Abstract
Objectives:
Writer's cramp (WC) is characterized by excessive cocontractions of agonist and antagonist hand and forearm muscles during writing. Changes in functional magnetic resonance imaging activation patterns in such conditions can be ambiguous as they might either reflect some aspect of the primary pathophysiological mechanism or, alternatively, may be the result of adaptive actions during task execution. To circumvent this problem, we examined WC patients during rest, i.e., without a task, using independent component analysis (ICA) applied to the blood oxygen level‐dependent time series.
Methods:
Functionally connected brain networks during rest were defined by ICA to assess differences between WC patients (n = 16) and healthy controls (n = 16). Analysis was carried out using FMRIB's Software Library.
Results:
Two functional networks showed between‐group differences, the sensorimotor network and the default‐mode network. In WC patients, the connectivity was reduced in the left postcentral area and increased in basal ganglia in contrast to healthy controls. These changes were not reversed after treatment with botulinum toxin.
Conclusions:
In line with other studies, the results show a dysfunction in cortico‐subcortical circuits in WC involving somatosensory cortex, areas interfacing the sensory and motor systems, and putamen contralateral to symptomatic hand. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: focal dystonia, resting state, fMRI, ICA, writer's cramp, connectivity, botulinum toxin
INTRODUCTION
Idiopathic dystonia is defined by involuntary and sometimes painful muscle contractions resulting in abnormal posture. It can be classified according to the anatomical distribution of the pathological movements as focal, segmental, or generalized dystonia [Albanese et al., 2006; Fahn et al., 1998; Geyer and Bressman, 2006]. Primary adult‐onset dystonia is the most common form. It is often focal and can be subdivided into a number of distinct clinical syndromes such as blepharospasm, cervical dystonia, or upper limb dystonia [Geyer and Bressman, 2006].
In a number of dystonic syndromes, including focal dystonias such as cervical dystonia [Dauer et al., 1998; Defazio et al., 2007], writer's cramp (WC) [Gasser et al., 1996], and musician's cramp [Schmidt et al., 2006], a genetic predisposition has been identified, though the pathophysiological changes in movement control are far from being understood. WC is the most common task‐specific idiopathic focal dystonia and is characterized by excessive cocontractions of agonist and antagonist hand and forearm muscles during writing [Marsden and Sheehy, 1990; Sheehy and Marsden, 1982]. Botulinum toxin (BTX) therapy is currently the treatment of choice and is reported to significantly improve symptoms of WC [Jankovic et al., 1990].
Functional magnetic resonance imaging (fMRI) might be useful to gain insight into the pathophysiology of dystonic syndromes. Activation studies have either used movements that induce dystonia or have used movements that do not. The former have generally shown increased activation in cerebellar and premotor areas [Berg et al., 2000; Preibisch et al., 2001; Pujol et al., 2000], whereas the latter have shown decreased activity of the primary sensorimotor cortex and the caudal part of SMA [Baker et al., 2003; Dresel et al., 2006; Oga et al., 2002]. As has been discussed with regard to other neurological diseases such as amyotrophic lateral sclerosis (ALS) [Konrad et al., 2002, 2006; Schoenfeld et al., 2005; Stanton et al., 2007], changes in fMRI activation patterns can be ambiguous, though. They might either reflect some aspect of the primary pathophysiological mechanism or, alternatively, may be the result of adaptive, potentially compensatory, mechanisms during task execution (such as increased effort or increased need for executive control).
As a further step in the elucidation of these changes, this study uses a rather novel fMRI method, network analysis based on independent component analysis (ICA), applied to blood oxygen level‐dependent (BOLD) time series obtained during rest, i.e., without a goal‐directed task. ICA methods are particularly suited to recover the sources (or components) underlying the observed signals, i.e., the spatiotemporal patterns of the fMRI BOLD signal, by assuming that the sources are statistically independent [Calhoun and Adali, 2006; Calhoun et al., 2001; Esposito et al., 2005; Garrity et al., 2007; McKeown et al., 1998]. A decisive advantage of the ICA method is that it can be applied easily to “resting‐state” scans as in this investigation. Such data only take minutes to acquire and do not suffer from performance confounds that may be present in patients with cognitive or motor impairments [Beckmann et al., 2005; Greicius et al., 2004].
Importantly, different typical resting‐state networks can be recovered from the BOLD signal with high reliability across individuals and studies [Beckmann et al., 2005; Damoiseaux et al., 2006; De Luca et al., 2006; van den Heuvel et al., 2008]. One of these consistently recovered networks is the default‐mode network (DMN) comprising a large frontal area (including ventral anterior cingulate cortex, medial prefrontal cortex, and orbitofrontal cortex), the posterior cingulate cortex, the inferior parietal cortex, and a temporal region involving the parahippocampal gyrus. It is conceptualized as a stand‐alone cognitive network [Raichle and Snyder, 2007; Raichle et al., 2001]. Another often reported network is the sensorimotor network (SMN) [Beckmann et al., 2005; Damoiseaux et al., 2006; De Luca et al., 2006], which typically includes primary motor cortex, premotor cortex, anterior section of cingulate cortex, and the somatosensory region.
A number of studies have provided initial evidence that resting‐state activity might be altered in neuropsychiatric conditions such as autism [Kennedy and Courchesne, 2008; Kennedy et al., 2006], Alzheimer's disease [Greicius et al., 2004], depression [Greicius et al., 2007], schizophrenia [Liang et al., 2006; Williamson, 2007], attention‐deficit hyperactivity disorder [Tian et al., 2006], and ALS [Mohammadi et al., 2009]. These studies underline the potential of resting‐state fMRI analysis to reveal impaired network activity in neuropsychiatric conditions.
In this study, we analyze resting‐state networks in WC using ICA to characterize the differences in the degree of functional connectivity within large‐scale networks. Given the “motor” nature of WC, we hypothesized that differences in the relative degree of functional connectivity within the SMN should be present between patients and healthy controls. Furthermore, we hypothesized that such sensitivity might permit the assessment of treatment effects for BTX, which is why patients were scanned twice, once before and once after treatment with BTX. As previous studies of conditions affecting the peripheral motor system, such as deafferentiation, have shown changes in resting‐state connectivity [Pawela et al., 2010], we were interested whether BTX would restore possible alterations of resting‐state activity in WC. Although activation changes after application of BTX in active tasks may either reflect the changed peripheral motor feedback or a cortical compensation because of peripheral weakness (i.e., not necessarily a real plastic change of the cortical motor network), resting‐state analysis circumvents this ambiguity. Given the absence of a task, any differences in the resting‐state SMN can not be attributed to differences in effort or task difficulty between patients and control subjects. Moreover, we also examined whether there are any differences in the DMN between the WC group and healthy controls.
METHODS
Patients
The study was approved by the local ethics committee. All participants gave their written informed consent before their inclusion in the study. Two groups were investigated using BOLD fMRI. The first group comprised 16 patients (10 women) with a mean age of 48.3 ± 6.8 suffering from WC. The second group included 16 healthy controls (11 women) with a mean age of 52.1 ± 5.4. All patients were right handed. The symptoms were exclusively on the right hand and occurred only during writing. The mean symptom duration was 6.2 ± 2.4 years. None of the patients showed another dystonic signs. During the scans there were no dystonic postures. Besides BTX, all patients were free of other medications. Especially, there was no history of medication for anticholinergics or other drugs.
Patients were scanned twice: once immediately before BTX treatment and a second time 4 weeks after injection. The mean time delay between BTX treatment and the first scan was 88 ± 9 days. Patients were treated with different BTX preparations (Dysport®, Botox®, or Xeomin®) with comparable clinical success. The mean duration of BTX therapy was 5.8 ± 3.1 years. The latency of response (7.3 ± 4.2 days) and the duration of treatment effect (10.6 ± 1.8 weeks) were very similar for all three products. The mean dose was 110 ± 65 U for Dysport® (n = 8), 40 ± 25 U for Botox® (n = 5), and 50 ± 15 U for Xeomin® (n = 3).
BTX‐related global clinical improvement (GCI) was assessed based on patient self‐report on a 0–3 scale (0 = no effect; 1 = slight decrease in severity and improvement of function; 2 = moderate decrease in severity and improvement of function; and 3 = marked decrease in severity and improvement of function). The severity of WC was measured according to the Arm Dystonia Disability Scale (ADDS). ADDS contains seven items that score the impairment of manual motor control. Patients who are subjectively free of any motor dysfunction have an ADDS of 100% [Fahn, 1989]. All patients were asked for any side effects after treatment with BTX.
The WC patients rated the improvement after BTX therapy with a mean GCI of 2.6 ± 0.3. The results for ADDS ranged from 17 to 69% (median 54%) before injection. The treatment decreased the severity of symptoms and increased the ADDS to a median value of 88% (range 70–100%). There were no significant side effects of treatment.
Experimental Design
During the data acquisition for functional connectivity, the subjects were instructed to neither engage in cognitive nor motor activity and to keep their eyes closed. The functional run took 6 min to complete.
Image Acquisition
Magnetic resonance images were acquired on a 3‐T Siemens Magnetom Allegra Scanner (Erlangen, Germany) equipped with a standard head coil. A total of 178 T2*‐weighted volumes of the whole brain (EPI sequence; TR = 2,000 ms, TE = 30 ms, flip angle = 80°, FOV = 192 mm, matrix = 64 × 64, 34 slices, slice thickness = 3 mm, and interslice gap = 0.75 mm) parallel to the AC‐PC line were recorded for functional imaging. A T1‐weighted high‐resolution data set was acquired using a 3D MPRAGE (three‐ dimensional magnetization‐prepared rapid acquisition gradient echo) sequence for anatomical information (matrix = 192 × 256, 1‐mm isovoxel). The subject's head was fixed during the entire measurement to avoid head movements.
FMRI Data Analysis
FMRI analysis at rest was carried out using Multivariate Exploratory Linear Optimized Decomposition into Independent Components Version 3.09, part of FSL (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl) [Beckmann et al., 2005].
Preprocessing consisted of motion correction, brain extraction, spatial smoothing using a Gaussian kernel of full width at half maximum of 6 mm, and high‐pass temporal filtering equivalent to a time constant of 150 s (0.007 Hz). FMRI volumes were registered to the individual's structural scan and standard space images using FMRIB's Nonlinear Image Registration Tool. Preprocessed functional data containing 178 time points for each subject were temporally concatenated across subjects to create a single 4D data set. The between‐subject analysis of the resting data was carried out using a regression technique (dual regression) that allows for voxel‐wise comparisons of resting functional connectivity [Filippini et al., 2009]. This approach proceeds in three stages. First, the concatenated multiple FMRI data sets are decomposed using ICA to identify large‐scale patterns of functional connectivity in the population of subjects. In this analysis, the data set was decomposed into 25 components, in which the model order was estimated using the Laplace approximation to the Bayesian evidence for a probabilistic principal component model [Beckmann and Smith, 2004].
Second, the dual‐regression approach is used to identify, within each subject's FMRI data set, subject‐specific temporal dynamics and associated spatial maps. This involves first using the full set of group‐ICA spatial maps in a linear model fit (spatial regression) against the separate fMRI data sets, resulting in matrices describing temporal dynamics for each component and subject, then using these time‐course matrices in a linear model fit (temporal regression) against the associated fMRI data set to estimate subject‐specific spatial maps.
These spatial maps characterize the subject‐ and voxel‐specific degree of integration into a given group component map. Finally, the different component maps are collected across subjects into single 4D files (one per original ICA map, with the fourth dimension being subject identification) and tested voxel wise for statistically significant differences between groups using nonparametric permutation testing [Nichols and Holmes, 2002]. This results in spatial maps characterizing the between‐subject/group differences. These maps were thresholded at P < 0.05 (family‐wise error corrected) using threshold‐free cluster enhancement [Smith and Nichols, 2009] to define clusters of significant changes in connectivity. Data were visualized in MNI standard space using FSLView.
RESULTS
The ICA decomposition resulted in 25 spatial maps, containing all classically identified resting‐state networks (e.g., visual, auditory, DMN, and somatosensory, see [Beckmann et al., 2005; Damoiseaux et al., 2006; De Luca et al., 2006]) as well as artifactual components. The complete set of 25 components was used for the dual‐regression analysis. Only two original components (the SMN and the DMN) showed any between‐group changes in the degree of functional connectivity.
Default‐Mode Network
The DMN was identified as the spatial map comprising prefrontal, anterior and posterior cingulate, lateral parietal, and inferior/middle temporal gyri (extending to the mesial temporal lobe), cerebellar areas, and thalamic nuclei [Boly et al., 2008; Raichle et al., 2001]. This network has been reviewed recently by Raichle and Snyder [ 2007], who have stressed that activity in this network is high during rest and reduced during cognitive activity.
Figure 1A shows this network for all subjects, patients and controls, together. Basal ganglia and cerebellar areas were strongly integrated into the DMN [Habas et al., 2009]. Importantly, using the step‐wise approach outlined in the methods, we could demonstrate a differential involvement of the basal ganglia into the DMN in the two groups. The between‐group analysis of this network showed a stronger connectivity in the WC group involving the left putamen (Fig. 1B). The MNI coordinates (maximum effect) were X = −26 mm, Y = −8 mm, and Z = 4 mm. The degree of network integration as measured by the magnitude of the regression coefficient of the voxels within the area against the relevant reference time course was found to be increased by a factor of 5. The clusters involved in DMN showed no significant difference between two groups.
Figure 1.
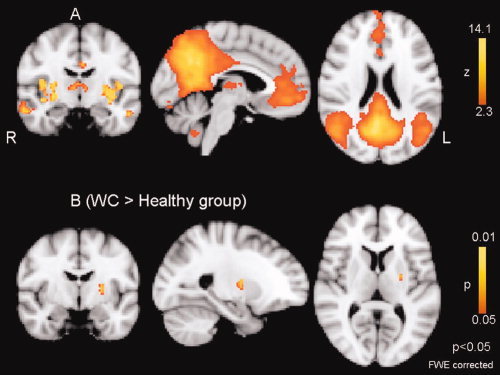
Default‐mode network (DMN). A: Spatial map representing DMN for the entire 32 participants (WC = 16 and controls = 16). B: Difference in network connectivity between the healthy group and WC calculated using the dual‐regression approach. There is a significantly increased connectivity in the left putamen in WC.
The putamen has not been described as being part of the DMN per se. That is, a simple primary contrast testing for significance of the correlation of the putamen and the DMN reference against zero correlation does not reach significance. Using unbiased permutation testing, we here show that the differential contrast (i.e., the difference in correlation between the two groups) is significantly different at the canonical level of P < 0.05. Basal ganglia network is identified in recent studies and suggested to correlate with DMN [Damoiseaux et al., 2008; Robinson et al., 2009].
Sensorimotor Network
The SMN was comprised precentral and postcentral gyri [Beckmann et al., 2005] as previously described by a number of authors [Biswal et al., 1997; De Luca et al., 2005, 2006; Jafri et al., 2008; Lowe et al., 2002]. Figure 2A shows this network in all subjects. The between‐group analysis showed a stronger connectivity in the healthy control group involving the left postcentral gyrus (Fig. 2B). The MNI coordinates (maximum of effect) were X = −44 mm, Y = −34 mm, and Z = 52 mm. Using the Juelich Histological Atlas (part of FSL), we identified the following regions: primary somatosensory cortex: BA2 (58%) and BA1 (14%), superior parietal lobe (8%), and inferior parietal lobe (2%). Figure 2C shows that the altered area involves also anterior intraparietal sulcus (aIPS) and partially the inferior parietal lobule (IPL). The degree of network integration was found to be increased by a factor of 6.
Figure 2.
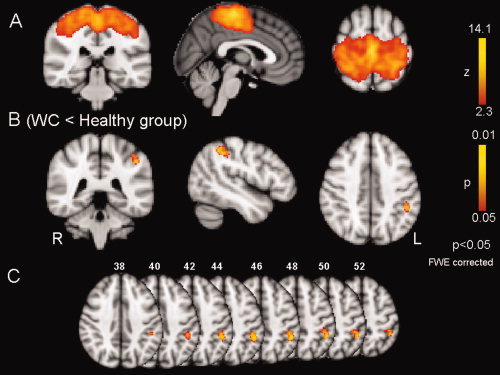
Sensorimotor network (SMN). A: Spatial map representing the SMN for the entire 32 participants (WC = 16 and controls = 16). B: Difference in SMN between healthy subjects and WC calculated using the dual‐regression approach. There is significantly decreased connectivity in left primary somatosensory cortex in WC. C: Axial slices of 2B, the coordinate of each slice is expressed in mm. It also shows areas involving anterior intraparietal sulcus (aIPS) and partially the inferior parietal lobule (IPL).
In 14 patients, we were able to register resting‐state data before and after successful administration of BTX. The comparison of the different networks between these two conditions showed no significant change in any network.
DISCUSSION
This study used ICA‐based resting‐state fMRI analysis to delineate possible functional changes in brain networks in WC. In line with previous fMRI studies of resting‐state activity [Beckmann et al., 2005; Damoiseaux et al., 2006; De Luca et al., 2006; van den Heuvel et al., 2008], we were able to identify multiple resting‐state networks, two of which showed focal changes in the functional connectivity between WC and control participants: DMN and SMN. This, once again, underscores the stability of the networks that can be retrieved from resting‐state BOLD activity. We found a significantly decreased connectivity in the left primary somatosensory area and an increased connectivity in left putamen in WC relative to the control group. These changes were thus contralateral to the symptomatic right hand.
One particular feature of dystonia is loss of inhibition at the spinal, brainstem, and cortical level [Berardelli et al., 1998]. In healthy subjects, when a specific voluntary movement is generated, the brain has to suppress other possible movements inducing surround inhibition [Mink, 1996]. This mechanism is altered in dystonic patients [Sohn and Hallett, 2004], as even imaginary movements can cause a reduction of surround inhibition in focal hand dystonia [Quartarone et al., 2005]. As inhibitory synaptic activity is associated with increased metabolism, decreased synaptic inhibition should be reflected by decreased regional cerebral blood flow [Kelly and McCulloch, 1983; Nudo and Masterton, 1986]. This mechanism may underlie the reductions in resting‐state connectivity in the SMN in this study. This would suggest that the reduction of cortical inhibition leads to a significant reduction of intrinsic activity particularly in the postcentral gyrus. The analyses used in this study can bring into evidence regions that are differentially integrated into the networks under study in the different groups. For instance, the somatosensory cortex was less integrated with the SMN in the dystonic patients. Figure 2C shows the altered area in primary somatosensory area involving also aIPS and partially the IPL. The aIPS has been shown to be involved in motor planning and is supposed to use sensory input to modulate the motor command when reaching a goal [Tunik et al., 2005], whereas the IPL has been supposed to act as a sensorimotor interface [Mattingley et al., 1998]. Thus, it appears that not only the somatosensory cortex but also two areas interfacing the sensory and motor systems show decreased network integrity in WC.
Importantly, the present maps depict differences in connectivity not in activity. In light of the known disinhibition in primary motor cortex in WC as revealed by TMS [Butefisch et al., 2005], it may come as a surprise that there was no change in connectivity in this area. However, the disinhibition has been shown during specific movements and not at rest. Therefore, we speculate that during rest there is no change in inhibition and consequently no difference in connectivity for this area. It is also noteworthy that sensory stimulation of the hand can induce increased excitability in primary motor cortex at rest in healthy persons but even more so in WC (affected hand) [Quartarone et al., 2005]. Another study investigated the effect of electrical stimulation of the fingers on the amplitude of TMS‐evoked motor potentials and found that MEP suppression in response to digital stimulation was preserved in dystonia, but that the somatotopically distributed input–output organization of the sensorimotor interactions was lost in dystonic patients' hands [Tamburin et al., 2002]. These findings might reflect a disintegration of the SMN as found in this study, in particular an altered connectivity in the somatosensory afferent system.
Previously, activation in sensorimotor cortex has been found to be reduced during nondystonia inducing motor tasks as a baseline situation [Baker et al., 2003; Dresel et al., 2006; Oga et al., 2002]. However, dystonic movements cause increased recruitment in sensorimotor cortex, i.e., increased activation of these areas in fMRI [Berg et al., 2000; Preibisch et al., 2001; Pujol et al., 2000] correlated with dystonic symptoms. Sensory dysfunction is a well‐known feature of WC [Nelson et al., 2009]. It is interesting to speculate that patients may experience a decreased reliance on sensory input resulting in reduced network integration of this area. A previous study showed gray matter decrease in the hand area of the left primary sensorimotor cortex, bilateral thalamus, and cerebellum in WC patients [Delmaire et al., 2007]. Whether this structural change contributes to the decreased connectivity in SMN in the current must remain speculative at this point and needs to be addressed by future investigations using multimodal imaging.
With regard to the DMN, an increased connectivity of the putamen to this network was found in WC. Disturbances of cortico‐subcortical circuits are supposed to be important factors in the pathophysiology of dystonia. Further examples for an alteration in these circuits are the increased basal ganglia activity during the discrimination of tactile stimuli [Peller et al., 2006] and changes in white matter microstructure in this circuit as demonstrated by diffusion tensor imaging [Delmaire et al., 2009]. Thus, the increased connectivity of the putamen with the DMN may present another instance of dysfunction of cortico‐subcortical circuits in WC. The increased connectivity may reflect the reduced selectivity and functional segregation within the basal ganglia. In particular, the increased output of the putamen to the globus pallidus and a concomitant decrease in the activity of the subthalamicopallidal and pallidothalamic pathways as described in primate models of upper limb dystonia [Mitchell et al., 1990]. Further research should address the relationship between resting‐state changes and activation changes. For example, is the increased connectivity in putamen a compensatory answer to reduced connectivity in somatosensory cortex? Considering the suggestion that the basal ganglia may be involved in focusing the motor command by gating sensory inputs for the performance of the movement [Hallett, 1998], the changed connectivity in both systems found in this study may explain the resulting overflow of motor action leading in dystonic posture.
We found no changes in network connectivity as a function of BTX treatment. This is in contrast to a PET activation study using dystonia‐inducing task, in which BTX treatment resulted in enhanced activation of premotor area, posterior (BA7) and inferior (BA40) parietal cortex. This could have reflected changes in movement strategy after BTX‐induced muscle weakness. However, this treatment failed to normalize the impaired activation of primary motor cortex [Ceballos‐Baumann et al., 1997]. The lack of BTX‐related changes in resting‐state networks in this study, therefore, suggests that BTX has no effect on the underlying cortical dysfunction in WC but rather becomes apparent only after task induction.
Our findings are novel in that they demonstrate altered function in somatosensory cortex and the basal ganglia in the absence of any task. This is important as any task‐dependent study is potentially confounded by possible differences in task difficulty and performance level between the dystonic and healthy subjects [Rosenkranz et al., 2005; Torres‐Russotto and Perlmutter, 2008].
As any method, analysis of resting‐state activity with fMRI has certain drawbacks: First, the situation during rest may be considered as insufficiently controlled. Also, resting‐state activity fails to uncover task‐related changes in movement strategies or functional compensation that occur only during motor performance. Analysis of resting‐state activity, therefore, should be considered as complementary to functional measures. Therefore, further studies are needed to relate changes in resting‐state activity to functional measures, such as movement‐induced fMRI activations or neurophysiological measures determined by transcranial magnetic stimulation [Beck et al., 2008; Koch et al., 2008; Pirio et al., 2009] on an interindividual basis. Such an approach will further strengthen the significance of the alterations found in this study.
REFERENCES
- Albanese A, Barnes MP, Bhatia KP, Fernandez‐Alvarez E, Filippini G, Gasser T, Krauss JK, Newton A, Rektor I, Savoiardo M, Valls‐Sole J ( 2006): A systematic review on the diagnosis and treatment of primary (idiopathic) dystonia and dystonia plus syndromes: Report of an EFNS/MDS‐ES Task Force. Eur J Neurol 13: 433–444. [DOI] [PubMed] [Google Scholar]
- Baker RS, Andersen AH, Morecraft RJ, Smith CD ( 2003): A functional magnetic resonance imaging study in patients with benign essential blepharospasm. J Neuro‐Ophthalmol 23: 11–15. [DOI] [PubMed] [Google Scholar]
- Beck S, Richardson SP, Shamim EA, Dang N, Schubert M, Hallett M ( 2008): Short intracortical and surround inhibition are selectively reduced during movement initiation in focal hand dystonia. J Neurosci 28: 10363–10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM ( 2004): Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging 23: 137–152. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM ( 2005): Investigations into resting‐state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360: 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD ( 1998): The pathophysiology of primary dystonia. Brain 121( Part 7): 1195–1212. [DOI] [PubMed] [Google Scholar]
- Berg D, Preibisch C, Hofmann E, Naumann M ( 2000): Cerebral activation pattern in primary writing tremor. J Neurol Neurosurg Psychiatry 69: 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Van Kylen J, Hyde JS ( 1997): Simultaneous assessment of flow and BOLD signals in resting‐state functional connectivity maps. NMR Biomed 10: 165–170. [DOI] [PubMed] [Google Scholar]
- Boly M, Phillips C, Tshibanda L, Vanhaudenhuyse A, Schabus M, Dang‐Vu TT, Moonen G, Hustinx R, Maquet P, Laureys S ( 2008): Intrinsic brain activity in altered states of consciousness: How conscious is the default mode of brain function? Ann N Y Acad Sci 1129: 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butefisch CM, Boroojerdi B, Chen R, Battaglia F, Hallett M ( 2005): Task‐dependent intracortical inhibition is impaired in focal hand dystonia. Mov Disord 20: 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T ( 2006): Unmixing fMRI with independent component analysis. IEEE Eng Med Biol Mag 25: 79–90. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ ( 2001): Spatial and temporal independent component analysis of functional MRI data containing a pair of task‐related waveforms. Hum Brain Mapp 13: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos‐Baumann AO, Sheean G, Passingham RE, Marsden CD, Brooks DJ ( 1997): Botulinum toxin does not reverse the cortical dysfunction associated with writer's cramp. A PET study. Brain 120( Part 4): 571–582. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF ( 2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103: 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA ( 2008): Reduced resting‐state brain activity in the “default network” in normal aging. Cereb Cortex 18: 1856–1864. [DOI] [PubMed] [Google Scholar]
- Dauer WT, Burke RE, Greene P, Fahn S ( 1998): Current concepts on the clinical features, aetiology and management of idiopathic cervical dystonia. Brain 121( Part 4): 547–560. [DOI] [PubMed] [Google Scholar]
- Defazio G, Berardelli A, Hallett M ( 2007): Do primary adult‐onset focal dystonias share aetiological factors? Brain 130: 1183–1193. [DOI] [PubMed] [Google Scholar]
- Delmaire C, Vidailhet M, Elbaz A, Bourdain F, Bleton JP, Sangla S, Meunier S, Terrier A, Lehericy S ( 2007): Structural abnormalities in the cerebellum and sensorimotor circuit in writer's cramp. Neurology 69: 376–380. [DOI] [PubMed] [Google Scholar]
- Delmaire C, Vidailhet M, Wassermann D, Descoteaux M, Valabregue R, Bourdain F, Lenglet C, Sangla S, Terrier A, Deriche R, Lehericy S ( 2009): Diffusion abnormalities in the primary sensorimotor pathways in writer's cramp. Arch Neurol 66: 502–508. [DOI] [PubMed] [Google Scholar]
- De Luca M, Smith S, De Stefano N, Federico A, Matthews PM ( 2005): Blood oxygenation level dependent contrast resting state networks are relevant to functional activity in the neocortical sensorimotor system. Exp Brain Res 167: 587–594. [DOI] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM ( 2006): fMRI resting state networks define distinct modes of long‐distance interactions in the human brain. Neuroimage 29: 1359–1367. [DOI] [PubMed] [Google Scholar]
- Dresel C, Haslinger B, Castrop F, Wohlschlaeger AM, Ceballos‐Baumann AO ( 2006): Silent event‐related fMRI reveals deficient motor and enhanced somatosensory activation in orofacial dystonia. Brain 129: 36–46. [DOI] [PubMed] [Google Scholar]
- Esposito F, Scarabino T, Hyvarinen A, Himberg J, Formisano E, Comani S, Tedeschi G, Goebel R, Seifritz E, Di Salle F ( 2005): Independent component analysis of fMRI group studies by self‐organizing clustering. Neuroimage 25: 193–205. [DOI] [PubMed] [Google Scholar]
- Fahn S ( 1989): Assessment of the primary dystonia In: Munsat T, editor. The Quantification of Neurologic Deficit. Boston: Butterworths; pp 241–270. [Google Scholar]
- Fahn S, Bressman SB, Marsden CD ( 1998): Classification of dystonia. Adv Neurol 78: 1–10. [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE ( 2009): Distinct patterns of brain activity in young carriers of the APOE‐epsilon4 allele. Proc Natl Acad Sci USA 106: 7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD ( 2007): Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry 164: 450–457. [DOI] [PubMed] [Google Scholar]
- Gasser T, Bove CM, Ozelius LJ, Hallett M, Charness ME, Hochberg FH, Breakefield XO ( 1996): Haplotype analysis at the DYT1 locus in Ashkenazi Jewish patients with occupational hand dystonia. Mov Disord 11: 163–166. [DOI] [PubMed] [Google Scholar]
- Geyer HL, Bressman SB ( 2006): The diagnosis of dystonia. Lancet Neurol 5: 780–790. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V ( 2004): Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci USA 101: 4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF ( 2007): Resting‐state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD ( 2009): Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 29: 8586–8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M ( 1998): The neurophysiology of dystonia. Arch Neurol 55: 601–603. [DOI] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD ( 2008): A method for functional network connectivity among spatially independent resting‐state components in schizophrenia. Neuroimage 39: 1666–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J, Schwartz K, Donovan DT ( 1990): Botulinum toxin treatment of cranial‐cervical dystonia, spasmodic dysphonia, other focal dystonias and hemifacial spasm. J Neurol Neurosurg Psychiatry 53: 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PA, McCulloch J ( 1983): The effects of the GABAergic agonist muscimol upon the relationship between local cerebral blood flow and glucose utilization. Brain Res 258: 338–342. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E ( 2008): The intrinsic functional organization of the brain is altered in autism. Neuroimage 39: 1877–1885. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E ( 2006): Failing to deactivate: Resting functional abnormalities in autism. Proc Natl Acad Sci USA 103: 8275–8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Schneider S, Baumer T, Franca M, Munchau A, Cheeran B, Fernandez dO, Cordivari C, Rounis E, Caltagirone C, Bhatia K, Rothwell JC ( 2008): Altered dorsal premotor‐motor interhemispheric pathway activity in focal arm dystonia. Mov Disord 23: 660–668. [DOI] [PubMed] [Google Scholar]
- Konrad C, Henningsen H, Bremer J, Mock B, Deppe M, Buchinger C, Turski P, Knecht S, Brooks B ( 2002): Pattern of cortical reorganization in amyotrophic lateral sclerosis: A functional magnetic resonance imaging study. Exp Brain Res 143: 51–56. [DOI] [PubMed] [Google Scholar]
- Konrad C, Jansen A, Henningsen H, Sommer J, Turski PA, Brooks BR, Knecht S ( 2006): Subcortical reorganization in amyotrophic lateral sclerosis. Exp Brain Res 172: 361–369. [DOI] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y ( 2006): Widespread functional disconnectivity in schizophrenia with resting‐state functional magnetic resonance imaging. Neuroreport 17: 209–213. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Phillips MD, Lurito JT, Mattson D, Dzemidzic M, Mathews VP ( 2002): Multiple sclerosis: Low‐frequency temporal blood oxygen level‐dependent fluctuations indicate reduced functional connectivity initial results. Radiology 224: 184–192. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Sheehy MP ( 1990): Writer's cramp. Trends Neurosci 13: 148–153. [DOI] [PubMed] [Google Scholar]
- Mattingley JB, Husain M, Rorden C, Kennard C, Driver J ( 1998): Motor role of human inferior parietal lobe revealed in unilateral neglect patients. Nature 392: 179–182. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, Jung TP, Kindermann SS, Bell AJ, Sejnowski TJ ( 1998): Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp 6: 160–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW ( 1996): The basal ganglia: Focused selection and inhibition of competing motor programs. Prog Neurobiol 50: 381–425. [DOI] [PubMed] [Google Scholar]
- Mitchell IJ, Luquin R, Boyce S, Clarke CE, Robertson RG, Sambrook MA, Crossman AR ( 1990): Neural mechanisms of dystonia: Evidence from a 2‐deoxyglucose uptake study in a primate model of dopamine agonist‐induced dystonia. Mov Disord 5: 49–54. [DOI] [PubMed] [Google Scholar]
- Mohammadi B, Kollewe K, Samii A, Krampfl K, Dengler R, Munte TF ( 2009): Changes of resting state brain networks in amyotrophic lateral sclerosis. Exp Neurol 217: 147–153. [DOI] [PubMed] [Google Scholar]
- Nelson AJ, Blake DT, Chen R ( 2009): Digit‐specific aberrations in the primary somatosensory cortex in Writer's cramp. Ann Neurol 66: 146–154 [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP ( 2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Masterton RB ( 1986): Stimulation‐induced [14C]2‐deoxyglucose labeling of synaptic activity in the central auditory system. J Comp Neurol 245: 553–565. [DOI] [PubMed] [Google Scholar]
- Oga T, Honda M, Toma K, Murase N, Okada T, Hanakawa T, Sawamoto N, Nagamine T, Konishi J, Fukuyama H, Kaji R, Shibasaki H ( 2002): Abnormal cortical mechanisms of voluntary muscle relaxation in patients with writer's cramp: An fMRI study. Brain 125: 895–903. [DOI] [PubMed] [Google Scholar]
- Pawela CP, Biswal BB, Hudetz AG, Li R, Jones SR, Cho YR, Matloub HS, Hyde JS ( 2010): Interhemispheric neuroplasticity following limb deafferentation detected by resting‐state functional connectivity magnetic resonance imaging (fcMRI) and functional magnetic resonance imaging (fMRI). Neuroimage 49: 2467–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peller M, Zeuner KE, Munchau A, Quartarone A, Weiss M, Knutzen A, Hallett M, Deuschl G, Siebner HR ( 2006): The basal ganglia are hyperactive during the discrimination of tactile stimuli in writer's cramp. Brain 129: 2697–2708. [DOI] [PubMed] [Google Scholar]
- Pirio RS, Bliem B, Voller B, Dang N, Hallett M ( 2009): Long‐latency afferent inhibition during phasic finger movement in focal hand dystonia. Exp Brain Res 193: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preibisch C, Berg D, Hofmann E, Solymosi L, Naumann M ( 2001): Cerebral activation patterns in patients with writer's cramp: A functional magnetic resonance imaging study. J Neurol 248: 10–17. [DOI] [PubMed] [Google Scholar]
- Pujol J, Roset‐Llobet J, Rosinés‐Cubells, Deus J, Narberhaus B, Valls S, Capdevila A, Pascual‐Leone A ( 2000): Brain cortical activation during guitar‐induced hand dystonia studied by functional MRI. Neuroimage 12: 257–267. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Morgante F, Sant'angelo A, Crupi D, Romano M, Messina C, Berardelli A, Girlanda P ( 2005): Corticospinal excitability during motor imagery of a simple tonic finger movement in patients with writer's cramp. Mov Disord 20: 1488–1495. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ ( 2007): A default mode of brain function: A brief history of an evolving idea. Neuroimage 37: 1083–1090. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Basso G, Soldati N, Sailer U, Jovicich J, Bruzzone L, Kryspin‐Exner I, Bauer H, Moser E ( 2009): A resting state network in the motor control circuit of the basal ganglia. BMC Neurosci 10: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Williamon A, Butler K, Cordivari C, Lees AJ, Rothwell JC ( 2005): Pathophysiological differences between musician's dystonia and writer's cramp. Brain 128: 918–931. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Jabusch HC, Altenmuller E, Hagenah J, Bruggemann N, Hedrich K, Saunders‐Pullman R, Bressman SB, Kramer PL, Klein C ( 2006): Dominantly transmitted focal dystonia in families of patients with musician's cramp. Neurology 67: 691–693. [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Tempelmann C, Gaul C, Kuhnel GR, Duzel E, Hopf JM, Feistner H, Zierz S, Heinze HJ, Vielhaber S ( 2005): Functional motor compensation in amyotrophic lateral sclerosis. J Neurol 252: 944–952. [DOI] [PubMed] [Google Scholar]
- Sheehy MP, Marsden CD ( 1982): Writers' cramp—A focal dystonia. Brain 105( Part 3): 461–480. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE ( 2009): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44: 83–98. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Hallett M ( 2004): Disturbed surround inhibition in focal hand dystonia. Ann Neurol 56: 595–599. [DOI] [PubMed] [Google Scholar]
- Stanton BR, Williams VC, Leigh PN, Williams SC, Blain CR, Jarosz JM, Simmons A ( 2007): Altered cortical activation during a motor task in ALS. Evidence for involvement of central pathways. J Neurol 254: 1260–1267. [DOI] [PubMed] [Google Scholar]
- Tamburin S, Manganotti P, Marzi CA, Fiaschi A, Zanette G ( 2002): Abnormal somatotopic arrangement of sensorimotor interactions in dystonic patients. Brain 125: 2719–2730. [DOI] [PubMed] [Google Scholar]
- Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, Sui M, Cao Q, Hu S, Peng M, Zhuo Y ( 2006): Altered resting‐state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett 400: 39–43. [DOI] [PubMed] [Google Scholar]
- Torres‐Russotto D, Perlmutter JS ( 2008): Task‐specific dystonias: A review. Ann N Y Acad Sci 1142: 179–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunik E, Frey SH, Grafton ST ( 2005): Virtual lesions of the anterior intraparietal area disrupt goal‐dependent on‐line adjustments of grasp. Nat Neurosci 8: 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M, Mandl R, Hulshoff PH ( 2008): Normalized cut group clustering of resting‐state FMRI data. PLoS ONE 3: e2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson P ( 2007): Are anticorrelated networks in the brain relevant to schizophrenia? Schizophr Bull 33: 994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]


