Abstract
Understanding the neural functional organization of swallowing in healthy elders is essential in diagnosing and treating older adults with swallowing difficulties. The primary aims of this investigation were to identify the neural activation sites of different components of deglutition in healthy elders using functional Magnetic Resonance Imaging (fMRI) and to investigate age differences in the neural control of swallowing. Ten young (age range 19–25 years of age) and nine older (age range 66–77 years of age) right‐handed healthy individuals were scanned in a 3‐Tesla MRI scanner. Subjects were visually cued for both a “Swallow” task and for component/control tasks (“Prepare to swallow,” “Tap your tongue,” and “Clear your throat”). Behavioral interleaved gradient (BIG) methodology was used to address movement related artifacts. Between‐group comparisons revealed statistically stronger activations in the primary somatosensory cortex of young adults during the motor tasks examined. Both groups showed activations in the major motor areas involved in the initiation and execution of movement; however, areas involved in sensory processing, sensorimotor integration and/or motor coordination and control, showed reduced or limited activity in the elderly. Potential implications of these findings for clinical practice are discussed. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: deglutition, aging, neurophysiology, functional Magnetic Resonance Imaging, deglutition disorders
INTRODUCTION
Deglutition or swallowing is one of the main processes that enable humans to sustain life. Swallowing disorders (a.k.a. dysphagia) are a global health care concern. Without effective treatment, dysphagia may lead to dehydration, malnutrition, pulmonary complications related to chronic aspiration, particularly pneumonia, and reductions in quality of life [Ney et al., 2009]. Despite this significant clinical problem, the critical underlying neurophysiological processes of dysphagia are complex and not well understood.
Dysphagia in older individuals is usually a result of age‐related disease, with stroke most commonly reported [Daniels et al., 1998; Martino et al., 2005; Robbins et al., 1993]. However, increased prevalence of swallowing difficulties in healthy aging in the absence of disease is frequently noted and is newly termed presbyphagia [Leslie et al., 2005; Ney et al., 2009; Robbins et al., 1992]. Physiological changes in the swallowing mechanism as people age are known to exist in both the motor and sensory components of deglutition [Cook et al., 1994; Daniels et al., 2004; Feldman et al., 1980; Logemann et al., 2000; Nicosia et al., 2000; Robbins et al., 1992, 1995; Shaker et al., 1994].
Regarding motor function, research reveals significant age‐related changes in lingual pressure generation [Robbins et al., 1995], an important contributor to the swallowing process as it enables food manipulation and propulsion from the oral cavity to the pharynx. Additionally, healthy elders need increased time to prepare the bolus in the oral cavity [Feldman et al., 1980], and are in general slower eaters than their younger counterparts [Cook et al., 1994; Robbins et al., 1992]. Etiological explanation of these motor swallowing declines, partially comes from evidence that sarcopenia (i.e., age‐related loss of muscle mass and selective muscle fibers) is also present in the muscles of the upper aerodigestive tract in old age [Cartee, 1995; Faulkner et al., 1995], thus attributing these motor declines to the end organ, i.e., the muscle.
Regarding sensory swallowing components, older healthy adults need larger volumes of material in order to trigger the pharyngeal swallow response, i.e., the response that is necessary for a complete and safe laryngeal and airway closure to be achieved during the swallow [Shaker et al., 1994]. They are also known to demonstrate a delay in the initiation of this response [Robbins et al., 1992], reduced taste perception [Calhoun et al., 1992; Fukunaga et al., 2005], increased sensory discrimination thresholds in the mouth and the laryngopharynx [Aviv, 1997], and increased instances of penetration (airway invasion up to the level of the true vocal folds) [Daniels et al., 2004; Robbins et al., 1999]. The underpinnings of these sensory declines are not well understood. It has been hypothesized, however, that the delay in the initiation of the pharyngeal response may be due to the fact that, as people age, the more voluntary—more cortically regulated—oral events of swallowing become neurologically “un‐coupled” from the more automatic—brainstem regulated—pharyngeal response, suggesting a neural component underlying these sensory declines [Ney et al., 2009].
Taking into consideration the physiological declines in aging deglutition and in lieu of the Ney et al. [ 2009] hypothesis, it is considered of great clinical interest to investigate potential changes in the neural processes that govern swallowing as people age. In fact, most studies that have focused on the neural control of swallowing have either used animals or relatively young healthy human subjects as their samples making it difficult to generalize their findings to the elderly population. Results from these studies report similar areas of neural activation during swallowing including pericentral and perisylvian areas, the cingulate gyrus, the insula, thalamus, basal ganglia nuclei, premotor and prefrontal regions, and parieto‐occipital areas [Hamdy et al., 1999; Kern et al., 2001a, b; Malandraki et al., 2009a, b; Martin et al., 2001, 2004; Mosier and Bereznaya, 2001; Mosier et al., 1999; Suzuki et al., 2003; Toogood et al., 2005]. Changes in the neural mechanisms that govern the complex sensorimotor act of swallowing with age have not been extensively investigated [Martin et al., 2007].
Recently, an fMRI study examined the neural activation of swallowing in nine older healthy females over 60 years of age [Martin et al., 2007]. Results revealed that deglutition in older females activated multiple cerebral areas, including the lateral pericentral, perisylvian, and anterior cingulate cortex with postcentral gyrus activation being more lateralized to the left for both dry and water swallows. This study did not make direct statistical comparisons of the neural swallowing activation between old and younger adults; it did, however, report larger areas of activations cortically than subcortically during both water and saliva swallows, in those nine older females, as well as lack of activation in the cerebellum and midbrain structures [Martin et al., 2007]. Interestingly, during water swallowing, a fourfold increase in the brain volume activated was seen when compared to the saliva swallow, especially in the right premotor and prefrontal cortex in this group. The authors suggested that this additional activation may represent a compensatory response for swallowing water in healthy elders, secondary to the age‐related declines seen in the oral sensorimotor functioning of elders.
The only fMRI study to date that has investigated age effects on the amplitude of neural activation during swallowing is the one conducted by Humbert et al. [ 2009]. These authors found that, for all swallow types examined (i.e., saliva, water and barium), older adults showed significantly higher BOLD activity than the younger group across a large region of the cortex, including the right pre and postcentral gyri, bilateral frontal lobe, bilateral parietal regions (inferior and superior gyri), and the right superior temporal gyrus. However, the younger group also exhibited higher BOLD activity in selected areas, including the left pre and postcentral gyri, left supplementary motor area (SMA), and right superior frontal gyrus. In contrast with Martin et al. [ 2007], this investigation found that saliva swallows elicited significantly higher BOLD responses in regions important for swallowing compared with water and barium. These discrepancies, however, may be explained by differences in the methodology of these studies. The authors conclude that the additional cortical activations seen in some brain areas in their elderly sample may designate that older adults need increased effort than younger individuals to swallow the same bolus types and amounts, and that young adults may be more efficient in cortical use for the same task than elders [Humbert et al., 2009]. Alternatively, it could also be hypothesized that this additional activation may not be compensatory, but rather may reflect inability to suppress activity in conflicting regions [Buckner, 2002].
Although in Humbert et al. [ 2009], the swallowing process resulted in larger activations in selected cortical areas of older adults, it is important to note that swallowing is a multi‐phase event with multiple components. In this work, we examined the swallowing process, as well as simple component tasks that approximate the phases of swallowing. By examining the level of cortical activation associated with these component tasks, we aim to gain greater insight into the functional significance of potential additional cortical activations in the older adult sample.
Swallowing is conventionally divided into three major phases: the oral phase, involving the entrance and manipulation of the food in the oral cavity; the pharyngeal phase, which involves the transportation of the food from the oropharynx into the esophagus around a well protected and closed laryngeal vestibule; and the esophageal phase, involving the transportation of the food through the esophagus and into the stomach [Logemann, 1998; Perlman and Christensen, 1997]. In addition to these conventionally described phases, swallowing also involves an initial motor planning and preparatory stage during which an individual prepares for the swallowing sequence that will follow. Planning of swallow is included in this study in order to investigate whether declines in motor performance with age include the intentional planning stage or if age‐related declines are associated only with motor output or sensorimotor integration areas. Additionally, a disorder of motor planning of swallowing known as swallowing apraxia is characterized by a delay in the initiation of bolus transfer with no lingual movement or by lingual searching movements before bolus is transferred posteriorly in the oral cavity [Daniels, 2000; Logemann, 1998]. Understanding the neural control of planning deglutition and all other physiological components in normal healthy adults is believed to provide greater insight as to how the entire swallowing sequence is implemented and initiated.
Swallowing is a complex sensorimotor process involving many physiological phases. It is logical to hypothesize that the multiple areas activated during swallowing in both young and elderly adults reflect regions responsible for different aspects of the swallowing process; such as areas responsible for lingual or mandibular control, for sensorimotor planning, or for pharyngeal components of deglutition [Hamdy et al., 1999; Huckabee et al., 2003; Suzuki et al., 2003]. Identification of the neural control of different swallowing components has been researched in healthy young adults and results indicated that pharyngeal components of swallowing (such as laryngeal closure) rely more heavily on subcortical networks, whereas oral components of swallowing (such as tongue elevation) depend more on cortical sensorimotor innervation [Malandraki et al., 2009a].
There were two main objectives of this study. First, we aimed to identify the neural activation during separate components of the swallowing process in older healthy adults, i.e., during swallowing, tongue tapping, throat clearing, and planning of deglutition without execution, following the same methodology as described in Malandraki et al. [ 2009a] that examined healthy young adults. This attempt was made to better understand the neural control of this complex sensorimotor process in old age. By studying these different components, we also aimed to determine whether simpler elements reflecting different swallowing events recruit additional areas of activation in older adults, based on previously reported increases in functional activations for swallowing with age [Humbert et al., 2009]. Our second objective was to examine potential differences in the amplitude of the neural control of deglutition and component tasks in eight selected Regions‐of‐Interest (ROIs) between two age groups, healthy young and elderly adults.
MATERIALS AND METHODS
Subjects
Nineteen right‐handed healthy individuals with no history of speech, or swallowing difficulties, or any neurological involvement participated in this study. An oral sensorimotor examination was performed with all subjects to ensure healthy normal oral sensorimotor function. Also, all subjects were right‐handed as measured by the Edinburgh Handedness Inventory [Oldfield, 1971] (Mean EHI score: 78, SD; 11), and gave written informed consent. The study protocol was approved by the University of Illinois at Urbana‐Champaign Institutional Review Board.
Participants were divided into two age groups. Group A included 10 young adults, five males and five females with a mean age 21.7 years of age (yoa) (SD: 2.1 yoa). Group B included nine older adults, six females and three males with a mean age 70.2 yoa (SD: 3.9 yoa). To ensure identification of a healthy elderly group, elderly individuals had to be high functioning, community‐dwelling older adults, who were a minimum of 65 years of age. Participants with a known history of stroke or other brain dysfunction, head and neck cancer or anatomic alterations in that area, and speech or swallowing difficulties were excluded from the study. Also, potential subjects on any medications that could affect their swallowing or neural function were excluded from the study. Subjects were asked to refrain from any alcohol use for 12 h before the experiment. To ensure that all subjects could reliably read the visual commands of the tasks, vision was corrected by use of MR‐compatible eyeglasses for five of the nine elderly participants. None of the young subjects needed corrected vision.
Tasks
The experimental design and the procedure have been previously described in detail in Malandraki et al. [ 2009a, b]. In short, this study followed an event‐related design with jittered Interstimulus Intervals (ISIs) ranging in duration from 7 to 32 s. Tasks were presented intermixed in randomized intervals in six different 6‐min functional scanning runs and each task was completed a total of 30 times during the experiment. Tasks included: voluntary swallowing of 3 ml of room temperature water, planning of a swallow without execution, tapping of the tip of the tongue against the alveolar ridge, and throat clearing. Participants were visually cued with instructions to “Swallow,” “Prepare to swallow,” “Tap your tongue,” and “Clear your throat” at randomized time intervals through an LCD projection screen. For the voluntary swallow task, participants were instructed to swallow 3 ml of water every time the visual cue “Swallow” (and a bolus thereafter) was presented. Immediately preceding the “Swallow” command, participants saw the visual command “Prepare to swallow.” For the planning (anticipatory) task, participants were instructed to anticipate the swallowing process every time the visual cue “Prepare to swallow” was presented; however, they neither did receive a bolus nor did they see the visual command “Swallow”; thus, they did not perform the motor task. Participants did not know if the command to swallow would follow the preparatory command. Controlled liquid release of 3 ml water boluses was achieved through a specially designed system (consisting of a clear plastic infusion tube, a hand‐held syringe, and a one‐way flow valve). The experimenter administered the liquid boluses.
Behavioral Interleaved Gradient (BIG) methodology was used to address movement‐related artifacts [Gracco et al., 2005]. With this method, a 3 s gap during which images were not acquired followed 2 s of image acquisition. During the 3‐s gap period, the subjects performed the task as instructed. A 45‐min training session generally occurred the day preceding the actual experiment, but had occurred as many as 3 days before. During this session, participants were trained to perform all tasks while lying down inside a mock magnet. Figure 1 summarizes the experimental design and shows a small portion of one of the six runs.
Figure 1.
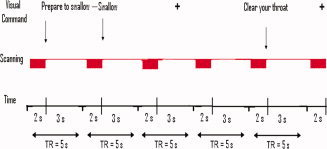
Schematic illustration of experimental design for two tasks. Subjects' eyes were fixated on cross until seeing the visual command. As soon as the command appeared on the screen they performed the task. Blocks represent periods of image acquisition. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Monitoring of Swallowing and Throat Clearing Trials
The swallowing and throat clearing trials were verified from their laryngeal movement patterns [Malandraki et al., 2009a, b]. Laryngeal movements were monitored by attaching an MR‐compatible infant respiratory belt (Lafayette Instruments Co) around the neck of the subject. Laryngeal movements associated with swallowing and throat clearing trials were distinguished by their distinct patterns for both groups of participants [Malandraki et al., 2009a, b].
Scanning Protocol
MRI experiments were performed at the Biomedical Imaging Center (BIC) of the University of Illinois at Urbana‐Champaign (UIUC), on a 3 T Siemens Allegra MRI head scanner. Two high‐resolution anatomic scans were acquired for the registration of the functional images and registration was performed using FEAT (FMRI Expert Analysis Tool) Version 5.4, part of FSL (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl). A T1‐weighted MPRAGE 3D image, (with 160 slices per slab, on a 256 × 256 matrix, 1.1 mm isotropic resolution and TR = 2,250 ms, TE = 2.62 ms and flip angle 9 degrees) was acquired. Also, a T2‐weighted turbo‐spin echo (TSE) scan was acquired to get anatomical information from the functional slice prescription. The TSE acquisition had the following parameters: FOV (Field of View) of 240 mm, TR of 3 s, 34 slices, with 4 mm slice thickness. FMRIB's Linear Image Registration Tool (FLIRT) was used for registration to high‐resolution structural and/or standard space images [Jenkinson et al., 2002].
Multi‐slice EPI acquisition used the following parameters: TE of 30 ms, TR of 5 s (including 3 s quiescent period), on a 64 × 64 matrix with a FOV of 240 mm. Seventy‐four volumes of 34 axial interleaved slices of 4‐mm thickness, were acquired during each run/session.
fMRI Data Analysis
FMRI Expert Analysis Tool (FEAT) Version 5.91, part of FMRIB's Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl) [Smith et al., 2004] was used for the fMRI data processing. The following prestatistics processing was used; motion correction using MCFLIRT [Jenkinson et al., 2002]; slice‐timing correction using Fourier‐space time‐series phase‐shifting; nonbrain removal using BET [Smith, 2002]; spatial smoothing using a Gaussian kernel of FWHM 9.0 mm; grand‐mean intensity normalization of the entire 4D dataset by a single multiplicative factor; high‐pass temporal filtering (Gaussian‐weighted least‐squares straight line fitting, with sigma = 50.0 s).
In the first level analysis, the task related responses were analyzed using multiple linear regression with a single regressor for each task convolved with a canonical hemodynamic response function and motion parameter estimates were used as additional regressors of no interest. A higher level group analysis was then used, using the FMRIB Local Analysis of Mixed Effects (FLAME) [Beckmann et al., 2003] module in FSL and a one‐sample t‐test was performed for each task to determine significant group activations in each and every task for each group. The group Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 2.3 at a corrected cluster significance threshold of P = 0.05 using the theory of Gaussian Random Fields (GRF) [Worsley et al., 1996]. For group analysis, the 3‐D anatomical data sets of each subject were spatially normalized and converted to the standard anatomical Montreal Neurological Institute (MNI) space.
Another mixed effects analysis was performed with subjects treated as random effects and tasks as fixed factors to examine differences between tasks in each group. Paired t‐tests were performed between all possible pairs of the three control tasks. A conjunction analysis was performed on the t‐test results between a component task and other tasks. Then the minimum z‐score image for the pair‐wise comparisons for each voxel was used to determine if a certain control task was significantly more activated than any other task. This procedure was also performed for the swallowing task versus the control tasks.
To compare the amplitude of neural activation between groups, Region of Interest (ROI) analyses were performed. The primary motor and somatosensory cortices and the premotor area have been most consistently reported as activated areas during swallowing tasks [Hamdy et al., 1999; Kern et al., 2001a, b; Malandraki et al., 2009a, b; Martin et al., 2001, 2004, 2007; Mosier and Bereznaya, 2001; Mosier et al., 1999; Suzuki et al., 2003; Toogood et al., 2005]. Thus, the ROI analyses performed used these areas of interest to enable comparisons between groups. Anatomical sections to define the primary motor cortex (areas 4a and 4p) [Geyer et al., 1996], the primary sensory cortex (areas 3a, 3b, 1, and 2) [Geyer et al., 1999, 2000; Grefkes et al., 2001], the premotor cortex (area 6) [Geyer, 2004], and the primary visual cortex (V1, BA 17) [Amunts et al., 2000] were based on the maximum probability maps (MPM) and macrolabels maps [Eickhoff et al., 2005] of the Statistical Parametric Mapping Anatomy Tool box. The primary visual cortex was used as a reference area for both groups of participants to examine if comparisons between the two groups were valid. Volumetric and functional imaging studies have indicated that the primary visual cortex is relatively well preserved in old age [Park et al., 2004; Raz et al., 2004]. Because all participants saw the same visual stimuli, it was expected that activation in V1 would be similar across subjects and tasks. Mean percent BOLD signal change was extracted per ROI in each subject and was used as the dependent variable. To compare the amplitude of neural activation between groups within each task and for each ROI, pairwise Wilcoxon tests were performed. Nonparametric statistical tools were used due to the potential lack of normal distribution in the data.
RESULTS
Healthy Elderly Adults
Response latency of the swallows and the throat clearing events
For the elderly group, the mean latency from the visual command to the completion of the laryngeal movement signal associated with the water swallows was 2.56 s (±0.27 SD) [Malandraki et al., 2009b]. The mean latency for the throat clearing events was 2.41 s (± 0.19 SD) [Malandraki et al., 2009b]. These latency data indicate that the elderly subjects responded promptly to the visual cues in both the water swallows and the throat clearing tasks. Young adults had 2.26 s and 2.56 s mean latency values for swallowing and throat clearing, respectively [Malandraki et al., 2009a].
Elderly Group Activations in the Four Tasks
The significant activations of the young group have been previously reported in Malandraki et al., [ 2009a]. Thus, this section presents the results for the older group of participants.
Swallowing of 3 ml water
In the elderly subjects, the 3 ml water swallow evoked significant activation in multiple brain regions (see Fig. 2) [Z = 2.3–4.6, P (corrected) ≤ 0.05]. The most prominent group activations were observed bilaterally in the precentral (BA 4) and postcentral gyrus (BA 1, 2, 3), the premotor cortex (BA 6) and the right frontal operculum. Significant activations were also seen in the insular cortex, the inferior parietal lobule, the superior and middle temporal gyri (including Heschl's gyri), the cuneus and precuneus and the cingulate gyrus (BAs 24, 31) bilaterally. Subcortical activations were observed in portions of the thalamus and the lentiform nuclei (putamen and lateral globus pallidus). Limited cerebellar activation and midbrain activation were also seen.
Figure 2.
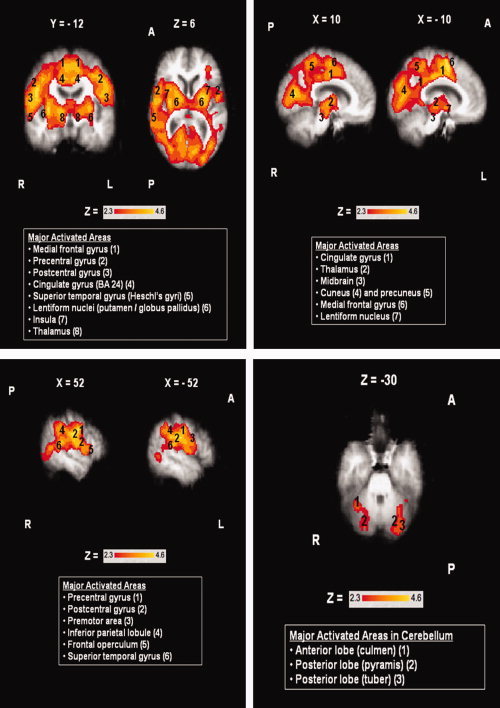
Areas of activation during swallowing in the elderly group (N = 9). Boxes report major activated areas. Images shown in radiological convention (right hemisphere is shown on the left). Coordinates are given in MNI space in mm. A = Anterior, P = Posterior, L = Left Hemisphere, R = Right Hemisphere. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Planning of deglutition
At the group level, planning of deglutition without execution did not show any significantly activated areas for the elderly group of participants. This is likely due to the very limited activations that were seen in the single subject level analysis during this task. Two of the nine elderly participants (both males) did not show any significant activation during this task. Of the seven remaining participants, three showed limited activation in areas of the visual cortex, and four limited activations in the premotor and primary motor areas and the visual cortex. With planning being a weakly activating task, the activations appear to be subthreshold for this group size. Adding more subjects in future studies may increase power and result in detectable activations in this area.
Tongue tapping
Tongue tapping significantly activated the anterior (BA 24) and posterior cingulate gyrus, the primary motor and somatosensory cortices (BA 1, 2, 3, 4), the premotor area (BA 6), the precuneus and cuneus, and portions of the medial and frontal gyri bilaterally in the elderly participants (see Fig. 3) [Z = 2.3–4.5, P (corrected) ≤ 0.05]. Activations were also observed in the putamen and the frontal operculum in the left hemisphere.
Figure 3.
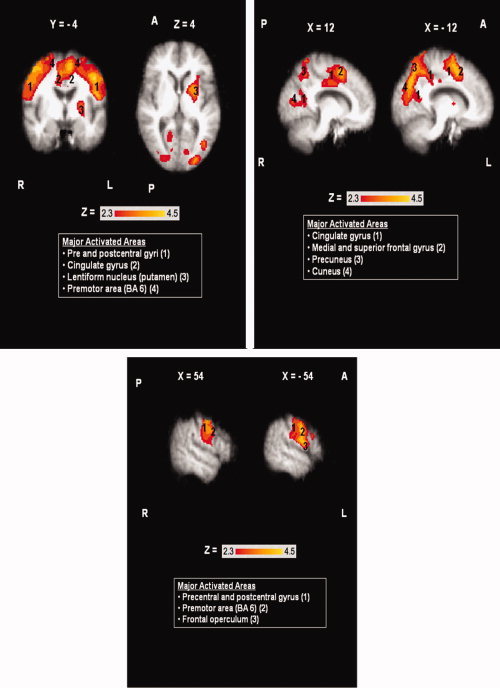
Areas of activation during tongue tapping in the elderly group (N = 9). Boxes report major activated areas. Images shown in radiological convention (the right hemisphere is shown on the left). Coordinates are given in MNI space in mm. A = Anterior, P = Posterior, L = Left Hemisphere, R = Right Hemisphere. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Throat clearing
Throat clearing resulted in significant bilateral activation [Z = 2.3–4.4, P (corrected) ≤ 0.05] of the medial frontal gyrus and the premotor area (BA 6), and the cuneus. In the left hemisphere significant activations were observed in the cingulate gyrus, the insula, the inferior frontal gyrus, the precentral gyrus, and the superior parietal lobule. Also, the right primary somatosensory cortex was significantly activated during throat clearing. Areas of activation during this task in the elderly group are shown in Figure 4.
Figure 4.
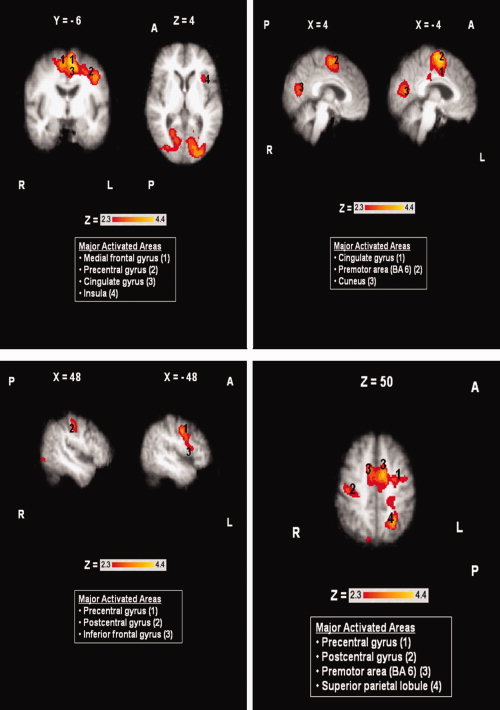
Areas of activation during throat clearing in the elderly group (N = 9). Boxes report major activated areas. Images shown in radiological convention (right hemisphere is shown on the left). Coordinates are given in MNI space in mm. A = Anterior, P = Posterior, L = Left Hemisphere, R = Right Hemisphere. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Statistical Comparison Between Tasks in Older Adults
When swallowing was compared with all control tasks, multiple areas were found to be more significantly activated during swallowing (Fig. 5a) than during the control tasks in the older adult group. These areas included portions of the primary motor and somatosensory cortices and the premotor area, the superior temporal gyrus (extending to Heschl's gyri), the lentiform nuclei (putamen and globus pallidus), the thalamus, the insula and the parietal lobules bilaterally.
Figure 5.
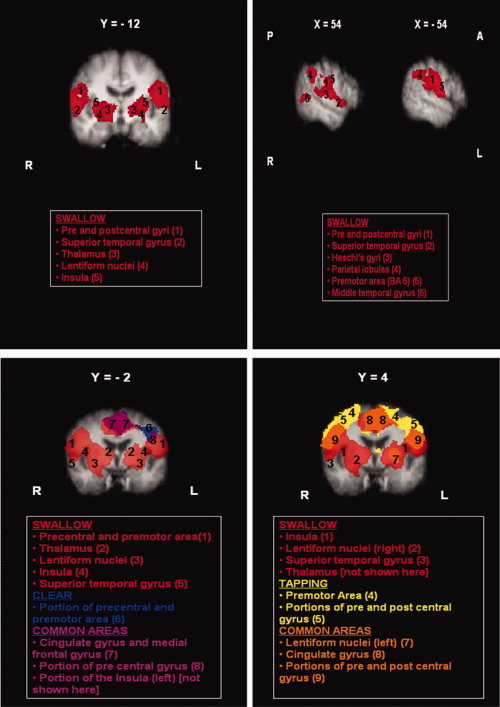
(a) Areas of most significant activation during swallowing (red) in Group B. (b) Overlaid statistical maps showing qualitative functional comparisons between tasks with areas of activation during swallowing (red), throat clearing (blue), both swallowing and throat clearing (purple), tongue tapping (yellow), and both swallowing and tongue tapping (orange). Images shown in radiological convention (right hemisphere is shown on the left). Coordinates are given in MNI space. A = Anterior, P = Posterior, L = Left Hemisphere, R = Right Hemisphere. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
When paired t‐tests were performed between all the possible pairs of the three control tasks, no areas were found to be more significantly activated in any of these tasks when compared to the others. This was probably due to the overall weaker activations that were seen in the elderly group of participants compared to their younger counterparts. Qualitative comparisons (Fig. 5b) show that the three motor tasks examined (swallowing, tongue tapping, throat clearing) share many common areas of activation, including portions of the primary motor and somatosensory cortex, the premotor area, and the cingulate gyrus. Swallowing, however, appears to elicit larger sites of activation than any in all the reported areas. Also, both tongue tapping and throat clearing show activations in a more dorsal area of the primary motor and premotor cortex than swallowing. Subcortical activations of the insula, the thalamus, and the lentiform nuclei are mainly seen during swallowing, however tongue tapping also elicited activation of the putamen in the left hemisphere and throat clearing activation of a small portion of the left insular cortex.
Comparisons Between Young and Elderly (ROI Analyses)
During swallowing, younger adults exhibited a trend of increased amplitude of activation in all ROIs examined compared with older adults. However, not all differences reached statistical significance. Specifically, younger adults showed significantly increased amplitude of activation in sensory Brodmann areas 2, 3A, and 3b (P = 0.041, 0.038, and 0.026 respectively) in motor BA 4p (P = 0.044) (Fig. 6a). During planning, differences were significant only in BA 1 (P = 0.014) (Fig. 6b). Tongue tapping also elicited greater activations in the younger group in the entire primary somatosensory cortex (P ≤ 0.04) and in the premotor cortex area BA 6 (P = 0.017) (Fig. 6c). During throat clearing, all sensorimotor areas examined, except BA 2, were significantly more activated in the younger group (P ≤ 0.003) (Fig. 6d). Differences in activation amplitude of the primary visual cortex area (reference area) were minimal between the two groups in all tasks, indicating that comparisons between the two groups are valid.
Figure 6.
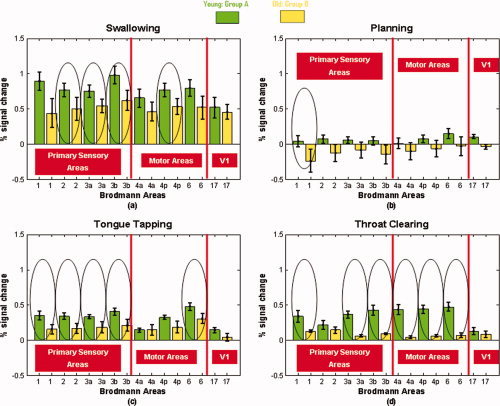
Average % signal change in all ROIs during (a) swallowing, (b) planning, (c) tongue tapping, and (d) throat clearing in both groups. Green = Young adults' activation (Group A); Yellow = Old adults' activation (Group B) circles indicate statistically significant amplitude differences between age groups (P < 0.05). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
Although changes in swallowing physiology as people age have been described, neurophysiological changes that may govern these processes are still unknown. This investigation had two main objectives. First, we aimed to identify the sites of neural activation during swallowing and three separate components of deglutition in healthy older adults to better understand the neural control of this complex sensorimotor process in old age. By studying these different components, we also aimed to determine whether these simpler elements recruit additional areas of activation in older adults, based on previously reported increases in neural activations for swallowing with age [Humbert et al., 2009]. The second objective was to examine potential differences in the amplitude of the neural control of deglutition and component tasks in eight common swallowing‐activated ROIs between healthy young and elderly participants.
Regarding the first aim, when swallowing was compared with all control tasks for the elderly group, multiple areas were found to be more significantly activated during swallowing, including portions of the primary motor and somatosensory cortices and the premotor area, the superior temporal gyrus (extending to Heschl's gyri), the lentiform nuclei (putamen and globus pallidus), the thalamus, the insula and the parietal lobules bilaterally. These areas have also been reported as significantly active during swallowing in older adults by previous fMRI investigations [Martin et al., 2007; Humbert et al., 2009]. Qualitative comparisons between activations during the three motor tasks (swallowing, tongue tapping and throat clearing) further revealed that these tasks share many common areas of activation, including portions of the primary motor and somatosensory cortex, the premotor area and the cingulate gyrus. However, swallowing appeared to elicit larger sites of activation. This finding is in agreement with results from the young group of participants [Malandraki et al., 2009a]. Interestingly, tongue tapping and throat clearing showed activations in a more dorsal area of the primary motor and premotor cortex than swallowing. Subcortical activations of the insula, the thalamus, and the lentiform nuclei were mainly seen during swallowing, however tongue tapping also elicited activation of the putamen in the left hemisphere and throat clearing activation of a small portion of the left insular cortex.
Tongue tapping and throat clearing also can be associated with other physiological events apart from swallowing. Specifically, tongue tapping, which is an oral manipulation task, is further involved in articulation. During production of alveolar sounds, the tip of the tongue contacts the alveolar ridge (e.g. for the production of sounds/t/and/d/) in a similar way that the tongue tip begins the transfer of the bolus from the anterior to the posterior oral cavity during the oral transport stage of swallowing. Additionally, throat clearing involves the abrupt approximation of the true vocal folds. True vocal fold approximation in a lighter form is also occurring during phonation. Thus, it may be speculated that some of the tasks we have examined can also explain neural activation areas for other physiological events and are not limited to swallowing. Since we did not directly test these tasks, this can only stated as an interesting assumption that needs further investigation.
Percent signal changes of the BOLD response in predefined ROIs were used to examine the second objective of comparing activations between young and old adults. During swallowing and tongue tapping, younger adults exhibited significantly increased amplitude of activation in portions of the primary somatosensory cortex and motor area 4p. During planning, differences were significant only in somatosensory BA 1, whereas during throat clearing, most sensorimotor areas examined were significantly more activated in the younger group. The fact that some mean differences between the two groups did not reach statistical significance, may be attributed to the overall small sample size used in the present study. Sensitivity to these differences would likely have been improved with a larger sample size.
Primary Motor Areas are Preserved Across Age
A closer look at all areas of activation during swallowing and related tasks in both age groups revealed an interesting pattern of preserved and declining functions. It appears that both young and elderly adults show significant activations in the major motor areas involved in the execution of movement, that is, the primary motor and premotor areas and the cingulate motor cortex, during swallowing, tongue tapping, and throat clearing. Additionally, amplitude differences between the two groups in the primary motor areas were not statistically significant for most of the tasks examined. These results indicate that the cortically regulated motor control of swallowing remains relatively well preserved in old age.
As discussed earlier, declines in the motor portions of the oropharyngeal swallowing mechanism have been noted as people age. However, these declines have been previously attributed to changes in muscle composition [Cartee, 1995; Faulkner et al., 1995; Newton et al., 1987; Price and Darvell, 1982]. The results of the present investigation are in agreement with this notion. The primary motor cortex areas appeared relatively functionally preserved in our elderly group across most tasks examined, which points toward the argument that motor declines in aging deglutition are usually associated to the end organs, i.e., the muscles and the loss of muscle mass and strength, while the cortical motor control of swallowing remains relatively preserved. Future research combining measurements of oropharyngeal muscle composition and strength with neuroimaging methods is needed to help elucidate how accurate this finding is.
Somatosensory and Sensorimotor Integration Declines With Age
In contrast to the findings in the primary motor areas, areas that are involved in sensory processing and integration between sensory inputs and motor outputs and or motor coordination and control, such as the parietal lobules, the thalamus, the insular cortex, the cerebellum, and the basal ganglia showed limited if any activation in the elderly group. These areas have been found to be activated in healthy young adults [Malandraki et al., 2009a; Suzuki et al., 2003; Toogood et al., 2005], This is partially in agreement with the results of both Martin et al. [ 2007] and Humbert et al. [ 2009] that did not report significant cerebellar or extensive subcortical activations in their elderly subjects. Furthermore, in this study, neural activity amplitude differences between the two groups were statistically significant in most of the primary somatosensory cortex areas examined across the three‐motor tasks.
The reduced neural activation in the primary somatosensory cortex, and in the sensory association and sensorimotor integration areas in the elderly observed in the present investigation may explain some of the sensory oropharyngeal age‐related declines seen in swallowing. As was previously described, healthy older adults exhibit increased sensory thresholds in order to trigger the swallowing pharyngeal response [Shaker et al., 1994; Robbins et al., 1992], significant delay in the pharyngeal response initiation [Robbins et al., 1992], reduced taste perception [Calhoun et al., 1992; Fukunaga et al., 2005], and increased instances of laryngeal penetration [Daniels et al., 2004; Robbins et al., 1999].
This could be a rather significant clinical finding in that it may suggest that swallowing treatments targeting sensory components of deglutition (e.g. sensory stimulation techniques) have to target the neurophysiological underpinnings of such a sensory decline in order to be effective for patients with dysphagia. Healthy aging is known to be accompanied by a delay in the onset of the pharyngeal swallowing events [Robbins et al., 1992]. With onset of additional age‐related conditions, (e.g. stroke, dementia etc.), including frailty [Ney et al., 2009; Robbins et al., 2005], elderly individuals frequently present with an abnormally long delay in the initiation of the pharyngeal swallowing response [Rosenbek et al., 1996] known to dramatically increase the risk for aspiration [Horner et al., 1990] and for aspiration pneumonia in these populations [Linden and Siebens, 1983]. This work on the pharyngeal response delay, in combination with our present findings, suggest the increasing need for future studies investigating effects of sensory treatments on swallowing physiology and neurophysiology for identification of optimal treatment targeting sensory deficits in swallowing.
Comparison With Previous Studies
In contrast with findings from previous functional imaging studies examining age‐related changes in the neural control of motor tasks [Humbert et al., 2009; Hutchinson et al., 2002; Mattay et al., 2002], this study observed less distributed activation in older adults in all ROIs in both hemispheres and for all four tasks examined. This difference in distribution was not statistically tested in this study and cannot be evaluated in terms of its significance; however, it contradicts the observation of “overactivation” (i.e., more distributed activations) in the sensorimotor cortex of the elderly seen in previous neuroimaging studies of simple motor control [Hutchinson et al., 2002; Mattay et al., 2002] and swallowing [Humbert et al., 2009].
There are, however, several experimental design and methodological differences between the present investigation and the study by Humbert et al. [ 2009] that could partially explain the different results. First, we used a 3 ml water bolus to elicit the swallow responses, whereas Humbert et al. [ 2009] used saliva swallows and 5 ml water swallows, as well as barium presentations. Additionally, although both studies used an event related design with randomized interstimulus intervals, the present investigation also used BIG methodology to correct for motion artifacts [Gracco et al., 2005]. If the statement of Humbert et al. [ 2009], that the cortical “overactivation” seen in their elderly sample may be caused by increased effort during swallowing, is valid, it becomes even more important to significantly control for motion artifacts that may be caused by that increased effort. Additionally, the saliva swallows that were tested in their study were cued differently (visually), than the water and barium swallows (tactile cueing through direct presentation of the material in the oral cavity), and that might have also affected the increased activations seen during saliva swallows. In this investigation, all subjects were cued with the same cueing method (visual cueing) for all tasks. Some of these differences may account for the discrepancies in neural activation seen in these two studies. A larger sample size would be needed for definitive conclusions to be made regarding these issues.
Limitations of This Study
There are several factors to consider in interpreting the results of this study. First, the data reported are based on two groups of 10 and 9 subjects each. Larger sample sizes are needed to further investigate the differences between neural activation of swallowing in young and old healthy adults. Furthermore, results reported for each group included both genders. Although structural and physiological differences do exist between male and female brains [Gur et al., 2002], gender comparisons were not completed at this time due to the limited number of subjects for each gender. Future studies on the neural control of swallowing should include a balanced number of males and females that would enable gender comparisons to be completed.
Another consideration is the fact that planning of deglutition without execution did not show any significantly activated areas for the elderly group of participants. This is likely due to the limited activations that were seen in the single subject level analysis during this task. With planning being a weakly activating task, the activations appear to be subthreshold for this group size. Adding more subjects in future studies may increase power and result in detectable activations.
CONCLUSION
Overall, this study added to the limited body of literature on the functional neural control of swallowing and related tasks in healthy elderly adults by (a) examining the identification of the neural activation during separate components of the swallowing process and (b) studying potential age differences in the amplitude of neural activation in selected ROIs. This study provides evidence that swallowing requires larger and more widespread areas for neural control than the combination of the component tasks examined (planning, tongue tap, and throat clearing tasks). In addition, we found, in general, decreased activations in older adults when compared to their younger counterparts during swallowing and component tasks. For swallowing, these reductions were significant in several primary somatosensory areas indicating a decline in the neural processing of sensory signals for coordinating the swallow response.
This work was performed at the Biomedical Imaging Center of the Beckman Institute at the University of Illinois at Urbana‐Champaign.
REFERENCES
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K ( 2000): Brodmann's areas 17 and 18 brought into stereotaxic space—Where and how variable? NeuroImage 11: 66–84. [DOI] [PubMed] [Google Scholar]
- Aviv JE ( 1997): Effects of aging on sensitivity of the pharyngeal and supraglottic areas. Am J Med 103: 74S–76S. [DOI] [PubMed] [Google Scholar]
- Beckmann C, Jenkinson M, Smith SM ( 2003): General multi‐level linear modelling for group analysis in FMRI. NeuroImage 20: 1052–1063. [DOI] [PubMed] [Google Scholar]
- Buckner RL ( 2002): Age‐Related Changes in Neural Activity During Episodic Memory. Paper presented at the Symposium on Neuroscience, Aging and Cognition, San Francisco.
- Calhoun KH, Gibson B, Hartley L, Minton J, Hokanson JA ( 1992): Age‐related changes in oral sensation. Laryngoscope 102: 109–116. [DOI] [PubMed] [Google Scholar]
- Cartee GD ( 1995): What insights into age‐related changes in skeletal muscle are provided by animal models? J Gerontol A Biol Sci Med Sci 50: 137–141. [DOI] [PubMed] [Google Scholar]
- Cook IJ, Weltman MD, Wallace K, Shaw DW, McKay E, Smart RC, Butler SP ( 1994): Influence of aging on oral‐pharyngeal bolus transit and clearance during swallowing: scintigraphic study. Am J Physiol Gastrointest Liver Physiol 29: G972–G977. [DOI] [PubMed] [Google Scholar]
- Daniels SK ( 2000): Swallowing apraxia: A disorder of the praxis system? Dysphagia 15: 159–166. [DOI] [PubMed] [Google Scholar]
- Daniels SK, Brailey K, Priestly DH, Herrington LR, Weisberg LA, Foundas AL ( 1998): Aspiration in patients with acute stroke. Arch Phys Med Rehabil 79: 14–19. [DOI] [PubMed] [Google Scholar]
- Daniels SK, Corey DM, Hadskey LD, Legendre C, Priestly DH, Rosenbek JC, Foundas AL ( 2004): Mechanism of sequential swallowing during straw drinking in healthy young and older adults. J Speech Hear Res 47: 33–45. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K ( 2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Faulkner JA, Brooks SV, Zerba E ( 1995): Muscle atrophy and weakness with aging: Contraction‐induced injury as an underlying mechanism. J Gerontol A Biol Sci Med Sci 50: 124–129. [DOI] [PubMed] [Google Scholar]
- Feldman RS, Kapur KK, Alman JE, Chauncey HH ( 1980): Aging and mastication: Changes in performance and in the swallowing threshold with natural dentition. J Am Geriatr Soc 28: 97–103. [DOI] [PubMed] [Google Scholar]
- Fukunaga A, Uematsu H, Sugimoto K ( 2005): Influences of aging on taste perception and oral somatic sensation. J Gerontol A Biol Sci Med Sci 60: 109–113. [DOI] [PubMed] [Google Scholar]
- Geyer S ( 2004): The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Adv Anat Embryol Cell Biol 174: 1–89. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, Klingberg T, Larsson J, Zilles K, Roland PE ( 1996): Two different areas within the primary motor cortex of man. Nature 382: 805–807. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schleicher A, Zilles K ( 1999): Areas 3a, 3b, and 1 of human primary somatosensory cortex. NeuroImage 10: 63–83. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schormann T, Mohlberg H, Zilles K ( 2000): Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2: Spatial normalization to standard anatomical space. NeuroImage 11: 684–696. [DOI] [PubMed] [Google Scholar]
- Gracco VL, Tremblay P, Pike B ( 2005): Imaging speech production using fMRI. NeuroImage 26: 294–301. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K ( 2001): Human somatosensory area 2: Observer‐independent cytoarchitectonic mapping, interindividual variability, and population map. NeuroImage 14: 617–631. [DOI] [PubMed] [Google Scholar]
- Gur RC, Gunning‐Dixon FM, Turetsky BI, Bilker WB, Gur RE ( 2002): Brain region and sex differences in age association with brain volume: A quantitative MRI study of healthy young adults. Am J Geriatr Psychiatry 10: 72–80. [PubMed] [Google Scholar]
- Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant E ( 1999): Cortical activation during human volitional swallowing: An event‐related fMRI study. Am J Physiol 277: G219–G225. [DOI] [PubMed] [Google Scholar]
- Horner J, Massey EW, Brazer SR ( 1990): Aspiration in bilateral stroke patients. Neurology 40: 1686–1688. [DOI] [PubMed] [Google Scholar]
- Huckabee ML, Deecke L, Cannito MP, Gould HJ, Mayr W ( 2003): Cortical control mechanisms in volitional swallowing: The Bereitschaftspotential. Brain Topogr 16: 3–17. [DOI] [PubMed] [Google Scholar]
- Humbert IA, Fitzgerald ME, McLaren DG, Johnson S, Porcaro E, Kosmatka K, Hind J, Robbins J ( 2009): Neurophysiology of swallowing: Effects of age and bolus type. NeuroImage 44: 982–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson S, Kobayashi M, Horkan CM, Pascual‐Leone A, Alexander MP, Schlaug G ( 2002): Age‐related differences in movement representation. NeuroImage 17: 1720–1728. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S ( 2002): Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17: 825–841. [DOI] [PubMed] [Google Scholar]
- Kern M, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, Shaker R ( 2001a): Swallow‐related cerebral cortical activity maps are not specific to deglutition. Am J Physiol Gastrointest Liver Physiol 280: G531–G538. [DOI] [PubMed] [Google Scholar]
- Kern MK, Jaradeh S, Arndorfer RC, Shaker R ( 2001b): Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol 280: G354–G360. [DOI] [PubMed] [Google Scholar]
- Leslie P, Drinnan MJ, Ford GA, Wilson JA ( 2005): Swallow respiratory patterns and aging: Presbyphagia or dysphagia? J Gerontol A Biol Sci Med Sci 60: 391–395. [DOI] [PubMed] [Google Scholar]
- Linden P, Siebens AA ( 1983): Dysphagia: Predicting laryngeal penetration. Arch Phys Med Rehabil 64: 281–284. [PubMed] [Google Scholar]
- Logemann JA ( 1998): Evaluation and Treatment of Swallowing Disorders, 2nd ed. Austin, TX: Pro‐Ed. [Google Scholar]
- Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH ( 2000): Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. J Speech Hear Res 43: 1264–1274. [DOI] [PubMed] [Google Scholar]
- Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C ( 2009a) Neural activation of swallowing and swallowing related tasks in healthy young adults: An attempt to separate the components of deglutition. Hum Brain Mapp 30: 3209–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malandraki GA, Sutton BP, Perlman AL, Karampinos DC ( 2009b): Age‐related differences in laterality of cortical activations in swallowing. Dysphagia. DOI, 10.1007/s00455‐009‐9250‐z. [DOI] [PubMed] [Google Scholar]
- Martin RE, Goodyear BG, Gati JS, Menon RS ( 2001): Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol 85: 938–950. [DOI] [PubMed] [Google Scholar]
- Martin RE, MacIntosh BJ, Smith RC, Barr AM, Stevens TK, Gati JS, Menon RS ( 2004): Cerebral areas processing swallowing and tongue movement are overlapping but distinct: A functional magnetic resonance imaging study. J Neurophysiol 92: 2428–2443. [DOI] [PubMed] [Google Scholar]
- Martin RE, Barr A, MacIntosh B, Smith R, Stevens TK, Taves D, Gati J, Menon R, Hachinski V ( 2007): Cerebral cortical processing of swallowing in older adults. Exp Brain Res 176: 12–22. [DOI] [PubMed] [Google Scholar]
- Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R ( 2005): Dysphagia after stroke: Incidence, diagnosis, and pulmonary complications. Stroke 36: 2756–2763. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR ( 2002): Neurophysiological correlates of age‐related changes in human motor function. Neurology 58: 630–635. [DOI] [PubMed] [Google Scholar]
- Mosier KM, Bereznaya I ( 2001): Parallel cortical networks for volitional control of swallowing in humans. Exp Brain Res 140: 280–289. [DOI] [PubMed] [Google Scholar]
- Mosier KM, Liu WC, Maldjian JA, Shah R, Modi B ( 1999): Lateralization of cortical function in swallowing: A functional MR imaging study. Am J Neuroradiol 20: 1520–1526. [PMC free article] [PubMed] [Google Scholar]
- Newton JP, Abel EW, Robertson EM, Yemm R ( 1987): Changes in human masseter and medial pterygoid muscles with age: a study by computed tomography. Gerodontics 3: 151–154. [PubMed] [Google Scholar]
- Ney DM, Weiss JM, Kind AJH, Robbins J ( 2009): Senescent swallowing: Impact, strategies, and interventions. Nut Clin Pract 24: 395–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia MA, Hind JA, Roecker EB, Carnes M, Doyle J, Dengel GA, Robbins J ( 2000): Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A Biol Sci Med Sci 55: M634–M640. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR ( 2004): Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci USA 101: 13091–13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman AL, Christensen J ( 1997): Topography and functional anatomy of the swallowing structures In: Perlman AL, Schulze‐Delrieu KS, editors. Deglutition and Its Disorders. San Diego, CA: Singular Publication Group; pp. 15–42. [Google Scholar]
- Price PA, Darvell BS ( 1982): Force and mobility in the aging human tongue. Med J Aust 1: 75–78. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning‐Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD ( 2004) Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiol Aging 25: 377–396. [DOI] [PubMed] [Google Scholar]
- Robbins J, Hamilton JW, Lof GL, Kempster G ( 1992): Oropharyngeal swallowing in normal adults of different ages. Gastroenterology 103: 823–829. [DOI] [PubMed] [Google Scholar]
- Robbins J, Levine R, Maser A, Rosenbek JC, Kempster GL ( 1993): Swallowing after unilateral cerebral stroke. Clin Commun Disord 3: 45–55.8111364 [Google Scholar]
- Robbins J, Levine R, Wood J, Roecker EB, Luschei E ( 1995): Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol A Biol Sci Med Sci 50: M257–M262. [DOI] [PubMed] [Google Scholar]
- Robbins J, Coyle J, Roecker E, Rosenbek J, Wood J ( 1999): Differentiation of normal and abnormal airway protection during swallowing using the penetration‐aspiration scale. Dysphagia 14: 228–232. [DOI] [PubMed] [Google Scholar]
- Rosenbek J, Roecker E, Wood J, Robbins J ( 1996): Thermal application reduces the duration of stage transition in dysphagia after stroke. Dysphagia 11: 225–233. [DOI] [PubMed] [Google Scholar]
- Robbins J, Gangnon R, Theis S, Kays SA, Hind J ( 2005): The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc 53: 1483–1489. [DOI] [PubMed] [Google Scholar]
- Shaker R, Ren J, Zamir Z, Sarna A, Liu J ( 1994): Effect of aging, position, and temperature on the threshold volume triggering pharyngeal swallows. Gastroenterology 107: 396–402. [DOI] [PubMed] [Google Scholar]
- Smith S ( 2002): Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy R, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM ( 2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23: 208–219. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Asada Y, Ito J, Hayashi K, Inoue H, Kitano H ( 2003): Activation of the cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia 18: 71–77. [DOI] [PubMed] [Google Scholar]
- Toogood JA, Barr AM, Stevens TK, Gati JS, Menon RS, Martin RE ( 2005): Discrete functional contributions of cerebral cortical foci in voluntary swallowing: A functional magnetic resonance imaging (fMRI) “Go, No‐Go” study. Exp Brain Res 161: 81–90. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans, AC ( 1996): A unified statistical approach for determining significant voxels in images of cerebral activation. Hum Brain Map 4: 58–73. [DOI] [PubMed] [Google Scholar]


