Abstract
Somatosensory signals modulate activity throughout a widespread network in both of the brain hemispheres: the contralateral as well as the ipsilateral side of the brain relative to the stimulated limb. To analyze the ipsilateral somatosensory brain areas that are engaged during limb stimulation, we performed functional magnetic resonance imaging (fMRI) in 12 healthy subjects during electrical median nerve stimulation using both a block‐ and an event‐related fMRI design. Data were analyzed through the use of model‐dependent (SPM) and model‐independent (ICA) approaches. Beyond the well‐known positive blood oxygenation level‐dependent (BOLD) responses, negative deflections of the BOLD response were found consistently in several ipsilateral brain areas, including the primary somatosensory cortex, the supplementary motor area, the insula, the dorsal part of the posterior cingulate cortex, and the contralateral cerebellum. Compared to their positive counterparts, the negative hemodynamic responses showed a different time course, with an onset time delay of 2.4 s and a peak delay of 0.7 s. This characteristic delay was observed in all investigated areas and verified by a second (purely tactile) event‐related paradigm, suggesting a systematic difference for brain areas involved in the processing of somatosensory information. These findings may indicate that the physiological basis of these deactivations differs from that of the positive BOLD responses. Therefore, an altered model for the negative BOLD response may be beneficial to further model‐dependent fMRI analyses. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: fMRI, BOLD, median nerve, tactile, inhibition
INTRODUCTION
Stimulation of the median nerve and tactile stimulation both activates the primary somatosensory cortex (SI), which comprises four different cytoarchitectonical subdivisions (areas 3a, 3b, 1, and 2) [Brodmann, 1909; Vogt and Vogt, 1919]. Electrophysiological and imaging studies have shown that in addition to the SI, a widespread brain matrix is activated. This activation involves the secondary somatosensory cortex (SII) bilaterally, the contralateral posterior parietal cortex (PPC), the insula, the posterior midcingulate gyrus (pMCG), the supplementary motor area (SMA), the thalamus, and the ipsilateral cerebellum [Arienzo et al., 2006; Backes et al., 2000; Boakye et al., 2000; Del Gratta et al., 2000; Deuchert et al., 2002; Ferretti et al., 2003; Kampe et al., 2000; Karhu and Tesche, 1999; Korvenoja et al., 1999; Nihashi et al., 2005; Schnitzler et al., 1999]. Within this network, several aspects of the sensory signal are processed, including the location, intensity, pleasantness, pain, preparation for action, and affective interpretation [Arienzo et al., 2006; Bingel et al., 2002; Del Gratta et al., 2000; Ferretti et al., 2003; Henderson et al., 2007; Lamm et al., 2007; Schnitzler and Ploner, 2000].
Information processing in the brain does not only involve activation of several brain regions, but also involves the deactivation of other regions. The physiological basis of these deactivations has only been partially characterized. It is well established that neuronal activation is associated with a positive blood oxygenation level‐dependent signal (BOLD) response [Logothetis et al., 2001; Mukamel et al., 2005; Niessing et al., 2005]. Whether strong inhibitions, in a manner similar to their association with increased brain metabolism [Bruehl et al., 1995, 1998], are associated with positive or negative BOLD responses is still controversial [Lipton et al., 2006; Sotero and Trujillo‐Barreto, 2007]. However, animal experiments have revealed a tight coupling between negative BOLD responses and decreases in neuronal activity [Shmuel et al., 2006] or enhanced inhibitions [Devor et al., 2007]. We recently demonstrated that the negative BOLD response observed in the ipsilateral somatosensory cortex following stimulation of the median nerve is associated with an elevation of the sensory threshold, suggesting an underlying inhibition or disfacilitation [Kastrup et al., 2008]. The negative BOLD response observed in the contralateral cortex following a subliminal stimulation was also accompanied by an elevation of the perception threshold [Blankenburg et al., 2003]. Thus, at least in some areas and under some conditions, the negative BOLD response appears to correspond to a net inhibition in task‐specific brain regions.
The interaction between both hemispheres during the processing of somatosensory information is not fully understood. Electrophysiological investigations of the somatosensory network in animals suggest an interhemispheric inhibition between homologous parts of area 3b [Clarey et al., 1996; Lipton et al., 2006]. Iwamura et al. [1994] reported bilateral excitatory receptive fields in nonhuman primates. A careful inspection of the data obtained by Iwamura et al. [1994] suggests, however, that the activation reported in that study concerned neurons in the anterior wall of the intraparietal sulcus, that is, more posterior brain areas (predominantly area 5) [Iwamura et al., 1994, 2001]. Studies addressing this issue in the human brain have arrived at ambiguous conclusions. There are reports of an activation of the ipsilateral PPC [Del Gratta et al., 2000; Nihashi et al., 2005] and area 2 [Nihashi et al., 2005]. Others have not reported significant BOLD responses within the ipsilateral SI or PPC [Backes et al., 2000; Boakye et al., 2000; Ferretti et al., 2003]. These studies have focused on positive BOLD responses, and it remains unclear whether all these studies have also tested for negative responses. A negative BOLD response following tactile stimulation was recently described within the ipsilateral SI and motor cortex (MI) [Hlushchuk and Hari, 2006]. In addition, there are reports of negative BOLD responses within the ipsilateral primary MI [Hamzei et al., 2002; Newton et al., 2005; Stefanovic et al., 2004]. Moreover, ipsilateral SI signal changes during median nerve stimulation were observed with magnetoencephalography [Kanno et al., 2003; Korvenoja et al., 1999], although these studies did not distinguish between activation and deactivation. Sutherland and Tang [2006] found bilateral SI activity with the use of electroencephalography (EEG).
In this present study, we systematically analyzed BOLD responses in the hemisphere ipsilateral to stimulation. Given the complexity of somatosensory information processing, we hypothesized that ipsilateral deactivations are not limited to the primary somatosensory area. We were interested in the time course of the negative BOLD response following event‐related stimulation. We also analyzed whether ipsilateral deactivations were similar in event‐related and blocked stimulation paradigms. The characteristic time course of the responses to median nerve stimulation was verified by using a second (purely tactile) stimulation paradigm.
MATERIALS AND METHODS
Subjects
Twelve healthy volunteers (mean age, 23.1 ± 1.6 years; range, 21–26 years) without any history of neurological or psychiatric diseases participated in this study. All subjects were right‐handed and female to avoid possible confounding effects due to gender‐specific activation patterns [Cosgrove et al., 2007]. Handedness was determined using the Edinburgh Handedness Inventory [Oldfield, 1971]. The study was approved by the local ethics committee, and all subjects gave their written informed consent according to the declaration of Helsinki.
Stimulation Procedures
In this study, we used two different forms of somatosensory stimuli: an electrical median nerve stimulation and a more physiological tactile stimulation. A median nerve stimulus was used to investigate activation‐ and deactivation patterns within the somatosensory system in all subjects. Although this stimulus may be considered unphysiological, we used it for two reasons. First, most previously reported investigations of the ipsilateral SI were based on such a stimulus, and we used the same approach to ensure optimal comparability to these studies. Second, after considering previous studies, we expected only small ipsilateral signal changes [Hlushchuk and Hari, 2006; Nihashi et al., 2005] and wanted to maximize the cortical response. The stimulus was applied unilaterally at the right wrist and consisted of clinical neurostimulator‐generated (Digitimer Constant Current Stimulator model DS7A) 40 Hz monophasic square wave pulses with a duration of 200 μs. The stimulus intensity was chosen at the lowest intensity that could trigger a motor response (5.1 mA ± 0.89) and presented in a block (30 s on/30 s off, 24 repetitions, experiment I) and event‐related regime (2 s on, 100 repetitions, experiment II). In an additional experiment (experiment III), we used a tactile stimulus in 8 of the 12 subjects to verify the characteristic time course of the negative BOLD response with a more physiological natural stimulus. Tactile stimuli were delivered to fingers 1–3 of the right hand by balloon diaphragms driven by compressed air. Each stimulus lasted for 100 ms (20‐ms rise time, 30‐ms plateau, and 50‐ms return to baseline pressure). The tactile stimuli were presented in an event‐related regime with the same configuration as experiment II (2 s on, 100 repetitions). No subject reported any painful perception in the tactile or electrical event‐related design, whereas subjects reported unpleasant to painful perceptions in the electrical block design. The event‐related interstimulus time was randomized between 8.7 and 15.8 s to avoid systematic errors in hemodynamic response function estimation.
Functional Magnetic Resonance Imaging Recordings
All experiments were performed on a 3.0‐T MR scanner (Trio, Siemens, Erlangen, Germany) to obtain echo‐planar T2*‐weighted image volumes (EPI) and transaxial T1‐weighted structural images. Functional data were acquired in two EPI sessions of 493 (block design) and 627 (event‐related design) volumes. The first three volumes were subsequently discarded due to equilibration effects. A functional‐image volume comprised 40 transaxial slices including the whole cerebrum and cerebellum (voxel size = 3 mm ×3 mm ×3 mm, repetition time = 3 s, TE 35 ms) for the block design and 20 transaxial slices including the cortex down to the SII (voxel size = 3 mm ×3 mm ×3 mm, repetition time = 2 s, TE 35 ms) for the event‐related design. The high‐resolution T1‐weighted structural images had a voxel size of 1 mm × 1 mm × 1 mm to allow for precise anatomical localization.
Data Analysis
Data analysis was performed on a PC using MATLAB (Mathworks, Natiek, MA) and SPM5 software (Wellcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm). For each subject, all images were realigned to the first volume using six parameter rigid‐body transformations to correct for motion artifacts [Friston et al., 1995]. The images were coregistered with the subject's corresponding anatomical (T1‐weighted) images, resliced to correct for acquisition delays (referenced to the tenth slice only in the event‐related design), normalized to the Montreal Neurological Institute (MNI) standard brain [Evans et al., 1993] to report MNI coordinates, and smoothed using a 6‐mm full‐width‐at‐half‐maximum Gaussian kernel.
Statistical analysis was performed using the general linear model to obtain statistical parametric maps by performing a multiple regression analysis. Statistical parametric maps for positive and negative T‐contrast were calculated for every condition and subject. Based on pilot experiments, there was evidence for a time delay of the negative BOLD response when compared with the positive BOLD response. Therefore, we used the inverse standard HRF function, available in SPM with a time lag of 1 s to account for a possible delay of the negative BOLD response in the event‐related design. Functional MRI signal time courses were high‐pass filtered (30‐s event‐related design, 128‐s block design) and modeled as experimental stimulus onset functions convolved by the canonical hemodynamic response function (low‐pass filter). Individual results were projected onto the coregistered individual high‐resolution, T1‐weighted 3‐D data set. The anatomical localizations of activations were analyzed by referencing the standard stereotaxic atlas and mapped by using the anatomical toolbox of the SPM software [Eickhoff et al., 2005, 2006] (http://www.fz-juelich.de/ime/spm_anatomy_toolbox). Furthermore, all activations were localized by visual inspection of the individual T1‐weighted structural data. The individual maps were used to perform a random effect analysis to obtain consistent group activation patterns. The resulting group statistical maps were thresholded by the false discovery rate (FDR) [Genovese et al., 2002]. Because of our anatomical a priori hypothesis (deactivations were assumed to occur in the ipsilateral somatosensory brain areas), deactivation T‐maps were thresholded at P < 0.001 uncorrected.
In addition to the model‐based analysis, we performed an independent component analysis (ICA) using the preprocessed images (realigned, coregistered, normalized, and smoothed). Twenty components were estimated for each experiment using the infomax algorithm implemented in the “gift” toolbox [Calhoun et al., 2001a, 2009]. The chosen number of components provided a reasonable trade‐off between preserving relevant variance in the data while easing the burden of interpretation [Calhoun et al., 2001b].
The independently obtained components were converted to T‐maps. Because we were interested in task‐related changes in the somatosensory cortex, we used this knowledge for a combined a priori temporal and spatial approach for the selection of independent components (ICs). The components were ranked by the correlation of their associated time courses with the presumed hemodynamic responses. The five ICs with the closest correlation were overlaid on coregistered 3D brain volumes from each subject and visually inspected. Components whose spatial location at least partly overlapped the primary or secondary somatosensory cortex in more than half of the subjects were selected for group analysis. According to these criteria, we selected three ICs in both event‐related designs and two ICs in the block design. Next, a voxel‐wise random effects analysis was performed on the component image by entering the single‐subject component images into a one‐sample t‐test. The resulting images were thresholded in analogy to the SPM T‐maps.
Area‐Specific BOLD‐Response Analysis
A Brodmann area (BA)‐specific analysis was performed in the somatosensory system to address area‐specific differences of the BOLD response following median nerve and tactile stimulation. For this purpose, we used unsmoothed data to discriminate between activations in distinct areas. To identify an area, we used population maps from the anatomical toolbox of SPM [Eickhoff et al., 2005, 2006]. The 30 voxels surrounding the point of maximum activation (SPM analysis) within an activated cluster in a specific individual BA were extracted from unsmoothed data. The mean peristimulus time course was estimated and averaged across all subjects who exhibited significantly activated voxels. To quantify the differences between the positive and the negative BOLD responses, we estimated two different parameters:
time to peak: time between stimulus onset and maximum amplitude
time to main response: time between stimulus onset and the end of the initial negative/positive dip where the BOLD response hit the baseline.
In order to compare these results with the ICA, we averaged the time course of the main IC of the right and left SI across all subjects.
RESULTS
Positive BOLD Response Following Median Nerve Stimulation
Electrical stimulation of the right median nerve in the event‐related design evoked highly significant activations (P = 0.003, FDR corrected) in the random effect group analysis (Fig. 1). These activations comprised the contralateral (left) SI/MI, the bilateral SII, the left SMA, and the pMCG. All these areas exhibited significant activity in every single subject in a subject‐specific analysis. Table I summarizes the MNI coordinates and t‐values of peak activation for the identified clusters in the event‐related stimulation group. In the subject‐specific analysis, the activation cluster within the SI/MI region revealed at least two local maxima that are reflected in the group analysis (Table I). The stronger activation was located in the anterior wall of the postcentral gyrus (BA 3b) and the crown of the postcentral gyrus (BA 1), whereas the second maximum was located in the precentral gyrus (BA 4/6). Furthermore, area 2 showed a spatially extended activation with a lower significance compared with areas 3b, 1, or 6.
Figure 1.
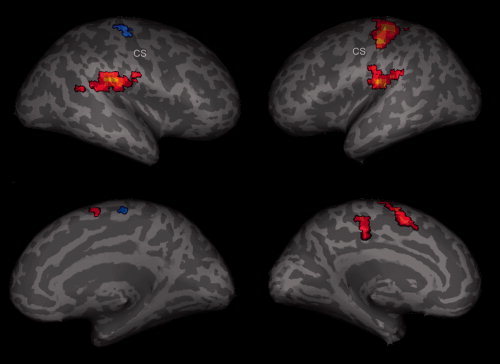
Random effect group (n = 12) analysis. Activations (P < 0.003, FDR corrected) and deactivations (P < 0.001, uncorrected) in response to event‐related (2 s) right median nerve stimulation are shown superimposed on an inflated brain, showing views on the right and left hemisphere. Yellow‐red encode positive BOLD signals whereas blue encode negative BOLD signals.
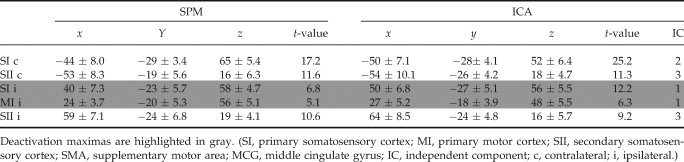
Table I.
MNI coordinates of activation/deactivation maxima with corresponding t‐value for the event‐related median nerve stimulation
| SPM | ICA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | t‐value | x | y | z | t‐value | IC | |
| SI c | −48 ± 5.6 | −21 ± 5.7 | 48 ± 7.3 | 15.27 | −36 ± 5.2 | −30 ± 3.4 | 60 ± 8.1 | 9.5 | 2 |
| MI c | −24 ± 5.2 | −18 ± 4.2 | 69 ± 4.5 | 11.65 | −33 ± 5.1 | −15 ± 4.2 | 63 ± 7.5 | 8.5 | 2 |
| SI/MI i | 48 ± 4.8 | −18 ± 4.3 | 57 ± 5.3 | 8.63 | 36 ± 6.2 | −27 ± 5.1 | 57 ± 5.4 | 5.4 | 1 |
| SII c | −45 ± 4.9 | −24 ± 3.1 | 18 ± 3.8 | 11.46 | −57 ± 7.6 | −21 ± 3.6 | 18 ± 3.4 | 8.1 | 3 |
| SII i | 48 ± 10.8 | −27 ± 5.6 | 21 ± 5.3 | 10.21 | 57 ± 8.8 | −21 ± 4.2 | 21 ± 3.9 | 9.1 | 3 |
| SMA c | −6 ± 3.4 | 6 ± 4.1 | 45 ± 5.6 | 8.18 | −9 ± 4.2 | 12 ± 5.6 | 54 ± 6.2 | 5.9 | 2 |
| SMA i | 9 ± 3.9 | −21 ± 3.1 | 57 ± 8.0 | 4.92 | 9 ± 4.2 | −3 ± 6.2 | 48 ± 9.1 | 6.2 | 1 |
| pMCG c | −9 ± 2.9 | −27 ± 2.3 | 48 ± 3.2 | 6.93 | −6 ± 3.5 | −27 ± 3.1 | 48 ± 2.8 | 7.7 | 2 |
Deactivation maximas are highlighted in gray. (SI, primary somatosensory cortex; MI, primary motor cortex; SII, secondary somatosensory cortex; SMA, supplementary motor area; MCG, middle cingulate gyrus; IC, independent component; c, contralateral; i, ipsilateral.)
In the hemisphere ipsilateral to the stimulated nerve (right cortex), no group activations were found in the primary somatosensory or primary MI, even if the significance level was reduced to an uncorrected P < 0.001. However, activated voxels in this region were found in 10 of 12 volunteers in the single‐subject analysis. These activations were mainly located in BA 2, but were also in the premotor area and in the PPC.
The median nerve stimulation in the block design revealed a slightly different activation pattern. As shown in Table II, the activation maximum in the group analysis was located more on the crown of the postcentral gyrus. The left SII was significantly activated, whereas the activation of the right SII and right midcingulate cortex observed in the event‐related design remained below the significance level in the block design. Furthermore, no significant SMA activation could be detected. Other activated areas were the left insula (not activated in the event‐related design) and some areas not covered in the event‐related design (left thalamus, left putamen, and right cerebellum) (Tab. II).
Table II.
MNI coordinates of activation/deactivation maxima with corresponding t‐value for the blocked median nerve stimulation
| SPM | ICA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | t‐value | x | y | z | t‐value | IC | |
| SI/MI c | −48 ± 5.1 | −21 ± 3.5 | 54 ± 6.5 | 12.83 | −45 ± 4.8 | 21 ± 3.7 | 54 ± 5.2 | 13.2 | 2 |
| SI/MI i | 48 ± 9.7 | −18 ± 3.4 | 60 ± 10.3 | 4.73 | 33 ± 8.2 | −27 ± 3.7 | 51 ± 9.1 | 6.1 | 1 |
| SII c | −57 ± 6.7 | −21 ± 8.3 | 21 ± 3.2 | 6.60 | −57 ± 8.1 | −24 ± 5.7 | 15 ± 4.2 | 14.4 | 2 |
| SII i | 42 ± 7.6 | −27 ± 7.2 | 18 ± 4.2 | 12.2 | 2 | ||||
| SMA c | −9 ± 2.9 | −6 ± 6.5 | 48 ± 3.7 | 12.2 | 2 | ||||
| SMA i | 9 ± 2.3 | −12 ± 3.1 | 63 ± 4.2 | 5.00 | 9 ± 3.1 | −15 ± 6.6 | 51 ± 4.6 | 5.9 | 1 |
| dPCC i | 3 ± 2.8 | −36 ± 10.7 | 48 ± 3.7 | 5.55 | 3 ± 4.1 | −39 ± 8.7 | 30 ± 5.2 | 4.0* | 1 |
| Cerebellum c | −33 ± 6.4 | −42 ± 11.3 | −33 ± 8.0 | 6.08 | −30 ± 5.7 | −48 ± 12.1 | −33 ± 7.4 | 3.9* | 1 |
| Cerebellum i | 18 ± 3.3 | −60 ± 6.1 | −21 ± 4.1 | 6.44 | 18 ± 4.1 | −54 ± 5.2 | −21 ± 8.3 | 8.9 | 2 |
| Insula c | −36 ± 2.8 | −18 ± 2.6 | 12 ± 4.3 | 4.66 | −39 ± 2.9 | −15 ± 3.7 | 12 ± 4.5 | 4.0 | 2 |
| Insula i | 30 ± 3.3 | −21 ± 6.4 | 15 ± 5.8 | 5.82 | 36 ± 3.1 | −18 ± 4.6 | 18 ± 4.2 | 5.1 | 1 |
| Putamen c | −27 ± 2.6 | 0 ± 4.1 | −3 ± 3.7 | 8.72 | −30 ± 2.1 | −6 ± 4.3 | −3 ± 2.3 | 6.2 | 2 |
Deactivation maximas are highlighted in gray. (SI, primary somatosensory cortex; MI, primary motor cortex; SII, secondary somatosensory cortex; SMA, supplementary motor area; MCG, middle cingulate gyrus; IC, independent component; c, contralateral; i, ipsilateral; *, not significant.)
Negative BOLD Response Following Median Nerve Stimulation
To address negative BOLD responses in the event‐related design data sets, we used the inverse standard HRF function with a time lag of 1 s, which revealed a significant (P < 0.05, familywise error corrected and FDR corrected) deactivation in the random effect group analysis in the right hemispheric SI (Table I). Deactivations were found in the right MI and right SMA at a significance of P = 0.001 uncorrected (see Fig. 1). At the individual level, significant (P = 0.001, uncorrected) right hemispheric SI/MI deactivations could be observed in all subjects. This deactivation comprised the anterior wall of the postcentral gyrus (BA 3b) and the crown of the postcentral gyrus (BA 1). Further deactivations were found on the precentral gyrus, and significant SMA deactivation was seen in 9 of 12 subjects.
The group analysis of the block design revealed a significant deactivation in the right SI/MI in a spatial localization highly congruent to the event‐related design. However, the t‐values were slightly smaller (Table II, Fig. 2). The deactivation cluster seen in the SMA exhibited a similar significance, but was located more anteriorly in the block design. Beyond the event‐related deactivations, we found an additional deactivation in the bilateral dorsal part of the posterior cingulate cortex (dPCC). Moreover, deactivations appeared in the right posterior insula and left cerebellum (Tab. II, Fig. 2).
Figure 2.
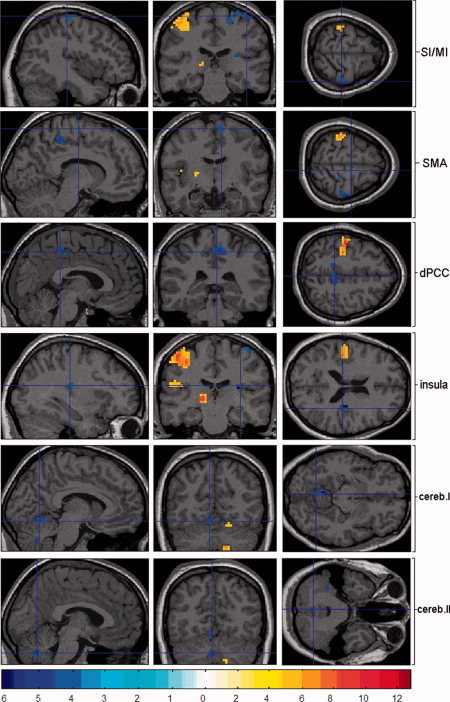
Random effect group (n = 12) analysis. Activations (P < 0.05, FDR corrected) and deactivations (P < 0.001, uncorrected) in response to blocked (30 s) right median nerve stimulation are shown superimposed on an individual brain in sagittal (left), coronal (middle), and axial (right) orientation. To investigate the network of negative BOLD signal changes, we focused the images on the deactivated clusters. Red encodes positive BOLD signals whereas blue encodes negative BOLD signals. Because of the use of different MRI‐sequences with different numbers of voxels, we used a different threshold for the activation t‐map compared to the event‐related design. Therefore, we are able to maintain a static t‐value threshold in order to provide a comparable visual impression.
BOLD Response Following Tactile Stimulation
Tactile stimulation of fingers 1–3 of the right hand in the event‐related design evoked significant activations in all subjects in the left SI and bilateral SII and significant deactivations in all subjects in the right SI. This deactivation comprised the anterior wall of the postcentral gyrus (BA 3b) and the crown of the postcentral gyrus (BA 1). Deactivations of area 4 were found ipsilateral in 6 of 8 subjects (SPM) and in all subjects using ICA (Table III).
Table III.
MNI coordinates of activation/deactivation maxima with corresponding t‐value for the event‐related tactile stimulation
|
Time Course of the BOLD Response
The peristimulus time course was investigated for distinct areas. Figures 3, 4, 5, 6 show the time course of the signal in the block and event‐related experiments for the 30 voxels surrounding the point of maximum activation and deactivation. The negative BOLD response showed a prolonged initial overshoot, resulting in a significant time delay (2.4 ± 0.71 s) of the negative BOLD response compared to the positive BOLD response (r = 0.76, P < 0.01; Pearson correlation coefficient). Correspondingly, a time‐to‐peak increase was observed for the negative BOLD response (7.38 ± 0.82 s) compared to the positive BOLD response (6.72 ± 0.71 s). Both seem to return to baseline at the same time (Figs. 3 and 4).
Figure 3.
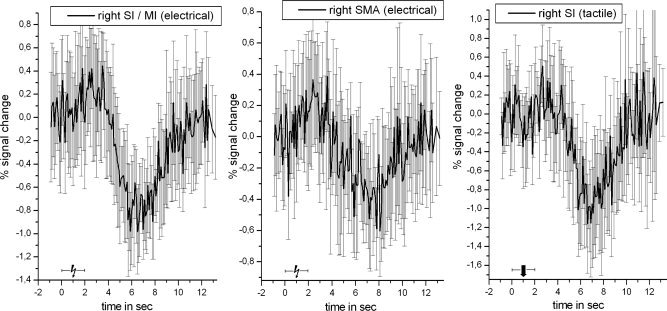
Event‐related negative BOLD signal time course for the most deactivated cluster (highest t‐value; inverse HRF) within right SI/MI (left), SMA (middle) following median nerve stimulation, and right SI following tactile stimulation (right). All data were averaged across 30 voxels surrounding the peak response. The time courses were extracted prior to spatial smoothing and were averaged across all subjects.
Figure 4.
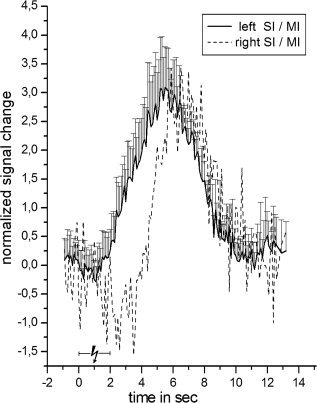
Event‐related positive BOLD signal time course for the most activated cluster (highest t‐value) within left SI (solid line) with standard deviation following median nerve stimulation. All data were averaged across 30 voxels surrounding the peak response. The time courses were extracted before spatial smoothing and were averaged across all subjects. Additionally, we plotted the inverse negative BOLD signal from Figure 3 relative (scaled) to the amplitude of the positive BOLD signal. Therefore, the y‐axis indicates the percent signal change solely for the positive BOLD signal (solid line).
Figure 5.
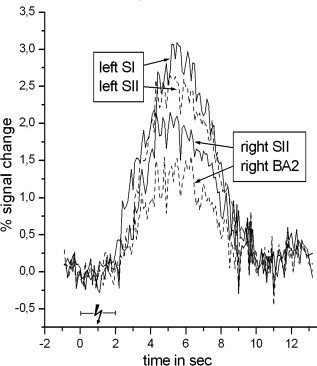
Event‐related positive BOLD signal time course for the most activated cluster (highest t‐value) within left SI, left SII, right SII, and right Brodmann area 2 following median nerve stimulation. All data were averaged across 30 voxels surrounding the peak response. The time courses were extracted before spatial smoothing and were averaged across all subjects.
Figure 6.
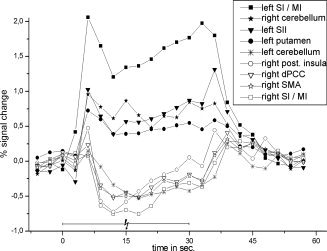
Blocked positive and negative BOLD signal time course for the most activated clusters (highest t‐value) within left/right SI/MI, left SII, left putamen, left/right cerebellum, right posterior insula, right SMA, and right dPCC during median nerve stimulation. All data were averaged across 30 voxels surrounding the peak response. The time courses were extracted prior to spatial smoothing and averaged across all subjects. The cerebellar activation/deactivation consisted of two/three different clusters, which were averaged.
The shape of the positive BOLD response was similar to the mirror image of its negative counterpart, with a larger amplitude and width (see Fig. 4). The mean maximum amplitude following median nerve stimulation in the left SI was a 3.09% ± 1.01% signal change, whereas the mean maximum negative amplitude in right SI was a −1.50% ± 0.39% signal change. We detected a small initial dip and a poststimulus undershoot for the positive BOLD response, whereas the negative response showed an extended initial positive overshoot. The positive BOLD response was plotted on top of the normalized and inverted negative response to provide a better visualization of differences in shape (Fig. 4).
Following tactile stimulation, the negative BOLD response in the ipsilateral SI cortex exhibited a time course comparable to that after median nerve stimulation. The amplitude in the SI amounted to −1.69% ± 0.46%, with a time to peak of 7.32 ± 0.93 s. Because of the signal averaging, the illustrated time course in Figure 3 shows slightly different amplitude values.
Figure 5 shows the positive BOLD responses following median nerve stimulation for the left SI, the bilateral SII, and the right BA 2. We found no substantial variation between the time courses beyond the differences in amplitude.
Because of the nonrandom onsets in the block design, the time course has a low‐temporal resolution compared to the event‐related design (Fig. 6). However, the time delay and the negative time course was reproducible and showed a maximum negative amplitude in the right SI of a −1.12% ± 0.28% signal change. Moreover, the amplitude of the negative BOLD response decreased during stimulation, and a poststimulus overshoot was observed.
ICA
The independent component analysis (ICA) revealed comparable results to the model‐based SPM analysis (Tables I, II, III). With all types of stimulation and in all subjects, we found different ICs for the right (IC‐1) and the left SI (IC‐2). The strongest positive correlation was revealed in the left SI, whereas the right SI component displayed the strongest negative temporal correlation to the stimulus presentation. The left SI IC also included bilateral SII activations and contralateral (left) activations of BA 40 and BA 2 in the block design, whereas a different IC (IC‐3) revealed these in both event‐related designs. The deactivations in the right SI and right SMA consistently shared the same IC (IC‐1) in all stimulation types. Moreover, the same IC also included the right insula and the bilateral MI in the block design. The group analysis of the two main ICs (right and left SI) of the block design is presented in Figure 7. The t‐values of the right dPCC and left cerebellum were estimated to be below the significance threshold. In addition to these two selected components, there were components that exhibited activation in the left SI and deactivation in the right SI in single subjects. However, none of these components revealed strong temporal correlations to the presented stimulus or displayed significant amplitudes within the somatosensory network in the group analysis. The temporal correlation between the stimulus onsets and the left SI IC was greater than for the right SI IC in all stimulation types. The event‐related averaged time courses for both of the main components are shown in Figure 8. The left SI IC was plotted on top of the normalized and inverted right SI response to provide a better visualization of differences in shape. The left SI IC revealed a time course very similar to the standard HRF, whereas the right SI IC showed a delayed negative time course. Furthermore, the right SI IC showed an extended initial positive overshoot.
Figure 7.
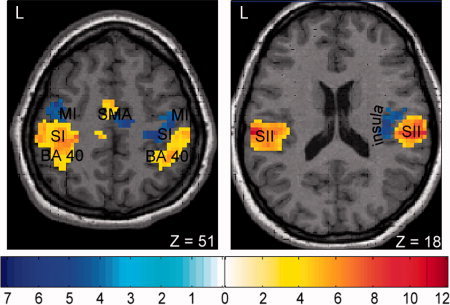
Results from the ICA group analysis (n = 12) for the blocked median nerve stimulation. The two main IC's are shown superimposed on an individual brain in axial orientation. Red encodes the IC with a positive correlation to the stimulation paradigm (activations; P < 0.05, FDR corrected), whereas blue encodes the IC with a negative correlation (deactivations; P < 0.001, uncorrected).
Figure 8.
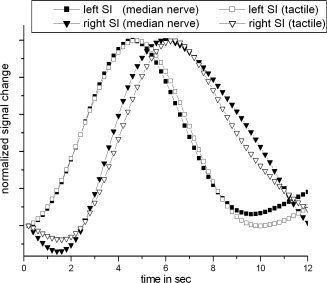
Event‐related estimated main components which correspond to left SI (squares) and right SI (triangle) following median nerve and tactile stimulation. All components were averaged across all subjects and normalized to a peak magnitude of one. Components that correspond to right SI were plotted with inverse sign to ensure a better visual comparability.
DISCUSSION
Median nerve stimulation activated the contralateral primary SI/MI as well as the contralateral or bilateral secondary somatosensory cortex (SII). This result is consistent with recent functional magnetic resonance imaging (fMRI), PET, MEG, and EEG studies [Backes et al., 2000; Boakye et al., 2000; Del Gratta et al., 2000; Deuchert et al., 2002; Ferretti et al., 2003; Kampe et al., 2000; Kanno et al., 2003; Karhu and Tesche, 1999; Korvenoja et al., 1999; Nihashi et al., 2005]. Moreover, activation of the contralateral pMCG and SMA was also found in recent fMRI studies of somatosensory stimulation [Arienzo et al., 2006; Christmann et al., 2007; Niddam et al., 2005; Nihashi et al., 2005; Vogt, 2005; Vogt and Laureys, 2005]. Also consistent with previous studies, the ipsilateral primary somatosensory cortex [Hlushchuk and Hari, 2006; Kastrup et al., 2008], the primary MI, and the contralateral cerebellum were deactivated [Hlushchuk and Hari, 2006]. In addition to the previous reports, we found significant negative BOLD responses in the ipsilateral SMA, the insula, and the bilateral dPCC. Deactivations were present in recordings obtained in the block design as well as in the event‐related design, and the deactivations were seen during both electrical and tactile stimulation. Notably, several deactivated brain areas were activated in the hemisphere contralateral to the stimulus, that is, the SI, the MI, the SMA, and the insula, with the expected inverse situation for the cerebellum that showed activation ipsilaterally and deactivation contralaterally. It is tempting to speculate that the ipsilateral deactivated areas observed in our study constitute a somatosensory network, because these areas were found mainly in one IC by the ICA, which identifies temporally coherent but spatially distinct networks [Calhoun et al., 2001a, 2009]. Moreover, inhibitory networks have also been described during motor tasks [Marchand et al., 2007], with tactile sensory processing in the visual cortex [Merabet et al., 2007] and with visual imagery, when compared with perception [Amedi et al., 2005]. However, to test this hypothesis, further studies that focus on the functional and effective connectivity of these areas are required.
SI
The ipsilateral deactivation in the SI after somatosensory stimulation seen in this study has also been described by Hlushchuk and Hari [2006] and Kastrup et al. [2008]. Other studies reported a positive BOLD response following median nerve stimulation in the ipsilateral SI; these responses were located in area 2 [Nihashi et al., 2005] or the posterior part of the SI [Korvenoja et al., 1999]. In animal experiments, Iwamura et al. [1994] found small ipsilateral activations between areas 2 and 5. We also observed such activations in this study (data not shown), although at a lower significance level than the deactivations. These activations were restricted to areas 2 and 5 by the model‐based approach. The present findings concerning the SI mirror those seen in motor studies. A deactivation of the ipsilateral MI was observed in several studies following finger movements [Hamzei et al., 2002; Newton et al., 2005; Stefanovic et al., 2004].
MI
The observed ipsilateral deactivation of area 4 is in line with a recent report [Hlushchuk and Hari, 2006]. However, Hlushchuk and Hari [2006] found bilateral deactivations in area 4, whereas significant contralateral MI deactivations were found only in the block design by ICA. For both event‐related types of stimulation (electrical and tactile), no contralateral MI deactivations were found. This different deactivation pattern of the MI compared to that found in the study of Hlushuck and Hari [2006] seems to indicate that contralateral MI deactivation is more dependent on the duration than on the type of stimulation.
dPCC
The predominantly ipsilateral deactivation of the cingulate cortex in our subjects comprised the rostral part of the dorsal part of the posterior cingulate cortex (dPCC) and the cingulate sulcus, near the marginal ramus. To the best of our knowledge, this is the first report of the detection of such a deactivation of the dPCC by the use of fMRI. It confirms a previous report of reduced rCBF in the PCC during painful stimulation using PET [Vogt et al., 1996]. It is noteworthy that studies of the cingulate cortex do not report positive BOLD responses following somatosensory stimulation at this location [Vogt and Laureys, 2005], indicating that this area is not activated by the processing of somatosensory information. Recently, it was suggested that pMCG and dPCC are involved in visuospatial orientation in response to sensory stimuli [Vogt, 2005]. These regions probably mediate rapid body orientation and movement toward somatosensory stimuli via the caudal cingulate motor area and the SMA [Vogt, 2005]. We therefore hypothesize that, in this context, the ability for rapid visuospatial orientation (dPCC) and movements (SMA) of the contralateral hand is reduced.
Insula
The insula most likely must be divided into two different parts: a granular posterior portion and an agranular anterior part [Dupont et al., 2003]. A significant activation of the contralateral posterior and ipsilateral anterior insula was found by the use of fMRI during different painful stimuli, whereas the contralateral anterior and ipsilateral posterior insula displayed marginal or no activation [Bingel et al., 2002, 2003; Ferretti et al., 2003; Henderson et al., 2007; Schnitzler and Ploner, 2000]. Consistent with these studies, we observed a significant activation of the contralateral posterior insula and a significant activation of the anterior ipsilateral insula in 3 of 12 subjects. In the complementary ipsilateral posterior insula, we found a significant negative BOLD response. In accordance with this, our subjects reported a painful or unpleasant perception during the block (but not during the event‐related) design. During tactile stimulation, no significant negative BOLD response was found in the insula. Therefore, we suggest that insula deactivation is related to pain processing rather than tactile processing. Moreover, with respect to the functional correlate of ipsilateral SI deactivation, one could hypothesize that the insula deactivation may alter the pain perception for the hand that is not stimulated. This could be one possible explanation for the effectiveness of electrical nerve stimulation in relieving chronic pain [Johnson and Martinson, 2007]. However, further studies are required to elucidate the role of the ipsilateral insula for pain processing.
Time Course
In this study, the ipsilateral deactivations observed in the event‐related design were time‐locked to the stimuli, while showing a characteristic time course with a delay of the negative BOLD response by 2.4 s. This delay was also found in the main IC of the ipsilateral SI. The time course of the main SI ICs roughly corresponded to the signal time course extracted from the unsmoothed data. However, differences in the width and time to the peak of the negative and positive BOLD responses point to a participation of other ICs that were not significant in the group analysis. Because of the time resolution of the recordings, the data from the block design experiments do not allow an exact quantification of the delay; however, we estimate the delay to be on the order of seconds. This delay was observed in all investigated areas during both electrical and tactile stimulation and was similar to that observed by Meltzer et al. [2008] in the hippocampus. Accordingly, the different deactivated brain areas were found mainly in one component by the ICA analysis. We therefore suggest that this characteristic time delay is a general phenomenon of the negative BOLD response, at least in the somatosensory system.
As a possible explanation, one could formally argue that this delay is caused by the interhemispheric information transfer time. However, it seems very unlikely that the transfer of information from one hemisphere to the other requires such a long time; in electrophysiological studies, callosal transfer times between 6 and 20 ms have been calculated [Duque et al., 2007]. Other arguments against this theory are the absence of a time delay for the ipsilateral positive BOLD response in SII and BA 2 and the initial overshoot of the negative BOLD response, which starts simultaneously with the negative dip of the positive BOLD response and indicates a nearly undelayed information processing.
This initial overshoot of the negative BOLD response could point to an initial reduction of oxygen consumption, causing an initial overshoot of oxyhemoglobin [Devor et al., 2007] before a reduction of the local cerebral blood volume (CBV) takes place [Devor et al., 2007; Harel et al., 2002]. Such a decreased CBV and the corresponding decreased oxyhemoglobin concentration may be caused by vasoconstriction [Devor et al., 2007], thereby resulting in the measured negative BOLD response [Devor et al., 2007; Harel et al., 2002]. Devor et al. [2007] observed a time delay of vasoconstriction and its associated negative BOLD response compared to vasodilatation and its associated positive BOLD response. It is important to emphasize that Devor et al. [2007] and Harel et al. [2002] measured the negative BOLD response in the surroundings of a positive response in animals. In this study, isolated negative BOLD responses are described, which may also be due to a different, hitherto unknown mechanism. Based on the observation of the characteristic time course, we hypothesize that the physiological basis of a negative BOLD response differs from that of a positive one. As a consequence, a model‐based analysis of negative BOLD responses requires a response function different from that used for the analysis of conventional positive responses. Therefore, we used a delayed inverse HRF in the model‐based SPM analysis. The validity of this approach was confirmed by ICA, which revealed comparable results without constraining the shape of the temporal response.
Interhemispheric Pathway
The pathways conveying the information from one hemisphere to the other require further consideration. The maximum activation in the left hemisphere was located in areas 3b and 1. According to morphological and electrophysiological studies, these areas do not have reciprocal transcallosal connections [Killackey et al., 1983] or bilateral receptive fields [Iwamura et al., 1994, 2001]. However, interhemispheric transcallosal connections within the most caudal part of the SI (BA 2) have been described [Killackey et al., 1983]. Moreover, the existence of bilateral receptive fields for the hand could also be shown in area 5 and possibly area 2 using electrophysiology [Iwamura et al., 1994, 2001]. We found slight activations of ipsilateral areas 2 and 5 that were possibly due to a transcallosal information transfer. These observations are in line with former results [Nihashi et al., 2005]. Area 2 has dense reciprocal connections to area 1, area 3b, and the MI and may, in turn, alter the activity in these areas, which may result in decreased activity. This model is supported by the fact that ipsilateral activation observed in healthy subjects was not detectable in callosotomized patients [Fabri et al., 1999, 2001]. Therefore, in agreement with Hlushchuk and Hari [2006], we suggest an interhemispheric transcallosal information transfer between areas 2 and 5 that results in an inhibition of ipsilateral areas 3b and 1.
CONCLUSION
This study shows for the first time that deactivations after somatosensory stimulation are not limited to the primary somatosensory cortex, but are also detectable in the ipsilateral SMA, the ipsilateral insula, and the bilateral dPCC. We suggest that not only are brain areas with a positive BOLD response involved in the processing of somatosensory information, but also multiple, mainly ipsilateral‐localized brain areas that are characterized by a negative BOLD response. Although the functional significance of these specific deactivations requires further investigation, preliminary functional studies as well as recent investigations of the underlying neuronal function suggest that these negative responses indicate neuronal deactivation or disfacilitation. A detailed investigation of the time courses revealed a different shape and a characteristic time delay of the negative BOLD responses compared to the positive ones. Considering these different time courses in hemodynamic models for the BOLD responses is likely to improve model‐dependent fMRI analyses.
Acknowledgements
The authors thank the reviewers for their helpful comments and insights.
REFERENCES
- Amedi A, Malach R, Pascual‐Leone A ( 2005): Negative BOLD differentiates visual imagery and perception. Neuron 48: 859–872. [DOI] [PubMed] [Google Scholar]
- Arienzo D, Babiloni C, Ferretti A, Caulo M, Del Gratta C, Tartaro A, Rossini PM, Romani GL ( 2006): Somatotopy of anterior cingulate cortex (ACC) and supplementary motor area (SMA) for electric stimulation of the median and tibial nerves: An fMRI study. Neuroimage 33: 700–705. [DOI] [PubMed] [Google Scholar]
- Backes WH, Mess WH, van Kranen‐Mastenbroek V, Reulen JP ( 2000): Somatosensory cortex responses to median nerve stimulation: fMRI effects of current amplitude and selective attention. Clin Neurophysiol 111: 1738–1744. [DOI] [PubMed] [Google Scholar]
- Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C ( 2002): Subcortical structures involved in pain processing: Evidence from single‐trial fMRI. Pain 99: 313–321. [DOI] [PubMed] [Google Scholar]
- Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C ( 2003): Single trial fMRI reveals significant contralateral bias in responses to laser pain within thalamus and somatosensory cortices. Neuroimage 18: 740–748. [DOI] [PubMed] [Google Scholar]
- Blankenburg F, Taskin B, Ruben J, Moosmann M, Ritter P, Curio G, Villringer A ( 2003): Imperceptible stimuli and sensory processing impediment. Science 299: 1864. [DOI] [PubMed] [Google Scholar]
- Boakye M, Huckins SC, Szeverenyi NM, Taskey BI, Hodge CJ Jr. ( 2000): Functional magnetic resonance imaging of somatosensory cortex activity produced by electrical stimulation of the median nerve or tactile stimulation of the index finger. J Neurosurg 93: 774–783. [DOI] [PubMed] [Google Scholar]
- Brodmann K ( 1909): Vergleichende Lokalisationslehre der Goßhirnrinde. Leipzig: J.A. Barth. [Google Scholar]
- Bruehl C, Kloiber O, Hossman KA, Dorn T, Witte OW ( 1995): Regional hypometabolism in an acute model of focal epileptic activity in the rat. Eur J Neurosci 7: 192–197. [DOI] [PubMed] [Google Scholar]
- Bruehl C, Hagemann G, Witte OW ( 1998): Uncoupling of blood flow and metabolism in focal epilepsy. Epilepsia 39: 1235–1242. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ ( 2001a): A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14: 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, McGinty VB, Pekar JJ, Watson TD, Pearlson GD ( 2001b): fMRI activation in a visual‐perception task: Network of areas detected using the general linear model and independent components analysis. Neuroimage 14: 1080–1088. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Liu J, Adali T ( 2009): A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage 45: 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmann C, Koeppe C, Braus DF, Ruf M, Flor H ( 2007): A simultaneous EEG‐fMRI study of painful electric stimulation. Neuroimage 34: 1428–1437. [DOI] [PubMed] [Google Scholar]
- Clarey JC, Tweedale R, Calford MB ( 1996): Interhemispheric modulation of somatosensory receptive fields: Evidence for plasticity in primary somatosensory cortex. Cereb Cortex 6: 196–206. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK ( 2007): Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 62: 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Gratta C, Della Penna S, Tartaro A, Ferretti A, Torquati K, Bonomo L, Romani GL, Rossini PM ( 2000): Topographic organization of the human primary and secondary somatosensory areas: An fMRI study. Neuroreport 11: 2035–2043. [DOI] [PubMed] [Google Scholar]
- Deuchert M, Ruben J, Schwiemann J, Meyer R, Thees S, Krause T, Blankenburg F, Villringer K, Kurth R, Curio G, Villringer A ( 2002): Event‐related fMRI of the somatosensory system using electrical finger stimulation. Neuroreport 13: 365–369. [DOI] [PubMed] [Google Scholar]
- Devor A, Tian P, Nishimura N, Teng IC, Hillman EM, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM ( 2007): Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level‐dependent signal. J Neurosci 27: 4452–4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Bouilleret V, Hasboun D, Semah F, Baulac M ( 2003): Functional anatomy of the insula: New insights from imaging. Surg Radiol Anat 25: 113–119. [DOI] [PubMed] [Google Scholar]
- Duque J, Murase N, Celnik P, Hummel F, Harris‐Love M, Mazzocchio R, Olivier E, Cohen LG ( 2007): Intermanual differences in movement‐related interhemispheric inhibition. J Cogn Neurosci 19: 204–213. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K ( 2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K ( 2006): Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage 32: 570–582. [DOI] [PubMed] [Google Scholar]
- Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM ( 1993): 3D statistical neuroanatomical models from 305 MRI volumes. IEEE Nuclear Science Symposium and Medical Imaging Conference, San Francisco, CA. p 1813–1817.
- Fabri M, Polonara G, Quattrini A, Salvolini U, Del Pesce M, Manzoni T ( 1999): Role of the corpus callosum in the somatosensory activation of the ipsilateral cerebral cortex: An fMRI study of callosotomized patients. Eur J Neurosci 11: 3983–3994. [DOI] [PubMed] [Google Scholar]
- Fabri M, Polonara G, Del Pesce M, Quattrini A, Salvolini U, Manzoni T ( 2001): Posterior corpus callosum and interhemispheric transfer of somatosensory information: An fMRI and neuropsychological study of a partially callosotomized patient. J Cogn Neurosci 13: 1071–1079. [DOI] [PubMed] [Google Scholar]
- Ferretti A, Babiloni C, Gratta CD, Caulo M, Tartaro A, Bonomo L, Rossini PM, Romani GL ( 2003): Functional topography of the secondary somatosensory cortex for nonpainful and painful stimuli: An fMRI study. Neuroimage 20: 1625–1638. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frackowiak RSJ, Heather JD, Frith CD, Poline JB, Ashburner J ( 1995): Human Brain Mapp 2; 165–189. [Google Scholar]
- Genovese CR, Lazar NA, Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Hamzei F, Dettmers C, Rzanny R, Liepert J, Buchel C, Weiller C ( 2002): Reduction of excitability (“inhibition”) in the ipsilateral primary motor cortex is mirrored by fMRI signal decreases. Neuroimage 17: 490–496. [DOI] [PubMed] [Google Scholar]
- Harel N, Lee SP, Nagaoka T, Kim DS, Kim SG ( 2002): Origin of negative blood oxygenation level‐dependent fMRI signals. J Cereb Blood Flow Metab 22: 908–917. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Gandevia SC, Macefield VG ( 2007): Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: A single‐trial fMRI study. Pain 128: 20–30. [DOI] [PubMed] [Google Scholar]
- Hlushchuk Y, Hari R ( 2006): Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. J Neurosci 26: 5819–5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamura Y, Iriki A, Tanaka M ( 1994): Bilateral hand representation in the postcentral somatosensory cortex. Nature 369: 554–556. [DOI] [PubMed] [Google Scholar]
- Iwamura Y, Taoka M, Iriki A ( 2001): Bilateral activity and callosal connections in the somatosensory cortex. Neuroscientist 7: 419–429. [DOI] [PubMed] [Google Scholar]
- Johnson M, Martinson M ( 2007): Efficacy of electrical nerve stimulation for chronic musculoskeletal pain: A meta‐analysis of randomized controlled trials. Pain 130: 157–165. [DOI] [PubMed] [Google Scholar]
- Kampe KK, Jones RA, Auer DP ( 2000): Frequency dependence of the functional MRI response after electrical median nerve stimulation. Hum Brain Mapp 9: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno A, Nakasato N, Hatanaka K, Yoshimoto T ( 2003): Ipsilateral area 3b responses to median nerve somatosensory stimulation. Neuroimage 18: 169–177. [DOI] [PubMed] [Google Scholar]
- Karhu J, Tesche CD ( 1999): Simultaneous early processing of sensory input in human primary (SI) and secondary (SII) somatosensory cortices. J Neurophysiol 81: 2017–2025. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Baudewig J, Schnaudigel S, Huonker R, Becker L, Sohns JM, Dechent P, Klingner C, Witte OW ( 2008): Behavioral correlates of negative BOLD signal changes in the primary somatosensory cortex. Neuroimage 41: 1364–1371. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Gould HJ III, Cusick CG, Pons TP, Kaas JH ( 1983): The relation of corpus callosum connections to architectonic fields and body surface maps in sensorimotor cortex of new and old world monkeys. J Comp Neurol 219: 384–419. [DOI] [PubMed] [Google Scholar]
- Korvenoja A, Huttunen J, Salli E, Pohjonen H, Martinkauppi S, Palva JM, Lauronen L, Virtanen J, Ilmoniemi RJ, Aronen HJ ( 1999): Activation of multiple cortical areas in response to somatosensory stimulation: Combined magnetoencephalographic and functional magnetic resonance imaging. Hum Brain Mapp 8: 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Nusbaum HC, Meltzoff AN, Decety J ( 2007): What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS ONE 2: e1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton ML, Fu KM, Branch CA, Schroeder CE ( 2006): Ipsilateral hand input to area 3b revealed by converging hemodynamic and electrophysiological analyses in macaque monkeys. J Neurosci 26: 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A ( 2001): Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157. [DOI] [PubMed] [Google Scholar]
- Marchand WR, Lee JN, Thatcher JW, Thatcher GW, Jensen C, Starr J ( 2007): Motor deactivation in the human cortex and basal ganglia. Neuroimage 38: 538–548. [DOI] [PubMed] [Google Scholar]
- Meltzer JA, Negishi M, Constable RT ( 2008): Biphasic hemodynamic responses influence deactivation and may mask activation in block‐design fMRI paradigms. Hum Brain Mapp 29: 385–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet LB, Swisher JD, McMains SA, Halko MA, Amedi A, Pascual‐Leone A, Somers DC ( 2007): Combined activation and deactivation of visual cortex during tactile sensory processing. J Neurophysiol 97: 1633–1641. [DOI] [PubMed] [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R ( 2005): Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science 309: 951–954. [DOI] [PubMed] [Google Scholar]
- Newton JM, Sunderland A, Gowland PA ( 2005): fMRI signal decreases in ipsilateral primary motor cortex during unilateral hand movements are related to duration and side of movement. Neuroimage 24: 1080–1087. [DOI] [PubMed] [Google Scholar]
- Niddam DM, Chen LF, Wu YT, Hsieh JC ( 2005): Spatiotemporal brain dynamics in response to muscle stimulation. Neuroimage 25: 942–951. [DOI] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA ( 2005): Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science 309: 948–951. [DOI] [PubMed] [Google Scholar]
- Nihashi T, Naganawa S, Sato C, Kawai H, Nakamura T, Fukatsu H, Ishigaki T, Aoki I ( 2005): Contralateral and ipsilateral responses in primary somatosensory cortex following electrical median nerve stimulation—An fMRI study. Clin Neurophysiol 116: 842–848. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Ploner M ( 2000): Neurophysiology and functional neuroanatomy of pain perception. J Clin Neurophysiol 17: 592–603. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Volkmann J, Enck P, Frieling T, Witte OW, Freund HJ ( 1999): Different cortical organization of visceral and somatic sensation in humans. Eur J Neurosci 11: 305–315. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK ( 2006): Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci 9: 569–577. [DOI] [PubMed] [Google Scholar]
- Sotero RC, Trujillo‐Barreto NJ ( 2007): Modelling the role of excitatory and inhibitory neuronal activity in the generation of the BOLD signal. Neuroimage 35: 149–165. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Pike GB ( 2004): Hemodynamic and metabolic responses to neuronal inhibition. Neuroimage 22: 771–778. [DOI] [PubMed] [Google Scholar]
- Sutherland MT, Tang AC ( 2006): Reliable detection of bilateral activation in human primary somatosensory cortex by unilateral median nerve stimulation. Neuroimage 33: 1042–1054. [DOI] [PubMed] [Google Scholar]
- Vogt BA ( 2005): Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt C, Vogt O ( 1919): Allgemeinere ergebnisse unserer hirnforschung. J Psychol Neurol 25: 279–462. [Google Scholar]
- Vogt BA, Laureys S ( 2005): Posterior cingulate, precuneal and retrosplenial cortices: Cytology and components of the neural network correlates of consciousness. Prog Brain Res 150: 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Derbyshire S, Jones AK ( 1996): Pain processing in four regions of human cingulate cortex localized with co‐registered PET and MR imaging. Eur J Neurosci 8: 1461–1473. [DOI] [PubMed] [Google Scholar]


