Abstract
STUDY QUESTION
What is the safety and efficacy profile during long-term (12–24 months) uninterrupted treatment with the selective progesterone receptor modulator asoprisnil, 10 and 25 mg in women with heavy menstrual bleeding (HMB) associated with uterine fibroids?
SUMMARY ANSWER
Uninterrupted treatment with asoprisnil should be avoided due to endometrial safety concerns and unknown potential long-term consequences.
WHAT IS KNOWN ALREADY
Asoprisnil was well tolerated in shorter-term studies and effectively suppressed HMB and reduced fibroid volume.
STUDY DESIGN, SIZE, DURATION
Women with uterine fibroids who had previously received placebo (n = 87) or asoprisnil 10 mg (n = 221) or 25 mg (n = 215) for 12 months in two double-blind studies entered this randomized uncontrolled extension study and received up to 12 additional months of treatment followed by 6 months of post-treatment follow-up. Women who previously received placebo were re-randomized to either asoprisnil 10 or 25 mg for the extension study. This report focuses on the 436 women who received asoprisnil in the double-blind studies and this extension study. Results for women who previously received placebo in the double-blind studies are not described.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Women ≥18 years of age who completed a 12-month, double-blind, placebo-controlled study, had estradiol levels indicating that they were not menopausal and had no endometrial hyperplasia or other significant endometrial pathology were eligible. The safety endpoints were focused on endometrial assessments. The composite primary efficacy endpoint was the proportion of women who demonstrated a response to treatment by meeting all three of the following criteria at the final month for participants who prematurely discontinued or at month 12 for those who completed the study: a reduction from initial baseline to final visit of ≥50% in the menstrual pictogram score, hemoglobin concentration ≥11 g/dl or an increase of ≥1 g/dl from initial baseline at the final visit, and no surgical or invasive intervention for uterine fibroids. Other efficacy endpoints included rates for amenorrhea and suppression of bleeding, changes in fibroid and uterine volume and changes in hematologic parameters. No statistical tests were planned or performed for this uncontrolled study.
MAIN RESULTS AND ROLE OF CHANCE
Imaging studies revealed a progressive increase in endometrial thickness and cystic changes that frequently prompted invasive diagnostic procedures. Endometrial biopsy results were consistent with antiproliferative effects of asoprisnil. Two cases of endometrial cancer were diagnosed. At the final month of this extension study (total duration of uninterrupted treatment up to 24 months), the primary efficacy endpoint was achieved in 86 and 92% of women in the asoprisnil 10- and 25-mg groups, respectively. During each month of treatment, amenorrhea was observed in the majority of women (up to 77 and 94% at 10 and 25 mg, respectively). There was a progressive, dose-dependent decrease in the volume of the primary fibroid with asoprisnil 10 and 25 mg (−55.7 and −75.2% median decrease, respectively, from baseline [i.e. the beginning of the placebo-controlled study] to month 12 [cumulative months 12–24] of this extension study). These effects were associated with improvements in quality of life measures.
LIMITATIONS, REASONS FOR CAUTION
This study was uncontrolled, which limits the interpretation of safety and efficacy findings. The study also had multiple protocol amendments with the addition of diagnostic procedures and, because no active comparator was included, the potential place of asoprisnil in comparison to therapies such as GnRH agonists and surgery cannot be determined.
WIDER IMPLICATIONS OF THE FINDINGS
Long-term, uninterrupted treatment with asoprisnil leads to prominent cystic endometrial changes that are consistent with the ‘late progesterone receptor modulator’ effects, which prompted invasive diagnostic procedures, although treatment efficacy is maintained. Although endometrial cancers were uncommon during both treatment and follow-up, these findings raise concerns regarding endometrial safety during uninterrupted long-term treatment with asoprisnil. This study shows that uninterrupted treatment with asoprisnil should be avoided due to safety concerns and unknown potential long-term consequences.
STUDY FUNDING/COMPETING INTEREST(S)
AbbVie Inc. (prior sponsor, TAP Pharmaceutical Products Inc.) sponsored the study and contributed to the design and conduct of the study, data management, data analysis, interpretation of the data and the preparation and approval of the manuscript. Financial support for medical writing and editorial assistance was provided by AbbVie Inc. M. P. Diamond received research funding for the conduct of the study paid to the institution and is a consultant to AbbVie. He is a stockholder and board and director member of Advanced Reproductive Care. He has also received funding for study conduct paid to the institution for Bayer and ObsEva. E. A. Stewart participated as a site investigator in the phase 2 study of asoprisnil and served as a consultant to TAP Pharmaceuticals during the time of design and conduct of the studies while on the faculty of Harvard Medical School and Brigham and Women’s Hospital, Boston, MA. In the last 3 years, she has received support from National Institutes of Health grants HD063312, HS023418 and HD074711. She has served as a consultant for AbbVie Inc., Allergan, Bayer HealthCare AG and Myovant for consulting related to uterine leiomyoma and to Welltwigs for consulting related to infertility. She has received royalties from UpToDate and the Med Learning Group. A.R.W. Williams has acted as a consultant for TAP Pharmaceutical Products Inc. and Repros Therapeutics Inc. He has current consultancies with PregLem SA, Gedeon Richter, HRA Pharma and Bayer. B.R. Carr has served as consultant and received research funding from AbbVie Inc. and Synteract (Medicines360). E.R. Myers has served as consultant for AbbVie Inc., Allergan and Bayer. R.A. Feldman received compensation for serving as a principal investigator and participating in the conduct of the trial. W. Elger was a co-inventor of several patents related to asoprisnil.
C. Mattia-Goldberg is a former employee of AbbVie Inc. and owns AbbVie stock or stock options. B.M. Schwefel and K. Chwalisz are employees of AbbVie Inc. and own AbbVie stock or stock options.
TRIAL REGISTRATION NUMBER
Keywords: asoprisnil, J867, uterine leiomyomata, uterine leiomyoma, uterine fibroids, selective progesterone receptor modulator
WHAT DOES THIS MEAN FOR PATIENTS?
Uterine fibroids are abnormal growths in the uterus (womb) that are not cancerous but can cause heavy bleeding and pain. Besides surgery to remove them, there are no good long-term treatments.
In earlier studies, the experimental drug asoprisnil reduced the size of uterine fibroids, improving patients’ discomfort and pain, while also greatly reducing excessive bleeding from the uterus. This study included women with uterine fibroids who took asoprisnil for up to 2 years in total (1 year in this study plus 1 year earlier).
Asoprisnil had a good safety profile overall. However, in some women, the lining of the uterus became thicker and looked different from normal. To find out whether these changes were cancerous, doctors had to do invasive tests of the uterus. There were a few cancers, including endometrial cancer, breast cancer and lymphoma, but it was not clear if asoprisnil caused them. Asoprisnil continued to control bleeding and keep the uterine fibroids smaller than before asoprisnil treatment had begun.
In most women, treatment with asoprisnil causes menstrual periods to stop, and there is a thickening (build-up) of the lining of the uterus. Because of the changes in the lining of the uterus, studies of asoprisnil were stopped.
Introduction
Uterine fibroids (leiomyomata) are sex-hormone-dependent benign neoplasms of the myometrium and the most common tumors in premenopausal women (Carr et al., 1993; Stewart, 2001; Bulun, 2013). The main symptom of uterine fibroids is heavy menstrual bleeding (HMB), which can lead to iron-deficiency anemia. Depending on their size and location, uterine fibroids may also be associated with pressure-related symptoms (Stewart, 2001; Stovall, 2001). Symptomatic uterine fibroids may lead to impairment of women’s quality of life (Stewart, 2001; Spies et al., 2002) and a significant health-economic burden (Cardozo et al., 2012; Fuldeore et al., 2015). Surgical interventions, such as hysterectomy and myomectomy, still remain the most widely used treatments for symptomatic uterine fibroids, and the medical treatment options are generally limited to short-term preoperative treatment with GnRH agonists or the management of HMB with anti-fibrinolytics or high-dose progestogens (Stewart, 2001). More recently, several selective progesterone receptor modulators (SPRMs), including mifepristone, asoprisnil and ulipristal acetate, have been evaluated in women with symptomatic uterine fibroids and showed high efficacy in controlling HMB and reducing fibroid volume (Eisinger et al., 2003; Chwalisz et al., 2007; Donnez et al., 2012a, 2012b). Intermittent treatment with ulipristal acetate was approved in the European Union and Canada for the long-term management of symptomatic uterine fibroids (Donnez et al., 2014), although use in both regions is now limited to a single preoperative course or intermittent treatment in women who are ineligible for surgery; in either situation, ulipristal should not be given for longer than 3 months continuously (Esmya, 2018; Fibristal®, 2018).
Asoprisnil is an 11β-benzaldoxime-substituted SPRM which showed mixed progesterone agonist/antagonist activity in both animal models and clinical studies in women (DeManno et al., 2003; Chwalisz et al., 2005). In two placebo-controlled phase 3 studies, asoprisnil (10 and 25 mg) was very effective in controlling HMB during uninterrupted treatment for 12 months (Stewart et al., 2019). These studies showed a small but statistically significant increase in endometrial thickness at month 12 and generally benign endometrial biopsy findings. However, cystic endometrial changes were observed on MRI and transvaginal ultrasound images in some women at the end of treatment, which led to an increase in invasive diagnostic procedures during these studies. We report findings from the extension study to these placebo-controlled studies, which primarily evaluated safety and efficacy of asoprisnil during uninterrupted treatment for an additional period of up to 12 months (i.e. up to 24 months in total) with a focus on endometrial safety. Safety and efficacy were also evaluated in women who received asoprisnil for 12 months who were initially treated with placebo in the placebo-controlled phase 3 trials.
Materials and Methods
Study design
This uncontrolled, 12-month, multicenter, parallel-group extension study included eligible women from the two, phase 3, placebo-controlled studies (Study 1 [NCT00152269] and Study 2 [NCT00160 381]). In the extension study, women who had been on active treatment during either of the placebo-controlled studies continued treatment with their initial asoprisnil dosage of 10 or 25 mg; women who previously had received placebo were randomized to either 10 or 25 mg via an interactive voice response system that referred to a randomization schedule previously created by the study sponsor (Fig. 1). This report focuses on patients who received asoprisnil both in the placebo-controlled studies and this extension study (the asoprisnil/asoprisnil population), thus receiving asoprisnil for up to 24 months. Results for women who previously received placebo in the double-blind studies are not described.
Figure 1.
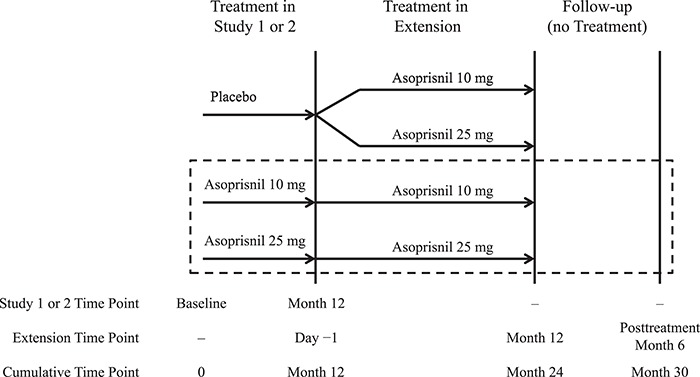
Design of a 12-month extension study to evaluate the safety and efficacy of asoprisnil in women with heavy menstrual bleeding and uterine fibroids. The dashed box indicates the population that is the primary focus of this report.
Study site personnel and participants remained blinded to the dose regimen throughout the extension period. Originally, this study consisted of a 12-month treatment period and 6-month follow-up period and included site visits every 3 months with monthly telephone calls to the participants during months when no site visits were scheduled. During the course of the study, the follow-up period was extended beyond 6 months for study participants with abnormal endometrial changes on ultrasound and/or the presence of ‘late progesterone receptor modulator (PRM)’ effects in endometrial biopsies at month 6 post-treatment to monitor endometrial changes.
Ethical approval
The study was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice Guidelines. The institutional review board or independent ethics committee for each clinic site reviewed and approved all relevant material related to the clinical study, including the clinical protocol, amendments and informed consent documents. Written informed consent was obtained from each woman before participation in the study. An independent data safety monitoring board and panel of endometrial pathologists regularly reviewed the safety aspects throughout this study.
Study population
This extension study included women who completed 12 months of treatment with asoprisnil or placebo in either placebo-controlled phase 3 Study 1 or 2 (Stewart et al., 2019), with no more than a 14-day interruption in dosing between the prior protocols and this study. Eligible women had initial baseline negative serum and urine pregnancy tests, estradiol levels ≥15 pg/ml and a normal mammogram (in women ≥41 years of age). Additionally, eligible women had endometrial biopsy results which showed no adverse endometrial findings (e.g. endometrial hyperplasia or cancer) at month 12 of the placebo-controlled phase 3 studies. Women were excluded if they had a surgical intervention for uterine fibroids in phase 3 studies, any clinically significant abnormal laboratory finding or abnormality on physical examination at day-1 (month 12 visit in the placebo-controlled phase 3 studies), had a decrease in bone mineral density (BMD) of ≥5% from baseline to month 12 in Study 1 or were diagnosed with diffuse adenomyosis at day-1 using MRI evaluation. Women with any endometrial thickness finding ≥25 mm or two findings of endometrial thickness ≥19 mm or focal endometrial abnormalities at any time during this study were discontinued from the study and entered the follow-up period, during which a thorough endometrial evaluation was conducted. Women who underwent any invasive or semi-invasive procedures (e.g. hysterectomy, myomectomy, uterine embolization, endometrial ablation, dilation and curettage [D&C] or polypectomy) because of uterine fibroids or suspicious endometrial findings during the study were also discontinued. Women were required to use two methods of non-hormonal contraception during the study. Women entering the study with anemia, as defined by the World Health Organization as a hemoglobin concentration below 12 g/dl (120 g/l) for non-pregnant women, received a supply of ferrous sulfate 300–325 mg at their next regularly scheduled visit following the diagnosis of anemia.
Safety evaluation and endpoints
Endometrial biopsies were performed at treatment months 6 and 12 and post-treatment months 3 and 6. All protocol-mandated endometrial biopsies were evaluated by a central as well as a local pathologist. If the two diagnoses were discrepant, or at least one of the diagnoses was abnormal, the biopsy was sent to a third central pathologist for arbitration and final diagnosis. All readings were conducted blinded to the participant’s treatment and to the other pathologist’s diagnosis. However, endometrial biopsies performed during unscheduled diagnostic procedures (hysteroscopy, polypectomy and D&C) were evaluated by both the local and central pathologist. Algorithms for dealing with cases of unsatisfactory biopsy specimens were prespecified in the study protocol. The endometrial biopsy results were assessed by the central laboratory according to diagnostic categories that were developed by a panel of expert endometrial pathologists specifically for asoprisnil clinical trials, including the ‘early PRM’ effect categories (‘non-physiologic secretory effect’ and ‘secretory pattern, mixed type’), as described in the publication of the placebo-controlled studies (Stewart et al., 2019). In addition, at the end of this extension study, the endometrial pathology panel defined the ‘late PRM’ effects which became evident in this study as the endometrial effects of asoprisnil changed after treatment for more than 12 months. These categories were only used to determine the reversibility of endometrial changes post-treatment and were not included in the main endometrial diagnostic categories (Supplementary Table SI). The major histologic criteria of this ‘late PRM syndrome’ included the presence of prominent large cystic glands, broad stromal columns lined by metaplastic epithelium and thick-walled vessels in the stratum functionalis. The minor criteria included the presence of prominent variegated metaplastic epithelium in a non-proliferative setting and patchy stromal prominence with plexiform vessels.
Endometrial thickness was measured using ultrasound at treatment months 3, 6 and 12 of the extension study (total treatment durations of up to 15, 18 and 24 months) and post-treatment months 3 and 6, and using MRI at treatment month 12 of the extension study (total treatment duration of up to 24 months) and post-treatment month 6. Saline infusion sonohysterogram (SIS) or hysteroscopy was performed in women with endometrial thickness ≥19 mm or ultrasound or MRI findings suggestive of a polyp. The MRI images, including endometrial thickness measurement, were assessed by a central reader. The ultrasound images and endometrial thickness measurements were evaluated by local radiologists. Other safety parameters included changes in BMD (measured by dual-energy x-ray absorptiometry [DXA] at treatment month 12 of the extension study: total treatment duration of up to 24 months) in the subset of women from Study 1, mammography at treatment month 12 of the extension study (total treatment duration of up to 24 months) and changes in biochemical parameters, including serum lipids, liver and endocrine parameters, at treatment months 3, 6 and 12 of the extension study (total treatment durations of up to 15, 18 and 24 months) and post-treatment months. A central reading service (DXA Resource Group, Inc., Worcester, MA, USA) was used for DXA evaluation. Adverse events (AEs) were recorded throughout the study. Serum and/or urine pregnancy tests were performed on a monthly basis.
Efficacy endpoints
The primary efficacy endpoint was identical to that in the placebo-controlled phase 3 studies, i.e. the proportion of women who demonstrated a response to treatment by meeting all three of the following criteria at month 24 or at the final month if they discontinued prematurely: a reduction from baseline (defined as the beginning of the placebo-controlled study) to final visit of ≥50% in the menstrual pictogram (MP) score, hemoglobin concentration ≥11 g/dl or an increase of ≥1 g/dl from baseline at the final visit and no surgical or invasive intervention for uterine fibroids, as described before (Stewart et al., 2019). Uterine bleeding was assessed on a daily basis by MP, as previously described (Larsen et al., 2013). Secondary efficacy endpoints included response rate to the primary endpoint criteria at month 6 (total treatment duration of up to 18 months), monthly amenorrhea rates, uterine and fibroid volumes (measured with ultrasound and MRI), uterine fibroid symptom assessment and quality of life measures using the Uterine Fibroid Symptom Health-Related Quality of Life Questionnaire (UFS-QOL), as described (Stewart et al., 2019).
Analyses
The safety analysis set included all women who received at least one dose of asoprisnil in the extension study. Safety results reported here are restricted to women who had received asoprisnil during both the placebo-controlled and extension periods (asoprisnil/asoprisnil). All efficacy summaries were performed on the modified intent-to-treat (mITT) population, defined as all women who had ≥1 dose of asoprisnil, had a screening menstrual cycle score >80 ml from the data available in the diary or a hemoglobin score ≤10.5 g/dl at baseline from the pivotal study and did not discontinue treatment before day 30 of the extension study unless they discontinued for fibroid surgery. The efficacy results reported here are only presented for women who received more than 12 and up to 24 months of treatment with asoprisnil 10 or 25 mg across the double-blind and extension studies (asoprisnil/asoprisnil population) such that women treated with placebo in the phase 3 placebo-controlled studies were excluded. No statistical tests were planned or performed for this uncontrolled study.
Data sharing
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (e.g. protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Results
Study population
A total of 436 (221 patients receiving asoprisnil 10 mg in Study 1 or 2 plus 215 patients receiving asoprisnil 25 mg in Study 1 or 2) women received asoprisnil during the placebo-controlled phase 3 studies, completed those studies and received ≥1 dose of asoprisnil in the extension study (Fig. 2). Thus, these ‘asoprisnil/asoprisnil’ participants received a total of between 12 and 24 months of asoprisnil treatment. Overall, 251 (58%) of the 436 asoprisnil/asoprisnil women completed the 12-month extension study, with AEs being the most common reason for premature discontinuation (8% in the asoprisnil/asoprisnil 10-mg group and 10% in the asoprisnil/asoprisnil 25-mg group). Endometrial procedures or the intention to have such procedures (other than scheduled study biopsies) resulted in premature study discontinuation in 6% of women in the asoprisnil/asoprisnil 10-mg group and 2% in the asoprisnil/asoprisnil 25-mg group. Baseline demographic and clinical characteristics pretreatment for the phase 3 placebo-controlled studies are presented in Supplementary Table SII (Stewart et al., 2019).
Figure 2.

Study disposition (N = 523) showing the number of women who entered the extension study, completed the study, prematurely discontinued from the treatment period, entered the follow-up periods and the reasons for premature discontinuation. Women from the two placebo-controlled studies continued treatment with their initial asoprisnil dosage of 10 (n = 221) or 25 (n = 215) mg (asoprisnil/asoprisnil participants; n = 436). Women who previously received placebo were randomized to asoprisnil 10 (n = 43) or 25 (n = 44) mg (placebo/asoprisnil participants; n = 87). This report focuses on the asoprisnil/asoprisnil participants.
Safety and tolerability
General safety
The percentage of asoprisnil/asoprisnil women who reported ≥1 AE during asoprisnil treatment in this extension study was no different between the 10- and 25-mg groups (83 and 80%, respectively); most AEs were considered to be mild or moderate in severity. The most frequently occurring AEs (≥5% in either group) are summarized in Table I. The most common were upper respiratory tract infection, musculoskeletal and connective tissue signs and symptoms, headaches and gastrointestinal and abdominal pains. Forty-eight women (21 [8%] asoprisnil 10-mg, 27 [10%] asoprisnil 25-mg) experienced AEs during the study that led to withdrawal from the study (Supplementary Table SIII). Serious AEs were experienced by nine (4%) and 17 (8%) women in the asoprisnil 10- and 25-mg groups, respectively, during either treatment or follow-up of the extension study (Supplementary Table SIV). Six women (one asoprisnil 10 mg, five asoprisnil 25 mg) experienced serious AEs of HMB (reported as ‘menorrhagia,’ ‘uterine hemorrhage,’ or ‘vaginal hemorrhage’), which were treated with medications (n = 4) or hysterectomy (n = 2). Twenty-one of 265 women (8%; 10 and 11 women in the asoprisnil 10- and 25-mg arms, respectively), experienced heavy uterine bleeding AEs during the post-treatment follow-up period (defined as ≥1 day following the last day of study drug). AEs included the following terms: dysfunctional uterine bleeding, menorrhagia, metrorrhagia, uterine hemorrhage and vaginal hemorrhage; some women required hospitalization for treatment. Treatment included medications (n = 13) and surgical procedures (n = 5: hysteroscopy, hysterectomy, D&C or endometrial ablation). The majority (57%; 12/21) of these women had endometrial thickness ≥19 mm one or more times during the study, and many had cystic changes.
Table I.
Most frequently reported adverse events by high-level term during the treatment period of the extension study in ≥5% of women in any group (asoprisnil/asoprisnil groups only; safety analysis set).
| Adverse event, n (%) | Asoprisnil 10 mg (n = 221) | Asoprisnil 25 mg (n = 215) |
|---|---|---|
| Any adverse event | 184 (83) | 172 (80) |
| Upper respiratory tract infections | 43 (19) | 47 (22) |
| Musculoskeletal and connective tissue signs and symptomsa | 36 (16) | 42 (20) |
| Headachesa | 34 (15) | 31 (14) |
| Gastrointestinal and abdominal painsb | 25 (11) | 19 (9) |
| Uterine lesions (non-neoplastic)c | 19 (9) | 22 (10) |
| Joint-related signs and symptoms | 24 (11) | 17 (8) |
| Vulvovaginal signs and symptoms | 8 (4) | 13 (6) |
| Peripheral vascular disordersa,d | 8 (4) | 12 (6) |
| Nausea and vomiting symptoms | 13 (6) | 14 (7) |
| Bacterial infectionsa | 13 (6) | 11 (5) |
| Breast signs and symptoms | 6 (3) | 10 (5) |
| Influenza viral infections | 9 (4) | 14 (7) |
| Bladder and urethral symptoms | 13 (6) | 10 (5) |
| Non-site-specific procedural complications | 15 (7) | 10 (5) |
| Lower respiratory tract and lung infections | 10 (5) | 9 (4) |
| Gastrointestinal atonic and hypomotility disordersa | 6 (3) | 11 (5) |
| Uterine disordersa | 9 (4) | 12 (6) |
| Vascular hypertensive disordersa | 4 (2) | 10 (5) |
aNot elsewhere classified.
bExcluding oral and throat pain.
cUterine polyp and uterine cyst.
dConsistsing entirely of hot flushes.
Serious AEs of neoplasm occurred in nine women, five of them were malignant, and one was unknown malignant potential (lung neoplasm; Supplementary Table SV). The malignancies included three cases of breast cancer in two women (grade 2/3 infiltrating ductal carcinoma, age 48 years, asoprisnil 10-mg; grade 2 invasive ductal adenocarcinoma and grade 3 ductal carcinoma in situ, age 51 years, asoprisnil 25-mg). Additionally, one woman, age 41 years, previously treated with placebo and re-randomized to receive asoprisnil 10 mg, had a serious AE of apocrine ductal carcinoma in situ of the breast. There was one case of B-cell lymphoma (age 44 years, asoprisnil 25-mg). In addition, there were two cases of endometrial cancer (asoprisnil 10 and 25 mg, 44 and 54 years, respectively). One woman was diagnosed with complex hyperplasia without atypia in the asoprisnil 25-mg asoprisnil/asoprisnil treatment group based on a hysteroscopy specimen obtained on study day 142. This woman underwent hysterectomy 5 months after discontinuing the study drug due to HMB. The histology of the endometrium obtained from the removed uterus revealed the presence of endometrial cancer (adenocarcinoma endometrioid type). A second case of endometrial cancer (well-differentiated adenocarcinoma, endometrioid type mixed with other types in the background of endometrial hyperplasia complex type with atypia) was diagnosed in an endometrial polyp removed on day 63 of this extension study (asoprisnil/asoprisnil 10-mg group). In addition to the malignancies, two cases of benign tumors were observed, with one case of acoustic neuroma (age 51 years, asoprisnil 25-mg), and one case of meningioma (age 42 years, asoprisnil 25-mg).
Endometrial safety
Endometrial biopsy results. The endometrial biopsy results at baseline (baseline of the placebo-controlled phase 3 studies), day-1 of this extension study (defined as the month-12 visit of the placebo-controlled phase 3 studies), months 6 and 12 of treatment in the extension study (18 and 24 months total treatment, respectively) and 3 months post-treatment are presented in Supplementary Table SI. At day-1, most of the asoprisnil/asoprisnil women had results in the subcategories of inactive, secretory pattern, mixed type, non-physiologic secretory effect and weakly proliferative endometrium. Similarly, at month 6 of this extension study, most of the asoprisnil/asoprisnil women had results in the subcategories of inactive, non-physiologic secretory effect, and weakly proliferative. Consistent results were observed at month 12 of this extension study (24 months total), except that more women had unsatisfactory tissue for diagnosis (18 and 30% in asoprisnil/asoprisnil 10- and 25-mg groups, respectively).
Changes in endometrial thickness by MRI and transvaginal ultrasound imaging. Endometrial thickness was measured using MRI and transvaginal ultrasound at different time points during the study (Figs 3A–C and 4A–C). At month 12 of the placebo-controlled phase 3 studies, there had been a mean increase from baseline of approximately 2 mm in endometrial thickness as measured by MRI in women treated with asoprisnil (Fig. 3B). At day-1 of the extension study, mean (SD) endometrial thickness as measured by MRI was 9.4 (8.8) and 9.7 (8.1) mm in the asoprisnil/asoprisnil 10-mg (n = 208) and 25-mg (n = 198) groups, respectively (Fig. 3A). There was a further dose-dependent increase from baseline (i.e. the start of the placebo-controlled phase 3 studies) in mean (SD) endometrial thickness at month 12 of the extension study (24 months total). In the asoprisnil/asoprisnil 10- and 25-mg groups, the mean (SD) endometrial thickness increased by 3.4 (9.8) and 6.2 (12.8) mm, respectively (mean (SD) thickness at month 12: 10.9 (9.3) and 13.9 (13.2) mm, respectively). During the follow-up period, endometrial thickness was slightly lower than the pretreatment baseline values in all asoprisnil groups, with a mean (SD) thickness at the 6 month follow-up of 6.4 (2.6) and 6.6 (3.9) mm in the asoprisnil/asoprisnil 10-mg (n = 78) and 25-mg groups (n = 93), respectively (Fig. 4A and B). There was a dose- and time-dependent increase in the percentage of women with endometrial thickness ≥19 mm (Figs 3C and 4C). The percentages of asoprisnil/asoprisnil 10- and 25-mg women with endometrial thickness ≥19 mm, as measured by MRI, increased from 13 and 12%, respectively, at day 1 (compared with baseline values of the placebo-controlled phase 3 studies) to 18 and 25% at month 12 of this extension study (24 months total; Fig. 3C). At month 3 of the follow-up period, 10 and 9% of women in the asoprisnil/asoprisnil 10- and 25-mg groups, respectively, had endometrial thickness ≥19 mm as measured by transvaginal ultrasound (Fig. 4C).
Figure 3.

Endometrial thickness during the extension study (safety analysis population). (A) Mean (SD) values at study visits. (B) Mean (SD) changes from baseline (start of the placebo-controlled, phase 3 studies) at study visits. (C) Proportion of women with endometrial thickness ≥19 mm at study visits and at any time during the treatment period. An absence of data indicates that the diagnostic procedure was not performed at that time point. TVU = transvaginal ultrasound.
Figure 4.

Endometrial thickness for women with post-treatment follow-up data (safety analysis population). (A) Mean (SD) values at study visits. (B) Mean (SD) changes from baseline (start of the placebo-controlled, phase 3 studies) at study visits. (C) Proportion of women with endometrial thickness ≥19 mm at study visits and at any time during the treatment period. An absence of data indicates that the diagnostic procedure was not performed at that time point.
Changes in endometrial texture by MRI images and invasive diagnostic procedures. In the asoprisnil/asoprisnil 10- and 25-mg groups there was an increase in the percentage of women with ‘heterogeneity suggestive of polyp’ (25 and 42%, respectively) and endometrial cysts (23 and 40%, respectively) at month 12 of this extension study (24 months total) compared with day 1, as assessed using MRI (Stewart et al., 2019). The majority of women with ‘heterogeneity suggestive of polyp’ also had endometrial cysts (Supplementary Table SVI).
These changes led to an increase in invasive diagnostic procedures, including hysteroscopy, D&C or polypectomies, during this extension study. Invasive procedures were performed during (i) the treatment period and (ii) treatment plus follow-up periods in 18 and 23% of women in the asoprisnil/asoprisnil 10-mg group, respectively, and in 24 and 30% of women in the asoprisnil/asoprisnil 25-mg group, respectively. Large endometrial cysts covered by thin endometrial layers and filled with translucent fluid (‘bubble-wrap’-like appearance) were also occasionally observed during the hysteroscopy procedures in women who received asoprisnil/asoprisnil 10 mg. However, benign endometrial polyps were confirmed by histological evaluation from samples obtained during a hysteroscopy, D&C or polypectomy only in 2% (5/221) of asoprisnil/asoprisnil 10-mg and 4% (8/215) asoprisnil/asoprisnil 25-mg women, incidences that are similar to those observed in asoprisnil-treated women in the placebo-controlled, phase 3 studies (Stewart et al., 2019).
Reversibility of endometrial changes during the follow-up period. After discontinuation of asoprisnil treatment, the endometrium showed a trend of regression toward the pattern observed at baseline of the pivotal studies. Endometrial biopsy data collected primarily at month 3 post-treatment demonstrated that the endometrium was primarily characterized by cyclic physiologic or proliferative patterns (Supplementary Table SV). Similarly, there was a reduction in mean endometrial thickness to the baseline values, and endometrial cysts and heterogeneity suggestive of polyp were evident on MRI images in four and nine women, respectively, of 171 total women at month 3 post-treatment. Women who experienced endometrial thickening and/or had evidence of ‘late PRM’ effects in endometrial biopsies during the post-treatment follow-up period were to be followed to assess reversal of effects. The data indicated that elevations in endometrial thickness trended to decrease over time; however, fluctuation within the same woman was observed due to differences in the method of assessment (MRI or transvaginal ultrasound) and stage of the menstrual cycle. Approximately 18 women, including 15 in the asoprisnil/asoprisnil groups, had a reported endometrial biopsy classified as having ‘late PRM’ effects. From the limited post-treatment follow-up biopsy data available, four women with ‘late PRM’ biopsy findings had a subsequent result that showed this finding to be absent.
Safety parameters for general chemistry and hepatic and renal markers
No clinically meaningful changes in general chemistry and hepatic and renal parameters were observed. Five women showed transient elevation of liver enzymes (alanine aminotransferase, aspartate aminotransferase, gamma-glutamyltransferase or lactate dehydrogenase) and one woman experienced elevated bilirubin at some point during the study; none of them were prematurely discontinued from the study because of abnormal liver function. Although there was little change in total cholesterol, asoprisnil reduced high-density lipoprotein cholesterol (HDL cholesterol) and increased low-density lipoprotein cholesterol (LDL cholesterol); these effects appeared to be greater at the higher asoprisnil dose (Supplementary Table SVII).
Endocrine parameters
No clinically significant treatment differences were observed for thyroxine, thyroid-stimulating hormone and cortisol. The gonadotropin data suggest a modest inhibitory effect of asoprisnil on basal FSH production that showed no clear dose-dependent effect. Furthermore, small but statistically significant decreases in prolactin levels were observed throughout the placebo-controlled studies (Stewart et al., 2019). Further dose-dependent small reductions in mean estradiol concentrations were measured at months 6 and 12 compared with the baseline values on day-1. However, in the majority of women treated with asoprisnil, estradiol levels still remained in the early follicular range. Dose-dependent reductions in total testosterone concentrations and sex hormone-binding globulin were observed, with no change observed in free testosterone (Supplementary Table SVII).
BMD
BMD of the lumbar spine (L1–L4) was only measured in Study 1 and in this extension study for women continuing on from that study. Mean (SD) baseline (i.e. last measurement before the first dose of the study) BMD measurements in Study 1 were 1.1 (0.1) units for both the asoprisnil/asoprisnil 10- and 25-mg groups. At day-1 of the extension study (i.e. after 12 months of treatment with asoprisnil in the placebo-controlled study), mean percentage change from baseline (i.e. start of the placebo-controlled study) in BMD was −0.2 and −0.5% for the asoprisnil/asoprisnil 10- and 25-mg groups, respectively. In the extension study, a small (−0.8%; n = 55) mean percentage reduction from baseline in mean BMD was recorded at month 12 (24 months of total exposure) in the asoprisnil/asoprisnil 25-mg group (Supplementary Table SVIII).
Efficacy endpoints
For the primary efficacy endpoint, 86 and 92% of women in the asoprisnil/asoprisnil 10- and 25-mg groups, respectively, responded to treatment at month 12 of the extension study (24 months total treatment) or the final visit if they discontinued prematurely. The response rates at month 6 of the extension study (18 months total treatment) were 92 and 96% for asoprisnil 10 and 25 mg, respectively (Fig. 5).
Figure 5.
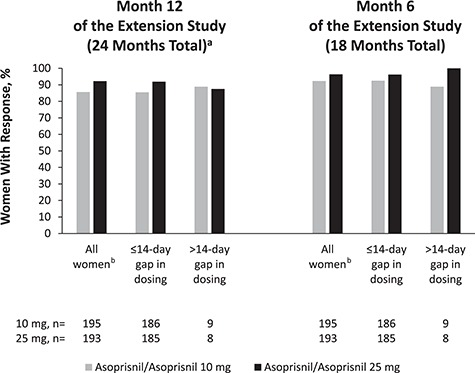
Responder rate (primary efficacy endpoint) at month 6 and month 12 of treatment in the extension study (18 and 24 months total treatment mITT population; asoprisnil/asoprisnil groups only). DB = double-blind; mITT = modified intent-to-treat. Women started with >80 ml of blood loss during screening menstrual cycle of the DB study or hemoglobin ≤10.5 g/dl during screening or at day-1 of the DB study. Response to treatment was the percentage of women who met all of the following criteria: ≥50% decrease in blood loss during month 12 or final visit if they discontinued prematurely; hemoglobin ≥11 g/dl or an increase ≥1 g/dl from baseline to month 12 or final visit if they discontinued prematurely and no surgery or withdrawal with a plan to have surgery for leiomyomata. aOr final visit if they discontinued prematurely. bWomen who qualified for the primary analysis were those in the mITT population who had baseline and on-treatment data for the primary endpoint, unless they prematurely discontinued to have surgery or invasive intervention for leiomyomata.
Amenorrhea rates
During each month of treatment, amenorrhea (defined as no bleeding during each 30-day treatment period) rates ranged from 64 to 77% of women in the asoprisnil/asoprisnil 10-mg group and from 80 to 94% of women in the asoprisnil/asoprisnil 25-mg group (Fig. 6).
Figure 6.

Percentage of women with monthly amenorrhea rates during each month of treatment in the extension study. The percentage of women with no bleeding during each 30-day treatment period was assessed for women who had ≥20 days of diary data for any month or who had bleeding during any month.
Monthly blood loss
The mean monthly blood loss (MBL) as assessed by MP score was consistently reduced in both treatment groups throughout this extension study. At month 12 of the extension study (24 months total treatment), mean MBL was 14.3 ml for the asoprisnil/asoprisnil 10-mg group and 5.1 ml for the asoprisnil/asoprisnil 25-mg group (Supplementary Fig. S1). At baseline (of the placebo-controlled studies), mean MP total score values in the asoprisnil/asoprisnil 10- and 25-mg groups were 285.6 and 298.1 ml, respectively (Supplementary Table SII).
In the follow-up period after asoprisnil treatment was discontinued, the return of menses occurred in most women (88 and 86% in the asoprisnil/asoprisnil 10- and 25-mg groups, respectively, based on a Kaplan-Meier estimate) within 90 days for women with available post-treatment data (n = 219). Median time to first follow-up menses was 22 days in the asoprisnil/asoprisnil 10-mg group and 26 days in the asoprisnil/asoprisnil 25-mg group (Supplementary Table SIX). MBL results during the first follow-up menses suggested that, although there was still a mean decrease from pretreatment baseline in blood loss, the scores represented a return to HMB (>80 ml). Mean (SD) MBL of the first menses during the follow-up period was 185.3 (183.6) ml in the asoprisnil/asoprisnil 10-mg asoprisnil/asoprisnil group and 246.6 (393.1) ml in the asoprisnil/asoprisnil 25-mg group (Supplementary Fig. S1).
Hematologic and iron parameters
Mean hemoglobin values during this extension study ranged from 13.1 to 13.5 g/dl for asoprisnil/asoprisnil 10 mg and from 13.3 to 13.4 g/dl for asoprisnil/asoprisnil 25 mg. Similar sustained benefits were observed as improvements in other hematologic parameters such as hematocrit, ferritin and total iron binding capacity (Supplementary Table SX).
Fibroid and uterine volume
Compared with baseline (pretreatment values of the placebo-controlled phase 3 studies), there was a further reduction in median primary uterine fibroid volume but not uterine volume at month 12 of this extension study (24 months total treatment) in both asoprisnil/asoprisnil groups (Table II). This effect was dose-dependent. Uterine fibroid regrowth was examined in the subset of women who had MRI performed following cessation of treatment. At month 6 of the post-treatment follow-up period, both asoprisnil/asoprisnil 10- and 25-mg groups maintained reductions in fibroid and uterine volumes compared with baseline values before any treatment with asoprisnil in randomized lead-in studies (Table II); however, from the end of treatment in extension study (24 months total treatment) to the 6-month post-treatment follow-up, there was an increase in both fibroid and uterine volumes (Table II).
Table II.
Median percentage change (from start of the placebo-controlled, phase 3 pivotal studies) in fibroid and uterine volume during the extension study (mITT population) and post-treatment period (mITT population for women with follow-up data)
| Extension study | ||||
| Primary fibroid | Uterus | |||
|---|---|---|---|---|
| n | Median change, % | n | Median change, % | |
| Asoprisnil/Asoprisnil 10 mg | ||||
| Day −1 (12 months total) | 143 | −45.0 | 151 | −26.6 |
| Month 12 (24 months total) | 123 | −55.7 | 151 | −28.0 |
| Asoprisnil/Asoprisnil 25 mg | ||||
| Day −1 (12 months total) | 128 | −65.0 | 136 | −37.6 |
| Month 12 (24 months total) | 121 | −75.2 | 128 | −40.8 |
| Post-treatment period | ||||
| Primary fibroid | Uterus | |||
| n | Median change, % | n | Median change, % | |
| Asoprisnil/Asoprisnil 10 mg | ||||
| From DB baseline to FU month 6 | 53 | −59.4 | 56 | −22.4 |
| From month 12 of the extension study (24 months total) to FU month 6 | 43 | 20.7 | 47 | 18.3 |
| Asoprisnil/Asoprisnil 25 mg | ||||
| From DB baseline to FU month 6 | 62 | −69.8 | 62 | −36.5 |
| From month 12 of the extension study (24 months total) to FU month 6 | 51 | 17.9 | 52 | 18.2 |
DB = double-blind; FU = follow-up; mITT = modified intent-to-treat.
Values are for fibroids ≥2 cm at baseline in the mITT.
UFS-QOL questionnaire
The improvements in symptom severity scores that were observed in the placebo-controlled phase 3 studies were maintained in this extension study. Similar trends were observed in the total and individual item health-related quality of life scores in the asoprisnil/asoprisnil population (Supplementary Fig. S2A–H).
Discussion
The primary objective of this extension study was the assessment of long-term safety of asoprisnil 10 and 25 mg during uninterrupted use treatment for an additional 12 months (up to 24 months of total duration of treatment) with a focus on endometrial safety. To our knowledge, this is the first study that describes the evolution of endometrial changes for up to 24 months of an uninterrupted treatment with an SPRM. Efficacy endpoints were also assessed through months 13–24 of asoprisnil treatment.
Both doses of asoprisnil were generally well tolerated, and most AEs were either mild or moderate in severity. The percentage of women with at least one AE was similar in both treatment groups. There were no concerning trends or findings in laboratory parameters, including general chemistry and renal and coagulation parameters. Similarly, no clinically meaningful changes in hepatic function parameters, including evidence of liver injury, were observed, and there were no discontinuations from the study because of liver AEs. Considering the extremely rare occurrence of liver damage, which may be due to use of SPRMs, the risk for liver damage can most probably only be assessed in large population-based observational studies using prescription registry data.
Mean estradiol concentrations were reduced further with asoprisnil/asoprisnil 25-mg treatment during this study, compared with the placebo-controlled phase 3 pivotal studies (Stewart et al., 2019), but the changes were relatively small and remained in the early follicular range in most women. It remains unclear whether this effect was due to treatment with asoprisnil or the occurrence of menopause in some women. Asoprisnil treatment for up to 2 years did not appear to affect BMD, which is consistent with its effects on estradiol concentrations. The observed decrease in SHBG may have been due to partial androgenic activity of asoprisnil (DeManno et al., 2003), a decrease in estradiol concentration or both. Small increases in LDL-cholesterol and decreases in HDL-cholesterol were observed in this study, particularly with asoprisnil/asoprisnil 25 mg, which also suggests a weak androgenic effect. These changes were not considered clinically meaningful in this population.
The overall pattern of endometrial biopsy results was consistent with an endometrial antiproliferative effect of asoprisnil, as evidenced by a progressive decrease in proliferative patterns and a progressive increase in quiescent patterns, and increase in the frequency of unsatisfactory biopsy results due to the lack of tissue. These patterns were considered as ‘late PRM’ biopsy categories by the endometrial pathologists involved in this study. The two specific ‘early PRM’ biopsy result categories (‘non-physiological secretory effect’ and ‘secretory pattern, mixed type’), later included in the PRM-associated endometrial changes (PAEC) spectrum (Mutter et al., 2008), were diagnosed less frequently after the exposure to asoprisnil for 24 months (approximately 12–14% and 1–2%, respectively), compared with 6 months of treatment (approximately 30% in the placebo-controlled, phase 3 studies (Stewart et al., 2019) or 3 months of treatment (71%) (Chwalisz et al., 2007). It should be noted that the ‘early PRM’ effects of asoprisnil were not associated with an increase in endometrial thickness during treatment for up to 6 months and only a small increase in mean endometrial thickness at 12 months (Chwalisz et al., 2007; Williams et al., 2007; Stewart et al., 2019).
Although the endometrial biopsy results suggest an antiproliferative effect of asoprisnil, the MRI and ultrasound data demonstrated a further progressive increase in both endometrial thickness and texture changes (heterogeneity suggestive of polyps and/or large endometrial cysts) during this additional treatment period compared with the 1-year treatment (Supplementary Table SX). There was a correlation between cystic changes on transvaginal ultrasound and MRI, and endometrial thickening >19 mm; however, this endometrial thickening could not be predicted during the study based on baseline characteristics (Stewart et al., 2019). These changes led to a further increase in invasive diagnostic procedures such as hysteroscopy, polypectomy or D&C compared with the placebo-controlled phase 3 studies. The review of endometrial samples obtained during these procedures conducted regularly by the panel of expert endometrial pathologists revealed the presence of large endometrial cysts covered by inactive epithelium, separated by stromal columns populated with thick-walled spiral arteries and designated as the ‘late PRM’ effects. Large endometrial cysts covered by thin endometrial layers and filled with translucent fluid (with a ‘bubble-wrap” appearance) were also occasionally observed during the hysteroscopy procedures. These changes explain the appearance of the endometrium in imaging studies (‘heterogeneity suggestive of polyps,’ endometrial cysts), and the increase in endometrial thickness, as well as a relatively high percentage of insufficient endometrial biopsies in this study. The polypoid changes (‘heterogeneity suggestive of polyps’) as observed on MRI or transvaginal ultrasound images were most likely secondary to the formation of large endometrial cysts, because benign endometrial polyps were confirmed by histologic diagnosis in only 2 and 4% women in the asoprisnil/asoprisnil 10- and 25-mg treatment groups, respectively. Overall, both histological and imaging data indicate that there was continuous time- and dose-dependent progression of endometrial changes during uninterrupted treatment with asoprisnil for an extended period of time, i.e. progression from ‘early PRM’ to ‘late PRM’ effects characterized by the presence of large endometrial cysts and low endometrial proliferation.
Two endometrial cancers (adenocarcinoma, endometrioid type) were diagnosed in the study, located within: an endometrial polyp removed during a hysteroscopy after exposure to 10 mg asoprisnil for a total of 15 months; the uterus of a woman who underwent hysterectomy 5 months post-treatment due to HMB (this woman was diagnosed with endometrial hyperplasia complex type with atypia at month 24). This second woman was treated with asoprisnil 25 mg for 22 months. The relationship of the development of endometrial cancers to asoprisnil treatment is unclear, as this study was uncontrolled, but a causal relationship cannot be ruled out. Although it is difficult to find a population exactly matching the one from our study (women with uterine fibroids and HMB), the study sponsor previously conducted analyses of malignancies across all phase 2 and 3 studies of asoprisnil in comparison with a large managed care database of women with uterine fibroids and abnormal menstrual bleeding (Supplementary Table SXI). That analysis found that three women treated with asoprisnil had endometrial adenocarcinoma (incidence rate [95% CI], 2.1 [0.44–6.28] per 1000 person-years), and one woman treated with asoprisnil had endometrial sarcoma (incidence rate [95% CI], 0.7 [0.02–3.89] per 1000 person-years). In the managed care database, the incidence rates (95% CIs) of endometrial adenocarcinoma and endometrial sarcoma were 0.83 (95% CI, 0.70–0.98) and 0.11 (95% CI, 0.06–0.17) per 1000 person-years, respectively. Thus, the rate of endometrial carcinoma was higher in the pooled asoprisnil studies than in the managed care database. However, it is difficult to draw any conclusion about significance based on such a small number of events. In addition, a recent systematic review estimated the prevalence of endometrial cancer in women with HMB with or without uterine fibroids to be 0.11% (95% CI, 0.04–0.32%) (Pennant et al., 2017). This estimate from the systematic review is lower than in our study population of asoprisnil/asoprisnil women (0.66%), although again the number of events was small and precludes definitive conclusions. Finally, patients in asoprisnil studies had an endometrial biopsy every 6 months, which is higher than in usual practice, and this could have increased the likelihood of finding cancers.
Because asoprisnil inhibits progesterone action in the endometrium, functional ‘unopposed’ estrogen effects should be considered as a potential cause of endometrial cancers in this study. It is well known that a main risk factor for endometrial cancer is exposure to endogenous or exogenous ‘unopposed’ estrogen or tamoxifen use, treatments that are associated with increased endometrial proliferation and endometrial hyperplasia with atypia (Morice et al., 2015). The literature data also suggest that premenopausal women with abnormal uterine bleeding have a higher incidence of endometrial hyperplasia and cancer, compared with premenopausal women without bleeding abnormalities (Farquhar et al., 1999). Moreover, some studies suggest that women with uterine fibroids are at increased risk of endometrial cancer (Fortuny et al., 2009; Rowlands et al., 2011). There are some similarities between endometrial changes observed in this study and those described for postmenopausal women treated long-term with tamoxifen. However, there are striking differences in endometrial morphology between these two treatments. Both drugs produce cystic dilatation of glands but against a different background. The cystic dilatation of glands associated with tamoxifen may be diffuse or localized within polyps (Ismail, 1994; Cohen, 2004). Endometrial polyps represent the most common (8–36%) endometrial pathology in postmenopausal women treated with tamoxifen (Cohen, 2004). These polyps are often multiple, and microscopically characterized by increased glandular and stromal proliferation and high frequency of endometrial hyperplasia with or without atypia (Nuovo et al., 1989; Cohen et al., 1996). Tamoxifen also leads to increased proliferation in both stromal and epithelial compartments of the endometrium, as evidenced by an increased mitotic activity and Ki67 expression (Ismail, 1994; Decensi et al., 1996). Imaging studies show that tamoxifen-induced endometrial cysts are localized in subendometrial layers and have an adenomyosis-like appearance (McCluggage et al., 2000; Fong et al., 2003). In contrast, asoprisnil treatment was associated with endometrial cysts, low frequency of endometrial polyps and low mitotic activity in the endometrial epithelium and stroma.
The endometrial biopsy results at month 3 post-treatment and imaging results from the limited number of women with follow-up data showed the presence of cyclic or physiologic endometrial patterns and a reversal of imaging findings (endometrial thickening or textural changes) in the vast majority of women. However, 15 women in the asoprisnil/asoprisnil groups required an extended follow-up period for up to 12 months post-treatment because of the presence of the ‘late PRM’ effects and/or endometrial thickening at month 3 or 6 post-treatment. This observation indicates that in some women, the complete reversal of the ‘late PRM’ effects in the endometrium may take a relatively long period of time, in spite of normal menstrual cycles during this period. This is important information for all SPRMs, because women in this age group may be perimenopausal and experience anovulatory cycles that may not reverse PAEC morphology during the interruption (off-drug) period of the intermittent regimens.
Three cases of breast cancers in two women treated continuously with asoprisnil were diagnosed in this study, one case during the treatment phase and two cases during the follow-up period. Similarly, it is unclear whether these cases were related to asoprisnil treatment or were incidental findings. Across all available asoprisnil phase 2 and 3 studies, the incidence rate (95% CI) of breast carcinoma was lower than in a comparable managed care population (1.4 [0.17–5.05] versus 2.33 [2.12–2.56] per 1000 person-years, respectively), whereas the incidence rate (95% CI) of breast carcinoma in situ was higher in the pooled asoprisnil studies than in the managed care population (2.1 [0.44–6.28] versus 0.53 [0.44–0.65] per 1000 person-years); the numbers of events were low for both kinds of breast neoplasm, making firm conclusions difficult (Supplementary Table SXI). Neoplasms originating from other tissues occurred only sporadically in women treated with asoprisnil. Interestingly, there is some evidence that ulipristal acetate, which shares a mechanism of action with asoprisnil, has an antiproliferative effect in several cell-based models of breast cancer (Esber et al., 2015). Similarly, the SPRM telapristone reduced cell proliferation in rat mammary tumors that had been treated with medroxyprogesterone acetate or progesterone (Lee et al., 2016). The progesterone receptor antagonist onapristone showed clinical benefit at a dose of 100 mg/day in women with breast cancer but was limited by hepatic toxicity (Robertson et al., 1999), whereas more modest clinical benefit in women with breast cancer was observed at doses of 10–50 mg/day (Cottu et al., 2018).
High efficacy of asoprisnil in controlling HMB was maintained during the entire treatment period. The primary efficacy endpoint was met by 86% of women in the asoprisnil/asoprisnil 10-mg group and 92% of women in the asoprisnil/asoprisnil 25-mg group at month 12 of this extension study (month 24 total) or the final visit if they discontinued prematurely. The efficacy of asoprisnil was also supported by various other secondary efficacy endpoints related to bleeding, including reductions in monthly MBL, high monthly amenorrhea rates and continued fibroid volume reduction. Improvements in hematologic parameters (hemoglobin, hematocrit, ferritin and total iron binding capacity) observed in the placebo-controlled phase 3 studies were maintained in this extension study. A further dose-dependent reduction in fibroid volume was observed in the extension study, whereas uterine volume and UFS-QOL measures remained stable. The reductions in fibroid volume persisted at 3 months after stopping treatment. Amenorrhea was reversible after stopping treatment, as the vast majority of women with available post-treatment data returned to menses within 90 days. However, the control of HMB, as observed during the treatment period, was not durable after stopping treatment, since the majority of women returned to HMB after the resumption of menses. In addition, after stopping treatment, 21 women (8%) experienced AEs indicative of HMB, which often required blood transfusion, hormonal therapy or surgical procedures; most of these women had increased endometrial thickness and cystic endometrial changes. This observation suggests that the ‘late PRM’ effects in the endometrium played a role in these events.
The strength of this study was thorough endometrial assessments during the entire treatment and follow-up periods and the involvement of expert endometrial pathologists in defining the PRM-induced endometrial changes. However, this study also has several limitations, including the lack of a control group and multiple protocol amendments with the addition of diagnostic procedures, for example, adding SIS and hysteroscopy for endometrial assessments that were not conducted at baseline as well as adding new withdrawal criteria for endometrial changes. Finally, because no active comparator was included in the placebo-controlled phase 3 studies and the present extension study, the potential place of asoprisnil in comparison to therapies such as GnRH agonists and surgery cannot be determined.
In conclusion, uninterrupted asoprisnil treatment for up to 2 years was very effective in controlling HMB and reducing fibroid volume. However, this treatment was associated with profound cystic endometrial changes that are consistent with the ‘late PRM’ effects and a progressive endometrial thickening that mimicked endometrial hyperplasia. These changes led to a further increase in diagnostic and therapeutic procedures during the study compared with the placebo-controlled studies and eventually led to the termination of asoprisnil studies with an uninterrupted treatment regimen (Abbott, 2018). The endometrial changes observed during this study were reversible after stopping treatment and resumption of menses. Two cases of endometrial cancer were diagnosed in this study. This study shows that uninterrupted treatment with asoprisnil should be avoided because of safety concerns and unknown potential long-term consequences.
Supplementary Material
Acknowledgements
The authors are grateful to late Craig A. Winkel, MD, of the Department of Obstetrics & Gynecology, Georgetown University, Washington, DC, USA, for his contributions to the study design that was submitted by TAP Pharmaceutical Products Inc and approved by the US FDA prior to commencing the clinical trial and for his critical review of the manuscript. Dr Winkel was a medical director for TAP during the clinical trials. Susan Hogan, PhD, and Michael Theisen, PhD, of Complete Publication Solutions, North Wales, PA, provided medical writing and editorial support to the authors in the development of this manuscript. Financial support to Complete Publication Solutions for medical writing and editorial assistance was provided by AbbVie Inc.
Authors’ roles
MPD was responsible for study conduct, data analysis, manuscript drafting and critical discussion. EAS participated in study design, data analysis, manuscript preparation and critical discussion. ARWW was a member of the independent endometrial pathology consultant panel in these studies. He was involved in manuscript drafting and review and critical discussion. BRC participated in collection, analysis and interpretation of the data and in reviewing and approving the scientific publication. ERM participated in interpretation of data and critical review of the manuscript. RAF served as a principal investigator and participated in the conduct of the trial and participated in critical review and discussion of the manuscript. WE was the chief executive officer of EnTec, in which role he was responsible for the collaboration with TAP Pharmaceutical Products Inc. to develop the concept of a PR-agonistic PRM, the discovery of asoprisnil and characterization of its hormonal properties, and consultation in clinical evaluation. He was involved in manuscript drafting and review and critical discussion. CM-G participated in study design, study execution, data clean-up and analysis and critical discussion of the manuscript. BMS participated in analysis, manuscript drafting and critical discussion. KC was the medical director of this study. He was involved in designing the study, evaluation of the data and manuscript drafting and review.
Funding
AbbVie Inc. (prior sponsor, TAP Pharmaceutical Products Inc.) sponsored the study and contributed to the design and conduct of the study, data management, data analysis, interpretation of the data and in the preparation and approval of the manuscript. Financial support for medical writing and editorial assistance was provided by AbbVie Inc.
Conflict of interest
MPD received research funding for the conduct of the study paid to the institution and is a consultant to AbbVie. He is a stockholder and board and director member of Advanced Reproductive Care. He has also received funding for study conduct paid to the institution for Bayer and ObsEva. EAS participated as a site investigator in the phase 2 study of asoprisnil and served as a consultant to TAP Pharmaceuticals during the time of design and conduct of the studies while on the faculty of Harvard Medical School and Brigham and Women’s Hospital, Boston, MA. In the last 3 years, she has received support from National Institutes of Health grants HD063312, HS023418 and HD074711. She has served as a consultant for AbbVie Inc., Allergan, Bayer HealthCare AG and Myovant for consulting related to uterine leiomyoma and to Welltwigs for consulting related to infertility. She has received royalties from UpToDate and the Med Learning Group. ARWW has acted as a consultant for TAP Pharmaceutical Products Inc. and Repros Therapeutics Inc. He has current consultancies with PregLem SA, Gedeon Richter, HRA Pharma, and Bayer. BRC has served as consultant and received research funding from AbbVie Inc. and Synteract (Medicines360). ERM has served as consultant for AbbVie Inc., Allergan and Bayer. RAF received compensation for serving as a principal investigator and participating in the conduct of the trial. WE was a co-inventor of several patents related to asoprisnil. CM-G is a former employee of AbbVie Inc. and owns AbbVie stock or stock options. BMS and KC are employees of AbbVie Inc. and own AbbVie stock or stock options.
References
- Abbott. Study to Evaluate the Safety of Asoprisnil in the Treatment of Uterine Fibroids. https://www.clinicaltrials.gov/ct2/show/NCT00156195 (8 January 2018, date last accessed).
- Bulun SE. Uterine fibroids. N Engl J Med 2013;369:1344–1355. [DOI] [PubMed] [Google Scholar]
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol 2012;206:211.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr BR, Marshburn PB, Weatherall PT, Bradshaw KD, Breslau NA, Byrd W, Roark M, Steinkampf MP. An evaluation of the effect of gonadotropin-releasing hormone analogs and medroxyprogesterone acetate on uterine leiomyomata volume by magnetic resonance imaging: a prospective, randomized, double blind, placebo-controlled, crossover trial. J Clin Endocrinol Metab 1993;76:1217–1223. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Larsen L, Mattia-Goldberg C, Edmonds A, Elger W, Winkel CA. A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertil Steril 2007;87:1399–1412. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev 2005;26:423–438. [DOI] [PubMed] [Google Scholar]
- Cohen I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol 2004;94:256–266. [DOI] [PubMed] [Google Scholar]
- Cohen I, Altaras MM, Shapira J, Tepper R, Rosen DJ, Cordoba M, Zalel Y, Figer A, Yigael D, Beyth Y. Time-dependent effect of tamoxifen therapy on endometrial pathology in asymptomatic postmenopausal breast cancer patients. Int J Gynecol Pathol 1996;15:152–157. [DOI] [PubMed] [Google Scholar]
- Cottu PH, Bonneterre J, Varga A, Campone M, Leary A, Floquet A, Berton-Rigaud D, Sablin MP, Lesoin A, Rezai K et al. Phase I study of onapristone, a type I antiprogestin, in female patients with previously treated recurrent or metastatic progesterone receptor-expressing cancers. PLoS One 2018;13:e0204973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decensi A, Fontana V, Bruno S, Gustavino C, Gatteschi B, Costa A. Effect of tamoxifen on endometrial proliferation. J Clin Oncol 1996;14:434–440. [DOI] [PubMed] [Google Scholar]
- DeManno D, Elger W, Garg R, Lee R, Schneider B, Hess-Stumpp H, Schubert G, Chwalisz K. Asoprisnil (J867): a selective progesterone receptor modulator for gynecological therapy. Steroids 2003;68:1019–1032. [DOI] [PubMed] [Google Scholar]
- Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, Ugocsai G, Mara M, Jilla MP, Bestel E et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 2012a;366:409–420. [DOI] [PubMed] [Google Scholar]
- Donnez J, Tomaszewski J, Vazquez F, Bouchard P, Lemieszczuk B, Baro F, Nouri K, Selvaggi L, Sodowski K, Bestel E et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med 2012b;366:421–432. [DOI] [PubMed] [Google Scholar]
- Donnez J, Vazquez F, Tomaszewski J, Nouri K, Bouchard P, Fauser BC, Barlow DH, Palacios S, Donnez O, Bestel E et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril 2014;101:1565–1573 e1561–1518. [DOI] [PubMed] [Google Scholar]
- Eisinger SH, Meldrum S, Fiscella K, le Roux HD, Guzick DS. Low-dose mifepristone for uterine leiomyomata. Obstet Gynecol 2003;101:243–250. [DOI] [PubMed] [Google Scholar]
- Esber N, Le Billan F, Resche-Rigon M, Loosfelt H, Lombes M, Chabbert-Buffet N. Ulipristal acetate inhibits progesterone receptor isoform A-mediated human breast cancer proliferation and BCl2-L1 expression. PLoS One 2015;10:e0140795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmya (Ulipristal Acetate). Summary of Product Characteristics. Budapest: Gedeon Richter Plc, 2018 [Google Scholar]
- Farquhar CM, Lethaby A, Sowter M, Verry J, Baranyai J. An evaluation of risk factors for endometrial hyperplasia in premenopausal women with abnormal menstrual bleeding. Am J Obstet Gynecol 1999;181:525–529. [DOI] [PubMed] [Google Scholar]
- Fibristal® (Ulipristal Acetate). Summary of Product Characteristics. Markham, ON: Allergan Inc, 2018 [Google Scholar]
- Fong K, Causer P, Atri M, Lytwyn A, Kung R. Transvaginal US and hysterosonography in postmenopausal women with breast cancer receiving tamoxifen: correlation with hysteroscopy and pathologic study. Radiographics 2003;23:137–150. discussion 151–155. [DOI] [PubMed] [Google Scholar]
- Fortuny J, Sima C, Bayuga S, Wilcox H, Pulick K, Faulkner S, Zauber AG, Olson SH. Risk of endometrial cancer in relation to medical conditions and medication use. Cancer Epidemiol Biomark Prev 2009;18:1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuldeore M, Yang H, Soliman AM, Winkel C. Healthcare utilization and costs among women diagnosed with uterine fibroids: a longitudinal evaluation for 5 years pre- and post-diagnosis. Curr Med Res Opin 2015;31:1719–1731. [DOI] [PubMed] [Google Scholar]
- Ismail SM. Pathology of endometrium treated with tamoxifen. J Clin Pathol 1994;47:827–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen L, Coyne K, Chwalisz K. Validation of the menstrual pictogram in women with leiomyomata associated with heavy menstrual bleeding. Reprod Sci 2013;20:680–687. [DOI] [PubMed] [Google Scholar]
- Lee O, Choi MR, Christov K, Ivancic D, Khan SA. Progesterone receptor antagonism inhibits progestogen-related carcinogenesis and suppresses tumor cell proliferation. Cancer Lett 2016;376:310–317. [DOI] [PubMed] [Google Scholar]
- McCluggage WG, Desai V, Manek S. Tamoxifen-associated postmenopausal adenomyosis exhibits stromal fibrosis, glandular dilatation and epithelial metaplasias. Histopathology 2000;37:340–346. [DOI] [PubMed] [Google Scholar]
- Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet 2016;387:1094–1108. [DOI] [PubMed] [Google Scholar]
- Mutter GL, Bergeron C, Deligdisch L, Ferenczy A, Glant M, Merino M, Williams AR, Blithe DL. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol 2008;21:591–598. [DOI] [PubMed] [Google Scholar]
- Nuovo MA, Nuovo GJ, McCaffrey RM, Levine RU, Barron B, Winkler B. Endometrial polyps in postmenopausal patients receiving tamoxifen. Int J Gynecol Pathol 1989;8:125–131. [DOI] [PubMed] [Google Scholar]
- Pennant ME, Mehta R, Moody P, Hackett G, Prentice A, Sharp SJ, Lakshman R. Premenopausal abnormal uterine bleeding and risk of endometrial cancer. BJOG 2017;124:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JF, Willsher PC, Winterbottom L, Blamey RW, Thorpe S. Onapristone, a progesterone receptor antagonist, as first-line therapy in primary breast cancer. Eur J Cancer 1999;35:214–218. [DOI] [PubMed] [Google Scholar]
- Rowlands IJ, Nagle CM, Spurdle AB, Webb PM, Australian National Endometrial Cancer Study Group, Australian Ovarian Cancer Study Group . Gynecological conditions and the risk of endometrial cancer. Gynecol Oncol 2011;123:537–541. [DOI] [PubMed] [Google Scholar]
- Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol 2002;99:290–300. [DOI] [PubMed] [Google Scholar]
- Stewart EA. Uterine fibroids. Lancet 2001;357:293–298. [DOI] [PubMed] [Google Scholar]
- Stewart EA, Diamond MP, Williams ARW, Carr BR, Myers ER, Feldman RA, Elger W, Mattia-Goldberg C, Schwefel BM, Chwalisz K. Safety and efficacy of the selective progesterone receptor modulator asoprisnil for heavy menstrual bleeding with uterine fibroids: pooled analysis of two 12-month, placebo-controlled, randomized trials. Hum Reprod 2019;34:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stovall DW. Clinical symptomatology of uterine leiomyomas. Clin Obstet Gynecol 2001;44:364–371. [DOI] [PubMed] [Google Scholar]
- Williams AR, Critchley HO, Osei J, Ingamells S, Cameron IT, Han C, Chwalisz K. The effects of the selective progesterone receptor modulator asoprisnil on the morphology of uterine tissues after 3 months treatment in patients with symptomatic uterine leiomyomata. Hum Reprod 2007;22:1696–1704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


