Abstract
Recent research suggests that the inference of others' intentions from their observed actions is supported by two neural systems that perform complementary roles. The human putative mirror neuron system (pMNS) is thought to support automatic motor simulations of observed actions, with increased activity for previously experienced actions, whereas the mentalizing system provides reflective, non‐intuitive reasoning of others' perspectives, particularly in the absence of prior experience. In the current fMRI study, we show how motor familiarity with an action and perceptual familiarity with the race of an actor uniquely modulate these two systems. Chinese participants were asked to infer the intentions of actors performing symbolic gestures, an important form of non‐verbal communication that has been shown to activate both mentalizing and mirror neuron regions. Stimuli were manipulated along two dimensions: (1) actor's race (Caucasian vs. Chinese actors) and (2) participants' level of experience with the gestures (familiar or unfamiliar). We found that observing all gestures compared to observing still images was associated with increased activity in key regions of both the pMNS and mentalizing systems. In addition, observations of one's same race generated greater activity in the posterior pMNS‐related regions and the insula than observations of a different race. Surprisingly, however, familiar gestures more strongly activated regions associated with mentalizing, while unfamiliar gestures more strongly activated the posterior region of the pMNS, a finding that is contrary to prior literature and demonstrates the powerful modulatory effects of both motor and perceptual familiarity on pMNS and mentalizing regions when asked to infer the intentions of intransitive gestures. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: mirror neuron system, mentalizing, familiarity, race, gestures, fMRI
INTRODUCTION
How do we efficiently infer other's intentions by observing their actions? Recent research indicates that intention understanding engages two complementary systems: the putative human mirror neuron system (pMNS) and the mentalizing system [de Lange et al., 2008; Hesse et al., 2009; Keysers and Gazzola, 2007; Uddin et al., 2007]. The pMNS, composed of motor‐related brain regions in the inferior frontal gyrus (IFG) and inferior parietal lobule (IPL), is activated both when an individual makes an action and when he or she observes another person make the same action [Aziz‐Zadeh et al., 2006; Gallese et al., 1996; Rizzolatti and Craighero, 2004]. It has been proposed that mapping observed actions onto one's own motor representations supports motor simulations of an observed action, allowing the observer to then predict others' intentions [Gallese et al., 2004; Iacoboni et al., 2005; Rizzolatti and Craighero, 2004]. In contrast, the mentalizing system is composed of regions in the medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), and the bilateral temporal‐parietal junctions (TPJ), and is thought to be involved in non‐intuitive reflections of others' mental states [Fletcher et al., 1995; Frith and Frith, 2006; Saxe and Powell, 2006; Saxe, 2006]. These regions have been linked to perspective‐taking and tend to be activated by a conscious effort to infer others' intentions, across a variety of stimuli including stories, cartoons, and images of others [Frith and Frith, 2006; Gallagher et al., 2000; Saxe and Kanwisher, 2003].
A recent meta‐analysis [Van Overwalle and Baetens, 2009] revealed that pMNS regions tend to be active when observing biological movement, with stronger activity when observing familiar actions for which one has a pre‐existing motor representation [Calvo‐Merino et al., 2005; Cross et al., 2006; Van Overwalle and Baetens, 2009]. In contrast, mentalizing regions are activated for higher level goal inferences, regardless of the presence of visual biological stimuli, and tend to be active in the absence of existing motor representations, such as during observation of movements that are unplanned, out‐of‐context, or biomechanically impossible [Brass et al., 2007; Kilner and Frith, 2008; Liepelt et al., 2008; Van Overwalle and Baetens, 2009]. Thus, the existing body of research suggests that these systems serve complementary roles, with mentalizing regions more active during novel contexts and pMNS regions more active during familiar contexts [Van Overwalle and Baetens, 2009].
Although the task of understanding others' actions is heavily social in nature, little is known about how social factors affect the contributions and interactions of pMNS and mentalizing regions. Prior research has demonstrated that one's race, culture, religion, and even political affiliation can modulate cognitive and sensorimotor processing during passive observation [Han and Northoff, 2008; Serino et al., 2009; Xu et al., 2009]. However, to date, no studies have explored how these powerful factors modulate neural activity when asked to infer intentions from observed human movements.
The current functional magnetic resonance imaging (fMRI) study manipulated two critical factors related to familiarity (perceptual familiarity with the race of the actor, motor familiarity from experience with the action) during the task of inferring an actor's intentions. The aim was to understand the specific contributions of individual regions within the pMNS and mentalizing systems when perceptual and motor familiarity with the stimuli are manipulated. In order to explore the social influences on action understanding in these two neural systems, the current study utilized symbolic, or intransitive, gestures (e.g., thumbs up), which are learned and familiarized through one's cultural experiences [Archer, 1997]. As these gestures require an integration of visuomotor representations with abstract intentions, they have been shown in separate studies to activate regions of both pMNS [Skipper et al., 2009; Straube et al., 2009; Villarreal et al., 2008] and mentalizing networks [Gallagher and Frith, 2004].
There is sparse existing research on how perceptual familiarity modulates action understanding. In the current study, we examined the effect of perceptual familiarity on action understanding by manipulating the race of the actors used in the stimuli (Chinese, Caucasian). To this end, we recruited Chinese individuals living in mainland China who have limited exposure to, and thus less perceptual familiarity with, Caucasian individuals compared to Chinese individuals. For the sake of clarity, in this article, we refer to this factor related to perceptual familiarity specifically as “race,” while experience with an action (motor familiarity) will be referred to simply as “familiarity.” However, we acknowledge that the construct of race may also include many other factors not explored here, most notably, in‐group/out‐group effects, and that these larger constructs related to race should be further studied using a design comparing participants from two or more racial groups.
Previous studies on the effects of race on the pMNS demonstrate conflicting evidence, with two transcranial magnetic stimulation (TMS) studies revealing opposite results: one demonstrated increased corticospinal excitability during observation of actors of one's own race versus a different race [Molnar‐Szakacs et al., 2007], whereas the other found a reverse pattern [Desy and Theoret, 2007]. In support of the former results, an fMRI study showed greater activation in regions associated with the pMNS for more physically similar others than physically dissimilar others [Buccino et al., 2004a]. In addition, there is increased activity in mentalizing regions when observing the eyes of one's own race versus the eyes of another race, suggesting increased higher level processing of one's own race [Adams et al., 2009]. Notably, these same‐race effects may also be due to one's increased perceptual familiarity with one's own race compared to another race. We thus hypothesized that observation of same‐race individuals, who are more perceptually familiar than different‐race individuals, ought to evoke stronger activity in regions associated with both motor simulation and mentalizing than observation of different‐race individuals.
Furthermore, research on passive observation of familiar or unfamiliar actions suggests that experience with the action increases pMNS activity for one's own expert skilled actions, such as expert dancers watching their own dance form versus an unfamiliar dance form [Calvo‐Merino et al., 2005; Cross et al., 2006]. On the other hand, passive observation of actions that are biomechanically impossible or that do not make sense within a context has been associated with increased activation in mentalizing regions [e.g., turning on a light‐switch with one's knee when one's hands are free versus when one's hands are occupied; Brass et al., 2007; Kilner and Frith, 2008; Liepelt et al., 2008]. Thus, we hypothesized that observations of familiar gestures, which are within one's own motor repertoire, would be easier to simulate and thus more strongly involve pMNS regions, while observations of unfamiliar gestures, which lack an existing motor representation and may require additional reasoning capabilities, would more strongly involve mentalizing regions.
MATERIALS AND METHODS
Participants
Eighteen healthy Chinese adults (10 males and 8 females, 18–30 years of age, mean ± SD = 23.0 ± 2.28), born in and living in China, were recruited in this study. Participants were scanned while observing familiar and unfamiliar gestures performed identically by two actors, one Caucasian and one Chinese. All participants were right‐handed, had normal or corrected‐to‐normal vision, and had no neurological or psychiatric history. Written informed consent was obtained from all participants before inclusion in the study. This study was approved by a local ethics committee and the University of Southern California Institutional Review Board and was performed in accordance with the 1964 Declaration of Helsinki.
Stimuli
Action observation
The visual stimuli consisted of 2‐s movie clips. Half of the clips depicted a Caucasian performing expressive hand gestures that were either familiar (i.e., thumbs up) or unfamiliar (i.e., “quail” in American Sign Language) with his right hand. The other half depicted a Chinese actor making identical gestures. Both actors were male, in their mid‐20s, right‐handed and of similar physical build. While performing gestures, actors maintained a neutral affect with gaze held directly forward and no additional eye movements to prevent provision of additional social cues. In addition, both actors were equally familiar or unfamiliar with the gestures they were asked to perform and rehearsed all gestures prior to filming. To assess the potential differences in familiarity with gestures performed by Chinese and Caucasian actors, in a separate behavioral study, we asked 64 Chinese participants to rate how familiar they were with the gestures performed by the actors using a 3‐point Likert scale (1 = familiar, 3 = unfamiliar). We found that there were no significant differences between the gestures performed by Chinese and Caucasian actors; familiar gestures performed by the two actors were judged as being equally familiar (Chinese: 1.35 ± 0.24, Caucasian: 1.38 ± 0.28, P = 0.43) while unfamiliar gestures performed by both actors were judged as being equally unfamiliar (Chinese: 2.80 ± 0.09, Caucasian: 2.77 ± 0.11, P = 0.51). Still photos of some of the different stimuli are illustrated in Figure 1. Each actor was filmed completing six different familiar gestures and six different unfamiliar gestures, resulting in 12 clips per actor and 24 different clips total. A control for action observation consisted of a 2‐s presentation of a still photo made from the first frame of the video clips (six stills per actor and 12 different stills total).
Figure 1.

Examples of still images of the stimuli. Participants observed 2‐s videos of familiar gestures (left panel), unfamiliar gestures (middle panel), and control still images (right panel). Each gesture and still image was performed by an actor of the participants' own race (Chinese) and an actor of a different race (Caucasian). Original videos were presented in full color.
Action execution
A cue for action execution trials consisted of a stimulus with 500 ms of a black box outlined in red, followed by 1500 ms of the red outline around the still images used in control trials.
Task Design and Procedure
Action observation
Prior to scanning, to try to engage both the pMNS and mentalizing regions [De Lange et al., 2008], participants were instructed to observe the video clips as though the actors were performing the gestures directly to them and were asked to think about the actor's intentions in doing each gesture. Also, prior to scanning, they were shown still photos of both actors for 30 s each to become familiar with the actors' faces. They were finally instructed to actively infer the actor's intentions by attending to the actor's hand movements, rather than the actor's face, for the duration of the clips shown during the scanning session and were informed that they would be asked the meaning of each gesture immediately after the scanning session, as an additional motivation to actively think about the intentions of each gesture clip.
Action execution
Participants were instructed to rest their right hand next to, but not on, a button box. When cued by the red‐outlined action execution stimuli, participants moved their hand to the button box, using their index fingers to repeatedly press the button for the duration of the clip.
General procedure and design
The video clips were presented through a projector onto a rear‐projection screen located at the subject's head. Each movie clip subtended a visual angle of 21.4° × 17.1° at a viewing distance of 80 cm. Each condition was shown for 18 trials per run for three runs, for a total of 54 trials per condition, with the exception of the action execution condition, which was shown for nine trials per run for a total of 27 trials. All conditions, including action observation and action execution conditions, were combined and evenly distributed across three functional runs of 340 s (170 TRs) each. Following an event‐related design, each run used an optimized random sequence generated in Optseq (http://surfer.nmr.mgh.harvard.edu/optseq/) with an interstimulus interval between successive clips that was jittered between 0 and 5 s, with a mean of 2 s. A schemata of the general design can be found in Figure S1.
Behavioral methods
Following scanning, subjects were shown the gesture stimuli on a computer outside the scanner and were asked to rate how familiar they were with each gesture, using a Likert‐type scale where 1 indicated extremely unfamiliar and 10 indicated extremely familiar. They were also asked how positive/negative they felt each gesture was and what they thought each gesture meant, using a 3‐point scale for positive, negative, or neutral for the former and an open response for the latter. Finally, they were asked to rate how much they liked each actor on a Likert‐type scale where 1 indicated not liking the actor at all and 10 indicated liking the actor very much. These scores were later computed to ensure the stimuli were accurately perceived as either familiar or unfamiliar and positive or negative and to ensure both actors were similarly perceived. In addition, participants were given the Multigroup Ethnic Identity Measure (MEIM), a self‐report measure designed to examine one's sense of ethnic identity [Roberts et al., 1999, modified from Phinney, 1992].
fMRI Image Acquisition and Analysis
Scanning was performed at Peking University First Hospital on a GE 3‐T scanner with a standard head coil. Thirty‐two transverse slices of functional images covering the whole brain were acquired using a gradient‐echo echo‐planar pulse sequence (64 × 64 × 32 matrix with a spatial resolution of 3.4 × 3.4 × 4.4 mm, repetition time = 2000 ms, echo time = 30 ms, FOV = 24 × 24 cm, flip angle = 90°). Anatomical images were obtained using a 3D FSPGR T1 sequence (256 × 256 × 128 matrix with a spatial resolution of 0.938 × 0.938 × 1.4 mm, TR = 7.4 ms, TI = 450 ms, TE = 3.0 ms, flip angle = 20°).
Imaging data was analyzed using SPM2 (Statistical Parametric Mapping 2; the Wellcome Department of Cognitive Neurology, London, United Kingdom) implemented in MATLAB (Mathworks, Sherborn, MA). The functional data were first time‐corrected to compensate for delays associated with acquisition time differences between slices during the sequential imaging. The functional images were then realigned to the first scan to correct for head motion between scans. All six movement parameters (translation: x, y, z and rotation: pitch, roll, yaw) were included in the statistical model. The anatomical image was co‐registered with the mean functional image produced during the process of realignment. All images were normalized to a 2 × 2 × 2 mm3 Montreal Neurological Institute (MNI) template. Functional images were spatially smoothed using a Gaussian filter with the full‐width/half‐maximum parameter (FWHM) set to 8 mm. In addition, high pass temporal filtering with a cut‐off of 180 s was applied. The event‐related neural activity was modeled using a canonical hemodynamic response function (HRF) with temporal derivative. Effects at each voxel were estimated and regionally specific effects were compared using linear contrasts in individual participants using a fixed effects analysis.
A group‐level random effects analysis was then conducted, taking into account between‐subject variability [Penny et al., 2004]. A priori regions of interest (ROIs) for the PMNS (left IFG and IPL) and the mentalizing systems (dmPFC, PCC, bilateral TPJ) were defined independently of the current dataset to avoid circularity [Kriegeskorte et al., 2009]. Functional definitions were taken from two relevant papers on the pMNS [Buccino et al., 2004b] and mentalizing system [Den Ouden et al., 2005], with the criteria that each paper contained activity from all ROIs within the given system and were well‐cited within the field. We used a small volume correction with a mask defining the six regions with 10‐mm radius spheres with centers at the peak activations from these papers. Results were reported at the P < 0. 05 level, FDR‐corrected for multiple comparisons over the six ROIs, and with a cluster threshold of eight contiguous voxels (k ≥ 8). Non‐apriori regions of significant activation were reported at the whole‐brain level using a threshold of P < 0.001 (uncorrected) and a cluster threshold of eight contiguous voxels (k ≥ 8).
ROI analyses were then performed by extracting beta‐values from group‐level results within each of the previously defined 10 mm ROIs. A 2 × 2 repeated measures ANOVA was performed on each ROI with the factors of familiarity and race using the R statistical package [Ihaka and Gentleman, 1996], and results were subjected to a Bonferroni correction for multiple comparisons.
RESULTS
Behavioral Results
Participants rated gestures from the category “familiar gestures” as significantly more familiar than gestures from the category “unfamiliar gestures” (familiar: 9.40 ± 1.27; unfamiliar: 3.17 ± 1.96; P < 0.001). Participants also accurately identified all familiar gestures and were unable to accurately identify any of the unfamiliar gestures. Participants also rated half of the gestures as neutral (51.7%), followed by positive (28.3%), and negative (20.0%). Furthermore, there was no significant difference in subjects' responses to the question “How much do you like [actor's name]?” as subjects reported liking Caucasian and Chinese actors equally (Caucasian: 6.78 ± 2.05; Chinese: 6.17 ± 1.54; P > 0.3). All of the participants reported having had limited interactions with Caucasian individuals, primarily through the media only. In addition, participants' scores on the MEIM were correlated with the fMRI data, as described below (see Fig. S2).
fMRI Results
All gestures versus control still images
Observation of all gestures versus control still images activated a priori pMNS regions of interest in the left dorsal inferior frontal gyrus (IFG) and the left inferior parietal lobe (IPL). At the whole brain level, the right posterior cingulate cortex, left middle temporal gyrus (MT/V5), right fusiform gyrus, bilateral superior parietal lobules, left precentral gryus, and left posterior superior temporal gyrus (pSTG), including Wernicke's area, were active (see Fig. 2 for fMRI results, Table I for all peak activations, and Fig. S3 for bar diagrams of beta values from ROIs).
Figure 2.
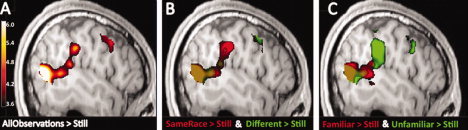
Brain responses to observations of gestures versus still images (all images displayed at P < 0.001 uncorrected for visualization purposes; x = −51). A: Observation of all gestures across familiarity and races versus still images evoked greater activity in components of the pMNS [the left dorsal inferior frontal gyrus (IFG) and dorsal premotor cortex and inferior parietal lobule (IPL)], as well as the posterior superior temporal sulcus (pSTS) and posterior cingulate cortex (PCC; not shown). B: Observation of the same race versus still (red) evoked activity in the left IPL and pSTS, while observation of a different race versus still (green) evoked activity in the left dorsal premotor cortex and pSTS. C: Observation of familiar gestures versus still images (red) evoked greater activity in the left pSTS, while unfamiliar gestures versus still images (green) evoked activity in dorsal IFG, IPL, and pSTS.
Table I.
Localization of brain activations from random effects analysis
| Anatomical region | BA | T‐Value | Cluster size | Coordinates [x, y, z] |
|---|---|---|---|---|
| All Gestures > Still Photo | ||||
| L Inferior parietal lobule | 40 | 4.80 | 515 | [−52, −32, 34] |
| L Inferior frontal gyrus | 44 | 4.09 | 336 | [−48, 10, 38] |
| L V5/MT | 18 | 11.86 | 1747 | [−48, −70, 0] |
| R Fusiform gyrus | 37 | 8.89 | 1867 | [50, −58, −18] |
| R Superior parietal lobule | 7 | 5.22 | 106 | [32, −56, 60] |
| L Superior parietal lobule | 7 | 5.17 | 156 | [−26, −56, 64] |
| L Precentral gyrus | 6 | 4.73 | 21 | [−46, 2, 50] |
| R Posterior cingulate cortex | 30/31 | 4.10 | 15 | [6, −38, 12] |
| L Inferior frontal gyrus | 44 | 4.09 | 26 | [−48, 10, 38] |
| R Posterior superior temporal gyrus | 22 | 3.93 | 10 | [68, −34, 14] |
| Same Race > Different Race | ||||
| R Insula | 4.387 | 20 | [38, −2, −6] | |
| L Inferior parietal lobule | 2 | 4.20 | 37 | [−58, −22, 32] |
| Different Race > Same Race | ||||
| L Middle occipital gyrus | 19 | 5.64 | 57 | [−28, −86, 2] |
| L Fusiform gyrus | 37 | 4.61 | 95 | [−32, −66, −16] |
| R Fusiform gyrus | 37 | 4.36 | 20 | [30, −78, −10] |
| Familiar Gestures > Unfamiliar Gestures | ||||
| R Posterior cingulate cortex | 23 | 5.38 | 498 | [6, −38, 32] |
| L Temporoparietal junction | 39 | 4.85 | 334 | [−50, −66, 38] |
| L Dorsal medial prefrontal cortex | 32/9 | 4.09 | 421 | [−4, 44, 26] |
| R Temporoparietal junction | 39 | 3.56 | 267 | [52, −68, 40] |
| R Posterior cingulate cortex | 23 | 7.39 | 178 | [6, −34, 36] |
| L Lingual gyrus | 17/18 | 6.02 | 505 | [−4, −82, 2] |
| L Posterior cingulate cortex | 31 | 5.38 | 113 | [−4, −16, 48] |
| L Temporoparietal junction | 39 | 4.85 | 101 | [−50, −66, 38] |
| R Angular gyrus | 40 | 4.36 | 33 | [62, −54, 34] |
| L Middle frontal gyrus | 10 | 4.30 | 35 | [−28, 48, 26] |
| R Posterior cingulate cortex | 23 | 4.17 | 19 | [10, −4, 48] |
| L Dorsal medial prefrontal cortex | 9 | 4.09 | 35 | [−4, 44, 26] |
| R Calcarine gyrus | 17 | 4.01 | 40 | [14, −80, 14] |
| Unfamiliar Gestures > Familiar Gestures | ||||
| L Inferior parietal lobule | 40 | 6.90 | 515 | [−52, −30, 36] |
| L Superior parietal lobule | 7 | 10.25 | 1817 | [−20, −70, 60] |
| L Middle occipital gyrus | 19/18 | 9.09 | 484 | [−38, −78, 4] |
| R V5/MT | 18 | 7.75 | 671 | [50, −72, 2] |
| L Thalamus | 4.50 | 13 | [−14, −28, 0] | |
| L Superior frontal gyrus | 6 | 4.23 | 9 | [−20, −6, 68] |
| R Superior parietal lobule | 7 | 4.16 | 54 | [18, −66, 60] |
A priori regions (in bold) reported at P < 0.05 FDR, whole brain results reported at P < 0.001 uncorrected at the voxel level, cluster threshold >8.
Same and different race observations
Observation of gestures performed by the same race (Chinese) and different race (Caucasian) actors were separately contrasted to the control condition (still images). The same race (Chinese) actors compared to stills displayed activity in a priori pMNS regions of interest (the left IFG and the left IPL) along with a large region of activity in the left postcentral gyrus and bilaterally in the pSTS, SPL and the V5/MT region. In contrast, the different race (Caucasian) versus stills resulted in no significant activity in a priori regions. Whole‐brain analyses at the P < 0.001 uncorrected level revealed activity in the dorsal precentral gyrus (BA 6) bilaterally, the left pSTS, and the middle occipital gyrus bilaterally.
The direct comparison of same race versus different race observations did not reveal any significant activation in a priori regions of interests. However, at the whole‐brain level, the same versus different race contrast demonstrated activity in the left anterior IPL (supramarginal gyrus) and the right posterior insula. Different race versus same race showed increased activity only in the bilateral fusiform gyri and left middle occipital gyrus extending into the middle temporal gyrus (V5/MT; see Fig. 3).
Figure 3.
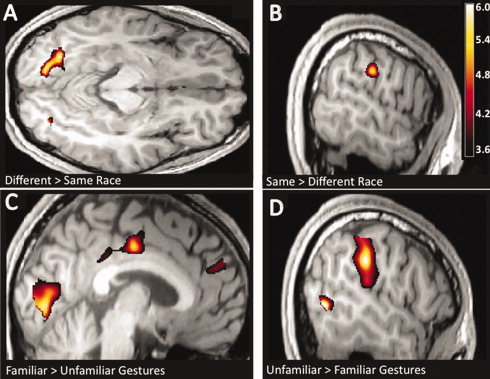
Race‐driven and experience‐driven brain responses (all images displayed at P < 0.001 uncorrected for visualization purposes). A: Observations of another race versus one's own race (DifferentRace > SameRace) evoked greater activity in the occipital cortex bilaterally in the fusiform gyrus and middle temporal gyrus (area V5/MT; not shown; z = −11). B: Observations of one's own race versus another race (SameRace > DifferentRace) evoked greater activity in the left IPL and right posterior insula (not shown; x = −59). C: Observations of familiar gestures versus unfamiliar gestures (Familiar > Unfamiliar) evoked greater activity in the dorsal medial prefrontal cortex (dMPFC), the posterior cingulate (PCC), the cuneus, and the bilateral temporoparietal junctions (not shown), regions associated with mentalizing and reasoning processes (x = −4). D: Observations of unfamiliar gestures versus familiar gestures (Unfamiliar > Familiar) evoked greater activity in the left IPL and postcentral gyrus and the bilateral middle temporal gyri (area V5/MT) in the putative extrastriate body area (EBA; x = −53).
In addition, scores on the MEIM were correlated with fMRI activity during observations of one's own race versus a different race (Fig. S2). Higher MEIM scores were positively correlated with activity in the dmPFC when observing one's own race versus a different race, and with activity in the left dorsal premotor cortex when observing a different versus same race.
Familiar and unfamiliar gestures
Familiar gestures compared to still images did not demonstrate significant activity in a priori regions of interest, but did reveal activity in the left dorsal IFG, left pSTS and bilateral visual cortices (V5/MT, middle occipital gyri) at the whole brain level. Unfamiliar gestures, compared to still images, activated a priori regions of interest in the left dorsal IFG and left IPL. They also activated the left pSTS, bilateral SPL, and bilateral lateral middle temporal gyrus (V5/MT) into the putative extrastriate body area (EBA), as well as in the fusiform gyri, at the whole brain level.
Familiar versus unfamiliar gestures revealed activity in a priori regions of interest, the posterior cingulate cortex (PCC), the dorsal portion of the medial prefrontal cortex (dmPFC) and the bilateral TPJ. Whole‐brain analysis further demonstrated activation in the bilateral occipital gyri within the primary visual cortex (BA 17/18). In contrast, unfamiliar versus familiar gestures generated activity in an a priori region of interest in the left IPL, along with additional activity at the whole brain level along a large vertical region of the left postcentral gyrus, from the dorsal aspect of the postcentral gyrus to the ventral portion of the supramarginal gyrus. Additional activity also appeared in the bilateral SPL and bilateral V5/MT in the putative EBA and fusiform gyri (see Fig. 3).
Region of interest analyses
Beta values from our regions of interest in the pMNS (L IFG, L IPL) and mentalizing systems (mPFC, PCC, L TPJ, R TPJ), as previously defined, were then extracted and analyzed in 2 × 2 repeated measures ANOVAs with factors of race and familiarity, corrected for multiple comparisons, to examine whether there was a main effect or interaction effect between race and familiarity in these regions (see Fig. S3). We found a main effect of familiarity, with beta values significantly greater for unfamiliar than familiar conditions in the IPL (F = 18.45, P < 0.00012), whereas there was a main effect of familiarity, with beta values significantly greater for familiar than unfamiliar conditions in three of the four mentalizing ROIs, with the last one nearing significance (mPFC: F = 15.35, P < 0.00058; L TPJ: F = 9.89, P < 0.010; R TPJ: F = 12.79, P < 0.0022; PCC: F = 5.67, P < 0.11). In addition, the IPL demonstrated a significant effect of race with beta values for observations of the same‐race actor greater than those of the different‐race actor (F = 7.0, P < 0.05). Although none of the ROIs demonstrated a significant interaction effect between race and familiarity with the gesture, two fMRI contrasts exploring interactions between race and familiarity (Same Race + Familiar > Different Race + Unfamiliar; Different Race + Unfamiliar > Same Race + Familiar) found significant results in regions of the pMNS and mentalizing systems (see Fig. S4).
In addition, post hoc analyses demonstrated a significant overlap between BOLD signal from action execution and action observation conditions, suggesting validation of the presence of pMNS activity as found by using independently defined ROIs in the small volume correction (see Fig. S5).
DISCUSSION
Abstract Gestures
Observations of all gestures compared to still images generated activity within both the left dorsal IFG and the left IPL, which comprise the human pMNS, as well as the right PCC, which is thought to be a component of the mentalizing system [Van Overwalle and Baetens, 2009], in line with our initial hypotheses. Previous studies have focused on the task‐dependent activity of either pMNS or mentalizing regions during gesture observation, with results reported in one system or the other [Gallagher and Frith, 2004; Skipper et al., 2009; Straube et al., 2009; Villarreal et al., 2008]. The current findings, however, support recent literature demonstrating activity of both systems during the general process of understanding the intentions behind an observed gesture [Schippers et al., 2009]. Furthermore, these data support previous findings indicating that regions of the human pMNS are involved in the processing of manual gestures and abstract communication [Corina and Knapp, 2006; Gentilucci and Dalla Volta, 2008; Willems et al., 2007]. The activation of the PCC, a region commonly associated with the mentalizing system as well as with episodic and autobiographical memory retrieval [Maddock et al., 2001], may be involved in interpreting the actor's intentions and/or comparing the observed stimulus to prior memories in order to understand the gesture's meaning. Altogether, our findings suggest that observing symbolic gestures requires the interplay between regions from both pMNS and mentalizing regions.
Processing Perceptual Familiarity in Individuals of the Same Versus a Different Race
Observations of the same race compared to still images demonstrated significant activity in pMNS regions of interest (IFG, IPL). Observations of a different race compared to still images generated no significant activity in any a priori regions of interest. However, there was activity in regions associated with the pMNS at the whole brain level [e.g., the dorsal premotor cortex and pSTS; Van Overwalle and Baetens, 2009], suggesting a less robust signal for observing a different race compared to one's own race, possibly in different regions of the pMNS from observations of one's own race. In addition, in accordance with our hypothesis, observations of same‐race actors directly contrasted with different‐race actors demonstrated greater activity in the posterior component of the pMNS (the anterior IPL), further contributing to the suggestion that actions of more perceptually familiar and/or physically similar individuals are more readily mapped onto sensorimotor representations of the self. Additional activity was found in the insula and may indicate enhanced emotional processing for individuals of the same race. These results are consistent with prior research suggesting that greater shared physical properties are associated with increased activity in the pMNS [Buccino et al., 2004a; Molnar‐Szakacs et al., 2007]. Furthermore, prior research has found that racial group membership increases emotional responses to members of one's own group [Xu et al., 2009].
In contrast, observations of different‐race actors versus same‐race actors generated greater visual activity within the fusiform gyrus bilaterally, which is thought to support processing of face stimuli [Kanwisher et al., 1997], as well as in the middle occipital gyrus extending into area V5/MT which is the putative extrastriate body area (EBA) and thought to support processing of body movements [Downing et al., 2001; Astafiev et al., 2004]. These findings are also in accordance with prior research demonstrating that physically different others generate greater activity in visual regions [Buccino et al., 2004a].
Interestingly, one's self‐reports of ethnic identification as being Chinese correlated with higher mentalizing activity for one's own race versus a different race and higher motor‐related activity for a different race versus one's own race. These results suggest that the more one identifies with one's ethnic group, the more one utilizes mentalizing regions to process one's own race versus another race, a finding that is in accordance with previous research [e.g., Adams et al., 2009]. In contrast, the more one identifies with one's own ethnic group, the more motor‐related activity they have when observing different‐race individuals. This result seems to conflict with our previous suggestion that we map those who are more perceptually familiar onto our own sensorimotor representations than those who are perceptually less familiar. Thus, it is likely that there are other variables at play when we begin to incorporate ethnic identification into the analysis.
All together, these results suggest that humans are more apt to process the actions of those more perceptually familiar by engaging their own sensory‐motor representations and emotional responses more strongly, as seen here when Chinese participants viewed their own race. Thus, it appears that activity in pMNS and mentalizing regions may be modulated by social factors such as perceptual familiarity and, in this case, race. This effect may be strengthened by one's daily life practice, particularly if one has limited experience or perceptual familiarity with another racial group, as found in our pool of participants.
By contrast, when observing actors that are perceptually less familiar from ourselves (e.g., actors of difference race), we may engage in increased visual processing, particularly of individuals' faces and body movements, as these often may provide additional information that might assist us in understanding the “other.” Notably, these results are seen despite the fact that, in the current study, participants were asked to attend to the hand gesture rather than to the face of the actor, thus decreasing the amount of direct attention to race, while in many prior studies on race, participants are instructed to observe the faces of actors, thus increasing the explicit attention to racial information.
Thus, although the effects of race may have been minimized by the task instructions of specifically asking participants to focus on the hand gestures rather than on the race of the actor, these results indicate an implicit, automatic difference in neural processing despite an attentional focus elsewhere. Further research using eye tracking may be useful to assess whether diverted attention to visual processing of different race individuals is in fact responsible for decreased pMNS activity. In addition, a better understanding of whether these neural patterns of activation can be correlated with stereotyping or prejudiced behavior would be beneficial.
Finally, it should be noted that these observed effects may be influenced by additional cultural or racial factors. Recent cultural neuroscience studies have shown increasing evidence that sociocultural contexts can influence or modulate neural substrates of human cognition [Chiao and Ambady, 2007; Han and Northoff, 2008; Ito and Bartholow, 2009]. Culture‐specific neural processes have been observed in many aspects of human cognition such as perception, attention, and emotion, as found in one recent study demonstrating the culture‐specific modulation of automatic fear responses [Chiao et al., 2008]. As our participants were all Chinese individuals, living in China, future research may use additional diverse subject pools to explore cross‐cultural differences in race‐related effects on action understanding networks to assess whether these results may be modulated by the culture or race of the participants.
Gesture Familiarity
Although both familiar and unfamiliar conditions activated regions of the pMNS when compared to observations of still images, during a direct comparison, familiar gestures more strongly activated all four a priori regions associated with the mentalizing network (mPFC, PCC, and bilateral TPJs), whereas unfamiliar gestures more strongly activated parietal sensory‐motor regions and the putative extrastriate body area (EBA). These findings, which are reversed from our initial hypothesis, seem to suggest that when observing, and likely trying to understand the intentions of, an actor making a familiar action, we activate the pMNS as well as additionally recruit components of the mentalizing network. One explanation is that familiarity with the movement may not only provide existing visually and motorically based representations but also existing semantic and episodic memories associated with the observed action. This is notable, as participants were not explicitly tested on their motoric familiarity with the observed gestures, and therefore may have seen and recognized—but never personally performed—the familiar gestures. However, as prior studies have found that both visual experience and personal motoric experience with an action sequence can increase motor representations in pMNS regions when observing familiar actions [Cross et al., 2009], it may be that either visual or motoric familiarity is enough to modulate the observed effect seen in these results. Thus, regardless of whether or not participants had physically performed the gestures themselves, it appears that as long as they were familiar with the gesture, they more heavily recruited activity in regions associated with mentalizing processes. This includes the dorsal mPFC, which is associated with general mentalizing tasks and triadic social interactions in which two people jointly attend to a third item or action [Saxe, 2006; Saxe and Powell, 2006]. In addition, the bilateral TPJ, involved in the direction of attention as well as perspective‐taking, emotional meaning, and linguistic associations [Saxe and Kanwisher, 2003], and the posterior cingulate cortex, associated with monitoring the external environment and episodic autobiographical memory retrieval [Gusnard and Raichle, 2001; Maddock et al., 2001], may assist in taking the perspective of the actor and possibly linking the familiar gesture with existing memories and experiences respectively. The recruitment of these areas may reflect the individual's ability to retrieve higher level intentions and goals from observed familiar actions based on prior experiences with the familiar actions.
By contrast, this additional cognitive processing may not occur when observing unfamiliar gestures, for which one has no prior experiences, memories, or knowledge of meanings. Instead, when we try to understand an actor making an unfamiliar action, motor‐related regions within the pMNS network, in particular the IPL, become more active. Interestingly, these findings are similar to previous studies demonstrating that observation of an unfamiliar action, with the intent to imitate the observed action, generates greater activity in pMNS regions than familiar actions (Vogt et al., 2007). While participants were not explicitly instructed to imitate the gestures, it is possible that understanding unfamiliar gestures may implicitly recruit regions involved in imitation of the novel action, in order to make sense of it. Thus, it may be that when possibly inferring the higher level goals of an action that is unfamiliar but that we are capable of performing, we attempt to simulate the observed action using the IPL and other sensorimotor regions to try to use our pre‐existing motor representations to generate a basic understanding of the observed action.
Interestingly, the IPL, which is a multi‐modal region commonly associated with grasp affordances, motor attention, body awareness, and action planning [Oztop and Arbib, 2002; Fogassi et al., 2005], showed increased activation both in response to unfamiliar gestures as well as to one's own race. Thus, the IPL may be involved in developing a sensorimotor representation of the observed action that is sensitive to both physical similarity and prior experiences with the movement. Activity in the IPL may be increased in an automatic process when there is increased physical similarity to oneself (i.e., simulating one's own race), as well as via a more effortful cognitive process when an action is unfamiliar, directing attention to the manner in which an unfamiliar action is performed in order to extract basic motor goals and intentions. In addition, the unpredictable nature of the unfamiliar gestures may also explain the increased activation in the bilateral superior parietal lobule, a region associated with error‐monitoring and thought to be involved in building on‐line motor predictions of observed actions [Wolperts et al., 1998]. Activity in this region bilaterally may indicate a reaction to unexpected, unfamiliar movements during internal simulation of the movement.
These results may seem contradictory to previous findings, as studies on passive observations of actions that are unusual or inconsistent within a context have demonstrated increased activity in mentalizing regions [Brass et al., 2007; Kilner and Frith, 2008; Liepelt et al., 2008; Van Overwalle and Baetens, 2009]. Such results suggest that the mentalizing regions become active when a passively observed goal does not have a matching motor representation, as in the case of an unfamiliar action. However, such results may be heavily influenced by task instructions, such as focusing on the goal of an action rather than on how the action is performed [De Lange et al., 2008; Hesse et al., 2009]. As our study is the first to our knowledge to employ a task instructing participants to make active inference of intentions during observation of both familiar and unfamiliar human movements, we propose that the unique social demands of inferring an unfamiliar action's intention—a scenario that occurs often in real life, such as when in a new country or learning a new sport—modulates neural activity in pMNS and mentalizing regions differently than during passive observation. Furthermore, observing familiar but contextually implausible actions [e.g., observing an actor turn on a light switch with their elbow; Brass et al., 2007] may not require additional sensorimotor representations but higher level reasoning capabilities instead. Thus, the current results suggest that while understanding a familiar action may involve regions associated with the mentalizing system, understanding an unfamiliar action, for which one has no abstract, experience‐specific, or contextually relevant information, may activate sensorimotor regions, such as in the IPL, more heavily. Additional research may help us to elucidate whether these results are due to the strategy employed in understanding the observed actor's gesture (e.g., trying to simulate the gesture versus trying to objectively reason about the gesture), and the extent to which sociocultural influences might determine the strategies involved.
CONCLUSION
Our results reveal the complex interplay between one's perceptual and motor familiarity with an actor or action on neural regions underlying both action observation and intention understanding. This interaction requires a contribution from both pMNS and mentalizing regions during gesture observations and may be strongly modulated by a variety of social factors. Specifically, our results suggest that observations of actors that are perceptually familiar are associated with increased sensory‐motor and emotion‐related processing, whereas observations of actors who are perceptually unfamiliar to oneself are associated with increased visual processing. Furthermore, our data indicate that understanding familiar gestures increases activity in the mPFC, PCC, and bilateral TPJs, suggesting an increased engagement of mentalizing processes during this task. In contrast, understanding unfamiliar gestures more strongly activates the posterior component of the pMNS (the IPL), possibly reflecting the generation of a motor‐based representation of the unfamiliar action, which may then provide basic information about the goal of the observed action. Activity in the parietal regions may also reflect violations of expectations during simulation, in accordance with the region's proposed role in the error‐monitoring of actions.
In line with prior research, our findings also demonstrate that the pMNS and mentalizing regions are differentially modulated, supporting the idea that the two systems largely perform complementary roles [Van Overwalle and Baetens, 2009]. Our data suggest that such roles can further be dissociated along the bases of familiarity with race or with actions, and that the activity in these regions may be heavily dependent on the particular task and stimuli used.
Supporting information
Additional supporting information may be found in the online version of this article.
Supporting Information
Acknowledgements
The authors thank Wang Gang, Xiaojing Xu, Savio Wong, and Alicia Johnson for their assistance in this project.
Contributor Information
Sook‐Lei Liew, Email: sliew@usc.edu.
Shihui Han, Email: shan@pku.edu.cn.
References
- Adams R, Rule N, Franklin R, Wang E, Stevenson M, Yoshikawa S, Nomura M, Sato W, Kveraga K, Ambady N ( 2010): Cross‐cultural reading the mind in the eyes: An fmri investigation. J Cogn Neurosci 27: 97–108. [DOI] [PubMed] [Google Scholar]
- Archer D ( 1997): Unspoken diversity: Cultural differences in gestures. Qualitative Sociology 20: 79–105. [Google Scholar]
- Astafiev S, Stanley CM, Shulman GL, Corbetta M ( 2004): Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nat Neurosci 7: 542–548. [DOI] [PubMed] [Google Scholar]
- Aziz‐Zadeh L, Koski L, Zaidel E, Mazziotta J, Iacoboni M ( 2006): Lateralization of the human mirror neuron system. J Neurosci 26: 2964–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Schmitt R, Spengler S, Gergely G ( 2007): Investigating action understanding: Inferential processes versus action simulation. Curr Biol 17: 2117–2121. [DOI] [PubMed] [Google Scholar]
- Buccino G, Lui F, Canessa N, Patteri I, Lagravinese G, Benuzzi F, Porro C, Rizzolatti G ( 2004a): Neural circuits involved in the recognition of actions performed by nonconspecifics: An fmri study. J Cogn Neurosci 16: 114–126. [DOI] [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink G, Zilles K, Freund HJ, Rizzolatti G ( 2004b): Neural circuits underlying imitation learning of hand actions an event‐related fmri study. Neuron 42: 323–334. [DOI] [PubMed] [Google Scholar]
- Calvo‐Merino B, Glaser D, Grezes J, Passingham R, Haggard P ( 2005): Action observation and acquired motor skills: An fmri study with expert dancers. Cereb Cortex 15: 1243–1249. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Ambady N ( 2007): Cultural neuroscience: Parsing universality and diversity across levels of analysis In: Kitayama S, Cohen D, editors. Handbook of Cultural Psychology. New York, NY: Guilford Press; pp 237–254. [Google Scholar]
- Chiao JY, Iidaka T, Gordon HL, Nogawa J, Bar M ( 2008): Cultural specificity in amygdala response to fear faces. J Cogn Neurosci 20: 2167–2174. [DOI] [PubMed] [Google Scholar]
- Corina D, Knapp H ( 2006): Special issue: Review sign language processing and the mirror neuron system. Cortex 42: 529–539. [DOI] [PubMed] [Google Scholar]
- Cross E, Hamilton A, Grafton S ( 2006): Building a motor simulation de novo: Observation of dance by dancers. Neuroimage 31: 1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross ES, Kraemer DJM, Hamilton AFC, Kelley WM, Grafton ST ( 2009): Sensitivity of the action observation network to physical and observational learning. Cerebral Cortex 19: 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lange FP, Spronk M, Willems R, Toni I, Bekkering H ( 2008): Complementary systems for understanding action intentions. Curr Biol 18: 454–457. [DOI] [PubMed] [Google Scholar]
- Den Ouden H, Frith U, Frith C, Blakemore S ( 2005): Thinking about intentions. Neuroimage 28: 787–796. [DOI] [PubMed] [Google Scholar]
- Desy M, Theoret H ( 2007): Modulation of motor cortex excitability by physical similarity with an observed hand action. PLoS One 2: e971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P, Happe F, Frith U, Baker S, Dolan R, Frackowiak R, Frith C ( 1995): Other minds in the brain: A functional imaging study of “theory of mind” in story comprehension. Cognition 57: 109–128. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G ( 2005): Parietal lobe: From action organization to intention understanding. Science 308: 662–667. [DOI] [PubMed] [Google Scholar]
- Frith C, Frith U ( 2006): The neural basis of mentalizing. Neuron 50: 531–534. [DOI] [PubMed] [Google Scholar]
- Gallagher H, Frith C ( 2004): Dissociable neural pathways for the perception and recognition of expressive and instrumental gestures. Neuropsychologia 42: 1725–1736. [DOI] [PubMed] [Google Scholar]
- Gallagher H, Happe F, Brunswick N, Fletcher P, Frith U, Frith C ( 2000): Reading the mind in cartoons and stories: An fmri study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia 38: 11–21. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G ( 1996): Action recognition in the premotor cortex. Brain 119: 593–609. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G ( 2004): A unifying view of the basis of social cognition. Trends Cogn Sci 8: 396–403. [DOI] [PubMed] [Google Scholar]
- Gentilucci M, Dalla Volta R ( 2008): Spoken language and arm gestures are controlled by the same motor control system. J Exp Psychol 61: 944–957. [DOI] [PubMed] [Google Scholar]
- Gusnard D, Raichle M ( 2001): Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694. [DOI] [PubMed] [Google Scholar]
- Han S, Northoff G ( 2008): Culture‐sensitive neural substrates of human cognition: A transcultural neuroimaging approach. Nat Rev Neurosci 9: 646–654. [DOI] [PubMed] [Google Scholar]
- Hesse M, Sparing R, Fink GR ( 2009): End or means: The “what” and “how” of observed intentional actions. J Cogn Neurosci 21: 776–790. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Molnar‐Szakacs I, Gallese V, Buccino G, Mazziotta J, Rizzolatti G ( 2005): Grasping the intentions of others with one's own mirror neuron system. PLoS Biol 3: e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihaka R, Gentleman R ( 1996): R: A language for data analysis and graphics. J Computat Graph Stat 299–314. [Google Scholar]
- Ito TA, Bartholow BD ( 2009): The neural correlates of race. Trends Cogn Sci 13: 524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Mcdermott J, Chun M ( 1997): The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, Gazzola V ( 2007): Integrating simulation and theory of mind: From self to social cognition. Trends Cogn Sci 11: 194–196. [DOI] [PubMed] [Google Scholar]
- Kilner J, Frith C ( 2008): Action observation: Inferring intentions without mirror neurons. Curr Biol 18: R32–R33. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons W, Bellgowan P, Baker C ( 2009): Circular analysis in systems neuroscience: The dangers of double dipping. Nat Neurosci 12: 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepelt R, Von Cramon DY, Brass M ( 2008): How do we infer others' goals from non‐stereotypic actions? The outcome of context‐sensitive inferential processing in right inferior parietal and posterior temporal cortex. Neuroimage 43: 784–792. [DOI] [PubMed] [Google Scholar]
- Maddock R, Garrett AS, Buonocore M ( 2001): Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104: 667–676. [DOI] [PubMed] [Google Scholar]
- Molnar‐Szakacs I, Wu A, Robles F, Iacoboni M ( 2007): Do you see what I mean? Corticospinal excitability during observation of culture‐specific gestures. PLoS One 2: e626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztop E, Arbib M ( 2002): Schema design and implementation of the grasp‐related mirror neuron system. Biol Cybern 87: 116–140. [DOI] [PubMed] [Google Scholar]
- Penny W, Holmes AP, Friston K ( 2004): Random effects analysis. Human Brain Function 843–850. [Google Scholar]
- Phinney J ( 1992): The multigroup ethnic identity measure: A new scale for use with adolescents and young adults from diverse groups. J Adolesc Res 7: 156–176. [Google Scholar]
- Rizzolatti G, Craighero L ( 2004): The mirror‐neuron system. Annu Rev Neurosci 27: 169–192. [DOI] [PubMed] [Google Scholar]
- Roberts R, Phinney J, Masse L, Chen Y, Roberts C, Romero A ( 1999): The structure of ethnic identity in young adolescents from diverse ethnocultural groups. J Early Adolesc 19: 301–322. [Google Scholar]
- Saxe R ( 2006): Uniquely human social cognition. Curr Opin Neurobiol 16: 235–239. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N ( 2003): People thinking about thinking people. The role of the temporo‐parietal junction in “theory of mind.” Neuroimage 19: 1835–1842. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell L ( 2006): It's the thought that counts: Specific brain regions for one component of theory of mind. Psychol Sci 17: 692–699. [DOI] [PubMed] [Google Scholar]
- Schippers M, Gazzola V, Goebel R, Keysers C ( 2009): Playing charades in the fmri: Are mirror and/or mentalizing areas involved in gestural communication? PLoS One 4: e6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serino A, Giovagnoli G, Ladavas E ( 2009): I feel what you feel if you are similar to me. PLoS One 4: e4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper J, Goldin‐Meadow S, Nusbaum H, Small S ( 2009): Gestures orchestrate brain networks for language understanding. Curr Biol 19: 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube B, Green A, Weis S, Chatterjee A, Kircher T ( 2009): Memory effects of speech and gesture binding: Cortical and hippocampal activation in relation to subsequent memory performance. J Cogn Neurosci 21: 821–836. [DOI] [PubMed] [Google Scholar]
- Uddin L, Iacoboni M, Lange C, Keenan J ( 2007): The self and social cognition: The role of cortical midline structures and mirror neurons. Trends Cogn Sci 11: 153–157. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K ( 2009): Understanding others' actions and goals by mirror and mentalizing systems: A meta‐analysis. Neuroimage 48: 564–584. [DOI] [PubMed] [Google Scholar]
- Villarreal M, Fridman E, Amengual A, Falasco G, Gerscovich E, Ulloa E, Leiguarda R ( 2008): The neural substrate of gesture recognition. Neuropsychologia 46: 2371–2382. [DOI] [PubMed] [Google Scholar]
- Vogt S, Buccino G, Wohlschläger A, Canessa N, Shah N, Zilles K, Eickhoff S, Freund H, Rizzolatti G, Fink G ( 2007): Prefrontal involvement in imitation learning of hand actions: Effects of practice and expertise. Neuroimage 37: 1371– 1383. [DOI] [PubMed] [Google Scholar]
- Willems R, Ozyurek A, Hagoort P ( 2007): When language meets action: The neural integration of gesture and speech. Cereb Cortex 17: 2322. [DOI] [PubMed] [Google Scholar]
- Wolperts D, Goodbody S, Husain M ( 1998): Maintaining internal representations: The role of the human superior parietal lobe. Nat Neurosci 1: 529–533. [DOI] [PubMed] [Google Scholar]
- Xu X, Zuo X, Wang X, Han S ( 2009): Do you feel my pain? Racial group membership modulates empathic neural responses. J Neurosci 29: 8525–8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Supporting Information


