Abstract
Premenstrual increases in negative mood are thought to arise from changes in gonadal hormone levels, presumably by influencing mood regulation and stress sensitivity. The amygdala plays a major role in this context, and animal studies suggest that gonadal hormones influence its morphology. Here, we investigated whether amygdala morphology changes over the menstrual cycle and whether this change explains differences in stress sensitivity. Twenty‐eight young healthy women were investigated once during the premenstrual phase and once during the late follicular phase. T1‐weighted anatomical images of the brain were acquired using magnetic resonance imaging and analyzed with optimized voxel‐based morphometry. To measure mood regulation and stress sensitivity, negative affect was assessed after viewing strongly aversive as well as neutral movie clips. Our results show increased gray matter volume in the dorsal part of the left amygdala during the premenstrual phase when compared with the late follicular phase. This volume increase was positively correlated with the premenstrual increase in stress‐induced negative affect. This is the first study showing structural plasticity of the amygdala in humans at the macroscopic level that is associated with both endogenous gonadal hormone fluctuations and stress sensitivity. These results correspond with animal findings of gonadal hormone‐mediated neural plasticity in the amygdala and have implications for understanding the pathogenesis of specific mood disorders associated with hormonal fluctuations. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: menstrual cycle, amygdala, gonadal hormones, stress, mood, structural MRI
INTRODUCTION
Gonadal hormone fluctuations covary with changes in mood and stress resilience. For example, during the premenstrual phase of the menstrual cycle, when progesterone and estradiol levels decline after a relatively stable period with high levels, women suffer more from negative mood and are more vulnerable to stress [Epperson et al., 2007; Kask et al., 2008; Ossewaarde et al., 2010; Sveindottir and Bäckström, 2000]. Although most women show some degree of cycle‐related mood changes, some women are more susceptible to these gonadal hormone fluctuations than others. In these women, negative mood symptoms and heightened stress sensitivity are aggravated so that diagnostic criteria of premenstrual syndrome (PMS) or premenstrual dysphoric disorder (PMDD) are met [Bäckström et al., 1983; Epperson et al., 2007; Kask et al., 2008]. One explanation for these mood changes in both patients and healthy women is that they might be due to the influence of gonadal hormones on the amygdala. This brain structure is involved in negative affect and depression [Drevets et al., 2008; Frodl et al., 2002; Siegle et al., 2002; van Eijndhoven et al., 2009] and also plays a major role in modulating the sympatho‐adrenomedullary and hypothalamo‐pituitary‐adrenocortical axis responses to stress [Herman et al., 2005; Phelps and LeDoux, 2005]. We and others have previously shown that the hormonal changes during the menstrual cycle as well as exogenous progesterone administration regulate amygdala responsivity [Derntl et al., 2008; Goldstein et al., 2005; Ossewaarde et al., 2010; van Wingen et al., 2007, 2008]. However, whether these functional changes in the amygdala are accompanied by morphological changes is currently unknown.
Animal and human research indicates that gonadal hormones also affect brain structure [Hagemann et al., 2011; Hermel et al., 2006; McEwen, 2002], with evidence for gonadal hormone‐mediated structural plasticity in the medial nucleus of the amygdala [Cooke, 2006]. Gonadal hormone receptors are expressed in large amounts in the medial portion of the amygdala [Cooke et al., 2003; Greco et al., 2001; Simerly et al., 1990], and dendritic spine density is modulated by gonadal hormones in this brain region [Cooke and Woolley, 2005]. In addition, estradiol administration increases medial amygdala volume [Fan et al., 2008] and in combination with progesterone, cell bodies of neurons located in the posterodorsal portion of this brain region expand [de Castilhos et al., 2010]. In addition to these structural changes, altered amygdala function has been associated with stress sensitivity changes across the menstrual cycle in humans [Ossewaarde et al., 2010]. Moreover, studies in healthy women measuring cognitive performance, cardiovascular reactivity, and secretion of stress hormones in response to physical and various psychological stressors suggest that stress sensitivity is increased during the premenstrual phase [Kumari and Corr, 1998; Marinari et al., 1976; Moldovanova et al., 2008; Tersman et al., 1991]. However, the association between putative morphological changes and stress sensitivity are unknown.
Therefore, we investigated whether human amygdala morphology changes across the menstrual cycle and whether this change is associated with stress sensitivity. Using voxel‐based morphometry (VBM), gray matter volume of the amygdala during the premenstrual phase, when women have the most premenstrual complaints, was compared with volume in the late follicular phase, when women have the least complaints [de Castilhos et al., 2010; Fan et al., 2008]. Additionally, we explored whether menstrual cycle‐dependent changes in amygdala volume are associated with concurrent menstrual cycle‐dependent changes in stress sensitivity. To assess changes in stress sensitivity, we measured negative mood both after the presentation of strongly aversive movie clips [Henckens et al., 2009; Ossewaarde et al., 2010; Qin et al., 2009; van Marle et al., 2009] as well as after neutral movie clips during both menstrual cycle phases. As gonadal hormone administration increases amygdala volume [de Castilhos et al., 2010; Fan et al., 2008], we hypothesized that amygdala gray matter would be larger in the premenstrual phase than in the late follicular phase and that this volume increase would be positively associated with increased stress‐induced negative mood states.
MATERIALS AND METHODS
Participants
Twenty‐eight healthy right‐handed women (mean age: 22.8 years, range: 18–38 years) participated in this study. None of the women had used hormonal contraceptives for the previous 3 months and all had regular menstrual cycles (26–32 days over the past year). Women were excluded when they consumed more than three alcoholic beverages per day on average or smoked more than 20 cigarettes per week. In addition, they were asked not to consume alcohol 24 h prior to the experiment and not to use psychoactive substances for 72 h. They had no history of psychiatric disorders (as determined with the Mini International Neuropsychiatric Interview [M.I.N.I.]; Sheehan et al., 1998) and were additionally screened specifically for PMDD or PMS using the Dutch version of the Moos Menstrual Distress Questionnaire (MDQ; Moos, 1968). To screen PMS/PMDD prospectively and qualitatively, we asked the women to keep a prospective record of their symptoms for 1 month on a Dutch version of the calendar of premenstrual experiences [Mortola et al., 1990].
All women were physically healthy and reported no magnetic resonance imaging (MRI) contraindications. Data of one woman were excluded from data analysis due to excessive head movement during scanning. The study was approved by the local ethical review board (Commissie Mensgebonden Onderzoek (CMO) Region Arnhem‐Nijmegen, The Netherlands), and all women provided written informed consent.
Design and Procedure
Women were tested once during the late follicular phase and once during the premenstrual phase in counterbalanced order. During a screening day prior to scanning, subjects completed the M.I.N.I [Sheehan et al., 1998] and the MDQ [Moos, 1968]. Each woman was scanned in the afternoon or evening, which was kept equal for both test sessions (within participants). Important to note is that other data of this experiment have been reported elsewhere (see Ossewaarde et al., 2010). Timing of test sessions within the menstrual cycle phase was ascertained as follows. For the late follicular phase, test sessions were planned between Days 8 and 12 (mean time point of test session: Day 10; SD: 1 day) with respect to the first day of the menstrual cycle (i.e., start of menstruation). To determine the premenstrual phase, two time points were used. First, participants contacted the experimenter when they had a positive result from their LH peak assessments (as determined using commercially available ovulation predictor kits; Wondfo Biotech, Guangzhou, China). Subsequently, an appointment was made for premenstrual scanning approximately 14 days after the start of ovulation. To ascertain that this test session took place in the premenstrual phase, participants were asked to contact the experimenter again when their next menstruation started (mean time point of test session: 2 days before menstruation started; SD: 1 day). Menstrual cycle phases could further be verified retrospectively using salivary neuroactive steroid concentrations (see below).
On testing days, participants arrived between 2 and 7 p.m. To measure salivary concentrations of allopregnanolone, 10 ml of saliva was collected at the beginning of each test session (5 min after arrival). The sampling procedure and assay of salivary allopregnanolone have been detailed previously [Ossewaarde et al., 2010]. The saliva concentrations of allopregnanolone were higher in the premenstrual phase (mean ± SD, 0.050 nmol/l ± 0.016) than in the late follicular phase (mean ± SD, 0.035 nmol/l ± 0.010; t 25 = 4.67, P < 0.001). Kolmogorov‐Smirnov tests confirmed that the distributions of allopregnanolone concentrations in the two cycle phases did not deviate from a normal distribution (both Z < 1). Moreover, distributions contained no outliers. Note that progesterone and allopregnanolone levels are strongly positively correlated with each other [Wang et al., 1996]. Even though estradiol and/or progesterone levels are more commonly used for confirmation of menstrual cycle phase, these data indicate that participants were tested during the intended menstrual cycle phase.
Stress Induction
To induce a stressful state, highly aversive movie clips were shown in the MRI scanner in counterbalanced order [Cousijn et al., 2010; Henckens et al., 2009; Ossewaarde et al., 2010; Qin et al., 2009; van Marle et al., 2009, 2010]. These clips consisted of scenes of a movie [Irréversible (2002), Gaspar Noé] containing maximally aggressive behavior and violence against men and women. As a control condition, neutral, nonarousing scenes of another movie [Comment j'ai tué mon père (2001), Anne Fontaine] were also shown in the scanner. The stressful and the neutral movie clips were similar in the amount of speech, human (face) presence, luminance, (scanner) environment, and language and were shown in a counterbalanced manner. During each test day, women saw a stressful and a neutral movie clip with approximately 105 min in between during which they performed other tasks (reported elsewhere, see Ossewaarde et al., 2010) and underwent the anatomical scan. As there were two scanning sessions (once during the premenstrual and once during the late follicular phase), women saw four movie clips in total (two stressful and two neutral movie clips) [Ossewaarde et al., 2010].
Changes in affect were assessed using the Positive and Negative Affect Scales [Watson et al., 1988] at three time points during each test day: at baseline (15 min after arrival and outside the scanner), right after the first movie clip (75 min after arrival and in the scanner), and right after the second movie clip at the end of the experiment (180 min after arrival and in the scanner).
Structural MRI Data Acquisition and Analysis
All MR images were collected using a Siemens (Erlangen, Germany) TIM Trio 3.0 Tesla MRI with a standard T1‐weighted 3D MP‐RAGE sequence (echo time/repetition time: 2.96/2,300 ms; flip angle: 8°; field of view: 256 mm × 256 mm × 192 mm; voxel size: 1 mm isotropic; GRAPPA acceleration factor 2).
Anatomical MR images were compared for differences in local gray matter volume using VBM within the SPM5 statistical software (SPM5; Wellcome Department of Imaging Neuroscience, London). Normalizing, bias correcting, and segmenting into gray matter, white matter, and cerebrospinal fluid were performed using the VBM toolbox (VBM5.1 Toolbox version 1.19; see also http://dbm.neuro.uni-jena.de/vbm/vbm5-for-spm5/) using priors (default settings). VBM was carried out in the following way. Diffeomorphic image registration was performed using the DARTEL toolbox in SPM [Ashburner, 2007]. First, all images were realigned to templates from the datasets of the 27 female subjects in this study. Second, Jacobian‐scaled (modulated) images were calculated and subsequently transformed to MNI space using affine transformation. Finally, all data were smoothed with an 8‐mm Gaussian FWHM kernel.
Data analysis was performed in SPM with the gray matter images using a GLM approach. A repeated measures ANOVA was used with menstrual cycle phase (premenstrual versus late follicular) as within‐subject factor. Statistical tests were familywise error rate corrected for multiple comparisons across the whole brain or across the region of interest using a small volume correction (SVC; Worsley et al., 1996). The amygdala was anatomically defined based on macroscopic anatomical parcellation of a canonical T1‐weighted MRI scan in MNI space (anatomical automatic labeling (AAL) atlas; Tzourio‐Mazoyer et al., 2002). All statistical analyses were corrected for nonstationarity. For illustrative purposes, figures of volume differences are shown at an uncorrected threshold of P < 0.001.
To assess whether amygdala volume changes were associated with premenstrual changes in stress‐induced negative affect, we performed an additional correlation analysis (Spearman's rank) between the difference in amygdala volume at the cluster maximum (peak voxel) and the difference in the stress‐induced change in negative affect (stress versus neutral) between the premenstrual and follicular phase (see also Ossewaarde et al., 2010).
RESULTS
Negative Affect
We first assessed whether baseline negative affect scores differed for the two menstrual cycle phases. As expected, the baseline scores that were obtained 15 min after arrival and outside the scanner environment were higher during the premenstrual phase (mean ± SD, 12.11 ± 2.20) than during the follicular phase [mean ± SD, 10.96 ± 1.11; F(1,26) = 7.26, P < 0.05]. Second, we tested whether the stress induction procedure led to increased negative mood. As expected, negative affect ratings were higher after the aversive movie clips than after the neutral control clips [mean± SD, 14.09 ± 3.42 and 12.73 ± 2.68, respectively; F(1,26) = 6.52, P < 0.05]. Next, we tested whether menstrual cycle phase changed the influence of stress on negative mood; however, the difference in stress‐induced negative affect between menstrual cycle phases was not consistent across individuals (P > 0.05).
Structural MRI
First, structural images were analyzed within a second‐level repeated measures ANOVA with menstrual cycle phase (premenstrual versus late follicular) as within‐subject factor. Amygdala gray matter volume was larger in the premenstrual phase when compared with the late follicular phase in its dorsal part (peak voxel: −15, −4, −9; P < 0.05, SVC corrected; see Fig. 1). We did not identify a brain region showing larger gray matter volume during the late follicular phase when compared with the premenstrual phase (P > 0.001, uncorrected).
Figure 1.
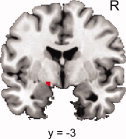
Coronal view showing the significant increase in dorsal amygdala gray matter volume in the premenstrual versus late follicular phase (P < 0.001 uncorrected for visualization purposes; MNI‐coordinates: −15, −3, −9; R = right). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Second, to investigate whether individual differences in amygdala gray matter enlargement were associated with individual differences in menstrual cycle‐related changes in stress‐induced negative affect, we performed a correlation analysis. The increase in amygdala gray matter volume from the follicular to the premenstrual phase correlated with the stress × menstrual cycle phase interaction in negative affect (r s = 0.39, P < 0.05; see Fig. 2). Specifically, the larger the increase in amygdala volume during the premenstrual phase, the larger is the stress‐induced enhancement of negative affect during this phase of the menstrual cycle.
Figure 2.
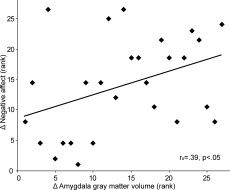
Rank correlation between gray matter increase in the amygdala across the menstrual cycle and stress‐induced negative affect scores. X‐axis: Δ amygdala gray matter increase extracted from the peak voxel at −15, −3, −9 (premenstrual > late follicular). Y‐axis: Δ stress‐induced increase in negative affect across the menstrual cycle (premenstrual [stress − neutral] > late follicular [stress – neutral]).
DISCUSSION
The aim of the this study was to identify morphological changes in the amygdala across the menstrual cycle and a possible association with individual differences in menstrual cycle‐related changes in stress sensitivity. As hypothesized, we observed premenstrual increases in gray matter volume in the amygdala. Despite the fact that we did not find any consistent changes in stress‐induced mood across the menstrual cycle, the premenstrual increase in gray matter in the amygdala was positively correlated with premenstrual changes in stress‐induced negative mood across individuals. Together, these findings support the notion that premenstrual increases in stress‐induced negative mood go along with individual differences in menstrual cycle phase‐induced morphological changes in the amygdala.
The observed increase in gray matter volume in the amygdala during the premenstrual phase (when gonadal hormone levels are increased and decline rapidly) may be explained by the effect that gonadal hormones have on dendritic spine density and neuronal size. These hormone effects on amygdala morphology occur in dorsal parts and more precisely in the medial nucleus of the amygdala [Cooke, 2006; Cooke and Woolley, 2005; Cooke et al., 2003], which has large amounts of gonadal hormone receptors [Simerly et al., 1990]. These effects appear to be mediated by progesterone and estradiol [Morris et al., 2008; Rocha et al., 2007]. For example, it has been shown that progesterone and estradiol administration to ovariectomized rats increases cell bodies and nuclei of neurons located in the posterodorsal portion of the medial amygdala to a larger extent when compared with vehicle or estradiol administration alone [de Castilhos et al., 2010]. Moreover, volumetric effects of estrogen appear to be specific to the medial amygdala [Fan et al., 2008]. Although the spatial resolution of 3T MRI scans is normally not sufficient for the localization of this particular nucleus, the anatomical location of this nucleus corresponds well with the location where we observed the premenstrual volume increase [Sah et al., 2003]. However, it is not evident what the change in regional gray matter volume, as measured with MRI, reflects. Probably, increases in gray matter are caused by the formation of new connections (i.e., increases in synaptic proteins) and dendrite and spine growth [Holtmaat et al., 2006; Kozorovitskiy et al., 2005; Yasumatsu et al., 2008]. This is in line with our suggestion that the observed increase in gray matter volume in the amygdala during the premenstrual phase may be explained by the effects of gonadal hormones on dendritic spine density.
The question arises whether functional amygdala changes related to premenstrual increases in negative mood and stress sensitivity [Ossewaarde et al., 2010; Protopopescu et al., 2008] are caused by the structural changes in this brain region. A recent study suggests that the association between amygdala responsivity and blood pressure reactivity to stress is mediated by individual differences in amygdala volume [Gianaros et al., 2008]. Moreover, reduced stress after a mindfulness‐based stress reduction intervention is associated with the decrease in the volume of amygdala gray matter [Holzel et al., 2010]. Finally, structural MRI studies in humans have shown that repeated activation of a neural region is related to an increase in the corresponding gray matter volume, whereas termination of activation has been associated with a decrease in gray matter [Draganski et al., 2004; Maguire et al., 2000]. Thus, increased amygdala reactivity during the premenstrual phase may result from hormonally regulated changes in amygdala morphology; however, direct evidence for this hypothesis is still lacking.
A potential limitation of this study is that we scanned women in the preovulatory phase, when estradiol levels could already be increasing. As estradiol also affects structural plasticity in the amygdala [Cooke, 2006], this may have reduced our effect size. However, the periovulatory phase is a very short period (i.e., only 2 or 3 days) and probably variable across subjects. Thus, consistent changes across subjects during this phase are highly unlikely.
In conclusion, this study shows increased gray matter volume in the dorsal part of the amygdala during the premenstrual phase when compared with the late follicular phase. This is the first study showing structural plasticity of the amygdala associated with endogenous gonadal hormone fluctuations in humans. The premenstrual increase in amygdala volume is associated with stress‐induced changes in mood. These results provide potential insight into how gonadal hormones affect neural plasticity and the pathogenesis of specific mood disorders associated with gonadal hormone fluctuations.
REFERENCES
- Ashburner J ( 2007): A fast diffeomorphic image registration algorithm. Neuroimage 38: 95–113. [DOI] [PubMed] [Google Scholar]
- Bäckström T, Sanders D, Leask R, Davidson D, Warner P, Bancroft J ( 1983): Mood, sexuality, hormones, and the menstrual cycle. II. Hormone levels and their relationship to the premenstrual syndrome. Psychosom Med 45: 503–507. [DOI] [PubMed] [Google Scholar]
- Cooke BM ( 2006): Steroid‐dependent plasticity in the medial amygdala. Neuroscience 138: 997–1005. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS ( 2005): Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol 64: 34–46. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Breedlove SM, Jordan CL ( 2003): Both estrogen receptors and androgen receptors contribute to testosterone‐induced changes in the morphology of the medial amygdala and sexual arousal in male rats. Horm Behav 43: 336–346. [DOI] [PubMed] [Google Scholar]
- Cousijn H, Rijpkema M, Qin S, van Marle HJ, Franke B, Hermans EJ, van Wingen G, Fernandez G ( 2010): Acute stress modulates genotype effects on amygdala processing in humans. Proc Natl Acad Sci USA 107: 9867–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castilhos J, Hermel EE, Rasia‐Filho AA, Achaval M ( 2010): Influence of substitutive ovarian steroids in the nuclear and cell body volumes of neurons in the posterodorsal medial amygdala of adult ovariectomized female rats. Neurosci Lett 469: 19–23. [DOI] [PubMed] [Google Scholar]
- Derntl B, Windischberger C, Robinson S, Lamplmayr E, Kryspin‐Exner I, Gur RC, Moser E, Habel U ( 2008): Facial emotion recognition and amygdala activation are associated with menstrual cycle phase. Psychoneuroendocrinology 33: 1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A ( 2004): Neuroplasticity: Changes in grey matter induced by training. Nature 427: 311–312. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML ( 2008): Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct Funct 213: 93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Pittman B, Czarkowski KA, Stiklus S, Krystal JH, Grillon C ( 2007): Luteal‐phase accentuation of acoustic startle response in women with premenstrual dysphoric disorder. Neuropsychopharmacology 32: 2190–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Hanbury R, Pandey SC, Cohen RS ( 2008): Dose and time effects of estrogen on expression of neuron‐specific protein and cyclic AMP response element‐binding protein and brain region volume in the medial amygdala of ovariectomized rats. Neuroendocrinology 88: 111–126. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl E, Zetzsche T, Bottlender R, Born C, Groll C, Jager M, Leinsinger G, Hahn K, Moller HJ ( 2002): Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry 51: 708–714. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR ( 2008): Individual differences in stressor‐evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci 28: 990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N ( 2005): Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci 25: 9309–9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco B, Allegretto EA, Tetel MJ, Blaustein JD ( 2001): Coexpression of ER beta with ER alpha and progestin receptor proteins in the female rat forebrain: Effects of estradiol treatment. Endocrinology 142: 5172–5181. [DOI] [PubMed] [Google Scholar]
- Hagemann G, Ugur T, Schleussner E, Mentzel HJ, Fitzek C, Witte OW, Gaser C ( 2011): Changes in brain size during the menstrual cycle. PLoS One 6: e14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJ, Hermans EJ, Pu Z, Joëls M, Fernández G ( 2009): Stressed memories: How acute stress affects memory formation in humans. J Neurosci 29: 10111–10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H ( 2005): Limbic system mechanisms of stress regulation: Hypothalamo‐pituitary‐adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29: 1201–1213. [DOI] [PubMed] [Google Scholar]
- Hermel EE, Ilha J, Xavier LL, Rasia‐Filho AA, Achaval M ( 2006): Influence of sex and estrous cycle, but not laterality, on the neuronal somatic volume of the posterodorsal medial amygdala of rats. Neurosci Lett 405: 153–158. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K ( 2006): Experience‐dependent and cell‐type‐specific spine growth in the neocortex. Nature 441: 979–983. [DOI] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, Pitman RK, Lazar SW ( 2010): Stress reduction correlates with structural changes in the amygdala. Soc Cogn Affect Neurosci 5: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kask K, Gulinello M, Bäckström T, Geyer MA, Sundström‐Poromaa I ( 2008): Patients with premenstrual dysphoric disorder have increased startle response across both cycle phases and lower levels of prepulse inhibition during the late luteal phase of the menstrual cycle. Neuropsychopharmacology 33: 2283–2290. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gross CG, Kopil C, Battaglia L, McBreen M, Stranahan AM, Gould E ( 2005): Experience induces structural and biochemical changes in the adult primate brain. Proc Natl Acad Sci USA 102: 17478–17482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Corr PJ ( 1998): Trait anxiety, stress and the menstrual cycle: Effects on Raven's Standard Progressive Matrices test. Pers Individ Differences 24: 615–623. [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD ( 2000): Navigation‐related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA 97: 4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinari KT, Leshner AI, Doyle MP ( 1976): Menstrual cycle status and adrenocortical reactivity to psychological stress. Psychoneuroendocrinology 1: 213–218. [DOI] [PubMed] [Google Scholar]
- McEwen B ( 2002): Estrogen actions throughout the brain. Recent Prog Horm Res 57: 357–384. [DOI] [PubMed] [Google Scholar]
- Moldovanova I, Schroeder C, Jacob G, Hiemke C, Diedrich A, Luft FC, Jordan J ( 2008): Hormonal influences on cardiovascular norepinephrine transporter responses in healthy women. Hypertension 51: 1203–1209. [DOI] [PubMed] [Google Scholar]
- Moos RH ( 1968): The development of a menstrual distress questionnaire. Psychosom Med 30: 853–867. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM ( 2008): Sexual dimorphism in neuronal number of the posterodorsal medial amygdala is independent of circulating androgens and regional volume in adult rats. J Comp Neurol 506: 851–859. [DOI] [PubMed] [Google Scholar]
- Mortola JF, Girton L, Beck L, Yen SS ( 1990): Diagnosis of premenstrual syndrome by a simple, prospective, and reliable instrument: The calendar of premenstrual experiences. Obstet Gynecol 76: 302–307. [PubMed] [Google Scholar]
- Ossewaarde L, Hermans EJ, van Wingen GA, Kooijman SC, Johansson IM, Bäckström T, Fernández G ( 2010): Neural mechanisms underlying changes in stress‐sensitivity across the menstrual cycle. Psychoneuroendocrinology 35: 47–55. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE ( 2005): Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 48: 175–187. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Tuescher O, Pan H, Epstein J, Root J, Chang L, Altemus M, Polanecsky M, McEwen B, Stern E, Silbersweig D ( 2008): Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord 108: 87–94. [DOI] [PubMed] [Google Scholar]
- Qin S, Hermans EJ, van Marle HJ, Luo J, Fernández G ( 2009): Acute psychological stress reduces working memory‐related activity in the dorsolateral prefrontal cortex. Biol Psychiatry 66: 25–32. [DOI] [PubMed] [Google Scholar]
- Rocha MI, Mestriner RG, Hermel EE, Xavier LL, Rasia‐Filho AA, Achaval M ( 2007): Neuronal somatic volume of posteroventral medial amygdala cells from males and across the estrous cycle of female rats. Neurosci Lett 420: 110–115. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J ( 2003): The amygdaloid complex: Anatomy and physiology. Physiol Rev 83: 803–834. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC ( 1998): The Mini‐International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 59: 22–33. [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS ( 2002): Can't shake that feeling: Event‐related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry 51: 693–707. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW ( 1990): Distribution of androgen and estrogen receptor mRNA‐containing cells in the rat brain: An in situ hybridization study. J Comp Neurol 294: 76–95. [DOI] [PubMed] [Google Scholar]
- Sveindottir H, Bäckström T ( 2000): Prevalence of menstrual cycle symptom cyclicity and premenstrual dysphoric disorder in a random sample of women using and not using oral contraceptives. Acta Obstet Gynecol Scand 79: 405–413. [DOI] [PubMed] [Google Scholar]
- Tersman Z, Collins A, Eneroth P ( 1991): Cardiovascular responses to psychological and physiological stressors during the menstrual cycle. Psychosom Med 53: 185–197. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- van Eijndhoven P, van Wingen G, van Oijen K, Rijpkema M, Goraj B, Jan Verkes R, Oude Voshaar R, Fernandez G, Buitelaar J, Tendolkar I ( 2009): Amygdala volume marks the acute state in the early course of depression. Biol Psychiatry 65: 812–818. [DOI] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Fernández G ( 2009): From specificity to sensitivity: How acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry 66: 649–655. [DOI] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Fernandez G ( 2010): Enhanced resting‐state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage 53: 348–354. [DOI] [PubMed] [Google Scholar]
- van Wingen G, van Broekhoven F, Verkes RJ, Petersson KM, Bäckström T, Buitelaar J, Fernández G ( 2007): How progesterone impairs memory for biologically salient stimuli in healthy young women. J Neurosci 27: 11416–11423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Bäckström T, Buitelaar JK, Fernández G ( 2008): Progesterone selectively increases amygdala reactivity in women. Mol Psychiatry 13: 325–333. [DOI] [PubMed] [Google Scholar]
- Wang M, Seippel L, Purdy RH, Bäckström T ( 1996): Relationship between symptom severity and steroid variation in women with premenstrual syndrome: Study on serum pregnenolone, pregnenolone sulfate, 5 alpha‐pregnane‐3,20‐dione and 3 alpha‐hydroxy‐5 alpha‐pregnan‐20‐one. J Clin Endocrinol Metab 81: 1076–1082. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A ( 1988): Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol 54: 1063–1070. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC ( 1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]
- Yasumatsu N, Matsuzaki M, Miyazaki T, Noguchi J, Kasai H ( 2008): Principles of long‐term dynamics of dendritic spines. J Neurosci 28: 13592–13608. [DOI] [PMC free article] [PubMed] [Google Scholar]


