lesions located in the infratentorial and sopratentorial white matter, in periventricular and in iuxtacortical location (mean lesion load: 1330.7 ± 696.7 mm3).
All patients were in a stable phase of the disease, without relapses in the last 3 months, without visual deficits and with an EDSS score lower than or equal to 2, which indicates minimal disability in only one functional system. At neurological examination, the majority of these patients had no clinically detected pyramidal or sensory sign; only six patients showed pyramidal signs, one patient showed tactile hypoesthesia on the palm of his left hand. We also recruited 10 gender‐ and age‐matched control subjects for comparison.
All the subjects included in this study were right‐handed according to a modified Italian‐translated Edinburgh Handedness Inventory [Oldfield, 1971] and naïve to the specific purpose of this study. Informed consent was obtained according to our institution policy and to the Declaration of Helsinki.
Behavioral Data
Subjects were instructed to respond as fast and accurately as possible to the presentation on a computer screen of a random sequence of red squares displayed on the fingertip of the index, middle, ring, and little finger of a pictured palm hand, by tapping the appropriate finger with the thumb. Subjects were seated facing the computer screen and wore a sensor‐engineered glove (patent number: TO2005A00368, 31/05/2005) on both hands [Bove et al., 2007]. Data were acquired at 1 KHz (USB‐1208FS; Measurement Computing); custom‐made software generated visual stimuli and recorded the time of each finger touch. If the subject was able to touch the correct finger, the corresponding square became blue, as a positive feedback to the subject. This feedback was not used for further analysis, but was used as reward motivating the subject to improve the performance. The session included 2‐min blocks: four with the right (R1–R4) and then two with the left (L1‐L2) hand, as shown in Figure 1. R1 and L1 were used to familiarize the subjects with the task, R2 and L2 measured the initial performance, with the right and the left hand, respectively. Stimuli lasted 500 ms and were presented at a frequency of 1 Hz, which was found to be comfortable for the patients involved in this study. The number of stimuli per block was 120 for both groups. The time intervals between blocks were as follows: 10–30 s between blocks performed with the same hand, 1–2 min between right‐ and left‐hand blocks.
Figure 1.
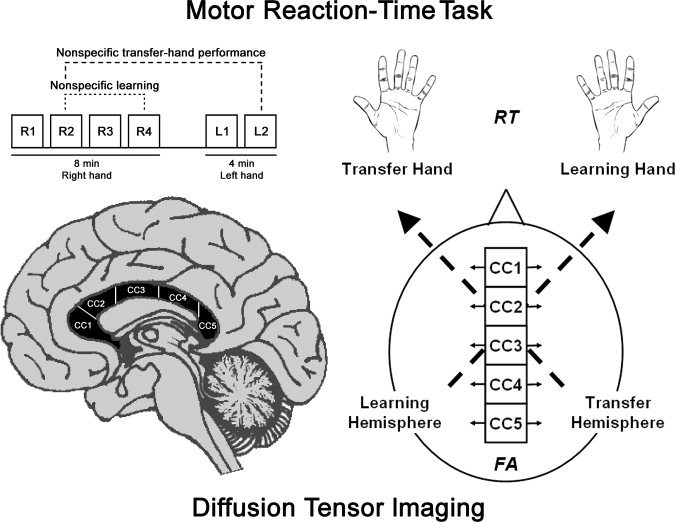
Experimental design including mRT Task and diffusion tensor imaging. The temporal order of random blocks of finger opposition movements performed with the right and then left hand is shown. For the right hand, nonspecific learning was calculated as the difference in RT between the random blocks R2 and R4 (dotted lines). The amount of nonspecific transfer from the right to the left hand was calculated as the difference in RT between the random blocks R2 and L2 (dashed lines). The subdivision of CC into five subregions (CC1–CC5) is displayed on the pictured brains. RT (response time) and FA (fractional anisotropy) indicate respectively the measures of motor performance and callosal damage in the five callosal subregions connecting the two hemispheres. Right hand is the “Learning Hand” and left hand is the “Transfer Hand”, controlled respectively by the learning and the transfer hemispheres.
For each subject, the mean RT (i.e., the time interval between stimulus presentation and the onset of the corresponding touch) and the total number of errors (i.e., wrong or absent responses) were calculated for each block. Error trials were discarded from analysis. Nonspecific learning, indicating right‐hand improvement, was calculated as the difference in RT between R2 and R4 (Delta_Right) and the amount of transfer as the difference in RT between R2 and L2 (Delta_Transfer). Subjects with positive mean value of Delta_Right were considered as showing right‐hand improvement; similarly, subjects with positive mean value of Delta_Transfer were considered as showing nonspecific transfer of learning. On the basis of these definitions, Delta_Right and Delta_Transfer had significance only if assuming positive values: negative values indicated that the process of learning and transfer, respectively, did not occur.
Magnetic Resonance Imaging
Patients underwent brain MR imaging examination on a 1.5‐Tesla MR system (Signa Excite General Electric, WI), including axial dual‐echo proton density/T2‐weighted imaging (slice thickness: 3 mm; TR: 3,000 ms; TE: 16.1/96.8 ms; flip angle: 90°; FOV: 250 mm; matrix: 256 × 256) and single‐shot spin‐echo echo‐planar diffusion tensor imaging (DTI) (slice thickness: 2 mm; TR: 16,000 ms; TE: 105 ms; flip angle: 90°; FOV: 240 mm; matrix: 256 × 256), with diffusion gradients applied in 15 noncollinear directions (b = 1,000 s/mm2) and two baseline acquisitions without diffusion gradients.
Hyperintense lesions on proton density/T2‐weighted images were outlined for each patient, and the total volume of lesions (“lesion load”) was calculated using commercially available software (Analyze 6.0, Mayo Clinic).
DTI data were processed by using FDT (FMRIB's Diffusion Toolbox), a software tool for analysis of diffusion‐weighted images, part of FSL (FMRIB's Software Library) [Smith et al., 2004]. After correction for eddy current distortions and motion artifacts, a diffusion tensor model was fitted at each voxel, then FA parametric maps were obtained to investigate microstructural damage in CC.
Correlations Between Behavioral Data and DTI
Analogously to a previous work [Bonzano et al., 2008], five regions of interest (ROI) were delineated along the CC, on the basis of a geometrical scheme [de Lacoste et al., 1985], on the midsagittal FA map in the native space for each patient; voxels at the edge were excluded to minimize partial volume effects.
To investigate the role of CC in intermanual transfer, mean FA values within the selected ROIs were calculated and correlated with the amount of nonspecific transfer (Fig. 1).
In this correlation analysis, we included only patients showing Delta_Right > 0 AND Delta_Transfer > 0. In fact, we first had to eliminate patients who did not reduce their RT with the right hand because they were not able to learn with the right hand and they could not transfer new skills to the left hand (by definition, these were the patients with Delta_Right < 0). Second, we had to select those patients showing a transfer process to study a possible role of CC in this process (Delta_Transfer > 0).
Statistics
A two‐way ANOVA for repeated measures (RM‐ANOVA) was performed on the parameter RT using the factors GROUP (PwMS and control subjects) as between subjects factor and BLOCK (R2, R3, and R4) as within subject factor. RM‐ANOVA was then conducted for the two groups separately using the factor BLOCK (R2, R3, and R4) as within subject factor. Significant main effects and interactions were further explored with Newman–Keuls post‐hoc test. The same analysis was conducted for the analysis of the number of errors.
To assess differences in the parameters describing performance improvement and transfer to the nondominant hand, Student's t‐test for independent samples was adopted for between‐group comparison of Delta_Right and Delta_Transfer. Levene's test was used to evaluate the variance homogeneity of these two variables between the two groups. Then, Mann–Whitney U‐test was performed in case of inhomogeneity of variance. Student's t‐test for dependent samples was conducted separately for the two groups to assess differences between mean RT in block R2 and L2.
Pearson's correlation coefficients were calculated for PwMS between the amount of nonspecific transfer and (i) the FA values in the whole CC and in each CC ROI, (ii) lesion load. Bonferroni's correction for multiple nonindependent comparisons was applied.
Data in the text are reported as mean ± SD
RESULTS
Right (Learning Hand)
Figure 2 shows the mean RT of the two groups in the different blocks following temporal order.
Figure 2.
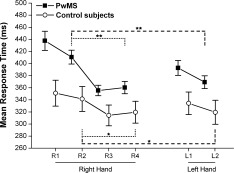
PwMS and control subjects performing the RT task with the right and left hand: mean response time. The abscissa shows blocks and performing hand in temporal order; variance is expressed as SE. Nonspecific learning is shown in dotted lines, nonspecific transfer‐hand performance is shown in dashed lines; *P < 0.05, **P < 0.001.
RM‐ANOVA conducted on the two groups together revealed a significant effect of GROUP on RT (RT averaged on the three learning blocks was 375.48 ± 53.19 ms in the PwMS group vs. 325.10 ± 58.24 ms in the control group (F(1,30) = 7.98, P = 0.008), indicating that, on average, PwMS showed longer RT than control subjects. Also the effect of BLOCK (R2, R3, and R4) on RT was statistically significant (RT averaged on the two groups shortened with blocks: R2 = 389.07 ± 65.11 ms, R3 = 342.57 ± 48.56 ms, R4 = 347.57 ± 53.40 ms (F(2,60) = 20.79, P < 0.001)); however, the interaction GROUP‐BLOCK was not statistically significant (F(2,60) = 2.73, P = 0.073), indicating a similar trend in the reduction of mean RT with blocks (R2, R3, and R4) in the two groups.
RM‐ANOVA separated for the two groups revealed again a significant effect of BLOCK in both the PwMS (F(2,42) = 24.19, P < 0.001) and the control group (F(2,18) = 8.59, P = 0.002).
Post‐hoc analysis showed that in both groups RT was significantly shorter in R3 and R4 with respect to R2 (PwMS: R3 vs. R2: P < 0.001 and R4 vs. R2: P < 0.001; controls: R3 vs. R2: P = 0.003 and R4 vs. R2: P = 0.006). These findings indicate a nonspecific performance improvement in both groups.
Delta_Right was higher in the PwMS group related to the control group, but Levene's test showed no variance homogeneity of Delta_Right between the two groups (50.40 ± 49.36 vs. 21.92 ± 23.11 ms; F(1,30) = 4.54, P = 0.04) because the PwMS group showed larger variance. Then, a nonparametric test revealed no significant difference in Delta_Right between the two groups (Mann–Whitney U‐test: U = 67, P = 0.08) suggesting that patients improved their performance with the right hand as the control subjects. Nineteen of 22 patients and 9 of 10 control subjects showed a nonspecific performance improvement (Delta_Right > 0). The three patients who did not improve their learning‐hand motor performance did not show significantly different clinical features with respect to the PwMS group. In fact, EDSS ranged between 1 and 2 and lesion load between 965.5 mm3 (minor than the mean value 1330.7 mm3) and 3024.6 mm3 (maximum value in the group). One of these three patients showed pyramidal signs at neurological examination; on the other hand, five of the six patients showing pyramidal signs were able to improve performance with the right hand.
RM‐ANOVA did not show significant effect of GROUP nor BLOCK on the mean total number of errors, summed across trials for each block for each subject.
Mean response time and total number of errors averaged on each group is reported for each right hand block (R1–R4) in Table I.
Table I.
Mean response time and mean total number of errors for the right and left blocks averaged on each group
| Mean response time (ms) | Mean total number of errors | |||
|---|---|---|---|---|
| Controls | PwMS | Controls | PwMS | |
| R1 | 351.06 ± 67.52 | 437.69 ± 73.92 | 12.10 ± 7.62 | 19.32 ± 13.13 |
| R2 | 341.48 ± 65.08 | 410.70 ± 53.62 | 11.70 ± 8.76 | 13.41 ± 7.91 |
| R3 | 314.25 ± 54.42 | 355.45 ± 40.69 | 11.50 ± 7.85 | 15.50 ± 10.30 |
| R4 | 319.56 ± 57.20 | 360.30 ± 47.59 | 13.70 ± 12.39 | 16.09 ± 12.04 |
| L1 | 334.30 ± 58.96 | 392.78 ± 57.52 | 23.90 ± 15.44 | 21.32 ± 12.23 |
| L2 | 319.42 ± 62.81 | 369.18 ± 49.81 | 19.60 ± 16.29 | 19.77 ± 10.27 |
Data are reported as mean ± standard deviation.
Left (Transfer Hand)
Figure 2 shows the mean RT in the PwMS and in the control group for each block. In both PwMS and controls, a statistically significant difference in RT was found in block R2 with respect to L2 (PwMS group: L2 = 369.18 ± 49.81 vs. R2 = 410.70 ± 53.62 ms; df = 21, t = 3.62, P = 0.002) and control group: L2 = 319.42 ± 62.81 vs. R2 = 341.48 ± 65.08 ms; df = 9, t = 3.75, P = 0.004), indicating a significant nonspecific transfer‐hand performance improvement in both groups.
Fourteen of the 19 patients and 8 of the 9 control subjects showing learning with the right hand had Delta_Transfer > 0. The five patients who showed pyramidal signs nevertheless showing nonspecific learning were able to transfer the acquired nonspecific motor information to the left hand. The patient presenting with tactile hypoesthesia on the palm of his left hand did not show nonspecific transfer. Delta_Transfer was higher in the PwMS group related to the control group, but Levene's test showed no variance homogeneity of Delta_Transfer between the two groups (65.87 ± 51.23 vs. 30.07 ± 8.84 ms; F(1,20) = 9.10, P = 0.007), due to larger variability among patients. Then, a nonparametric test revealed no significant difference in Delta_Transfer between the two groups (Mann–Whitney U‐test: U = 31, P = 0.09), suggesting that PwMS showed a nonspecific improvement of the skill to the transfer hand similarly to the control subjects.
Mean response time and mean total number of errors in the left hand averaged on each group is reported for each left hand block (L1 and L2) in Table I.
MRI Data and Amount of Nonspecific Transfer in PwMS
Behavioral data showed that PwMS were able to learn and transfer the finger motor skill defined in the proposed mRT protocol. However, patients showed large intra‐group variability of Delta_Transfer. Therefore, we asked whether this finding could be related to a different ability among patients to transfer information about motor performance through callosal fibers. Thus, we assessed if different values of Delta_Transfer among patients could correlate with different CC integrity as measured by FA.
Fourteen of 22 patients improved performance with the right hand and transferred the acquired motor skill to the left hand; these patients were included in the correlation analysis. The mean FA values in the CC ROIs of these patients are reported in Table II.
Table II.
Mean fractional anisotropy in the different ROIs (CC1–CC5) and in the whole corpus callosum and total lesion load for the patients with multiple sclerosis included in the correlation analysis
| Patient | CC1 | CC2 | CC3 | CC4 | CC5 | Whole CC | Lesion load (mm3) |
|---|---|---|---|---|---|---|---|
| 1 | 0.77 | 0.66 | 0.68 | 0.71 | 0.68 | 0.70 | 2467.83 |
| 2 | 0.65 | 0.69 | 0.65 | 0.78 | 0.83 | 0.72 | 815.67 |
| 3 | 0.74 | 0.64 | 0.77 | 0.89 | 0.85 | 0.78 | 1202.70 |
| 4 | 0.65 | 0.52 | 0.83 | 0.55 | 0.73 | 0.66 | 1963.96 |
| 5 | 0.69 | 0.66 | 0.58 | 0.63 | 0.80 | 0.67 | 1635.51 |
| 6 | 0.72 | 0.62 | 0.58 | 0.59 | 0.80 | 0.66 | 2164.03 |
| 7 | 0.63 | 0.60 | 0.66 | 0.71 | 0.82 | 0.68 | 836.48 |
| 8 | 0.66 | 0.55 | 0.53 | 0.48 | 0.77 | 0.60 | 1340.04 |
| 9 | 0.73 | 0.65 | 0.69 | 0.72 | 0.78 | 0.71 | 2193.95 |
| 10 | 0.75 | 0.59 | 0.63 | 0.69 | 0.82 | 0.70 | 295.47 |
| 11 | 0.70 | 0.59 | 0.57 | 0.60 | 0.84 | 0.66 | 291.31 |
| 12 | 0.69 | 0.69 | 0.68 | 0.71 | 0.74 | 0.70 | 1127.79 |
| 13 | 0.58 | 0.61 | 0.62 | 0.68 | 0.75 | 0.65 | 895.30 |
| 14 | 0.76 | 0.66 | 0.75 | 0.70 | 0.71 | 0.72 | 1003.20 |
| Mean | 0.69 | 0.62 | 0.66 | 0.67 | 0.78 | 0.69 | 1302.37 |
| SD | 0.05 | 0.05 | 0.08 | 0.10 | 0.05 | 0.04 | 692.34 |
A significant linear correlation between Delta_Transfer and FA was found only in CC3 (r = 0.74, P = 0.003), as shown in Figure 3C. This correlation survived Bonferroni's correction for multiple nonindependent comparisons (statistical threshold: P = 0.01). No correlation was found between Delta_Transfer and FA in the other CC ROIs and in the whole CC (Fig. 3A–F).
Figure 3.
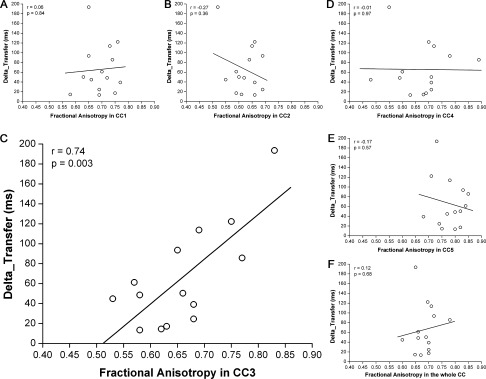
Linear correlation between the amount of nonspecific transfer (Delta_Transfer) and fractional anisotropy in (A) CC1, (B) CC2, (C) CC3, (D) CC4, (E) CC5, and (F) whole CC. Solid line represents the linear fitting. Pearson's correlation coefficient r and P value are reported; as shown, Delta_Transfer correlated with FA only in CC3.
White matter fibers obtained by probabilistic tractography using CC3 as seed mask are displayed in Figure 4. As a result, CC3 (Fig. 4A) revealed white matter connections with the precentral gyri, thus including M1 hand areas, just anterior to the central sulci, and with the superior frontal gyri (axial plane, Fig. 4D). On the sagittal plane, the connection to the medial frontal surface, above the cingulate sulcus and anterior to the pars marginalis and the central sulcus is visualized (Fig. 4B). On the coronal plane, the passage of white matter fibers through the internal capsules can be noticed (Fig. 4C).
Figure 4.
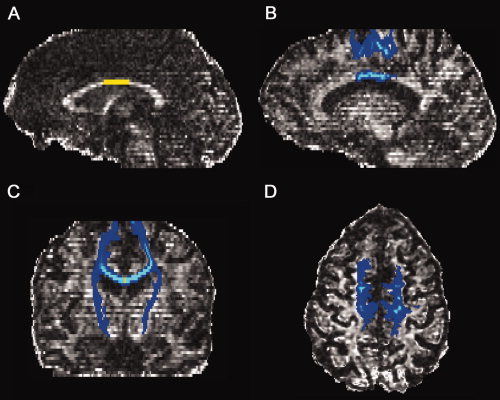
Cortical projections of callosal fibers originating from CC3 obtained by probabilistic tractography (yellow: CC3; blue and light blue: traced fibers), represented on the fractional anisotropy map of a representative patient. (A) the selected callosal ROI, CC3; (B–D) obtained tracts displayed respectively on sagittal, coronal, and axial plane.
No correlation was found between lesion load and the amount of transfer (P = 0.37).
We also analyzed the FA of the patients showing Delta_Transfer < 0, and we observed that the FA values in CC3 varied in a wide range from 0.56 to 0.77. This finding could indicate that in these patients the white matter integrity of CC3 was not the only problem in nonspecific intermanual transfer. On the other hand, it should be noted that also a control subject had a Delta_Transfer < 0.
DISCUSSION
We studied the motor performance of a group of PwMS with minimal disability (EDSS ≤ 2) in a pure mRT task based on blocks of randomly presented visual stimuli (nonspecific learning). We found that, although PwMS showed on average longer RT in the learning phase with respect to control subjects, with motor training they were able to reduce their RT with a similar trend to that observed in control subjects. In general, nonspecific performance improvements probably reflect learning to optimize the procedure for selecting the correct finger after stimulus presentation and, together, the general skill necessary to appropriately execute the finger opposition movement [Perez et al., 2007; Willingham, 1999].
Therefore, this finding might indicate that these patients, tested in the early phase of learning, did not show significant abnormalities in higher‐order visuomotor integration processes, known to be driven by cortico‐striatal and cortico‐cerebellar networks [Doyon and Benali, 2005]. The intact learning capability shown by the PwMS involved in this study could be explained by the presence of only subtle functional impairments in these neural structures that, with a simple motor training, could be reduced or completely abolished. Also, we can propose that cortico‐striatal and cortico‐cerebellar networks could be able, by their interaction, to compensate each other, as cortico‐cerebellar networks are suggested to be capable of compensating an impairment of the nigro‐striatal pathway in early Parkinson's disease during a trial‐and‐error sequence learning task [Mentis et al., 2003].
An indication of the presence of subtle learning impairments in these patients is the significantly larger variability in right‐hand motor performance improvement at the end of the learning phase (Delta_Right) detected in the PwMS group with respect to the control group. Indeed, although the majority of patients improved with training, the progress level was highly variable among them indicating some possible minor fails in the functioning of the neural circuits involved in the motor learning processes. In a recent work, it has been shown that PwMS with no clinical disability (EDSS lower than 1), although showing an impairment in reaching task execution, had an intact force field adaptation in a reaching task with robot‐generated forces [Casadio et al., 2008]. However, not always PwMS have an adaptation capability similar to controls. Indeed, it has been shown that PwMS with an EDSS score around 5 had a significantly lower ability of adaptation than control subjects, especially for task features requiring a complex integration of sensory information [Leocani et al., 2007]. On the other hand, in another work, a group of PwMS with minimal disability (median EDSS lower than 2.5) exhibited short‐term adaptation to a simple finger motor task similar to normal subjects [Mancini et al., 2009]. All these findings could indicate that motor adaptation is a physiological process which is preserved in PwMS with low disability. Therefore, we cannot completely exclude alterations in motor adaptation in our group of patients but we are quite confident that in PwMS with minimal disability, if present, the impairment in motor adaptation is subtle.
Also, we cannot exclude the role of cerebellum in inducing longer RT values in PwMS because damage to the cerebellum or the cerebellar peduncles is very common in multiple sclerosis [Anderson et al., 2009]. However, it has been shown that in a pure mRT task without sequence presentation the role of cerebellum is drastically reduced, in fact patients with cerebellar lesions are able to easily manage a random mRT task showing response times comparable with normal subjects [Molinari et al., 1997]. Some impairments could likely refer to a reduction in the attention capabilities that can occur in PwMS early in the course of the disease [Dujardin et al., 1998]. Moreover, white matter, which can be diffusely damaged also in the normal‐appearing tissue in earliest MS stage, is composed of myelinated fibers that are crucial for the rapid transfer of information between distant brain regions and specifically for novel working memory and complex attentional tasks [Sanfilipo et al., 2006; Santa Maria et al., 2004]. We could speculate that the important reduction of RT towards values similar to the control group occurring at the end of the right‐hand learning phase might indicate the use of compensatory neural circuits located at the corticostriatal regions as those adopted to cope with a decline of the efficiency of visuomotor processing by older healthy subjects, in which a reduction of white matter integrity has been demonstrated [Madden et al., 2004].
We also investigated the possibility that in PwMS nonspecific learning could be transferred from the learning hand to the other hand (transfer hand) as occurs in normal subjects [Perez et al., 2007]. The behavioral protocol we proposed included four blocks with the right hand, and the motor performance improvement was calculated as the difference in RT between R2 and R4. Conversely, only two blocks were asked to be performed with the left hand; thus, a nonspecific learning process could not be completely elicited for the left hand as for the right hand. Following this approach, if RT in L2 was shorter than in R2 in a right‐handed subject, it could indicate that the subject was able to take advantage of the transfer of the information acquired when practicing with the right hand. The testing of the left hand after the complete experimental session performed with the right hand could give an explanation of why the mean response time for L1 was markedly reduced in PwMS when compared with R1. This was not evident in control subjects maybe because their initial response time values with the learning hand were close to their floor, thus providing a smaller range for transfer‐hand performance improvement.
Interestingly, our results showed that our group of PwMS preserved the ability to transfer the acquired motor skills from the learning hand to the transfer hand. In fact, we found that the mean value of Delta_Transfer, indicating the amount of nonspecific transfer, was similar between PwMS and control subjects. Similarly to Delta_Right, also Delta_Transfer showed higher variability in the PwMS group with respect to the control group suggesting possible subtle impairments in the communication between the cortical areas of the two hemispheres in some patients.
CC is the main structure involved in interhemispheric transfer of information, and intermanual transfer has been found to be impaired in split‐brain and acallosal patients, showing evidence of a role of CC in intermanual transfer [de Guise et al., 1999; Lassonde et al., 1995]. Microscopic fiber tract injury of CC has been demonstrated by water diffusion changes in PwMS since the early phase of disease [Ge et al., 2004], and CC abnormalities in PwMS have been related to decreased sensorimotor performance, deficit in bimanual coordination and cognitive dysfunction [Bonzano et al., 2008; Larson et al., 2002; Mesaros et al., 2009; Pelletier et al., 1993; Schnider et al., 1993]. In recent studies, decreased movement‐associated blood oxygenation level‐dependent (BOLD) signal deactivation in the ipsilateral sensorimotor cortex has been found in PwMS [Lenzi et al., 2007; Manson et al., 2006, 2008] and the duration of transcallosal inhibition, measured by a paired‐pulse transcranial magnetic stimulation (TMS) protocol, was correlated with the activation in the ipsilateral M1 [Lenzi et al., 2007]. Therefore, we could assume that an increase of relative activation in the ipsilateral sensorimotor cortex could affect the interhemispheric integration of information in the intermanual transfer process.
IHI has been found to correlate with the ability to intermanually transfer information in a pure mRT task (nonspecific transfer) [Perez et al., 2007] and with the microstructural integrity of motor callosal fibers [Wahl et al., 2007] in healthy subjects. These findings, together with previous ones in patients with circumscript surgical lesions within different CC areas [Meyer et al., 1998], drew the common conclusion that sensorimotor information transfer between the two hemispheres is mainly regulated by the body of CC. However, these studies could not demonstrate the possible involvement of other regions of CC. In fact, these studies were based on TMS, that measures the functional connectivity between the M1 areas of the two hemispheres, or on the investigation of the integrity of only the body of CC.
In this work, to investigate the function of CC in nonspecific intermanual transfer, we adopted an mRT task and studied the relationship between the transfer of motor information and the integrity of different portions of CC, correlating the amount of nonspecific transfer with the gradation of microstructural damage in the subgroup of PwMS that provided evidence of nonspecific learning and transfer. Indeed, our intent was to investigate the possibility of a relationship between the degree of CC structural integrity and the amount of transfer, which can be estimated only when patients learned with the right hand and transferred the acquired skills to the left hand (Delta_Right > 0 and Delta_Transfer > 0). In fact, we had to select those patients showing a transfer process to study a possible role of CC in this process. On the basis of our definition, the amount of transfer was given by the parameter Delta_Transfer, which had significance only if it assumed positive values: negative values of Delta_Transfer indicated that the process of transfer did not occur. Actually, it is common that right‐handed people have more difficulties in performing a motor task with the left hand, due to the lateralization of hand movements, and this finding could also be detected in healthy subjects. As a consequence, if the left‐hand RT is shorter than the right‐hand RT it means that a training with the right hand really improved the left‐hand performance through the intermanual transfer of the acquired motor skills, but the contrary is not true because a less ability in the left hand can drive the process leading to Delta_Transfer < 0. In this case, a negative value of Delta_Transfer cannot be attributable to problems in the interhemispheric transfer process, but it could simply mean that the left hand showed a motor performance worst than the right hand at the same level of training.
Also, as shown in the Results, the FA values in CC3 of the patients showing Delta_Transfer < 0 widely ranged from 0.56 (the lowest FA in CC3 in our group of 22 PwMS) to 0.77 (a value which can be considered normal [Bonzano et al., 2008]). Therefore, this finding suggested that the reasons of a Delta_Transfer < 0 can include different aspects, as mentioned before: one is the topic of this work, which is a degradation of CC hampering a correct transfer of information between the two cortical hemispheres; the other one deals with the motor performance with the left hand. Actually, as we observed a high variability in the group of PwMS in the right‐hand performance, the same could have occurred also in the case of the left hand. In general, we can consider that Delta_Transfer can be influenced, in a subtle way, by an inferior natural performance with the nondominant hand but, notwithstanding this, a significant role of CC3 integrity in nonspecific intermanual transfer has been demonstrated.
Furthermore, in our study, one patient did not show nonspecific transfer likely because he presented with tactile hypoesthesia on the palm of his left hand.
To quantify structural damage in CC in this group of PwMS, we used diffusion tensor imaging, since this MRI technique has been demonstrated to be able to detect pathological processes in acute and chronic MS plaques and in normal‐appearing brain, reflecting biophysical changes in the underlying pathology of the demyelinating process [Tievsky et al., 1999]. Abnormal anisotropy and apparent diffusion coefficient values were found to be abnormal also in periplaque regions, and DTI has been considered more accurate than T2‐weighted MR imaging for assessment of disease burden [Guo et al., 2002]. Injury to the normal‐appearing white matter has been found in CC [Coombs et al., 2004], and fractional anisotropy can be particularly indicated to study pathological processes involving the CC [Bonzano et al., 2008; Ge et al., 2004; Hasan et al., 2005; Oh et al., 2004]. On the basis of these findings, we calculated mean FA values in five different CC subregions in each patient and correlated these indexes of damage with motor performance measurements.
We demonstrated that PwMS can have damage in these CC subregions, as indicated by significantly lower FA values with respect to control subjects [Bonzano et al., 2008]. Then, also in this work, with this DTI analysis we were able to study PwMS with potentially a wide range of callosal damage from the normal‐appearing white matter to the conventional MS plaque. Therefore, we did not include control subjects in the correlation analysis between RT and CC structural integrity, also because normal fiber tracts are more intact than in patients and this could create a cluster of data around higher FA values, thus attenuating the correlation result by a ceiling effect [Schulte et al., 2005].
Our results demonstrated that only CC3 is involved in the nonspecific transfer process, excluding the influence of other CC subregions and definitely confirming the results obtained in other studies [Meyer et al., 1998; Perez et al., 2007].
Interestingly, in a previous work we found that bimanual coordination is mainly influenced by the anterior part of CC (CC1 and CC2), which is deputized to motor control and planning. Similar results were obtained in another work showing that, in alcoholics, the crossed‐uncrossed difference, testing visuomotor interhemispheric transfer, was best predicted by diffusivity in the genu [Schulte et al., 2005]. Conversely, in the present work we found an involvement of a more posterior CC subregion (CC3), which mainly matches posterior midbody [Hofer and Frahm, 2006] and includes fibers connecting M1 hand areas (Fig. 4D).
All these results show evidence of the high specificity of callosal subregions for the execution of different functions. Also, it can be noticed that recent studies proposed a posterior shift of callosal motor fibers crossing the human CC compared with monkeys, likely because of an increase in prefrontal white matter volume suggesting connectional elaboration [Hofer and Frahm, 2006; Meyer et al., 1998; Wahl et al., 2007; Zarei et al., 2006].
Future investigations should be required to give an answer about the role of CC in sequence‐specific intermanual transfer. In fact, a first attempt to find a correlation between motor CC and sequence‐specific intermanual transfer was not successful [Perez et al., 2007]. We could make the hypothesis that this transfer process is more complex than that of mRT tasks and likely regulated by the simultaneous cooperation of different callosal subregions. Also, above all if including patients with greater disability, electrophysiological studies to measure central motor and sensory conduction times should be conducted, since dysfunction of central motor and sensory pathways could affect motor performance.
We can conclude that callosal posterior midbody seems to be essential for the interhemispheric transfer of information related to a pure sensorimotor task. Importantly, we demonstrated that patients affected by MS, showing a low level of disability, are able to improve their motor performance with practice. Further, patients maintaining integrity in the callosal posterior midbody have the possibility to accurately transfer the sensorimotor information necessary to start with a higher level of performance when executing the same task with the nontrained hand. Then, it seems feasible to suggest the use of protocols requiring the interhemispheric communication in a rehabilitative treatment for PwMS, as already proposed in the rehabilitation of stroke patients [Floel et al., 2008; Hummel and Cohen, 2006; Nowak et al., 2009; Takeuchi et al., 2005]. More generally, identifying the brain areas involved in the processes underlying the ability to transfer a learnt motor skill from one side of the body to another may be of great importance for the development of treatments for unilateral movement disorders [Birbaumer, 2007].
Acknowledgements
Data were collected and processed with Glove Analyzer System (GAS) provided by eTT s.r.l.
REFERENCES
- Anderson V, Fisniku L, Altmann D, Thompson A, Miller D ( 2009): MRI measures show significant cerebellar gray matter volume loss in multiple sclerosis and are associated with cerebellar dysfunction. Mult Scler 15: 811–817. [DOI] [PubMed] [Google Scholar]
- Birbaumer N ( 2007): Motor learning: Passing a skill from one hand to the other. Curr Biol 17: R1024–R1026. [DOI] [PubMed] [Google Scholar]
- Bonzano L, Tacchino A, Roccatagliata L, Abbruzzese G, Mancardi GL, Bove M ( 2008): Callosal contributions to simultaneous bimanual finger movements. J Neurosci 28: 3227–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove M, Tacchino A, Novellino A, Trompetto C, Abbruzzese G, Ghilardi MF ( 2007): The effects of rate and sequence complexity on repetitive finger movements. Brain Res 1153: 84–91. [DOI] [PubMed] [Google Scholar]
- Casadio M, Sanguineti V, Morasso P, Solaro C ( 2008): Abnormal sensorimotor control, but intact force field adaptation, in multiple sclerosis subjects with no clinical disability. Mult Scler 14: 330–342. [DOI] [PubMed] [Google Scholar]
- Coombs BD, Best A, Brown MS, Miller DE, Corboy J, Baier M, Simon JH ( 2004): Multiple sclerosis pathology in the normal and abnormal appearing white matter of the corpus callosum by diffusion tensor imaging. Mult Scler 10: 392–397. [DOI] [PubMed] [Google Scholar]
- de Guise E, del Pesce M, Foschi N, Quattrini A, Papo I, Lassonde M ( 1999): Callosal and cortical contribution to procedural learning. Brain 122 ( Part 6): 1049–1062. [DOI] [PubMed] [Google Scholar]
- de Lacoste MC, Kirkpatrick JB, Ross ED ( 1985): Topography of the human corpus callosum. J Neuropathol Exp Neurol 44: 578–591. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC ( 1999): Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp Brain Res 124: 520–524. [DOI] [PubMed] [Google Scholar]
- Doyon J, Benali H ( 2005): Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol 15: 161–167. [DOI] [PubMed] [Google Scholar]
- Dujardin K, Donze AC, Hautecoeur P ( 1998): Attention impairment in recently diagnosed multiple sclerosis. Eur J Neurol 5: 61–66. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD ( 1992): Interhemispheric inhibition of the human motor cortex. J Physiol 453: 525–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floel A, Hummel F, Duque J, Knecht S, Cohen LG ( 2008): Influence of somatosensory input on interhemispheric interactions in patients with chronic stroke. Neurorehabil Neural Repair 22: 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Law M, Johnson G, Herbert J, Babb JS, Mannon LJ, Grossman RI ( 2004): Preferential occult injury of corpus callosum in multiple sclerosis measured by diffusion tensor imaging. J Magn Reson Imaging 20: 1–7. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Cohen LG, Floeter MK, Chen R, Corwell B, Hallett M ( 1998): Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. J Physiol 510 ( Part 1): 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein M, Berlucchi G ( 2008): Classical disconnection studies of the corpus callosum. Cortex 44: 914–927. [DOI] [PubMed] [Google Scholar]
- Guo AC, MacFall JR, Provenzale JM ( 2002): Multiple sclerosis: Diffusion tensor MR imaging for evaluation of normal‐appearing white matter. Radiology 222: 729–736. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Gupta RK, Santos RM, Wolinsky JS, Narayana PA ( 2005): Diffusion tensor fractional anisotropy of the normal‐appearing seven segments of the corpus callosum in healthy adults and relapsing‐remitting multiple sclerosis patients. J Magn Reson Imaging 21: 735–743. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J ( 2006): Topography of the human corpus callosum revisited–comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 32: 989–994. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG ( 2006): Non‐invasive brain stimulation: A new strategy to improve neurorehabilitation after stroke? Lancet Neurol 5: 708–712. [DOI] [PubMed] [Google Scholar]
- Japikse KC, Negash S, Howard JH Jr, Howard DV ( 2003): Intermanual transfer of procedural learning after extended practice of probabilistic sequences. Exp Brain Res 148: 38–49. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF ( 1983): Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- Larson EB, Burnison DS, Brown WS ( 2002): Callosal function in multiple sclerosis: Bimanual motor coordination. Cortex 38: 201–214. [DOI] [PubMed] [Google Scholar]
- Lassonde M, Sauerwein HC, Lepore F ( 1995): Extent and limits of callosal plasticity: Presence of disconnection symptoms in callosal agenesis. Neuropsychologia 33: 989–1007. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Conte A, Mainero C, Frasca V, Fubelli F, Totaro P, Caramia F, Inghilleri M, Pozzilli C, Pantano P ( 2007): Effect of corpus callosum damage on ipsilateral motor activation in patients with multiple sclerosis: A functional and anatomical study. Hum Brain Mapp 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani L, Comi E, Annovazzi P, Rovaris M, Rossi P, Cursi M, Comola M, Martinelli V, Comi G ( 2007): Impaired short‐term motor learning in multiple sclerosis: Evidence from virtual reality. Neurorehabil Neural Repair 21: 273–278. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM ( 2004): Diffusion tensor imaging of adult age differences in cerebral white matter: Relation to response time. Neuroimage 21: 1174–1181. [DOI] [PubMed] [Google Scholar]
- Mancini L, Ciccarelli O, Manfredonia F, Thornton JS, Agosta F, Barkhof F, Beckmann C, De Stefano N, Enzinger C, Fazekas F, Filippi M, Gass A, Hirsch JG, Johansen‐Berg H, Kappos L, Korteweg T, Manson SC, Marino S, Matthews PM, Montalban X, Palace J, Polman C, Rocca M, Ropele S, Rovira A, Wegner C, Friston K, Thompson A, Yousry T ( 2009): Short‐term adaptation to a simple motor task: A physiological process preserved in multiple sclerosis. Neuroimage 45: 500–511. [DOI] [PubMed] [Google Scholar]
- Manson SC, Palace J, Frank JA, Matthews PM ( 2006): Loss of interhemispheric inhibition in patients with multiple sclerosis is related to corpus callosum atrophy. Exp Brain Res 174: 728–733. [DOI] [PubMed] [Google Scholar]
- Manson SC, Wegner C, Filippi M, Barkhof F, Beckmann C, Ciccarelli O, De Stefano N, Enzinger C, Fazekas F, Agosta F, et al. ( 2008): Impairment of movement‐associated brain deactivation in multiple sclerosis: Further evidence for a functional pathology of interhemispheric neuronal inhibition. Exp Brain Res 187: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis MJ, Dhawan V, Nakamura T, Ghilardi MF, Feigin A, Edwards C, Ghez C, Eidelberg D ( 2003): Enhancement of brain activation during trial‐and‐error sequence learning in early PD. Neurology 60: 612–619. [DOI] [PubMed] [Google Scholar]
- Mesaros S, Rocca MA, Riccitelli G, Pagani E, Rovaris M, Caputo D, Ghezzi A, Capra R, Bertolotto A, Comi G, et al. ( 2009): Corpus callosum damage and cognitive dysfunction in benign MS. Hum Brain Mapp 30: 2656–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BU, Roricht S, Woiciechowsky C ( 1998): Topography of fibers in the human corpus callosum mediating interhemispheric inhibition between the motor cortices. Ann Neurol 43: 360–369. [DOI] [PubMed] [Google Scholar]
- Molinari M, Leggio MG, Solida A, Ciorra R, Misciagna S, Silveri MC, Petrosini L ( 1997): Cerebellum and procedural learning: Evidence from focal cerebellar lesions. Brain 120 ( Part 10): 1753–1762. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P ( 1987): Attentional requirements of learning: Evidence from performance measures. Cognit Psychol 19: 1–32. [Google Scholar]
- Nowak DA, Grefkes C, Ameli M, Fink GR ( 2009): Interhemispheric competition after stroke: Brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair 23: 641–656. [DOI] [PubMed] [Google Scholar]
- Oh J, Henry RG, Genain C, Nelson SJ, Pelletier D ( 2004): Mechanisms of normal appearing corpus callosum injury related to pericallosal T1 lesions in multiple sclerosis using directional diffusion tensor and 1H MRS imaging. J Neurol Neurosurg Psychiatry 75: 1281–128s6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Parlow SE, Kinsbourne M ( 1989): Asymmetrical transfer of training between hands: Implications for interhemispheric communication in normal brain. Brain Cognit 11: 98–113. [DOI] [PubMed] [Google Scholar]
- Pelletier J, Habib M, Lyon‐Caen O, Salamon G, Poncet M, Khalil R ( 1993): Functional and magnetic resonance imaging correlates of callosal involvement in multiple sclerosis. Arch Neurol 50: 1077–1082. [DOI] [PubMed] [Google Scholar]
- Perez MA, Wise SP, Willingham DT, Cohen LG ( 2007): Neurophysiological mechanisms involved in transfer of procedural knowledge. J Neurosci 27: 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfilipo MP, Benedict RH, Weinstock‐Guttman B, Bakshi R ( 2006): Gray and white matter brain atrophy and neuropsychological impairment in multiple sclerosis. Neurology 66: 685–692. [DOI] [PubMed] [Google Scholar]
- Santa Maria MP, Benedict RH, Bakshi R, Coad ML, Wack D, Burkard R, Weinstock‐Guttman B, Roberts S, Lockwood AH ( 2004): Functional imaging during covert auditory attention in multiple sclerosis. J Neurol Sci 218: 9–15. [DOI] [PubMed] [Google Scholar]
- Schnider A, Benson F, Rosner LJ ( 1993): Callosal disconnection in multiple sclerosis. Neurology 43: 1243–1245. [DOI] [PubMed] [Google Scholar]
- Schulte T, Sullivan EV, Muller‐Oehring EM, Adalsteinsson E, Pfefferbaum A ( 2005): Corpus callosal microstructural integrity influences interhemispheric processing: A diffusion tensor imaging study. Cereb Cortex 15: 1384–1392. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. ( 2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 ( Suppl 1): S208‐S219. [DOI] [PubMed] [Google Scholar]
- Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K ( 2005): Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke 36: 2681–2686. [DOI] [PubMed] [Google Scholar]
- Tievsky AL, Ptak T, Farkas J ( 1999): Investigation of apparent diffusion coefficient and diffusion tensor anisotrophy in acute and chronic multiple sclerosis lesions. Am J Neuroradiol 20: 1491–1499. [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Ziemann U ( 2008): The human motor corpus callosum. Rev Neurosci 19: 451–466. [DOI] [PubMed] [Google Scholar]
- Wahl M, Lauterbach‐Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U ( 2007): Human motor corpus callosum: Topography, somatotopy, and link between microstructure and function. J Neurosci 27: 12132–12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham DB ( 1999): Implicit motor sequence learning is not purely perceptual. Mem Cognit 27: 561–572. [DOI] [PubMed] [Google Scholar]
- Zarei M, Johansen‐Berg H, Smith S, Ciccarelli O, Thompson AJ, Matthews PM ( 2006): Functional anatomy of interhemispheric cortical connections in the human brain. J Anat 209: 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]


