Abstract
A previous preliminary investigation based on a novel MRI approach to map anatomical connectivity revealed areas of increased connectivity in Alzheimer's disease (AD) but not in mild cognitive impairment patients. This prompted the hypothesis tested here, that these areas might reflect phenomena of brain plasticity driven by acetylcholinesterase inhibitors (AChEIs). Thirty‐eight patients with probable AD (19 under medication with AChEIs and 19 drug‐naïve) were recruited together with 11 healthy controls. All subjects had MRI scanning at 3T, including volumetric and diffusion‐weighted scans. Probabilistic tractography was used to initiate streamlines from all parenchymal voxels, and anatomical connectivity maps (ACMs) were obtained by counting, among the total number of streamlines initiated, the fraction passing through each brain voxel. After normalization into standard space, ACMs were used to test for between‐group comparisons, and for interactions between the exposure to AChEIs and global level of cognition. Patients with AD had reduced ACM values in the fornix, cingulum, and supramarginal gyri. The ACM value was strongly associated with the AChEI dosage‐x‐duration product in the anterior limb (non‐motor pathway) of the internal capsule. Tractography from this region identified the anterior thalamic radiation as the main white matter (WM) tract passing through it. The reduced connectivity in WM bundles connecting the hippocampi with the rest of the brain (fornix/cingulum) suggests a possible mechanism for the spread of AD pathology. An intriguing explanation for the interaction between AChEIs and ACM is related to the mechanisms of brain plasticity, partially driven by neurotrophic properties of acetylcholine replacement. Hum Brain Mapp 34:3158–3167, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: Alzheimer disease, anatomical connectivity mapping, AChE inhibitor, brain plasticity, diffusion MRI
INTRODUCTION
It is becoming increasingly clearer that the clinical manifestations of Alzheimer's disease (AD) are not only associated with regional gray matter (GM) loss [Bozzali et al., 2008; Frisoni et al., 2007; Karas et al., 2003; Raji et al., 2009; Serra et al., 2010a], but also with abnormal integration between cortical regions by disconnection mechanisms [Bozzali et al., 2011a; Gili et al., 2011; Villain et al., 2005]. This concept is supported by both associations found between measures of white matter (WM) integrity and measures of cognitive disability in AD [Bozzali et al., 2002; Bozzali et al., 2011a; Serra et al., 2010a], and by functional imaging data [Ibáñez et al., 1998; Nestor et al., 2003]. It has been recently shown that disconnection may precede the occurrence of GM loss in certain regions of AD brains, suggesting that GM atrophy is partially due to neuronal deafferentation [Gili et al., 2011]. We recently employed a novel approach based on diffusion MRI and tractography, namely anatomical connectivity mapping (ACM), to obtain a scalar measure of structural brain connectivity [Bozzali et al., 2011b]. An exploratory investigation in patients with AD and mild cognitive impairment (MCI) revealed, as expected, some brain areas of decreased anatomical connectivity at different clinical stages. Additionally, unexpected regions of increased connectivity were also observed in AD but not in MCI patients. There are many possible reasons that may account for this difference in ACM between AD and MCI patients. MCI is known to represent quite a heterogeneous population, which includes individuals at a “prodromal” AD stage, individuals who will convert to non‐AD forms of dementia, and individuals who will remain stable or will even revert back to normality [Perri et al., 2007, 2009; Petersen, 2004]. Our MCI patients were all of amnestic type and, according to the inclusion criteria, they had not to show any remarkable sign of vascular pathology [Bozzali et al., 2011b]. They have been clinically followed‐up (at 6 months intervals) for 2 years after recruitment, revealing a higher annual rate of conversion to AD (50%) than expected from previous literature [Petersen, 2004]. This suggests that our cohort of MCI patients was in large part belonging to the stage of “prodromal” AD. In this perspective, the two major discriminating factors between our AD and MCI patients were: (1) the disease duration and (2) the administration of acetylcholinestarase inhibitors (AChEIs) to the patients with AD only. With respect to the latter discriminating factor, there is increasing evidence that replacement of acetylcholine levels (by inhibition of cholinesterase enzymes) produces neurobiological effects, such as modulation of the β‐amyloid cascade, neuroprotection, anti‐inflammation, and also promyelination, which exceed those strictly related with improvement of cognitive efficiency. Despite the difficulty of translating basic research findings into therapeutic effects in humans, AChEIs have been demonstrated to reduce, by muscarinic stimulation (M1 and M3 receptors), unbalances in the metabolism of the APP (i.e., Amyloid Precursor Protein), thus impacting on the deposition of Aβ proteins [Svensson and Giacobini, 2000]. Protection against glutamate neurotoxicity has been also attributed to AChEIs, as an effect due to stimulation of α4‐ and α7 nicotinic receptors [Pepeu and Giovanni, 2010]. As mentioned earlier, it has also been proposed that part of AChEIs efficacy may be due to non‐synaptic effects, such as anti‐inflammation and promyelination. With respect to anti‐inflammation, it has been proposed that AChEIs may affect astrocytes and microglia in the same way as they affect T cells, by harnessing the anti‐inflammatory effect of α7 nicotinic receptors [Reale et al., 2004, 2006]. With respect to promyelination, which is particularly interesting for the current study, cholinergic stimulation of oligodendrocytes has been demonstrated to promote myelination during development, and myelin repair in older age [Bartzokis et al., 2007].
Against this background, the increased ACM we previously observed in patients with AD only [Bozzali et al., 2011b] can be interpreted as a consequence of the occurrence of phenomena of brain plasticity, which might be either spontaneous or driven by AChEIs. To address this issue, we planned the current study with the following specific aims: (i) to confirm the patterns of altered structural connectivity in a larger population of patients with AD and (ii) to assess, by correlation analysis, the effect of AChEIs on ACM in AD brains, and its interaction with cognitive disability.
MATERIALS AND METHODS
Subjects
Thirty‐eight patients diagnosed with probable AD by NINCDS‐ADRDA consensus criteria [McKhann et al., 1984] were recruited. Nineteen patients were under medication with AChEIs (8 Rivastigmine and 11 Donepezil), whereas 19 were drug‐naïve. Thirteen out of these 19 patients (68.4%) were enrolled in the study at their first clinical assessment (and for this reason were drug‐naïve). The remaining six patients (31.6%) had clinical conditions which prevented the administration of AChEIs (cardiac ischemia, or history of peptic ulcer). Patients being treated with AChEIs had to be under therapy for an interval between 6 months and 2 years at the time of enrollment. The AChEIs dosage could range between 5 and 10 mg daily of Donepezil or 6 and 12 mg daily of Rivastigmine. As shown in Table 1, there was no significant difference between the two subgroups of patients (those under medication and those drug‐naïve) with respect to mean age, disease duration (calculated starting from the beginning of cognitive deficits as reported by patients' assistants), mini mental state examination [MMSE; Folstein et al., 1975; Measso et al., 1993] scores, or education. Eleven healthy subjects (HS) matched with patients with AD for age and gender were also enrolled in the study and served as controls. All subjects underwent a neuropsychological assessment to obtain the cognitive profile of patients, and to exclude any remarkable cognitive impairment in controls, who were screened using a shorter neuropsychological battery than the patients. None of the subjects included in this study took part in our previous study on ACM [Bozzali et al., 2011b]. Subjects were excluded if they had either two or more hyperintense lesions with a diameter ≥10 mm or more than eight hyperintense lesions with a diameter between 5 and 10 mm on dual‐echo MR images, to minimize the risk of concomitant vascular pathology [Bozzali et al., 2002; Gili et al., 2011; Serra et al., 2010a]. HS had to show no evidence of cognitive deficits on neuropsychological testing. Major systemic, psychiatric, and other neurological illnesses were carefully investigated and excluded in all subjects. Local Ethical Committee approval and written informed consent (either from the subjects or from their responsible guardians if incapable) were obtained before study initiation.
Table 1.
Principal demographic and clinical characteristics of studied subjects
| Patients with AD | |||
|---|---|---|---|
| Subjects under AChEIs (N = 19) | Subjects drug‐naïve (N = 19) | HS (N = 11) | |
| Mean (SD) age [years] | 72.1 (7.4) | 74.7 (7.6) | 71.3 (8.4) |
| Gender (F/M) | 10/9 | 12/7 | 7/4 |
| Mean (SD) years of education | 11.0 (3.9) | 9.5 (2.1) | 12.4 (3.0) |
| Mean (SD) MMSE score | 18.4 (4.8) | 18.3 (4.0) | 28.9 (1.6) |
| aMedian (range) disease duration (months) | 31.5 (9–58) | 32.0 (10–57) | – |
Disease duration was calculated starting from the beginning of cognitive deficits as reported by patients' assistants.
AChEIs, Acetylcholinesterase inhibitors; HS, healthy subjects; MMSE, mini mental state examination; SD, standard deviation.
Neuropsychological Assessment
The neuropsychological battery was administered to patients with AD by two trained neuropsychologists 48 h before MRI. It included a global cognitive evaluation, using the MMSE [Folstein et al., 1975; Measso et al., 1993], and tests specific for each cognitive domain: (1) Verbal episodic long‐term memory: Immediate and Delayed recall of a 15‐Word List [Carlesimo et al., 1996]; Short Story Recall [Carlesimo et al., 2002]; (2) Visuo‐spatial episodic long‐term memory: Delayed recall of Complex Rey's Figure [Carlesimo et al., 2002]; (3) Short‐term memory: Digit span and Corsi Block Tapping task [Orsini et al., 1987]; (4) Executive functions and attention: Phonological Word Fluency [Carlesimo et al., 1996]; (5) Language: Naming subtest of BADA [Miceli et al., 1991]; (6) Problem‐solving: Raven's Coloured Progressive Matrices [Carlesimo et al., 1996]; (7) Praxis: Copy of drawings and Copy of drawings with Landmarks [Carlesimo et al., 1996]; Copy of Complex Rey's Figure [Carlesimo et al., 2002]. HS were screened using a shorter neuropsychological battery, including: MMSE; delayed recall of a 15‐Word List; Digit span and Corsi Block Tapping task; Phonological Word Fluency; Raven's Colored Progressive Matrices; copy of drawings. For each administered test appropriate adjustments for sex, age and education were applied according to the Italian normative data (reported in the references listed next to each test).
MRI Acquisition and Pre‐Processing
Subjects were scanned using a head‐only 3T MRI system (Magnetom Allegra Siemens, Erlangen, Germany). The following sequences were acquired in a single session: (1) dual‐echo turbo spin echo (TSE; TR = 6190 ms, TE = 12/109 ms); (2) fast‐fluid‐attenuated‐inversion‐recovery (FLAIR; TR = 8170 ms, TE = 96 ms); (3) 3D Modified‐Driven‐Equilibrium‐Fourier‐Transform (MDEFT) scan (TR = 1338 ms, TE = 2.4 ms, Matrix = 256 × 224 × 176, in‐plane FOV = 250 × 250 mm2, slice thickness = 1 mm); (4) Diffusion weighted (DW) twice‐refocused [Reese et al., 2003] spin echo echo‐planar imaging (SE EPI; TR = 7 s, TE = 85 ms, b factor = 1000 s/mm2, isotropic resolution = 2.3 mm3), collecting seven images with no diffusion weighting (b = 0) and 61 images with diffusion gradients applied along 61 non‐collinear directions.
According to the inclusion criteria, TSE and FLAIR scans were reviewed to exclude the presence of macroscopic brain abnormalities. Diffusion data were corrected for misalignment between volumes according to the following steps: (i) b = 0 images were realigned to the first volume with a rigid body transformation computed using the FMRIB's Linear Image Registration Tool [FLIRT; Jenkinson and Smith, 2001], and averaged; (ii) the 61 DW volumes were averaged and coregistered to the scalp stripped mean b = 0 image, to yield an average transformation (Tx 1) matching the mean DW image to the mean b = 0; (iii) each DW volume was realigned to the mean DW image (with a rigid body transformation, described by Tx 2), and the transformation matching each DW volume with the b = 0 image was obtained by combining Tx 2 with Tx 1. The b matrices were rotated accordingly [Leemans and Jones, 2009]. All the remaining processing was done using the Camino toolkit (http://www.camino.org.uk), if not otherwise specified. The diffusion tensor was estimated in every voxel [Basser et al., 1994], and maps of fractional anisotropy (FA) were obtained. Each FA map was used as a reference for affine registration of the corresponding T1‐weighted anatomical image using Statistical Parametric Mapping 8 (SPM8; Wellcome Department of Imaging Neuroscience; http://www.fil.ion.ucl.ac.uk/spm/). Once registered, the anatomical images were segmented into WM, GM, and cerebrospinal fluid using SPM8. For every subject a binary parenchymal mask was obtained by adding GM and WM segments. The resulting image, whose intensity reflects each voxel's probability of containing parenchyma, was thresholded to retain only those voxels with intensity greater than 0.8, and binarized. As in our preliminary study [Bozzali et al., 2011b], we used the Q‐ball algorithm [Tuch, 2004] to process the DTI data, as it provides a model of diffusion able to resolve more than one direction per voxel. Tuch's original radial‐basis function formulation [Tuch, 2004] was employed, as implemented in Camino with the default parameter settings [Seunarine et al., 2007].
Computation of the Anatomical Connectivity Maps
To generate the anatomical connectivity maps (ACMs), probabilistic tractography based on the probabilistic index of connectivity (PICo) method [Parker et al., 2003], was used. The algorithm accounts for the uncertainty associated with the determination of the principal directions of diffusion in every voxel by generating a probability density function (PDF) of estimated fiber alignment from the average principal directions derived from Q‐ball modelling of each voxel [Seunarine et al., 2007]. This provides voxel‐wise estimates of the confidence in fiber tract alignment, which are then used in the probabilistic tract‐tracing procedure. Streamline tracking was then performed, with 10 Monte Carlo iterations from all voxels in the parenchymal mask.
ACMs were obtained as previously described in detail [Bozzali et al., 2011b]. Briefly, the total number of streamlines passing through each voxel was counted. Then, connectivity maps were divided by the maximum possible count (sum of the number of voxels tracked, weighted by the number of diffusion directions per voxel times the number of Monte Carlo iterations) to normalize for brain volume. The resultant ACMs were smoothed using an 8 mm3 Gaussian kernel before statistical analysis.
Statistical Analysis
Voxel‐wise statistics was carried out using SPM8. Three separate models were used to assess: (1) the presence of between‐group ACM differences (adjusting for age, gender, education, and number of voxels in the parenchymal mask); (2) the correlation between regional ACM and the dosage‐x‐duration of therapy product (in patients under AChEIs treatment); (3) the relationship between ACM and the global level of cognition (as measured by MMSE score) in patients, classifying them into two separate groups (drug naïve or under medication, adjusting for age and number of parenchymal voxels).
For the between‐group comparison (1), three groups were modelled: HS, patients with AD under AChEI, and patients with AD drug‐naïve. T‐contrasts were used for testing specific hypotheses.
For the correlation between regional ACM value and the dosage‐x‐duration of therapy (2), only the ACM maps of the 19 patients under medication were used, and we had to convert dosages of Donepezil and Rivastigmine into a common unit. As the typical therapeutic approach consists, in both cases, of the prescription of a low dosage, followed by a progressive increase, up to a maximum dose, we used the maximum (12 mg for Rivastigmine and 10 for Donepezil) as the reference for rescaling all dosages. Therefore, each dosage of Rivastigimane was rescaled as 10/12. Furthermore, the type of drug was modelled as an additional covariate of no interest. The threshold for statistical significance was set at P < 0.001.
Finally, for the ACM versus MMSE analysis, two groups were modelled (drug naïve and under medication), and the group by MMSE interaction was explored.
Post Hoc WM Tract Reconstruction
To interpret the findings of the voxel‐wise analysis, we repeated the ACM computation, this time only accepting those streamlines that passed through the region we found to be significantly associated with drug exposure in patients (see Results). The same parameters used for the main analysis (same seeding mask, number of iterations = 10) were employed. The resulting images were warped into standard space and averaged across subjects to yield a representative map of the tract for each group (HS, patients with AD under AChEIs, and drug‐naïve patients).
RESULTS
Subject Characteristics
There were no differences between patients with AD and HS with respect to age, gender, and education (Table 1). As expected, all patients with AD showed a widespread cognitive impairment, with no significant differences between subgroups (those drug‐naïve and those under AChEI medication; Table 2).
Table 2.
Neuropsychological assessment of studied subjects
| Cognitive domain test | Cut‐off for normality | Mean (SD) scores | ||
|---|---|---|---|---|
| AD under AChEIs (n = 19) | Drug‐naïve AD (n = 19) | HS (n = 11) | ||
| Verbal episodic long‐term memory | ||||
| 15‐Words List | Immediate recall (cut‐off ≥ 28.5) | 20.4 (10.3)a | 24.2 (7.1)b | 47.7 (10.3) |
| Delayed recall (cut‐off ≥ 4.6) | 1.5 (2.0)a | 2.6 (2.7)b | 9.9 (2.1) | |
| Recognition: hit rates | 8.4 (4.2) | 7.7 (4.8) | – | |
| Recognition: false | 7.4 (7.6) | 7.1 (7.2) | – | |
| Visuo‐spatial episodic long‐term memory | ||||
| Complex Rey's figure | Immediate recall (cut‐off > 6.4) | 5.3 (1.5) | 6.0 (5.3) | – |
| Delayed recall (cut‐off > 6.3) | 3.0 (2.5) | 2.9 (4.2) | – | |
| Visuo‐spatial short‐term memory | ||||
| Digit span | (cut‐off > 3.7) | 4.5 (1.1)b | 4.9 (1.3)b | 6.0 (1.1) |
| Corsi Block Tapping task | (cut‐off ≥ 3.5) | 3.6 (1.4)a | 3.4 (1.6)b | 5.22 (0.8) |
| Executive functions | ||||
| Phonological Word Fluency | (cut‐off > 17.3) | 20.3 (8.0)a | 23.4 (10.5)b | 35.9 (8.4) |
| Language | ||||
| Naming of objects | (cut‐off > 22) | 24.0 (5.8) | 24.3 (5.8) | – |
| Reasoning | ||||
| Raven's Coloured Progressive Matrices | (cut‐off > 18.9) | 20.5 (7.1)a | 20.4 (6.8)b | 34.0 (2.3) |
| Constructional praxis | ||||
| Copy of drawings | (cut‐off > 7.1) | 6.8 (3.5)a | 7.0 (3.2)b | 11.2 (1.0) |
| Copy of drawings with landmarks | (cut‐off > 61.8) | 54.5 (19.7) | 49.5 (19.9) | – |
| Copy of Complex Rey's Figure | (cut‐off > 23.7) | 13.5 (12.1) | 14.8 (9.7) | – |
For each administered test appropriate adjustments for sex, age and education were applied according to the Italian normative data. For each test, cut‐off scores of normality (≥95% of the lower tolerance limit of the normal population distribution) are reported in brackets.
AD, Alzheimer's disease; AChEIs, acetylcholinesterase inhibitors; HS, healthy subjects.
P ≤ 0.05 AD under AChEIs versus HS.
P ≤ 0.05 Drug‐naïve AD versus HS.
ACM
Between group comparisons
When comparing all patients with AD with HS, ACM values were found to be reduced (P < 0.001) in AD in the fornix, in the supramarginal gyrus (bilaterally) and in the cingulum (Fig. 1A), whereas they were increased along the cortico‐spinal tracts (Fig. 1B). When contrasting either subgroup of patients with AD (those drug‐naïve and those under AChEI medication) against HS separately, ACM was found to be significantly altered in the same areas as when considering all patients as a single group (data not shown).
Figure 1.
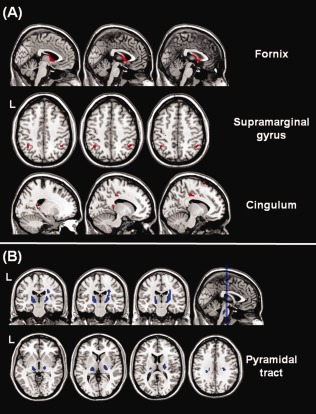
Voxel‐wise comparison of ACM values between patients with AD and HS. Reductions of structural connectivity in patients are shown in red (panel A), whereas increases are shown in blue (panel B). The reductions located in the fornix, in the supramarginal gyri and in the cingulum are consistent with a progressive disconnection between the medial temporal lobes and the rest of the brain. The increased ACM values in the cortico‐spinal tract might reflect a relative sparing of this WM tract compared to association bundles (i.e., the superior longitudinal fasciculus). See text for further details.
An additional comparison was carried out by contrasting only the patients with AD (N = 7) who were treated with the maximum dose of AChEIs (either 10 mg daily for Donepezil or 12 mg daily for Rivastigmine) with HS, highlighting a region of increased ACM values in the right hemisphere, centred at MNI coordinates: (28, 13, and 16), comprising the putamen and the anterior limb of the internal capsule (Fig. 2A). The location of this area is consistent with previously reported findings [Bozzali et al., 2011b].
Figure 2.
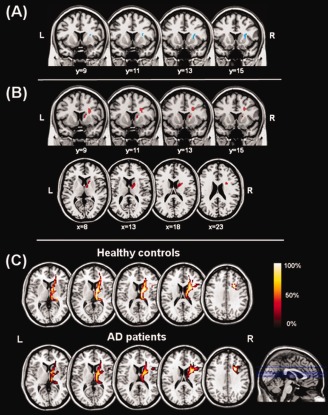
Panel A: Region of increased ACM values (light blue area) in patients with AD treated with the maximum dose of AChEIs compared to healthy controls; this area includes the putamen and the anterior limb (non motor portion) of the right internal capsule. Panel B: Direct associations (red areas) between ACM values and dosage‐x‐duration of therapy product in the group of patients under treatment with Acetylcholinesterase inhibitors (AChEIs). The area of significant association is located again within the anterior limb of the internal capsule. This area is proximal to the cholinergic basal forebrain, supporting a putative neurotrophic and neurorestorative role of AChEIs. Panel C: “Selective” ACM maps (area shown in red in panel B) obtained when using the area shown in panel B as way‐point (i.e., retaining only streamlines passing through it). The figure shows the average maps for healthy controls and Alzheimer's disease (AD) patients. The main tract coincides with the known anatomical pathway of the anterior thalamic radiation. Spatial coordinates in the figure are in MNI space. See text for further details.
Correlation analyses
When analyzing the correlation between ACM and dose × duration of therapy, a strong positive association was observed in the right anterior limb of the internal capsule (Fig. 2B). When looking at the relationship between MMSE and ACM, a significant (P < 0.001) MMSE score × group (drug naive vs. under therapy patients) interaction was observed in the same area (Fig. 3), indicating that drug‐free patients show a direct correlation between their MMSE scores and ACM values in that area, which is lost in patients under AChEIs.
Figure 3.
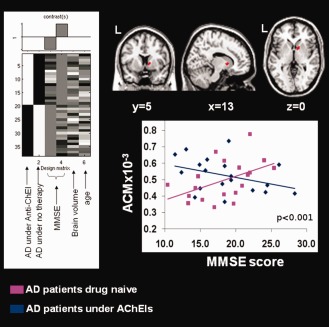
Effect of the interaction between the administration of Acetylcholinesterase inhibitors (AChEIs; yes or no) and the global level of cognition [as measured by mini mental state examination (MMSE) score] on anatomical connectivity (ACM) of patients with AD. This area (shown in red) largely overlaps with the one found to be directly associated with exposure to AChEIs, as shown in Figure 2 (panel B). The design matrix used to test the interaction is reported on the left. It includes the two groups of patients modelled separately (those under treatment and those drug‐naïve), their correspondent MMSE scores (covariate of interest), and their age and brain volumes (covariates of no interest). In the graph, there is reported association between individual MMSE scores and ACM values derived from the brain area of interaction for the two groups of patients, those drug‐naïve (violet) and those under medication (blue). This direct correlation, which is present in the former group of patients, is lost in the latter one. See text for further details.
Post hoc “selective” ACM reconstruction
The ACM reconstruction obtained only retaining the connections passing through the region of positive correlation observed between ACM values and dose × duration product of AChEIs (Fig. 2B) was consistent across subjects. The group‐averaged images of these maps (Fig. 2C), indicate that the main WM tract crossing that region is the right anterior thalamic radiation.
DISCUSSION
In this study, we recruited a cohort of patients with probable AD, with equal numbers under AChEIs and drug‐naïve. The former subgroup included patients who had been under treatment for a variable time, ranging from 6 months up to 2 years. The shortest treatment duration was chosen to ensure an exposure time that could be compatible with putative processes of brain plasticity. The longest duration was chosen to avoid a significant gap in clinical staging between treated and untreated patients. Within‐subject variability in treatment exposure (dose and duration) was welcomed to allow a correlation analysis with imaging data.
When contrasting all patients with AD against HS, ACM values were reduced in the supramarginal gyri, in the cingulum, and in the fornix of the former group. This anatomical distribution confirms and extends our exploratory findings [Bozzali et al., 2011b], and supports, on a “structural” basis, the critical role of brain disconnection in AD pathophysiology [Gili et al., 2011; Villain et al., 2005]. Reduced ACM values in the supramarginal gyrus and cingulum of patients with AD represents a robust finding, which is highly consistent with previous literature [Gili et al., 2011; Greicius et al., 2004; Ibáñez et al., 1998; Nestor et al., 2003]. It has been demonstrated that functional disconnection of the posterior cingulate cortex in AD, precedes the occurrence of GM atrophy in the same area [Gili et al., 2011], and different combinations of structural damage to the cingulum and GM loss around it, respond to different clinical stages [Bozzali et al., 2011]. On the other hand, the fornix was previously reported to be critical for the conversion from MCI to AD [Copenhaver et al., 2006].
ACM was increased in the corticospinal tracts of patients with AD. Because of the normalisation of ACM maps by the total number of streamlines initiated, the overall reduction in connectivity may translate into an artificial increase of connectivity of those tracts (i.e., corticospinal tracts) which are relatively spared by AD pathology [Bozzali et al., 2002]. An alternative explanation is provided with reference to a recent study by another group [Douaud et al., 2011], investigating the “mode of anisotropy,” an index reflecting how “linear” (as opposed to “planar”) the shape of diffusion tensor is in a voxel. This study revealed, unexpectedly, an increase of this index in patients with AD, located in the area where the corticospinal tract crosses the superior longitudinal fasciculus. The Authors concluded that this paradoxical result is due to a selective sparing of the corticospinal tracts. In the current study, it is likely that the tractography algorithm used for ACM, encountered less alternative, confounding, pathways from the corticospinal tract through the superior longitudinal fasciculus or corpus callosum in patients with AD than in controls, thus generating an artificial increase of ACM values confined to the corticospinal tracts. Similar “unmasking” effects have previously been reported in apparently paradoxical increases in FA observed in normal appearing deep gray matter in multiple sclerosis [Ciccarelli et al., 2001].
When contrasting the two AD subgroups (patients under treatment and patients drug‐naïve) in isolation against HS, patterns of reduced ACM values were similar to those observed in the whole AD population. Moreover, no significant difference was observed in the ACM between the two subgroups of patients. This suggests that reduced brain connectivity in the cingulum, in the supramarginal gyri and in the fornix may be responsible for patients' cognitive disabilities, independently of AChEI medication. This aspect does not necessarily mean that AChEIs are ineffective in modulating AD pathophysiology. The two groups of patients were indeed matched for cognitive decline and disease duration. Interestingly, when limiting the analysis to those patients under maximum dosage of AChEIs, we were able to replicate our previous findings of increased ACM [Bozzali et al., 2011b]. Consistently, the most striking result of the current study is the positive impact of AChEIs (dosage‐x‐time) on AD brains. Such an effect was found in a region between the putamen and the caudate, identified by tractography as belonging to the right anterior thalamic radiation. The anterior thalamic radiation connects the thalamus to the prefrontal cortex, does not carry motor pathways, and is relevant for higher level functions. Interestingly, the correlation analysis performed here, which intrinsically accounts for different treatment dosages and durations, returned a similar anatomical localisation for the changes in ACM values previously observed in a independent group of patients [Bozzali et al., 2011b]. We speculate that this drug related effect may reflect a putative neurotrophic and neurorestorative role that AChEIs have been reported to exert on cholinergic neurons [Ginestet et al., 2007], as suggested by long‐term stabilizing effects of AChEIs in AD [Giacobini, 2002]. Although preliminary, this interpretation is also supported by the positive effect that, according to our results, medication plays in modulating the relationship between MMSE scores and structural connectivity, again, along the anterior thalamic radiation. This area of drug modulation (obtained in a data‐driven fashion) is particularly intriguing for its proximity to the cholinergic basal forebrain, as shown by others [Grothe et al., 2010; Hanyu et al., 2002]. The cholinergic modulation found in the anterior thalamic radiation is interesting also for the connection of this structure to the medial‐dorsal nucleus of the thalamus [Wakana et al., 2004], which, in turn, is known to project to brain areas implicated in the control of behavior [e.g., the anterior cingulate and the dorsolateral prefrontal cortex; Eckert et al., 2011]. In recent years, great interest has been dedicated to clarify the neurobiological substrate underling the neuropsychiatric and behavioral aspects (also known as behavioural and psychological symptoms of dementia [BPSD], see IPA 1996) of AD [Bruen et al., 2008; Rosen et al., 2005; Serra et al., 2010]. The pathophysiology of BPSD in AD has not been clearly delineated yet. However, a direct association between the occurrence and severity of dysinhibition and neurodegeneration of the anterior cingulate cortex was recently reported [Serra et al., 2010b]. Although future studies are needed to assess whether a direct association exists between changes in anterior thalamic radiation and BPSD, we speculate that the modulation of ACM we observed here might be related to a potential interaction between AChEIs and BPSD. A clinical effect of AChEIs on BPSD in patients with AD has been recently suggested by Lockhart and coworkers 2011. Moreover, consistent with the specific effect of interaction we found between AChEIs administration (yes/no) and the MMSE score on patients ACM in the anterior thalamic radiation, other Authors have suggested that these reduced neuropsychiatric symptoms in AD may help to improve the results detectable on global cognitive measures [Brousseau et al., 2007]. In this perspective, our findings might reflect, on a neurobiological basis, a potential explanation for the some clinical effects of AChEI therapy.
With respect to a more specific interpretation of ACM changes in terms of the neurobiological substrate, we hypothesize that an increased number of regenerating axons and sprouting of collateral fibers might explain the observed modulation in the brain of patients under AChEIs. This finding seems relatively robust as the same area of ACM modulation came out from two independent analyses involving patients with AD under medication [see Bozzali et al., 2011b]. In support to this hypothesis, which remains speculative, it has been proposed that the cholinergic stimulation of oligodendrocytes might play a critical role in promoting brain myelination during brain development, and might also contribute to mechanisms of myelin repair in older age [Bartzokis et al., 2007].
In a recent study, Likitjaroen and coworkers investigated, in a longitudinal placebo‐controlled study of patients with AD, the potential effect of Galantamine in preserving the regional FA in a set of selected WM regions (i.e., the corpus callosum, the cingulum, and the cerebellum), and reported only a limited impact of the treatment [Likitjaroen et al., 2011]. This is only apparently inconsistent with our findings for several reasons. First, the anterior thalamic radiation was not included in the set of regions investigated by Likitjaroen et al., thus making a direct comparison between the two studies difficult. Moreover, in contrast to ACM, FA expresses only an index of regional WM integrity, and does not provide any information on the structural brain connectivity. Finally, if our interpretation of sprouting of collateral fibers is correct, this phenomenon is expected to reduce rather than increase the FA locally. This might have mitigated the expected effect of Galantamine (FA preservation) in the study conducted by Likitjaroen et al. [Likitjaroen et al., 2011].
One limitation of this study is the inclusion of patients being treated with either Donepezil or Rivastigmine, the former inhibiting the acetylcholinesterase enzyme only, the latter inhibiting both the cholinesterase and the butyrylcholinesterase enzymes. Because of the partially different pharmacodynamics of Donepezil and Rivastigmine, there are no available conversion tools to make the two drug doses interchangeable. On the other hand, the common effect of both drugs is to increase the availability of acetylcholine in neuronal synapses, and adjusted indirect comparisons found drugs to be similar, at least with respect to their cognitive outcomes [Hansen et al., 2008; Ritchie et al., 2004]. Additionally, within the obvious oversimplification of including patients under both Donepezil or Rivastigmine, the type of drug were modelled as a covariate of no interest in our analyses. Nevertheless, a differential investigation of Donepezil and Rivastigmine is of great interest and needs to be addressed by future studies. Another limitation of the current study is the small sample size. This is particularly evident when attempting to replicate previous findings [Bozzali et al., 2011b] through a between‐group comparison of patients under AChEIs and patients drug naïve, which reached statistical significance only when including those patients assuming AChEIs at the highest doses (n = 7). However, as already mentioned, the area of ACM modulation found in this between group contrast was similar to that found in both a previous independent study [Bozzali et al., 2011b] and in an independent statistical analysis of correlation (dosage‐x‐duration of therapy product), which included patients under medication only. Nevertheless, these findings need to be confirmed in future studies on larger populations.
Finally, as ACM is based on probabilistic tractography, it is important to reiterate that it suffers from all the well‐known limitations of the technique. In particular, tractography is sensitive only to the larger pathways of the brain and, even when probabilistic methods are combined with multi‐fibre decomposition methods, as used here, the results of tractography can be confounded by fibre crossings, kissings, and divergence.
In conclusion, this study further demonstrates how brain disconnection is relevant for the understanding of AD pathophysiology. Moreover, it demonstrates in vivo the effect of AChEIs in determining brain plasticity and proposes a new method for monitoring clinical trials in AD.
DISCLOSURE STATEMENT
G.J.M.P. is a director and shareholder in Bioxydyn Limited, a company with an interest in ACM. None of the other Authors has any financial interest related to the publication of the present manuscript. The study was approved by the local ethics committee before initiation. All subjects gave written informed consent before taking part.
AUTHOR CONTRIBUTIONS
M.B., G.J.M.P., and M.C. equally contributed to conceive the study; B.S. and L.S. did MR scanning and image analysis; R.P., C.M., and M.G.V. recruited patients and controls; L.S. and M.G.V. did neuropsychological testing of patients and controls; C.C. contributed to interpretation; M.B. and M.C. supervised study design and image analysis; M.C. and G.G. conducted the statistical analysis; M.B., G.J.M.P., and M.C. wrote the manuscript. All Authors commented on the manuscript.
ACKNOWLEDGMENTS
The Neuroimaging Laboratory of the Santa Lucia Foundation is supported in part by the Italian Ministry of Health.
REFERENCES
- Andersson JL, Jenkinson M, Smith S (2007): Non‐Linear Registration, Aka Spatial Normalization FMRIB Technical Report TR07JA2 from wwwfmriboxacuk/analysis/techrep 2007.
- Bartzokis G (2007): Acetylcholinesterase inhibitors may improve myelin. Biol Psychiatry 62:294–301. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D (1994): Estimation of the effective self‐diffusion tensor from the NMR spin echo. J Magn Reson B 103:247–254. [DOI] [PubMed] [Google Scholar]
- Bozzali M, Falini A, Franceschi M, Cercignani M, Zuffi M, Scotti G, Comi G, Filippi M (2002): White matter damage in Alzheimer's disease assessed in vivo using diffusion tensor magnetic resonance imaging. J Neurol Neurosurg Psychiatry 72:742–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzali M, Cercignani M, Caltagirone C (2008): Brain volumetrics to investigate aging and the principal forms of degenerative cognitive decline: A brief review. Magn Reson Imaging 26:1065–1070. [DOI] [PubMed] [Google Scholar]
- Bozzali M, Giulietti G, Basile B, Serra L, Spanò B, Perri R, Giubilei F, Marra C, Caltagirone C, Cercignani M (2011a): Damage to the cingulum contributes to Alzheimer's disease pathophysiology by deafferentation mechanism. Hum Brain Mapp (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzali M, Parker GJ, Serra L, Embleton K, Gili T, Perri R, Caltagirone C, Cercignani M (2011b)Anatomical connectivity mapping: a new tool to assess brain disconnection in Alzheimer's disease. Neuroimage 54:2045–2051. [DOI] [PubMed] [Google Scholar]
- Brousseau G, Rourke BP, Burke B (2007): Acetylcholinesterase inhibitors, neuropsychiatric symptoms, and Alzheimer's disease subtypes: An alternate hypothesis to global cognitive enhancement. Exp Clin Psychopharmacol 15:546–554. [DOI] [PubMed] [Google Scholar]
- Bruen PD, McGeown WJ, Shanks MF, Venneri A (2008): Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer's disease. Brain 131:2455–2463. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Caltagirone C, Gainotti G (1996): The mental Deterioration Battery: Normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The group of the Standardization of the Mental Deterioration Battery. Eur Neurol 36:378–384. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Buccione I, Fadda L, Graceffa A, Mauri M, Lo russo S, Bevilacqua G, Caltagirone C (2002): Standardizzazione di due test di memoria per uso clinico: Breve Racconto e Figura di Rey. Nuova Rivista di Neurologia 12:1–13. [Google Scholar]
- Ciccarelli O, Werring DJ, Wheeler‐Kingshott CA, Barker GJ, Parker GJ, Thompson AJ, Miller DH (2001): Investigation of MS normal‐appearing brain using diffusion tensor MRI with clinical correlations. Neurology 56:926–933. [DOI] [PubMed] [Google Scholar]
- Copenhaver BR, Rabin LA, Saykin AJ, Roth RM, Wishart HA, Flashman LA, Santulli RB, McHugh TL, Mamourian AC (2006): The fornix and mammillary bodies in older adults with Alzheimer's disease, mild cognitive impairment, and cognitive complaints: A volumetric MRI study. Psychiatry Res 147:93–103. [DOI] [PubMed] [Google Scholar]
- Douaud G, Jbabdi S, Behrens TE, Menke RA, Gass A, Monsch AU, Rao A, Whitcher B, Kindlmann G, Matthews PM, Smith S (2011): DTI measures in crossing‐fibre areas: Increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer's disease. Neuroimage 55:880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert U, Metzger CD, Buchmann JE, Kaufmann J, Osoba A, Li M, Safron A, Liao W, Steiner J, Bogerts B, Walter M (2011): Preferential networks of the mediodorsal nucleus and centromedian‐parafascicular complex of the thalamus: A DTI tractography study. Hum Brain Mapp (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975): Mini‐mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Pievani M, Testa C, Sabattoli F, Bresciani L, Bonetti M, Beltramello A, Hayashi KM, Toga AW, Thompson PM (2007): The topography of gray matter involvement in early and late onset Alzheimer's disease. Brain 130:720–730. [DOI] [PubMed] [Google Scholar]
- Giacobini E (2002): Long‐term stabilizing effect of cholinesterase inhibitors in the therapy of Alzheimer' disease. J. Neural Transm Suppl ( 62):181–187. [DOI] [PubMed] [Google Scholar]
- Gili T, Cercignani M, Serra L, Perri R, Giove F, Maraviglia B, Caltagirone C, Bozzali M (2011): Regional brain atrophy and functional disconnection across Alzheimer's disease evolution. J Neurol Neurosurg Psychiatry 82:58–66. [DOI] [PubMed] [Google Scholar]
- Ginestet L, Ferrario JE, Raisman‐Vozari R, Hirsch EC, Debeir T (2007): Donepezil induces a cholinergic sprouting in basocortical degeneration. J Neurochem 102:434–440. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V (2004): Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 101:4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe M, Zaborszky L, Atienza M, Gil‐Neciga E, Rodriguez‐Romero R, Teipel SJ, Amunts K, Suarez‐Gonzalez A, Cantero JL (2010): Reduction of basal forebrain cholinergic system parallels cognitive impairment in patients at high risk of developing Alzheimer's disease. Cereb Cortex 20:1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen RA, Gartlehner G, Webb AP, Morgan LC, Moore CG, Jonas DE (2008): Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer's disease: a systematic review and meta‐analysis. Clin Interv Aging 3:211–225. [PMC free article] [PubMed] [Google Scholar]
- Hanyu H, Asano T, Sakurai H, Tanaka Y, Takasaki M, Abe K (2002): MR analysis of the substantia innominata in normal aging, Alzheimer disease, and other types of dementia. AJNR Am J Neuroradiol 23:33–34. [PMC free article] [PubMed] [Google Scholar]
- Ibáñez V, Pietrini P, Alexander GE, Furey ML, Teichberg D, Rajapakse JC, Rapoport SI, Schapiro MB, Horwitz B (1998): Regional glucose metabolic abnormalities are not the result of atrophy in Alzheimer's disease. Neurology 50:1585–1593. [DOI] [PubMed] [Google Scholar]
- IPA (1996): Behavioural and psychological signs and symptoms in dementia: Implication for research and treatment. Int Psychogeriatr 8:215–552. [PubMed] [Google Scholar]
- Jenkinson M, Smith S (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Karas GB, Burton EJ, Rombouts SA, van Schijndel RA, O'Brien JT, Scheltens P, McKeith IG, Williams D, Ballard C, Barkhof F (2003): A comprehensive study of gray matter loss in patients with Alzheimer's disease using optimized voxel‐based morphometry. Neuroimage 18:895–907. [DOI] [PubMed] [Google Scholar]
- Leemans A, Jones DK (2009): The B‐matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 61:1336–1349. [DOI] [PubMed] [Google Scholar]
- Likitjaroen Y, Meindl T, Friese U, Wagner M, Buerger K, Hampel H, Teipel SJ (2011): Longitudinal changes of fractional anisotropy in Alzheimer's disease patients treated with galantamine: a 12‐month randomized, placebo‐controlled, double‐blinded study. Eur Arch Psychiatry Clin Neurosci (in press). [DOI] [PubMed] [Google Scholar]
- Lockhart IA, Orme ME, Mitchell SA (2011): The efficacy of licensed‐indication use of donepezil and memantine monotherapies for treating behavioural and psychological symptoms of dementia in patients with Alzheimer's disease: systematic review and meta‐analysis. Dement Geriatr Cogn Dis Extra 1:212–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984): Clinical diagnosis of Alzheimer's disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34:939–944. [DOI] [PubMed] [Google Scholar]
- Measso G, Cavartezan F, Zappalà G, Lebowitz DB, Crook TH, Pirozzolo FJ, Amaducci L, Massari D, Grigoletto F (1993): The Mini Mental State Examination: Normative study of a random sample of Italian population. Dev Neuropsychol 9:77–85. [Google Scholar]
- Miceli G, Laudanna A, Burani C, Capasso R (1991):Batteria per l'analisi dei deficit afasici. Vol.1Milano:ASRN. Berdata. [Google Scholar]
- Nestor PJ, Fryer TD, Smielewski P, Hodges JR (2003): Limbic hypometabolism in Alzheimer's disease and mild cognitive impairment. Ann Neurol 54:343–351. [DOI] [PubMed] [Google Scholar]
- Orsini A, Grossi D, Capitani E, Laiacona M, Papagno C, Vallar G (1987): Verbal and spatial immediate memory span: Normative data from 1355 adults and 1112 children. Ital J Neurol Sci 8:539–548. [DOI] [PubMed] [Google Scholar]
- Parker GJ, Haroon HA, Wheeler‐Kingshott CA (2003): A framework for a streamline based probabilistic index of connectivity (PICo) using a structural interpretation of MRI diffusion measurements. J Magn Reson Imaging 18:242–254. [DOI] [PubMed] [Google Scholar]
- Pepeu G, Giovannini MG (2010): Cholinesterase inhibitors and memory. Chem Biol Interact 187:403–408. [DOI] [PubMed] [Google Scholar]
- Perri R, Serra L, Carlesimo GA, Caltagirone C, Early Diagnosis Group of the Italian Interdisciplinary Network on Alzheimer's Disease (2007): Amnestic mild cognitive impairment: difference of memory profile in subjects who converted or did not convert to Alzheimer's disease. Neuropsychology 21:549–558. [DOI] [PubMed] [Google Scholar]
- Perri R, Carlesimo GA, Serra L, Caltagirone C, Early Diagnosis Group of the Italian Interdisciplinary Network on Alzheimer's Disease (2009): When the amnestic mild cognitive impairment disappears: characterisation of the memory profile. Cogn Behav Neurol 22:109–116. [DOI] [PubMed] [Google Scholar]
- Petersen RC (2004): Mild cognitive impairment as a diagnostic entity. J Int Med 256:183–194. [DOI] [PubMed] [Google Scholar]
- Raji CA, Lopez OL, Kuller LH, Carmichael OT, Becker JT (2009): Age, Alzheimer disease, and brain structure. Neurology 73:1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale M, Iarlori C, Gambi F, Feliciani C, Salone A, Toma L, DeLuca G, Salvatore M, Conti P, Gambi D (2004): Treatment with an acetylcholinesterase inhibitor in Alzheimer patients modulates the expression and production of the pro‐inflammatory and anti‐inflammatory cytokines. J Neuroimmunol 148:162–171. [DOI] [PubMed] [Google Scholar]
- Reale M, Iarlori C, Gambi F, Feliciani C, Isabella L, Gambi D (2006): The acetylcholinesterase inhibitor, Donepezil, regulates a Th2 bias in Alzheimer's disease patients. Neuropharmacology 50:606–613. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ.Reduction of eddy‐current‐induced distortion in diffusion MRI using a twice‐refocused spin echo. Magn Reson Med 2003;49:177–182. [DOI] [PubMed] [Google Scholar]
- Ritchie CW, Ames D, Clayton T, Lai R (2004): Metaanalysis of randomized trials of the efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer disease. Am J Geriatr Psychiatry 12:358–369. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno‐Tempini ML, Weiner MW, Miller BL (2005): Neuroanatomical correlates of behavioural disorders in dementia. Brain 128:2612–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra L, Cercignani M, Lenzi D, Perri R, Fadda L, Caltagirone C, Macaluso E, Bozzali M (2010a)Gray and white matter changes at different stages of Alzheimer's disease. J Alzheimers Dis 19:147–159. [DOI] [PubMed] [Google Scholar]
- Serra L, Perri R, Cercignani M, Spanò B, Fadda L, Marra C, Carlesimo GA, Caltagirone C, Bozzali M (2010b): Are the behavioral symptoms of Alzheimer's disease directly associated with neurodegeneration? J Alzheimers Dis 21:627–639. [DOI] [PubMed] [Google Scholar]
- Seunarine K, Cook P, Hall M, Embleton K, Parker G, Alexander D (2007): Exploiting peak anisotropy for tracking through complex structures. IEEE ICCV Workshop on MMBIA. [Google Scholar]
- Svensson AL, Giacobini E (2000): Cholinesterase Inhibitors Do More than Inhibit Cholinesterase In: Giacobini E, editor.Cholinesterases and Cholinesterase Inhibitors.London:Martin Dunitz; pp227–235. [Google Scholar]
- Tuch DS (2004): Q‐ball imaging. Magn Reson Med 52:1358–1372. [DOI] [PubMed] [Google Scholar]
- Villain N, Desgranges B, Viader F, de la Sayette V, Mézenge F, Landeau B, Baron JC, Eustache F, Chételat G (2008): Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer's disease. J Neurosci 28:6174–6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae‐Poetscher LM, van Zijl PC, Mori S (2004): Fiber tract‐based atlas of human white matter anatomy. Radiology 230:77–87. [DOI] [PubMed] [Google Scholar]


