Abstract
The aim of this study was to analyze correlations among the annual rate of gray matter volume change, age, gender, and cerebrovascular risk factors in 381 healthy community‐dwelling subjects with a large age range by applying a longitudinal design over 6 years using brain magnetic resonance images (MRIs). Brain MRI data were processed with voxel‐based morphometry using a custom template by applying diffeomorphic anatomical registration using the exponentiated lie algebra procedure. The annual rate of regional gray matter volume change showed significant positive correlations with age in several regions, including the bilateral temporal pole, caudate nucleus, ventral and dorsolateral prefrontal cortices, insula, hippocampus, and temporoparietal cortex, whereas significant negative correlations with age were observed in several regions including the bilateral cingulate gyri and anterior lobe of the cerebellum. Additionally, a significant age‐by‐gender interaction was found for the annual rate of regional gray matter volume change in the bilateral hippocampus. No significant correlations were observed between the annual rate of regional gray matter volume change and body mass index or systolic blood pressure. A significant positive correlation between the annual rate of gray matter volume change and age indicates that the region shows not linear but accelerated gray matter loss with age. Therefore, evaluating the annual rate of the gray matter volume change with age in healthy subjects is important in understanding how gray matter volume changes with aging in each brain region and in anticipating what cognitive functions are likely to show accelerated decline with aging. Hum Brain Mapp 34:2292–2301, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: aging, gray matter, magnetic resonance imaging, voxel‐based morphometry, longitudinal, cerebrovascular risk factor
INTRODUCTION
Structural neuroimaging studies applying a longitudinal design have shown that global and regional gray matter volume declines with age in healthy subjects [Driscoll et al., 2009; Du et al., 2006; Fotenos et al., 2005; Raz et al., 2005; Resnick et al., 2003; Scahill et al., 2003; Taki et al., 2011a; Thambisetty et al., 2010] and the same finding has been made by studies applying a cross‐sectional design [Cherubini et al., 2009; Fjell et al., 2009; Grieve et al., 2005; Good et al., 2001; Jernigan et al., 2001; Raz et al., 2004; Smith et al., 2007; Sowell et al., 2003; Taki et al., 2004]. Using a longitudinal design, it is possible to measure direct changes in gray matter volume and to overcome confounding factors from which cross‐sectional studies suffer, such as secular changes in nutrition and medical care. Several longitudinal studies have focused on the correlation between regional gray matter volume decline and age in healthy subjects [Du et al., 2006; Raz et al., 2005; Thambisetty et al., 2010]. For example, a recent study analyzed regional differences of gray matter volume decline with age in 72 healthy adults aged from 20 to 77 years by applying manual region‐of‐interest (ROI) analysis [Raz et al., 2005]. The study showed that longitudinal gray matter volume decline is not uniform among brain regions, and that more volume decline was observed in regions such as the caudate nucleus and the cerebellum, whereas less volume decline was observed in regions such as the fusiform gyrus, entorhinal cortex, and the primary visual cortex. More recently, longitudinal changes in cortical thickness were investigated among 66 healthy older adults aged from 60 to 84 years [Thambisetty et al., 2010]. The study showed that age‐related decline in cortical thickness is widespread, and that there is an anterior–posterior gradient with frontal and parietal regions exhibiting greater rates of decline than temporal and occipital regions. Because these studies showed that several gray matter regions such as the hippocampus, orbitofrontal gyrus, and the cerebellum exhibited a curvilinear correlation with age in ROI analyses [Raz et al., 2005; Thambisetty et al., 2010], it has been suggested that rate of gray matter volume change with age differs among gray matter regions. Thus, although several longitudinal studies have focused on regional gray matter volume change with age, to the best of our knowledge, no studies have shown correlations between the annual rate of gray matter volume change and age and gender differences in whole gray matter regions. A significant positive correlation between the annual rate of the gray matter volume change and age indicates that a region shows not linear, but inverted‐U‐shaped curvilinear gray matter loss with age, and a significant negative correlation indicates that the region shows U‐shaped curvilinear gray matter loss. Therefore, evaluating the annual rate of gray matter volume change with age in healthy subjects is important in understanding how gray matter volume changes with age in each brain region. These findings help not only to understand the mechanisms of normal brain aging but also to distinguish neurodegenerative diseases from normal aging, as neurodegenerative diseases such as Alzheimer's disease (AD) show regional gray matter volume decline compared with age‐matched healthy subjects in several regions such as the hippocampus and cingulate gyrus [Busatto et al., 2008; Raji et al., 2009]. Additionally, evaluating the annual rate of gray matter volume change with age in healthy subjects is also important to anticipate which cognitive functions show accelerated decline with age in healthy subjects, as the regional gray matter volume in several regions is associated with several cognitive functions including attention, executive function, and semantic memory [Kramer et al., 2007; Taki et al., 2011b; Zimmerman et al., 2006].
Furthermore, recent neuroimaging studies have shown that not only age but also several cerebrovascular risk factors are significantly correlated with regional gray matter volume. For example, hypertension is associated with regional gray matter volume decline in several regions, including the prefrontal cortex [Raz et al., 2003; Salerno et al., 1992; Strassburger et al., 1997]. In addition, obesity is associated with gray matter volume decline in several regions including the frontal lobe and the hippocampus [Pannacciulli et al., 2006; Raji et al., 2010; Taki et al., 2008; Ward et al., 2005]. However, it has not yet been clarified whether these cerebrovascular risk factors are significantly correlated with the annual rate of gray matter volume change, that is, whether these cerebrovascular risk factors are associated with accelerated rather than linear gray matter volume change.
Therefore, the purpose of this study was to analyze the correlations among the annual rate of gray matter volume change, age, gender, and cerebrovascular risk factors by applying a longitudinal design over 6 years and using brain magnetic resonance images (MRIs) in healthy, community‐dwelling subjects with a large age range (21–80 years). We applied voxel‐based morphometry (VBM) [Ashburner and Friston, 2000] in MRI analysis. VBM is an established automated neuroimaging technique that enables the global analysis of brain structure without a priori identification of a region of interest. VBM is not biased toward any specific brain region and permits the identification of unsuspected potential brain structural differences or abnormalities. We hypothesized that the prefrontal regions would show a positive correlation between the rate of the gray matter volume decline and age, because several higher cognitive functions, such as executive function, exhibit accelerated decline with age [Baudouin et al., 2009; Nilsson, 2003], and because there are significant positive correlations between executive function and gray matter volume levels in the prefrontal cortex [Kramer et al., 2007; Zimmerman et al., 2006]. In terms of cerebrovascular risk factors, we hypothesized that body mass index (BMI), one of the most commonly used indices of obesity, calculated by dividing weight in kilograms by the square of height, would show a significant positive correlation with the annual rate of regional gray matter volume change, because we have previously shown that global gray matter volume decline rate is significantly and positively associated with BMI [Taki et al., 2011a].
METHODS
Subjects
All subjects were Japanese and recruited from our previous brain‐imaging project, a part of the Aoba Brain Imaging Project [Sato et al., 2003]. Recruitment of subjects was described previously [Taki et al., 2011a]. Briefly, from an initial 1,604 potential participants, we selected individuals who lived in the city of Sendai (the center of the previous project) at the time of the previous study. Then, eligible people were contacted by mail and telephone and invited to participate in the longitudinal follow‐up, and 442 completed the follow‐up study. Screening criteria applied to the follow‐up sample were the same as those that had been used to determine eligibility at entry. Persons who reported a history of any malignant tumor, head trauma with loss of consciousness for >5 min, cerebrovascular disease, epilepsy, any psychiatric disease, or claustrophobia were excluded from the study. Of the 442 subjects who completed the follow‐up, 54 (12.2%) were excluded from this study because they no longer met the health screening criteria. All subjects were screened for dementia using the mini‐mental state examination (MMSE) [Folstein et al., 1975], with a cutoff of 26. Two subjects were excluded from the study based on the MMSE (one male and one female, who each scored 25). All subjects underwent measurements of height and weight in indoor clothing without shoes to calculate BMI. Blood pressure in the right brachial artery was measured in the sitting position after a 10‐min rest. An experienced neuroradiologist examined the MR scans for any tumors and cerebrovascular disease. Follow‐up MRI data on an additional eight subjects (1.8%) were unsuitable for the longitudinal analysis (four subjects showed brain tumors and four subjects showed cerebral infarct). Thus, the final sample consisted of 381 participants (40.1% of the eligible cohort: 158 men, 223 women). The mean ± standard deviation (SD) follow‐up interval was 7.42 ± 0.55 years (range, 6.2–9.0). The mean ± SD age of the participants at baseline was 51.2 ± 11.8 years (range, 21–80 years). We collected present history of several cerebrovascular risk factors such as hypertension and diabetes mellitus using a questionnaire. Characteristics of the subjects and history of disease and medication for each gender at follow‐up are shown in previously [Taki et al., 2011a]. A total of 11 subjects (mean age, 65.3 years; range, 57.7–73.4 years at follow‐up; three men, eight women) were scanned twice on the same day to permit an estimation of measurement reliability, and no significant difference in the calculated gray matter volume or intracranial volume estimated using both baseline and follow‐up MRI was observed between the two scans. Details of the measurement reliability are reported elsewhere [Taki et al., 2011a]. We also collected data on body height and weight from the subjects using height and weight scales. We calculated the BMI using body height and weight data for each subject at the time of the follow‐up scan.
Written informed consent according to the Declaration of Helsinki (1991) was obtained from each subject prior to MRI scanning, after a full explanation of the purpose and procedures of the study. Approval for these experiments was obtained from the institutional review board of Tohoku University.
Image Acquisition
All images were collected using the same 0.5‐T MR scanner (Signa contour; GE‐Yokogawa Medical Systems, Tokyo, Japan) for both baseline and follow‐up images. The scanner was routinely calibrated using the same standard GE phantom between baseline and follow‐up. During this period, no major hardware upgrade occurred. At baseline and follow‐up, all subjects were scanned with identical pulse sequences: 124 contiguous, 1.5‐mm‐thick axial planes of three‐dimensional T 1‐weighted images (spoiled gradient recalled acquisition in steady state: repetition time, 40 ms; echo time, 7 ms; flip angle, 30; voxel size, 1.02 × 1.02 × 1.5 mm3).
Image Analysis
We applied VBM to conduct the image analyses. Specifically, we used Statistical Parametric Mapping 8 software (SPM8, Wellcome Department of Cognitive Neurology, London, UK) for the structural segmentation, longitudinal registration, and group statistics, and the diffeomorphic anatomical registration using exponentiated lie algebra (DARTEL) [Ashburner, 2007] deformation framework for intersubject spatial normalization. Because DARTEL produces more accurate registration [Klein et al., 2009], it allows improved sensitivity of findings such as the correlation between regional gray matter volume and age. The overall processing, which is further detailed below, consisted of three main steps. First, the first time point images were segmented, and their gray matter volume was warped to Montreal Neurological Institute (MNI) space by creating DARTEL deformation fields. Second, the follow‐up scan images were registered to their baseline scan using high‐dimensional warping, and their gray matter volume was warped accordingly. Then, the images were further warped to the MNI space using the deformation fields computed before. Last, a weighted‐difference image of the (smoothed, modulated) gray matter tissues from the two time points was computed so that the group analysis could be conducted using the general linear model framework.
Image analysis of the baseline scan
First, the “New Segmentation” algorithm from SPM8 was applied to every T 1‐weighted MRI from the baseline scan to extract tissue maps corresponding to gray matter, white matter, and cerebrospinal fluid (CSF). This algorithm, which is an improvement on “Unified Segmentation” [Ashburner and Friston, 2005], uses a Bayesian framework to iteratively perform the probabilistic tissue classification and the spatial deformation to normalized space. Although we were only interested in the probabilistic tissue segmentation, the new Bayesian segmentation‐and‐warping algorithm, which includes an improved set of tissues priors [Ashburner and Friston, 2009] for regularization, increased the robustness and accuracy of the segmentation compared with previous standard VBM algorithms. This step allowed us to obtain probability maps for the three aforementioned tissues of every subject and to have them all rigidly registered to a common space. The temporary common space of rigidly registered tissues is necessary as a starting point for the DARTEL algorithm. Next, the segmented tissues maps were used to create a custom template and associated warping fields using the DARTEL template‐creation tool [Ashburner, 2007]. This tool estimates a best set of smooth, nonlinear deformations from every subject's tissues to their common average, applies the deformations to create a new average, and then reiterates until convergence. Smoothness and reversibility of the deformation are obtained from the diffeomorphic property of DARTEL transformations and ensure the accuracy of the mapping between the original and final spaces. The resulting template space was matched to the MNI space using an affine‐only registration, which enabled us to match our image custom coordinate space to the more standard MNI space [Bergouignan et al., 2009]. The final affine registration was provided and conducted by SPM by minimizing a multiclass divergence metric from our tissue set to its own bundled MNI‐space tissue set derived from the ICBM Tissue Probabilistic Atlas. At the end of that process, each subject's gray matter map was warped using its corresponding smooth, reversible deformation parameters to the custom template space and then to the final MNI standard space. To preserve the amount of gray matter in the process, we applied modulation by locally multiplying gray matter values by the determinant of the Jacobian of the (reverse) warping field, which has the effect of compensating for the volume change induced by the warping [Good et al., 2001]. Finally, the warped gray matter images were smoothened by convolving an 8‐mm full‐width at half maximum (FWHM) isotropic Gaussian kernel.
Image analysis of the follow‐up scan
For every subject, the follow‐up scan image was also initially segmented using the “New Segmentation” algorithm of SPM8, a process that yielded individual segmented tissue probability maps in native space. Then, using the “Coregister” algorithm in SPM8, a rigid spatial transformation was estimated to best match every image of the follow‐up scan to its corresponding image of the baseline scan. This rigid transformation was also applied to tissues maps. After that, a fine‐grained, nonlinear, high‐frequency warping field was computed using the “High Dimensional Warping” tool in SPM8 [Ashburner et al., 2000], which estimates possible local changes of the image at one time point image relative to that at another time point. The high‐resolution warping field was then used to spatially transform the gray matter tissue map from the second time point to match that from the first time point. Here again, tissue volume modulation was used to correct the scaling effect of the warping. From the 381 initial subjects' images, images of four subjects were excluded because the coregistration or warping algorithm failed to converge to a reasonable output. Then, for each subject's data, the DARTEL flow field computed on the baseline images was applied to the registered follow‐up scan image, again using modulation, to produce a final gray matter amount image in the MNI space, which we smoothed at 8 mm FWHM. Finally, to calculate the annual rate of regional gray matter volume change, we subtracted the normalized, modulated, smoothed gray matter image of the baseline scan from that of the follow‐up scan and then divided the result by the interval between baseline and follow‐up (in years) in each subject.
Statistical Analysis
To investigate the correlations among the annual rate of regional gray matter volume change, age, gender, and several cerebrovascular risk factors including hypertension and obesity, we performed a multiple regression analysis with age, gender, intracranial volume, systolic blood pressure, and BMI as independent variables, with the annual rate of the regional gray matter volume change as a dependent variable. The intracranial volume was regarded as a covariate of no interest. We created a design matrix, which included two pairs of regressors (i.e., age and intercept), one for each gender, to linearly regress the age onto the male and female groups independently. All regressors were centered on their group mean. This design allowed us to test for a linear relationship of age to annual rate of regional gray matter volume change all over the brain as well as a difference in slope for the male and female groups. We used data on age, systolic blood pressure, and BMI at follow‐up in the analysis. To remove the influence of the partial volume effect between gray and white matter and between gray matter and the CSF space, we applied an inclusive mask consisting of voxels whose value was higher than an empirically determined value of 0.5 in the average image derived from the normalized, modulated, and smoothed gray matter images in all the subjects in all analyses. Statistical testing was conducted at every voxel, and we considered significant all the voxels whose t statistic was above a threshold set for a family‐wise error (FWE) rate of α = 0.05 computed using random fields theory to account for multiple‐comparisons. The P values reported below are peak‐level FWE corrected.
RESULTS
The annual rate of regional gray matter volume change showed a significant positive correlation with age in several regions, including the bilateral temporal pole, caudate nucleus, ventral and dorsolateral prefrontal cortices, insula, hippocampus, posterior lobe of the cerebellum, temporoparietal cortex, adjusted for gender, intracranial volume, systolic blood pressure, and BMI (Fig. 1). In particular, the annual rate of regional gray matter volume change in the right temporal pole (t = 7.10, P < 0.001), the right caudate nucleus (t = 6.39, P < 0.001), the left ventral prefrontal cortex (t = 6.23, P < 0.001), the right insula (t = 6.11, P < 0.001), the right hippocampus (t = 6.05, P < 0.001), the right posterior lobe of the cerebellum (t = 6.01, P < 0.001), and the right inferior parietal lobule (t = 5.90, P < 0.001) showed strong significant positive correlations with age. These results suggest that the aforementioned regions show not linear but significant accelerated volume decline with age. In other words, these regions show a significant inverted‐U‐shaped, second‐order polynomial function with age. On the other hand, the annual rate of regional gray matter volume change was significantly and negatively correlated with age in several regions, including the bilateral cingulate cortices, anterior lobe of the cerebellum, and the bilateral orbitofrontal cortices adjusted for gender, intracranial volume, systolic blood pressure, and BMI (Fig. 2). In particular, the annual rate of regional gray matter volume change showed significant negative correlations with age in several regions including the right posterior cingulate cortex (t = 8.26, P < 0.001), the right anterior lobe of the cerebellum (t = 7.71, P < 0.001), and the bilateral anterior cingulate cortices (left, t = 6.10, P < 0.001; right, t = 6.18, P < 0.001) showed significant strong negative correlations with age. A significant age‐by‐gender interaction was found for the correlation between the annual rate of change in regional gray matter volume and age in the bilateral hippocampus. Females showed a stronger correlation between the annual rate of regional gray matter volume change and age in the bilateral hippocampus than did males (left, t = 5.43, P = 0.005; right, t = 5.70, P = 0.001) (Fig. 3); in no region did males show a significantly larger annual rate of gray matter volume change compared with females. As for the cerebrovascular risk factors, no factor showed significant positive or negative correlations with the annual rate of regional gray matter volume change.
Figure 1.
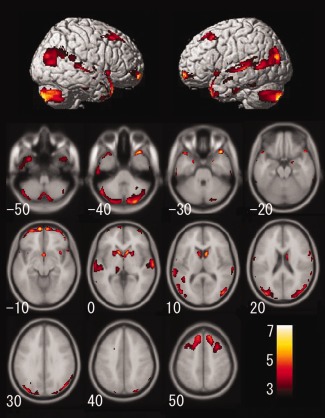
Gray matter regions where annual rate of volume change showed a significant positive correlation with age adjusted for gender, intracranial volume, systolic blood pressure, and body mass index. The results of the correlation were superimposed onto structural MRIs. The left side of the image represents the left side of the brain. The number at the bottom of the left side of each axial image indicates the value of the z‐axis in Talairach stereotaxic space. The color scale indicates the t score. The significance level was set at P < 0.05, corrected for FWE rate.
Figure 2.
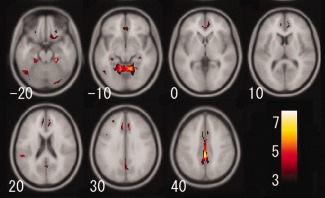
Gray matter regions where annual rate of volume change showed a significant negative correlation with age adjusted for gender, intracranial volume, systolic blood pressure, and body mass index. Details are the same as for Figure 1.
Figure 3.
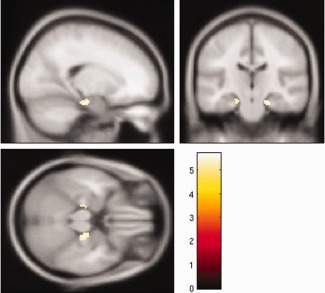
Gray matter regions where females showed a stronger correlation between annual rate of regional gray matter volume and age, intracranial volume, systolic blood pressure, and body mass index. The color scale indicates the t score. The significance level was set at P < 0.05, corrected for FWE rate.
DISCUSSION
This study provides the first longitudinal findings showing correlations among the rate of regional gray matter volume change, age, gender, and cerebrovascular risk factors by applying a longitudinal design over 6 years using brain MRIs in healthy, community‐dwelling subjects with a large age range. We found that several gray matter regions had significant positive correlations with the annual rate of regional gray matter volume change with age, and other gray matter regions showed significant negative correlations with age. These results suggest that several gray matter regions show not linear but accelerated gray matter volume decline with age, whereas other gray matter regions first show a steep gray matter volume decline but later rather stabilize in volume. Additionally, a significant age‐by‐gender interaction was found for the annual rate of regional gray matter volume change in the bilateral hippocampus. No significant correlations were observed between the annual rate of regional gray matter volume change and BMI or systolic blood pressure.
We demonstrated that the annual rate of regional gray matter volume change showed a significant positive correlation with age in several regions including the bilateral temporal pole, caudate nucleus, ventral and dorsolateral prefrontal cortices, insula, hippocampus, posterior lobe of the cerebellum, and temporoparietal cortex. Overall, these regions showed a significant negative linear correlation with age using both cross‐sectional and longitudinal designs. For example, accelerated gray matter loss with advancing age has been observed in regions of the prefrontal cortex including the superior and middle frontal gyri and superior parietal cortex in nondemented elderly people [Driscoll et al., 2009]. Other studies have also shown that prefrontal volume decline is common with advancing age [Fjell et al., 2009; Good et al., 2001; Raz et al., 2004, 1997, 2005; Resnick et al., 2003; Sowell et al., 2003; Taki et al., 2004]. Additionally, although most gray matter regions show at least linear gray matter volume decline with age [Taki et al., 2004], we have revealed in this study that the aforementioned several gray matter regions show accelerated rather than linear gray matter volume decline with age. Actually, the accelerated gray matter volume decline with age in the hippocampus is consistent with recent studies [Du et al., 2006; Raz et al., 2005], although some studies have suggested that the volumes in the hippocampus are preserved with age [Good et al., 2001; Grieve et al., 2005; Sowell et al., 2003]. This inconsistency in the results may arise from differences in the age range of subjects in each study; the age range was wider in studies that showed relative preservation of hippocampal volume and included young adults [Good et al., 2001; Grieve et al., 2005; Sowell et al., 2003], whereas studies that found accelerated volume loss in the hippocampus were observing elderly subjects [Du et al., 2006; Raz et al., 2005]. Therefore, a wide age range among subjects may diminish the effect of age‐related gray matter volume decline in the hippocampus, which seems to occur primarily in elderly individuals. In this study, we demonstrated that the hippocampal gray matter shows accelerated volume reduction with age across a wide age range by focusing on the annual rate of regional gray matter volume change and age. Thus, we showed that the annual rate of gray matter volume decline with age is not linear or uniform and that several regions such as the bilateral temporal pole, caudate nucleus, ventral and dorsolateral prefrontal cortices, insula, hippocampus, posterior lobe of the cerebellum, and temporoparietal cortex show an accelerated annual rate of gray matter volume decline with age.
We demonstrated that the annual rate of regional gray matter volume change showed a significant negative correlation with age in several regions, including the bilateral cingulate cortices, anterior lobe of the cerebellum, and orbitofrontal cortices. These regions have been reported to show relatively preserved gray matter volume with age [Fjell et al., 2009; Grieve et al., 2005]. Actually, our results are consistent with a cross‐sectional study showing that the trajectory of the cingulate gyral volume with age had a rather steep decline in young adults and then became more stable [Grieve et al., 2005]. The cingulate cortex and the orbitofrontal cortex are known to be rather early‐maturing regions [Gogtay et al., 2004; Sowell et al., 1999; Thompson et al., 2001; Whitford et al., 2007], and early‐maturing regions are thought to be more stable across age [Grieve et al., 2005], although the mechanism has not been fully clarified. This may be a reason that the annual rate of regional gray matter volume change in those regions showed a significant negative correlation with age. Thus, we showed that several regions with relative preservation of gray matter volume demonstrate first an accelerated and then a slowed annual rate of gray matter volume decline with age.
We found a significant age‐by‐gender interaction in the correlation between the annual rate of regional gray matter volume loss and age in the bilateral hippocampus. In these regions, females showed a stronger correlation between the annual rate of regional gray matter volume change and age than did males. The hippocampus has been confirmed to generate new neurons even in human adults [Gould et al., 1999]. Additionally, estrogen is known to upregulate adult hippocampal neurogenesis via cell proliferation [Barha and Galea, 2010]. Because the average age of menopause is thought to be about 50 years in Japanese women [Amagai et al., 2006], the effect of estrogen on hippocampal neurogenesis would be expected to decrease in middle‐aged woman. As a result, the annual rate of regional gray matter volume loss in the bilateral hippocampus may increase with age only in women. This may explain our finding that females showed a stronger correlation between the annual rate of regional gray matter volume loss and age than did males.
We demonstrated that the annual rate of regional gray matter volume change was not significantly negatively correlated with BMI or systolic blood pressure. However, several recent studies, including our own, have revealed a significant correlation between obesity or elevated systolic blood pressure and brain or gray matter volume decline [Gustafson et al., 2004; Pannacciulli et al., 2006; Raji et al., 2010; Taki et al., 2004, 2011a, 2008; Ward et al., 2005] due to obesity‐ or hypertension‐related vascular pathologies including carotid artery‐wall thickening [De Michele et al., 2002], vascular and coronary endothelial dysfunction [Brook et al., 2001; Sorisky, 2002; Williams et al., 2002], peripheral resistance, and arterial stiffness [Yki‐Jarvinen and Westerbacka, 2000]. The findings of this study and the aforementioned studies indicate that increasing BMI or systolic blood pressure affects linear, but not accelerated, gray matter volume decline.
Focusing on gray matter regions whose annual rate of volume change shows significant positive or negative correlations with age is thought to be important for the following reasons. Recent studies have shown that there are significant structure–function correlations affecting several cognitive functions in healthy subjects (i.e, larger gray matter volume may be associated with higher cognitive function in specific fields). For example, regional gray matter volume in the prefrontal cortex is associated with attention and executive function in healthy elderly people [Kramer et al., 2007; Zimmerman et al., 2006]. In fact, recent studies and our results show accelerated gray matter volume loss with advancing age in the prefrontal cortex [Good et al., 2001; Fjell et al., 2009; Raz et al., 2004, 1997, 2005; Resnick et al., 2003; Sowell et al., 2003; Taki et al., 2004], and recent studies show executive function decline is one of the cognitive functions that shows prominent decline with age [Connelly et al., 1991; Schretlen et al., 2000]. Therefore, detecting regions that show significant positive correlations between annual rate of gray matter volume change and age is important to provide some evidence for the mechanisms of cognitive aging in healthy elderly people. Similarly, focusing on regions that show significant negative correlations between the annual rate of the gray matter volume change and age, such as the cingulate cortex and orbitofrontal cortex, is important, as these regions are reported to be affected early on in the progression of AD [Kantarci and Jack, 2004; Scahill et al., 2003]. Therefore, detecting the gray matter regions where the annual rate of gray matter volume change shows significant negative correlations with age may help to distinguish neurodegenerative diseases such as AD from normal aging.
This study had some limitations. First, related to the MR scanner, tissue contrast at 0.5 T was substantially lower than that of higher field strength magnets, so that the use of a less powerful scanner may have affected the results of the tissue segmentation process. Generally, brain MRIs derived from a relatively low field strength generally show rather small contrast between gray matter and white matter, possibly affecting segmentation accuracy. Thus, before we began collecting brain MRI data, we tried several image collection protocols and checked the accuracy of segmentation by visual inspection. Additionally, we checked all segmented gray matter images of the baseline and follow‐up images. As a result, although minimal parts of nongray matter structures, such as the meninges, are segmented as gray matter, we ensured that there were no apparent major segmentation errors by visual inspection. Representative images are shown in Supporting Information Figure S1. Moreover, it is also a strength of this study that we used the same MR scanner for the baseline and follow‐up images because a different MR scanner or a different pulse sequence of MRI acquisition could also have affected the tissue segmentation process. Second, also regarding the MR scanner, it is possible that scanner drift may result in signal changes and altered image quality over time, although the MR scanner that we used was the same for the baseline and follow‐up images, and the scanner was routinely calibrated using the same standard GE phantom at both baseline and follow‐up and was appropriately maintained. Thus, we checked all T 1‐weighted images and gray matter segment images and subtracted gray matter image of the baseline scan from that of the follow‐up scan. Although we did not find any obvious misclassifications, we realized that the contrast of gray matter and white matter in the frontoparietal regions, such as the motor and sensory areas, are weak as compared with other regions. As a result, those regions are thought to be more vulnerable to artifacts, which may have made it difficult to find a decrease in gray matter volume around frontoparietal regions, such as the motor area, as shown in Supporting Information Figure S2. Third, regarding the subjects, selection biases may have occurred in that subjects who were concerned about their medical or psychological condition remained in the follow‐up study. Fourth, regarding image processing, the possibility of misclassification during tissue segmentation, such as the classification of white matter hyperintensities as gray matter, cannot be dismissed. Although we cannot rule out misclassification in tissue segmentation using our fully automated method, to reduce the possibility of tissue misclassification, we used not only voxel intensity itself but also a priori knowledge of the normal location of gray matter, white matter, and CSF to instruct the segmentation process. Considering these limitations, a fully automated method of MRI processing was a strength in dealing with a large quantity of data objectively and efficiently. Fifth, although we applied 26 as the cutoff score of MMSE in the dementia screening, and the mean and SD of the score of MMSE was 29.2 ± 1.19 in men and 29.2 ± 1.15 in women, we cannot deny the possibility that participants with incipient dementia were included among the present participants. Sixth, although we performed a longitudinal analysis by analyzing the correlation between the annual rate of gray matter volume change and age, we collected brain MRI data at only two time points. Thus, in discussing the acceleration or preservation of gray matter volume change, it is thought to be affected by secular changes, such as nutrition and medical care.
In summary, using a longitudinal design over 6 years with 381 community‐dwelling healthy individuals, we examined the correlations among the rate of the gray matter volume change, age, gender, and several cerebrovascular risk factors by applying a longitudinal design over 6 years using brain MRIs in 381 healthy community‐dwelling subjects with a large age range using VBM. As a result, the annual rate of regional gray matter volume change showed significant positive correlations with age in several regions, including the bilateral temporal pole, caudate nucleus, and ventral and dorsolateral prefrontal cortices, whereas significant negative correlations with age were found in several regions including the bilateral cingulate gyri and anterior lobe of the cerebellum. Additionally, a significant age‐by‐gender interaction was found for the annual rate of regional gray matter volume change in the bilateral hippocampus. No significant correlations were observed between the annual rate of regional gray matter volume change and BMI or systolic blood pressure. Evaluating the annual rate of gray matter volume change with age in healthy subjects is important to understand how gray matter volume changes with aging in each brain region and to anticipate which cognitive functions are likely to show accelerated decline with aging.
Supporting information
Supporting Information Figure 1
Supporting Information Figure 2
Acknowledgments
The authors thank K. Inoue and K. Okada for their insightful comments and K. Inaba, K. Saito, N. Ishibashi, and H. Masuyama for their technical help in collecting data.
REFERENCES
- Amagai Y, Ishikawa S, Gotoh T, Kayaba K, Nakamura Y, Kajii E (2006): Age at menopause and mortality in Japan: The Jichi Medical School Cohort Study. J Epidemiol 16:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Andersson JL, Friston KJ (2000): Image registration using a symmetric prior—In three dimensions. Hum Brain Mapp 9:212–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2000): Voxel‐based morphometry—The methods. Neuroimage 11:805–821. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. Neuroimage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2009): Computing average shaped tissue probability templates. Neuroimage 45:333–341. [DOI] [PubMed] [Google Scholar]
- Barha CK, Galea LA (2010): Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochim Biophys Acta 1800:1056–1067. [DOI] [PubMed] [Google Scholar]
- Baudouin A, Clarys D, Vanneste S, Isingrini M (2009): Executive functioning and processing speed in age‐related differences in memory: Contribution of a coding task. Brain Cogn 71:240–245. [DOI] [PubMed] [Google Scholar]
- Bergouignan L, Chupin M, Czechowska Y, Kinkingnehun S, Lemogne C, Le Bastard G, Lepage M, Garnero L, Colliot O, Fossati P (2009): Can voxel based morphometry, manual segmentation and automated segmentation equally detect hippocampal volume differences in acute depression? Neuroimage 45:29–37. [DOI] [PubMed] [Google Scholar]
- Brook RD, Bard RL, Rubenfire M, Ridker PM, Rajagopalan S (2001): Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol 88:1264–1269. [DOI] [PubMed] [Google Scholar]
- Busatto GF, Diniz BS, Zanetti MV (2008): Voxel‐based morphometry in Alzheimer's disease. Expert Rev Neurother 8:1691–1702. [DOI] [PubMed] [Google Scholar]
- Cherubini A, Peran P, Caltagirone C, Sabatini U, Spalletta G (2009): Aging of subcortical nuclei: Microstructural, mineralization and atrophy modifications measured in vivo using MRI. Neuroimage 48:29–36. [DOI] [PubMed] [Google Scholar]
- Connelly SL, Hasher L, Zacks RT (1991): Age and reading: The impact of distraction. Psychol Aging 6:533–541. [DOI] [PubMed] [Google Scholar]
- De Michele M, Panico S, Iannuzzi A, Celentano E, Ciardullo AV, Galasso R, Sacchetti L, Zarrilli F, Bond MG, Rubba P (2002): Association of obesity and central fat distribution with carotid artery wall thickening in middle‐aged women. Stroke 33:2923–2928. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM (2009): Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology 72:1906–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Chao LL, Kornak J, Jagust WJ, Kramer JH, Reed BR, Miller BL, Norman D, Chui HC, Weiner MW (2006): Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiol Aging 27:733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB (2009): High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex 19:2001–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975): “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL (2005): Normative estimates of cross‐sectional and longitudinal brain volume decline in aging and AD. Neurology 64:1032–1039. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM (2004): Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ (2001): A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14:21–36. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E (1999): Hippocampal neurogenesis in adult old world primates. Proc Natl Acad Sci USA 96:5263–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E (2005): Preservation of limbic and paralimbic structures in aging. Hum Brain Mapp 25:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I (2004): A 24‐year follow‐up of body mass index and cerebral atrophy. Neurology 63:1876–1881. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema‐Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR (2001): Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging 22:581–594. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Jack CRJr (2004): Quantitative magnetic resonance techniques as surrogate markers of Alzheimer's disease. NeuroRx 1:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV (2009): Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 46:786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, Weiner MW, Chui HC (2007): Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology 21:412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson LG (2003): Memory function in normal aging. Acta Neurol Scand Supplementum. 17:7–13. [DOI] [PubMed] [Google Scholar]
- Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA (2006): Brain abnormalities in human obesity: A voxel‐based morphometric study. Neuroimage 31:1419–1425. [DOI] [PubMed] [Google Scholar]
- Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM (2010): Brain structure and obesity. Hum Brain Mapp 31:353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji CA, Lopez OL, Kuller LH, Carmichael OT, Becker JT (2009): Age, Alzheimer disease, and brain structure. Neurology 73:1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning‐Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD (2004): Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiol Aging 25:377–396. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD (1997): Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cereb Cortex 7:268–282. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD (2005): Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex 15:1676–1689. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Acker JD (2003): Hypertension and the brain: Vulnerability of the prefrontal regions and executive functions. Behav Neurosci 117:1169–1180. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C (2003): Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J Neurosci 23:3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno JA, Murphy DG, Horwitz B, DeCarli C, Haxby JV, Rapoport SI, Schapiro MB (1992): Brain atrophy in hypertension. A volumetric magnetic resonance imaging study. Hypertension 20:340–348. [DOI] [PubMed] [Google Scholar]
- Sato K, Taki Y, Fukuda H, Kawashima R (2003): Neuroanatomical database of normal Japanese brains. Neural Networks 16:1301–1310. [DOI] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC (2003): A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol 60:989–994. [DOI] [PubMed] [Google Scholar]
- Schretlen D, Pearlson GD, Anthony JC, Aylward EH, Augustine AM, Davis A, Barta P (2000): Elucidating the contributions of processing speed, executive ability, and frontal lobe volume to normal age‐related differences in fluid intelligence. J Int Neuropsychol Soc 6:52–61. [DOI] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Markesbery WR (2007): Age and gender effects on human brain anatomy: A voxel‐based morphometric study in healthy elderly. Neurobiol Aging 28:1075–1087. [DOI] [PubMed] [Google Scholar]
- Sorisky A (2002): Molecular links between obesity and cardiovascular disease. Am J Ther 9:516–521. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW (2003): Mapping cortical change across the human life span. Nat Neurosci 6:309–315. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW (1999): Localizing age‐related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage 9:587–597. [DOI] [PubMed] [Google Scholar]
- Strassburger TL, Lee HC, Daly EM, Szczepanik J, Krasuski JS, Mentis MJ, Salerno JA, DeCarli C, Schapiro MB, Alexander GE (1997): Interactive effects of age and hypertension on volumes of brain structures. Stroke 28:1410–1417. [DOI] [PubMed] [Google Scholar]
- Taki Y, Goto R, Evans A, Zijdenbos A, Neelin P, Lerch J, Sato K, Ono S, Kinomura S, Nakagawa M, Sugiura M, Watanabe J, Kawashima R, Fukuda H (2004): Voxel‐based morphometry of human brain with age and cerebrovascular risk factors. Neurobiol Aging 25:455–463. [DOI] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Goto R, Kawashima R, Fukuda H (2011a): A longitudinal study of gray matter volume decline with age and modifying factors. Neurobiol Aging 32:907–915. [DOI] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Goto R, Wu K, Kawashima R, Fukuda H (2011b): Correlation between gray/white matter volume and cognition in healthy elderly people. Brain Cogn 75:170–176. [DOI] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K, Uchida S, Kawashima R, Fukuda H (2008): Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity 16:119–124. [DOI] [PubMed] [Google Scholar]
- Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM (2010): Longitudinal changes in cortical thickness associated with normal aging. Neuroimage 52:1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, Toga AW, Rapoport JL (2001): Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early‐onset schizophrenia. Proc Natl Acad Sci USA 98:11650–11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC (2005): The effect of body mass index on global brain volume in middle‐aged adults: A cross sectional study. BMC Neurol 5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford TJ, Rennie CJ, Grieve SM, Clark CR, Gordon E, Williams LM (2007): Brain maturation in adolescence: Concurrent changes in neuroanatomy and neurophysiology. Hum Brain Mapp 28:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams IL, Wheatcroft SB, Shah AM, Kearney MT (2002): Obesity, atherosclerosis and the vascular endothelium: Mechanisms of reduced nitric oxide bioavailability in obese humans. Int J Obes Relat Metab Disord 26:754–764. [DOI] [PubMed] [Google Scholar]
- Yki‐Jarvinen H, Westerbacka J (2000): Vascular actions of insulin in obesity. Int J Obes Relat Metab Disord 24(Suppl 2):S25–S28. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Brickman AM, Paul RH, Grieve SM, Tate DF, Gunstad J, Cohen RA, Aloia MS, Williams LM, Clark CR, Whitford TJ, Gordon E (2006): The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. Am J Geriatr Psychiatry 14:823–833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1
Supporting Information Figure 2


