Abstract
Human brain pathways required for language processing are poorly known. A new white matter tract in humans, the middle longitudinal fascicle, has recently been anatomically determined by diffusion tensor imaging and suggested to be essential for language. Our aim is to determine the importance of the middle longitudinal fascicle for language processing. This study is based on 8 patients with glioma resection at least involving the superior temporal gyrus of the left dominant hemisphere. Language is systematically examined pre‐ and postoperatively at 3 months. Intraoperative electrostimulation is used to map cortical and subcortical structures as functional boundaries of the glioma resection, including those essential for language processing. The resections are extensive (on average 62 ml, ranging from 21 to 111 ml) and include a large part of the middle longitudinal fascicle in all patients. Intraoperatively, no interference with picture naming is observed by electrostimulation of the middle longitudinal fascicle, while in all patients the inferior fronto‐occipital fascicle is identified by eliciting semantic paraphasia as functional boundary. Postoperatively, no new permanent language deficits are detected by systematic language examination. Therefore, we suggest that the middle longitudinal fascicle may participate but is not essential for language processing. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: language, middle longitudinal fascicle, electrostimulation, brain mapping, connectivity
INTRODUCTION
The anatomical determination of white matter tracts in man is leaping forward compared to their functional assignment. This can be explained by recent advances in neuroimaging with diffusion tensor imaging (DTI) that provides detailed anatomy of fascicles but no functional information [Catani et al.,2002; Catani and Thiebaut de Schotten,2008; Fernandez‐Miranda et al.,2008; Wakana et al.,2004]. Function of fascicles is largely determined by lesion studies, for instance in stroke patients, or indirectly by DTI connectivity of cortical areas of which the function was assigned using functional MR imaging, positron emission tomography or magneto‐encephalography, or scarcely by single cell recordings in patients. Lesion studies are limited by the fact that the areas involved are often larger than single fascicles. Functional neuroimaging techniques such as functional MR imaging, PET and MEG are limited by temporal, spatial, and chemical resolution and a low signal‐to‐noise ratio due to artifacts from various sources such as venous effects, patient motion and task compliance [Kim and Ogawa,2002; Kim and Singh,2003; Logothetis,2008; Turner and Jones,2003; Wheless et al.,2004]. A customary approach to directly map function of white matter tracts is electrostimulation of fascicles in patients under local anesthesia for resective brain surgery, as demonstrated for the superior longitudinal fascicle [Duffau et al.,2002], the inferior fronto‐occipital fascicle [Duffau et al.,2005] and the inferior longitudinal fascicle [Mandonnet et al.,2007b]. This allows for detailed anatomofunctional correlation of fascicles, basically only limited by the time available during brain surgery. The present study is an adjunct to these earlier works.
Recently, a new white matter tract for the human brain was anatomically defined by DTI, which was termed the middle longitudinal fascicle (MdLF) [Makris and Pandya,2009; Makris et al.,2009]. In earlier studies using autoradiographic histological tract tracing the MdLF was identified for macaque [Seltzer and Pandya,1984] and in later studies diffusion spectrum imaging was validated to autoradiographic tracing of tracts in nonhuman primates, which included the MdLF [Schmahmann et al.,2007]. The MdLF connects the angular gyrus [AG, Brodmann's area (BA) 39] with the superior temporal gyrus (STG, BA 22) up to the temporal pole (BA 38) and courses in the white matter within the STG. Several fascicles are in proximity to the MdLF. The MdLF is localized medial and ventral with respect to the superior longitudinal fascicle segment II (SLF‐II) and arcuate fascicle (AF) [Makris and Pandya,2009; Makris et al.,2009]. The MdLF is located lateral and superior from the inferior fronto‐occipital fascicle (IFOF). Posterior the MdLF and IFOF are closely‐related though well differentiated in parallel at the sagittal stratum and anterior the MdLF and IFOF are separated by the temporal ramus of the sylvian fissure and the cortex of the insula and the STG. The MdLF is located dorsal in relation to the inferior longitudinal fascicle (ILF) [Makris and Pandya,2009; Makris et al.,2009].
Because of the anatomical connectivity of the MdLF between the AG and the STG, the MdLF in has been hypothesized to participate in language function in particular comprehension of speech in the dominant hemisphere [Makris et al.,2009]. Even more so, the MdLF has been speculated as the main connection between “Geschwind's territory” and “Wernicke's territory” [Makris and Pandya,2009], that may be closely related to the vertical segment of the AF [Catani et al.,2005; Frey et al.,2008; Makris et al.,2005; Makris et al.,2009]. In the nondominant hemisphere the MdLF has been hypothesized to be involved in spatial attention, attention processing and episodic memory [Cabeza et al.,2008]. Dysfunction of spatial attention may result in the hemi‐inattention of the neglect syndrome [Committeri et al.,2007; Mort et al.,2003].
Here, we present functional assignment of the MdLF in the left dominant hemisphere using intraoperative electrostimulation of cortical and subcortical connections in relation to postoperative language outcome in patients with glioma resection involving the STG.
MATERIALS AND METHODS
Participants
Patients were selected which had a glioma resection that involved the STG in the left dominant hemisphere. Eight patients had intraoperative language mapping and follow‐up with postoperative language examination and were eligible for retrospective analysis. In these patients, gliomas were resected until interference with the tasks performed was encountered, so that functional boundaries determined the resection cavity. Preoperative characteristics of the patients are detailed in Table I. Handedness was verified by the Edinburgh inventory [Oldfield,1971].
Table I.
Patient and tumor characteristics
| Id | Age/ gender | Handedness | Presenting symptoms | Tumor localization | Tumor volume in cm3 | Histopathological diagnosis |
|---|---|---|---|---|---|---|
| 1 | 38 F | R | Partial seizures with aphasia | L temporal | 95 | Low grade glioma |
| 2 | 27 F | R | Partial seizures with vertigo and dysesthesia | L temporoinsular | 185 | Anaplastic glioma |
| 3 | 61 F | R | Partial seizures with aphasia | L temporoinsular | 45 | Low grade glioma |
| 4 | 31 M | L | Partial jacksonian seizures with aphasia, interictal dysphasia, working and episodic memory deficit | L temporoinsular | 210 | Anaplastic glioma |
| 5 | 32 M | R | Partial seizures with dysphasia | L mesiotemporal | 98 | Low grade glioma |
| 6 | 55 M | R | Partial seizures with aphasia, interictal working memory, attention and concentration deficit | L temporoinsular | 32 | Low grade glioma |
| 7 | 27 M | R | Partial seizures | L temporoinsular | 75 | Low grade glioma |
| 8 | 54 F | R | Single complex seizure | L temporal | 50 | Anaplastic glioma |
Language Assessment
Language functionality was determined for all patients in a standardized way utilizing the DO‐80 [Metz‐Lutz et al.,1991] and the Boston Diagnostic Aphasia Examination [Goodglass et al.,2000] by an independent speech therapist. The preoperative examination was performed on the day of admission. The immediate postoperative analysis was scheduled in the week after surgery before hospital discharge and the final postoperative analysis was obtained at least three months after surgery using the same tests.
Intraoperative Mapping
The method of intraoperative mapping was previously described [Duffau et al.,2002,2005; Mandonnet et al.,2007b].
A wide craniotomy completely exposing the sylvian fissure, the frontal operculum and the superior, middle, and part of the inferior temporal gyrus was performed under sedation. The glioma margins were verified in relation to the sulcal and gyral brain surface anatomy with intraoperative ultrasonography. Sterile tags labeled with letters marked the glioma contour on the cortex.
Prior to glioma resection, the cortex was mapped for language and sensorimotor interference sites in all patients in awake conditions under local anaesthesia. A bipolar electrode (Nimbus*; Newmedic, Labège, France) with 5 mm tip spacing was utilized for mapping to apply electrostimulation with a biphasic current intensity between 1.5 and 5 mA (60 Hz pulse frequency, 1 ms single pulse phase, ∼4 s tissue contact) while naming picture objects presented every 4 s on a computer monitor. The DO‐80 set was used for the picture naming task. A speech therapist determined the language responses of patients in terms of anarthria, anomia, phonological, or semantic paraphasia and perseveration, while the patient and the speech therapist were unaware of the timing of electrostimulation application. At the ventral premotor cortex, speech arrest could be induced in all patients while counting aloud. This served as a mandatory positive reference site for optimizing the stimulation intensity parameter for each individual increasing from a baseline of 1.5 mA in cumulative steps of 0.5 mA until a reproducible response was obtained. A cortical site of 5 × 5 mm was considered positive for language function when any interference with picture naming was met at three nonsequential stimulations followed by normalization after stimulation. Sterile numbered tags marked the positive stimulation sites and a photograph before resection was taken capturing the cortical map for each individual.
After completion of cortical mapping, the glioma resection was started during which the subcortical structures, such as the white matter tracts and grey nuclei, were systematically electrostimulated while the patient performed continuous picture naming to identify language pathways. The resection cavity was extended up to the connecting fascicles as functional boundaries so that a maximal glioma resection was obtained while preserving essential cortical and subcortical functional structures. The essential functional structures, that are customarily encountered in temporal resections of the language dominant hemisphere, are the vertical part of the AF posterolaterally inducing phonological paraphasia, the IFOF medially inducing semantic paraphasia and the optic radiation dorsoposteriorly inducing phosphenes or visual neglect. When a part of the insula invaded by glioma is removed, the striatum is encountered medially inducing articulation deficits. After completion of the resection, a second photograph was taken containing sterile numbered tags for the subcortical positive sites.
The positive stimulation sites on the intraoperative photographs were compared in relation to sulcal anatomy and summarized on the image of a brain from a single subject.
Neuroimaging
Routine pre‐ and postoperative MR imaging included T1‐weighted images before and after gadolinium infusion in the axial, sagittal and coronal orthogonal planes, T2‐weighted and fluid attenuated inversion recovery sequences.
The anatomical definitions used were according to generally‐accepted criteria [Scarabino and Duvernoy,2006]. The STG was defined by the sylvian fissure superiorly, the superior temporal sulcus (STS) inferiorly and the inferior circular sulcus of the insula inferomedially. To determine the extent of resection of the STG, the overall length of the STG was measured in the sagittal plane from the temporal pole to the terminal ascending segment of the sylvian fissure according to standard anatomical criteria [Crespo‐Facorro et al.,2000; Kim et al.,2000]. Then, the length of the resected part of the STG from the temporal pole to the posterior margin of the resection cavity also measured in the sagittal plane was measured and the percentage of the resected length of the STG was calculated. Measures were taken from the MR imaging at least three months postoperatively.
The MdLF can be clearly located on standard coronal MR imaging, because this fascicle is traversing from anterior to posterior within the white matter of the STG in between the inferior insular sulcus and the STS.
The resection cavity was outlined for each patient using MRIcron software (http://www.sph.sc.edu/comd/rorden/mricron/) on the Montreal Neurological Institute single subject brain template (http://www.bic.mni.mcgill.ca/cgi/icbm_view/) based on the postoperative images as detailed in Mandonnet et al.,2007a. The resection cavity volumes were calculated from the outlines. In this way a probabilistic map of the resection cavities was obtained. For orientation of the slices of the probabilistic map, the DTI maps with the outline of the MdLF were used with permission as published by Makris et al.,2009.
RESULTS
The study population consisted of 4 females and 4 males, on average 41 years of age with a range from 27 to 61. All presented with epilepsy, 5 patients had ictal language deficits. Two patients presented with interictal dysphasia and cognitive dysfunction. The gliomas were limited to the temporal lobe in 3 patients and had extension from the temporal lobe to the insula in 5 patients. The tumor volumes ranged from 32 to 210 cm3, on average 99 cm3. The observations of language outcome, electrostimulation mapping and neuroimaging for the 8 patients are listed in Tables II, III, IV.
Table II.
Language Outcome and examination preoperative, immediately postoperative and at 3 months after surgery
| DO‐80a | Boston diagnostic aphasia examination | ||||||
|---|---|---|---|---|---|---|---|
| Id | Final language outcome | Preoperative | Immediately postoperative | At 3 months | Preoperative | Immediately postoperative | At 3 months |
| 1 | Improvement | 77 (1/1/1/0) | 80 (0/0/0/0) | 80 (0/0/0/0) | Normal | Normal | Normal |
| 2 | Stable | 80 (0/0/0/0) | na | 80 (0/0/0/0) | Normal | na | Normal |
| 3 | Stable | 80 (0/0/0/0) | 68 (4/8/0/0) | 78 (1/1/0/0) | na | Syntactic comprehension | normal |
| Semantics | |||||||
| 4 | Improvement | 72 (5/2/1/0) | 77 (3/0/0/0) | na | Syntactic comprehension | Syntactic comprehension, reading | na |
| 5 | Stable | 78 (0/1/1/0) | na | 78 (0/1/1/0) | Normal | na | Normal |
| 6 | Stable | 78 (2/0/0/0) | 69 (6/4/1/0) | 77 (0/3/0/0) | na | na | Normal |
| 7 | Stable | 79 (0/1/0/0) | 63 (3/9/2/3) | 80 (0/0/0/0) | Normal | Syntactic comprehension | Normal |
| Reading, writing | |||||||
| 8 | Slight decrease | 77 (1/1/1/0)) | 64 (12/2/2/0) | 66 (9/4/1/0) | Normal | Syntactic comprehension | Discrete dysnomia, semantic paraphasia, dyssyntaxia |
Total (anomia/semantic paraphasia/phonological paraphasia/perseveration).
na, not available.
Table III.
Cortical and subcortical electrostimulation mapping observations
| Cortical | Subcortical | |||||||
|---|---|---|---|---|---|---|---|---|
| Id | IFG | VPMC | STG | MTG | ITG | IFOF | SLF‐II/AF | Other |
| 1 | nd | Speech arrest | None | None | none | Dysnomia | Phonological paraphasia, vertigo | Perseveration (striatum) |
| 2 | None | Speech arrest | Hesitation | nd | nd | Semantic paraphasia | Phonological paraphasia | Pseudohallucination, visual blurring in right hemifield (OR) |
| 3 | None | Speech arrest | Dysnomia | nd | nd | Semantic paraphasia | Phonological paraphasia | Speech arrest (striatum) |
| 4 | Phonological paraphasia | Speech arrest | None | Semantic paraphasia | nd | Semantic paraphasia, anomia | Phonological paraphasia | |
| 5 | nd | Speech arrest | Semantic paraphasia | Anomia | nd | Semantic paraphasia | Phosphenes in right hemifield (OR) | |
| 6 | None | Speech arrest | None | None | nd | Semantic paraphasia | Phonological paraphasia | Speech arrest, dysarticulation (striatum) |
| 7 | nd | Speech arrest | Semantic paraphasia | nd | nd | Semantic paraphasia | ||
| 8 | nd | Speech arrest | Anomia | nd | nd | Semantic paraphasia | ||
nd, not determined; IFG, inferior frontal gyrus; VPMC, ventral premotor cortex; STG, superior temporal gyrus; MTG, medial temporal gyrus; ITG, inferior temporal gyrus; IFOF, inferior fronto‐occipital fascicle; SLF‐II/AF, superior longitudinal fascicle segment II/arcuate fascicle; OR, optic radiation.
Table IV.
Postoperative neuroimaging results
| Id | Resection cavity volume in cm3 | Distance from temporal pole to posterior resection margin in mm | Distance from temporal pole to ascending ramus of sylvian fissure in mm | Percentage of superior temporal gyrus length resected (%) |
|---|---|---|---|---|
| 1 | 111 | 65 | 68 | 96 |
| 2 | 54 | 37 | 75 | 49 |
| 3 | 55 | 39 | 76 | 51 |
| 4 | 21 | 24 | 85 | 28 |
| 5 | 71 | 36 | 72 | 50 |
| 6 | 70 | 43 | 78 | 55 |
| 7 | 43 | 34 | 79 | 43 |
| 8 | 69 | 38 | 66 | 58 |
Language Outcome
Full language examination immediately after surgery was omitted in two patients due to logistic reasons, but postoperative aphasia was not observed. One patient has not had language examination at three months, because of a language improvement at language examination immediately after surgery. Language examination before surgery was confirmed to be within normal ranges in 7 patients. Six patients experienced less than maximal language scores with between 1 and 8 deficits out of 80 items. Immediately after surgery, language performance was normal in one patient and a variety of temporary language deficits were recognized in 5 patients, predominantly anomia and semantic paraphasia and only incidentally phonological paraphasia and perseveration. For all patients except one, immediate postoperative language deficits resolved to preoperative levels or better at final language examination at 3 months postoperatively after completion of dedicated speech rehabilitation. One patient had a slight decrease in language scores, which did not have an impact on her quality of life, because it is barely noticeable in everyday verbal communication according to the patient. Therefore, no new permanent language deficits were induced by these glioma resections involving the STG using systematic electrostimulation language mapping.
Electrostimulation Mapping Findings
In all 8 patients, language dominance of the left hemisphere was confirmed by the positive language sites obtained by the cortical mapping, including the 31‐year‐old left‐handed male (Id 4). After identification of the ventral premotor cortex, the frontal operculum was not further cortically‐mapped when this was of no consequence to the resection strategy. At least one positive language site was identified on the STG in 5 patients, on the MTG in 2 patients and no language sites were identified on the ITG (see Fig. 1). In 2 patients no positive language sites were identified over the exposed temporal cortex. Subcortical mapping determined the posteromedial margin of the resection by language interference in all cases, usually inducing semantic paraphasia, which in correlation with the postoperative MR images indicated the IFOF. The posterolateral margin of the resection was established by phonemic paraphasia in 5 patients, in correlation with the postoperative images indicating the vertical portion of the AF. In 2 patients phosphenes were induced, which suggested the optic radiation. In 3 patients with an insular extension of glioma invasion speech arrest or perseveration were induced at the medial margin of the resection, corresponding to the striatum. Although identification of the MdLF was not an explicit part of the electrostimulation and resection strategy, no language interference was observed intraoperatively neither during stimulating at the location of the MdLF nor during resection, while the patient continuously performed picture naming. In all patients the first subcortical language interference at the level of the STG consisted of semantic paraphasia at the level of the IFOF, medial from the MdLF as confirmed by the postoperative MR imaging.
Figure 1.
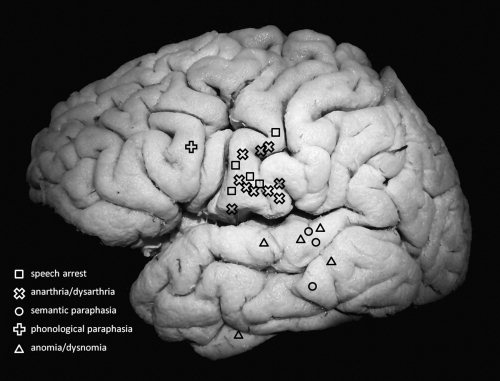
Summary of language interference sites identified by cortical electrostimulation during picture naming in 8 patients with a glioma involving the superior temporal gyrus.
A case illustrating the typical findings of cortical and subcortical electrostimulation mapping is shown in Figure 2.
Figure 2.
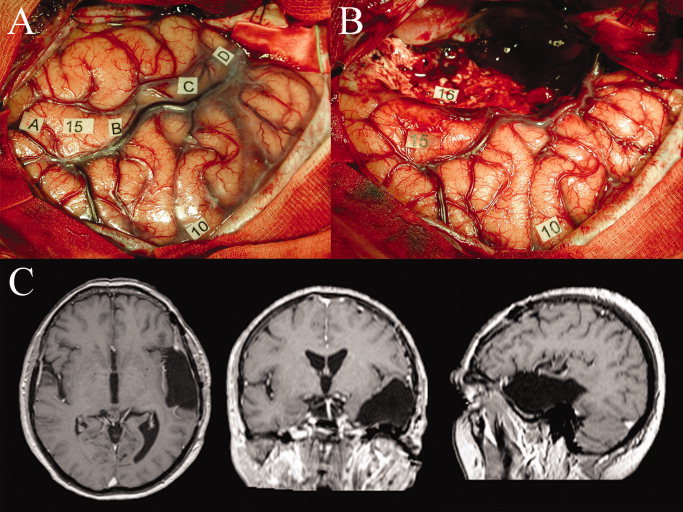
Patient example (Id 8) with typical cortical and subcortical mapping observations and anatomofunctional correlation of intraoperative photographs before and after resection with postoperative neuroimages in axial, coronal, and sagittal views. Panel A: Intraoperative photograph with cortical exposure after left‐sided craniotomy showing a large draining sylvian vein that divides the temporal operculum (left‐upper half of panel) from the frontal operculum (right‐lower half of image). A small superior temporal gyrus, a medial temporal gyrus with tumoral enlargement and a small part of the inferior temporal gyrus are visible. The glioma contour has been verified by ultrasonography and is marked with letter tags A to D. Cortical language interference sites are located at tag number 10 indicating speech arrest at the ventral premotor cortex and at tag number 15 indicating anomia posterior on the superior temporal gyrus. Panel B: Photograph after resection involving part of the temporal lobe. A subcortical language interference site is located at tag number 16 indicating semantic paraphasia at the inferior fronto‐occipital fascicle. Panel C: The postoperative MR imaging demonstrates that a large part of the superior temporal gyrus is resected, including the middle longitudinal fascicle located within the white matter of the superior temporal gyrus. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Postoperative Imaging
The volumes of resection were substantial and ranged from 21 to 111 cm3, on average 62 cm3. The full length of the STG was resected in one patient, approximately half of the STG in 6 patients and approximately a quarter of the STG in one patient. The resection was not extended posterior from the positive language sites of the temporal cortex in any of the patients. Heschl's gyrus was completely resected in one patient. The probabilistic map of the resection cavity volumes in the sagittal, coronal and axial orthogonal planes is shown in Figure 3. It is clear that in all cases a major part of the MdLF was resected as part of temporal lobe resections.
Figure 3.
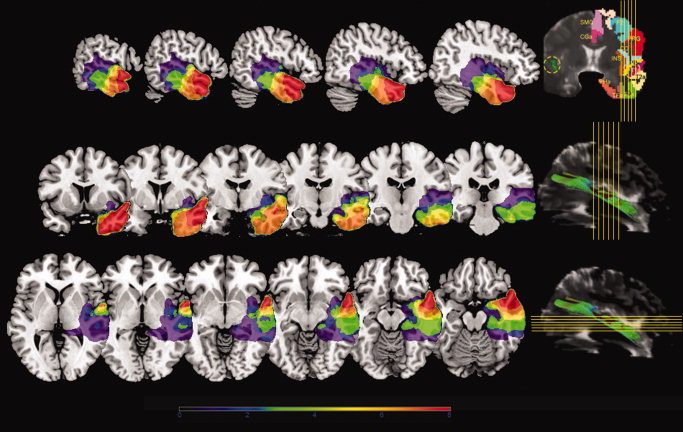
Probabilistic map of resection volumes. The cumulative extent of resection for 8 patients is shown on the Montreal Neurological Institute single subject brain template in the color range as indicated below the figure (see “Materials and Methods” section). Sagittal (top), coronal (middle) and axial (bottom) sections were selected that cover the middle longitudinal fascicle to demonstrate the overlap between the volumes of resection and the middle longitudinal fascicle. Yellow reference lines of the sections are plotted on a coronal and a sagittal T2‐weighted‐EPI section of the diffusion tensor MR imaging as reprinted with permission from Makris et al. [2009]. In the coronal reference section (top‐right), cortical region parcellation is overlaid on the left hemisphere and the middle longitudinal fascicle is outlined on the right hemisphere in green within the yellow dotted circle, as detailed in Makris et al. [2009] (From Makris et al., Cereb Cortex,2009, 19, 777–785, Oxford University Press, reproduced by permission.). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
This study is the first to address function to the recently‐identified MdLF in humans. The main findings are (1) that no language interference is identified intraoperatively during electrostimulation and resection of the MdLF in the language dominant hemisphere and (2) that no permanent language deficits are induced despite resection of a large part of the MdLF. Based on these two observations in glioma patients, we postulate that the MdLF is not essential for language in humans. Still, it should be noted that the MdLF was not completely resected in all patients. Therefore, our findings do not exclude that the part of the MdLF posterior from the resection margin of the STG can be essential for language, because this posterior resection margin was determined by positive language sites in all but one patient. Hence, our results demonstrate that the anterior part of the MdLF is not essential for language function.
The existence of the MdLF is firmly established in primates. In monkeys, the discovery of the MdLF was done by Seltzer and Pandya [1978,1984] using autoradiography, a fact that has also been confirmed recently with diffusion spectrum imaging [Schmahmann et al.,2007], for a review, see [Schmahmann and Pandya,2006]. In another recent review, Petrides and Pandya [2009] say that “It should be pointed out here that the inferior parietal lobule and adjacent caudal superior temporal sulcus (origins of the dorsal stream) and the intermediate‐to‐anterior superolateral temporal region (origin of the ventral stream) are known to be massively interconnected via the middle longitudinal fasciculus.” However, a human equivalent of the MdLF, that consists of a single fascicle connecting the AG with the STG and is distinct from the SLF‐II/AF, can certainly be debated. The MdLF in humans has so far been observed in two DTI studies from the same group with four and five subjects, respectively [Makris and Pandya,2009; Makris et al.,2009] and using histological observations in 1999 [cited in [Makris et al.,2009]). However, the MdLF has not been described in the work of pioneers using anatomical white matter dissections or myelin staining of the human brain.
Electrostimulation mapping in glioma patients during surgical resection has facilitated the correlation of language function with several anatomically defined white matter fascicles, such as the ILF [Mandonnet et al.,2007b], the IFOF [Duffau et al.,2005], the AF [Duffau et al.,2002], the frontoparietal articulatory loop [Duffau et al.,2003], and the uncinate fascicle [Duffau et al.,2009]. Direct mapping using electrostimulation has been considered the gold standard for validating non‐invasive functional localization mapping techniques [Fandino et al.,1999; Hanakawa et al.,2001; Henry et al.,2004; Kinoshita et al.,2005; Korvenoja et al.,2006; Lehericy et al.,2000; Mikuni et al.,2007; Okada et al.,2006; Roessler et al.,2005; Sobottka et al.,2002]. Nevertheless, some limitations have to be considered for its use as a functional localization technique. First, the temporal resolution of electrostimulation is limited due to time restrictions during surgery. The current paradigm allows for a binary interpretation of stimulation interference in ∼4 s. Second, the minimal spatial resolution of electrostimulation is by definition 5 mm due to the bipolar stimulator probe configuration. And the sensitivity of electrostimulation is determined by the stimulation intensity. So that by increasing the stimulus intensity, the sensitivity increases because the stimulus covers a larger tissue volume, at the same time the specificity of electrostimulation decreases and the risk of intraoperative seizures increases [Haglund et al.,1993]. In the current paradigm, the minimal stimulus intensity required for speech arrest at the ventral premotor cortex is identified in order to maximize specificity of localization with electrostimulation. The specificity is further increased by non‐successively repeating the stimulation at least three times at the same location [Ojemann et al.,1989]. Third, the current paradigm is limited to the aspects of language processing that are involved in picture naming. This task is optimized for valid and efficient intraoperative assessment of global language function during brain tumor resection. As such, picture naming involves attention, visual perception, visual‐conceptual integration, semantic comprehension, motor initiation, phonologic expression and articulation [DeLeon et al.,2007; Glaser,1992; Hamberger et al.,2005; Johnson et al.,1996], but not semantic processing of sentences or syntax. Fourth, valid interpretation of language interference depends to a large extent on patient cooperation and motivation. If the attention level of the patient decreases, for instance due to fatigue, erroneous interpretation of electrostimulation may result, increasing the number of false‐positive sites. Fifth, subcortical electrostimulation does not allow for examination of genuine normal brain, because there is evidently always an indication for resective brain surgery. Despite these limitations, we consider the intraoperative language localization by electrostimulation valid including the observation of absence of language function in the MdLF. This is further supported by the fact that the final language examination showed results equivalent to or better than baseline for all patients, indicating that essential language structures were preserved.
Our observation of absence of essential language function is reinforced by another anatomical argument. The MdLF has no significant lateralization in volume or fractional anisotropy as determined by quantitative DTI [Makris et al.,2009]. Several structures that are essential for language display substantial asymmetry in concordance with language lateralization, such as the volume of the pars opercularis [Keller et al.,2007], the volume of the planum temporale [Dorsaint‐Pierre et al.,2006; Watkins et al.,2001], the volume of the primary auditory cortex [Catani et al.,2007; Penhune et al.,1996], the volume and fractional anisotropy of the AF [Catani et al.,2007; Eluvathingal et al.,2007; Matsumoto et al.,2008] and the volume and fractional anisotropy of the SLF [Makris et al.,2005].
Several alternative speculative explanations for our findings can be considered. First, our observations were done in patients harboring gliomas, so that theoretically plasticity mechanisms may have resulted in a relocation of essential language structures such as the MdLF. However, despite the presence of a glioma, another structure that is considered essential for language, i.e., the IFOF [Duffau et al.,2005], was identifiable in all patients and its function was clearly not relocated. It means that stimulation of the white matter tract running mesially within the roof of the temporal ventricle elicited semantic disturbances in the 8 patients, in agreement with our previous report which showed that this pathway corresponded to the IFOF, a bundle crucial for semantic processing. This tract is easily identifiable since the inferior longitudinal fasciculus is located below the temporal ventricle and was demonstrated to not be essential for language [Mandonnet et al.,2007b], while the uncinate fasciculus is located anteriorly to the IFOF and was also demonstrated to not be essential for language [Duffau et al.,2009]. Conversely, the IFOF was still crucial for language in all patients. Second, the MdLF may be involved in language function of humans, but the contribution of the MdLF could be compensated after resection. Third, perhaps the MdLF is part of a redundant language network, including the IFOF as well as the extreme/external capsule fiber system that has been demonstrated in recent studies [Frey et al.,2008], so that the MdLF would only portray essentiality when other crucial nodes in the language network are lost, which would otherwise not have resulted in language deficits. Such a compensation or redundancy of language involvement has been demonstrated for the ILF and the uncinate fascicle [Duffau et al.,2009; Mandonnet et al.,2007b].
If the MdLF is not essential for language, then which function pertains to it? From the perspective of associationism, the MdLF could bear one of many higher cognitive functions recruited by the STG/STS or AG. Several cognitive functions have been related to the STG/STS according to functional neuroimaging studies and lesion studies [Karnath,2001; Hein and Knight,2008], such as intelligible speech processing [Leff et al.,2008; Narain et al.,2003; Scott et al.,2000; Wise et al.,2001], syntactic language processing [Friederici and Kotz,2003], visuospatial processing [Ellison et al.,2004; Mayer et al.,2004; Pelphrey et al.,2005], audiospatial processing [Makris and Pandya,2009], spatial awareness [Karnath et al.,2001], face recognition [Vuilleumier and Pourtois,2007], audiovisual integration [Mottonen et al.,2004; Stevenson and James,2009; Wright et al.,2003], crossmodal processing [Beauchamp et al.,2008; Friederici and Kotz,2003], and mentalization [Frith and Frith,1999]. Cognitive functions projected in the AG include mental arithmetic [Ischebeck et al.,2009], audiovisual integration [Bernstein et al.,2008], semantic language processing [Humphries et al.,2006], spatial awareness and attention [Husain and Nachev,2007]. In case appropriate intraoperative stimulation paradigms were to be developed for any of these functions, the anatomofunctional correlate of the MdLF could possibly be pinpointed using electrostimulation. However, paradigms that provide a binary interpretation in a timeframe of ∼4 s are not available for any of these functions. Although these cognitive functions have not been formally tested with postoperative neuropsychological examination in the present population, all patients have resumed a normal social and professional life.
Perhaps seeking for a disconnection syndrome resulting from severance of the MdLF will not provide a proper answer. If the MdLF exists, it might be a pathway participating in a parallel transmodal neurocognitive network and apparently it is not one of its critical epicentres [Catani et al.,2005; Mesulam,2008]. One critical epicentre for the language network, which is in concordance with our findings, is the cortex of the STG superficial of the MdLF at the level of the positive language sites that determine the posterior margin of the resection cavity and that cover the “Wernicke territory.” We speculate that in case the MdLF is a noncritical epicentre of a neurocognitive network, its severance will result in a reshaping of the processing of the other components in the network, resulting in compensation after resection of the MdLF.
CONCLUSIONS
We postulate that the MdLF may participate but it is not essential for language, based on absence of language interference during intraoperative electrostimulation at the level of the MdLF and absence of new permanent language deficits after resection of a large part of the MdLF in patients with glioma resections of the STG in the language dominant hemisphere.
REFERENCES
- Beauchamp MS, Yasar NE, Frye RE, Ro T ( 2008): Touch, sound and vision in human superior temporal sulcus. Neuroimage 41: 1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein LE, Auer ET Jr, Wagner M, Ponton CW ( 2008): Spatiotemporal dynamics of audiovisual speech processing. Neuroimage 39: 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M ( 2008): The parietal cortex and episodic memory: An attentional account. Nat Rev Neurosci 9: 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK ( 2002): Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17: 77–94. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH ( 2005): Perisylvian language networks of the human brain. Ann Neurol 57: 8–16. [DOI] [PubMed] [Google Scholar]
- Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK ( 2007): Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci USA 104: 17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M ( 2008): A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44: 1105–1132. [DOI] [PubMed] [Google Scholar]
- Committeri G, Pitzalis S, Galati G, Patria F, Pelle G, Sabatini U, Castriota‐Scanderbeg A, Piccardi L, Guariglia C, Pizzamiglio L ( 2007): Neural bases of personal and extrapersonal neglect in humans. Brain 130: 431–441. [DOI] [PubMed] [Google Scholar]
- Crespo‐Facorro B, Kim J, Andreasen NC, Spinks R, O'Leary DS, Bockholt HJ, Harris G, Magnotta VA ( 2000): Cerebral cortex: A topographic segmentation method using magnetic resonance imaging. Psychiatry Res 100: 97–126. [DOI] [PubMed] [Google Scholar]
- DeLeon J, Gottesman RF, Kleinman JT, Newhart M, Davis C, Heidler‐Gary J, Lee A, Hillis AE ( 2007): Neural regions essential for distinct cognitive processes underlying picture naming. Brain 130: 1408–1422. [DOI] [PubMed] [Google Scholar]
- Dorsaint‐Pierre R, Penhune VB, Watkins KE, Neelin P, Lerch JP, Bouffard M, Zatorre RJ ( 2006): Asymmetries of the planum temporale and Heschl's gyrus: relationship to language lateralization. Brain 129: 1164–1176. [DOI] [PubMed] [Google Scholar]
- Duffau H, Capelle L, Sichez N, Denvil D, Lopes M, Sichez JP, Bitar A, Fohanno D ( 2002): Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo‐functional study. Brain 125: 199–214. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Denvil D, Lopes M, Capelle L ( 2003): The articulatory loop: Study of the subcortical connectivity by electrostimulation. Neuroreport 14: 2005–2008. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio‐Mazoyer N, Capelle L ( 2005): New insights into the anatomo‐functional connectivity of the semantic system: A study using cortico‐subcortical electrostimulations. Brain 128: 797–810. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Moritz‐Gasser S, Mandonnet E ( 2009): Is the left uncinate fasciculus essential for language? A cerebral stimulation study. J Neurol 256: 382–389. [DOI] [PubMed] [Google Scholar]
- Ellison A, Schindler I, Pattison LL, Milner AD ( 2004): An exploration of the role of the superior temporal gyrus in visual search and spatial perception using TMS. Brain 127: 2307–2315. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing‐Cobbs L ( 2007): Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex 17: 2760–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandino J, Kollias SS, Wieser HG, Valavanis A, Yonekawa Y ( 1999): Intraoperative validation of functional magnetic resonance imaging and cortical reorganization patterns in patients with brain tumors involving the primary motor cortex. J Neurosurg 91: 238–250. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Miranda JC, Rhoton AL Jr, Alvarez‐Linera J, Kakizawa Y, Choi C, de Oliveira EP ( 2008): Three‐dimensional microsurgical and tractographic anatomy of the white matter of the human brain. Neurosurgery 62: 989–1026. [DOI] [PubMed] [Google Scholar]
- Frey S, Campbell JS, Pike GB, Petrides M ( 2008): Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci 28: 11435–11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Kotz SA ( 2003): The brain basis of syntactic processes: Functional imaging and lesion studies. Neuroimage 20 ( Suppl 1): S8–S17. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U ( 1999): Interacting minds—A biological basis. Science 286: 1692–1695. [DOI] [PubMed] [Google Scholar]
- Glaser WR. 1992. Picture naming. Cognition 42: 61–105. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, Barressi B ( 2000): The Assessment of Aphasia and Related Disorders, 3rd ed. Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Haglund MM, Ojemann GA, Blasdel GG ( 1993): Optical imaging of bipolar cortical stimulation. J Neurosurg 78: 785–793. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT, McKhann GM II, Perrine K, Goodman RR ( 2005): Brain stimulation reveals critical auditory naming cortex. Brain 128: 2742–2749. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Ikeda A, Sadato N, Okada T, Fukuyama H, Nagamine T, Honda M, Sawamoto N, Yazawa S, Kunieda T, Ohara S, Taki W, Haschimoto N, Yonekura Y, Konishi J, Shibasaki H ( 2001): Functional mapping of human medial frontal motor areas. The combined use of functional magnetic resonance imaging and cortical stimulation. Exp Brain Res 138: 403–409. [DOI] [PubMed] [Google Scholar]
- Hein G, Knight RT ( 2008): Superior temporal sulcus—It's my area: or is it? J Cogn Neurosci 20: 2125–2136. [DOI] [PubMed] [Google Scholar]
- Henry RG, Berman JI, Nagarajan SS, Mukherjee P, Berger MS ( 2004): Subcortical pathways serving cortical language sites: Initial experience with diffusion tensor imaging fiber tracking combined with intraoperative language mapping. Neuroimage 21: 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E ( 2006): Syntactic and semantic modulation of neural activity during auditory sentence comprehension. J Cogn Neurosci 18: 665–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M, Nachev P ( 2007): Space and the parietal cortex. Trends Cogn Sci 11: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischebeck A, Zamarian L, Schocke M, Delazer M ( 2009): Flexible transfer of knowledge in mental arithmetic—An fMRI study. Neuroimage 44: 1103–1112. [DOI] [PubMed] [Google Scholar]
- Johnson CJ, Paivio A, Clark JM ( 1996): Cognitive components of picture naming. Psychol Bull 120: 113–139. [DOI] [PubMed] [Google Scholar]
- Karnath HO ( 2001): New insights into the functions of the superior temporal cortex. Nat Rev Neurosci 2: 568–576. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Ferber S, Himmelbach M ( 2001): Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature 411: 950–953. [DOI] [PubMed] [Google Scholar]
- Keller SS, Highley JR, Garcia‐Finana M, Sluming V, Rezaie R, Roberts N ( 2007): Sulcal variability, stereological measurement and asymmetry of Broca's area on MR images. J Anat 211: 534–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PE, Singh M ( 2003): Functional magnetic resonance imaging for brain mapping in neurosurgery. Neurosurg Focus 15: E1. [DOI] [PubMed] [Google Scholar]
- Kim SG, Ogawa S ( 2002): Insights into new techniques for high resolution functional MRI. Curr Opin Neurobiol 12: 607–615. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Crespo‐Facorro B, Andreasen NC, O'Leary DS, Zhang B, Harris G, Magnotta VA ( 2000): An MRI‐based parcellation method for the temporal lobe. Neuroimage 11: 271–288. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Yamada K, Hashimoto N, Kato A, Izumoto S, Baba T, Maruno M, Nishimura T; Yoshimine T ( 2005): Fiber‐tracking does not accurately estimate size of fiber bundle in pathological condition: initial neurosurgical experience using neuronavigation and subcortical white matter stimulation. Neuroimage 25: 424–429. [DOI] [PubMed] [Google Scholar]
- Korvenoja A, Kirveskari E, Aronen HJ, Avikainen S, Brander A, Huttunen J, Ilmoniemi RJ, Jääskeläinen JE, Kovala T, Mäkelä JP, Salli E, Seppä M ( 2006): Sensorimotor cortex localization: Comparison of magnetoencephalography, functional MR imaging, and intraoperative cortical mapping. Radiology 241: 213–222. [DOI] [PubMed] [Google Scholar]
- Leff AP, Schofield TM, Stephan KE, Crinion JT, Friston KJ, Price CJ ( 2008): The cortical dynamics of intelligible speech. J Neurosci 28: 13209–13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehéricy S, Duffau H, Cornu P, Capelle L, Pidoux B, Carpentier A, Auliac S, Clémenceau S, Sichez JP, Bitar A, Valéry CA, Van Effenterre R, Faillot T, Srour A, Fohanno D, Philippon J, Le Bihan D, Marsault C ( 2000): Correspondence between functional magnetic resonance imaging somatotopy and individual brain anatomy of the central region: Comparison with intraoperative stimulation in patients with brain tumors. J Neurosurg 92: 589–598. [DOI] [PubMed] [Google Scholar]
- Logothetis NK ( 2008): What we can do and what we cannot do with fMRI. Nature 453: 869–878. [DOI] [PubMed] [Google Scholar]
- Makris N, Pandya DN ( 2009): The extreme capsule in humans and rethinking of the language circuitry. Brain Struct Funct 213: 343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS Jr, Pandya DN ( 2005): Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT‐MRI study. Cereb Cortex 15: 854–869. [DOI] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, Kaiser JR, Sorg S, Kennedy DN, Pandya DN ( 2009): Delineation of the middle longitudinal fascicle in humans: A quantitative, in vivo, DT‐MRI study. Cereb Cortex 19: 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandonnet E, Jbabdi S, Taillandier L, Galanaud D, Benali H, Capelle L, Duffau H ( 2007a): Preoperative estimation of residual volume for WHO grade II glioma resected with intraoperative functional mapping. Neuro Oncol 9: 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H ( 2007b): Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain 130: 623–629. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Okada T, Mikuni N, Mitsueda‐Ono T, Taki J, Sawamoto N, Hanakawa T, Miki Y, Hashimoto N, Fukuyama H, Takahashi R, Ikeda A ( 2008): Hemispheric asymmetry of the arcuate fasciculus: A preliminary diffusion tensor tractography study in patients with unilateral language dominance defined by Wada test. J Neurol 255: 1703–1711. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Dorflinger JM, Rao SM, Seidenberg M ( 2004): Neural networks underlying endogenous and exogenous visual‐spatial orienting. Neuroimage 23: 534–541. [DOI] [PubMed] [Google Scholar]
- Mesulam M ( 2008): Representation, inference, and transcendent encoding in neurocognitive networks of the human brain. Ann Neurol 64: 367–378. [DOI] [PubMed] [Google Scholar]
- Metz‐Lutz M, Kremin H, Deloche G, Hannequin D, Ferrand L, Perrier D ( 1991): Standardisation d'un test de de'nomination orale: Contrôle des effets de l'âge, du sexe et du niveau de scolarite' chez les sujets adultes normaux. Rev Neuropsychol 1: 73–95. [Google Scholar]
- Mikuni N, Okada T, Nishida N, Taki J, Enatsu R, Ikeda A, Miki Y, Hanakawa T, Fukuyama H, Hashimoto N ( 2007): Comparison between motor evoked potential recording and fiber tracking for estimating pyramidal tracts near brain tumors. J Neurosurg 106: 128–133. [DOI] [PubMed] [Google Scholar]
- Mort DJ, Malhotra P, Mannan SK, Rorden C, Pambakian A, Kennard C, Husain M ( 2003): The anatomy of visual neglect. Brain 126: 1986–1997. [DOI] [PubMed] [Google Scholar]
- Mottonen R, Schurmann M, Sams M ( 2004): Time course of multisensory interactions during audiovisual speech perception in humans: a magnetoencephalographic study. Neurosci Lett 363: 112–115. [DOI] [PubMed] [Google Scholar]
- Narain C, Scott SK, Wise RJ, Rosen S, Leff A, Iversen SD, Matthews PM ( 2003): Defining a left‐lateralized response specific to intelligible speech using fMRI. Cereb Cortex 13: 1362–1368. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M ( 1989): Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg 71: 316–326. [DOI] [PubMed] [Google Scholar]
- Okada T, Mikuni N, Miki Y, Kikuta K, Urayama S, Hanakawa T, Fushimi Y, Yamamoto A, Kanagaki M, Fukuyama H, Hashimoto N, Togashi K ( 2006): Corticospinal tract localization: Integration of diffusion‐tensor tractography at 3‐T MR imaging with intraoperative white matter stimulation mapping—Preliminary results. Radiology 240: 849–857. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, Michelich CR, Allison T, McCarthy G ( 2005): Functional anatomy of biological motion perception in posterior temporal cortex: an FMRI study of eye, mouth and hand movements. Cereb Cortex 15: 1866–1876. [DOI] [PubMed] [Google Scholar]
- Penhune VB, Zatorre RJ, MacDonald JD, Evans AC ( 1996): Interhemispheric anatomical differences in human primary auditory cortex: Probabilistic mapping and volume measurement from magnetic resonance scans. Cereb Cortex 6: 661–672. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN ( 2009): Distinct parietal and temporal pathways to the homologues of Broca's area in the monkey. PLoS Biol 7: e1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler K, Donat M, Lanzenberger R, Novak K, Geissler A, Gartus A, Tahamtan AR, Milakara D, Czech T, Barth M, Knosp E, Beisteiner R ( 2005): Evaluation of preoperative high magnetic field motor functional MRI (3 Tesla) in glioma patients by navigated electrocortical stimulation and postoperative outcome. J Neurol Neurosurg Psychiatry 76: 1152–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarabino T, Duvernoy H ( 2006): Atlas of Morphology and Functional Anatomy of the Brain, 1st ed. New York, NY: Springer. [Google Scholar]
- Schmahmann JD, Pandya DN, editors. (2006): Middle longitudinal fascicle In: Fiber pathways of the brain. Oxford: Oxford University Press; pp 318–320. [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, Wedeen VJ ( 2007): Association fibre pathways of the brain: Parallel observations from diffusion spectrum imaging and autoradiography. Brain 130: 630–653. [DOI] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJ ( 2000): Identification of a pathway for intelligible speech in the left temporal lobe. Brain 123: 2400–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN ( 1978): Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res 149: 1–24. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN ( 1984): Further observations on parieto‐temporal connections in the rhesus monkey. Exp Brain Res 55: 301–312. [DOI] [PubMed] [Google Scholar]
- Sobottka SB, Bredow J, Beuthien‐Baumann B, Reiss G, Schackert G, Steinmeier R ( 2002): Comparison of functional brain PET images and intraoperative brain‐mapping data using image‐guided surgery. Comput Aided Surg 7: 317–325. [DOI] [PubMed] [Google Scholar]
- Stevenson RA, James TW ( 2009): Audiovisual integration in human superior temporal sulcus: Inverse effectiveness and the neural processing of speech and object recognition. Neuroimage 44: 1210–1223. [DOI] [PubMed] [Google Scholar]
- Turner R, Jones T ( 2003): Techniques for imaging neuroscience. Br Med Bull 65: 3–20. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G ( 2007): Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia 45: 174–194. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae‐Poetscher LM, van Zijl PC, Mori S ( 2004): Fiber tract‐based atlas of human white matter anatomy. Radiology 230: 77–87. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P, Taylor J, Worsley KJ, Evans AC ( 2001): Structural asymmetries in the human brain: A voxel‐based statistical analysis of 142 MRI scans. Cereb Cortex 11: 868–877. [DOI] [PubMed] [Google Scholar]
- Wheless JW, Castillo E, Maggio V, Kim HL, Breier JI, Simos PG, Papanicolaou AC ( 2004): Magnetoencephalography (MEG) and magnetic source imaging (MSI). Neurologist 10: 138–153. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA ( 2001): Separate neural subsystems within ‘Wernicke's area’. Brain 124: 83–95. [DOI] [PubMed] [Google Scholar]
- Wright TM, Pelphrey KA, Allison T, McKeown MJ, McCarthy G ( 2003): Polysensory interactions along lateral temporal regions evoked by audiovisual speech. Cereb Cortex 13: 1034–1043. [DOI] [PubMed] [Google Scholar]


