Abstract
Distinct thalamic nuclei, like the mediodorsal (MD) nucleus and the centromedian/parafascicular complex (CM/Pf), are embedded in different basal ganglia—thalamocortical loops, which were shown to integrate cognitive and emotional aspects of human behavior. Despite well described connections on a microscopic scale, derived from tracing studies in animals, little is known about the intrinsic anatomical connections of these nuclei in humans. This lack of knowledge limits not only interpretation of functional imaging studies but also estimation of direct effects of deep brain stimulation which treats diseases as different as epilepsy or major depression. Therefore, non‐invasive diffusion tensor imaging (DTI) studies are key to analyzing connectivity patterns and elaborate approaches to close this gap. For our study, we explored the structural connectivity of the MD thalamic nuclei and the CM/Pf complex towards five cortical and six subcortical regions by using a preferential fiber calculation. We found both thalamic nuclei to be preferentially associated to distinct networks: whereas the MD is preferentially connected to prefrontal and limbic cortical regions, the CM is linked to subcortical regions. The anterior insula was the only cortical region associated with the subcortical network of the CM and the cortical network of the MD comprised one subcortical hub, the caudate nucleus, suggesting an integrative role of these two regions. Adding to predescribed anatomical tract tracing connectivities in animal studies, our finding lends support to the existence of similar basal ganglia‐thalamocortical circuits in humans and we could show a robust distinction of preferential connectivity for both thalamic nuclei. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: mediodorsal thalamus, centromedian thalamus, cortical networks, subcortical network, diffusion tensor imaging (DTI), quantitative tractography, structural connectivity, human neuroanatomy, deep brain stimulation
INTRODUCTION
There is clear consensus that different human behaviors are mediated through the complex interaction of heterogeneous brain networks. The continuing debate on segregated networks generally aims at cortical regions [Barabasi and Albert, 1999; Bullmore and Sporns, 2009; Hagmann et al., 2008], but it is likely that similar network segregation appears in subcortical regions—especially in the basal ganglia‐thalamocortical circuits as suggested by Alexander et al. [ 1986].
One of the main thalamic nuclei involved in those loops is the mediodorsal (MD) nucleus, whose functional specificity has also been suggested in recent functional magnetic resonance imaging (fMRI) studies [Walter et al., 2008]. In high‐resolution fMRI studies, MD activation during affective processing could be segregated from attention‐related processes, which were mediated in the nearby centromedian (CM) and parafascicular (Pf) nuclei [Metzger et al., 2010].
This preliminary evidence describing MD functioning in humans corresponds with its previously described roles in cognition, memory, motivation, and emotions [Oyoshi et al., 1996]; for reviews see [Haber and McFarland 2001; Haber and Knutson, 2010]. The MD is mainly connected to limbic [Groenewegen, 1988; Ray and Price, 1993; Vogt et al., 1987] and prefrontal regions, as reviewed by Barbas [ 2000] or Price [ 1999], and local activations as well as volumes and cell numbers have been shown to be impaired in depression and schizophrenia [ Anand et al., 2009; Minzenberg et al., 2009; Steiner et al., 2008].
In a recent diffusion tensor imaging (DTI) tractography study, Gutman et al. [ 2009] described a convergence of fiber tracts from different sites of deep brain stimulation as applied in therapy resistant depression. Although exact localization remains one of the primary challenges in MRI‐guided neurosurgical approaches to brain function, subcortical structures present anatomically well‐defined targets with limited intersubject variability, as compared to potential cortical targets. Sartorius et al. [ 2010] recently demonstrated the antidepressant effectiveness of deep brain stimulation for electrode placements at the level of the habenular complex. However, the high functional diversity of neighboring nuclei within the thalamus requires more exact knowledge of individual connection profiles for these structures. Non‐invasive tractography in humans has so far focused on gradients of connectivity, which were oriented on lobar preference of intrathalamic regions. This approach limited the integration of functionally specific, anticorrelated networks with adjacent cortical nodes, especially within the frontal lobes. Such adjacency of cortical targets would affect the discrimination of the neighboring MD and CM nuclei, for which a clear functional distinction could be made in humans [Metzger et al., 2010]. The CM, an anatomically circumscribed region for deep brain stimulation in epilepsy [Cukiert et al., 2009], has been historically classified as a part of the so‐called nonspecific thalamocortical projections that have been reviewed by Haber [ 2001].
Together with the Pf, the CM/Pf complex is mainly involved in attentional processing [Metzger et al., 2010; Minamimoto and Kimura, 2002] as also suggested by Haber and Calzavara [ 2009]. The CM/Pf has also been considered to be involved in motor control [Smith et al., 2009], pain [Krauss et al., 2002], and sexual processing [Coolen et al., 2003; Temel et al., 2004] and lesions seem to be related to complex attentional deficits representing its function in modulating attention towards motivationally relevant stimuli [Matsumoto et al., 2001]. The CM/Pf seems to have especially strong connections to subcortical regions, as reviewed by Haber and Calzavara [ 2009], such as the caudate nucleus [Macchi et al., 1984], putamen, and pallidum [Berendse and Groenewegen, 1991; Krettek and Price, 1977], as well as the nucleus accumbens [Jayaraman, 1985]. Projections described from the CM/Pf to amygdala [Ottersen and Ben‐Ari, 1979] and hippocampus [Cavdar et al., 2008] are sparser and of lower density. Further projections to anterior cingulate cortex (ACC) and insula have also been reported for the CM/Pf [van der Werf et al., 2002].
Both structures, the MD and the CM/Pf, have been associated with salience processing [Metzger et al., 2010; Seeley et al., 2007] and seem to be mediators between specific regions involved in their respective functional networks. Thus, the interactions of subcortical and cortical networks follow preferences in anatomical connectivity to possible hubs within the thalamus [Liao et al., 2011]. Preferential connectivity more likely represents actual selectivity in anatomical connections, since thalamic nuclei—despite having little direct connections between each other—seem to connect to a large number of cortical regions within different functional networks. This methodological variation in previous DTI investigations of the thalamus seemed to suggest an optimization of the functional architecture of the thalamus in terms of specified cell assemblies (i.e., nuclei), which are normally intersected by intrathalamic fiber bundles. Such architecture allows an efficient yet flexible processing of cognitive content and basic emotions as well as their interaction.
The gap in knowledge about anatomical correlates of distinct functional networks provided the inspiration for the current study. At first, we tested whether the previously described connectivities, as revealed by animal models, can also be found in humans using non‐invasive fiber tracking as measured by DTI. For that purpose, we used the MD and CM/Pf complex as seeds to perform a tractography towards 11 cortical and subcortical regions. We then tested whether segregated networks involving cortical and subcortical regions can be consistently found in humans and whether they can be assigned to either the MD or the CM/Pf, in healthy male subjects. The selection of these neighboring nuclei followed the previous functional characterization from fMRI data, their different roles in processing salience information, and their potential roles as anatomically defined targets of deep brain stimulation.
As a primary objective of our investigation, we aimed at defining specific networks of these two nuclei in humans based on non‐invasive imaging of anatomical connectivity. Given the high ontogenetic variability of brain development for males and females [Huster et al., 2009] related to differences in gray matter (GM) and white matter (WM) in adults [Bellis et al., 2001], as well as in volumes of subcortical structures, we decided to initially examine only healthy male subjects.
METHODS
Subjects
We recruited 23 healthy, male subjects without history of neurological or psychiatric disorders (age range: 24–44 years, mean = 30.7, SD = 5.2). In accordance with the requirements of the Declaration of Helsinki and the University of Magdeburg Ethical Committee, all participants provided written and informed consent before MRI examinations.
MRI‐Data Acquisition
Data were acquired using a 3 Tesla Siemens MAGNETOM Trio scanner (Siemens, Erlangen, Germany) with an 8‐channel phased‐array head coil for signal reception and Syngo MR2004A software.
The MRI protocol included a T1‐weighted sagittal 3D scan (MPRAGE sequence, 192 slices, slice thickness: 1.0 mm, echo time (TE): 4.77 ms, repetition time (TR): 2,500 ms, inversion time (TI): 1,100 ms, flip angle: 7°, bandwidth: 140 Hz/pixel, scan time: 9:20 min) a T2‐weighted axial 2D scan (turbo spin echo (TSE) sequence, 72 slices, slice thickness: 2 mm, TE: 78 ms, TR: 3300 ms, acquisition matrix: 256 × 192 × 72, voxel size: 1.0 × 1.0 × 2.0 mm3, scan time: 4:24 min) and a DTI‐scan with an EPSE sequence [Reese et al., 2003]. The parameters of the DTI‐scan were 68 axial slices with the same center position of the image block as in the T2‐weighted scan, TR: 8,200 ms, TE: 89 ms, PAT‐modus: GRAPPA (acceleration factor 3, 25% phase oversampling), slice thickness: 2.0 mm, acquisition matrix: 128 × 128 × 68, voxel size: 2.0 × 2.0 × 2.0 mm3, 4 runs each with two averages and frequency adjustment for each run, total scan time: 4 × 4:39 min, each run with one non‐diffusion weighted volume and 12 diffusion weighted volumes (non‐collinear diffusion gradient directions from Siemens MDDW mode), b‐values of 0 and 1,000 s/mm2.
Defining the Regions of Interest
Using MRIcron [Rorden et al., 2007] with thalamic seed regions: the MD and the CM/Pf were defined based on high‐resolution images utilizing anatomical observations in human brain atlases [Mai et al., 2004]. Target regions consisted of cortical and subcortical regions of interests (ROIs). The cortical target regions were drawn spherically in both hemispheres and made of the main subregions of the four region model of the cingulate cortex [Vogt and Laureys, 2005]: pregenual anterior cingulate cortex (pgACC), dorsal anterior cingulate cortex (dACC), posterior cingulate cortex (PCC), and the anterior insula/frontal operculum (ai/fo), given its important functional connectivity with the cingulate subregions [Dosenbach et al., 2008; Horn et al., 2010]. We also included a spherical target bilaterally in the dorsolateral prefrontal cortex (dlPFC) at the level of Brodmann Area (BA) 46 given its central involvement in basal ganglia‐thalamocortical circuits [Alexander et al., 1986]. The subcortical regions involved: caudate nucleus, amygdala, nucleus accumbens, hippocampus, putamen, and pallidum. These structures were provided by the fsl 4.0 package [http://www.fmrib.ox.ac.uk/fsl/] as separated anatomical masks [Mazziotta et al., 2001]. We used them bilaterally as target regions on the same MNI skull‐striped T1 template also supplied by fsl 4.0 [Smith, 2002]. Fiber estimation was performed for each thalamic seed region separately. For graphical display of the anatomical localization and spatial extent of all ROIs please refer to Figure 1.
Figure 1.
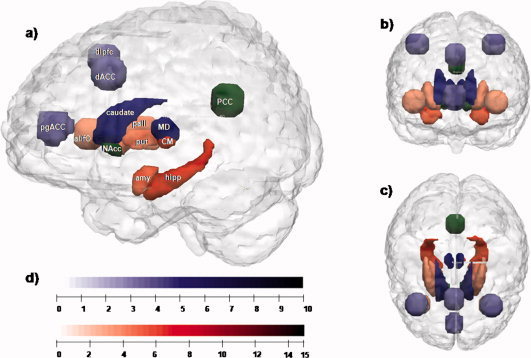
Network segregation based on relative fiber counts. (a) Sagittal plane; (b) coronal plane; (c) transversal plane (d) color bars: indicating the level of T‐values for each region shown in (a)–(c). Regions with preferential connectivity to the MD are shown in blue and those connecting stronger to the CM/Pf complex are shown in red, the strength of the connectivity are visualized in the brightness of the blue and red colors. The PCC and the nucleus accumbens do not show significant preferences and appear in green. Abbreviations: MD, mediodorsal thalamic nucleus; CM, centromedian/parafascicular complex of the thalamus; amy, left amygdala; hipp, left hippocampus; PCC, posterior cingulate cortex; put, right putamen; pall, right pallidum; NAcc, right nucleus accumbens; caudate, right caudate nucleus; dlPFC, right dorsolateral prefrontal cortex; dACC, dorsal anterior cingulate cortex; pgACC, pregenual anterior cingulate cortex; aI/fo, left anterior insula‐frontal operculum.
DTI‐Data Preprocessing and Fiber Tracking
For DTI images, head motion was removed by aligning all diffusion‐weight scans to the non‐diffusion‐weighted images (b0, b = 0 s/mm2) of the first run based on the b0‐images using SPM5 (Wellcome Dept. of Cognitive Neurology, London, UK). We minimized eddy current distortions existing in the single‐shot spin‐echo planar imaging sequence by utilizing the implementation developed by Reese et al. [ 2003]. Additionally, corrections were made by affine registration to the b0‐image of the corresponding run.
Diffusion tensors were calculated for each voxel and further decomposed into eigenvalues and eigenvectors using the SPM diffusion toolbox provided by Glauche (URL: http://sourceforge.net/projects/spmtools). Furthermore, the apparent diffusion coefficient (ADC) and fractional anisotropy (FA) maps were computed. The fiber tract reconstruction was carried out in DTI native space for each subject. Briefly, a Monte Carlo simulation algorithm [Bodammer et al., 2009] that repeatedly searches for probable paths through the determined diffusion tensor matrix was implemented in MATLAB (MathWorks, Natick, MA). An estimate of the voxel‐specific probability distribution of axonal connections was used to calculate the probabilities of all allowable propagation steps. Specifically, each step was chosen by drawing randomly from this distribution. We used a probabilistic instead of a deterministic approach for our calculations, which is less prone to false negative results [Bodammer et al., 2009; Guye et al., 2008].
As the ROIs with morphometric alterations were derived from the normalized MNI space, the inverse transformation of the spatial normalization was applied to acquire the seed ROI in the native DTI space [Gong et al., 2009; Liao et al., in press]. More precisely, the inverse transformation was applied to the ROIs in the normalized MNI to the individual skull‐stripped T1 volume, which was coregistered with the individual T2 volume and consecutively to the b0 volume of every subject, resulting in the subject‐specific ROI in the native DTI space. The ROIs were added to the MNI template and together they were transformed to the individual skull‐stripped T1 volume, which was coregistered with the individual T2 volume and consecutively to the b0 volume of every subject. After visual verification of the transformation process, the thalamic regions were used as seed regions for fiber tracking towards the cortical and subcortical targets. The tractography analysis was performed independently with 5,000 starts for each start voxel in each seed region, which resulted in a calculation time of nearly 8 weeks for a 16‐core Linux server. Within each predefined seed region, the number of paths to a predefined target region detected for a given number of path calculation starts was used as a measure of neuronal connectivity within the axonal tract being considered.
Statistical Analysis
The analysis was run in two steps: firstly an ANOVA for the detection of main effects of seed, target, and hemisphere on the number of detected fiber tracts was applied with a significance cutoff value of P<0.05,; secondly post hoc pairwise t‐tests were calculated (P<0.05) for significant effects. Fiber counts between the two thalamic seed nuclei under study and the cortical and subcortical targets were set relative to each other after taking into account the different volumes of the MD and the CM/Pf.
RESULTS
Both MD and CM/Pf showed countable fiber tracts to all target regions.
General Preference of MD and CM/Pf Seeds
ANOVA calculation revealed two single significant main effects—one for target (F(10, 140) = 41.519, P < 0.001) and one for hemisphere (F(1, 14) = 5.504, P = 0.034)—as well as a significant interaction effect for the two factors of seed and target (F(1, 140) = 50.546, P<0.001):
Mediodorsal thalamus
Relative preferences in fiber counts were found bilaterally for the MD (relative to the CM/Pf seeded tracts) with cortical targets in pgACC (T = 5.203; P<0.001), dACC (T = 4.758; P<0.01), and dlPFC (T = 4.020; P<0.01). The only subcortical target with a numerical preference of the MD tracts was the caudate nucleus (T = 8.007; P<0.001) (see Fig. 1, blue voxels).
CM/Pf complex
The CM/Pf seeded tracts, in contrast to the MD, showed a stronger numerical preference towards subcortical regions of the hippocampus (T = 9.347; P<0.001), amygdala (T = 7.704; P<0.001), pallidum (T = 5.926; P<0.001), and putamen (T = 4.326; P<0.001). In contrast, only one cortical target in the anterior insula/frontal operculum (ai/fo) (T = 3.476; P<0.001) showed relatively stronger connections, in terms of relative fiber counts, towards the CM/Pf (see Fig. 1, red voxels) as compared to the MD (see Fig. 1, blue voxels).
Nucleus accumbens and PCC showed no preference to the MD or the CM/Pf (Fig. 1, green voxels).
Notably, even though strong differences in counted fibers between the thalamic seeds and our ROIs generate highly significant T‐values, they might simply be based on low absolute fiber counts. For a quantitative characterization of the connections please see Table I.
Table I.
Counted DTI fibers of each thalamic seed region (n = 23)
| Target regions | Left MD | Right MD | Left CM/Pf | Right CM/Pf |
|---|---|---|---|---|
| Cortical | ||||
| ai/fo | 7 (±6.2) | 5 (±3.9) | 20 (±23)*** | 16 (±15.3)*** |
| dACC | 11 (±11.4)** | 8 (±7.3)** | 3 (±3.5) | 3 (±3.3) |
| dlPFC | 4 (±5.1)** | 10 (±10.2)** | 2 (±3.4) | 3 (±3.6) |
| PCC | 5 (±4.7) | 7 (±6.9) | 6 (±7.7) | 6 (±8.8) |
| pgACC | 6 (±5.2)*** | 4 (±2.9)*** | 3 (±3.1) | 2 (±1.7) |
| Subcortical | ||||
| Amygdala | 30 (±21.2) | 19 (±16.8) | 125 (±72.8)*** | 64 (±45.1)*** |
| Caudate nucleus | 341 (±164.5)*** | 334 (±176.4)*** | 151 (±94.5) | 138 (±106.4) |
| Hippocampus | 19 (±12.8) | 25 (±17.3) | 58 (±25.3)*** | 59 (±45.3)*** |
| NAcc | 68 (±32.2) | 54 (±41.3) | 71 (±42.7) | 52 (±39.4) |
| Pallidum | 124 (±62.6) | 94 (±61.4) | 202 (±104.2)*** | 153 (±99.2)*** |
| Putamen | 64 (±35.7) | 61 (±50.9) | 101 (±64.3)*** | 89 (±58.4)*** |
Quantitative depiction of the DTI fiber counts for each connection between the thalamic seeds and the target regions. The numbers indicate the means (±SD) of counted connections for all 23 subjects, corrected for seed volumes.
Please note that the less numerous cortical connections might be affected by technical limitations of DTI, and do not necessarily represent the same degree of anatomical connectivity. Statistical thresholds:
P < 0.001,
P < 0.01 indicate significantly greater fiber counts for one thalamic region (MD or CM/Pf) compared to the other.
ai/fo: anterior insula/frontal operculum; dACC: dorsal anterior cingulate cortex; dlPFC: dorsolateral prefrontal cortex; PCC: posterior cingulate cortex; pgACC: pregenual anterior cingulate cortex.
Validation
According to the work of Anderson [ 2001], noise effects limit the comparability of DTI data. Different fiber lengths as well as different tissues, which the fibers pass through, are not comparable. The algorithm we used allows for selective filtering of the fiber count for each step of computed fiber length. Using a pairwise t‐test, no significant differences of fiber length between MD and CM/Pf for each hemisphere could be found. The close vicinity of both thalamic structures made it most likely that they use the same pathways to reach their targets, which we verified using the fiber atlas of Schmahmann and Pandya [ 2006]. Once the connecting fibers follow a curved pathway, the path length is divergent to the linear distance. To exclude a possible bias of selected path length and to examine our results beyond the chosen fiber length, we performed the above‐described analysis over a range of path lengths for each connection, which vary from the first detected distance to 50 voxels with an increment of 5 voxels (Fig. 2). Similar patterns were found over a large interval and even when using extreme values—which do not necessarily represent meaningful direct anatomical connections—and the described preference for the MD or the CM/Pf remained stable. More importantly, no effect for seed preference was reversed to the alternative nucleus when changing the path length.
Figure 2.
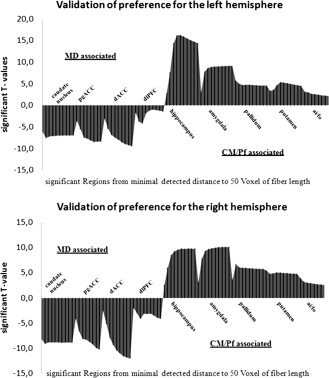
For the left and right hemispheres, the above histograms show significant T‐values (P <0.05) between seed regions and different target regions. Fibers were counted on steps of 5‐voxel‐path length differences, from minimal detected distance to 50 voxels. For each counting step, the CM‐associated regions are shown above T value = 0 and the MD corresponding regions are shown below T value = 0. T values are given for relative preferences significantly different from zero. Real anatomical distance was verified in the range of 10–19 voxels of path length between seeds and target regions. Even beyond anatomical distance, the fiber count revealed significant effects of constant relative preference.
DISCUSSION
Based on our findings, for the first time, a preferential connectivity pattern of the MD and the CM/Pf thalamic nuclei—as suggested by the postmortem and animal literatures—could be derived from non‐invasive human MRI data. The observed pattern of relative preference of fiber tract distributions for the MD and the CM/Pf reflected a difference in cortical and subcortical target regions for these structures. This pattern was robust across hemispheres and across variable fiber lengths. In general, we found preferential connections of the MD with cortical target‐regions, with the exception of the caudate nucleus, and preferential connections of the CM/Pf with subcortical structures, with the exception of the anterior insula. This is consistent with the cortical/subcortical division of target regions described in a number of animal studies.
Mediodorsal Thalamic Nucleus
The MD, as a main part of the limbic thalamus [Vogt and Pandya, 1987], is essentially understood as a relay nucleus, linking basal ganglia and cortex [Haber and Calzavara, 2009]. Strong connections of the MD have been described to prefrontal areas including the dlPFC in humans and monkeys [Klein et al., 2010; Ray and Price, 1993], as reviewed by Groenewegen [ 1988]. Additional connections have been described to pgACC and dACC in humans, primates, cats, and rats [Jones and Leavitt, 1974; Klein et al., 2010; Krettek and Price, 1977; Ray and Price, 1993] as well as to subcortical structures such as the caudate nucleus in monkeys [Nakano et al., 1990]. In addition to showing these reported connections in humans with DTI, our study also demonstrated the preference of the MD in contrast to the CM/Pf towards the dlPFC, dACC, and pgACC, and the caudate nucleus (Fig. 3). This preferential connectivity is further supported by reports of sparse fiber tracts between the CM/Pf and cortical regions [Herkenham 1980; van der Werf et al., 2002], which stands in contrast to their heavy striatal projections as found in rats, cats, and monkeys, as reviewed by Haber and Calzavara [ 2009] and Haber and McFarland [ 2001], and van der Werf et al. [ 2002].
Figure 3.
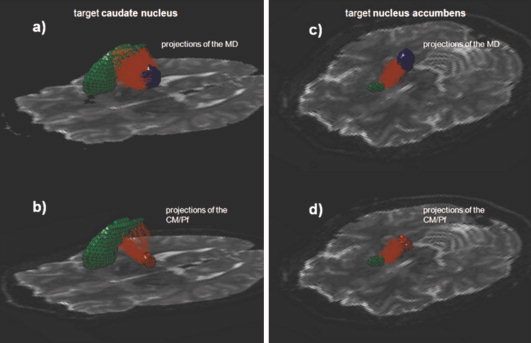
The fiber tracts (light red) from the thalamic seeds (Mediodorsal thalamic nucleus (MD) in blue and Centromedian and Parafascicular Complex (CM/Pf) in red) towards two target regions (green) are depicted above. In (a) and (b) the MD and the CM/Pf complex project towards the caudate nucleus–showing the difference in the counted fibers whereas in (c) and (d) the projections toward the nucleus accumbens illustrate the lack of differences in the projections of the MD and the CM/Pf. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
This specific preference of the MD towards the cortex and the caudate nucleus is well in line with functionally described thalamo‐striato‐cortical loops [Alexander et al., 1986; Haber and Calzavara, 2009]. The MD, the caudate nucleus, and the dlPFC are involved in dorsolateral‐prefrontal loops associated with cognition, motivation, and execution of movements [Haber and McFarland, 2001; Li et al., 2008]. The link to the cingulate cortex forms a part of the limbic network [Ongür and Price, 2000; Price, 1999], which is fundamental in processing reward, motivation, and emotion [Haber and Calzavara, 2009; Oyoshi et al., 1996].
Centromedian and Parafascicular Complex
The CM and the Pf thalamic nuclei have been investigated together in most anatomical studies because of their close vicinity and were combined as a complex. Therefore, our discussion of the regions that are preferentially connected to those intralaminar nuclei relies on prior reports of joint connectivity of both nuclei.
The CM/Pf complex forms a main part of the intralaminar nuclei [van der Werf et al., 2002], providing strong connections to subcortical structures and especially the basal ganglia [Jones and Leavitt, 1974; Royce and Mourey, 1985]. Well‐described subcortical connectivity is found with the putamen [Johnson, 1961; van der Werf et al., 2002] as well as the nucleus accumbens and pallidum, forming the ventral striatum [Berendse and Groenewegen, 1991; Krettek and Price, 1977; Nakano et al., 1990]. Based on a review by Haber and McFarland [ 2001], it seems that the ventral striatum receives a major part of its projections from the CM/Pf complex. Our findings show that the MD is more strongly connected with the caudate nucleus, which is in line with findings critically evaluated by Haber and Calzavara [ 2009], who report that the input from the MD and the CM/Pf complex to dorsal striatum, consisting of caudate nucleus and putamen, are equally large. Less frequent subcortical connectivity for CM/Pf is described with amygdala [ Ottersen and Ben‐Ari, 1979; Su and Bentivoglio, 1990] and hippocampus [Green and Adey, 1956], which is in concordance with our data. The large thalamostriatal projections as well as the lower fiber count for CM/Pf to the other subcortical regions of interest are depicted in Table I.
Projections to cortical areas from the CM/Pf complex have been reported as weak and more non‐specific, as critically appraised by Haber and McFarland [ 2001] and van der Werf [ 2002]. At least in the case of frontal regions, the cortical projections seem to be limited to agranular cortex and do not exceed into prefrontal areas [Finch et al., 1984; Hsu and Price, 2007; Jasmin et al., 2004], which were preferentially connected to the MD. This finding is also reflected by our results, which show that the anterior insula, consisting in part of agranular cortex [Mesulam and Mufson, 1982], was the only cortical region in our analysis that showed preferential connectivity with the CM/Pf. This is supported by direct connections between the CM/Pf and the insula in rats [Berendse and Groenewegen, 1991; Jasmin et al., 2004; Mufson and Mesulam, 1984].
The results of our study lend support to the existence of connections of the CM/Pf in humans that have hitherto only been described by animal studies. Furthermore, our study reveals a significant preferential connectivity for the CM/Pf compared to the MD in humans with primarily subcortical regions: pallidum, putamen, hippocampus, and amygdala. The only cortical exception to this pattern of CM/Pf‐subcortical connectivity is the anterior insular/frontal operculum.
On the functional level, a strong connection of the CM/Pf to basal ganglia is consistent with its described involvement in motor preparation and control [Smith et al., 2009], see also van der Werf et al. 2002 for an extensive review. More specifically, the involvement of the CM in attentional processing has been shown [Kimura et al., 2004; Kinomura et al., 1996], suggesting a role in directing attention and modulating a directed response. Preferential connection of the CM/Pf complex to the anterior insula as a core structure of attentional processing [Nelson et al., 2010] supports the recently found functional connectivity of both structures during attentional tasks in humans. Dosenbach et al. [ 2008] revealed its cortico‐cortical connectivity with a dorsal portion of ACC (BA 32) and recently, Metzger et al. [ 2010] reported its connectivity with the CM during internally generated attention towards an upcoming salient stimulus. While the role of the CM/Pf in functions like motor control and attentional processing is of high significance, no such evidence exists for a specific involvement of the MD, which lends further support to the observed structural preference from a functional point of view.
Regions Without Preference
No preferential connection of either the MD or CM/Pf could be found to the nucleus accumbens and PCC (Fig. 3). Notably, whereas strong fiber connections to the nucleus accumbens from the MD and the CM/Pf were observed, we detected only weak connections to the PCC from both thalamic nuclei (see Table I). These findings are in accordance with previously described sparse interprojections between the MD [Krettek and Price, 1977] as well as the CM/Pf [Vogt and Laureys, 2005] to the PCC and strong fiber connections from the MD [Guillery, 1959] and the CM/Pf [Su and Bentivoglio, 1990] to the nucleus accumbens. A functional relationship between the MD and nucleus accumbens has been hypothesized for the field of reward guided behavior by Haber and Knutson [ 2010] and for limbic functions like emotional processing by Haber and McFarland [ 2001] and also for mood regulation by Price [ 1999], based on other reports. Functional implications of connections from the CM/Pf to the nucleus accumbens have been described as reward modulating but they are not necessarily involved in reward processing itself [Matsumoto et al., 2001].
Overall, our results support an anatomical integration of the MD in a network relevant for emotional and salience processing and of the CM/Pf in a network which may modulate attentional processes. Such a segregation of the two thalamic structures in terms of distinct connections to networks either processing internal representation of emotion or external attention has been described on a functional level and remains an important part of the ongoing discussion of specialized cortical networks [Dosenbach et al., 2008; Raichle et al., 2001; Zhang et al., 2010]. Hitherto, the ongoing debate of anticorrelated networks involved mainly cortical regions. However, this may have primarily reflected the technical difficulty of reliably isolating smaller subcortical structures—especially intrathalamic nodes—because of comparably poor resolution of most fMRI studies.
Given the functional specificity of subcortical and especially thalamic structures, failure to consider cortical integration might underestimate or oversimplify this network model, whereas a close interaction of subcortical and cortical structures forming integrated loops might be more likely and would reflect what has been previously hypothesized based on translational work [Alexander et al., 1986; Haber and Calzavara, 2009; Price, 1999].
Our study, as is the case with non‐invasive DTI studies more generally, suffers from limited spatial resolution when compared to established invasive tracing methods and is unable to consider the impact of non‐Gaussian diffusion processes [Hagmann et al., 2006; Landman et al., 2007]. Also, this method cannot detect axons, nor can it differentiate between efferent and afferent fiber bundles. Additionally, it is worth noting that within those relatively large voxels, intermingling axonal tracts with multiple orientations often exist. This diminished anisotropy causes reduced fiber counts in DTI, and it is important that low fiber counts are not interpreted as the absence of anatomical connections. However, the opposite is not necessarily true for high fiber counts. Furthermore, due to a DTI isovoxel size of 2 mm, we combined the CM and the Pf into one seed. However, previous studies have shown that both intralaminar nuclei are closely connected in their projections and functions [Kimura et al., 2004; Royce and Mourey, 1985]. The methodological approach used in this article was guided by our research questions about thalamic nuclei. We were specifically interested in the connectivity profile of two nuclei of high functional and clinical interest, such as for deep brain stimulation. Here, a method focusing on the exact anatomical boundaries of the start regions may be ideal, especially if direct, long‐range effects of focal stimulation have to be estimated. The choice of a priori targets that have shown specific activations in tasks activating the CM or the MD, should however not exclude the notion that other regions—especially in the frontal cortex—could be defined using appropriate tasks of interest. While the CM and Pf have historically been described as “nonspecific” [Jones and Leavitt, 1974] in contrast to the MD [Haber and McFarland, 2001], our study cannot contribute to this ongoing debate regarding the specificity of thalamic projections [Vogt et al., 2008]. Given the inherent limitation of DTI studies, as well as the specific limitation of our study which used a highly selective set of circumscribed target regions, we are unable to determine laminar specificity, nor can we distinguish regionally specific from diffuse connections. Lastly, we only included male subjects in our study based on reported effects of sexual differences in brain development and architecture [Bellis et al., 2001; Huster et al., 2009]. The investigation of potential gender‐specific differences represents an interesting subject for future studies.
CONCLUSIONS
Our DTI study provides direct evidence of the different preferential anatomical connectivities of the thalamic MD and the CM/Pf. To the authors' knowledge, this is the first study to assess preferential connectivity for distinct anatomically defined thalamic substructures towards anatomically defined cortical and subcortical regions in humans.
We observed similar connections in humans as characterized previously using invasive methods in animal studies. Based on preferential connectivities, we could indicate the structural basis of putative networks, showing that the MD connects mainly to prefrontal cortical regions, whereas the CM/Pf complex is more closely related to subcortical regions. Exceptions to these cortico‐subcortical preferential networks are the association of the MD with the caudate nucleus and the CM/Pf complex with anterior insular cortex, which is in line with previous tracing studies. Moreover, this might suggest two important hubs of structurally and functionally segregated networks for these neighboring thalamic nuclei.
Exceeding prior knowledge of thalamic networks in humans, our DTI results support the segregation of thalamic nuclei into distinct functional units of cortico‐subcortical systems, which play an important role in shaping behavior. In the context of a distinct involvement of cingulate cortex and anterior insula during attentional set and switches of attention [Yamasaki et al., 2002; Menon and Uddin, 2010], these thalamico‐cortical loops provide further evidence of segregated yet integrated information processing.
Future work will have to explore such networks with regard to their functional meaning by using combined high‐resolution fMRI and DTI approaches, as well as by investigating patient populations.
Acknowledgements
We thank Guido Behlau for his skillful technical support during the data processing and the technical staff at the Department of neurology for their great support during the MR scans. The authors have declared that no competing interests exist.
REFERENCES
- Alexander GE, DeLong MR, Strick PL ( 1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 9: 357–381. [DOI] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Lowe MJ, Dzemidzic M ( 2009): Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatr Res 171: 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AW ( 2001): Theoretical analysis of the effects of noise on diffusion tensor imaging. Magn Reson Med 46: 1174–1188. [DOI] [PubMed] [Google Scholar]
- Barabasi AL, Albert R ( 1999): Emergence of scaling in random networks. Science 286: 509–512. [DOI] [PubMed] [Google Scholar]
- Barbas H ( 2000): Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull 52: 319–330. [DOI] [PubMed] [Google Scholar]
- Bellis MD de, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM ( 2001): Sex differences in brain maturation during childhood and adolescence. Cereb Cortex 11: 552–557. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ ( 1991): Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience 42: 73–102. [DOI] [PubMed] [Google Scholar]
- Bodammer NC, Kaufmann J, Kanowski M, Tempelmann C ( 2009): Monte Carlo‐based diffusion tensor tractography with a geometrically corrected voxel‐centre connecting method. Phys Med Biol 54: 1009–1033. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O ( 2009): Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature reviews. Neuroscience 10: 186–198. [DOI] [PubMed] [Google Scholar]
- Cavdar S, Onat FY, Cakmak YO, Yananli HR, Gülçebi M, Aker R ( 2008): The pathways connecting the hippocampal formation, the thalamic reuniens nucleus and the thalamic reticular nucleus in the rat. J Anat 212: 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen LM, Veening JG, Petersen DW, Shipley MT ( 2003): Parvocellular subparafascicular thalamic nucleus in the rat: Anatomical and functional compartmentalization. J Comp Neurol 463: 117–131. [DOI] [PubMed] [Google Scholar]
- Cukiert A, Burattini JA, Cukiert CM, Argentoni‐Baldochi M, Baise‐Zung C, Forster CR, Mello VA ( 2009): Centro‐median stimulation yields additional seizure frequency and attention improvement in patients previously submitted to callosotomy. Seizure 18: 588–592. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE ( 2008): A dual‐networks architecture of top‐down control. Trends Cogn Sci 12: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch DM, Derian EL, Babb TL ( 1984): Afferent fibers to rat cingulate cortex. Exp Neurol 83: 468–485. [DOI] [PubMed] [Google Scholar]
- Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, Beaulieu C ( 2009): Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex 19: 524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JD, Adey WR ( 1956): Electrophysiological studies of hippocampal connections and excitability. Electroencephalogr Clin Neurophysiol 8: 245–263. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ ( 1988): Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal‐prefrontal topography. Neuroscience 24: 379–431. [DOI] [PubMed] [Google Scholar]
- Guillery RW ( 1959): Afferent fibres to the dorso‐medial thalamic nucleus in the cat. J Anat 93: 403–419. [PMC free article] [PubMed] [Google Scholar]
- Gutman DA, Holtzheimer PE, Behrens TEJ, Johansen‐Berg H, Mayberg HS ( 2009): A tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry 65: 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guye M, Bartolomei F, Ranjeva J ( 2008): Imaging structural and functional connectivity: Towards a unified definition of human brain organization? Curr Opin Neurol 21: 393–403. [DOI] [PubMed] [Google Scholar]
- Haber S, McFarland NR ( 2001): The place of the thalamus in frontal cortical‐basal ganglia circuits. Neuroscientist 7: 315–324. [DOI] [PubMed] [Google Scholar]
- Haber SN, Calzavara R ( 2009): The cortico‐basal ganglia integrative network: the role of the thalamus. Brain Res Bull 78: 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B ( 2010): The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35: 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Jonasson L, Maeder P, Thiran J, van Wedeen J, Meuli R ( 2006): Understanding diffusion MR imaging techniques: From Scalar diffusion‐weighted imaging to diffusion tensor imaging and beyond. Radiographics 26 ( Suppl 1): S205–S223. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, van Wedeen J, Sporns O ( 2008): Mapping the structural core of human cerebral cortex. PLoS Biol 6: e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M ( 1980): Laminar organization of thalamic projections to the rat neocortex. Science (New York, N Y) 207: 532–535. [DOI] [PubMed] [Google Scholar]
- Horn DI, Yu C, Steiner J, Buchmann J, Kaufmann J, Osoba A, Eckert U, Zierhut KC, Schiltz K, He H, Biswal B, Bogerts B, Walter M ( 2010): Glutamatergic and resting‐state functional connectivity correlates of severity in major depression—The role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci 4: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Price JL ( 2007): Midline and intralaminar thalamic connections with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol 504: 89–111. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Westerhausen R, Kreuder F, Schweiger E, Wittling W ( 2009): Hemispheric and gender related differences in the midcingulum bundle: A DTI study. Hum Brain Mapp 30: 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin L, Burkey AR, Granato A, Ohara PT ( 2004): Rostral agranular insular cortex and pain areas of the central nervous system: A tract‐tracing study in the rat. J Comp Neurol 468: 425–440. [DOI] [PubMed] [Google Scholar]
- Jayaraman A ( 1985): Organization of thalamic projections in the nucleus accumbens and the caudate nucleus in cats and its relation with hippocampal and other subcortical afferents. J Comp Neurol 231: 396–420. [DOI] [PubMed] [Google Scholar]
- Johnson TN ( 1961): Fiber connections between the dorsal thalamus and corpus striatum in the cat. Exp Neurol 3: 556–569. [DOI] [PubMed] [Google Scholar]
- Jones EG, Leavitt RY ( 1974): Retrograde axonal transport and the demonstration of non‐specific projections to the cerebral cortex and striatum from thalamic intralaminar nuclei in the rat, cat and monkey. J Comp Neurol 154: 349–377. [DOI] [PubMed] [Google Scholar]
- Kimura M, Minamimoto T, Matsumoto N, Hori Y ( 2004): Monitoring and switching of cortico‐basal ganglia loop functions by the thalamo‐striatal system. Neurosci Res 48: 355–360. [DOI] [PubMed] [Google Scholar]
- Kinomura S, Larsson J, Gulyás B, Roland PE ( 1996): Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science (New York, N Y) 271: 512–515. [DOI] [PubMed] [Google Scholar]
- Klein JC, Rushworth MFS, Behrens TEJ, Mackay CE, Crespigny AJ de, D'Arceuil H, Johansen‐Berg H ( 2010): Topography of connections between human prefrontal cortex and mediodorsal thalamus studied with diffusion tractography. NeuroImage 51: 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss JK, Pohle T, Weigel R, Burgunder J ( 2002): Deep brain stimulation of the centre median‐parafascicular complex in patients with movement disorders. J Neurol Neurosurg Psychiatry 72: 546–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettek JE, Price JL ( 1977): The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol 171: 157–191. [DOI] [PubMed] [Google Scholar]
- Landman BA, Farrell JAD, Jones CK, Smith SA, Prince JL, Mori S ( 2007): Effects of diffusion weighting schemes on the reproducibility of DTI‐derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5T. NeuroImage 36: 1123–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CR, Yan P, Sinha R, Lee T ( 2008): Subcortical processes of motor response inhibition during a stop signal task. NeuroImage 41: 1352–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Ding J, Marinazzo D, Xu Q, Wang Z, Yuan C, Zhang Z, Lu G, Chen H ( 2011): Small‐world directed networks in the human brain: multivariate Granger causality analysis of resting‐state fMRI. NeuroImage 54: 2683–2694. [DOI] [PubMed] [Google Scholar]
- Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, Luo C, Wang Z, Tan Q, Lu G, Chen H: Default mode network abnormalities in mesial temporal lobe epilepsy: A study combining fMRI and DTI. Hum Brain Mapp (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchi G, Bentivoglio M, Molinari M, Minciacchi D ( 1984): The thalamo‐caudate versus thalamo‐cortical projections as studied in the cat with fluorescent retrograde double labeling. Exp Brain Res 54: 225–239. [DOI] [PubMed] [Google Scholar]
- Mai JK, Assheuer J, Paxinos G ( 2004): Atlas of the Human Brain, 2nd ed. San Diego: Academic Press. [Google Scholar]
- Matsumoto N, Minamimoto T, Graybiel AM, Kimura M ( 2001): Neurons in the thalamic CM‐Pf complex supply striatal neurons with information about behaviorally significant sensory events. J Neurophysiol 85: 960–976. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald DIacoboni M, Schormann T, Amunts K, Palomero‐Gallagher N, Geyer S, Parsons L, Narr K, Kabani N, Le Goualher G, Boomsma D, Cannon T, Kawashima R, Mazoyer B ( 2001): A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos Trans Roy Soc Lond Series B Biol Sci 356: 1293–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ ( 2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214: 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ ( 1982): Insula of the old world monkey. I. Architectonics in the insulo‐orbito‐temporal component of the paralimbic brain. J Comp Neurol 212: 1–22. [DOI] [PubMed] [Google Scholar]
- Metzger CD, Eckert U, Steiner J, Sartorius A, Buchmann JE, Stadler J, Tempelmann C, Speck O, Bogerts B, Abler B, Walter M ( 2010): High field FMRI reveals thalamocortical integration of segregated cognitive and emotional processing in mediodorsal and intralaminar thalamic nuclei. Front Neuroanat 4: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamimoto T, Kimura M ( 2002): Participation of the thalamic CM‐Pf complex in attentional orienting. J Neurophysiol 87: 3090–3101. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC ( 2009): Meta‐analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 66: 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM ( 1984): Thalamic connections of the insula in the rhesus monkey and comments on the paralimbic connectivity of the medial pulvinar nucleus. J Comp Neurol 227: 109–120. [DOI] [PubMed] [Google Scholar]
- Nakano K, Hasegawa Y, Tokushige A, Nakagawa S, Kayahara T, Mizuno N ( 1990): Topographical projections from the thalamus, subthalamic nucleus and pedunculopontine tegmental nucleus to the striatum in the Japanese monkey, Macaca fuscata . Brain Res 537: 54–68. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NUF, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE ( 2010): Role of the anterior insula in task‐level control and focal attention. Brain Struct Funct 214: 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongür D, Price JL ( 2000): The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10: 206–219. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Ben‐Ari Y ( 1979): Afferent connections to the amygdaloid complex of the rat and cat. I. Projections from the thalamus. J Comp Neurol 187: 401–424. [DOI] [PubMed] [Google Scholar]
- Oyoshi T, Nishijo H, Asakura T, Takamura Y, Ono T ( 1996): Emotional and behavioral correlates of mediodorsal thalamic neurons during associative learning in rats. J Neurosci 16: 5812–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL ( 1999): Prefrontal cortical networks related to visceral function and mood. Ann NY Acad Sci 877: 383–396. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JP, Price JL ( 1993): The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 337: 1–31. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ ( 2003): Reduction of eddy‐current‐induced distortion in diffusion MRI using a twice‐refocused spin echo. Magn Reson Med 49: 177–182. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H, Bonilha L ( 2007): Improving lesion‐symptom mapping. J Cogn Neurosci 19: 1081–1088. [DOI] [PubMed] [Google Scholar]
- Royce GJ, Mourey RJ ( 1985): Efferent connections of the centromedian and parafascicular thalamic nuclei: An autoradiographic investigation in the cat. J Comp Neurol 235: 277–300. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Kiening KL, Kirsch P, Gall CC von, Haberkorn U, Unterberg AW, Henn FA, Meyer‐Lindenberg A ( 2010): Remission of major depression under deep brain stimulation of the lateral habenula in a therapy‐refractory patient. Biol Psychiatry 67: e9–e11. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN ( 2006): Fiber Pathways of the Brain. New York: Oxford University Press; [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD ( 2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27: 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM ( 2002): Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Raju D, Nanda B, Pare J, Galvan A, Wichmann T ( 2009): The thalamostriatal systems: Anatomical and functional organization in normal and parkinsonian states. Brain Res Bull 78: 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein H, Bogerts B ( 2008): Immunological aspects in the neurobiology of suicide: Elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatric Res 42: 151–157. [DOI] [PubMed] [Google Scholar]
- Su HS, Bentivoglio M ( 1990): Thalamic midline cell populations projecting to the nucleus accumbens, amygdala, and hippocampus in the rat. J Comp Neurol 297: 582–593. [DOI] [PubMed] [Google Scholar]
- Temel Y, Visser‐Vandewalle V, Ackermans L, Beuls EAM ( 2004): Thalamus and penile erection. Int J Impotence Res 16: 505–511. [DOI] [PubMed] [Google Scholar]
- van der Werf YD, Witter MP, Groenewegen HJ ( 2002): The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev 39: 107–140. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN ( 1987): Cingulate cortex of the rhesus monkey. II. Cortical afferents. J Comp Neurol 262: 271–289. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Laureys S ( 2005): Posterior cingulate, precuneal and retrosplenial cortices: Cytology and components of the neural network correlates of consciousness. Prog Brain Res 150: 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL ( 1987): Cingulate cortex of the rhesus monkey. I. Cytoarchitecture and thalamic afferents. J Comp Neurol 262: 256–270. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Hof PR, Friedman DP, Sikes RW, Vogt LJ ( 2008): Norepinephrinergic afferents and cytology of the macaque monkey midline, mediodorsal, and intralaminar thalamic nuclei. Brain Struct Funct 212: 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Bermpohl F, Mouras H, Schiltz K, Tempelmann C, Rotte M, Heinze HJ, Bogerts B, Northoff G ( 2008): Distinguishing specific sexual and general emotional effects in fMRI‐subcortical and cortical arousal during erotic picture viewing. NeuroImage 40: 1482–1494. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G ( 2002): Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci USA 99: 11447–11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME ( 2010): Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb cortex 20: 1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]


