Abstract
On the basis of findings in normative samples that different cortical brain regions covary in gray matter volume, most likely as a result of mutually trophic influences during cortical development, we aimed to study whether patterns of covariation in regional gray matter, i.e., structural covariance, differed between adolescents who were born very preterm and full‐term controls. Optimized voxel‐based morphometry was used to study structural magnetic resonance imaging scans from 218 very preterm adolescents (gestational age <33 weeks) and 127 controls at 14–15 years of age. Local gray matter volumes were obtained for 18 regions of interest involved in sensorimotor and higher‐order cognitive functions. These were then used to predict local volumes in the remaining areas of the cortex, with total gray matter volume, age and gender used as confounding variables. Very preterm adolescents compared with controls demonstrated differential (i.e., both increased and decreased) structural covariance between medial, frontal and cingulate gyri, caudate nucleus, thalamus, primary visual cortex, cerebellum and several other cortical and subcortical regions of the cortex. These findings support previous research indicating that preterm birth is associated with altered cortical development, and suggest that developmental changes in one brain region may result in a cascade of alterations in multiple regions. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: preterm birth, magnetic resonance imaging, cortical development, brain, adolescent, caudate nucleus, thalamus, cerebellum
INTRODUCTION
An increased risk of structural brain alterations in very preterm (VPT) and very low birth weight individuals compared with controls has been described from childhood into young adulthood. These include smaller cortical volumes, larger lateral ventricles [Allin et al.,2004; Kesler et al.,2004; Nosarti et al.,2002], and reduced total gray matter volumes [Nosarti et al.,2002], specifically in sensorimotor and parieto‐occipital regions in childhood [Peterson et al.,2003] and in temporal, frontal and occipital cortices in adolescence [Nosarti et al.,2008]. White matter alterations in several brain regions, including frontal, parietal, temporal, and occipital lobes have also been documented [Nosarti et al.,2008].
Magnetic resonance imaging (MRI) studies looking at specific regions of interest have demonstrated decreases in size in VPT and very low birth weight individuals compared to controls in hippocampus [Nosarti et al.,2002], cerebellum [Allin et al.,2001], corpus callosum [Nosarti et al.,2004], and thalamus [Gimenez et al.,2006]. Taken together, these findings suggest that the brains of VPT individuals may be substantially different in structure, an observation which is compatible with the idea of neuroplastic reorganization in response to early brain lesions.
The study of structural brain alterations in vulnerable groups may be important for understanding their neurodevelopmental outcomes, and to date MRI findings have been used to predict motor delay and cerebral palsy [Woodward et al.,2006] and working memory deficits [Woodward et al.,2005] in VPT infants. In adolescence, we previously reported that gray and white matter alterations in brainstem, frontal, temporal, and limbic regions mediated the relationship between very preterm birth and adolescent cognitive impairment, with specific reference to language and executive function [Nosarti et al.,2008]. Investigation of the inter‐relationships between areas in which between‐group white and gray matter volumes differences were observed, revealed that such regionally specific alterations were all significantly associated, suggesting that abnormalities in one brain area may be reciprocally connected to abnormalities in other areas.
In this article, instead, we present the results of a study investigating patterns of covariation between a priori selected regions of interest and gray matter volume (i.e., cubic millimeters of gray matter per voxel) throughout the whole brain, i.e., structural covariance, in the same group of VPT adolescents and controls we studied earlier [Nosarti et al.,2008]. Structural covariance between regions of the human brain is thought to be the result of mutually trophic influences during cortical development [Mechelli et al.,2005]. Studies in adult normative samples have identified consistent patterns of positive and negative covariance in gray matter volume in brain regions involved in sensorimotor and higher‐order cognitive functions which are similar for males and females with the exception of the left amygdala [Mechelli et al.,2005]. These findings highlight the mutually dependent influences that proximal and distal brain structures might exert over one another during development in addition to minor differential variation according to an individual's gender. Recent investigations have also demonstrated that patterns of structural covariance may be altered in individuals with schizophrenia [Mitelman et al.,2005b], a disorder which is considered to be developmental in nature, and that such alterations are dependent on symptom profile [Modinos et al.,2009].
In this study, we hypothesized that differential patterns of structural covariance would be observed between VPT adolescents and full‐term controls, particularly in the prefrontal and temporal cortices, on the basis of known structural alterations associated with very preterm birth.
METHODS
Participants
VPT individuals belonged to two cohorts, each born before 33 weeks of gestation and admitted consecutively to the Neonatal Unit of University College London Hospital (UCLH). The first cohort (n = 223) was comprised of infants born in 1979–1982 that were enrolled for long‐term follow‐up, and received neurological and cognitive assessment at 1, 4, and 8 years [Roth et al.,1994]. At 14–15 years of age 156 individuals (76.1%) agreed to undergo assessment, including 128 that underwent MRI scans [Nosarti et al.,2002,2004,2008]. The second cohort (n = 147) was comprised of a subsample of infants born in 1983–1984 and admitted to UCLH. This selection was necessitated by an expansion in capacity at UCLH in 1983, which prevented the inclusion of the entire consecutive series due to limited research resources. At 14–15 years of age 113 individuals (76.9%) were assessed, including 90 who underwent MRI scans. The inclusion criteria were infants born at 28 weeks or less of gestation and a random sample of one in four infants born from 29 weeks to 33 weeks of gestation [Nosarti et al.,2008]. In both cohorts, VPT participants did neither differ from non‐participants in family socio–economic status, birth weight, gestational age, mode of delivery, condition at birth, the need for mechanical ventilation, neonatal ultrasonographic findings, nor in neurodevelopmental status when assessed at 1, 4, and 8 years of age.
Full‐term individuals (n = 47) delivered at UCLH in 1979–1980 were enrolled to act as age‐matched controls for assessment. Inclusion criteria were full‐term birth (38–42 weeks) and birth weight greater than 2,500 grams. At 14–15 years of age, 45 full‐term controls were traced and 21 (44.6%) agreed to assessment including MRI. Additional full‐term controls (n = 106), matched for year of birth and socio–economic status, were recruited through the press. The inclusion criteria were the same as for the UCLH controls; exclusion criteria were any history of neurological conditions including meningitis, head injury, and cerebral infections. Ethical approval for the study was obtained from local ethical committees. Written consent for the assessment, including MRI, was obtained from an accompanying parent and verbal consent was obtained from the participants.
MRI Acquisition
Magnetic resonance imaging was performed using a 1.5 Tesla machine (General Electric Medical Systems, Milwaukee, WI). The following sequences were obtained: sagittal T2‐weighted fast spin‐echo, 27 × 4 mm contiguous slices (TR, repetition time 2,500 ms, TEef 85 ms); axial T2‐weighted double‐echo fast spin‐echo, 28 × 5 mm contiguous slices (TR 2,900 ms, TEef 19 and 95 ms); and three‐dimensional T1‐weighted gradient‐echo sequence that allowed reconstruction in any plane of 124 1.5 mm slices (TR 35 ms, TEef 5 ms, flip angle 3°).
MRI Data Preprocessing
Structural images were preprocessed using optimized voxel based morphometry (VBM) implemented in Statistical Parametric Mapping version 2 (SPM2) (http://www.fil.ion.ucl.ac.uk/spm), running under Matlab 7.1 (Mathworks, Natick, Massachusetts). Voxel‐based morphometry is a whole‐brain, unbiased, semiautomated technique for characterizing brain differences in vivo using magnetic resonance images [Ashburner and Friston,2000]. A customized template of the whole brain was obtained with a standard VBM protocol [Ashburner and Friston,2000] through normalization of the GRASS T1‐weighted gradient‐echo sequences for all study participants (VPT and controls, n = 310) to the T1‐weighted stereotactic template of SPM2 using a 12 parameter affine transformation, followed by smoothing with an 8 mm FWHM isotropic Gaussian kernel (to reduce the effects of individual variation in sulcal/gyral anatomy) [Nosarti et al.,2008]. All spatially normalized images were resliced with a voxel size of 1 × 1 × 1 mm3 to reduce the partial volume problem and insure optimal tissue segmentation. Finally all smoothed normalized images were averaged. Starting estimates for the registration were assigned by specifying the position of the anterior commissure [Ashburner et al.,1997]. This method uses a Bayesian framework, whereby the construction of posterior probability maps (PPM) based on prior knowledge of the normal variability in brain size [Good et al.,2001] allows inferences about regionally specific effects.
The normalized averaged whole brain images were segmented into gray matter, white matter, and cerebrospinal fluid using a modified mixture model cluster algorithm which identifies voxels assigned to a particular tissue type combined with an a priori knowledge of the spatial distribution of the these tissue types in normal populations derived by probability maps. Segmentation includes a correction for intensity nonuniformity in order to account for image intensity variations due to differing positions of cranial structures in the MRI scanner [Ashburner and Friston,2000]. Finally, the segmented images were smoothed with an 8 mm full‐width half‐maximum (FWHM) isotropic Gaussian filter. Smoothing is used to improve the signal‐to‐noise ratio, as it reduces the influences of individual variations in gyral anatomy after spatial normalization and permits application of Gaussian random‐field theory for corrected statistical inference [Friston et al.,1995].
Separate stereotactic customized gray and white matter templates were created with an optimized VBM protocol [Good et al.,2001], by averaging all the 310 smoothed normalized gray/white matter images from the standard VBM protocol described earlier using affine transformation with sinc interpolation algorithm. Images from the whole group were chosen in order to minimize any potential bias for spatial normalization. Customized priors were computed from these templates, which were used to improve the accuracy of the extraction of gray and white matter of each subject in the normalization process described in the text [Good et al.,2001].
All original MRI images (in native space) were segmented into GM and WM using a SPM2 script. Non‐brain voxels were removed from the segmented images. The extracted GM and WM images were spatially normalized onto the customized GM and WM templates using the optimum 12‐parameter affine transformations. The spatially normalized images were segmented into GM and WM and subjected to a second extraction of normalized segmented GM and WM images. A brain extraction and cleaning procedure was applied and the cleaned GM and WM images were modulated, i.e., the spatially normalized GM and WM were multiplied by their relative volumes before and after spatial normalization, in order to adjust for the expansion and shrinkage of voxels that can occur during spatial normalization [Ashburner and Friston,2000]. Finally, the segmented images were smoothed using an 8 mm FWHM isotropic Gaussian kernel [Ashburner and Friston,2000]. All the described SPM2 steps were fully automated.
Statistical Analysis
A voxel‐wise statistical analysis was performed on the gray matter images using the general linear model as applied in SPM2. Regional gray matter densities were extracted from 18 regions of interest. The following 15 were chosen on the basis of structural alterations in ex‐VPT adolescents compared to controls we previously reported (both gray matter decreases and increases) [Nosarti et al.,2008]: middle temporal gyrus (BA 21; x = ±48, y = 2, z = −18), fusiform gyrus (BA 20; x = ±33, y = −38, z = −17), precentral gyrus (BA 4; x = ±38, y = −18, z = 42), lingual gyrus (BA 18; x = ±18, y = −84, z = −12), precuneus (BA 7; x = ±15, y = −75, z = 35), superior temporal gyrus (BA 20; x = ±21, y = −33, z = −3), superior frontal gyrus (BA10; x = ±21, y = 63, z = 2), medial frontal gyrus (BA 11; x = ±6, y = 62, z = −17), inferior frontal gyrus (BA 9; x = ±47, y = 8, z = 26), precentral gyrus (BA 4; x = ±65, y = −3, z = 15), cingulate gyrus (BA 31; x = ±8, y = −35, z = 35), caudate nucleus (x = ±14, y = 8, z = 17), thalamus, medial dorsal nucleus (x = ±3, y = −17, z = 3), posterior cerebellum, semilunar lobule (x = ±27, y = −87, z = −38), anterior cerebellum, culmen (x = ±12, y = −44, z = −3). The remaining three regions of interest were chosen because they had been widely characterized at the functional level by previous studies, they had been anatomically validated using the standard T1‐weighted template in Montreal Neurological Institute stereotactic space, and they had been used in a study of structural covariance in healthy adults [Mechelli et al.,2005]. These were: primary visual cortex (V1; x = ±24, y = −95, z = −6), primary auditory cortex (x = ±43, y = −24, z = 13), and primary somatosensory cortex (S1; x = ±27, y = −36, z = 60).
The regional gray matter densities extracted from the regions of interest were used to model regional densities in all voxels of the preprocessed gray matter segments. Separate correlation analyses were conducted for each a priori region of interest, resulting in 36 (18 × 2, left and right side) statistical models. To identify the effects associated with gestational age, scans of VPT adolescents and full‐term controls were modeled separately in all analyses. Global gray matter volume, gender and chronological age at assessment were modeled as covariates of no interest to identify regionally specific associations that could not be attributed to the effects of these variables. These statistical analyses identified, for each a priori region of interest, voxels which expressed either decreased or increased covariance with that region in VPT born adolescents relative to full‐term controls. Inferences were made at P < 0.05 after False Discovery Rate (FDR) correction for multiple comparisons across the whole brain.
RESULTS
Sample Characteristics
As previously reported, 35 scans could not be analyzed due to movement (3 VPT, 14 controls), signal artefact (6 VPT, 10 controls) or ventricular enlargement (2 VPT). Thus, a total of 207 VPT individuals and 103 controls were studied [Nosarti et al.,2008]. There were no differences between the 35 excluded subjects and those that were included in terms of head circumference [F(309) = 2.3, P = 0.13]; age [F(309) = 2.7, P = 0.10]; global executive function scores [F(309) = 0.02, P = 0.89]; and global language scores [F(309) = 0.6, P = 0.43] at assessment (please see Nosarti et al.,2008 for further details on these assessments). Furthermore, there were no differences between the excluded and the included VPT participants in terms of neonatal ultrasound classification (χ 2 = 1.8, P = 0.40). No participant had received a diagnosis of periventricular leucomalacia. Table I displays the socio‐demographic, perinatal, anthropometric, and neurodevelopmental characteristics of all study participants. VPT adolescents and controls did not differ in terms of gender distribution (χ 2 = 2.1, P = 0.45), socio–economic status as measured by Her Majesty's Stationary Office Standard Occupational Classification criteria (χ 2 = 3.1, P = 0.38), height [F(309) = 2.1, P = 0.15], or body mass index (BMI) calculated as [weight/height2] [F(309) = 1.2, P = 0.28]. However, the two groups differed in head circumference [F(309) = 17.1, P < 0.001], and chronological age at time of assessment [F(309) = 16.2, P < 0.001].
Table I.
Characteristics of preterm‐born adolescents and controls
| Cases (N = 207) | Controls (N = 103) | |
|---|---|---|
| Neonatal characteristicsa | ||
| Birth‐weight (g) | 1276.0 ± 353.8, (552–2,390) | 3358.4 ± 394.3, (3,200–4120) |
| Gestation at birth (weeks) | 29.1 ± 2.2, (24–32) | 40.1 ± 1.3, (38–41) |
| Males/Females (number) | 115/92 | 59/45 |
| Anthropometric data at assessment | ||
| Head circumference (cm)** | 55.1 ± 1.9, (50.0–60.0) | 56.1 ± 1.7, (52.0–61.2) |
| Height (cm) | 164.7 ± 8.4, (145–184) | 166.9 ± 7.4, (150–184) |
| Body Mass Index | 20.5 ± 3.9, (12.8–35.0) | 21.0 ± 4.0, (14.5–37.4) |
| Chronological age (years)** | 15.2 ± 0.5, (13.9–16.7) | 15.0 ± 0.7, (14.1–16.8) |
| Parental socio‐–economic status at assessment—number, percent | ||
| I‐II | 81 (39%) | 48 (46%) |
| III | 65 (31%) | 24 (23%) |
| IV‐V | 49 (24%) | 25 (24%) |
| Missing | 12 (6%) | 7 (7%) |
| Neonatal ultrasound results—number, percent | ||
| Normal | 106 (51%) | n/a |
| Uncomplicated periventricular haemorrhage | 67 (32%) | n/a |
| Periventricular haemorrhage and ventricular dilatation | 34 (17%) | n/a |
Mean, standard deviation (SD) and range are given, unless otherwise specified.
**P<0.001.
aFor controls: birth weight (n = 67) and gestational age (n = 72).
Structural Covariance
In the investigation of structural covariance, two regions were defined as being positively correlated if an increase in gray matter in one area was associated with an increase in gray matter in another area. Conversely, two regions were defined as being negatively correlated if an increase in gray matter in one area was associated with a decrease in gray matter in another area. We referred to homotopic regions to indicate the same areas of the cortex in opposite hemispheres (e.g., left and right superior frontal gyrus), ipsilateral regions to indicate different areas of the cortex in the same hemisphere (e.g., left superior frontal gyrus and left fusiform gyrus), and heterotopic regions to indicate different areas of the cortex in opposite hemispheres (e.g., left superior frontal gyrus and right precentral gyrus) [Mechelli et al.,2005]. As positive and negative associations between homotopic regions, between ipsilateral regions, and between heterotopic regions have been previously described in healthy controls [Mechelli et al.,2005], here we only report patterns of structural covariance which differed between VPT adolescents and full‐term controls, as well as Talairach coordinates and Z‐scores for within group correlations where significant between group differences were observed.
VPT > Control
We first report structural associations which were significantly stronger in VPT adolescents compared to full‐term controls (Table II and Fig. 1).
Table II.
Increased structural covariance in preterm‐born adolescents compared with controls between selected regions of interest and other areas of the cortex
| VPT > controls | VPT | Controls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Z | x | y | z | Z | x | y | z | Z | |
| Medial frontal gyrus, left | ||||||||||||
| Left lingual gyrus (IP) | −20 | −62 | 3 | 5.05 | −20 | −60 | 3 | 2.42 | −15 | −60 | 2 | −1.11 |
| Cingulate gyrus, left | ||||||||||||
| Right cingulate gyrus (HO) | 11 | −24 | 39 | 3.18 | 11 | −24 | 39 | 13.47 | 11 | −24 | 39 | 9.02 |
| Left cuneus (IP) | −11 | −71 | 8 | 4.34 | −11 | −66 | 6 | 2.56 | −8 | −60 | 2 | 0.57 |
| Left inferior temporal gyrus (IP) | −63 | −15 | −17 | 3.42 | −63 | −15 | −17 | 0.56 | −59 | −21 | −18 | 0.53 |
| Left middle occipital gyrus (IP) | −29 | −95 | 14 | 3.39 | −29 | −95 | 14 | 2.61 | −37 | −81 | 19 | 0.55 |
| Left superior temporal gyrus (IP) | −47 | 17 | 9 | 3.32 | −48 | 18 | 9 | 0.61 | −51 | 17 | 2 | 0.79 |
| Right posterior cingulate gyrus (HE) | 15 | −59 | 20 | 4.46 | 15 | −59 | 20 | 3.11 | 11 | −54 | 22 | 0.72 |
| 12 | −66 | 9 | 4.29 | 12 | −66 | 9 | 0.91 | 8 | −57 | 6 | 0.49 | |
| Right inferior temporal gyrus (HE) | 60 | −20 | −14 | 3.33 | 60 | −20 | −14 | 2.63 | 60 | −22 | −16 | 0.56 |
| Cingulate gyrus, right | ||||||||||||
| Left cingulate gyrus (HO) | −8 | −27 | 39 | 5.15 | −8 | −27 | 39 | 18.75 | −8 | −27 | 39 | 12.73 |
| Caudate nucleus, right | ||||||||||||
| Right posterior cerebellum (Hemisphere, Lobule VI) (IP) | 23 | −59 | −15 | 4.54 | 23 | −59 | −15 | 2.95 | 23 | −54 | −22 | 0.17 |
| 24 | −71 | −12 | 4.08 | 24 | −71 | −12 | 2.23 | 21 | −75 | −9 | 0.22 | |
| 11 | −59 | −45 | 4.01 | 11 | −59 | −45 | 4.76 | 11 | −59 | −46 | 0.17 | |
| 0 | −54 | −35 | 3.98 | 0 | −54 | −35 | 5.60 | 0 | −54 | −35 | 0.59 | |
| Thalamus, right | ||||||||||||
| Right superior frontal gyrus (IP) | 30 | 53 | 32 | 4.46 | 30 | 53 | 32 | 1.76 | 30 | 53 | 15 | 0.30 |
| Left superior frontal gyrus (HE) | −26 | 62 | 15 | 4.81 | −23 | 57 | 25 | 0.33 | −44 | 45 | −14 | 0.36 |
| Left insula (HE) | −44 | −41 | 20 | 4.70 | −45 | −39 | 21 | 0.31 | −51 | −41 | 25 | 0.54 |
| Posterior cerebellum, left | ||||||||||||
| Posterior cerebellum, right (Hemisphere, Lobule IX) (HO) | 12 | −57 | −48 | 5.04 | 12 | −57 | −48 | 7.25 | 12 | −57 | −49 | 0.90 |
| Posterior cerebellum, right | ||||||||||||
| Posterior cerebellum, left (Hemisphere, Lobule VIIIA) (HO) | −8 | −74 | −48 | 4.30 | −8 | −74 | −48 | 7.58 | −8 | −74 | −48 | 1.32 |
| −21 | −72 | −38 | 3.87 | −21 | −72 | −38 | 4.87 | −21 | −74 | −38 | 1.07 | |
IP, Ipsilateral effects; HO, homotopic effects; HE, heterotopic effect. Z scores greater than 3.0 are significant at P < 0.05 (FDR corrected).
Figure 1.
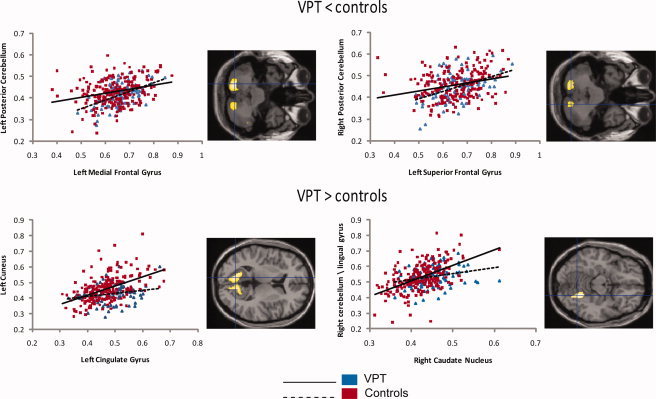
Gray matter correlations between selected regions of interest (x axis) and other areas of the human cortex (y axis) in VPT adolescents and controls. On the right of each correlation graph the local maxima for the significant regional clusters are displayed at the centre of the crosshair. The x and y axes report regional densities, measured as cubic millimeters of gray matter per voxel.
Homotopic effects
Our data showed that the gray matter volume of selective regions in one hemisphere was associated with gray matter volume of the homotopic region in the contralateral hemisphere more strongly in VPT‐born adolescents than controls. For instance VPT‐born individuals compared to controls showed increased covariance between the volume of an area of the cingulate gyrus in the left hemisphere and the same region in the right hemisphere and vice versa. Likewise, VPT‐born individuals showed stronger homotopic effects compared to controls in posterior cerebellum bilaterally.
Ipsilateral effects
Our results showed increased structural covariance between the volume of selective regions in one hemisphere and the volume of one or more regions in the same hemisphere in VPT‐born adolescents compared to controls. For instance, VPT‐born individuals demonstrated increased covariance compared to controls between the left medial frontal gyrus and the left lingual gyrus, as well as between the left cingulate gyrus and the left cuneus, left middle occipital gyrus, left inferior and left superior temporal gyri. Furthermore, the right caudate nucleus expressed differential positive associations in VPT adolescents compared to full‐term controls with the right posterior cerebellum. Increased ipsilateral covariance in VPT adolescents compared to controls was also demonstrated between the right thalamus and the right superior frontal gyrus.
Heterotopic effects
The left cingulate gyrus expressed differential positive heterotopic associations for VPT adolescents and full‐term controls. Specifically, this region was positively correlated with the right posterior cingulate gyrus and right inferior temporal gyrus in VPT adolescents more than in controls. Differential heterotopic effects were also observed in the right thalamus, which was positively correlated with the left superior frontal gyrus and left insula in VPT adolescents more than in controls.
VPT < Control
Analysis of our data revealed patterns of structural associations in selective regions of interest where VPT adolescents demonstrated decreased structural covariance compared to controls (Table III and Fig. 1).
Table III.
Decreased structural covariance in preterm‐born adolescents compared with controls between selected regions of interest and other areas of the cortex
| VPT < controls | VPT | Controls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Z | x | y | z | Z | x | y | z | Z | |
| Superior frontal gyrus, left | ||||||||||||
| Left inferior parietal lobule (IP) | −51 | −32 | 39 | 4.90 | −51 | −38 | 39 | 1.15 | −51 | −32 | 39 | 3.71 |
| −51 | −35 | 50 | 4.41 | −51 | −41 | 44 | 0.77 | −51 | −35 | 50 | 2.19 | |
| Left postcentral gyrus (IP) | −41 | −20 | 41 | 4.38 | −54 | −29 | 29 | 1.01 | −41 | −20 | 41 | 2.73 |
| Left posterior cerebellum (Hemisphere, Lobule VIIA, Crus II) (IP) | −20 | −77 | −38 | 4.25 | −24 | −66 | −36 | 0.70 | −20 | −77 | −38 | 0.74 |
| Right posterior cerebellum (Hemisphere, Lobule VIIA) (HE) | 17 | −75 | −41 | 3.84 | 20 | −68 | −33 | 0.70 | 17 | −75 | −41 | 1.27 |
| Medial frontal gyrus, left | ||||||||||||
| Left posterior cerebellum (Hemisphere, Lobule VIB) (IP) | −21 | −74 | −42 | 4.97 | −8 | −88 | −23 | 0.62 | −21 | −74 | −42 | 1.32 |
| Left postcentral gyrus (IP) | −39 | −21 | 42 | 3.97 | −51 | −35 | 39 | 0.66 | −39 | −21 | 42 | 1.93 |
| Left inferior parietal lobule (IP) | −56 | −30 | 36 | 3.81 | −54 | −33 | 38 | 0.52 | −56 | −30 | 36 | 5.09 |
| Right lateral cerebellum (HE) | 50 | −48 | −38 | 3.43 | 50 | −51 | −29 | 0.77 | 50 | −48 | −38 | 0.85 |
| Right posterior cerebellum (HE) | 14 | −72 | −36 | 5.33 | −2 | −80 | −39 | 0.36 | 14 | −72 | −36 | 2.18 |
| Thalamus, left | ||||||||||||
| Left middle temporal gyrus (IP) | −60 | −3 | −24 | 5.13 | −51 | −3 | −24 | −0.04 | −60 | −3 | −24 | 4.75 |
| Thalamus, right | ||||||||||||
| Left middle temporal gyrus (HE) | −60 | −3 | −24 | 5.34 | −51 | −5 | −21 | 0.01 | −60 | −3 | −24 | 4.90 |
| −54 | −9 | −15 | 4.79 | −51 | −9 | −14 | 0.11 | −54 | −9 | −15 | 5.01 | |
| Primary visual cortex, right | ||||||||||||
| Left superior occipital gyrus (HE) | −35 | −81 | 27 | 4.90 | −32 | −83 | 26 | 0.10 | −35 | −81 | 27 | 5.37 |
| Posterior cerebellum, right | ||||||||||||
| Left cingulate gyrus (HE) | −14 | −38 | 42 | 5.49 | −33 | −50 | 45 | 0.64 | −14 | −38 | 42 | 2.22 |
IP, ipsilateral effects; HO, homotopic effects; HE, heterotopic effect. Z scores greater than 3.0 are significant at P < 0.05 (FDR corrected).
Homotopic effects
We did not observe differential positive associations in homotopic regions for full‐term controls and VPT adolescents that survived our statistical threshold (P < 0.05, FDR corrected).
Ipsilateral effects
Controls and VPT adolescents demonstrated differential ipsilateral effects in the left superior frontal gyrus. VPT adolescents showed decreased structural covariance between this region and the left inferior parietal lobule, left postcentral gyrus, and left posterior cerebellum. The left medial frontal gyrus showed decreased structural covariance in VPT adolescents compared to controls with left posterior cerebellum, left postcentral gyrus and left parietal lobule. Decreased structural covariance in VPT adolescents was observed between the left thalamus and the left middle temporal gyrus.
Heterotopic effects
The left superior frontal gyrus expressed decreased structural covariance with the right posterior cerebellum in VPT adolescents compared to full‐term controls. Decreased structural covariance in VPT individuals was also observed between the left medial frontal gyrus and two areas of the right cerebellum. The right thalamus also evidenced differential heterotopic effects, specifically a weaker positive correlation with the left middle temporal gyrus in VPT adolescents compared to controls. Furthermore, decreased structural covariance in VPT adolescents was observed between the right primary visual cortex and the left superior occipital gyrus and between the right posterior cerebellum and the left cingulate gyrus.
DISCUSSION
The current investigation demonstrates for the first time increased as well as decreased structural covariance in gray matter volumes of a priori selected regions of interest and several other brain regions in VPT adolescents compared to full‐term controls. Significantly increased structural covariance was observed between homotopic, heterotopic and ipsilateral regions, whereas decreased covariance was found in heterotopic and ipsilateral regions only. Differential patterns of structural covariance were observed between several gray matter regions we previously reported in the same sample to be structurally altered in adolescence in VPT individuals compared to full‐term controls, including superior and media frontal gyri, cingulate gyrus, thalamus, caudate nucleus and cerebellum [Nosarti et al.,2008] and other frontal, parietal, occipital, temporal and cerebellar gray matter regions. As was the case with normative data [Mechelli et al.,2005], these findings cannot be explained by overall gray matter volume, which is known to be reduced in VPT adolescents compared to controls [Nosarti et al.,2002], as total gray matter volume was used as a confounding variable in the analysis. Furthermore, these results cannot be solely explained by differences in age or gender between the groups [Mechelli et al.,2005], as the two groups were selected on the basis of age and gender and in addition these variables were modeled as variables of no interest in the statistical analyses.
We suggest that the findings of several homotopic, heterotopic and ipsilateral regions expressing differences in their patterns of regional covariance in VPT adolescents compared to controls can be partly explained by the fact that early perinatal adversities may results in cortical and subcortical alterations of both gray and white matter and subsequent changes in dynamic processes of gray and white matter maturation [Allin et al.,2007; Ment et al.,2009]. The brain is particularly vulnerable to damage during the prenatal and early postnatal period, when cortical development is dominated by continuing processes of synaptic expansion and connectivity. At the time VPT infants are born, the germinal matrix, a transient embryonic structure of the telencephalon (it involutes at 34 weeks), proliferates glial precursor cells that are migrating to different layers of the cerebral cortex. These cells are implicated in the origination of oligodendroglia, which play a critical role in postnatal myelination and guidance molecules [Weiss et al.,2004], and astrocytes, which define the functional architecture of the cortex [Gressens et al.,1992]. Haemorrhages into the germinal matrix or lateral ventricles, or both, may lead to local destruction of glia precursor cells, stopping further neuronal migration from the germinal matrix to cerebral cortex in this area. As a consequence, neuronal precursors in transit along the radial glia may alter the course of their migration, and consequently reach the “wrong” layer of the cortex. Abnormal gray matter architecture may have subsequent effects on both distant and adjacent brain regions, which fail to receive input from the damaged cortex. Perinatal brain damage following very preterm birth further appears to disrupt GABAergic (γ‐amino butyric acid) interneuron development, the majority of which occurs during the third trimester of gestation when these interneurons migrate through white matter and the subplate. GABAergic neurons play a critical role on cortical synaptic development and white matter lesions have been associated with altered GABAergic pathway expression in VPT infants [Robinson,2005]. As there is evidence that white matter injury is associated with impaired gray matter development [Inder et al.,2003,2005], the adolescent differences in patterns of gray matter regional covariance in VPT individuals compared to controls we are reporting could reflect the long‐term effects of microstructural changes in connectivity due to disruptions of oligodendrocyte development and interneuron migration and consequent alteration in dynamic processes of gray matter maturation.
Whereas, as far as we are aware, gray matter structural covariance in very VPT samples in adolescence has not been previously studied, focal and distributed abnormalities of white matter connectivity in VPT adolescents have been documented, especially in relation to the corpus callosum, across which the majority of the inter‐hemispheric communication of the brain is conducted [Cooke and Abernethy,1999; Nosarti et al.,2004]. Altered white matter microstructure has been reported in VPT children without evidence of major neonatal brain injury in corpus callosum and within regions of the internal capsule [Nagy et al.,2003], whereas periventricular white matter damage has been associated with alterations in afferent and efferent projections fibers, callosal and major ipsilateral cortico‐cortical connections [Judas et al.,2005].
The development of positive associations between homotopic cortical regions, that is, same areas of the cortex in opposite hemispheres, may be dependent on mutually trophic effects mediated by the corpus callosum, which increases linearly from the age of 4 to 20 years [Giedd,2008]. In an adult normative sample, it was found that the gray matter volume of a region was a predictor of the volume of the homotopic region in the opposite hemisphere, except for the visual cortex [Mechelli et al.,2005]. Our data demonstrate differential structural covariance between homotopic regions in VPT adolescents compared to controls, which can be interpreted in the context of disordered structural connectivity. Specifically, VPT adolescents displayed increased covariance in homotopic regions of the cingulate gyrus and cerebellum. Fibers traversing the different callosal segments connect different cortical areas: the anterior corpus callosum connects frontal and temporal cortex, whilst the posterior regions connect occipital and parietal cortex [Bloom et al.,2005]. Increased homotopic structural covariance in anterior cingulate gyrus in VPT adolescents compared to controls may be compensatory in nature and reflect sparing of damage to the early developing mid‐anterior portion of the corpus callosum, and/or subsequent enlargement due to neuroplastic processes [Nosarti et al.,2004]. An alternative explanation could be that functional neuroanatomical differences described in VPT samples during performance of high‐order cognitive operations in anterior cingulate cortex [Narberhaus et al.,2009; Nosarti et al.,2009] may be secondary to structural alterations of this region.
The observed between‐group differences in structural covariance between heterotopic and ipsilateral regions, including the left superior, medial frontal, cingulate gyri and primary visual cortex (V1) and areas of the parietal, occipital, temporal and cerebellar cortex, could also be explained by a combination of region‐specific anatomical alterations and modification of connectivity.
We have previously reported altered functional neuroanatomical changes in fronto‐parieto‐occipital networks and caudate nucleus in VPT adolescents compared to controls during visual paired associates learning [Narberhaus et al.,2009], as well as fronto‐striatal‐cerebellar alterations during completion of a response inhibition task [Nosarti et al.,2006]. In addition, changes in fronto‐parietal and fronto‐occipital volumetric intercorrelations have been previously reported in schizophrenia [Mitelman et al.,2005b]. These between‐group alterations in structural covariance could underlie some of the deficits in high‐order cognitive functions observed in VPT individuals.
Another region we reported to display differential structural covariance (i.e. both increased and decreased) with other cortical areas in VPT adolescents compared to controls was the thalamus, via which the majority of sensory input to the cortex is received. The development of thalamo‐cortical topography is regulated by mechanisms involved in axonal guidance and pathway refinement [Price et al.,2006]. In the period from the 24th to the 31st postconceptional week the thalamo‐cortical axons (involved in cortical differentiation) reach their targets in the cortical plate, forming first sensory expectant circuitry [Kostovic and Vasung,2009]. During the same preterm period, gyrification progresses with the emergence of primary sulci. It is therefore plausible that changes in white matter connectivity following very preterm birth may result in altered thalamo‐cortical topography and contribute to determine cortical gyrification, which is reported to be altered in VPT samples [Kesler et al.,2006]. Differential volumetric intercorrelations have been reported in clinical samples. For instance, individuals with schizophrenia were found not to show the significant correlations observed in controls between thalamus and middle temporal gyrus [Mitelman et al.,2005a], whereas we reported decreased covariance between thalamus and middle temporal gyrus heterotopically and ipsilaterally and increased covariance between right thalamus and superior frontal gyrus ipsilaterally and heterotopically. As the thalamus is composed of several nuclei with afferent and efferent projections to different areas of the cortex, to further explore between‐group changes in structural covariance it would be important to investigate patterns of covariance between specific thalamic nuclei and other cortical areas.
During the preterm period the basal ganglia, a collection of subcortical nuclei comprising the caudate nucleus, putamen, globus pallidus, subthalamic nucleus, and substantia nigra, shows transient modular and proliferative activity, which decreases only after the 34th postconceptual week. Birth before this period may result in injury to the basal ganglia resulting in later neuromotor impairment [Krageloh‐Mann,2004]. In this study we found that the volume of the caudate nucleus showed increased ipsilateral covariance with gray matter volume in the cerebellum in VPT adolescents compared to controls, in the left cerebellar hemisphere, lobule VI, which is reportedly associated with cognition [Makris et al.,2003]. Caudate nucleus and cerebellum have been found to be structurally and functionally altered the following preterm birth [Allin et al.,2001; Nosarti et al.,2006,2008]. The cerebellum also showed decreased covariance with left superior frontal gyrus and left medial frontal gyrus. These alterations were all localised to three subdivisions of the cerebellar hemispheres (rather than vermis), all of which have also been associated with cognition, rather than motor or sensory function [Makris et al.,2003]. Given the likely role of the cerebellum in cognition, the altered patterns of covariance in fronto‐striatal‐cerebellar networks may underlie some of the cognitive deficits reported in VPT adolescents [Allin et al.,2001].
The findings of the current study need to be interpreted in the context of brains which are still developing. Normative data suggests that cortical gray matter volumes peak at ∼10 years of age in the frontal and temporal lobes, and ∼8 years of age in the parietal lobes and are subsequently followed by gray matter decreases [Giedd,2008]. Developmental trajectories of subcortical gray matter, such as the caudate nucleus, also follow an inverted U shape which is similar to that of the frontal and parietal gray matter, suggesting that brain regions which share widespread connections may also share similar developmental patterns [Giedd,2008]. Post‐adolescent studies of aging have demonstrated considerable variability in the regional volumes of the brain, including declines in gray matter volume in the parietal lobes, anterior cingulate cortex, frontal and middle temporal gyrus, caudate, cerebellum, as well as prefrontal white matter [Good et al.,2001]. Differential patterns of structural covariance in adolescence following very preterm birth could thus reflect differential regional rates of gray and white matter development [Allin et al.,2007; Ment et al.,2009]; although the extent of previously documented anatomical differences observed in this group suggests that gray matter development following very preterm birth may be both different and delayed [Nosarti et al.,2008].
To summarize, the present study demonstrated differential structural covariance between several regional gray matter volumes in VPT adolescents compared to controls. These findings can be interpreted as reflecting the effects of early perinatal lesions of both gray and white matter, which may interact with ongoing developmental processes and adversely affect adjacent as well as uninjured distant areas of the brain. In addition to potential alterations in the cytoarchitecture of the brain following very preterm birth, we speculate that neuroplastic compensatory mechanisms could contribute to explaining our findings. These structural differences in the “preterm brain” may result in differences in the localization of cognitive functions [Lawrence et al.,2010; Narberhaus et al.,2009; Nosarti et al.,2006,2008], and functional connectivity in the absence of behavioral differences [Schafer et al.,2009], as suggested by functional MRI data. The functional significance of differential structural covariance following very preterm birth has yet to be established, and may be an area of interest for future longitudinal studies.
REFERENCES
- Allin M, Matsumoto H, Santhouse AM, Nosarti C, AlAsady MH, Stewart AL, Rifkin L, Murray RM ( 2001): Cognitive and motor function and the size of the cerebellum in adolescents born very pre‐term. Brain 124: 60–66. [DOI] [PubMed] [Google Scholar]
- Allin M, Henderson M, Suckling J, Nosarti C, Rushe T, Fearon P, Stewart AL, Bullmore ET, Rifkin L, Murray R ( 2004): Effects of very low birthweight on brain structure in adulthood. Dev Med Child Neurol 46: 46–53. [DOI] [PubMed] [Google Scholar]
- Allin M, Nosarti C, Narberhaus A, Walshe M, Frearson S, Kalpakidou A, Wyatt J, Rifkin L, Murray R ( 2007): Growth of the corpus callosum in adolescents born preterm. Arch Pediatr Adolesc Med 161: 1183–1189. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ ( 2000): Voxel‐based morphometry—The methods. NeuroImage 11: 805–821. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Neelin P, Collins DL, Evans A, Friston K ( 1997): Incorporating prior knowledge into image registration. NeuroImage 6: 344–352. [DOI] [PubMed] [Google Scholar]
- Bloom JS, Hynd GW ( 2005): The role of the corpus callosum in interhemispheric transfer of information: Excitation or inhibition? Neuropsychol Rev 15: 59–71. [DOI] [PubMed] [Google Scholar]
- Cooke RW, Abernethy LJ ( 1999): Cranial magnetic resonance imaging and school performance in very low birth weight infants in adolescence. Arch Dis Child Fetal Neonatal Ed 81: F116–F121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J‐B, Heather JD, Frackowiak RSJ ( 1995): Spatial registration and normalisation of images. Hum Brain Mapp 2: 1–25. [Google Scholar]
- Giedd JN ( 2008): The teen brain: insights from neuroimaging. J Adolesc Health 42: 335–343. [DOI] [PubMed] [Google Scholar]
- Gimenez M, Junque C, Narberhaus A, Botet F, Bargallo N, Mercader JM ( 2006): Correlations of thalamic reductions with verbal fluency impairment in those born prematurely. Neuroreport 17: 463–466. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS ( 2001): A voxel‐based morphometric study of ageing in 465 normal adult human brains. NeuroImage 14: 21–36. [DOI] [PubMed] [Google Scholar]
- Gressens P, Richelme C, Kadhim HJ, Gadisseux JF, Evrard P ( 1992): The germinative zone produces the most cortical astrocytes after neuronal migration in the developing mammalian brain. Biol Neonate 61: 4–24. [DOI] [PubMed] [Google Scholar]
- Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ ( 2003): Defining the nature of the cerebral abnormalities in the premature infant: A qualitative magnetic resonance imaging study. J Pediatr 143: 171–179. [DOI] [PubMed] [Google Scholar]
- Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ ( 2005): Abnormal cerebral structure is present at term in premature infants. Pediatrics 115: 286–294. [DOI] [PubMed] [Google Scholar]
- Judas M, Rados M, Jovanov‐Milosevic N, Hrabac P, Stern‐Padovan R, Kostovic I ( 2005): Structural, immunocytochemical, and mr imaging properties of periventricular crossroads of growing cortical pathways in preterm infants. AJNR Am J Neuroradiol 26: 2671–2684. [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Ment LR, Vohr B, Pajot SK, Schneider KC, Katz KH, Ebbitt TB, Duncan CC, Makuch RW, Reiss AL ( 2004): Volumetric analysis of regional cerebral development in preterm children. Pediatr Neurol 31: 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Vohr B, Schneider KC, Katz KH, Makuch RW, Reiss AL, Ment LR ( 2006): Increased temporal lobe gyrification in preterm children. Neuropsychologia 44: 445–453. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Vasung L ( 2009): Insights from in vitro fetal magnetic resonance imaging of cerebral development. Semin Perinatol 33: 220–233. [DOI] [PubMed] [Google Scholar]
- Krageloh‐Mann I ( 2004): Imaging of early brain injury and cortical plasticity. Exp Neurol 190 ( Suppl 1): S84–S90. [DOI] [PubMed] [Google Scholar]
- Lawrence EJ, McGuire PK, Allin MP, Walshe M, Giampietro V, Murray RM, Rifkin L, Nosarti C ( 2010): The very preterm brain in young adulthood: The neural correlates of verbal paired associate learning. J Pediatr 156: 889–895. [DOI] [PubMed] [Google Scholar]
- Makris N, Hodge SM, Haselgrove C, Kennedy DN, Dale A, Fischl B, Rosen BR, Harris G, Caviness VS Jr, Schmahmann JD ( 2003): Human cerebellum: surface‐assisted cortical parcellation and volumetry with magnetic resonance imaging. J Cogn Neurosci 15: 584–599. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Friston KJ, Frackowiak RS, Price CJ ( 2005): Structural covariance in the human cortex. J Neurosci 25: 8303–8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment LR, Kesler S, Vohr B, Katz KH, Baumgartner H, Schneider KC, Delancy S, Silbereis J, Duncan CC, Constable RT, Makuch RW, Reiss AL ( 2009): Longitudinal brain volume changes in preterm and term control subjects during late childhood and adolescence. Pediatrics 123: 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman SA, Brickman AM, Shihabuddin L, Newmark R, Chu KW, Buchsbaum MS ( 2005a) Correlations between MRI‐assessed volumes of the thalamus and cortical Brodmann's areas in schizophrenia. Schizophr Res 75: 265–281. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Buchsbaum MS, Brickman AM, Shihabuddin L ( 2005b) Cortical intercorrelations of frontal area volumes in schizophrenia. NeuroImage 27: 753–770. [DOI] [PubMed] [Google Scholar]
- Modinos G, Vercammen A, Mechelli A, Knegtering H, McGuire PK, Aleman A ( 2009): Structural covariance in the hallucinating brain: A voxel‐based morphometry study. J Psychiatry Neurosci 34: 465–469. [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Skare S, Andersson JL, Lilja A, Flodmark O, Fernell E, Holmberg K, Bohm B, Forssberg H, Lagercrantz H, Klingberg T ( 2003): Preterm children have disturbances of white matter at 11 years of age as shown by diffusion tensor imaging. Pediatr Res 54: 672–679. [DOI] [PubMed] [Google Scholar]
- Narberhaus A, Lawrence E, Allin MP, Walshe M, McGuire P, Rifkin L, Murray R, Nosarti C ( 2009): Neural substrates of visual paired associates in young adults with a history of very preterm birth: Alterations in fronto‐parieto‐occipital networks and caudate nucleus. NeuroImage 47: 1884–1893. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Al Asady MH, Frangou S, Stewart AL, Rifkin L, Murray RM ( 2002): Adolescents who were born very preterm have decreased brain volumes. Brain 125: 1616–1623. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Rushe TM, Woodruff PW, Stewart AL, Rifkin L, Murray RM ( 2004): Corpus callosum size and very preterm birth: Relationship to neuropsychological outcome. Brain 127: 2080–2089. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Rubia K, Smith A, Frearson S, Williams SC, Rifkin L, Murray RM ( 2006): Altered functional neuroanatomy of response inhibition in adolescent males who were born very preterm. Dev Med Child Neurol 48: 265–271. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Giouroukou E, Healy E, Rifkin L, Walshe M, Reichenberg A, Chitnis X, Williams SC, Murray RM ( 2008): Gray and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain 131: 205–217. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Shergill SS, Allin MP, Walshe M, Rifkin L, Murray RM, McGuire PK ( 2009): Neural substrates of letter fluency processing in young adults who were born very preterm: Alterations in fronto‐occipital‐cerebellar networks. NeuroImage 47: 1904–1913. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Anderson AW, Ehrenkranz R, Staib LH, Tageldin M, Colson E, Gore JC, Duncan CC, Makuch R, Ment LR ( 2003): Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics 111: 939–948. [DOI] [PubMed] [Google Scholar]
- Price DJ, Kennedy H, Dehay C, Zhou L, Mercier M, Jossin Y, Goffinet AM, Tissir F, Blakey D, Molnar Z ( 2006): The development of cortical connections. Eur J Neurosci 23: 910–920. [DOI] [PubMed] [Google Scholar]
- Robinson S ( 2005): Systemic prenatal insults disrupt telencephalon development: implications for potential interventions. Epilepsy Behav 7: 345–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth SC, Baudin J, Pezzani‐Goldsmith M, Townsend J, Reynolds EO, Stewart AL ( 1994): Relation between neurodevelopmental status of very preterm infants at one and eight years. Dev Med Child Neurol 36: 1049–1062. [DOI] [PubMed] [Google Scholar]
- Schafer RJ, Lacadie C, Vohr B, Kesler SR, Katz KH, Schneider KC, Pugh KR, Makuch RW, Reiss AL, Constable RT, Ment LR ( 2009): Alterations in functional connectivity for language in prematurely born adolescents. Brain 132: 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J, Takizawa B, McGee A, Stewart WB, Zhang H, Ment L, Schwartz M, Strittmatter S ( 2004): Neonatal hypoxia suppresses oligodendrocyte Nogo‐A and increases axonal sprouting in a rodent model for human prematurity. Exp Neurol 189: 141–149. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Edgin JO, Thompson D, Inder TE ( 2005): Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain 128: 2578–2587. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE ( 2006): Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 355: 685–694. [DOI] [PubMed] [Google Scholar]


