Abstract
Cerebral 18F‐deoxyglucose positron emission tomography (FDG‐PET) has shown altered auditory pathway activity in tinnitus. However, the corresponding studies involved only small samples and analyses were restricted to the auditory cortex in most studies. Evidence is growing that also limbic, frontal, and parietal areas are involved in the pathophysiology of chronic tinnitus. These regions are considered to mediate perceptual, attentional, and emotional processes. Thus, the aim of the present study was the systematic evaluation of metabolic brain activity in a large sample of tinnitus patients. Ninety one patients with chronic tinnitus underwent FDG‐PET. The effects of tinnitus severity (assessed by a tinnitus questionnaire score), duration and laterality were evaluated with statistical parametric mapping (SPM) in whole brain analyses. In addition, region of interest analyses were performed for primary auditory areas. Tinnitus duration correlated positively with brain metabolism in right inferior frontal, right ventro‐medial prefrontal, and right posterior cingulate cortex. Tinnitus distress correlated positively with activation of left and right posterior inferior temporal gyrus as well as left and right posterior parahippocampal–hippocampal interface. Region of interest analysis demonstrated an overactivation of left in contrast to right Heschl's gyrus independently from tinnitus laterality and anatomical hemispheric differences. Tinnitus duration and distress were associated with areas involved in attentional and emotional processing. This is in line with recent findings indicating the relevance of higher order areas in the pathophysiology of tinnitus. Earlier results of asymmetric activation of the auditory cortices in tinnitus were confirmed, i.e., left‐sided overactivation was found independently from tinnitus laterality. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: tinnitus, positron emission tomography, auditory cortex, tinnitus network
INTRODUCTION
Tinnitus constitutes the perception of sound in the absence of an external auditory stimulus and is experienced by 5–15% of the general population. The manifestation of tinnitus can vary, ranging from intermittent tinnitus perception with little impact on daily life to a very bothersome tinnitus preventing the ability to do intellectual work and leading to social isolation. It is estimated that the condition severely affects quality of life in 1% of the population [Axelsson and Ringdahl, 1989; Khedr et al., 2010b]. In these patients tinnitus is often accompanied by other symptoms such as anxiety, depression, irritability, agitation, stress, depression, and/or insomnia [Crönlein et al., 2007; Langguth et al., 2007]. In the light of this symptomatology of high diversity, heterogeneous pathophysiological mechanisms within tinnitus patients are to be expected.
Functional and structural imaging techniques have been applied to identify brain structures involved in tinnitus [for reviews see Adjamian et al., 2009; Lanting et al., 2009]. On the cortical level tinnitus is accompanied by an overactivation of auditory cortical areas as revealed by functional magnetic resonance imaging [fMRI; Smits et al., 2007], single photon emission computed tomography [SPECT; Gardner et al., 2002; Marcondes et al., 2006], and positron emission tomography [PET; Arnold et al., 1996; Langguth et al., 2006; Plewnia et al., 2007]. In addition to the altered activity in auditory pathways, imaging studies also revealed an involvement of nonauditory brain areas. Functional imaging demonstrated alterations in prefrontal and parietal areas which seem to be involved in conscious perception and attention allocation, and also alterations in limbic areas which seem to be involved in emotional processes [for an review see Adjamian et al., 2009]. Structural abnormalities were detected in the subgenual anterior cingulate cortex [Leaver et al., 2011; Mühlau et al., 2006], hippocampus [Landgrebe et al., 2009], and auditory cortex [Husain et al., 2011; Schneider et al., 2009]. Furthermore, magnetoencephalography (MEG) indicated that tinnitus is related to abnormal neuronal connectivity in a larger network involving auditory and nonauditory areas [Schlee et al., 2008, 2009a, b].
It remains speculative to which extent the observed changes in nonauditory areas are genuinely related to tinnitus perception or to which extent they might only reflect the impact of tinnitus distress, since in most studies patient groups were not differentiated according to the amount of distress. Moreover, most investigated samples were rather small and not stratified according to clinical characteristics [Adjamian et al., 2009]. Up to now, only one imaging study specifically addressed the neuronal correlates of tinnitus‐related distress. In this study EEG source localization was used to compare tinnitus patients with low and high levels of distress, respectively. The results suggest that tinnitus distress was related to increased synchronized α‐activity in the subcallosal anterior cingulate cortex, insula, parahippocampal areas, and the amygdala [Vanneste et al., 2010]. An MEG study explored the changes of neuronal network activity occurring with increasing tinnitus duration [Schlee et al., 2009a]. This analysis showed that tinnitus with a duration of more than 4 years is related to a more widespread and distributed network activity as compared to shorter lasting tinnitus.
Here, we conducted an 18F‐deoxyglucose (FDG) PET study with a large sample size. We aimed to investigate the neuronal activation patterns associated with specific clinical tinnitus characteristics and to replicate previous findings of asymmetric auditory cortex activation in tinnitus patients [Arnold et al., 1996; Langguth et al., 2006].
First, we examined correlations of tinnitus duration, laterality, and distress with brain metabolism in whole brain analyses. We expected correlations of tinnitus duration and distress in auditory cortex as well as in frontal, parietal, and limbic areas processing conscious perception, attention allocation, and emotional processes related to tinnitus. With respect to laterality we had no particular hypotheses except the assumption that the overactivation of left auditory areas occurs independently from perceived tinnitus laterality. This assumption was based on previous FDG‐PET studies [Arnold et al., 1996; Langguth et al., 2006] and tested in the present large sample by region of interest (ROI) analyses.
MATERIALS AND METHODS
Sample
We included 91 patients [65 males; 49 ± 12 (20–65) years] with chronic tinnitus [duration 85 ± 102 (6–420) months]. Thirty patients reported a predominantly left‐sided, 23 a predominantly right‐sided tinnitus, and 38 patients described their tinnitus as bilateral or originating within the head. Tinnitus distress was assessed by the German version [Goebel and Hiller, 1994] of the Tinnitus questionnaire [TQ; Hallam et al., 1988] at the day of PET measurement. TQ overall scores ranged from 3 to 78 (40 ± 17) indicating a large variety in tinnitus distress.
Patients suffering from Meniere's disease, presenting conductive hearing loss or displaying hints of objective tinnitus were not included. Eighty‐three patients underwent a complete otologic and audiologic examination including pure tone audiometry, tympanometry, stapedius reflex tests, and otoscopy. As patients were measured in the context of clinical trials investigating PET‐guided neuronavigated repetitive transcranial magnetic stimulation [rTMS; Kleinjung et al., 2005; Langguth et al., in preparation] only patients were included that were eligible for rTMS treatment. Thus, patients with cardiac pacemakers, history of seizures, suspected diagnosis of organic brain damage or any other severe somatic, neurologic, or psychiatric diagnosis were not included. The study was approved by the Ethics Committee at the University of Regensburg. All procedures involved were conducted in accordance with the last revision of the Declaration of Helsinki. All participants gave written informed consent after a comprehensive explanation of the procedures.
Measurement and Analysis
Neuroimaging data were assessed by 18F‐deoxyglucose (FDG)‐PET measurements. All patients rested in a supine position with closed eyes in a quiet, dimly lit room. External acoustic stimulation was eliminated by plugging both ears hermetically. After 10–20 min resting PET neuroimaging was performed for 15 min, beginning 25–60 min after the injection of 112‐265 MBq FDG. An ECAT EXACT 47 PET Scanner (Siemens, Germany) was used for this study. The imaging protocol employed a 3D acquisition of three frames (5 minutes/frame). Image reconstruction was done on the frame plane sum by filtered back projection (Shepp filter with axial filtering and scatter correction, matrix size 256 × 256 pixels, zoom 2.5) after automated attenuation correction.
Preprocessing and statistical analysis (one scan per subject) were done with statistical parametric mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm/). After normalization to the SPM PET template, data were smoothed with a 12‐mm full‐width at half maximum (FHWM) kernel. For the first issue of the study (“tinnitus characteristics”) we used multiple regression designs to associate tinnitus duration, laterality, and distress with cerebral glucose utilization in separate design matrices. We used a relative threshold masking of 0.8 and an implicit mask (exclusion of voxels with zero values in any of the subjects), proportional scaling, and for global normalization a grand mean scaled value of 50 ml/dl/min. Every statistical design was done without and also including the covariates age, gender, area under the audiogram curve [area between 0 dB hearing level (HL) and audiogram curve] of the left and right ear. Nearly 77 of the 91 patients were included in the covariance analyses as in 8 patients no audiology data were available and 6 additional patients had to be excluded due to severe hearing loss (hearing loss above 35 dB in every frequency of a standard audiogram in at least one ear). As covariates can produce or extinguish statistical effects [Miller and Chapman, 2001] we present only voxels that were significant in both analyses. This was done by masking the contrast of one analysis with the contrast of the other analysis. A statistical threshold of 0.001 uncorrected for multiple comparisons was used. Localization of significant clusters was indicated by the MNI (Montreal Neurological Institute) coordinates of the peak voxel. Anatomical labeling of significant clusters was done by means of the WFU_PickAtlas [Maldjian et al., 2003], Anatomy toolbox [Eickhoff et al., 2007], and anatomical automatic labeling [AAL; Tzourio‐Mazoyer et al., 2002]. Correlation coefficients of significant clusters were calculated with SPSS 18.0.0 (SPSS, USA) after extracting data based on the above mentioned design matrix from significant clusters with the SPM toolbox MarsBar (http://marsbar.sourceforge.net/). T values of significant associations are related to the peak voxels of the cluster as obtained by SPM and r values are related to the whole cluster as obtained by SPSS. Cluster sizes are indicated by k E.
For the second issue (region of interest “primary auditory cortex,” anatomically defined), we extracted the mean metabolic activation for the left and right Heschl's gyrus (primary auditory cortex) with the SPM toolbox MarsBar (http://marsbar.sourceforge.net/) according to AAL using the same design matrix specification as for issue 1 (relative threshold masking of 0.8, implicit mask, proportional scaling, and grand mean scaled value of 50 ml dl−1 min−1). Data were further analyzed with SPSS 18.0.0 (SPSS, USA). We calculated an analysis of variance (ANOVA) with the repeated measures factor side (activation in left and right Heschl's gyrus) and the between subjects factor tinnitus laterality (left, both, right) and focused on the side main effect and the side by laterality interaction effect. In accordance to the analysis procedure of issue 1 of this study we repeated the ANOVA with covariates. To counter the argument that side effects are due to differences in the extent of the auditory cortices [Ide et al., 1999] we repeated the same analysis for spherical regions of interest covering the left and right Heschl's gyrus (radius of 20 mm around x = ±45; y = −15; z = 9 MNI coordinates) generated with the WFU_PickAtlas (http://www.nitrc.org/projects/wfu_pickatlas/).
RESULTS
Tinnitus Characteristics
Significant positive correlations of glucose metabolism with tinnitus duration were found in the right inferior frontal cortex (MNI: k E = 68; x = 44, y = 42, z = 10; T = 3.7; r = 0.350), in the border region of the right ventro‐medial prefrontal cortex and the temporal pole rather accentuated in the frontal cortex (MNI: k E = 170; x = 24, y = 16, z = −28; T = 4.1; r = 0.436), and right posterior cingulate cortex (MNI: k E = 41; x = 12, y = −44, z = 34; T = 3.9; r = 0.414; Fig. 1A and Fig. 2). Tinnitus distress as assessed by the tinnitus questionnaire score was positively correlated with activity in the left and right posterior inferior temporal gyrus (MNI: k E = 150; x = −56, y = −56, z = −22; T = 4.0; r = 0.415; k E = 72; x = 60, y = −54, z = −26; T = 4.0; r = 0.433) as well as left and right posterior parahippocampal–hippocampal interface (MNI: k E = 67; x = −24, y = −24, z = −10; T = 3.6; r = 0.434; k E = 129; x = 40, y = −20, z = −6; T = 3.5; r = 0.430; Fig. 1B and Fig. 2). Negative correlations of tinnitus duration and distress, and differences between groups with left, right, and nonlateralized tinnitus were not significantly associated with brain metabolism. Neither tinnitus duration, nor tinnitus lateralization, nor tinnitus distress were significantly associated with activity in auditory areas in the whole‐brain analysis.
Figure 1.
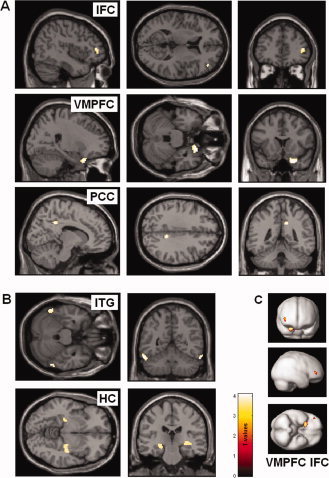
Brain areas with positive correlations of glucose metabolism with tinnitus duration (A, C) and tinnitus distress (B) on an uncorrected significance threshold of 0.001 masked with the contrast of the same design matrix additionally including the covariates age, gender, and audiometric data. MNI coordinates indicate the peak of significant effects. (A, C) Significant associations for tinnitus duration were found in right inferior frontal cortex (IFC), (MNI: x = 44, y = 42, z = 10; T = 3.7; r = 0.350), right ventro‐medial prefrontal cortex (VMPFC), (MNI: x = 24, y = 16, z = −28; T = 4.1; r = 0.436), and right posterior cingulate cortex (PCC), (MNI: x = 12, y = −44, z = 34; T = 3.9; r = 0.414). (B) Significant associations for tinnitus distress as measured with tinnitus questionnaire were found in left and right posterior inferior temporal gyrus (ITG), (MNI: x = −56, y = −56, z = −22; T = 4.0; r = 0.415; x = 60, y = −54, z = −26; T = 4.0; r = 0.433) and left and right posterior parahippocampal–hippocampal interface (HC), (MNI: x = −24, y = −24, z = −10; T = 3.6; r = 0.434; x = 40, y = −20, z = −6; T = 3.5; r = 0.430).
Figure 2.
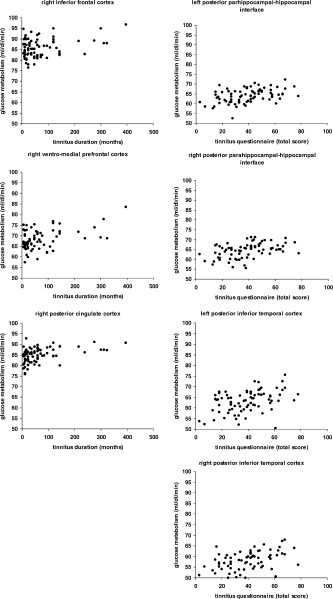
Scatter plots of significant correlations of the mean cluster glucose metabolism with tinnitus duration and tinnitus distress on an uncorrected significance threshold of 0.001 masked with the contrast of the same design matrix additionally including the covariates age, gender, and audiometric data.
Primary Auditory Cortex
A 2 × 3 ANOVA showed a significant main effect of hemisphere (F = 13.410; df = 1.88; P < 0.001) but no significant interaction effect between hemisphere and tinnitus laterality (F = 2.577; df = 2.88; P = 0.082). ANCOVA with age, gender, and audiogram data as covariates indicated again a significant effect of hemisphere (F = 11.362; df = 1.70; P = 0.001) and no significant interaction effect (F = 0.773; df = 2.70; P = 0.465). Analyses with spherical region of interests showed the same results. In summary, metabolism was higher in left in contrast to right auditory cortex independently from tinnitus laterality not influenced by anatomical asymmetry in auditory areas.
DISCUSSION
Steady state FDG‐PET measurements in 91 patients with subjective tinnitus indicated an association of tinnitus duration with activity in the right inferior frontal, right ventro‐medial prefrontal, and right posterior cingulate cortex and an association of tinnitus distress with brain metabolism in the posterior parahippocampal–hippocampal interface and posterior inferior temporal gyrus in both hemispheres.
Tinnitus Duration
The longer the tinnitus had been present the more brain metabolism was found in right inferior frontal (IFC), right ventro‐medial prefrontal (VMPFC), and right posterior cingulate cortex (Fig. 1A and Fig. 2). These results are in line with recent MEG studies investigating long range functional connectivity in tinnitus patients. Schlee et al. described a fronto‐parietal network accented in the right hemisphere [Schlee et al., 2008] with increasing relevance of frontal and parietal areas in the course of prolonged tinnitus duration [Schlee et al., 2009a, b]. However, it should be considered that functional connectivity and activity are not necessarily associated, i.e., increases in connectivity are not necessarily accompanied by increases in activity.
An important role for the prefrontal cortex in tinnitus pathophysiology was already postulated long ago in theoretical frameworks [Jastreboff, 1990]. Neuroimaging studies confirmed prefrontal cortex involvement [for a review see Adjamian et al., 2009; Lanting et al., 2009] without illustrating the role of the distinct prefrontal areas. However, a recent framework paper points out the relevance of the VMPFC. The VMPFC is closely connected to limbic areas [Öngür and Price, 2000] and involved in the autonomic response to emotional music [Johnsen et al., 2009]. Lesions of the VMPFC result in failure to extinguish aversive reactions associated with an auditory stimulus [Quirk et al., 2000]. Structural differences in this area have been identified in tinnitus patients by voxel‐based morphometry and it has been suggested that this finding might reflect reduced function of the VMPFC resulting in inefficient inhibitory control of the tinnitus signal on thalamic level [Leaver et al., 2011; Mühlau et al., 2006]. In this context the increase of metabolic activity in the VMPFC with increasing tinnitus duration may reflect the unsuccessful attempt to extinguish the tinnitus signal.
Unlike VMPFC, IFC was not identified as a tinnitus‐related brain area in previous neuroimaging studies. However, an activation of the inferior frontal cortex has been reported during verbal auditory hallucinations in schizophrenic patients [Kühn and Gallinat, 2010]. The right IFC constitutes a network involved in the detection of behaviorally relevant stimuli together with the temporo‐parietal cortex [Corbetta and Shulman, 2002]. Furthermore, the right IFC serves as the core region of response inhibition [Aron et al., 2004] and IFC activity might mirror the attempt to control the bottom‐up attention allocation to the tinnitus percept in a top‐down manner. The posterior cingulate cortex (PCC) is part of a self‐referential network involved in conscious perception and stimulus evaluation [Vogt and Laureys, 2005] and has already been shown to be involved in tinnitus [Schlee et al., 2009a, b] and tinnitus distress [Vanneste et al., 2010].
Summarizing our findings, patients suffering from chronic tinnitus showed higher brain metabolism in frontal regions and PCC, which is probably reflecting evaluation of and attention allocation to the tinnitus percept as well as an attempt of top‐down control of the tinnitus signal. Confirmatively, we found this association only for areas in the right hemisphere, which constitutes the dominant hemisphere for selective attention [Schlee et al., 2009a, b; Shulman et al., 2010].
Tinnitus Distress
Tinnitus distress has been described to be related to increased glucose metabolism in bilateral posterior parahippocampal–hippocampal interface. The vital involvement of hippocampal areas in tinnitus was documented by the transient tinnitus diminution after suppression of the amygdalo‐hippocampal complex by amobarbital [De Ridder et al., 2006]. Hippocampal deficits have been described in animal models of tinnitus [Goble et al., 2009; Kraus et al., 2010], and structural imaging in tinnitus patients has demonstrated a decrease in grey matter in the hippocampus [Landgrebe et al., 2009]. EEG studies suggest the involvement of the parahippocampal area as well. Tinnitus patients differed from health controls by increased delta and theta activity in the parahippocampal gyrus [Moazami‐Goudarzi et al., 2010]. Moreover, highly distressed tinnitus patients showed increased alpha activity in a larger network including the parahippocampal gyrus compared to patients with lower distress levels. The parahippocampal area has been hypothesized to play a central role in memory recollection, sending information from the hippocampus to the association areas [Diederen et al., 2010]. A dysfunction in this mechanism has been posited as an explanation for complex auditory phantom percepts such as auditory hallucinations [Diederen et al., 2010]. The converging findings of hippocampal and parahippocampal involvement in tinnitus and tinnitus distress suggest a similar mechanism for tinnitus. One may speculate that tinnitus distress results from a continuous learning process, where—in the absence of an external input—the phantom percept is reinforced and the connection with aversive emotional associations is updated continuously.
In addition to metabolism in posterior parahippocampal–hippocampal interface, increased activity in the left and right posterior inferior temporal gyrus was also associated with higher tinnitus distress. The left and the right inferior temporal gyrus are involved in sensory memory and serve as multisensory integration areas. A correlation between increased activity in these areas and tinnitus severity has already been documented in two previous neuroimaging studies [Marcondes et al., 2006; Plewnia et al., 2007]. As tinnitus distress is associated with concomitant increased glucose metabolism in hippocampal areas and posterior inferior temporal cortex the involvement of the posterior inferior temporal gyri fits well with the above mentioned mechanism of maladaptive memory consolidation. Recently, interplay of the hippocampus and the posterior inferior temporal cortex could be shown in an experiment investigating memory consolidation during acute stress [Henckens et al., 2009].
Alternatively the increased glucose metabolism in these areas could reflect unsuccessful attempts to integrate the phantom sound in a multisensory experience. Failure of multisensory integration has been recently proposed as a potential mechanism involved in the generation and maintenance of aversive phantom perceptions [De Ridder et al., 2011]. In this sense, the auditory percept of tinnitus without any visual percept of a sound source may represent a multisensory conflict.
We did not find any differences in brain activation when patients with left sided, right sided, and bilateral tinnitus were compared. Irrespectively of tinnitus laterality, ROI analyses of the primary auditory cortex showed an overactivation of the left in contrast to the right auditory cortex. In order to account for the larger volume of the left Heschl's gyrus as a potential confounding factor (i.e., larger Heschl's gyrus = higher brain metabolism) we conducted two analyses, one with equal sized spheres and one based on an anatomical atlas. Both analyses confirmed the left‐lateralization in tinnitus. Thus, our study is in line with previous findings of increased left sided metabolic activity of the auditory cortex [Arnold et al., 1996; Langguth et al., 2006; Wang et al., 2001], whereas other methods suggest that abnormalities of neuronal activity predominantly occur contra‐laterally to the perceived tinnitus side [De Ridder, 2010; Khedr et al., 2010a; Weisz et al., 2007]. This discrepancy may be mainly due to the fact that the different brain imaging methods identify different aspects of neuronal activation. The goal of this study was to detect alterations of metabolic brain activity which are related to specific tinnitus characteristics. Therefore only tinnitus patients were investigated and no control group without tinnitus. For this reason we cannot draw any conclusions from our data on metabolic alterations related to tinnitus per se. For example we cannot preclude the possibility that the observed left‐lateralization of auditory cortex activation is unspecific and not related to tinnitus at all.
Furthermore it remains to be elucidated by interventional studies whether the observed correlations reflect causal relations or pure epiphenomena. Moreover, we analyzed only the effects of selected clinical characteristics. Other clinically relevant factors such as tinnitus loudness or hyperacousis [Gu et al., 2010] may influence metabolic brain activity as well.
The effects detected in the present study were rather moderate (liberal significance threshold, big sample size, medium correlation coefficients). However, they were in accordance with the tinnitus literature [Gu et al., 2010]. Nevertheless, the moderate effects suggest that steady state PET measurements are not very sensitive for highlighting the role of cortical networks in tinnitus in more detail. Radiation exposure due to the application of radioactive tracers is a further limiting factor for the use of PET both in diagnostic and scientific applications. Preferable alternative methods could be soundless tools such as EEG/MEG and perhaps near‐infrared spectroscopy [Fallgatter et al., 2004; Obrig and Villringer, 2003], an optical approach to measure blood flow changes in cortical areas, as soundless tools. Compared to fMRI, which bears other handicaps (scanner noise) in the investigation of tinnitus patients [Eichhammer et al., 2007], all these methods are limited in spatial resolution.
The FDG‐PET scans analyzed in this study were performed for identifying the target for rTMS treatment. Whether FDG‐PET is useful in this context has to be elucidated by further investigations. Recent preliminary data suggest that steady state FDG‐PET is not best suited for target identification [Mennemeier et al., 2011] and has no advantages over coil positioning based on anatomical landmarks [Langguth et al., in preparation].
CONCLUSION
In conclusion, we could confirm that tinnitus duration and distress are related to activation of brain areas involved in attentional, emotional, and memory processes. In contrast, tinnitus characteristics were not associated with activity in auditory areas. These findings are in line with recent suggestions of involvement of higher order areas in generation and maintenance of severe tinnitus.
REFERENCES
- Adjamian P, Sereda M, Hall DA ( 2009): The mechanisms of tinnitus: Perspectives from human functional neuroimaging. Hear Res 253: 15–31. [DOI] [PubMed] [Google Scholar]
- Arnold W, Bartenstein P, Oestreicher E, Romer W, Schwaiger M ( 1996): Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: A PET study with [18F]deoxyglucose. ORL J Otorhinolaryngol Relat Spec 58: 195–199. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA ( 2004): Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8: 170–177. [DOI] [PubMed] [Google Scholar]
- Axelsson A, Ringdahl A ( 1989): Tinnitus—A study of its prevalence and characteristics. Br J Audiol 23: 53–62. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Crönlein T, Langguth B, Geisler P, Hajak G ( 2007): Tinnitus and insomnia. Prog Brain Res 166: 227–233. [DOI] [PubMed] [Google Scholar]
- De Ridder D ( 2010): Should rTMS for tinnitus be performed left‐sided, ipsilaterally or contralaterally, and is it a treatment or merely investigational? Eur J Neurol 17: 891–892. [DOI] [PubMed] [Google Scholar]
- De Ridder D, Elgoyhen AB, Romo R, Langguth B ( 2011): Phantom percepts: Tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci USA 108: 8075–8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D, Fransen H, Francois O, Sunaert S, Kovacs S, Van De Heyning P ( 2006): Amygdalohippocampal involvement in tinnitus and auditory memory. Acta Otolaryngol Suppl 556: 50–53. [DOI] [PubMed] [Google Scholar]
- Diederen KM, Neggers SF, Daalman K, Blom JD, Goekoop R, Kahn RS, Sommer IE ( 2010): Deactivation of the parahippocampal gyrus preceding auditory hallucinations in schizophrenia. Am J Psychiatry 167: 427–435. [DOI] [PubMed] [Google Scholar]
- Eichhammer P, Hajak G, Kleinjung T, Landgrebe M, Langguth B ( 2007): Functional imaging of chronic tinnitus: The use of positron emission tomography. Prog Brain Res 166: 83–88. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K ( 2007): Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 36: 511–521. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Ehlis A, Wagener A, Michel T, Herrmann MJ ( 2004): [Near‐infrared spectroscopy in psychiatry]. Nervenarzt 75: 911–916. [DOI] [PubMed] [Google Scholar]
- Gardner A, Pagani M, Jacobsson H, Lindberg G, Larsson SA, Wagner A, Hallstrom T ( 2002): Differences in resting state regional cerebral blood flow assessed with 99mTc‐HMPAO SPECT and brain atlas matching between depressed patients with and without tinnitus. Nucl Med Commun 23: 429–439. [DOI] [PubMed] [Google Scholar]
- Goble TJ, Moller AR, Thompson LT ( 2009): Acute high‐intensity sound exposure alters responses of place cells in hippocampus. Hear Res 253: 52–59. [DOI] [PubMed] [Google Scholar]
- Goebel G, Hiller W ( 1994): [The tinnitus questionnaire. A standard instrument for grading the degree of tinnitus results of a multicenter study with the tinnitus questionnaire] HNO 42: 166–172. [PubMed] [Google Scholar]
- Gu JW, Halpin CF, Nam EC, Levine RA, Melcher JR ( 2010): Tinnitus, diminished sound‐level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J Neurophysiol 104: 3361–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam RS, Jakes SC, Hinchcliffe R ( 1988): Cognitive variables in tinnitus annoyance. Br J Clin Psychol 27 ( Part 3): 213–222. [DOI] [PubMed] [Google Scholar]
- Henckens MJ, Hermans EJ, Pu Z, Joels M, Fernandez G ( 2009): Stressed memories: How acute stress affects memory formation in humans. J Neurosci 29: 10111–10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain FT, Medina RE, Davis CW, Szymko‐Bennett Y, Simonyan K, Pajor NM, Horwitz B ( 2011): Neuroanatomical changes due to hearing loss and chronic tinnitus: A combined VBM and DTI study. Brain Res 1369: 74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide A, Dolezal C, Fernandez M, Labbe E, Mandujano R, Montes S, Segura P, Verschae G, Yarmuch P, Aboitiz F ( 1999): Hemispheric differences in variability of fissural patterns in parasylvian and cingulate regions of human brains. J Comp Neurol 410: 235–242. [PubMed] [Google Scholar]
- Jastreboff PJ ( 1990): Phantom auditory perception (tinnitus): Mechanisms of generation and perception. Neurosci Res 8: 221–254. [DOI] [PubMed] [Google Scholar]
- Johnsen EL, Tranel D, Lutgendorf S, Adolphs R ( 2009): A neuroanatomical dissociation for emotion induced by music. Int J Psychophysiol 72: 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, Abo‐Elfetoh N, Rothwell JC, El‐Atar A, Sayed E, Khalifa H ( 2010a): Contralateral versus ipsilateral rTMS of temporoparietal cortex for the treatment of chronic unilateral tinnitus: Comparative study. Eur J Neurol 17: 976–983. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Shawky OA, Mohamed ES, El Attar GS, Mohammad KA ( 2010b): Epidemiological study of chronic tinnitus in Assiut, Egypt. Neuroepidemiology 35: 45–52. [DOI] [PubMed] [Google Scholar]
- Kleinjung T, Eichhammer P, Langguth B, Jacob P, Marienhagen J, Hajak G, Wolf SR, Strutz J ( 2005): Long‐term effects of repetitive transcranial magnetic stimulation (rTMS) in patients with chronic tinnitus. Otolaryngol Head Neck Surg 132: 566–569. [DOI] [PubMed] [Google Scholar]
- Kraus KS, Mitra S, Jimenez Z, Hinduja S, Ding D, Jiang H, Gray L, Lobarinas E, Sun W, Salvi RJ ( 2010): Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience 167: 1216–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Gallinat J ( 2010): Quantitative meta‐analysis on state and trait aspects of auditory verbal hallucinations in schizophrenia. Schizophr Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgrebe M, Langguth B, Rosengarth K, Braun S, Koch A, Kleinjung T, May A, de Ridder D, Hajak G ( 2009): Structural brain changes in tinnitus: Grey matter decrease in auditory and non‐auditory brain areas. Neuroimage 46: 213–218. [DOI] [PubMed] [Google Scholar]
- Langguth B, Eichhammer P, Kreutzer A, Maenner P, Marienhagen J, Kleinjung T, Sand P, Hajak G ( 2006): The impact of auditory cortex activity on characterizing and treating patients with chronic tinnitus—First results from a PET study. Acta Otolaryngol Suppl 556: 84–88. [DOI] [PubMed] [Google Scholar]
- Langguth B, Kleinjung T, Fischer B, Hajak G, Eichhammer P, Sand PG ( 2007): Tinnitus severity, depression, and the big five personality traits. Prog Brain Res 166: 221–225. [DOI] [PubMed] [Google Scholar]
- Lanting CP, de Kleine E, van Dijk P ( 2009): Neural activity underlying tinnitus generation: Results from PET and fMRI. Hear Res 255: 1–13. [DOI] [PubMed] [Google Scholar]
- Leaver AM, Renier L, Chevillet MA, Morgan S, Kim HJ, Rauschecker JP ( 2011): Dysregulation of limbic and auditory networks in tinnitus. Neuron 69: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH ( 2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Marcondes R, Fregni F, Pascual‐Leone A ( 2006): Tinnitus and brain activation: Insights from transcranial magnetic stimulation. Ear Nose Throat J 85: 233–234, 236–238. [PubMed] [Google Scholar]
- Mennemeier M, Chelette KC, Allen S, Bartel TB, Triggs W, Kimbrell T, Crew J, Munn T, Brown GJ, Dornhoffer J ( 2011): Variable changes in PET activity before and after rTMS treatment for tinnitus. Laryngoscope 121: 815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Chapman JP ( 2001): Misunderstanding analysis of covariance. J Abnorm Psychol 110: 40–48. [DOI] [PubMed] [Google Scholar]
- Moazami‐Goudarzi M, Michels L, Weisz N, Jeanmonod D ( 2010): Temporo‐insular enhancement of EEG low and high frequencies in patients with chronic tinnitus. QEEG study of chronic tinnitus patients. BMC Neurosci 11: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlau M, Rauschecker JP, Oestreicher E, Gaser C, Rottinger M, Wohlschlager AM, Simon F, Etgen T, Conrad B, Sander D ( 2006): Structural brain changes in tinnitus. Cereb Cortex 16: 1283–1288. [DOI] [PubMed] [Google Scholar]
- Obrig H, Villringer A ( 2003): Beyond the visible—Imaging the human brain with light. J Cereb Blood Flow Metab 23: 1–18. [DOI] [PubMed] [Google Scholar]
- Öngür D, Price JL ( 2000): The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10: 206–219. [DOI] [PubMed] [Google Scholar]
- Plewnia C, Reimold M, Najib A, Brehm B, Reischl G, Plontke SK, Gerloff C ( 2007): Dose‐dependent attenuation of auditory phantom perception (tinnitus) by PET‐guided repetitive transcranial magnetic stimulation. Hum Brain Mapp 28: 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K ( 2000): The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci 20: 6225–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee W, Weisz N, Bertrand O, Hartmann T, Elbert T ( 2008): Using auditory steady state responses to outline the functional connectivity in the tinnitus brain. PLoS One 3: e3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee W, Hartmann T, Langguth B, Weisz N ( 2009a): Abnormal resting‐state cortical coupling in chronic tinnitus. BMC Neurosci 10: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee W, Mueller N, Hartmann T, Keil J, Lorenz I, Weisz N ( 2009b): Mapping cortical hubs in tinnitus. BMC Biol 7: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Andermann M, Wengenroth M, Goebel R, Flor H, Rupp A, Diesch E ( 2009): Reduced volume of Heschl's gyrus in tinnitus. Neuroimage 45: 927–939. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Pope DL, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M ( 2010): Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci 30: 3640–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits M, Kovacs S, de Ridder D, Peeters RR, van Hecke P, Sunaert S ( 2007): Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus. Neuroradiology 49: 669–679. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Vanneste S, Plazier M, der Loo E, de Heyning PV, Congedo M, De Ridder D ( 2010): The neural correlates of tinnitus‐related distress. Neuroimage 52: 470–480. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Laureys S ( 2005): Posterior cingulate, precuneal and retrosplenial cortices: Cytology and components of the neural network correlates of consciousness. Prog Brain Res 150: 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tian J, Yin D, Jiang S, Yang W, Han D, Yao S, Shao M ( 2001): Regional glucose metabolic increases in left auditory cortex in tinnitus patients: A preliminary study with positron emission tomography. Chin Med J (Engl) 114: 848–851. [PubMed] [Google Scholar]
- Weisz N, Dohrmann K, Elbert T ( 2007): The relevance of spontaneous activity for the coding of the tinnitus sensation. Prog Brain Res 166: 61–70. [DOI] [PubMed] [Google Scholar]


