Abstract
The purpose of this study was to investigate the predictive function of sleep spindles in motor sequence consolidation. BOLD responses were acquired in 10 young healthy subjects who were trained on an explicitly known 5‐item sequence using their left nondominant hand, scanned at 9:00 pm while performing that same task and then were retested and scanned 12 h later after a night of sleep during which polysomnographic measures were recorded. An automatic algorithm was used to detect sleep spindles and to quantify their characteristics (i.e., density, amplitude, and duration). Analyses revealed significant positive correlations between gains in performance and the amplitude of spindles. Moreover, significant increases in BOLD signal were observed in several motor‐related areas, most of which were localized in the right hemisphere, particularly in the right cortico‐striatal system. Such increases in BOLD signal also correlated positively with the amplitude of spindles at several derivations. Taken together, our results show that sleep spindles predict neural and behavioral changes in overnight motor sequence consolidation. Hum Brain Mapp 34:2918–2928, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: motor sequence consolidation, cortico‐striatal system, sleep spindles, EEG, fMRI
INTRODUCTION
In recent years, a large body of evidence has demonstrated that the consolidation of motor sequence learning (MSL), as measured through offline gains in performance on a task, is sleep‐dependent [Doyon et al., 2009b; Fischer et al., 2002; Korman et al., 2003; Walker et al., 2002]. More specifically, studies have shown that specific electroencephalographic (EEG) oscillations such as sleep spindles contribute to MSL, consolidation. Spindles consist of short (0.5–2.5 s) synchronous bursts of fast EEG activity (11–15 Hz) with a fusiform shape (waxing and waning amplitude) observed during non‐rapid‐eye movement (NREM) sleep. They originate from the cyclic inhibition of thalamo‐cortical neurons, and they show low intraindividual variability across nights [Gaillard and Blois, 1981; Steriade et al., 1987]. They can also be divided into slow spindles (11–13 Hz), which are more prominent in frontal regions, and fast spindles (13–15 Hz), which are more frequent in parietal areas [see De Gennaro and Ferrara, 2003; Schabus et al., 2007 for a review]. Spindles are thought to be related to synaptic changes and long‐term potentiation (LTP), both involved in learning [Rioult‐Pedotti et al., 2000; Rosanova and Ulrich, 2005; Steriade and Timofeev, 2003].
Support for the role of sleep spindles in motor memory consolidation has come from two lines of evidence. On the one hand, several groups of researchers including ours have reported sizeable alterations in spindle characteristics on the night following training on a procedural motor memory task [Barakat et al., 2010; Fogel and Smith, 2006; Fogel et al., 2007b; Morin et al., 2008; Peters et al., 2007, 1968; Tamaki et al., 2008, 2009]. On the other hand, another research group reported positive correlations between spindle activity during an unaltered night of sleep and subsequent overnight gains of performance on a procedural task [Tamaki et al., 2008].
In addition, the neural correlates underlying motor sequence acquisition and sleep consolidation has been the object of much interest in recent studies. By comparing online brain activity during learning and re‐testing after a night of sleep, several authors have identified task‐related changes in activity in the cortico‐striatal system [Debas et al., 2010; Doyon et al., 2009a, 2002; Doyon and Benali, 2005; Fischer et al., 2005; Walker et al., 2005]. More specifically, and in support of this idea, our group recently reported increases in brain activity following MSL consolidation in subjects who underwent a night of sleep as compared with others who did not, the main differences of activity being located specifically in the globus pallidus and in the putamen ventrally [Debas et al., 2010]. Similar increases in MSL consolidation after sleep were also reported in M1 and the medial prefrontal cortex [Albouy et al., 2008; Peters et al., 2007; Walker et al., 2005], whereas only one study described reductions in brain activity in prefrontal, premotor and M1 areas after motor sequence sleep consolidation [Fischer et al., 2005]. However, the correlations between sleep EEG (e.g., sleep spindles), offline gains in performance and changes in brain activation reflecting overnight motor sequence consolidation remain unexplored.
The aim of this study was thus to better understand the mechanisms underlying motor sequence consolidation. To do so, we carried out an fMRI study using a within‐subject protocol. After training on a 5‐item finger‐tapping task, participants were scanned in the evening while performing that same task and were retested and scanned 12 h later after a night of sleep during which polysomnographic (PSG) measures were recorded. We first evaluated correlations between NREM sleep spindles characteristics (i.e., duration, amplitude, and density) during the post‐learning night and off‐line gains in performance in the MSL task. We then calculated the relationships between NREM sleep spindles characteristics during the post‐learning night, the off‐line gains in performance in the MSL task, and the changes of brain activity before and after sleep that reflect motor sequence consolidation. We hypothesized that sleep spindle characteristics would predict hemodynamic changes in the cortico‐striatal system, and also performance gains. Lastly, we expected to find significant inter‐correlations between the two latter measures.
METHOD
Subjects
Fifteen young healthy subjects (20–30 years, mean 23.0, 8 females) participated in this study. Subjects were right‐handed as assessed by the Edinburgh Handedness Inventory [Oldfield, 1971], in good health, and medication‐free. They also had a normal body mass index (<27) and reported no sleep complaints nor any psychiatric or neurological illnesses. All subjects scored lower than 4 on the short version of the Beck Depression Scale [Beck and Steer, 1987], and all women were tested in their follicular hormonal cycle. Subjects were nonsmokers, were not regular coffee or alcohol drinkers and were asked to be alcohol and caffeine‐free at least 12 h before each experimental session. Subjects were excluded if they worked night shifts, were engaged in a trans‐meridian trip three months preceding the study, were regular nappers or were extreme evening or morning‐type individuals [assessed by Morningness‐Eveningness Questionnaire from Horne & Östberg, 1976]. All subjects were instructed to maintain a regular cycle of sleep (bedtime around 11:00 p.m. [±1 h] and wake‐up around 7:00 [±1 h]) for seven days prior to and during the study, and their compliance was verified using a sleep diary. All subjects slept between 7 and 9 h per night, and had no disruption of their regular sleep‐wake cycle four weeks prior to their participation. Subjects underwent an adaptation/screening night in the lab with polysomnographic evaluations of their sleep. Those who presented sleep disturbances such as <85% sleep efficiency, >30 min sleep latency, sleep apnea, hypopnea, or periodic leg movements (>5 events per hour associated with cortical arousal) were excluded. Moreover, musicians and professional typists were excluded to avoid subjects with previous expertise on sequential motor types of tasks. All subjects gave their written informed consent to participate in the study and received financial compensation for their participation. The project was approved by the “Regroupement Neuroimagerie/Québec” Ethics Committee at the Montreal Geriatric Institute.
Experimental Design and Behavioral Paradigm
The present study uses a subset of the behavioral and fMRI data previously published in Debas et al. study [2010]. Thus briefly, memory consolidation of motor sequence learning was measured using a within‐subject design (see Fig. 1), in which participants were trained and scanned using a modified version of the sequence finger‐tapping task initially developed by Karni et al. 1995. In this task, subjects are asked to repeat an explicitly known sequence of five finger movements (4‐1‐3‐2‐4) with their left, non‐dominant hand using a MRI‐compatible custom made response box. After being familiarized with the sequence and having produced it three times without making errors, participants underwent a first training session in a mock scanner. Once training was completed, subjects were then positioned supine into the MRI bore, where they had to perform the same sequence task (i.e., immediate test session) while being scanned using a block design. Subjects were again tested on the sequence task 12 h later (i.e., delayed retest session) using the same scanning protocol. Each block started with a 2.5‐s period of instruction where the word “Sequence” appeared in the middle of the screen. This was followed by the display of a green square indicating that subjects could start producing the known sequence as fast as possible, while making as few errors as possible. After having completed 100 finger movements (i.e., 20 sequences) per block, subjects had 15 s to rest and these alternating experimental conditions were repeated eight times during each session. The same number of blocks of practice and sequences (i.e., 8 blocks of 20 5‐item sequences) was required in the training [mock scanner], immediate test [scanner] and delayed retest [scanner] sessions in order to control for motor outputs. As the accuracy was very stable across blocks (18 to 19/20 correct sequences), the mean time per sequences (TpS) was computed for each block, as it better reflected the subject's improvement on the task. Subjects were trained and scanned in the evening (9:00 p.m. approximately), and were re‐scanned in the morning (9:00 a.m. approximately), following a night of sleep with polysomnographic (PSG) recordings.
Figure 1.

Experimental design used in the present experiment.
Polysomnographic Recordings and Analysis
All participants underwent a night of sleep at the laboratory and PSG acquisitions were carried out during the entire night. EEG electrodes were applied to the subject's head according to the International 10‐20 System, using a referential montage with linked ears, right and left electrooculogram (EOG), and three chin electromyograms (EMG). Signals were recorded using a digital ambulatory sleep recorder (Vitaport‐3 System; TEMEC Instruments, Kerkrade, Netherlands). EEG signal impedance was set at 5 kΩ for all electrodes, and recorded signals were filtered at 70 Hz (low pass) with 1‐s time constant and digitized at a sampling rate of 256 Hz using commercial software (Colombus). Sleep stages were visually scored by a trained technician, unaware of hypotheses. Scoring was done according to standard criteria (Rechtschaffen and Kales, 1968) modified to 20‐sec epochs using an EEG layout (C3 derivation) displayed on a computer screen (Harmonie, Stellate System, Montreal, Canada). Artifacts were detected automatically and rejected from the analysis. Further artifacts were also eliminated by visual inspection.
Sleep spindles were detected for each subject separately on the frontal (F3, F4, Fz), central (C3, C4, Cz), and parietal (P3, P4, Pz) derivations during NREM sleep (stages 1‐2‐3 and 4) using an automatic algorithm based on an adapted version of the criteria used by Molle et al. 2002. The use of such an algorithm allows a more standardized approach and a more precise way to extract sleep spindles characteristics. The raw EEG data were first filtered using a band‐pass finite‐impulse response (FIR) filter between 11 and 14.9 Hz (‐3 dB at filter limits). The RMS (Root Mean Square) amplitude of the filtered signal was then calculated by 0.25 second epochs. Artifactual epochs and those corresponding to REM periods were discarded. Then, an amplitude threshold, corresponding to the 95th percentile of the RMS amplitude for the selected epochs was determined for each derivation. Spindles with amplitude higher than this threshold and with a minimum duration of 0.5 sec were marked. Three dependent variables were calculated for each derivation: spindle density (number of spindle per minute of N‐REM sleep), spindle amplitude (difference in voltage between negative peak and positive peak expressed in μV), and spindle duration (in sec). Finally, and to further explore a possible differential implication of the two types of spindles, fast (13‐15 Hz) and slow (11‐13 Hz) spindles were detected using the same algorithm and approach.
MRI Acquisition and Analysis
Brain imaging data was acquired using a 3T scanner (Magnetom Trio Siemens AG, Germany), equipped with an 8‐channel head coil. A high resolution anatomical T1‐weighted scan was acquired for each subject (voxel size = 1 × 1 × 1 mm3, TR = 23 ms, TE = 2.98 ms, FA = 9°; FoV 256 × 240 mm2; matrix 256 × 256; 176 slices). Functional T2*‐weighted images were also acquired with a gradient echo‐planar sequence sensitive to the blood oxygenation level dependent (BOLD) signal (voxel size = 3.75 × 3.75 × 5 mm3; TR = 2.5 s; TE = 30 ms; FA = 90°; FOV = 240 × 240 mm2, matrix size = 64 × 64; 28 slices).
Data were then analyzed with SPM 2 software (Wellcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/) implemented in Matlab 7 (Mathworks, Sherbom, MA). Preprocessing steps included the realignment, coregistration of functional and anatomical images, slice timing correction, spatial normalization into the MNI‐152 stereotactic space, and smoothing using a Gaussian Kernel of 6 mm full‐width at half‐maximum (FWHM). A cut‐off of 3 mm in each direction was set for movement excursions.
Statistics were derived based upon the general linear model. We first tested the effects of interest at an intraindividual level, using linear contrasts on a design matrix convolved with a standard canonical hemodynamic response function (HRF), generating statistical parametric maps. Movement parameters derived from realignment of the functional volumes were not included as this is not recommended when using a block design and manual responses [Johnstone et al., 2006]. Linear contrasts estimated the main effects of the MSL task relative to its baseline (i.e., rest blocks), as well as the main effect of sleep (Delayed retest – Immediate test). These contrasts were used to investigate increases in brain activity related to MSL consolidation. The individual statistical maps were then entered in a random effect analysis at the group level. Because of the small sample of subjects (n = 10) in this study, the statistical tests only had very limited detection power for a full brain search. We therefore formulated strong a priori hypotheses on the brain areas that were expected to play a role in MSL consolidation, i.e. SMA, M1, and Striatum. We then searched the literature for coordinates of activation peaks in these areas with relevant tasks [Doyon et al., 2003; Fischer et al., 2005; Walker et al., 2005]. Small volumes (sphere radius = 10mm) centered at these coordinates were generated and the multiple comparison problem was addressed using a stringent family wise error (FWE) correction restricted to the small volumes. There is however a bias in that procedure, in that only the expected effects are tested, and there is potentially a dramatic inflation of the rate of false negatives. To avoid this bias, we also inspected all significant results in a full brain analysis that survived a liberal threshold of P < 0.001, uncorrected for multiple comparisons. We listed all the areas where peaks were detected with that liberal (but sensitive) procedure, and reviewed again the literature to select peaks and implement a small volume correction similar to what was done for areas selected using an a priori knowledge [Doyon et al., 2003; Fischer et al., 2005; Albouy and Maquet, 2008; Grafton et al., 1998; Penhune and Doyon, 2002; Van Der Graaf et al., 2004]. Note that for three peaks, no related coordinates could be identified in the articles we reviewed from the literature. The coordinates of the peaks themselves were thus used to define the small volumes in these cases. There is some degree of circularity in the definition of the small volumes defined on the basis of the peaks identified with an uncorrected threshold [Vul et al., 2009]. Results that were found significant at the level of p<0.005 FWE‐corrected for small volumes are strong statistical evidence for peaks selected on pure a priori knowledge, while areas selected on the basis of uncorrected statistics should be regarded as exploratory findings, with only weak evidence for statistical significance. This second, liberal analysis was however critical to ensure that our a priori hypothesis did not grossly conflict with the overall picture conveyed by our neuroimaging dataset. Table 3 recapitulate the coordinates that were used to perform small volume correction, making a clear distinction between the areas of strong a priori hypothesis (with rigorous statistical validity) (i.e. Table 3) and those based on uncorrected statistics (with exploratory value) (i.e. Table 3). Note that correction for small volumes was implemented for all selected peaks at once, not just those specified on a strong a priori hypothesis.
Table 3.
MNI coordinates and significance scores of the brain regions showing an increase in BOLD activity for the delayed retest – immediate post‐training contrasts
| Areas | BA | x,y,z | svc coordinates | Reference author | Z corr |
|---|---|---|---|---|---|
| A—Delayed retest – immediate post‐training | |||||
| Right putamen/globus pallidus | 19, −8, −5 | 23, 0, 0 | Doyon, 2003 | 3.36 | |
| Right precentral gyrus | 6 | 34, −30, 70 | 36, −22, 66 | Fisher, 2005 | 3.29 |
| B—Delayed retest – immediate post‐training | |||||
| Right cingulate gyrus | 24 | 0, −4, 45 | 0, −10, 48 | Penhume, 2002 | 3.23 |
| Right cingulate gyrus | 24 | 4, −11, 40 | −3, −3, 45 | Grafton, 1998 | 3.13 |
| Right ventro‐lateral pre‐frontal gyrus | 47 | 41, 19, 0 | 38, 18, 6 | Albouy, 2008 | 3.22 |
| Right temporal pole | 22 | 60, 11, 5 | 56, 12, 8 | Albouy, 2008 | 3.10 |
| Left postcentral gyrus | 40 | −56, −23, 20 | −62, −18, 16 | Van Der Graaf, 2004 | 3.13 |
A: Significant brain activations after correction over a small volume (svc) of interest based on a priori hypothesis. BA, Broadman area. B: Exploratory results of significant brain activations after correction over a small volume (svc) of interest, following a whole brain analysis with a threshold uncorrected for multiple comparisons (P < 0.001). BA, Broadman area.
Correlation Analysis
Because the aim of this study was to examine the relationship between sleep, behavioral and imaging data sets, Pearson product‐moment correlations were first carried out between sleep spindle characteristics (i.e., amplitude, density, and duration) and percentage of performance gains in TpS between the first block of the delayed retest and the last block of the immediate post‐training sessions [((TpS immediate test − TpSdelayed retest)/TpS immediate test) × 100]. No correction for multiple analyses was used because of the small number of subjects. Then, and to further assess the relationship between brain activity changes following MSL consolidation and sleep data, simple regression analyses were carried out between sleep spindle characteristics and the (Delayed retest – Immediate test) BOLD contrast over the whole brain. More specifically, only spindle characteristics that significantly correlated with motor gains in performance were used as predictors for the activity related to the MSL task. Finally, effects of consolidation on brain activity changes were explored through simple regression analysis using performance gains in TpS as predictors of the activity related to the MSL task. Similar to the previous contrast analysis, small volume corrections were carried out (svc, sphere radius = 10mm) using the same approach, first with a liberal threshold of p<0.005, uncorrected for multiple comparisons, and then correcting using corresponding published coordinates [i.e., Doyon et al., 2003; Fischer et al., 2005; Albouy and Maquet, 2008; Grafton et al., 1998; Penhune and Doyon, 2002; Toni et al., 1998; Van Der Graaf et al., 2004, Oishi et al., 2005] and FWE corrections. Table 4 recapitulate the coordinates that were used to perform small volume correction, making a clear distinction again between the areas of strong a priori hypothesis (i.e., Table 4) and those based on uncorrected statistics (i.e., Table 4).
Table 4.
MNI coordinates and significance scores of the brain regions in which changes in BOLD activity correlated significantly with the amplitude of spindles
| Areas | BA | x,y,z | svc coordinates | Reference author | Z corr |
|---|---|---|---|---|---|
| A—Delayed retest – immediate post‐training | |||||
| F3 | |||||
| Right putamen/globus pallidus | 19, −8, −5 | 23, 0, 0 | Doyon, 2003 | 2.97 | |
| Right precentral gyrus | 6 | 30, −23, 70 | 36, −22, 66 | Fisher, 2005 | 2.82 |
| F4 | |||||
| Right putamen/globus pallidus | 19, −8, −5 | 23, 0, 0 | Doyon, 2003 | 3.01 | |
| Right precentral gyrus | 6 | 30, −23, 70 | 36, −22, 66 | Fisher, 2005 | 2.98 |
| C4 | |||||
| Right putamen/globus pallidus | 19, −8, −5 | 23, 0, 0 | Doyon, 2003 | 2.70 | |
| Right precentral gyrus | 6 | 30, −23, 70 | 36, −22, 66 | Fisher, 2005 | 2.71 |
| P3 | |||||
| Right putamen/globus pallidus | 19, −8, −5 | 23, 0, 0 | Doyon, 2003 | 2.69 | |
| B—Delayed retest – immediate post‐training | |||||
| F3 | |||||
| Right postcentral gyrus | 5 | 41, −45, 65 | 40, −38, 62 | Fisher, 2005 | 2.98 |
| Right supra−marginal gyrus | 40 | 49, −60, 50 | 44, −64, 46 | Oishi, 2005 | 2.85 |
| Right inferior frontal gyrus | 47 | 45, 38, −15 | 40, 42, −10 | Penhume, 2002 | 2.79 |
| Left postcentral gyrus | 3 | −26, −30, 75 | −34, −32, 68 | Toni, 1998 | 3.07 |
| Left Postcentral Gyrus | 1−3 | −45, −30, 65 | −40, −38, 62 | Fisher, 2005 | 2.72 |
| Left cerebellum (Lobule 9) | −15, −49, −10 | −16, −42, −40 | Albouy, 2008 | 2.99 | |
| F4 | |||||
| Right postcentral gyrus | 5 | 41, −45, 65 | 40, −38, 62 | Fisher, 2005 | 3.06 |
| Right inferior frontal gyrus | 40 | 49, −60, 50 | 44, −64, 46 | Oishi, 2005 | 2.79 |
| Right temporal pole | 22 | 56, 8, 0 | 56, 12, 8 | Albouy, 2008 | 2.80 |
| Left middle temporal gyrus | 21 | −68, −41, 0 | −68, −40, −12 | Albouy, 2008 | 2.86 |
| Left postcentral gyrus | 5 | −38, −45, 65 | −40, −38, 62 | Fisher, 2005 | 2.92 |
| Left postcentral gyrus | 3 | −26, −30, 75 | −34, −32, 68 | Toni, 1998 | 3.21 |
| Left superior frontal gyrus | 10 | −22, 56, 0 | −16, 50, −6 | Albouy, 2008 | 2.77 |
| Left cerebellum (Lobule 9) | −11, −49, −10 | −16, −42, −40 | Albouy, 2008 | 2.88 | |
| Fz | |||||
| Right inferior frontal gyrus | 47 | 45, 38, −15 | 40, 42, −10 | Penhume, 2002 | 2.73 |
| Left postcentral gyrus | 3 | −26, −30, 75 | −34, −32, 68 | Toni, 1998 | 2.82 |
| C3 | |||||
| Right inferior frontal gyrus | 47 | 45, 38, −15 | 40, 42, −10 | Penhume, 2002 | 2.71 |
| Left postcentral gyrus | 3 | −26, −30, 75 | −34, −32, 68 | Toni, 1998 | 2.81 |
| C4 | |||||
| Right postcentral gyrus | 5 | 41, −45, 65 | 40, −38, 62 | Fisher, 2005 | 2.74 |
| Right inferior frontal gyrus | 47 | 45, 38, −15 | 40, 42, −10 | Penhume, 2002 | 2.78 |
| Left postcentral gyrus | 3 | −26, −30, 75 | −34, −32, 68 | Toni, 1998 | 2.94 |
| P3 | |||||
| Right postcentral gyrus | 5−7 | 15, −45, 75 | 16, −42, 78 | Albouy, 2008 | 2.72 |
| Right postcentral gyrus | 5 | 41, −45, 65 | 40, −38, 62 | Fisher, 2005 | 2.80 |
| Right inferior frontal gyrus | 47 | 45, 38, −15 | 40, 42, −10 | Penhume, 2002 | 2.86 |
| Left middle temporal gyrus | 21 | −68, −41, 0 | −68, −40, −12 | Albouy, 2008 | 2.80 |
| Left postcentral gyrus | 5 | −38, −45, 65 | −40, −38, 62 | Fisher, 2005 | 2.74 |
| Left postcentral gyrus | 3 | −26, −30, 75 | −34, −32, 68 | Toni, 1998 | 3.03 |
| Left cerebellum (Lobule 9) | −11, −49, −10 | −16, −42, −40 | Albouy, 2008 | 2.74 | |
A: Significant brain activations after correction over a small volume (svc) of interest based on a priori hypothesis; BA, Broadman area. B: Exploratory results of significant brain activations after correction over a small volume (svc) of interest, following a whole brain analysis with a threshold uncorrected for multiple comparisons (P < 0.001). BA, Broadman area.
Five subjects were excluded from all statistical analyses because of technical issues in behavioral or polysomnographic recordings. Thus, analyses using these data sets were carried out with a sample size of 10 subjects (6 females).
RESULTS
Behavioral Measures
A detailed description of the behavioral findings have been previously published [Debas et al., 2010]. These results show that subjects reached asymptotic performance at the end of the training session, and that they showed offline gains of performance on the motor task after a night of sleep. In fact, compared with their performance on the last block of the immediate post‐training in the evening, subjects started off the delayed retest session in the morning with an average decrease in TpS of 112 ms (132 SD) (Cohen's d = 0.52) (Fig. 2). Furthermore, when compared with a group of subjects that were trained and retested 12 h later without intervening sleep [see (Debas et al., 2010), the night/sleep group (Mean = 1.24 ms, SD = 0.30) at retest was significantly faster than the day/awake group (Mean = 1.29 ms, SD = 0.35): t(38) = 12.4, P < 0.0001 (Cohen's d = 0.15)].
Figure 2.
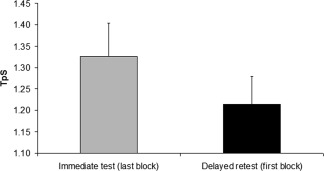
Behavioral Results: Comparison of the time per sequence in seconds (TpS) on the last block of practice during the immediate test and the first block of practice during the delayed retest.
Polysomnographic Sleep Variables
PSG parameters (i.e., sleep latency, total sleep time, sleep efficiency, and percentage of sleep stages) and spindle characteristics are reported in Tables 1 and 2. All subjects included in this study had normal sleep parameters that lasted for 446.70 min (±34.56 min), on average.
Table 1.
Mean (SD) polysomnographic sleep parameters
| Sleep parameter | |
|---|---|
| Sleep latency (min) | 26.67 (18.34) |
| Total sleep time (min) | 446.70 (34.56) |
| Sleep efficiency (%) | 95.56 (4.99) |
| Stage 1 (%) | 4.70 (2.39) |
| Stage 2 (%) | 60.17 (6.03) |
| Stage 3 (%) | 7.62 (2.92) |
| Stage 4 (%) | 3.00 (3.88) |
| REM sleep (%) | 24.51 (2.94) |
Table 2.
Mean (SD) sleep spindles characteristics on selected derivations
| F4 | C4 | P4 | Fz | Cz | Pz | F3 | C3 | P3 | |
|---|---|---|---|---|---|---|---|---|---|
| Density (spindles/mn) | 3.41 (0.11) | 3.29 (0.15) | 3.20 (0.30) | 3.29 (0.1) | 3.34 (0.13) | 3.27 (0.20) | 3.42 (0.56) | 3.31 (0.46) | 3.24 (0.37) |
| Amplitude (in μV) | 35.71 (6.58) | 33.55 (6.20) | 32.54 (9.33) | 35.30 (6.73) | 44.02 (8.32) | 41.98 (10.11) | 34.08 (6.35) | 34.16 (7.11) | 34.13 (9.18) |
| Duration (in s) | 0.68 (0.04) | 0.69 (0.05) | 0.73 (0.05) | 0.67 (0.03) | 0.70 (0.04) | 0.74 (0.05) | 0.69 (0.03) | 0.69 (0.04) | 0.73 (0.05) |
Functional MRI Data
The effects of sleep‐dependent consolidation on the MSL task were assessed through the difference in activations between the delayed retest and immediate test sessions. Because the task was performed with the left nondominant hand, changes in activity in controlateral brain regions were expected. As predicted, significant increases in BOLD signal following sleep were mainly found in the right basal ganglia (putamen/globus pallidus) and right primary motor cortex (see Fig. 3A,B). All significant results at P svc < 0.05 are reported in Tables 3.
Figure 3.
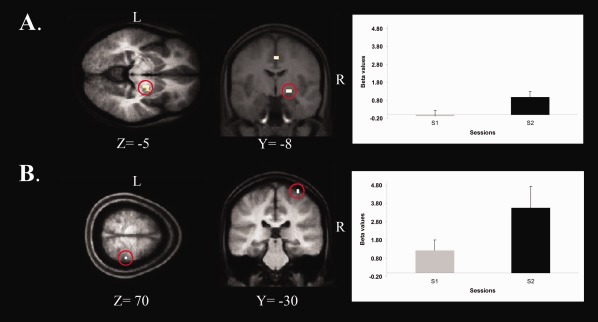
Statistical parametric maps revealing brain regions that showed significant changes in BOLD activity following a night of sleep. A: Right Putamen/Globus Pallidus, B: Right Primary Motor Cortex. On the right: graph depicting strength of BOLD signal in the Putamen/Globus Pallidus and Right Primary Motor Cortex during both the Immediate test (S1) and the Delayed Retest (S2) sessions.
Correlation Analyses
EEG: behavioral data
We also assessed whether sleep spindle characteristics during the experimental night predicted gains in performance occurring after sleep. These analyses yielded significant correlations between the decrease in TpS and spindle amplitude on F3, F4, Fz, C3, C4, and P3 derivations (r[9] = 0.76, P = 0.011; r[10] = 0.73, P = 0.025; r[9] = 0.77, P = 0.015; r[9] = 0.92, P < 0.001; r[9] = 0.83, P = 0.006; r[9] = 0.67, P = 0.046 respectively) (see Fig. 4). No other significant correlations were found. Furthermore, correlations analyses with both fast and slow spindles yielded very similar results, and accordingly, this distinction was dropped for the rest of the analyses.
Figure 4.
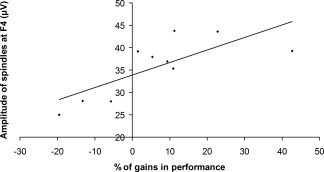
Correlation between the amplitude of spindles at F4 and percentage of performance gains (r[10] = 0.73, P = 0.025).
EEG: imaging data
To investigate whether sleep spindles predicted changes in BOLD activity in brain regions related to MSL consolidation, spindle characteristics that significantly correlated with gains in performance (i.e., amplitude values on F3, F4, Fz, C3, C4, and P3 for the NREM sleep) were regressed against results from the Delayed retest – Immediate test fMRI contrast data of the whole brain. Interestingly, spindle amplitude on F3, F4, C4, and P3 correlated significantly with increased BOLD activity in the right basal ganglia, and in the right putamen/globus pallidus, in particular (r[9] = 0.78, P = 0.013; r[10] = 0.70, P = 0.024; r[9] = 0.72, P = 0.030; r[10] = 0.70, P = 0.025 respectively). Spindle amplitude at F3, F4, and C4 correlated also with changes in the right primary motor cortex (r[9] = 0.75, P = 0.021; r[10] = 0.65, P = 0.041; r[9] = 0.68, P = 0.046 respectively) (see Fig. 5A,B). Additional significant correlations were found between changes in BOLD activity in other motor‐related brain regions and spindle amplitude on all derivations (Tables 4).
Figure 5.
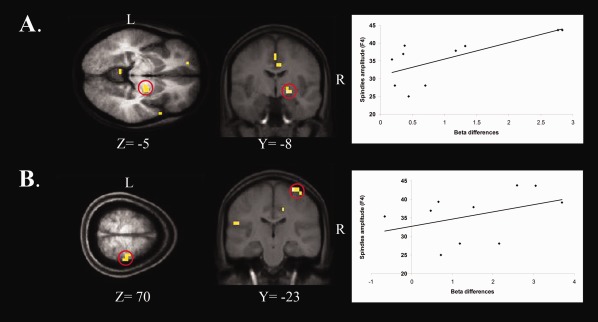
Brain regions showing a significant correlation between spindles amplitude on F4 and BOLD activity changes after the experimental night in the (A) Right Putamen /Globus Pallidus (r[10] = 0.70, P = 0.024), and (B) Right Primary Motor Cortex (r[10] = 0.65, P = 0.041). On the right: plot graph depicting a positive correlation between the strength of the BOLD signal (x axis) and the spindle amplitude on F4 (y axis).
Behavioral data: imaging data
The simple regression analysis between gains of performance and BOLD activity were not significant, and thus our findings revealed only a two‐way pattern of correlations (spindles/gains in performance and spindles/BOLD data).
DISCUSSION
Consistent with previous reports [Doyon et al., 2009b; Fischer et al., 2002; Morin et al., 2008; Walker et al., 2002], subjects performance on the MSL task spontaneously improved after a night of sleep. This suggests that consolidation occurred over night, and thus reaffirms once again the importance of sleep for this type of mnemonic process to take place. Importantly, the present study revealed significant positive correlations between sleep spindle characteristics on the one hand, and both offline improvement in MSL performance and differences in cerebral activity reflecting the consolidation of a new MSL task on the other hand. Subjects with higher spindle amplitude showed greater MSL consolidation. Moreover, significant increases in BOLD signal were observed in several motor‐related areas, most of which were localized in the right hemisphere particularly in the right cortico‐striatal system (i.e. M1 and putamen/globus pallidus). Interestingly, such increases in BOLD signal correlated positively with the amplitude of spindles that were detected on several derivations.
Although behavioral and fMRI data were acquired at different times of day (night vs morning) preventing us from controlling for possible circadian effects, significant gains in performance on a similar sequence task have previously been shown after diurnal sleep [Fischer et al., 2002] or following a daytime nap [Korman et al., 2007], thus suggesting that the pattern of results described here are consistent with results from studies controlling for time‐of‐day effects. Moreover, prior studies have reported similar changes in brain activity related to long‐term motor sequence learning, even when scanning sessions took place at the same time of day [Lehericy et al., 2005; Penhune and Doyon, 2002; Walker et al., 2005]. Thus the results of such studies imply that the observed differences in BOLD signal activity before and after a night of sleep are most likely due to neuronal changes related to MSL consolidation.
EEG and Behavior
The present study also revealed significant correlations between the subjects' spindle amplitude recorded during the experimental night and their offline MSL gains in performance. These results are in agreement with several previously published studies where a significant role has been attributed to sleep spindles in a diversity of procedural [Fogel and Smith, 2006; Fogel et al., 2007b; Morin et al., 2008; Peters et al., 2008; Peters et al., 2007] and declarative memory consolidation paradigms [Clemens et al., 2005; Schabus et al., 2004]. Taken together, these studies corroborate the role of sleep spindles in long‐term potentiation and synaptic plasticity [Rioult‐Pedotti et al., 2000; Rosanova and Ulrich, 2005; Steriade and Timofeev, 2003], and thus support the notion that such physiological characteristics contribute to motor procedural memory consolidation processes. Yet our results did not reveal differential and specific role for fast spindles. In fact, both fast and slow spindles yielded similar patterns of correlations which appear to be inconsistent with the results from Tamaki et al. [2008, 2009]. However, task differences (i.e. motor adaptation vs motor sequence learning) can explain such a discrepancy.
Given that the sequence task was executed using the left hand, correlations between spindles and performance gains were expected to be stronger in the right hemisphere. Contrary to this hypothesis, our results did not show any lateralized (i.e. left vs. right) effects as correlations were found with derivations on both sides of the brain (e.g., F3, F4, C3 and C4). The latter pattern of results might be due to the fact that correlations were carried out with data collected during the post‐training night only, and not based on changes in spindle activity before and after practice on the motor sequence learning task [Barakat et al., 2010; Tamaki et al., 2009]. Yet, it supports the predictive role of sleep spindles during the experimental night, subjects with higher spindle amplitude in either hemisphere showing higher gains of performance in the MSL task. Nevertheless, significant EEG/behavior correlations were mainly observed on derivations overlying different motor cortical areas (e.g., the premotor and primary motor cortices), suggesting that spindle activity during the experimental night may relate to the learning‐induced brain plasticity that occurs in these brain regions; a hypothesis that is supported by recent EEG/fMRI study in which spindles have been proposed to participate in the processing of sensorimotor information [Schabus et al., 2007].
fMRI Data
Analysis of the BOLD data revealed significant increases of activity in several regions of the brain after a night of sleep, mostly in the right primary motor area (M1) and lentiform nucleus (putamem/globus pallidus). Although the protocol used here does not allow us to determine whether changes in these brain regions occurred during sleep or as a consequence of it, there is cumulating evidence that such functional changes are facilitated by a period of sleep following initial motor learning [Albouy et al., 2008; Debas et al., 2010; Fischer et al., 2005]. It is worth mentioning that, unlike the EEG results, BOLD activity changes were clearly lateralized, and were almost exclusive to the right brain regions (i.e. contralateral to the hand used for task execution), showing more functional specificity in the latter imaging technique, and thus falling in line with our expectations. By showing increased neuronal activity in the lentiform nuclei following a post‐training night of sleep, and consistent with the results of previous studies, our results further confirm the critical role of the cortico‐striatal system in the consolidation of a newly learned sequence of finger movements [Debas et al., 2010; Hikosaka et al., 1999; Doyon et al., 2009a, 2003, 2002; Doyon and Benali, 2005].
EEG, Behavioral, and fMRI Data
Importantly, significant two‐way correlations were observed in this study. The amplitude of sleep spindles on several derivations (F3, F4, C4, and P3) correlated with both gains in performance and significant changes of BOLD signal in the putamen of the cortico‐striatal system. Not only do such correlations confirm the relation between sleep and common functional brain alterations underlying motor sequence consolidation, but they also suggest that sleep spindles might play a critical role in the reactivation of brain regions involved in the initial procedural learning during sleep, the latter being thought to constitute a possible physiological mechanism responsible for creating a more stable and robust motor memory trace following training [Maquet et al., 2000]. The latter hypothesis is supported by several studies in rodents [Pennartz et al., 2004] and humans [Maquet et al., 2000; Peigneux et al., 2003], in which recorded brain activity during training on a procedural task was re‐expressed during post‐training sleep. The possible implication of sleep spindles in memory process is also in accord with results of a recent study conducted over a regular night of sleep [Andrade et al., 2001]. Using a 1.5T fMRI along with an EEG recording, these authors reported an increase in the hippocampal/neocortical connectivity during the occurrence of Stage 2 spindles, as well as a significant interaction between spindles and hippocampal functional connectivity, hence supporting the idea that such sleep rhythms can mediate changes in a memory network. Finally, our results show that subjects who had greater spindle amplitude, also showed higher gains in performance and larger changes of activity in the cortico‐striatal system. This supports a possible predictive role for sleep spindles in memory consolidation [Clemens et al., 2005; Schabus et al., 2004; Tamaki et al., 2009], and is consistent with other viewpoints, which consider these rhythms as a physiological index of intelligence and a reflection of more general cognitive abilities [Bodizs et al., 2005; Brière, 2000; Fogel, 2001; Fogel et al., 2007a; Gais et al., 2002; Nader, 2001; Patterson et al., 1983; Schabus, 2009; Schabus et al., 2006]. Yet although interesting, such an interpretation remains a working hypothesis awaiting further experimental investigation.
CONCLUSIONS
Our results are the first direct evidence proposing a functional link between sleep spindles and both overnight gains in performance and brain correlates of motor memory consolidation of a newly acquired sequence of movements. Such findings thus suggest that alterations in spindle activity with age or in sleep disorders might explain some of the motor memory deficits observed in those conditions.
ACKNOWLEDGMENTS
The authors are grateful to Sonia Frenette and Vo An Nguyen for their technical assistance.
REFERENCES
- Albouy G, Maquet P (2008): Hippocampus, striatum and sequences. Med Sci (Paris) 24:921–922. [DOI] [PubMed] [Google Scholar]
- Albouy G, Sterpenich V, Balteau E, Vandewalle G, Desseilles M, Dang‐Vu T, Darsaud A, Ruby P, Luppi PH, Degueldre C, et al. (2008): Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron 58:261–272. [DOI] [PubMed] [Google Scholar]
- Andrade KC, Spoormaker VI, Dresler M, Wehrle R, Holsboer F, Samann PG, Czisch M (2011): Sleep spindles and hippocampal functional connectivity in human NREM sleep. J Neurosci 31:10331–10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat M, Doyon J, Debas K, Vandewalle G, Morin A, Poirier G, Martin N, Lafortune M, Karni A, Ungerleider LG, et al. (2010): Fast and slow spindle involvement in the consolidation of a new motor sequence. Behav Brain Res 217:117–121. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R (1987): The Beck Depression Inventory. Psychological Corporation. [Google Scholar]
- Bodizs R, Kis T, Lazar AS, Havran L, Rigo P, Clemens Z, Halasz P (2005): Prediction of general mental ability based on neural oscillation measures of sleep. J Sleep Res 14:285–292. [DOI] [PubMed] [Google Scholar]
- Brière M, Forest G, Lussier I, Godbout R (2000): Implicit verbal recall correlates positively with EEG sleep spindle activity. Sleep 23 ( Suppl 2):A219. [Google Scholar]
- Clemens Z, Fabo D, Halasz P (2005): Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience 132:529–535. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M (2003): Sleep spindles: an overview. Sleep Med Rev 7:423–440. [DOI] [PubMed] [Google Scholar]
- Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G, Hadj Tahar A, Bellec P, Karni A, Ungerleider LG, et al. (2010): Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc Natl Acad Sci USA 107:17839–17844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Benali H (2005): Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol 15:161–167. [DOI] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehericy S, Benali H (2009a)Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res 199:61–75. [DOI] [PubMed] [Google Scholar]
- Doyon J, Korman M, Morin A, Dostie V, Hadj Tahar A, Benali H, Karni A, Ungerleider LG, Carrier J (2009b)Contribution of night and day sleep vs. simple passage of time to the consolidation of motor sequence and visuomotor adaptation learning. Exp Brain Res 195:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG (2003): Distinct contribution of the cortico‐striatal and cortico‐cerebellar systems to motor skill learning. Neuropsychologia 41:252–262. [DOI] [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG (2002): Experience‐dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci USA 99:1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Hallschmid M, Elsner AL, Born J (2002): Sleep forms memory for finger skills. Proc Natl Acad Sci USA 99:11987–11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Nitschke MF, Melchert UH, Erdmann C, Born J (2005): Motor memory consolidation in sleep shapes more effective neuronal representations. J Neurosci 25:11248–11255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel S, Jacob J, Smith C (2001): Increased sleep spindle activity following simple motor procedural learning in humans. Actas de Fisiolgia 7:123. [Google Scholar]
- Fogel SM, Nader R, Cote KA, Smith CT (2007a)Sleep spindles and learning potential. Behav Neurosci 121:1–10. [DOI] [PubMed] [Google Scholar]
- Fogel SM, Smith CT (2006): Learning‐dependent changes in sleep spindles and Stage 2 sleep. J Sleep Res 15:250–255. [DOI] [PubMed] [Google Scholar]
- Fogel SM, Smith CT, Cote KA (2007b)Dissociable learning‐dependent changes in REM and non‐REM sleep in declarative and procedural memory systems. Behav Brain Res 180:48–61. [DOI] [PubMed] [Google Scholar]
- Gaillard JM, Blois R (1981): Spindle density in sleep of normal subjects. Sleep 4:385–391. [DOI] [PubMed] [Google Scholar]
- Gais S, Molle M, Helms K, Born J (2002): Learning‐dependent increases in sleep spindle density. J Neurosci 22:6830–6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry RB (1998): Abstract and effector‐specific representations of motor sequences identified with PET. J Neurosci 18:9420–9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, Miyachi S, Doya K (1999): Parallel neural networks for learning sequential procedures. Trends Neurosci 22:464–471. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Ores Walsh KS, Greischar LL, Alexander AL, Fox AS, Davidson RJ, Oakes TR (2006): Motion correction and the use of motion covariates in multiple‐subject fMRI analysis. Hum Brain Mapp 27:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG (1995): Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377:155–158. [DOI] [PubMed] [Google Scholar]
- Korman M, Doyon J, Doljansky J, Carrier J, Dagan Y, Karni A (2007): Daytime sleep condenses the time course of motor memory consolidation. Nat Neurosci 10:1206–1213. [DOI] [PubMed] [Google Scholar]
- Korman M, Raz N, Flash T, Karni A (2003): Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance. Proc Natl Acad Sci USA 100:12492–12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S, Benali H, Van de Moortele PF, Pelegrini‐Issac M, Waechter T, Ugurbil K, Doyon J (2005): Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci USA 102:12566–12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquet P, Laureys S, Peigneux P, Fuchs S, Petiau C, Phillips C, Aerts J, Del Fiore G, Degueldre C, Meulemans T, et al. (2000): Experience‐dependent changes in cerebral activation during human REM sleep. Nat Neurosci 3:831–836. [DOI] [PubMed] [Google Scholar]
- Molle M, Marshall L, Gais S, Born J (2002): Grouping of spindle activity during slow oscillations in human non‐rapid eye movement sleep. J Neurosci 22:10941–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin A, Doyon J, Dostie V, Barakat M, Hadj Tahar A, Korman M, Benali H, Karni A, Ungerleider LG, Carrier J (2008): Motor sequence learning increases sleep spindles and fast frequencies in post‐training sleep. Sleep 31:1149–1156. [PMC free article] [PubMed] [Google Scholar]
- Nader R, Smith C (2001): Intelligence and stage 2 sleep spindles. Actas Fisiol 7:125. [Google Scholar]
- Oishi K, Toma K, et al. (2005): Activation of the precuneus is related to reduced reaction time in serial reaction time tasks. Neurosci Res 52:37–45. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Patterson MB, Gluck H, Mack JL (1983): EEG activity in the 13–15 Hz band correlates with intelligence in healthy elderly women. Int J Neurosci 20( 3–4):161–171. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Destrebecqz A, Collette F, Delbeuck X, Phillips C, Aerts J, Del Fiore G, Degueldre C, et al. (2003): Learned material content and acquisition level modulate cerebral reactivation during posttraining rapid‐eye‐movements sleep. Neuroimage 20:125–134. [DOI] [PubMed] [Google Scholar]
- Penhune VB, Doyon J (2002): Dynamic cortical and subcortical networks in learning and delayed recall of timed motor sequences. J Neurosci 22:1397–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CM, Lee E, Verheul J, Lipa P, Barnes CA, McNaughton BL (2004): The ventral striatum in off‐line processing: Ensemble reactivation during sleep and modulation by hippocampal ripples. J Neurosci 24:6446–6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters KR, Ray L, Smith V, Smith C (2008): Changes in the density of stage 2 sleep spindles following motor learning in young and older adults. J Sleep Res 17:23–33. [DOI] [PubMed] [Google Scholar]
- Peters KR, Smith V, Smith CT (2007): Changes in sleep architecture following motor learning depend on initial skill level. J Cogn Neurosci 19:817–829. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A (1968): A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles:UCLA. [DOI] [PubMed] [Google Scholar]
- Rioult‐Pedotti MS, Friedman D, Donoghue JP (2000): Learning‐induced LTP in neocortex. Science 290:533–536. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Ulrich D (2005): Pattern‐specific associative long‐term potentiation induced by a sleep spindle‐related spike train. J Neurosci 25:9398–9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M (2009): Still missing some significant ingredients. Sleep 32:291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M, Gruber G, Parapatics S, Sauter C, Klosch G, Anderer P, Klimesch W, Saletu B, Zeitlhofer J (2004): Sleep spindles and their significance for declarative memory consolidation. Sleep 27:1479–1485. [DOI] [PubMed] [Google Scholar]
- Schabus M, Hodlmoser K, Gruber G, Sauter C, Anderer P, Klosch G, Parapatics S, Saletu B, Klimesch W, Zeitlhofer J (2006): Sleep spindle‐related activity in the human EEG and its relation to general cognitive and learning abilities. Eur J Neurosci 23:1738–1746. [DOI] [PubMed] [Google Scholar]
- Schabus M, Dang‐Vu TT, Albouy G, Balteau E, Boly M, Carrier J, Darsaud A, Degueldre C, Desseilles M, Gais S, et al. (2007): Hemodynamic cerebral correlates of sleep spindles during human non‐rapid eye movement sleep. Proc Natl Acad Sci USA 104:13164–13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I (2003): Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron 37:563–576. [DOI] [PubMed] [Google Scholar]
- Steriade M, Domich L, Oakson G, Deschenes M (1987): The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol 57:260–273. [DOI] [PubMed] [Google Scholar]
- Tamaki M, Matsuoka T, Nittono H, Hori T (2008): Fast sleep spindle (13–15 Hz) activity correlates with sleep‐dependent improvement in visuomotor performance. Sleep 31:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki M, Matsuoka T, Nittono H, Hori T (2009): Activation of fast sleep spindles at the premotor cortex and parietal areas contributes to motor learning: A study using sLORETA. Clin Neurophysiol 120:878–886. [DOI] [PubMed] [Google Scholar]
- Toni I, Krams M, Turner R, Passingham RE (1998): The time course of changes during motor sequence learning: A whole‐brain fMRI study. Neuroimage 8:50–61. [DOI] [PubMed] [Google Scholar]
- Van Der Graaf FH, De Jong BM, Maguire RP, Meiners LC, Leenders KL (2004): Cerebral activation related to skills practice in a double serial reaction time task: Striatal involvement in random‐order sequence learning. Brain Res Cogn Brain Res 20:120–131. [DOI] [PubMed] [Google Scholar]
- Vu VQ, Yu B, Kass RE (2009): Information in the non‐stationary case. Neural Computation 21:688–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R (2002): Practice with sleep makes perfect: Sleep‐dependent motor skill learning. Neuron 35:205–211. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R, Alsop D, Gaab N, Schlaug G (2005): Sleep‐dependent motor memory plasticity in the human brain. Neuroscience 133:911–917. [DOI] [PubMed] [Google Scholar]


