Abstract
The ability to resist immediate rewards is crucial for lifetime success and individual well‐being. Using functional magnetic resonance imaging, we assessed the association between trait impulsivity and the neural underpinnings of the ability to control immediate reward desiring. Low and high extreme impulsivity groups were compared with regard to their behavioral performance and brain activation in situations, in which they had to forego immediate rewards with varying value to achieve a superordinate long‐term goal. We found that highly impulsive (HI) individuals, who successfully compensated for their lack in behavioral self‐control, engaged two complementary brain mechanisms when choosing actions in favor of a long‐term goal, but at the expense of an immediate reward. First, self‐controlled decisions led to a general attenuation of reward‐related activation in the nucleus accumbens, which was accompanied by an increased inverse connectivity with the anteroventral prefrontal cortex. Second, HI subjects controlled their desire for increasingly valuable, but suboptimal rewards through a linear reduction of activation in the ventromedial prefrontal cortex (VMPFC). This was achieved by an increased inverse coupling between the VMPFC and the ventral striatum. Importantly, the neural mechanisms observed in the HI group differed from those in extremely controlled individuals, despite similar behavioral performance. Collectively, these results suggest trait‐specific neural mechanisms that allow HI individuals to control their desire for immediate reward. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: impulse control, Barratt Impulsiveness Scale, neuroimaging, desire‐reason dilemma, effective connectivity, fMRI
INTRODUCTION
In a modern world, in which people often have to pursue long‐term goals to achieve high benefits, the ability to restrain impulsive desires is crucial for lifetime success. A lack of self‐control contributes to many contemporary problems, such as overeating or suboptimal financial management, which has led to substantial interest in the mechanistic understanding of the impulsive phenotype [Congdon and Canli,2005]. Impulsivity has been conceptualized as a heterogeneous personality trait that consists of multiple dimensions [Evenden,1999]. Its central characteristics are the increased seeking of immediate reward, a reduced delay tolerance, and an inability to plan ahead [Kalenscher et al.,2006; Patton et al.,1995]. Highly impulsive (HI) individuals show difficulties in maximizing reward on the long run and in adapting to changes in stimulus‐reward contingencies [Franken et al.,2008]. Neurophysiologically, trait impulsivity has been characterized by an increased responsiveness of the nucleus accumbens (Nacc) and ventromedial prefrontal cortex (VMPFC) to predictors of reward [Hahn et al.,2009; Hariri et al.,2006]. This was accompanied by altered dopaminergic, opioidergic, and serotoninergic transmission in these structures in nonclinical impulsive subjects [Cools et al.,2005; Gjedde et al.,2010; Lee et al.,2009; Love et al.,2009; Oswald et al.,2007; Zald et al.,2008]. Conversely, the capacity to decouple behavior from a strongly desired, but suboptimal reward option, which is a hallmark of self‐controlled behavior, depends on prefrontal control of reward‐related activation. Self‐controlled decisions that favored a superordinate long‐term goal, but required rejection of immediate reward, were mediated by the anteroventral prefrontal cortex (avPFC), which restricted reward‐related activation in the Nacc [Diekhof and Gruber,2010]. Similarly, declining a liked, but unhealthy aliment was accompanied by a down‐regulation of activation in the VMPFC [Hare et al.,2009].
The present study was intended to further explore the association between trait impulsivity and the neural underpinnings of the ability to successfully deploy self‐control. We specifically investigated, which neural processes enable nonclinical HI individuals to successfully decouple behavior from desired reward options to achieve a higher‐order long‐term goal. Low and high extreme impulsivity groups, as defined by the Barratt Impulsiveness Scale (BIS‐11) [Patton et al.,1995], were compared with regard to their performance and brain activation in situations, in which they had to forego an immediate bonus to achieve a long‐term goal (i.e., a “desire‐reason dilemma”) [Diekhof and Gruber,2010]. We predicted that, firstly, the increased desire of HI individuals for immediate gratification should interfere with the ability to work for a long‐term goal in the face of instantly available reward. HI individuals, who were able to compensate for this trait‐specific shortcoming, were expected to engage the avPFC more strongly to down‐regulate reward‐related mesolimbic activation during rejection of immediate reward than subjects with low impulsivity scores (LO subjects) [Diekhof and Gruber,2010]. Second, an increasing value of the immediate reward option should make it increasingly difficult to deploy self‐control. HI subjects with normal performance should recruit compensatory brain mechanisms to devaluate the suboptimal immediate reward option and to successfully resolve the “desire‐reason dilemma.”
MATERIALS AND METHODS
Subjects
Participants were 24 right‐handed healthy undergraduate students half of which scored either high or low on the German version of the BIS‐11. Subjects were preselected from a larger sample of undergraduate students (see below) and further qualified for inclusion if they did not fulfill any of the following exclusion criteria: (a) previous medical diagnosis of neurological or psychiatric disorders; (b) previous or current use of psychotropic medication (e.g., antidepressants); or (c) any contraindications for an MRI scan (e.g., an implanted cardiac pace maker).
After analysis of the behavioral data, five of the 24 preselected participants had to be excluded, because they failed to sufficiently meet the requirements of the task (e.g., did not successfully finish the conditioning phase or failed to acquire any of the 10 point bonuses during MRI scanning; see Experimental Procedures below). Of the remaining 19 participants, 10 (five females) subjects were “highly impulsive individuals” (HI individuals), while 9 subjects (five females) were “controlled individuals” with extremely low scores on the BIS‐11 (LO individuals) (see Table I for mean BIS‐11 scores of the two extreme groups).
Table I.
Personality and demographic characteristics of the participants
| HI (n = 10) | LO (n = 9) | P value | |
|---|---|---|---|
| BIS‐11 total score (mean ± SD) | 71.0 ± 4.4 | 52.8 ± 3.2 | <0.0001 |
| BIS‐11 cognitive impulsiveness (mean ± SD) | 19.3 ± 2.8 | 12.9 ± 1.7 | <0.0001 |
| BIS‐11 motor impulsiveness (mean ± SD) | 24.6 ± 2.1 | 20.4 ± 1.8 | 0.0003 |
| BIS‐11 nonplanning impulsiveness (mean ± SD) | 27.1 ± 2.2 | 19.4 ± 2.4 | <0.0001 |
| Age (yr) (mean ± SD) | 23.9 ± 2.0 | 24.7 ± 1.8 | 0.398 |
| Gender | 5f/5m | 5f/4m | 0.809 |
Ethical approval from the local ethical committee at the University Medical Center of the Georg August University Goettingen and written informed consent were obtained before the investigation. The nature of the experimental procedures was explained to all subjects and subjects were paid for participation.
EXPERIMENTAL PROCEDURES
The experiment started with an operant conditioning task outside of the scanner, during which subjects learned to associate specific colors and responses with either an immediate reward or a neutral outcome [see also Diekhof and Gruber,2010]. Button choice was free and subjects were encouraged to explore the stimulus‐response‐reward contingencies to maximize their overall outcome. Three different colors led to an immediate reward (i.e., 10, 25, or 40 points, respectively) when collected with a left button press, while all other colors always led to a neutral outcome (i.e., 0 points) regardless of button choice. The potentially rewarded colors and the colors that always led to a neutral outcome were presented in a random sequence of trials, occurring 30 times each within this sequence. The goal of the operant conditioning task was to acquire stimulus‐response‐reward contingencies, which were relevant for the second phase of the experiment. Since the stimulus‐response‐reward contingency for selection of a rewarded color was 100% all subjects reliably acquired the reward contingencies.
During the second phase of the experiment, which took part in the MR scanner, participants performed a modified version of the sequential forced‐choice task introduced by Diekhof and Gruber [2010]. In this task, subjects had to pursue a superordinate long‐term goal during task blocks of four to seven trials to acquire 50 points at the end of the block. The superordinate goal of an individual task block was to collect two target colors that were defined by a cue at the beginning of each block. Apart from this, subjects also had to incorporate one of two context rules into their decisions, which determined how to treat the nontarget colors to successfully finish a block (i.e., to gain 50 points for achievement of the long‐term goal). In the “reason context” all nontarget colors had to be rejected regardless of their immediate reward association to achieve the superordinate goal. Conversely, in the “desire context” subjects were free to also collect the three conditioned nontarget colors for an immediate bonus, whereas all remaining neutral nontarget colors had to be rejected (see Fig. 1). Bonuses acquired in this context were added to the 50 points at the end of a block, if the long‐term goal was successfully reached. Although subjects were free to decide whether to collect or reject conditioned nontargets in the “desire context,” the optimal strategy for reward maximization was (apart from collecting the targets and rejecting neutral nontargets) to give into the “desire” and acquire the immediately rewarded nontarget colors. Conversely, during the “reason context” subjects were forced to overcome the tendency to acquire immediate rewards, which contradicted the superordinate long‐term goal (i.e., this condition constituted a “desire‐reason dilemma”) [Diekhof and Gruber,2010]. Since the three conditioned (rewarding) stimuli were associated with parametrically increasing values of immediate reward (i.e., 10, 25, or 40 points, respectively), they were further expected to interfere with the increased sensitivity of HI individuals for the value of immediate reward [e.g., Hariri et al.,2006], which should make it increasingly difficult for HI subjects to reject the immediate reward in the “reason context.”
Figure 1.
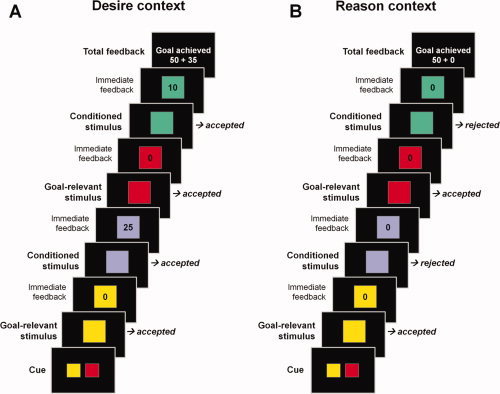
Experimental design of the modified sequential forced‐choice task. (A) “Desire context”: Subjects had to select all targets and had to reject all neutral nontargets. They were further free to select the conditioned (rewarding) nontargets (i.e., to follow previously acquired stimulus‐response‐reward associations) to acquire bonuses during pursuit of the superordinate long‐term task goal. (B) “Reason context”: Subjects had to select all targets and had to reject all neutral nontargets. In addition, participants always had to reject the conditioned (rewarding) nontargets in this context. This led to a “desire‐reason dilemma” during which participants had to counteract the behavioral bias oriented toward immediate reward and had to abandon the potential immediate bonuses to achieve the superordinate long‐term task goal. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The context changed every 10th block, which facilitated the correct implementation of the different context rules and was expected to make it easier for HI subjects to correctly reject rewarded trials during the dilemma. The first five blocks in a context incorporated four trials, in which subjects had to collect the target colors and reject or collect the other trials as required by the current context (short blocks; see Fig. 1). The remaining five blocks contained seven trials each (long blocks; not shown here). This task structure was different from the one used in our previous study [Diekhof and Gruber,2010], in which blocks with bonus trials or trials that required the rejection of an immediate reward were pseudorandomly presented and it was more difficult for HI subjects to retrieve the appropriate action, in particular during a “desire‐reason dilemma.”
In all, the participants completed 180 blocks over the course of three fMRI runs (the total duration of the three runs excluding breaks was approximately 50 min). Half of the blocks were performed in the “desire context” and the “reason context”, respectively (see also Fig. 1). Cues indicating a change in context always appeared for 2,600 ms. The relevant target colors changed every block. A block started with a blank screen of 200 ms duration followed by a cue displaying the two target colors (duration = 1,500 ms) and a subsequent blank screen delay of 200 ms. Individual squares within the blocks appeared for 900 ms after which a blank screen appeared for 200 ms before a feedback for the current choice the subject had made was given. This feedback had a duration of 700 ms and was followed by a blank screen with a duration of 100 ms. The total feedback, which indicated the overall outcome of a block (including bonuses), was always presented at the end of a block (for 3,900 ms) before the next block began. Failure to implement the superordinate task goal or failure to answer within 900 ms led to termination of the current block and zero outcome (goal failure).
Throughout the three functional runs, 180 cues with the two relevant target colors were presented at the beginning of each block. Within a context (“desire” or “reason”), the individually presented trials comprised maximally 279 target trials, 90 trails with a neutral nontarget, and 126 trials with a conditioned nontarget that was (potentially) rewarded with 10 points (42 trials), 25 points (42 trials), or 40 points (42 trials). If subjects committed an error (e.g. selected a neutral nontarget) before the actual end of the block, the block ended immediately and subjects received the “goal failure” feedback. If a block was successfully finished, the overall block outcome was displayed (see Fig. 1). In all, there were 180 trials with a total feedback, whereby the number of “goal failure” trials depended on the subject's performance. Trials within blocks were presented pseudorandomly and counter‐balanced for trial‐type transitions.
All points acquired in the second part of the experiment were cashed into real money. Subjects could receive up to €30, which were added to the general reimbursement of €15 for participation.
Assessment of Trait Impulsivity
Participants completed the German version of the BIS‐11, which was used to determine interindividual differences in trait impulsivity in the nonclinical population of undergraduate students. The BIS‐11 contains 30 items asking about impulsivity‐related behaviors and cognitions. Items are presented as questions that are scored on a 4‐point Likert scale [Barratt,1994]. The BIS‐11 total score is a combined measure of motor and decision impulsiveness (e.g., acting without thinking and making decisions “on the spur of the moment”) as well as the inability to plan ahead. The three subtraits (i.e., motor impulsiveness with 11 items, cognitive impulsiveness with 8 items, and nonplanning impulsiveness with 11 items) can also be measured independently. The BIS‐11 has a high internal consistency [Cronbach's [alpha] of 0.82 in a sample of undergraduate students; Patton et al.,1995] and individual scores are very stable over time (e.g. retest reliability of 0.85 over 3 years) [Manuck et al.,1998]. There is also some evidence that high levels of impulsivity as measured by BIS‐11 are inversely correlated with serotoninergic and dopaminergic responsivity [Buckholtz et al.,2010; Manuck et al.,1998]. Further, increased BIS‐11 scores have been observed in patients with substance abuse disorders [Lee et al.,2009; O'Boyle and Barratt,1993]. Finally, BIS‐11 scores were found to be correlated with the ability to control the behavioral impulse during a “desire‐reason dilemma” and also significantly scaled with the degree of the functional interaction between avPFC and Nacc, when the desire for immediate reward and the long‐term goal competed for action control [Diekhof and Gruber,2010].
In the present study, participants were part of a larger sample of undergraduate students of the Georg August University in Goettingen (n = 67) with comparable socioeconomic status and educational background, who were screened with the BIS‐11. The mean BIS‐11 total score of the complete student sample was 62.4 ± 8.6, which was similar to the population mean reported by Patton et al. [1995] and also resembled the mean of the group assessed by Diekhof and Gruber [2010], which had been 61.9 ± 7.2 (n = 18). The present study was restricted to two age‐ and gender‐matched subgroups selected from the student sample of 67 individuals. The mean BIS‐11 total scores of these groups were located at the extreme ends of the distribution of trait impulsivity in the healthy population (i.e., at least one standard deviation above or below the mean of the student sample; see Table I).
Behavioral Data Analyses
Statistical analyses of the reaction times (RTs) and error rates for conditioned (rewarding) stimuli in the two contexts were done using the software package SPSS for windows (version 17.0; SPSS Inc.). Analyses of variance (ANOVAs) were performed on the delta values of RTs and error rates from the two contexts (i.e., the difference in RTs and percentage of erroneous responses observed in the “reason context” vs. the “desire context”), which correspond to the respective subtraction contrasts used in the fMRI analysis, by introducing “value” (10, 25, or 40 points) as within‐subject factor and “group” (HI vs. LO) as between‐subject factor.
FMRI Data Acquisition and Analyses
Imaging was performed on a 3T system (Magnetom TRIO, Siemens Healthcare, Erlangen, Germany) equipped with the standard eight channel phased‐array head coil. First, a T1‐weighted anatomical dataset at 1 mm isotropic resolution was acquired. For fMRI, 27 axial slices parallel to the anterior commissure‐posterior commissure line were obtained in ascending acquisition order (slice thickness = 3 mm; interslice gap = 0.6 mm) using a gradient‐echo echo‐planar imaging (EPI) sequence (echo time of 33 ms; flip angle of 70°; field‐of‐view of 192 mm; interscan repetition time (TR) of 2,000 ms).
A total of 1,602 volumes was acquired in three functional runs. At the start of each run a fixation cross was presented for the duration of four TRs to allow time for magnetization to reach steady state. The images acquired during this period were discarded from data analysis. Stimuli were viewed through goggles (Resonance Technology, Northridge) during fMRI acquisition and subjects responded via button press on a fiber optic computer response device (Current Designs Inc., Philadelphia). The head was stabilized by small cushions to avoid head movements during scanning. Triggering of the visual stimulation by the scanner impulse during functional data acquisition and generation of stimuli was conducted through the Presentation® Software (Neurobehavioral Systems, Albany).
Functional images were preprocessed and analyzed with SPM2 (Wellcome Department of Cognitive Neurology, University College London, London, UK). Preprocessing comprised coregistration, correction of movement‐related artifacts (realignment and unwarping), corrections for slice‐time acquisition differences and low‐frequency fluctuations, normalization into standard stereotactic space (skull‐stripped EPI template by the Montreal Neurological Institute (MNI)), and spatial smoothing with an isotropic Gaussian kernel filter of 9 mm full‐width half‐maximum.
Statistical analyses used a general linear model (GLM), which comprised three regressors [i.e., goal‐relevant targets, neutral nontargets, conditioned (rewarding) nontargets] for the “desire context” and for the “reason context,” respectively. The cues and the block feedback for either successful goal completion or overall goal failure were also modeled as independent regressors, which resulted in a total of nine onset regressors. In addition, we also modeled two linear parametrical modulators that included the individual reward values (i.e., 10, 25, and 40 points) of the conditioned (rewarding) nontargets in either the “desire context” or the “reason context.” These parametric modulators signified how well the blood oxygenation level‐dependent (BOLD) signal correlated with the continuous variable “reward value” on a trial‐by‐trial basis. Erroneous trials and trials in which a conditioned (rewarding) stimulus was not selected for an immediate bonus in the “desire context” were excluded from the analyses.
A vector representing the temporal onsets of stimulus presentation was convolved with a canonical hemodynamic response function (hrf) to produce a predicted hemodynamic response to each experimental condition and the parametric modulators. Linear t contrasts were defined for assessing the specific effects elicited by the conditioned (rewarding) nontargets and the parametrical modulators in the two contexts in each of the two groups. To test for differences in reward‐related activation in the two contexts, we compared conditioned (rewarding) nontargets in the “desire context” with the same stimuli when being presented in the “reason context.” This contrast assessed the down‐regulation of activation during a competition between the superordinate goal and the proximal reward option in the “reason context” (i.e., during a “desire‐reason dilemma,” which required subjects to reject the immediate reward to achieve the superordinate goal; see also Diekhof and Gruber [2010]), when compared with activation observed during reward acquisition in the “desire context.” To assess the effect of the linear increase of the “reward value” of conditioned (rewarding) nontargets on brain activation in the two contexts, we further compared each parametric modulator against implicit baseline. In addition, we also calculated a direct contrast between the two parametric modulators. We thereby sought to identify brain regions that were differentially correlated with trial‐by‐trail changes in “reward value” during the “desire context” and the “reason context.”
For extraction of the parameter estimates of the three regressors representing immediate “reward value” (10, 25, and 40 points) it was necessary to construct and estimate a second GLM, which included three onset‐regressors for each value in the “desire context” and in the “reason context,” respectively. Parameter estimates from the local activation maximum in the VMPFC (0 24 −9) for each of these six regressors were extracted with marsbar (available at: http://marsbar.sourceforge.net/).
Single‐subject contrast images were taken to the second level to assess group effects with random‐effects analyses. ANOVAs were used to test for differences between HI and LO participants. The standard statistical criterion for group statistics was P < 0.001, uncorrected, with a minimum cluster size of 10 voxels, if not otherwise indicated. For brain regions with a specific a priori hypothesis based on previous observations [i.e., Diekhof and Gruber,2010; Hare et al.,2009], we used small‐volume‐corrections to account for the multiple comparisons problem [Worsley et al.,1996]. Activations corrected for small volume are reported at a threshold of P < 0.05, corrected for family‐wise error (FWE). For display purposes, we applied the more lenient criterion of P < 0.005, uncorrected, to all figures in the article.
Psychophysiological Interaction Analyses
To test the first prediction and as a replication of Diekhof and Gruber [2010], we first assessed the functional interactions between the right Nacc and the avPFC, when the immediate reward contingency and the superordinate goal competed for action control (i.e., during a “desire‐reason dilemma”) using psychophysiological interaction (PPI) analyses [Friston et al.,1997]. The local maximum in the right Nacc (15 12 −6), which showed a significant reduction of reward‐related activation in the “reason context” (see Table II; Fig. 2A), was selected as seed area for the first PPI analysis (see Diekhof and Gruber [2010], for a similar procedure).
Table II.
Down‐regulation of reward‐related activation during the “desire‐reason dilemma”
| Region | Brain regions showing reduced activation during the “desire‐reason dilemma” [conditioned (rewarding) nontargets in reason context vs. desire context] | |
|---|---|---|
| HI | LO | |
| MNI coordinates (t‐value) | MNI coordinates (t‐value) | |
| R Nacc | 15 12 −6 (−3.80) | n.s. |
| L/R VTA/midbrain | −6 −18 −21 (−3.87)a | n.s. |
Activations are reported at P < 0.05, corrected for small volume, if not otherwise indicated.
Activation is reported at P < 0.001, uncorrected.
Figure 2.
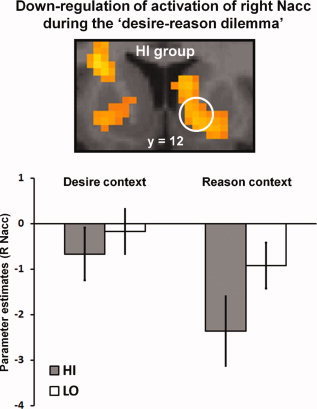
Down‐regulation of reward‐related striatal activation in highly impulsive subjects during the “desire‐reason dilemma.” Significant down‐regulation of activation in the right Nacc during the desire‐reason dilemma” in HI subjects (dilemma‐related deactivations are displayed on a coronal slice of the MNI template; see also Table II). The bar graph shows the mean parameter estimates for the reward signal in the right Nacc (in the comparison against implicit baseline) in the “desire context,” in which the conditioned (rewarding) stimulus was selected when it occurred as a nontarget, and during a “desire‐reason dilemma,” which required a rejection of the (same) conditioned stimulus in the “reason context.” Error bars depict the standard error of the mean. A significant down‐regulation of striatal activation during the dilemma could only be observed in the HI group. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In a second PPI analysis we further explored any changes in connectivity pattern arising from the VMPFC during the successful resolution of the “desire‐reason dilemma.” The VMPFC plays an important role in the representation of incentive value during decision making [e.g., Hare et al.,2008, Plassman et al.,2008]. Further, both in the present study and in a previous one [Hare et al.,2009] activity in the VMPFC was found to be significantly attenuated when impulsive subjects exercised self‐control in the face of increasingly desirable incentives. The second PPI analysis thus sought to identify those brain regions, whose functional connectivity with the VMPFC was modulated by the immediate reward value of conditioned (rewarding) stimuli when these had to be rejected during a “desire‐reason dilemma.” As seed area for this PPI analysis we selected the maximum in the VMPFC (0 24 −9), which showed a significant decrease in activation with increasing immediate reward value in the “reason context” (see Table IV below).
Table IV.
Parametrical down‐regulation of value‐related activation during the “desire‐reason dilemma”
| Region | Activation decrease with parametrically increasing immediate reward value during the “desire‐reason dilemma” | |||||
|---|---|---|---|---|---|---|
| Parametrical contrast [conditioned (rewarding) nontargets in reason context vs. desire context] | Parametrical contrast [conditioned (rewarding) nontargets in reason context vs. implicit baseline] | |||||
| HI | LO | HI > LO | HI | LO | HI > LO | |
| MNI coordinates (t‐value) | MNI coordinates (t‐value) | MNI coordinates (t‐value) | MNI coordinates (t‐value) | MNI coordinates (t‐value) | MNI coordinates (t‐value) | |
| L/R VMPFC | 0 33 −12 (−4.33) | n.s. | 0 30 −12 (−3.33)a | 0 24 −9 (−5.04)b | n.s. | −3 24 −9 (−4.94)b |
Activations are reported at P < 0.05, corrected for small volume, if not otherwise indicated.
Activation is reported at P < 0.005, uncorrected.
Activation is reported at P < 0.001, uncorrected, with a minimum clustersize of 10 voxels.
Individual BOLD signal time courses were extracted from the two local activation maxima, which served as physiological vectors in the two PPI analyses (see above). In the first PPI analysis the psychological vector consisted of the contrast that compared conditioned (rewarding) stimuli presented in the “reason context” with those in the “desire context”. In the second PPI analysis the psychological vector consisted of the comparison between the two parametrical modulators reflecting trial‐by‐trial changes in immediate reward value in the two contexts (i.e., the comparison of the parametrical modulator of increasing value in the “reason context” with that in the “desire context”).
Using Matlab and SPM2, the hemodynamic signals were first deconvolved using a parametric empirical Bayesian formulation [Gitelman et al.,2003] and mean‐corrected. Then the PPI term was built separately for each of the regions by multiplying the deconvolved and mean‐corrected BOLD signal with the respective psychological vector. After convolution with the hrf, mean correction, and orthogonalization, the three regressors (PPI term, physiological vector, and psychological vector) went into the statistical analysis to determine context‐dependent changes of functional connectivity over and above any main effect of task or any main effect of activity in the corresponding brain areas. In the two PPI contrasts, the PPI term was computed against implicit baseline. Random‐effects analyses (P < 0.001, uncorrected) were performed on single‐subject PPI contrast images. ANOVAs were used to test for differences in functional interactions in HI and LO participants.
In addition, we also performed two additional PPI analyses, in which we separately assessed the functional interactions between the right Nacc (as the seed) and the left avPFC either when conditioned (rewarding) stimuli were presented in the “reason context” or in the “desire context”. For this purpose we extracted the signal time courses from the Nacc and used the respective comparisons against implicit baseline as psychological vector (e.g., the contrast of “bonus acquisition vs. implicit baseline in the desire context”). We then further proceeded as described above. These additional analyses were intended to assess whether the difference in functional connectivity between the two contexts resulted (1) from a more positive PPI in the “desire context,” meaning that the slope of the regression line was more positive in the bonus condition than the general connectivity in the implicit baseline, or (2) from a more negative PPI in the “reason context”. The results from these PPI analyses are reported in the Supporting Information of this article.
The parameter estimates of the subject‐specific coupling strength between ventral striatum/Nacc and VMPFC for the three values of the immediately available, but rejected rewards are the results of three additional PPI analyses. These analyses explored the connectivity of the VMPFC in the individual comparisons of the three value regressors in the “reason context” and the “desire context” used in the second GLM (see above). This means that the psychological vector of the first PPI consisted of the contrast that tested for activation elicited by the rejection of an immediate option offering 10 points in the “reason context” versus acquisition of 10 points in the “desire context.” The second and third PPI analyses were also based on the comparison between the two contexts, but for the immediate reward values of 25 or 40 points, respectively. As seed area for these PPI analyses we again selected the maximum in VMPFC (0 24 −9; see above for the further procedures used in the PPI analyses).
RESULTS
Behavioral Results
Mean reaction times and the mean percentage of correct responses to conditioned (rewarding) stimuli in the two contexts are reported in Supporting Information, Table S1. The analysis of the delta values of RTs and error rates from the two contexts (i.e., the difference in RTs and rates of correct responses observed in the “reason context” vs. the “desire context”) revealed a significant, relative performance decline in both groups during the “desire‐reason dilemma.” This deterioration of behavioral performance thereby scaled with the increasing value of immediate reward in the “reason context,” when participants had to reject the immediate reward for the long‐term goal. This performance decline with increasing value was significant (main effect of value on performance: F RTs = 15.92, P < 0.0001; F error = 4.00, P = 0.025), whereas a significant main effect of group (F RTs = 1.12, P = 0.296; F error = 0.35, P = 0.555) and a significant “group × value interaction” (F RTs = 0.05, P = 0.960; F error = 0.55, P = 0.583) were absent. These results suggest that for both highly impulsive and extremely controlled individuals it became increasingly difficult to abstain from the increasingly valuable immediate reward option during pursuit of the superordinate goal.
fMRI Results
On the neural level, decisions that favored the superordinate long‐term goal at the expense of immediate reward led to a significant attenuation of hemodynamic responses to the conditioned (rewarding) stimuli in the right Nacc and ventral tegmental area (VTA) in HI subjects (Table II). Notably, a relative decline of reward‐related activation in the Nacc in relation to the implicit baseline was already evident in the “desire context” in the HI group, and the “desire‐reason dilemma” led to a further suppression of the reward‐related response (see Fig. 2). Additionally, in line with our previous findings [Diekhof and Gruber,2010], this context‐sensitive down‐regulation of reward‐related activation in the right Nacc during the dilemma was accompanied by a negative functional connectivity with the left avPFC also when compared with LO individuals (see Table III; Fig. 3A,B). HI subjects thereby displayed a significant change (i.e., a decrease) in the prefrontostriatal connectivity when comparing the “reason context” with the “desire context” (see Supporting Information, Table S2; Fig. 3C).
Table III.
Inverse functional connectivity between the right Nacc and left avPFC during the “desire‐reason dilemma” in HI subjects
| Region | Brain regions exhibiting an inverse functional connectivity with the right Nacc during the “desire‐reason dilemma” [conditioned (rewarding) nontargets in reason context vs. desire context] | ||
|---|---|---|---|
| HI | LO | HI > LO | |
| MNI coordinates (t‐value) | MNI coordinates (t‐value) | MNIcoordinates (t‐value) | |
| L avPFC | −24 45 9 (4.76); −27 51 12 (3.67) | n.s. | −24 48 9 (4.94); −39 51 3 (4.70) |
Activations are reported at P < 0.001, uncorrected, with a minimum cluster size of 10 voxels.
Figure 3.
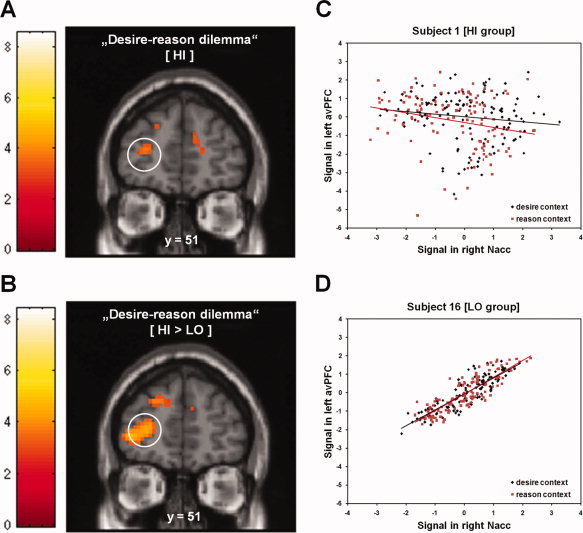
Context‐related changes in prefrontostriatal connectivity during the “desire‐reason dilemma.” (A) Reduced connectivity between right Nacc and left avPFC during the dilemma in the HI group (dilemma‐related reductions in prefrontostriatal connectivity are displayed on a coronal slice of the MNI template, color bars show associated t‐values; see also Table III). Importantly, on the group level this inverse functional connectivity was a result of a reduced positive coupling, rather than an increase in the negative functional connectivity between the two brain regions (see also Supporting Information, Table S2). (B) Reduced connectivity between right Nacc and left avPFC during the “desire‐reason dilemma,” which was more negative for HI compared with LO subjects. (C) PPI of the right Nacc for a representative single subject from the HI group. Mean‐corrected activity (in arbitrary units) in left avPFC (MNI coordinates (x y z): −24 48 9; box of 3 × 3 × 3 mm3) is displayed as a function of mean‐corrected activity in right Nacc (MNI coordinates (x y z): 15 12 −6; box of 3 × 3 × 3 mm3). Black diamonds represent measurements during bonus acquisition in the “desire context,” red squares denote measurements during rejection of immediate reward in the “reason context.” To take into account the hemodynamic lag in the BOLD response when assigning data points to conditions, the onset of conditions was delayed by 6 s (for a similar procedure please see Stephan et al. [2003]. (D) PPI of the right Nacc for a representative single subject from the LO group. Mean‐corrected activity (in arbitrary units) in left avPFC is displayed as a function of mean‐corrected activity in right Nacc. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In the LO group a down‐regulation of reward‐related activation in the right Nacc and VTA during the dilemma was only observed when lowering the statistical threshold to P < 0.05, uncorrected for multiple comparisons [MNI coordinates x y z (t‐value): Nacc 12 12 −6 (−1.89); VTA −9 −18 −24 (−2.21)]. Further, in controlled individuals the functional interaction between the avPFC and Nacc failed to decrease in the “reason context” (i.e., the relative decline of the prefrontostriatal coupling was absent when rejecting an immediate bonus for the higher‐order long‐term goal; see Supporting Information, Table S2 and Fig. 3D).
To test the second prediction that especially HI individuals need to control the interference of the increasing value of the immediate reward option when deploying self‐control, we assessed the effect of the linearly increasing reward value on neural processing during the successful resolution of the “desire‐reason dilemma.” We predicted that brain activation representing the immediate reward value would be significantly reduced when HI individuals successfully rejected the conditioned (rewarding) stimuli in the “reason context” [see for example Hare et al.,2009]. This analysis identified one brain region, which exhibited a significant linear decrease in activity that scaled with increasing reward value during the dilemma. Accordingly, the rejection of conditioned (rewarding) stimuli with linearly increasing reward value concurrently led to a reduction of activation in the subgenual anterior cingulate cortex and adjacent gyrus rectus in the HI group (Table IV; Fig. 4A,B). We will refer to this brain region as “VMPFC” in the remainder of the paper. Interestingly, when lowering the statistical criterion to P < 0.05, uncorrected, we further observed a relative increase of the ventromedial prefrontal signal in HI individuals in the “desire context” (i.e., a reduction of the degree of deactivation in the VMPFC) [MNI coordinates x y z (t‐value): 0 27 0 (2.84)], which scaled with the value of immediate reward. Conversely, in the LO group a value‐sensitive attenuation of activation in the VMPFC was absent during the dilemma and LO subjects also failed to show the linear value‐related increase during successful bonus acquisition, even when lowering the statistical threshold to P < 0.05, uncorrected (see also Fig. 4B).
Figure 4.
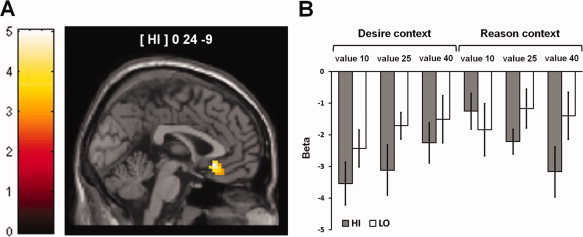
Parametrical deactivation of the VMPFC during the rejection of immediate reward options scaling with increasing reward value during the “desire‐reason dilemma” in HI subjects. (A) Significant parametrical decrease in the value‐related signal in the VMPFC during the “desire‐reason dilemma” in HI subjects (value‐related deactivations are displayed on a coronal slice of the MNI template, color bar shows associated t‐values; see also Table IV). (B) Parameter estimates for the reward signal tied to individual values in the VMPFC (in the comparison against implicit baseline). In the HI group the parameter estimates exhibited a significant decrease with increasing reward value in the “reason context.” [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To further examine whether this value‐sensitive decrease in ventromedial frontal activation during the “desire‐reason dilemma” was accompanied by changes in functional connectivity with other regions of the brain's reward system or higher‐order prefrontal regions as suggested by Hare et al. [2009], we explored the functional connectivity of the VMPFC. We observed that the VMPFC exhibited an increased inverse functional interaction with only one brain region, which comprised the Nacc and the adjacent ventromedial caudate nucleus. But again, the change in functional connectivity could only be observed in the HI group (Table V; Fig. 5A). We will refer to this brain region as “ventral striatum” in the remainder of the article. By plotting the parameter estimates of individual value regressors from the PPI, we found that functional coupling strength negatively scaled with the value of the immediately available but ultimately rejected reward option in HI individuals, but not in the LO group (Fig. 5B).
Table V.
Parametrical increase in the inverse connectivity between the VMPFC and right ventral striatum during the “desire‐reason dilemma” in HI subjects
| Region | Brain regions exhibiting a parametrical increase in the inverse connectivity with the VMPFC during rejection of increasingly valuable immediate rewards (i.e., during the “desire‐reason dilemma”) | ||
|---|---|---|---|
| Parametrical contrast [conditioned (rewarding) nontargets in reason context vs. desire context] | |||
| HI | LO | HI > LO | |
| MNI coordinates (t‐value) | MNI coordinates (t‐value) | MNI coordinates (t‐value) | |
| R ventral striatum | 12 21 −6 (4.44); 12 12 −6 (2.96)a | n.s. | 15 24 −6 (2.93)a |
Activations are reported at P < 0.001, uncorrected, with a minimum clustersize of 10 voxels, if not otherwise indicated.
Activation is reported at P < 0.005, uncorrected.
Figure 5.
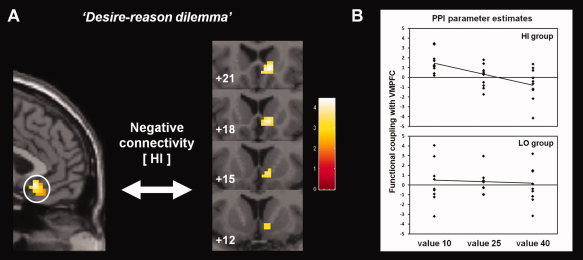
Value‐related increase in the inverse functional interaction between the VMPFC and the right ventral striatum with increasing value of the foregone reward option during the “desire‐reason dilemma” in the HI group. (A) Parametrical increase in the inverse connectivity between VMPFC and ventral striatum with increasing value of conditioned (rewarding) nontargets that had to be rejected in the “reason context” (dilemma‐related reductions in prefrontostriatal connectivity are displayed on a coronal slice of the MNI template, color bar shows associated t‐values; see also Table V). (B) Contrast values of the psychophysiological interaction parameters from the ventral striatum in the HI and in the LO group (in the comparison of individual bonus values presented in the “reason context” vs. the “desire context”). Only in HI individuals the parameter estimates of functional interaction strength between the VMPFC and the ventral striatum exhibited a significant decrease with increasing reward value in the “reason context” relative to the “desire context”. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For completeness, brain regions, whose activation positively scaled with the increasing value of immediate reward in either the “desire context” or the “reason context,” can be found in Supporting Information, Table S3. We observed that both HI and LO groups showed a similar increase of brain activation with increasing value of the immediate option in the right parietal cortex and the right middle frontal gyrus (MFG) in the “desire context” (Supporting Information, Table S3A). All remaining regions were exclusively activated in the HI group in either the “desire context” (Supporting Information, Table S3A) or the “reason context” (Supporting Information, Table S3B), apart from a single area in the right IFS, which was activated by increasing reward value in both contexts in the HI group (Supporting Information, Table S3C).
DISCUSSION
The ability to restrain immediate reward desiring is crucial for lifetime success and individual well‐being. Here we show that nonclinical individuals, who rated themselves as highly impulsive on the BIS‐11, succeeded in controlling their desire for immediate reward by engaging two complementary brain mechanisms. First of all, decisions that successfully counteracted immediate reward desiring led to a general attenuation of reward‐related activation in mesolimbic structures of the reward system (Table II). Replicating our previous findings [Diekhof and Gruber,2010] this was accompanied by an inverse functional interaction between the avPFC and the right Nacc (see Fig. 3). Second, highly impulsive subjects exhibited a linear attenuation of activation in the VMPFC, when they had to reject the increasingly valuable immediate rewards in the “reason context” (see Fig. 4). This devaluation of the foregone reward option scaled with changes in functional connectivity between the VMPFC and the right ventral striatum (i.e., the Nacc and adjacent ventrodmedial nucleus caudatus), which probably reduced the desire to collect the suboptimal immediate rewards (see Fig. 5).
Even more importantly, we observed that the neural mechanisms characteristic for the HI group were distinct from those employed by individuals that classified themselves as extremely controlled. Notably, LO subjects showed no inverse functional connectivity between the Nacc and avPFC during the “desire‐reason dilemma.” Apart from that, the LO group also exhibited no linear decrease in ventromedial frontal activation in response to the value of the foregone reward option (Table IV; Fig. 4B), and also failed to show a context‐ and value‐sensitive change in the functional interaction with the ventral striatum (Table V; Fig. 5B). Collectively, these results suggest that nonclinical individuals from the extreme ends of the continuum of trait impulsivity may recruit somewhat different neural mechanisms that may reflect diverse cognitive strategies, when facing a situation that requires the deployment of self control.
A Trait‐Specific Mechanism to Control the General Desire for Immediate Reward in the Highly Impulsive Phenotype
It has been assumed that a fundamental difference between individuals classified as either high or low in trait impulsivity is their ability to deploy self‐control (e.g., the ability to achieve higher‐order plans when being tempted by immediately available, but suboptimal reward) [Evenden,1999; Patton et al.,1995]. A lack of self‐control has been associated with abnormalities in frontostriatal connectivity [Diekhof and Gruber,2010; Hare et al.,2009; Jentsch and Taylor,1999; Sonuga‐Barke,2002], alterations in dopaminergic autoregulation and reward‐related activity in mesolimbic structures [Abler et al.,2006; Buckholtz et al.,2010; Hariri et al.,2006; Zald et al.,2008]; see also Marinelli and White [2000], as well as structural changes in the VMPFC [Matsuo et al.,2008]. In the present study, the two extreme impulsivity groups did not differ in their (behavioral) ability to control the desire for an immediate reward (see Behavioral results). This suggests that nonclinical HI subjects were able to control their lack of self‐control, which enabled them to successfully resolve the “desire‐reason dilemma.” Given the behavioral data, it follows that significant group‐differences in functional brain activation and connectivity during the successful resolution of the dilemma probably reflected trait‐specific and supposedly in part compensatory brain mechanisms. In clinical populations compensation for disorder‐specific deficits is often accompanied by an increased recruitment of task‐relevant brain regions [Manoach,2003]. Consistent with this hypothesis, we found a stronger inverse functional coupling between the avPFC and Nacc in the nonclinical HI group than in the LO sample (Table III; Fig. 3A,B). In HI subjects the inverse connectivity further accompanied the successful down‐regulation of reward‐related activation in the Nacc (Table II; Fig. 2). This conforms well with previous evidence including our own that implicated the avPFC in the successful promotion of behavioral flexibility, which enables decisions decoupled from the impact of immediately available reward [Boorman et al.,2009; Daw et al.,2006; Diekhof and Gruber,2010].
However, there are also some apparent inconsistencies with our previous results [Diekhof and Gruber,2010] that need to be addressed in detail: First, in contrast to our previous observations [Diekhof and Gruber,2010] the right Nacc was deactivated in both groups and across contexts, and only in the HI group the dilemma led to a further down‐regulation of an already negative signal change that was also statistically significant (see Fig. 2). Second, in the present study the inverse prefrontostriatal coupling during the dilemma was only observed in the HI group (Table III), who, notably, exhibited a normal performance in the decision task. Finally, this inverse functional connectivity was a result of a reduced positive coupling (see Supporting Information, Table S2), rather than an increase in the negative functional connectivity between the two regions like in our previous study [Diekhof and Gruber,2010]. Modifications of the sequential forced choice task used in the present study may probably account for these discrepancies: First of all, it should be noted that the modified version of the sequential forced‐choice task was less demanding than the original one used by Diekhof and Gruber [2010]. In the modified task, subjects used the same decision rule over the course of 10 consecutive blocks (i.e., either rejected all conditioned (rewarding) nontargets in the “reason context” or collected the same conditioned stimuli to acquire bonuses in the “desire context”). This was probably the reason why even HI subjects achieved a normal behavioral performance when facing the dilemma in the present study. In the original version “desire” and “dilemma trials” could occur within identical blocks, namely, when conditioned (rewarding) targets were repeatedly presented during a block, which required their rejection (see Diekhof and Gruber for further details on the original design). This made it more difficult to prepare the correct action when being confronted with conditioned (rewarding) stimuli, which may also explain why impulsive subjects failed to recruit the avPFC and performed poorly in our previous study [Diekhof and Gruber,2010]. In that way, our observations also conform with the notion that compensation is only possible, if task requirements match individual capacity. If task demands exceed this capacity, like in the HI subjects in our original study, a failure to recruit the relevant brain regions and a decline in behavioral performance may be inevitable consequences [Jansma et al.,2004; Manoach,2003]. Taken together, the discrepant findings in the present study may thus be rather understood as a consequence of differences in task design and difficulty than representing true inconsistencies.
At the same time, presenting consecutive blocks with similar task requirements in the present study may have further affected the underlying functional neural architecture necessary to cope with reoccurring “desire‐reason dilemmas.” There is already evidence that behavioral enhancement following practice is often accompanied by a functional reorganization of brain activity in task‐relevant regions [Kelly and Garavan,2005], which can also reflect the refinement of cognitive strategies. Such a functional reorganization may change levels of inter‐regional connectivity, for example through strengthening of inhibitory connections [Fletcher et al.,1999], and can sharpen the response in task‐relevant regions of the network [Poldrack,2000]. One could speculate that in order to cope with the demands of this self‐control task in particular HI subjects may have maintained a sustained negative connectivity between the avPFC and the Nacc throughout the course of the experiment, which may have controlled reward‐related mesolimbic activation across the whole experiment. A release of this increased control of mesolimbic activation was then only necessary on individual bonus trials in the “desire context,” which could have led to the relative decrease in negative functional connectivity that appeared as a relative increase in positive connectivity in the comparison against implicit baseline (see Supporting Information, Table S2). This would also explain why we found that (1) the Nacc was deactivated across the whole experiment (see Fig. 2), and (2) that the observed inverse functional connectivity during the dilemma actually reflected a reduced positive connectivity between the avPFC and Nacc in the HI group (Table III and Supporting Information, Table S2). Since our experiment lacked a low‐level control condition [like those used in common mixed designs, see for example Visscher et al. 2003], we cannot directly test this theory of a sustained negative prefrontostriatal connectivity across desire and reason contexts in HI subjects. However, from the above described observations in this group it seems plausible to put forward this possibility.
A Trait‐Specific Mechanism to Allow Highly Impulsive Subjects to Overcome Their Desire for Increasing Reward Value
Our data further show that the response of the VMPFC parametrically decreased with the increasing value of the rejected immediate reward option in subjects scoring high on the BIS‐11 (Table IV; Fig. 4). This is consistent with previous evidence suggesting a general role for the VMPFC in the representation of relative reward value especially when choosing between different reward options [Boorman et al.,2009; DeMartino et al.,2009; Grabenhorst et al.,2008; Hare et al.,2008; Kable and Glimcher,2007; McClure et al.,2004,2007; Plassmann et al.,2008; Smith et al.,2010]. Moreover, a similar reduction of ventromedial frontal activation has already been observed during the successful deployment of self‐control in nonclinical populations [Campbell‐Meiklejohn et al.,2008; Hare et al.,2009]. Similarly, the devaluation of food reward following sensory‐specific satiety [Small et al.,2001] as well as the devaluation of low calorie items in reference to high calorie aliments in the hungry state [Goldstone et al.,2009] resulted in a significant decrease in activation in the VMPFC. In animals, a maladaptive assignment of incentive value to conditioned stimuli that are no longer predictive of immediate reward has been described as a defining attribute of impulsivity [Flagel et al.,2009]. It is therefore plausible to assume that the attenuation of activation in the VMPFC in the “reason context” was vital to cope with this maladaptive trait in HI subjects. By supporting their capacity to decouple behavior from the conditioned reward contingency, the value‐sensitive reduction in ventromedial frontal activity probably enabled HI individuals to execute the necessary behavioral reversal. Conversely, in the “desire context” we observed a relative—although nonsignificant—increase in the ventromedial frontal signal in the HI group, which positively scaled with the magnitude of the immediate reward option and thus might have promoted successful bonus acquisition (see Fig. 4B). In that way, our data indeed support the view that the VMPFC codes the relative rather than the absolute value of a chosen option [e.g., Boorman et al.,2009; Daw et al.,2006], representing the relative devaluation of rejected immediate incentives in the “reason context” as well as the relative incentive value of the increasingly desirable immediate bonuses in the “desire context.” Further, one may speculate that the observed decline in the ventromedial prefrontal response in the “reason context” may have also reflected a relative reduction of positive affect experienced (e.g., disappointment). Especially for HI subjects it should have been extremely frustrating to reject the increasingly valuable immediate rewards for the higher‐order long‐term goal. This assumption would also be consistent with data from emotion regulation studies, which found that the extent of deactivation in the VMPFC signal scaled with the degree of negative affect experienced [e.g., Delgado et al.,2008; Diekhof et al.,2011].
In addition to that, the present data demonstrate that the ability to devaluate the suboptimal reward option was further accompanied by an inverse functional coupling between the VMPFC and the ventral striatum (Table V; Fig. 5). Such a functional relationship accords well with previous human fMRI and diffusion tensor imaging data [DiMartino et al.,2008; Draganski et al.,2008; Lehéricy et al.,2004] as well as data from tracer studies in nonhuman primates [Alexander and Crutcher,1990; Haber and Knutson,2010; Haber et al.,1995,2006; Schilman et al.,2008] demonstrating tight anatomical connections between these brain regions. The identified area in the ventral striatum coincides with the part of the ventral striatum that receives inputs from the VMPFC and adjacent orbitofrontal cortex [Haber and Knutson,2010]. Our data show for the first time that the degree of the inverse prefrontostriatal coupling increased with the value of the foregone reward option, which probably helped HI subjects to overcome the desire to exploit the suboptimal immediate reward during the dilemma. This is also in line with the assumption that the ventral striatum plays an essential role in action control oriented toward reward maximization. Increased activation in similar parts of the ventral striatum has previously been observed to represent stimulus‐action‐reward contingencies [Lauwereyns et al.,2002; Schlund and Cataldo,2005; Tricomi et al.,2004] and reward magnitude [Ballard and Knutson, 2008; Cromwell and Schultz,2003; Delgado et al.,2003; Knutson and Cooper,2005; Knutson et al.,2001]. Regional activation, especially in the human ventromedial caudate nucleus has further been found to increase during reversal of reward‐related choice [Cools et al.,2004; Rogers et al.,2000; Watanabe and Hikosaka,2005] and new learning of stimulus‐response‐reward associations [Delgado et al.,2005; Haruno and Kawato,2006; O'Doherty et al.,2004]. In rats and nonhuman primates, it has been demonstrated that lesions of the ventral and medial striatum, which correspond to the region identified in the present study, as well as the VMPFC disrupted the ability to reverse response‐reward‐contingencies [Clarke et al.,2008; Ferry et al.,2000; Li and Shao,1998]. Given these findings and the particular role of the caudate nucleus in the focused inhibition of competing actions [Jiang et al.,2003; Mink,1996; Redgrave et al.,1999], it follows that in the present study the reciprocal interaction with the VMPFC was probably crucial to overcome the maladaptive behavioral impulse to collect the increasingly valuable immediate rewards in the “reason context” In that way, the present data may also support the view that these interconnected regions may modify currently suboptimal reward contingencies to allow that actions associated with higher long‐term reward are chosen more frequently [O'Doherty et al.,2004]. Future studies using model‐based methods to determine causality in measures of connectivity [e.g., dynamic causal modeling; Friston et al.,2003; Stephan et al.,2008] have to further examine whether (1) a lack of prefrontal input into the ventral striatum or (2) an increased influence of the ventral striatum on the VMPFC may have helped subjects to overcome the suboptimal reward contingencies during the dilemma, or whether (3.) a third brain region may have mediated the frontostriatal interplay.
Brain Mechanisms Mediating Self‐Control in the Highly Controlled Phenotype
In the present study the highly controlled sample (LO) was characterized by comparably small changes in regional brain activation between the two decision contexts, in particular as compared to the HI group (Tables II, IV, and Supporting Information, Table S3). One possibility is that trait‐specific differences in individual processing efficiency or variations in capacity limitations may explain the discrepancies between the impulsive and the controlled phenotype. Given similar behavioral performance, it has already been shown that different populations (e.g., psychiatric patients and healthy controls) can vary in the extent of neural activation in task‐relevant regions, whereby a higher processing capacity was often accompanied by decreased activation [Jansma et al.,2004; Manoach et al.,2003]. In addition, controlled individuals may also differ from the HI group in the (neural) efficiency with which fundamental cognitive operations are performed [i.e., less task‐related activity in subjects with higher efficiency; see Rypma and Prabhakaran,2009]. The observation that LO subjects show the down‐regulation of reward‐related activation during the “desire‐reason dilemma” only at a more lenient statistical threshold of P < 0.05, uncorrected, and further failed to show the expected change in the prefrontostriatal connectivity (Table III) strongly argues for this assumption. Alternatively, these trait‐specific differences may have arisen from discrepant cognitive strategies or differences in the ability to keep up a once chosen cognitive strategy [Miller et al.,2002], or may have simply reflected a differential sensitivity of mesolimbic brain regions to the value of immediate reward [Bodi et al.,2009]. This latter assumption would also conform with the observation made by Hare et al. [2009], who found that subjects with a strong ability to control their eating behavior failed to exhibit a value‐sensitive ventromedial frontal response when viewing food items of different caloric value. Future studies should concern themselves in more detail with this highly controlled phenotype, which has been widely ignored by psychological and neuroimaging research so far. It will thereby be important to examine, which of the above described reasons for trait‐specific differences may hold true. From the present data we can only infer that HI and LO subjects differed in the neural mechanisms employed to resolve the “desire‐reason dilemma.”
Shared Neural Mechanisms Underlying Bonus Acquisition or Rejection in High and Low Impulsivity
In contrast to the abovementioned differences between HI and LO subjects, we found that activation in the right MFG and the parietal cortex linearly increased during bonus acquisition with the value of immediate reward in both groups (Supporting Information, Table S3). Given the proposed roles of these regions in higher‐order cognitive processing (e.g., value integration) and mental calculation [e.g., Gruber et al.,2001; Hoshi,2006; Kahnt et al.,2011; van Eimeren et al.,2010], one may assume that the value‐sensitive increase of activation in these brain regions most likely reflected cognitive operations during the decision in favor of the available bonuses. This accords well with previous findings in nonhuman primates that put forward a role for the parietal cortex in voluntary value‐based choice [Platt and Glimcher,1999; Sugrue et al.,2004] and reward‐related attention [Peck et al.,2009]. Similarly, findings in humans suggest these brain regions were activated during intertemporal choice in general [McClure et al.,2004,2007], during multiattribute decision making [Kahnt et al.,2011; Zysset et al.,2006], and also during voluntary value‐based choice [Dorris and Glimcher,2004; Kable and Glimcher,2007].
Finally, we identified one brain region in the right inferior frontal sulcus (IFS), which tracked the increasing reward value independent of context, but only in HI subjects (Supporting Information, Table S3). This suggests a complementary role of this brain region in value representations. One possibility is that the IFS contributed in the (automatic) retrieval or active maintenance of the reward value of individual conditioned stimuli. This would be consistent with previous findings that had implicated this brain region in working memory and mental calculation [Goldman‐Rakic,1996; Gruber et al.,2001; Ungerleider,1995]. However, value tracking or retrieval was only important during bonus acquisition in the “desire context,” whereas it was rather maladaptive in the “reason context.” The observation that only HI subjects showed a linear increase of activation in this brain region may be another indicator of their heightened sensitivity for the value of immediate reward that was more directly tied to the conditioned stimuli than in the LO group.
CONCLUSION
Taken together our findings support the view that nonclinical HI undergraduate students can engage trait‐specific and supposedly in part compensatory brain mechanisms to successfully deploy self‐control. The observed reduction of reward‐related activation in subcortical mesolimbic structures and the VMPFC thereby probably enabled highly impulsive subjects to counteract the desire for immediate reward when this was required by the superordinate long‐term goal. Interestingly, the neural mechanisms (i.e., the activation and connectivity patterns) underlying this ability in HI subjects differed from those in extremely controlled individuals, despite similar behavioral performance. This further suggests that the two extreme impulsivity groups could have used different cognitive strategies to achieve long‐term goals in the face of instantly available incentives.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Acknowledgements
The authors thank T. Graen for programming the test protocols and I. Pfahlert as well as the staff of the unit “MR‐Research in Neurology and Psychiatry” at the University Medical Center Goettingen (Germany) for help with data acquisition.
REFERENCES
- Abler B, Walter H, Erk S, Kammerer H, Spitzer M ( 2006): Prediction error as a linear function of reward probability is coded in human nucleus accumbens. Neuroimage 31: 790–795. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD ( 1990): Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends Neurosci 13: 266–271. [DOI] [PubMed] [Google Scholar]
- Ballard K, Knutson B ( 2009): Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage 45: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt ES ( 1994): Impulsiveness and aggression In: Monahan J, Steadman HJ, editors. Violence and Mental Disorder: Developments in Risk Assessment. Chicago: University of Chicago Press; pp 61–79. [Google Scholar]
- Bódi N, Kéri S, Nagy H, Moustafa A, Myers CE, Daw N, Dibó G, Takáts A, Bereczki D, Gluck MA ( 2009): Reward‐learning and the novelty‐seeking personality: A between‐ and within‐subjects study of the effects of dopamine agonists on young Parkinson's patients. Brain 132: 2385–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman ED, Behrens TE, Woolrich MW, Rushworth MF ( 2009): How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron 11: 733–743. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Cole D, Kessler RM, Zald DH ( 2010): Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci 13: 419–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell‐Meiklejohn DK, Woolrich MW, Passingham RE, Rogers RD ( 2008): Knowing when to stop: The brain mechanisms of chasing losses. Biol Psychiatry 63: 293–300. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Robbins TW, Roberts AC ( 2008): Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. J Neurosci 28: 10972–10982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E, Canli T ( 2005): The endophenotype of impulsivity: Reaching consilience through behavioral, genetic, and neuroimaging approaches. Behav Cogn Neurosci Rev 4: 262–281. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Robbins TW ( 2004): Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci 24: 1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Blackwell A, Clark L, Menzies L, Cox S, Robbins TW ( 2005): Tryptophan depletion disrupts the motivational guidance of goal‐directed behavior as a function of trait impulsivity. Neuropsychopharmacology 30: 1362–1373. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Schultz W ( 2003): Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J Neurophysiol 89: 2823–2838. [DOI] [PubMed] [Google Scholar]
- Daw ND, O'Doherty JP, Dayan P, Seymour B, Dolan RJ ( 2006): Cortical substrates for exploratory decisions in humans. Nature 441: 876–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Locke HM, Stenger VA, Fiez JA ( 2003): Dorsal striatum responses to reward and punishment: Effects of valence and magnitude manipulations. Cogn Affect Behav Neurosci 3: 27–38. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Miller MM, Inati S, Phelps EA ( 2005): An fMRI study of reward‐related probability learning. Neuroimage 24: 862–873. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, Phelps EA ( 2008): Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron 59: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Holt B, Dolan RJ ( 2009): The neurobiology of reference‐dependent value computation. J Neurosci 29: 3833–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Gruber O ( 2010): When desire collides with reason: Functional interactions between anteroventral prefrontal cortex and nucleus accumbens underlie the human ability to resist impulsive desires. J Neurosci 30: 1488–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Kipshagen HE, Falkai P, Dechent P, Baudewig J, Gruber O ( 2011): The power of imagination—How anticipatory mental imagery alters perceptual processing of fearful facial expressions. Neuroimage 54: 1703–1714. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP ( 2008): Functional connectivity of human striatum: A resting state FMRI study. Cereb Cortex 18: 2735–2747. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Glimcher PW ( 2004): Activity in posterior parietal cortex is correlated with the relative subjective desirability of action. Neuron 44: 365–378. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Klöppel S, Cook PA, Alexander DC, Parker GJ, Deichmann R, Ashburner J, Frackowiak RS ( 2008): Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci 28: 7143–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL ( 1999): Varieties of impulsivity. Psychopharmacology (Berl) 146: 348–361. [DOI] [PubMed] [Google Scholar]
- Ferry AT, Ongür D, An X, Price JL ( 2000): Prefrontal cortical projections to the striatum in macaque monkeys: Evidence for an organization related to prefrontal networks. J Comp Neurol 425: 447–470. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE ( 2009): Individual differences in the attribution of incentive salience to reward‐related cues: Implications for addiction. Neuropharmacology 56( Suppl 1): 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P, Buchel C, Josephs O, Friston K, Dolan R ( 1999): Learning‐related neuronal responses in prefrontal cortex studied with functional neuroimaging. Cereb Cortex 9: 168–178. [DOI] [PubMed] [Google Scholar]
- Franken IH, van Strien JW, Nijs I, Muris P ( 2008): Impulsivity is associated with behavioral decision‐making deficits. Psychiatry Res 158: 155–163. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ ( 1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6: 218–229. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W ( 2003): Dynamic causal modelling. Neuroimage 19: 1273–1302. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ ( 2003): Modeling regional and psychophysiologic interactions in fMRI: The importance of hemodynamic deconvolution. Neuroimage 19: 200–207. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Kumakura Y, Cumming P, Linnet J, Møller A ( 2010): Inverted‐U‐shaped correlation between dopamine receptor availability in striatum and sensation seeking. Proc Natl Acad Sci USA 107: 3870–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman‐Rakic PS ( 1996): The prefrontal landscape: Implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci 351: 1445–1453. [DOI] [PubMed] [Google Scholar]
- Goldstone AP, de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, Durighel G, Hughes E, Waldman AD, Frost G, Bell JD ( 2009): Fasting biases brain reward systems towards high‐calorie foods. Eur J Neurosci 30: 1625–1635. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET, Bilderbeck A ( 2008): How cognition modulates affective responses to taste and flavor: Top‐down influences on the orbitofrontal and pregenual cingulate cortices. Cereb Cortex 18: 1549–1559. [DOI] [PubMed] [Google Scholar]
- Gruber O, Indefrey P, Steinmetz H, Kleinschmidt A ( 2001): Dissociating neural correlates of cognitive components in mental calculation. Cereb Cortex 11: 350–359. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd‐Balta E ( 1995): The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci 15: 4851–4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R ( 2006): Reward‐related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive‐based learning. J Neurosci 26: 8368–8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B ( 2010): The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35: 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T, Dresler T, Ehlis AC, Plichta MM, Heinzel S, Polak T, Lesch KP, Breuer F, Jakob PM, Fallgatter AJ ( 2009): Neural response to reward anticipation is modulated by Gray's impulsivity. Neuroimage 46: 1148–1153. [DOI] [PubMed] [Google Scholar]
- Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A ( 2008): Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci 28: 5623–5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A ( 2009): Self‐control in decision‐making involves modulation of the vmPFC valuation system. Science 324: 646–648. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB ( 2006): Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci 26: 13213–13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruno M, Kawato M ( 2006): Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus‐action‐reward association learning. J Neurophysiol 95: 948–959. [DOI] [PubMed] [Google Scholar]
- Hoshi E ( 2006): Functional specialization within the dorsolateral prefrontal cortex: A review of anatomical and physiological studies of non‐human primates. Neurosci Res 54: 73–84. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, van der Wee NJ, Kahn RS ( 2004): Working memory capacity in schizophrenia: A parametric fMRI study. Schizophr Res 68: 159–171. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR ( 1999): Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward‐related stimuli. Psychopharmacology (Berl) 146: 373–390. [DOI] [PubMed] [Google Scholar]
- Jiang H, Stein BE, McHaffie JG ( 2003): Opposing basal ganglia processes shape midbrain visuomotor activity bilaterally. Nature 423: 982–986. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW ( 2007): The neural correlates of subjective value during intertemporal choice. Nat Neurosci 10: 1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T, Heinzle J, Park SQ, Haynes JD ( 2011): Decoding different roles for vmPFC and dlPFC in multi‐attribute decision making. Neuroimage 56: 709–715. [DOI] [PubMed] [Google Scholar]
- Kalenscher T, Ohmann T, Güntürkün O ( 2006): The neuroscience of impulsive and self‐controlled decisions. Int J Psychophysiol 62: 203–211. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Garavan H ( 2005): Human functional neuroimaging of brain changes associated with practice. Cereb Cortex 15: 1089–1102. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC ( 2005): Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol 18: 411–417. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D ( 2001): Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21: RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwereyns J, Watanabe K, Coe B, Hikosaka OA ( 2002): Neural correlate of response bias in monkey caudate nucleus. Nature 418: 413–417. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA ( 2009): Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci 29: 14734–14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehéricy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim DS ( 2004): Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol 55: 522–529. [DOI] [PubMed] [Google Scholar]
- Li L, Shao J ( 1998): Restricted lesions to ventral prefrontal subareas block reversal learning but not visual discrimination learning in rats. Physiol Behav 65: 371–379. [DOI] [PubMed] [Google Scholar]
- Love TM, Stohler CS, Zubieta JK ( 2009): Positron emission tomography measures of endogenous opioid neurotransmission and impulsiveness traits in humans. Arch Gen Psychiatry 66: 1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS ( 2003): Prefrontal cortex dysfunction during working memory performance in schizophrenia: Reconciling discrepant findings. Schizophr Res 60: 285–298. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, McCaffery JM, Matthews KA, Mann JJ, Muldoon MF ( 1998): Aggression, impulsivity, and central nervous system serotonergic responsivity in a nonpatient sample. Neuropsychopharmacology 19: 287–299. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JCA ( 2008): Voxel‐based morphometry study of frontal gray matter correlates of impulsivity. Hum Brain Mapp 30: 1188–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, White FJ ( 2000): Enhanced vulnerability to cocaine self‐administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci 20: 8876–8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD ( 2004): Separate neural systems value immediate and delayed monetary rewards. Science 306: 503–507. [DOI] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD ( 2007): Time discounting for primary rewards. J Neurosci 27: 5796–5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Van Horn JD, Wolford GL, Handy TC, Valsangkar‐Smyth M, Inati S, Grafton S, Gazzaniga MS ( 2002): Extensive individual differences in brain activations associated with episodic retrieval are reliable over time. J Cogn Neurosci 14: 1200–1214. [DOI] [PubMed] [Google Scholar]
- Mink JW ( 1996): The basal ganglia: Focused selection and inhibition of competing motor programs. Prog Neurobiol 50: 381–425. [DOI] [PubMed] [Google Scholar]
- O'Boyle MO, Barratt ES ( 1993): Impulsivity and DSM‐III‐R personality disorders. Person Indiv Diff 14: 609–611. [Google Scholar]
- O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ ( 2004): Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 304: 452–454. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, Zhou Y, Kumar A, Brasic J, Alexander M, Ye W, Kuwabara H, Hilton J, Wand GS ( 2007): Impulsivity and chronic stress are associated with amphetamine‐induced striatal dopamine release. Neuroimage 36: 153–166. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES ( 1995): Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol 51: 768–774. [DOI] [PubMed] [Google Scholar]
- Peck CJ, Jangraw DC, Suzuki M, Efem R, Gottlieb J ( 2009): Reward modulates attention independently of action value in posterior parietal cortex. J Neurosci 29: 11182–11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty J, Shiv B, Rangel A ( 2008): Marketing actions can modulate neural representations of experienced pleasantness. Proc Natl Acad Sci USA 105: 1050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ML, Glimcher PW ( 1999): Neural correlates of decision variables in parietal cortex. Nature 400: 233–238. [DOI] [PubMed] [Google Scholar]
- Poldrack RA ( 2000): Imaging brain plasticity: Conceptual and methodological issues–A theoretical review. Neuroimage 12: 1–13. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K ( 1999): The basal ganglia: A vertebrate solution to the selection problem? Neuroscience 89: 1009–1023. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW ( 2000): Contrasting cortical and subcortical activations produced by attentional‐set shifting and reversal learning in humans. J Cogn Neurosci 12: 142–162. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V ( 2009): When less is more and when more is more: The mediating roles of capacity and speed in brain‐behavior efficiency. Intelligence 37: 207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilman EA, Uylings HB, Galis‐de Graaf Y, Joel D, Groenewegen HJ ( 2008): The orbital cortex in rats topographically projects to central parts of the caudate‐putamen complex. Neurosci Lett 432: 40–45. [DOI] [PubMed] [Google Scholar]
- Schlund MW, Cataldo MF ( 2005): Integrating functional neuroimaging and human operant research: Brain activation correlated with presentation of discriminative stimuli. J Exp Anal Behav 84: 505–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones‐Gotman M ( 2001): Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain 124: 1720–1733. [DOI] [PubMed] [Google Scholar]
- Smith DV, Hayden BY, Truong TK, Song AW, Platt ML, Huettel SA ( 2010): Distinct value signals in anterior and posterior ventromedial prefrontal cortex. J Neurosci 30: 2490–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga‐Barke EJ ( 2002): Psychological heterogeneity in AD/HD—A dual pathway model of behaviour and cognition. Behav Brain Res 130: 29–36. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Marshall JC, Friston KJ, Rowe JB, Ritzl A, Zilles K, Fink GR ( 2003): Lateralized cognitive processes and lateralized task control in the human brain. Science 301: 384–386. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Kasper L, Harrison LM, Daunizeau J, den Ouden HE, Breakspear M, Friston KJ ( 2008): Nonlinear dynamic causal models for fMRI. Neuroimage 42: 649–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue LP, Corrado GS, Newsome WT ( 2004): Matching behavior and the representation of value in the parietal cortex. Science 304: 1782–1787. [DOI] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA ( 2004): Modulation of caudate activity by action contingency. Neuron 41: 281–292. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG ( 1995): Functional brain imaging studies of cortical mechanisms for memory. Science 270: 769–775. [DOI] [PubMed] [Google Scholar]
- van Eimeren L, Grabner RH, Koschutnig K, Reishofer G, Ebner F, Ansari D ( 2010): Structure‐function relationships underlying calculation: A combined diffusion tensor imaging and fMRI study. Neuroimage 52: 358–363. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Hikosaka O ( 2005): Immediate changes in anticipatory activity of caudate neurons associated with reversal of position‐reward contingency. J Neurophysiol 94: 1879–1887. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC ( 1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]
- Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, Shelby ES, Smith CE, McHugo M, Kessler RM ( 2008): Midbrain dopamine receptor availability is inversely associated with novelty‐seeking traits in humans. J Neurosci 28: 14372–14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zysset S, Wendt CS, Volz KG, Neumann J, Huber O, von Cramon DY ( 2006): The neural implementation of multi‐attribute decision making: A parametric fMRI study with human subjects. Neuroimage 31: 1380–1388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


