Abstract
Microbial symbionts can play critical roles when their host attempts to colonize a new habitat. The lack of symbiont adaptation can in fact hinder the invasion process of their host. This scenario could change if the exotic species are able to acquire microorganisms from the invaded environment. Understanding the ecological factors that influence the take-up of new microorganisms is thus essential to clarify the mechanisms behind biological invasions. In this study, we tested whether different forest habitats influence the structure of the fungal communities associated with ambrosia beetles. We collected individuals of the most widespread exotic (Xylosandrus germanus) and native (Xyleborinus saxesenii) ambrosia beetle species in Europe in several old-growth and restored forests. We characterized the fungal communities associated with both species via metabarcoding. We showed that forest habitat shaped the community of fungi associated with both species, but the effect was stronger for the exotic X. germanus. Our results support the hypothesis that the direct contact with the mycobiome of the invaded environment might lead an exotic species to acquire native fungi. This process is likely favored by the occurrence of a bottleneck effect at the mycobiome level and/or the disruption of the mechanisms sustaining co-evolved insect-fungi symbiosis. Our study contributes to the understanding of the factors affecting insect-microbes interactions, helping to clarify the mechanisms behind biological invasions.
Keywords: Exotic pest, Metabarcoding, Xyleborinus saxesenii, Xylosandrus germanus
Introduction
Insect invasions represent one of the most demanding challenges today (Leemans & De Groot, 2003). Preventive measures adopted so far (Ormsby & Brenton-Rule, 2017) have slowed down but not stopped these events (Haack et al., 2014), and further invasions are expected to occur (Seebens et al., 2017). One reason for the limited efficacy of existing biosecurity systems is the still overlooked role of microorganisms in invasion ecology (Lu, Hulcr & Sun, 2016; Amsellem et al., 2017; Linnakoski & Forbes, 2019). Insects, like many other organisms, live in association with bacterial and fungal symbionts (Douglas, 2015; Gurung, Wertheim & Falcao Salles, 2019), which can have a positive (i.e., mutualistic), negative (i.e., parasitic) or neutral (i.e., commensalistic) impact on their host’s fitness. These symbionts can also facilitate (Lu et al., 2010; Himler et al., 2011; Adams et al., 2011; Vilcinskas et al., 2013) or limit (Zhou et al., 2018; Umeda & Paine, 2019) the invasion process of their insect host. When invading a new environment, insects and their microorganisms experience biotic and abiotic forces that can lead to the loss of part of the microbiome (Lester et al., 2017). This “bottleneck effect” may predispose exotic insects to acquire microorganisms from the invaded environment (Hajek et al., 2013; Wooding et al., 2013; Taerum et al., 2013; Wingfield et al., 2017). These microorganisms may confer important ecological adaptations, such as heat tolerance or parasite defense, influencing insects’ ability to establish and spread in the invaded environment (Oliver et al., 2010; Henry et al., 2013). Clarifying the ecological factors and dynamics behind the acquisition of microorganisms during insect invasions is an essential step to plan effective biosecurity programs.
One of the most complex examples of symbiosis in forest ecosystems occurs between wood-boring ambrosia beetles (Coleoptera; Scolytinae and Platypodinae) and ambrosia fungi (Ascomycota: Microascales, Ophiostomatales) (Hulcr & Stelinski, 2017; Vanderpool, Bracewell & McCutcheon, 2018). Adult females acquire mutualistic ambrosia fungi from the parental nest and transport them to newly established nests inside specific organs (i.e., mycetangia) or inside their guts (Francke-Grosmann, 1963; Francke-Grosmann, 1967). Then, beetles farm the fungi within the wood galleries they live in (Biedermann & Taborsky, 2011), and feed on them as both larvae and adults (Batra, 1966). Besides these obligate nutritional mutualists, ambrosia beetles carry several other fungal symbionts in both the mycetangium and other body parts (Kostovcik et al., 2015; Freeman et al., 2016; Bateman et al., 2016; Malacrinò et al., 2017; Miller et al., 2019). These can be commensals, parasites or facultative mutualists (Skelton et al., 2018). The complexity of these symbioses is still largely unresolved, in particular considering the potential interactions among exotic insects and native fungi occurring in the invaded environment.
Several ambrosia beetle species have successfully established outside their native range in the last two decades (Rassati, Lieutier & Faccoli, 2016; Rabaglia et al., 2019). Nonetheless, the spread of several species has been limited by climatic conditions (e.g., humidity, temperature). Exotic ambrosia beetles are indeed able to survive only in areas suitable for the growth of their fungal symbionts (Marini et al., 2011; Rassati et al., 2016a; Rassati et al., 2016b; Zhou et al., 2018; Umeda & Paine, 2019). This scenario could however change if an exotic beetle is able to acquire native fungi from the invaded environment. This acquisition can occur through (i) the exchange of fungi between native and exotic species, and/or (ii) the direct contact with the mycobiome of the invaded environment. The exchange of fungi between native and exotic ambrosia beetles can occur between two species with neighboring galleries, when fungi grow from the gallery of one species to that of the other (Carrillo et al., 2014). This mechanism is expected to involve primary or facultative mutualists and may not be unusual, particularly because different species of ambrosia beetles select their host plant in a similar way, so different species may colonize the same tree (Ranger et al., 2015). The second mechanism, instead, may occur when adult females searching for a new host come in contact with native fungi present in the environment (Seibold et al., 2019). This mechanism should involve fungi that mainly establish commensalistic relationships with the beetles, but plant pathogens can also be involved (Juzwik et al., 2016; Ploetz et al., 2017; Chahal et al., 2019). Currently, the frequency and the extent of these associations is largely unclear.
In this study, we tested the hypothesis that different habitats influence the composition of the fungal community associated with ambrosia beetles, reflecting a potential acquisition of fungi from the environment. We used a metabarcoding approach for identification of the fungal community of the most widespread exotic (Xylosandrus germanus) and native (Xyleborinus saxesenii) ambrosia beetle species in European forests. Individuals of both species were collected in two forest habitats: old-growth forests and restored forests. Old-growth forests are expected to host more complex fungal communities than restored forests (Blaser et al., 2013; Pioli et al., 2018); thus, we hypothesized that ambrosia beetles should reflect these differences in their mycobiome. Furthermore, the mechanisms regulating insect-fungus symbioses resulting from a long co-evolutionary history (Biedermann, De Fine Licht & Rohlfs, 2019) might be disrupted by the interaction with microbiomes of the invaded habitat. Therefore, when comparing the fungal communities associated to individuals collected in the two forest habitats, we expected to observe larger differences for the exotic than for the native ambrosia beetle species.
Materials & Methods
Ambrosia beetle species
We selected two ambrosia beetle species: the exotic X. germanus and the native X. saxesenii. Xylosandrus germanus is a species native to Asia that was first reported in Europe in the 1950s and since then rapidly spread, becoming one of the dominant ambrosia beetles in European forest ecosystems (Galko et al., 2018). Xylosandrus germanus’ fungal mutualist is Ambrosiella grosmanniae (Mayers et al., 2015). Xyleborinus saxesenii is instead a species of Palaearctic origin and its main fungal mutualist is Raffaelea sulfurea, although other fungi have been found in association with this beetle species (Biedermann et al., 2013).
Sampling locations and procedure
Beetles were collected in 2016 in ten forest stands located in the Northeast of Italy (Fig. S1 and Table S1), across two forest habitats: old-growth forests (n = 5) and restored forests (n = 5). With “old-growth forests”, we refer to the remnants of the old oak–hop-hornbeam forest (Quercus spp. and Ostrya carpinifolia Scop.) that covered the vast majority of Veneto and Friuli Venezia Giulia regions after the last ice age. With “restored forests”, we refer to mixed forests that were planted over the last 30 years to restore forests across agricultural landscapes. Both forest habitats are dominated by oak (Quercus spp.), ash (Fraxinus spp.), maple (Acer spp.), and hop-hornbeam (O. carpinifolia). In addition, both forest habitats are present in relatively small patches embedded in an agriculture-dominated landscape (min = 2.65 ha, max = 165.15 ha for old-growth forests; min = 2.37 ha, max = 37.41 ha for restored forests).
Beetles were trapped using green and purple 12-multi-funnel traps (Synergy Semiochemicals, Burnaby, Canada) baited with ultra-high release rate ethanol pouches (99% purity, release rate of 300–400 mg/day at 20 °C, Contech Enterprises). Although ethanol is attractive for a wide range of wood-borers (Miller, 2006), it is also the most commonly used volatile for trapping ambrosia beetles (Reding et al., 2011). The ethanol pouch was always attached to the sixth funnel and hung outside the trap. Traps were set in the understory at about 1.5 m above the ground and were suspended at least 1m from the tree bole. Trap collecting cups were half-filled with 1:1 solution (v/v) of ethylene glycol:water to kill and preserve captured beetles (known as “wet system”) (Steininger et al., 2015). At each trap check, collecting cups were emptied and the solution was renewed. Traps were set up in mid-May and emptied every three weeks until the beginning of August. At each visit, all insects were collected, put in tubes filled with ethanol, and brought to the laboratory where ambrosia beetles were separated from other trapped insects. Each individual was then morphologically identified to species level and kept in separate vials filled with ethanol until they were processed. Then, we retained for the analysis only individuals of the two ambrosia beetle species that were simultaneously collected during the same trapping period and in the same trap. This allowed an intra-trap comparison to test for cross-contamination between beetle species (see ‘Results’, Table S2). Our sampling procedure did create the possibility of microbial cross-contamination among the different insect specimens simultaneously present in the trap collector cup (Viiri, 1997). However, in our previous work we demonstrated that individuals collected in the same trap do not show evidence of cross-contamination (Malacrinò et al., 2017). In an effort to reduce possible environmental contamination, we also sterilized the external surface of the insect body. First, we put each insect in a vial with ddH2O in a water bath and sonicated them for 1 min. After sonication, we washed each insect by vortexing once in ethanol (100%), twice in sodium hypochlorite (5%), and twice in ddH2O for 1 min following each wash step. For each ambrosia beetle species, we processed 15 individuals per sampling site (total of 300 individuals).
DNA extractions, libraries preparation and amplicon sequencing
Single individuals were crushed in an extraction buffer (10 mM Tris, 100 mM NaCl, 10 mM EDTA, 0.5% SDS) using three one mm ∅ stainless steel beads per tube, with the aid of a bead mill homogenizer set at 30 Hz for 5 min (TissueLyzer II, Qiagen, UK). The mixture was treated with proteinase K (5Prime GmbH, Germany) following the producer’s instructions. Total DNA was extracted using the MoBio PowerSoil Kit (Mo Bio Laboratories, Inc., Carlsbad, CA, USA) following the manufacturer’s protocol. DNA concentration and purity were assessed with a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific Inc., USA).
The fungal community associated with each individual was characterized by amplicon sequencing targeting the ITS2 region using gITS7 and ITS4 primers, as previously indicated by Kostovcik et al. (2015). We selected the ITS2 region (Nilsson et al., 2019) to ensure we captured the diversity of fungi with which beetles come in contact during host searching. We are aware that the ITS2 region can lead to an amplification bias for Microascales and Ophiostomatales (Kostovcik et al., 2015), the two orders including the main mutualists of X. germanus and X. saxesenii. Here, however, we are interested in the entire mycobiota, which has been less frequently described and might explain important aspects of beetle ecology. PCR reactions were performed in a total volume of 25 µl, containing about 50 ng of DNA, 0.5 µM of each primer, 1X KAPA HiFi HotStart ReadyMix (KAPA Biosystems, USA) and nuclease-free water. Amplifications were performed in a Mastercycler Ep Gradient S (Eppendorf, Germany) set at 95 °C for 3 min, 98 °C for 30 s, 56 °C for 30 s and 72 °C for 30 s, repeated 30 times, and ended with 10 min of extension at 72 °C. Each amplification was carried out in technical triplicate, including three non-template controls with nuclease-free water instead of DNA. Nuclease-free water (100 µl) was also processed using the same procedure as the experimental samples (from DNA extraction to sequencing) in order to exclude contamination of reagents and instruments. Amplification success was checked by electrophoresis on 1.5% agarose gel stained with GelRed (Biotium Inc., Fremont, CA, USA). Although we did not observe any amplification bands for the negative/non-template control samples, these were processed and sequenced together with experimental samples. PCR products from the same sample were then pooled together, and cleaned using Agencourt AMPure XP kit (Beckman Coulter, Brea, CA, USA) following the producer’s instructions. A further short-run PCR was performed to integrate Illumina i7 and i5 indexes following the producer’s protocol (Nextera XT, Illumina, San Diego, CA, USA), and amplicons were purified again as explained above. Libraries were then quantified with the Invitrogen Qubit HS dsDNA kit (Invitrogen, Carlsbad, CA, USA), normalized to a concentration of 10 ng/µl using nuclease-free water, pooled together and sequenced with an Illumina MiSeq sequencer, using the MiSeq Reagent Kit v3 300PE chemistry (Illumina, San Diego, CA, USA) following the producer’s protocol.
Data analysis
Demultiplexed paired-end reads were merged using the PEAR 0.9.1 algorithm using default parameters (Zhang et al., 2014). Raw data handling was carried out using QIIME 1.9 (Caporaso et al., 2012), quality filtering reads with default parameters, binning Operational Taxonomic Units (OTUs) using open-reference OTU-picking through UCLUST algorithm (97% similarity), and discarding chimeric sequences discovered with USEARCH 6.1 (Edgar, 2010). All non-fungal OTUs were discarded using ITSx (Bengtsson-Palme et al., 2013). Taxonomy assignment was performed using the BLAST method (default parameters) by querying towards a custom database built using all ITS2 reference sequences deposited at NCBI GenBank (accessed on July 2017). R statistical environment v3.5.1 (R Core Team, 2013) plugged with the packages vegan (Dixon, 2003), phyloseq (McMurdie & Holmes, 2013), picante (Kembel et al., 2010) and DESeq2 (Love, Huber & Anders, 2014) was used for data analysis. First, singletons and samples with fewer than 1,000 counts were removed. Data processing resulted in a dataset of 3,634,647 reads clustered into 19,744 OTUs. Then, comparisons of fungal community composition between ambrosia beetle species and between forest habitats within the same beetle species were performed using PERMANOVA analysis (999 permutations stratified at site level) calculated on a UniFrac distance matrix. Non-metric multidimensional scaling (NMDS) procedure was performed to visualize differences in the structure of fungal communities. The diversity of fungal communities was assessed using Chao1 (total diversity) (Chao, 1984), Faith’s phylogenetic diversity (which considers both total diversity and the phylogenetic relationship between taxa within the community) (Faith, 1992), and 1-Simpson (dominance) (Simpson, 1949) indexes. Comparisons were performed using mixed-effects models (one model for each diversity index) with the lmer function under the lme4 R package (Bates et al., 2015) using ambrosia beetle species and forest habitat as factors, and sampling site as a random variable. The package emmeans was used to infer pairwise contrasts within mixed-effects models (FDR corrected). The use of “sampling site” for stratification in PERMANOVA and as a random variable in the mixed-effects model allowed the control of both non-homogeneity in sample number at each trap (Table S2) and potential spatial effects. The differential presence of OTUs between forest habitats and within the same beetle species was assessed using the package DESeq2, by contrasting the two forest types within each species. The association of each fungal genus to a functional guild was performed by searching against the FUNGuild database (Nguyen et al., 2016) and manually curating the results in case of multiple results from the same query.
Results
Fungal communities associated with X. germanus and X. saxesenii
The reconstruction of the fungal communities showed that the exotic ambrosia beetle X. germanus and the native ambrosia beetle X. saxesenii are associated with different fungi (F1,211 = 10.5; P < 0.001). The absence of cross-contamination was shown by a multiple comparison procedure following PERMANOVA: differences between ambrosia beetle species were found at all sites (P < 0.01 FDR corrected–Table S2). In case of cross-contamination we would expect an overlap of the fungal communities and, thus, no differences.
The fungal communities of both ambrosia beetle species were dominated by unidentified taxa (83.97% in X. germanus and 73.91% in X. saxesenii–Table S3). Instead, we identified 26 genera that include plant pathogens (4.75% in X. germanus and 5% in X. saxesenii) mainly represented by the genus Cladosporium. The rest of the communities were represented by saprotrophs (2.73% in X. germanus and 4.12% in X. saxesenii), yeasts (5.35% in X. germanus and 15.96% in X. saxesenii) (Table S3) and at low relative abundances insect pathogens, mycorrhizal fungi, endophytes and lichen parasites (Table S3). In addition, in both ambrosia beetle species we found sequences that can likely be assigned to the respective main mutualists: Ambrosiella sp. in X. germanus (2.25%) and Raffaelea sp. in X. saxesenii (0.01%).
Effect of forest habitat on fungal communities
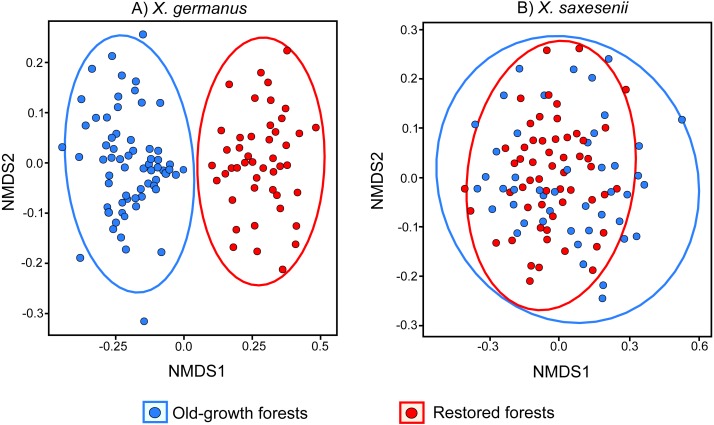
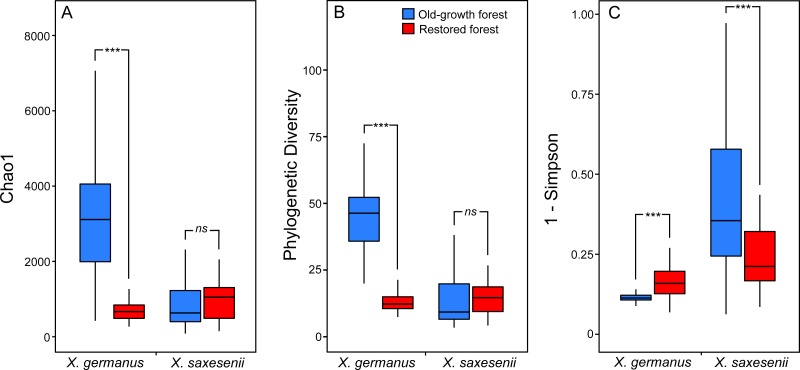
Using a PERMANOVA analysis we found that the fungal community segregated by forest habitat both in the exotic X. germanus (F1,10 = 21.8; P < 0.001—Fig. 1A) and in the native X. saxesenii (F1,10 = 1.6; P = 0.004—Fig. 1B), although the effect was much more evident in X. germanus (R2 = 0.16) than in X. saxesenii (R2 = 0.01). A different pattern between X. germanus and X. saxesenii also emerged when looking at the diversity of the fungal communities. For X. germanus, individuals collected in old-growth forests were associated with a richer and more diverse fungal community than those collected in restored forests (P < 0.001 for both Chao1 and phylogenetic diversity—Figs. 2A and 2B, Table S4), whereas these differences were not observed in the native X. saxesenii (P > 0.05 for both Chao1 and phylogenetic diversity—Figs. 2A and 2B, Table S4). On the contrary, in both X. germanus and X. saxesenii the dominance index (1-Simpson) significantly differed between the two forest habitats (P < 0.001—Fig. 2C, Table S4). In particular, we observed a higher dominance index in restored forests compared to old-growth forests for X. germanus, and a higher dominance index in old-growth forests versus restored forests for X. saxesenii.
Figure 1. NMDS (Non-metric Multi Dimensional Scaling) analysis of the fungal communities associated with the exotic ambrosia beetle X. germanus (A) and the native ambrosia beetle X. saxesenii (B) in old-growth forests and restored forests.
Figure 2. Alpha-diversity indices (A, Chao 1; B, Faith’s Phylogenetic Diversity; and (C) 1-Simpson) for fungal communities associated with the exotic ambrosia beetle X. germanus and the native ambrosia beetle X. saxesenii in old-growth and restored forests.
∗∗∗ = P < 0.001; ns = P > 0.05. Full results from mixed-effect models are reported in Table S4.
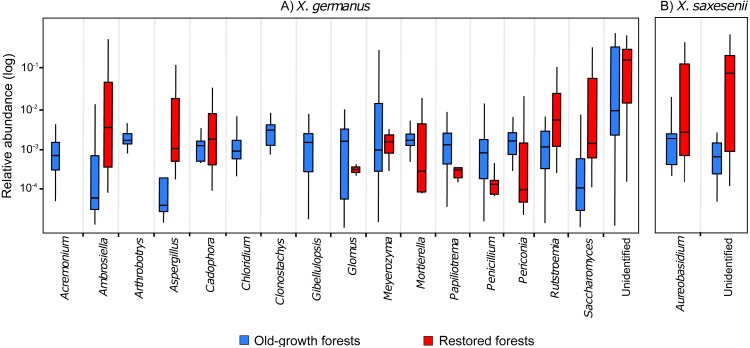
Comparing the fungal community associated with individuals collected in the two forest habitats, for X. germanus we found 121 differentially abundant OTUs: 4 of them were more abundant in restored than in old-growth forests (1 Ambrosiella sp., 1 Aspergillus sp., 1 Saccharomyces sp. and 1 unidentified, Fig. 3A), whereas 117 were more abundant in old-growth than in restored forests (102 unidentified OTUs, and 15 genera, Fig. 3A). The same analysis on X. saxesenii resulted in 4 differentially abundant OTUs (1 Aureobasidium sp. and 3 unidentified), all of them more abundant in restored than in old-growth forests (Fig. 3B).
Figure 3. Relative abundance (log-scale) of fungal genera in the exotic ambrosia beetle X. germanus (A) and the native ambrosia beetle X. saxesenii (B) associated to individuals collected in old-growth forests vs restored forests.
Genera represented in this figure are those that resulted to be significantly differentially abundant between the two forest habitats (cutoff P = 0.05 FDR corrected). Each genus is represented by a single OTU (excluding Glomus and Mortierella, each with 2 OTUs), while the category ‘Unidentified’ is represented by 103 OTUs in X. germanus and 3 OTU in X. saxesenii.
Discussion
Absence of adaptation, or low plasticity, in the microbiota of an exotic species can limit its establishment in a new environment (Rassati et al., 2016b; Umeda & Paine, 2019). The acquisition of microorganisms native to the invaded environment may however help the exotic species to overcome these ecological barriers. Yet, this topic is still in its infancy and the mechanisms leading to the acquisition of new microorganisms are still understudied. We found that forest habitat shaped the mycobiome associated with the exotic ambrosia beetle X. germanus, potentially reflecting the acquisition of fungi from the invaded environment. In addition, we showed a stronger effect of forest habitat on the fungal community associated with the exotic X. germanus compared to the native ambrosia beetle X. saxesenii. This suggests that two (non-mutually exclusive) mechanisms may have occurred: (i) a bottleneck effect that caused the loss of part of the original microorganisms; and (ii) the disruption of the mechanisms sustaining co-evolved insect-fungi symbiosis.
In our study, the exotic ambrosia beetle X. germanus and the native X. saxesenii were associated with different fungal communities. Although both species are highly polyphagous and have overlapping phenology, they can show different preferences in host tree species (Rassati et al., 2016a), ethanol content in host tissues (Rassati et al., 2019) or vertical strata (Menocal et al., 2018), which could lead to interactions with different fungal communities. We were unable to taxonomically identify the majority of OTUs due to the lack of reliable taxonomic information (Stielow et al., 2015; Abdelfattah et al., 2018). Among the identified taxa, however, we found a large cohort of plant pathogens, saprotrophs and yeasts, of which many have already been reported to establish a commensalistic relationship with both bark and ambrosia beetles (Kostovcik et al., 2015; Davis, 2015; Miller et al., 2016; Malacrinò et al., 2017).
We found that forest habitat greatly influenced the diversity and dominance of fungal communities associated with the exotic ambrosia beetle X. germanus. A similar pattern was previously shown only for the invasive ambrosia beetle Xyleborus glabratus, where sampling location influenced the structure of the symbiotic fungal community (Campbell et al., 2016). Here, we show that individuals of the exotic X. germanus were associated with a richer, more diverse and more even community of fungi in old-growth forests than in restored forests. This pattern reflects the different fungal community structures likely inhabiting the two forest habitats and suggests the occurrence of a direct acquisition of fungi from the environment during invasion. Future research efforts should directly compare the mycobiome associated with ambrosia beetles to the environmental fungal communities, proving empirical evidence that such acquisition occurs. After introduction in a new environment, an exotic insect and its microbiome experience a series of biotic and abiotic forces that may lead the insect to lose part of its original community of microorganisms. This “bottleneck effect” challenging the microbiome (e.g., Lester et al., 2017) may favor the acquisition of microorganisms from the invaded habitat. Given that we do not have data on the community of fungi associated with X. germanus in its native area, we cannot state whether a bottleneck effect occurred. Along with the depletion of the original mycobiome, we speculate that an exotic species may be prone to acquire new microorganisms due to the potential mismatch of the mechanisms maintaining symbioses with the invaded ecosystems. Symbioses are the result of a long co-evolution, and both the host and the symbionts present a series of chemical, structural, and genomic co-adaptations (Blaz et al., 2018; Mayers et al., 2019; Skelton et al., 2019; Biedermann, De Fine Licht & Rohlfs, 2019; Veselská et al., 2019). The mechanisms that serve to maintain existing symbiosis may be challenged by the newly encountered microbiomes and might not work properly, leading to the establishment of new associations. While most of the fungi may represent transient associations, it is possible that some can compete for resources with the primary mutualists present in the mycentangium (Castrillo, Griggs & Vandenberg, 2016; Menocal et al., 2017). Whether such a mechanism occurred, however, cannot be stated. Indeed, by analyzing the whole insect, we are not able to determine if the fungal taxa we identified inhabited the mycetangium or the insect’s guts. This is an important aspect to investigate in future studies as a switch in the fungal symbionts in the mycentangium may lead to important consequences for the beetle fitness (Skelton et al., 2019).
We also found a weak environmental effect on the native species. The microbiome of native insects have been shown to vary with habitat (Yun et al., 2014; Kudo et al., 2019), thus we expected some differences among the fungal communities associated with X. saxesenii individuals collected in the different forest habitats. In our study, however, differences were very small compared to those observed for X. germanus, and were found only in terms of dominance. Specifically, the community of fungi associated with X. saxesenii was more even in restored than old-growth forests. This pattern can be explained by the different microclimatic conditions and nutrient availability present in the two forest habitats which may have favored certain fungi rather than others.
Conclusions
A timely topic in invasion ecology is the understanding of the mechanisms by which exotic species establish novel symbiotic associations in the invaded environment (Lu, Hulcr & Sun, 2016; Amsellem et al., 2017). Despite we analyzed only one exotic ambrosia beetle species, our results support the hypothesis that the direct acquisition of microorganisms from the environment can modify the microbiome of an exotic species. Species distribution models are commonly used to plan invasive species surveillance programs and decide where to concentrate efforts and resources (Lantschner, De la Vega & Corley, 2019). These models are based on known occurrence records and the environmental conditions at occurrence localities to predict where a certain species can establish outside its native range. The acquisition of novel microorganisms in the invaded environment, however, may alter predictions for the establishment and spread of exotic species. Incorporating the role of microbes into ecological theories is thus fundamental to clarify the mechanisms behind insect invasions and aid in biosecurity surveillance.
Supplemental Information
Blue dots indicate old-growth forests, red dots indicate restored forests. All sites were located in north-eastern Italy. Each site is identified by the name of the locality. Map Data 2019 Google.
The main parameters were measured at each site in two circular areas (15 m radius) located close to the boundary of the point where trap was placed.
For each sampling site. P-value as adjusted for multiple comparisons using FDR method. *, number of samples after filtering
The guild column represents the prediction of the functional role of each genus, according to the FUNGuild database and manually curated.
Acknowledgments
The authors thank Peter Biedermann, Alison Bennett, Philip Smith and the two anonymous reviewers for their insightful comments on an earlier draft of this manuscript, and Matteo Marchioro for field assistance.
Funding Statement
This study was supported by the Fund for Basic Research Activities (FBAR)–ANVUR –Italian National Agency for the Evaluation of the University and Research Systems, and European Union’s Horizon 2020 research and innovation programme under grant agreement No 771271. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Davide Rassati, Email: davide.rassati@unipd.it.
Antonino Malacrinò, Email: malacrino.1@osu.edu, antonino.malacrino@gmail.com.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Davide Rassati conceived and designed the experiments, performed the experiments, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Lorenzo Marini conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Antonino Malacrinò conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Raw reads are available at NCBI SRA under the BioProject PRJNA524707.
References
- Abdelfattah et al. (2018).Abdelfattah A, Malacrinò A, Wisniewski M, Cacciola SO, Schena L. Metabarcoding: a powerful tool to investigate microbial communities and shape future plant protection strategies. Biological Control. 2018;120:1–10. doi: 10.1016/j.biocontrol.2017.07.009. [DOI] [Google Scholar]
- Adams et al. (2011).Adams AS, Jordan MS, Adams SM, Suen G, Goodwin LA, Davenport KW, Currie CR, Raffa KF. Cellulose-degrading bacteria associated with the invasive woodwasp Sirex noctilio. The ISME Journal. 2011;5:1323–1331. doi: 10.1038/ismej.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsellem et al. (2017).Amsellem L, Brouat C, Duron O, Porter SS, Vilcinskas A, Facon B. Importance of microorganisms to macroorganisms invasions: is the essential invisible to the eye? (The Little Prince, A. de Saint-Exupéry, 1943) In: Bohan DA, Dumbrell AJ, Massol F, editors. Networks of invasion: empirical evidence and case studies. Academic Press; 2017. pp. 99–146. [DOI] [Google Scholar]
- Bateman et al. (2016).Bateman C, Šigut M, Skelton J, Smith KE, Hulcr J. Fungal associates of the Xylosandrus compactus (Coleoptera: Curculionidae, Scolytinae) are spatially segregated on the insect body. Environmental Entomology. 2016;45:883–890. doi: 10.1093/ee/nvw070. [DOI] [PubMed] [Google Scholar]
- Bates et al. (2015).Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Batra (1966).Batra LR. Ambrosia fungi: extent of specificity to ambrosia beetles. Science. 1966;153:193–195. doi: 10.1126/science.153.3732.193. [DOI] [PubMed] [Google Scholar]
- Bengtsson-Palme et al. (2013).Bengtsson-Palme J, Ryberg M, Hartmann M, Branco S, Wang Z, Godhe A, De Wit P, Sánchez-García M, Ebersberger I, De Sousa F, Amend AS, Jumpponen A, Unterseher M, Kristiansson E, Abarenkov K, Bertrand YJK, Sanli K, Eriksson KM, Vik U, Veldre V, Nilsson RH. Improved software detection and extraction of ITS1 and ITS2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods in Ecology and Evolution. 2013;4:914–919. doi: 10.1111/2041-210X.12073. [DOI] [Google Scholar]
- Biedermann, De Fine Licht & Rohlfs (2019).Biedermann PHW, De Fine Licht HH, Rohlfs M. Evolutionary chemo-ecology of insect-fungus interactions: still in its infancy but advancing. Fungal Ecology. 2019;38:1–6. doi: 10.1016/j.funeco.2018.11.010. [DOI] [Google Scholar]
- Biedermann et al. (2013).Biedermann PHW, Klepzig KD, Taborsky M, Six DL. Abundance and dynamics of filamentous fungi in the complex ambrosia gardens of the primitively eusocial beetle Xyleborinus saxesenii Ratzeburg (Coleoptera: Curculionidae, Scolytinae) FEMS Microbiology Ecology. 2013;83:711–723. doi: 10.1111/1574-6941.12026. [DOI] [PubMed] [Google Scholar]
- Biedermann & Taborsky (2011).Biedermann PHW, Taborsky M. Larval helpers and age polyethism in ambrosia beetles. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:17064–17069. doi: 10.1073/pnas.1107758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser et al. (2013).Blaser S, Prati D, Senn-Irlet B, Fischer M. Effects of forest management on the diversity of deadwood-inhabiting fungi in Central European forests. Forest Ecology and Management. 2013;304:42–48. doi: 10.1016/J.FORECO.2013.04.043. [DOI] [Google Scholar]
- Blaz et al. (2018).Blaz J, Barrera-Redondo J, Vázquez-Rosas-Landa M, Canedo-Téxon A, Aguirre von Wobeser E, Carrillo D, Stouthamer R, Eskalen A, Villafán E, Alonso-Sánchez A, Lamelas A, Ibarra-Juarez L, Pérez-Torres C, Ibarra-Laclette E. Genomic signals of adaptation towards mutualism and sociality in two ambrosia beetle complexes. Life. 2018;9 doi: 10.3390/life9010002. Article 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell et al. (2016).Campbell AS, Ploetz RC, Dreaden TJ, Kendra PE, Montgomery WS. Geographic variation in mycangial communities of Xyleborus glabratus. Mycologia. 2016;108:657–667. doi: 10.3852/15-133. [DOI] [PubMed] [Google Scholar]
- Caporaso et al. (2012).Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. The ISME Journal. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo et al. (2014).Carrillo D, Duncan RE, Ploetz JN, Campbell AF, Ploetz RC, Peña JE. Lateral transfer of a phytopathogenic symbiont among native and exotic ambrosia beetles. Plant Pathology. 2014;63:54–62. doi: 10.1111/ppa.12073. [DOI] [Google Scholar]
- Castrillo, Griggs & Vandenberg (2016).Castrillo LA, Griggs MH, Vandenberg JD. Competition between biological control fungi and fungal symbionts of ambrosia beetles Xylosandrus crassiusculus and X. germanus (Coleoptera: Curculionidae): mycelial interactions and impact on beetle brood production. Biological Control. 2016;103:138–146. doi: 10.1016/J.BIOCONTROL.2016.09.005. [DOI] [Google Scholar]
- Chahal et al. (2019).Chahal K, Gazis R, Klingeman W, Hadziabdic D, Lambdin P, Grant J, Windham M. Assessment of alternative candidate subcortical insect vectors from walnut crowns in habitats quarantined for thousand cankers disease. Environmental Entomology. 2019;48:882–893. doi: 10.1093/ee/nvz064. [DOI] [PubMed] [Google Scholar]
- Chao (1984).Chao A. Nonparametric estimation of the number of classes in a population. Scandinavian Journal of Statistics. 1984;11:265–270. [Google Scholar]
- Davis (2015).Davis TS. The ecology of yeasts in the bark beetle holobiont: a century of research revisited. Microbial Ecology. 2015;69:723–732. doi: 10.1007/s00248-014-0479-1. [DOI] [PubMed] [Google Scholar]
- Dixon (2003).Dixon P. VEGAN, a package of R functions for community ecology. Journal of Vegetation Science. 2003;14:927–930. doi: 10.1111/j.1654-1103.2003.tb02228.x. [DOI] [Google Scholar]
- Douglas (2015).Douglas AE. Multiorganismal insects: diversity and function of resident microorganisms. Annual Review of Entomology. 2015;60:17–34. doi: 10.1146/annurev-ento-010814-020822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar (2010).Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Faith (1992).Faith DP. Conservation evaluation and phylogenetic diversity. Biological Conservation. 1992;61:1–10. doi: 10.1016/0006-3207(92)91201-3. [DOI] [Google Scholar]
- Francke-Grosmann (1963).Francke-Grosmann H. Some new aspects in forest entomology. Annual Review of Entomology. 1963;8:415–438. doi: 10.1146/annurev.en.08.010163.002215. [DOI] [Google Scholar]
- Francke-Grosmann (1967).Francke-Grosmann H. Ectosymbiosis in wood-inhabiting insects. In: Henry SM, editor. Symbiosis, vol 2. Associations of invertebrates, birds, ruminants and other biota. Academic Press; New York: 1967. pp. 141–206. [DOI] [Google Scholar]
- Freeman et al. (2016).Freeman S, Sharon M, Dori-Bachash M, Maymon M, Belausov E, Maoz Y, Margalit O, Protasov A, Mendel Z. Symbiotic association of three fungal species throughout the life cycle of the ambrosia beetle Euwallacea nr. fornicatus. Symbiosis. 2016;68:115–128. doi: 10.1007/s13199-015-0356-9. [DOI] [Google Scholar]
- Galko et al. (2018).Galko J, Dzurenko M, Ranger C, Kulfan J, Kula E, Nikolov C, Zúbrik M, Zach P. Distribution, habitat preference, and management of the invasive ambrosia beetle Xylosandrus germanus (Coleoptera: Curculionidae, Scolytinae) in European forests with an emphasis on the West Carpathians. Forests. 2018;10 doi: 10.3390/f10010010. Article 10. [DOI] [Google Scholar]
- Gurung, Wertheim & Falcao Salles (2019).Gurung K, Wertheim B, Falcao Salles J. The microbiome of pest insects: it is not just bacteria. Entomologia Experimentalis et Applicata. 2019;167:156–170. doi: 10.1111/eea.12768. [DOI] [Google Scholar]
- Haack et al. (2014).Haack RA, Britton KO, Brockerhoff EG, Cavey JF, Garrett LJ, Kimberley M, Lowenstein F, Nuding A, Olson LJ, Turner J, Vasilaky KN. Effectiveness of the international phytosanitary standard ISPM (15) on reducing wood borer infestation rates in wood packaging material entering the United States. PLOS ONE. 2014;9:e96611. doi: 10.1371/journal.pone.0096611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek et al. (2013).Hajek AE, Nielsen C, Kepler RM, Long SJ, Castrillo L. Fidelity among Sirex woodwasps and their fungal symbionts. Microbial Ecology. 2013;65:753–762. doi: 10.1007/s00248-013-0218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry et al. (2013).Henry LM, Peccoud J, Simon J-C, Hadfield JD, Maiden MJC, Ferrari J, Godfray HCJ. Horizontally transmitted symbionts and host colonization of ecological niches. Current Biology. 2013;23:1713–1717. doi: 10.1016/j.cub.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himler et al. (2011).Himler AG, Adachi-Hagimori T, Bergen JE, Kozuch A, Kelly SE, Tabashnik BE, Chiel E, Duckworth VE, Dennehy TJ, Zchori-Fein E, Hunter MS. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science. 2011;332:254–256. doi: 10.1126/science.1199410. [DOI] [PubMed] [Google Scholar]
- Hulcr & Stelinski (2017).Hulcr J, Stelinski LL. The ambrosia symbiosis: from evolutionary ecology to practical management. Annual Review of Entomology. 2017;62:285–303. doi: 10.1146/annurev-ento-031616-035105. [DOI] [PubMed] [Google Scholar]
- Juzwik et al. (2016).Juzwik J, McDermott-Kubeczko M, Stewart TJ, Ginzel MD. First report of Geosmithia morbida on ambrosia beetles emerged from thousand cankers-diseased Juglans nigra in Ohio. Plant Disease. 2016;100:1238–1238. doi: 10.1094/PDIS-10-15-1155-PDN. [DOI] [Google Scholar]
- Kembel et al. (2010).Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO. Picante: R tools for integrating phylogenies and ecology. Bioinformatics. 2010;26:1463–1464. doi: 10.1093/bioinformatics/btq166. [DOI] [PubMed] [Google Scholar]
- Kostovcik et al. (2015).Kostovcik M, Bateman CC, Kolarik M, Stelinski LL, Jordal BH, Hulcr J. The ambrosia symbiosis is specific in some species and promiscuous in others: evidence from community pyrosequencing. The ISME Journal. 2015;9:126–138. doi: 10.1038/ismej.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo et al. (2019).Kudo R, Masuya H, Endoh R, Kikuchi T, Ikeda H. Gut bacterial and fungal communities in ground-dwelling beetles are associated with host food habit and habitat. The ISME Journal. 2019;13:676–685. doi: 10.1038/s41396-018-0298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantschner, De la Vega & Corley (2019).Lantschner MV, De la Vega G, Corley JC. Predicting the distribution of harmful species and their natural enemies in agricultural, livestock and forestry systems: an overview. International Journal of Pest Management. 2019;65:190–206. doi: 10.1080/09670874.2018.1533664. [DOI] [Google Scholar]
- Leemans & De Groot (2003).Leemans R, De Groot RS. Millennium ecosystem assessment: ecosystems and human well-being: a framework for assessment. Island Press; Washington: 2003. [Google Scholar]
- Lester et al. (2017).Lester PJ, Sébastien A, Suarez AV, Barbieri RF, Gruber MAM. Symbiotic bacterial communities in ants are modified by invasion pathway bottlenecks and alter host behavior. Ecology. 2017;98:861–874. doi: 10.1002/ecy.1714. [DOI] [PubMed] [Google Scholar]
- Linnakoski & Forbes (2019).Linnakoski R, Forbes KM. Pathogens—the hidden face of forest invasions by wood-boring insect pests. Frontiers in Plant Science. 2019;10 doi: 10.3389/fpls.2019.00090. Article 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, Huber & Anders (2014).Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15 doi: 10.1186/s13059-014-0550-8. Article 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Hulcr & Sun (2016).Lu M, Hulcr J, Sun J. The role of symbiotic microbes in insect invasions. Annual Review of Ecology, Evolution, and Systematics. 2016;47:487–505. doi: 10.1146/annurev-ecolsys-121415-032050. [DOI] [Google Scholar]
- Lu et al. (2010).Lu M, Wingfield MJ, Gillette NE, Mori SR, Sun J-H. Complex interactions among host pines and fungi vectored by an invasive bark beetle. New Phytologist. 2010;187:859–866. doi: 10.1111/j.1469-8137.2010.03316.x. [DOI] [PubMed] [Google Scholar]
- Malacrinò et al. (2017).Malacrinò A, Rassati D, Schena L, Mehzabin R, Battisti A, Palmeri V. Fungal communities associated with bark and ambrosia beetles trapped at international harbours. Fungal Ecology. 2017;28:44–52. doi: 10.1016/j.funeco.2017.04.007. [DOI] [Google Scholar]
- Marini et al. (2011).Marini L, Haack RA, Rabaglia RJ, Petrucco Toffolo E, Battisti A, Faccoli M. Exploring associations between international trade and environmental factors with establishment patterns of exotic Scolytinae. Biological Invasions. 2011;13:2275–2288. doi: 10.1007/s10530-011-0039-2. [DOI] [Google Scholar]
- Mayers et al. (2019).Mayers CG, Harrington TC, Masuya H, Jordal BH, McNew DL, Shih H-H, Roets F, Kietzka GJ. Patterns of coevolution between ambrosia beetle mycangia and the Ceratocystidaceae, with five new fungal genera and seven new species. Persoonia—Molecular Phylogeny and Evolution of Fungi. 2019;44:41–66. doi: 10.3767/persoonia.2020.44.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayers et al. (2015).Mayers CG, McNew DL, Harrington TC, Roeper RA, Fraedrich SW, Biedermann PHW, Castrillo LA, Reed SE. Three genera in the Ceratocystidaceae are the respective symbionts of three independent lineages of ambrosia beetles with large, complex mycangia. Fungal Biology. 2015;119:1075–1092. doi: 10.1016/J.FUNBIO.2015.08.002. [DOI] [PubMed] [Google Scholar]
- McMurdie & Holmes (2013).McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLOS ONE. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menocal et al. (2017).Menocal O, Cruz LF, Kendra PE, Crane JH, Ploetz RC, Carrillo D. Rearing Xyleborus volvulus (Coleoptera: Curculionidae) on media containing sawdust from avocado or silkbay, with or without Raffaelea lauricola (Ophiostomatales: Ophiostomataceae) Environmental Entomology. 2017;46:1275–1283. doi: 10.1093/ee/nvx151. [DOI] [PubMed] [Google Scholar]
- Menocal et al. (2018).Menocal O, Kendra PE, Montgomery WS, Crane JH, Carrillo D. Vertical distribution and daily flight periodicity of ambrosia beetles (Coleoptera: Curculionidae) in Florida avocado orchards affected by Laurel wilt. Journal of Economic Entomology. 2018;111:1190–1196. doi: 10.1093/jee/toy044. [DOI] [PubMed] [Google Scholar]
- Miller (2006).Miller DR. Ethanol and (−)- α-Pinene: attractant kairomones for some large wood-boring beetles in Southeastern USA. Journal of Chemical Ecology. 2006;32:779–794. doi: 10.1007/s10886-006-9037-8. [DOI] [PubMed] [Google Scholar]
- Miller et al. (2016).Miller KE, Hopkins K, Inward DJG, Vogler AP. Metabarcoding of fungal communities associated with bark beetles. Ecology and Evolution. 2016;6:1590–1600. doi: 10.1002/ece3.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller et al. (2019).Miller KE, Inward DJ, Gomez-Rodriguez C, Baselga A, Vogler AP. Predicting the unpredictable: how host specific is the mycobiota of bark and ambrosia beetles? Fungal Ecology. 2019;42 doi: 10.1016/j.funeco.2019.07.008. Article 100854. [DOI] [Google Scholar]
- Nguyen et al. (2016).Nguyen NH, Song Z, Bates ST, Branco S, Tedersoo L, Menke J, Schilling JS, Kennedy PG. FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecology. 2016;20:241–248. doi: 10.1016/J.FUNECO.2015.06.006. [DOI] [Google Scholar]
- Nilsson et al. (2019).Nilsson RH, Anslan S, Bahram M, Wurzbacher C, Baldrian P, Tedersoo L. Mycobiome diversity: high-throughput sequencing and identification of fungi. Nature Reviews Microbiology. 2019;17:95–109. doi: 10.1038/s41579-018-0116-y. [DOI] [PubMed] [Google Scholar]
- Oliver et al. (2010).Oliver KM, Degnan PH, Burke GR, Moran NA. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annual Review of Entomology. 2010;55:247–266. doi: 10.1146/annurev-ento-112408-085305. [DOI] [PubMed] [Google Scholar]
- Ormsby & Brenton-Rule (2017).Ormsby M, Brenton-Rule E. A review of global instruments to combat invasive alien species in forestry. Biological Invasions. 2017;19:3355–3364. doi: 10.1007/s10530-017-1426-0. [DOI] [Google Scholar]
- Pioli et al. (2018).Pioli S, Antonucci S, Giovannelli A, Traversi ML, Borruso L, Bani A, Brusetti L, Tognetti R. Community fingerprinting reveals increasing wood-inhabiting fungal diversity in unmanaged Mediterranean forests. Forest Ecology and Management. 2018;408:202–210. doi: 10.1016/J.FORECO.2017.10.052. [DOI] [Google Scholar]
- Ploetz et al. (2017).Ploetz RC, Konkol JL, Narvaez T, Duncan RE, Saucedo RJ, Campbell A, Mantilla J, Carrillo D, Kendra PE. Presence and prevalence of Raffaelea lauricola, cause of laurel wilt, in different species of ambrosia beetle in Florida, USA. Journal of Economic Entomology. 2017;110:347–354. doi: 10.1093/jee/tow292. [DOI] [PubMed] [Google Scholar]
- R Core Team (2013).R Core Team . R Foundation for Statistical Computing; Vienna: 2013. [Google Scholar]
- Rabaglia et al. (2019).Rabaglia RJ, Cognato AI, Hoebeke ER, Johnson CW, LaBonte JR, Carter ME, Vlach JJ. Early detection and rapid response: a 10-Year summary of the USDA forest service program of surveillance for non-native bark and ambrosia beetles. American Entomologist. 2019;65:29–42. doi: 10.1093/ae/tmz015. [DOI] [Google Scholar]
- Ranger et al. (2015).Ranger CM, Schultz PB, Frank SD, Chong JH, Reding ME. Non-native ambrosia beetles as opportunistic exploiters of living but weakened trees. PLOS ONE. 2015;10:e0131496. doi: 10.1371/journal.pone.0131496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassati et al. (2019).Rassati D, Contarini M, Ranger CM, Cavaletto G, Rossini L, Speranza S, Faccoli M, Marini L. Fungal pathogen and ethanol affect host selection and colonization success in ambrosia beetles. Agricultural and Forest Entomology. 2019 doi: 10.1111/afe.12351. Epub ahead of print Aug 01 2019. [DOI] [Google Scholar]
- Rassati et al. (2016a).Rassati D, Faccoli M, Battisti A, Marini L. Habitat and climatic preferences drive invasions of non-native ambrosia beetles in deciduous temperate forests. Biological Invasions. 2016a;18:2809–2821. doi: 10.1007/s10530-016-1172-8. [DOI] [Google Scholar]
- Rassati et al. (2016b).Rassati D, Faccoli M, Haack RA, Rabaglia RJ, Petrucco Toffolo E, Battisti A, Marini L. Bark and ambrosia beetles show different invasion patterns in the USA. PLOS ONE. 2016b;11:e0158519. doi: 10.1371/journal.pone.0158519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassati, Lieutier & Faccoli (2016).Rassati D, Lieutier F, Faccoli M. Insects and diseases of Mediterranean forest systems. Springer International Publishing; Cham: 2016. Alien wood-boring beetles in Mediterranean regions; pp. 293–327. [DOI] [Google Scholar]
- Reding et al. (2011).Reding ME, Schultz PB, Ranger CM, Oliver JB. Optimizing ethanol-baited traps for monitoring damaging ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) in ornamental nurseries. Journal of Economic Entomology. 2011;104:2017–2024. doi: 10.1603/EC11119. [DOI] [PubMed] [Google Scholar]
- Seebens et al. (2017).Seebens H, Blackburn TM, Dyer EE, Genovesi P, Hulme PE, Jeschke JM, Pagad S, Pyšek P, Winter M, Arianoutsou M, Bacher S, Blasius B, Brundu G, Capinha C, Celesti-Grapow L, Dawson W, Dullinger S, Fuentes N, Jäger H, Kartesz J, Kenis M, Kreft H, Kühn I, Lenzner B, Liebhold A, Mosena A, Moser D, Nishino M, Pearman D, Pergl J, Rabitsch W, Rojas-Sandoval J, Roques A, Rorke S, Rossinelli S, Roy HE, Scalera R, Schindler S, Štajerová K, Tokarska-Guzik B, Van Kleunen M, Walker K, Weigelt P, Yamanaka T, Essl F. No saturation in the accumulation of alien species worldwide. Nature Communications. 2017;8 doi: 10.1038/ncomms14435. Article 14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibold et al. (2019).Seibold S, Müller J, Baldrian P, Cadotte MW, Štursová M, Biedermann PHW, Krah F-S, Bässler C. Fungi associated with beetles dispersing from dead wood—Let’s take the beetle bus! Fungal Ecology. 2019;39:100–108. doi: 10.1016/J.FUNECO.2018.11.016. [DOI] [Google Scholar]
- Simpson (1949).Simpson EH. Measurement of diversity. Nature. 1949;163:688–688. doi: 10.1038/163688a0. [DOI] [Google Scholar]
- Skelton et al. (2019).Skelton J, Johnson AJ, Jusino MA, Bateman CC, Li Y, Hulcr J. A selective fungal transport organ (mycangium) maintains coarse phylogenetic congruence between fungus-farming ambrosia beetles and their symbionts. Proceedings of the Royal Society B: Biological Sciences. 2019;286:20182127. doi: 10.1098/rspb.2018.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton et al. (2018).Skelton J, Jusino MA, Li Y, Bateman C, Thai PH, Wu C, Lindner DL, Hulcr J. Detecting symbioses in complex communities: the fungal symbionts of bark and ambrosia beetles within asian pines. Microbial Ecology. 2018;76:839–850. doi: 10.1007/s00248-018-1154-8. [DOI] [PubMed] [Google Scholar]
- Steininger et al. (2015).Steininger S, Storer C, Hulcr J, Lucky A. Alternative preservatives of insect DNA for citizen science and other low-cost applications. Invertebrate Systematics. 2015;29:468–472. doi: 10.1071/IS15003. [DOI] [Google Scholar]
- Stielow et al. (2015).Stielow JB, Lévesque CA, Seifert KA, Meyer W, Iriny L, Smits D, Renfurm R, Verkley GJM, Groenewald M, Chaduli D, Lomascolo A, Welti S, Lesage-Meessen L, Favel A, Al-Hatmi AMS, Damm U, Yilmaz N, Houbraken J, Lombard L, Quaedvlieg W, Binder M, Vaas LAI, Vu D, Yurkov A, Begerow D, Roehl O, Guerreiro M, Fonseca A, Samerpitak K, Van Diepeningen AD, Dolatabadi S, Moreno LF, Casaregola S, Mallet S, Jacques N, Roscini L, Egidi E, Bizet C, Garcia-Hermoso D, Martín MP, Deng S, Groenewald JZ, Boekhout T, De Beer ZW, Barnes I, Duong TA, Wingfield MJ, De Hoog GS, Crous PW, Lewis CT, Hambleton S, Moussa TAA, Al-Zahrani HS, Almaghrabi OA, Louis-Seize G, Assabgui R, McCormick W, Omer G, Dukik K, Cardinali G, Eberhardt U, De Vries M, Robert V. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia—Molecular Phylogeny and Evolution of Fungi. 2015;35:242–263. doi: 10.3767/003158515X689135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taerum et al. (2013).Taerum SJ, Duong TA, De Beer ZW, Gillette N, Sun J-H, Owen DR, Wingfield MJ. Large shift in symbiont assemblage in the invasive red turpentine beetle. PLOS ONE. 2013;8:e78126. doi: 10.1371/journal.pone.0078126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda & Paine (2019).Umeda C, Paine T. Temperature can limit the invasion range of the ambrosia beetle Euwallacea nr. fornicatus. Agricultural and Forest Entomology. 2019;21:1–7. doi: 10.1111/afe.12297. [DOI] [Google Scholar]
- Vanderpool, Bracewell & McCutcheon (2018).Vanderpool D, Bracewell RR, McCutcheon JP. Know your farmer: ancient origins and multiple independent domestications of ambrosia beetle fungal cultivars. Molecular Ecology. 2018;27:2077–2094. doi: 10.1111/mec.14394. [DOI] [PubMed] [Google Scholar]
- Veselská et al. (2019).Veselská T, Skelton J, Kostovčík M, Hulcr J, Baldrian P, Chudíčková M, Cajthaml T, Vojtová T, Garcia-Fraile P, Kolařík M. Adaptive traits of bark and ambrosia beetle-associated fungi. Fungal Ecology. 2019;41:165–176. doi: 10.1016/j.funeco.2019.06.005. [DOI] [Google Scholar]
- Viiri (1997).Viiri H. Fungal associates of the spruce bark beetle Ips typographus L. (Col. Scolytidae) in relation to different trapping methods. Journal of Applied Entomology. 1997;121:529–533. doi: 10.1111/j.1439-0418.1997.tb01444.x. [DOI] [Google Scholar]
- Vilcinskas et al. (2013).Vilcinskas A, Stoecker K, Schmidtberg H, Rohrich CR, Vogel H. Invasive harlequin ladybird carries biological weapons against native competitors. Science. 2013;340:862–863. doi: 10.1126/science.1234032. [DOI] [PubMed] [Google Scholar]
- Wingfield et al. (2017).Wingfield MJ, Barnes I, De Beer ZW, Roux J, Wingfield BD, Taerum SJ. Novel associations between ophiostomatoid fungi, insects and tree hosts: current status—future prospects. Biological Invasions. 2017;19:3215–3228. doi: 10.1007/s10530-017-1468-3. [DOI] [Google Scholar]
- Wooding et al. (2013).Wooding AL, Wingfield MJ, Hurley BP, Garnas JR, De Groot P, Slippers B. Lack of fidelity revealed in an insect-fungal mutualism after invasion. Biology Letters. 2013;9 doi: 10.1098/rsbl.2013.0342. Article 20130342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun et al. (2014).Yun J-H, Roh SW, Whon TW, Jung M-J, Kim M-S, Park D-S, Yoon C, Nam Y-D, Kim Y-J, Choi J-H, Kim J-Y, Shin N-R, Kim S-H, Lee W-J, Bae J-W. Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Applied and Environmental Microbiology. 2014;80:5254–5264. doi: 10.1128/AEM.01226-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2014).Zhang J, Kobert K, Flouri T, Stamatakis A. PEAR: a fast and accurate illumina paired-end reAd mergeR. Bioinformatics. 2014;30:614–620. doi: 10.1093/bioinformatics/btt593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al. (2018).Zhou Y, Avery PB, Carrillo D, Duncan RH, Lukowsky A, Cave RD. Identification of the Achilles heels of the laurel wilt pathogen and its beetle vector. Applied Microbiology and Biotechnology. 2018;102:5673–5684. doi: 10.1007/s00253-018-9037-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Blue dots indicate old-growth forests, red dots indicate restored forests. All sites were located in north-eastern Italy. Each site is identified by the name of the locality. Map Data 2019 Google.
The main parameters were measured at each site in two circular areas (15 m radius) located close to the boundary of the point where trap was placed.
For each sampling site. P-value as adjusted for multiple comparisons using FDR method. *, number of samples after filtering
The guild column represents the prediction of the functional role of each genus, according to the FUNGuild database and manually curated.
Data Availability Statement
The following information was supplied regarding data availability:
Raw reads are available at NCBI SRA under the BioProject PRJNA524707.