ABSTRACT
Yeast bioactives (YB) may stimulate broiler breeders (BB) to increase deposition of immunoglobulins (Ig) in eggs. We investigated the effects of feeding YB (mixture of derivatives from whole yeast subjected to enzymatic hydrolysis) to BB and/or their offspring on growth performance, gut development, and immune function in broiler chickens challenged with Eimeria. The BB (Ross 708 ♀ and Ross ♂) were assigned to 2 groups (60 ♀ and 10 ♂) and fed basal or basal diet supplemented with 500 g of YB/Mt. A total of 250 fertile eggs per treatment were collected, incubated, hatched, and sexed. Additional egg samples were analyzed for IgA and IgY contents. A total of 160 broiler chicks (80 ♀ and 80 ♂) from each breeder experimental group were placed in cages based on sex and BW resulting in 32 cages for each BB treatment group. Cages (16 per BB treatment group) were allocated to basal broiler chick diet or basal diet supplemented with 500 g of YB/Mt. On day 9, half of each BB by broiler chick dietary treatments was challenged with 1 mL of Eimeria culture (100,000 oocysts of Eimeria acervulina and 25,000 oocysts of Eimeria maxima). On day 14, all birds were necropsied for intestinal lesion scores and samples. Feeding YB to BB increased (P < 0.05) IgA concentration in egg yolk. Eimeria challenge decreased (P < 0.05) pancreas weight, jejunal villus height (VH), and growth performance but increased spleen weight, intestinal mass and jejunal mucosa IgA concentration. Independent of Eimeria challenge, feeding YB to BB and/or to chicks resulted in higher (P < 0.001) jejunal VH compared with feeding it to BB only or not at all. In conclusion, Eimeria challenge reduced growth performance and had negative effects on indices of intestinal function and health. Feeding YB to BB increased deposition of IgA in hatching eggs and improved jejunal VH independent of Eimeria challenge when fed to BB and/or to broiler chicks.
Keywords: broiler chickens, Eimeria, gut function and health, immunity, yeast bioactives
INTRODUCTION
Studies have shown that during the first few days of chick life, the small intestine accounts for a larger percentage of whole body weight (BW) (Wijtten et al., 2012). This developmental pattern is believed to reflect a survival strategy in which growth of digestive system is prioritized in early post-hatch period in order to support growth and development in grower and finisher phases (Lilja, 1983; Ferket, 2012). A rapid development of the gut associated lymphoid tissue occurs in concomitant with development of digestive structures and functions (Uni et al., 1999; Geyra et al., 2001). The gut microbes also facilitate immunoglobulin (Ig) responses, which in turn regulate the composition and function of the gut microbiota (Friedman et al., 2012). Thus, the reciprocal regulation of the gut microbiota and the host immune system establishes intestinal homeostasis in newly hatched chicks (Klasing, 2007; Friedman et al., 2012). However, modern poultry production practices do not usually allow for the natural microbial succession and optimal development of gut mucosal immune system (Friedman et al., 2012; Stanley et al., 2013). Traditionally, the poultry industry has relied on prophylactic broad spectrum anti-microbial growth promoters (AGP) in feed or water to enhance intestinal development and control enteric diseases (Niewold, 2007; Huyghebaert et al., 2011). However, in recent years, the emergence of microbes that are resistant to antibiotics used to treat human and animal infections, along with increasing consumer demand for antibiotic and drug free poultry products have initiated a search for effective non-antibiotic functional feed additives as alternatives to AGP (Huyghebaert et al., 2011; Kiarie et al., 2013; Kiarie and Mills, 2019). In addition, a widely accepted definition of antibiotic-free poultry is that there is no use of antibiotics (including ionophores) (Cervantes, 2015). Therefore, coccidiosis may result in more negative effects in antibiotic-free poultry industry.
The peculiarity of modern broilers is that they reach market weight before they achieve peak physiological and immune development (Ferket, 2012). This means they lack a fully functioning gastrointestinal tract (GIT) during grow-out period suggesting provision of adequate measures to support gut development in early life is critical (Bar-Shira and Friedman, 2005). The advances in developmental programming have demonstrated the long-term impact of perinatal management and nutrition (Calder et al., 2006; Murdoch et al., 2016). For instance, investigations have indicated that nutritional and environmental stimuli of broiler breeders (BB) and/or hatching eggs during incubation have impact on progeny health and growth performance (Uni and Ferket, 2004; Uni et al., 2005). The protective role of maternal antibodies is of particular interest due to the precocial nature of chicken (Friedman et al., 2012). These maternal antibodies are provided for during the process of egg formation, and continue to function in the hatchling until its own immune response can take over (Friedman et al., 2012).
Various studies have investigated transfer of pathogen-specific and non-pathogen antibodies from hens to offspring and demonstrated that the maternal antibodies are imperative for protecting the chicks (Tini et al., 2002; Cook, 2004; Hamal et al., 2006). However, there is dearth of information on manipulating BB nutrition to increase natural antibodies particularly IgA in hatching eggs (Gadde et al., 2017). Yeast bioactives (YB, cell contents and wall) have been shown to increase concentration of IgA in plasma and intestinal mucosal in poultry (Munyaka et al., 2012; Yitbarek et al., 2013; Alizadeh et al., 2016b; Leung et al., 2019a). However, little is known about the impact of offering (BB) diets containing YB on concentration of IgA in hatching eggs and subsequent effects on GIT development and responses to an enteric pathogen challenge. We therefore investigated the effects of feeding YB to BB or/and their offspring on growth performance, gut development and immune function in Eimeria challenged broiler chickens.
MATERIALS AND METHODS
All experimental design and procedures involving the use of animals were approved by the University of Guelph Animal Care Committee, and birds were cared for in accordance with the Canadian Council on Animal Care guidelines (CCAC, 2009).
Broiler Breeder Experimentation and Sample Collection
Broiler breeders (32-wk-old, Ross 708 ♀ and Ross ♂) were assigned to two groups (60 ♀ and 10 ♂) and fed a basal diet (Table 1) formulated according to Aviagen specifications (Aviagen, 2018) or the basal diet supplemented with 500 g of YB/Mt (Figure 1). Yeast bioactives were derived from hydrolysis of whole yeast by β-1,3-glucan hydrolase (Canadian Bio-Systems Inc., Calgary, AL, Canada). The product has 30% crude protein and 40% total non-starch polysaccharides of which more than 95% are mannans and β-1,3 glucans. The birds were kept in floor pens covered with wood shavings and received 14-h of incandescent light (20 lux, 0800 to 2200 hrs) and 10-h of dark per d. The birds were fed daily based on weekly BW according to Aviagen guidelines. After 14 D of exposure to feed, a total of 270 fertile eggs were collected (within 7 D) from each group, individually marked and subsequently stored at 4°C until incubation. A total of 250 eggs were incubated and hatched in a commercial grade incubator and hatcher (Nature Form, Jacksonville, FL) at the Arkell Poultry Research Station. The incubator was set at 37.5°C with 55% humidity to day 19 upon when eggs were candled for viable embryos and transferred to the hatcher set at 36.9°C with 66% humidity. The balance of 20 eggs per treatment was stored at −20°C for analyses of IgA and IgY.
Table 1.
Composition of the basal diet for broiler breeders, as fed basis.
| Ingredients, % | Amount |
|---|---|
| Corn | 38.0 |
| Wheat | 12.5 |
| Wheat middlings | 20.0 |
| Barley | 5.00 |
| Soybean meal | 13.1 |
| Limestone | 5.23 |
| Monocalcium phosphate | 1.62 |
| Limestone cocarse | 1.57 |
| Poultry vitamin premix1 | 1.00 |
| Corn oil | 0.79 |
| DL-methionine-99% | 0.27 |
| Salt | 0.26 |
| L-lysine | 0.21 |
| Sodium bicarbonate | 0.21 |
| L-threonine-98% | 0.16 |
| Multi-carbohydrases | 0.05 |
| L-tryptophan-98% | 0.02 |
| Ethoxyquin | 0.02 |
| Calculated provisions | |
| AME, kcal/kg | 2,800 |
| Crude protein, % | 15.0 |
| Crude fat, % | 3.44 |
| Linoleic acid, % | 1.38 |
| SID Lys, % | 0.60 |
| SID Met, % | 0.42 |
| SID Met + Cys, % | 0.59 |
| SID Trp, % | 0.14 |
| SID Thr, % | 0.49 |
| Ca, % | 3.00 |
| Available P, % | 0.35 |
| Na, % | 0.18 |
| Cl, % | 0.23 |
Vitamin mineral premix provided per kilogram of premix: vitamin A, 1,200,000 IU; vitamin D3, 500,000 IU; vitamin E, 8,000 IU; vitamin B12, 1,700 mcg; biotin, 22,000 mcg; menadione, 330 mg; thiamine, 400 mg; riboflavin, 860 mg; pantothenic acid, 2,000 mg; pyridoxine, 430 mg; niacin, 6,500 mg; folic acid, 220 mg; choline, 60,000 mg; iron, 6,000 mg; and copper, 1,000 mg.
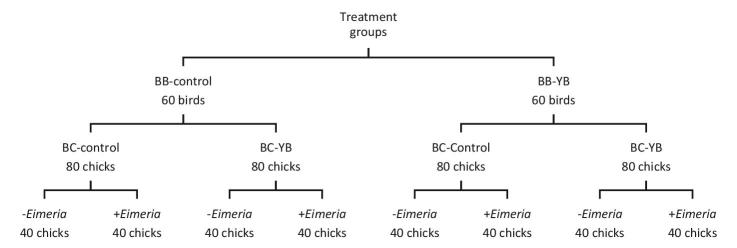
Figure 1.
Treatment structure in broiler breeder (BB) and broiler chicks (BC) experiments. Legend: YB: yeast bioactives.
Broiler Chick Experimentation and Sample Collection
On day 21 of incubation, chicks were hatched and sexed. A total of 160 broiler chicks (80 ♂ and 80 ♀) from each breeder experimental group were placed in cages (5 birds/cage) by sex making 32 cages for each maternal treatment group (Figure 1). A basal diet was formulated in a 2-phase feeding program: starter day 0 to 9 and grower day 10 to 14 (Aviagen, 2014) (Table 2). An additional diet was made by top-dressing the basal diet with 500 g of YB/Mt. Each diet was allocated to 16 cages (8 for each sex) of maternal treatment group (Figure 1). Chicks were allowed access to feed and water for ad libitum consumption for 14 D. The cages 20 in × 30 in (Ford Dickson Inc., Mitchell, Ontario, Canada) were housed in room whose temperature was initially set at 32˚C on day 1 and gradually diminished to 29˚C by day 14. The lighting program was 23L:1D (20+ LUX) from 1 to 3 D, and subsequently 20L:4D until day 14. On day 9, half of each maternal by broiler diet replicates were challenged with 1 mL of Eimeria culture (100,000 oocysts of Eimeria acervulina and 25,000 oocysts of Eimeria maxima) in distilled water suspension via oral gavage using 1-mL Norm-Ject syringe (Ottawa, ON) as described in our previous studies Kim et al., 2017 (Akbari Moghaddam Kakhki et al., 2019; Leung et al., 2019a,b). The syringe was emptied in the crop. The other half of cages were designated un-challenged control and received a sham (i.e., distilled water) challenge of equal volume. The Eimeria culture and challenge protocols were provided by Dr. John Barta of the Department of Pathobiology, University of Guelph. The culture propagation and preparation approaches were previously described by Leung et al. (2019a,b). Body weight and feed intake were measured on the days 9 and 14 for calculation of average daily feed intake (ADFI), average daily gain (ADG) and feed conversion ratio (FCR) during pre- (day 0 to 9) and post- (day 10 to 14) challenge periods.
Table 2.
Composition of the basal diet for broiler chicks, as fed basis.
| Ingredients, % | Day 0 to 9 | Day 9 to 14 |
|---|---|---|
| Corn | 45.5 | 42.6 |
| Soybean meal | 34.4 | 20.4 |
| Wheat | 10.0 | 15.0 |
| Pork meal | 3.00 | 6.00 |
| Corn DDGS | – | 10.0 |
| Soy oil | 2.85 | 2.96 |
| Poultry vitamin premix1 | 1.00 | 1.00 |
| Monocalcium phosphate | 1.42 | 0.27 |
| Limestone fine | 0.70 | 0.29 |
| DL-methionine-99% | 0.36 | 0.31 |
| L-lysine | 0.32 | 0.47 |
| Salt | 0.24 | 0.15 |
| L-threonine-98% | 0.17 | 0.20 |
| Sodium bicarbonate | 0.11 | 0.11 |
| Titanium dioxide | – | 0.25 |
| Calculated provisions | ||
| AME, kcal/kg | 3,000 | 3,100 |
| Crude protein, % | 23.0 | 21.5 |
| Crude fat, % | 5.12 | 5.57 |
| Linoleic acid, % | 2.61 | 2.85 |
| SID Lys, % | 1.28 | 1.15 |
| SID Met, % | 0.66 | 0.60 |
| SID Met + Cys, % | 0.95 | 0.85 |
| SID Trp, % | 0.25 | 0.20 |
| SID Thr, % | 0.86 | 0.77 |
| Ca, % | 0.96 | 0.87 |
| Available P, % | 0.48 | 0.44 |
| Na, % | 0.16 | 0.16 |
| Cl, % | 0.23 | 0.23 |
Vitamin mineral premix provided per kilogram of premix: vitamin A, 1,200,000 IU; vitamin D3, 500,000 IU; vitamin E, 8,000 IU; vitamin B12, 1,700 mcg; biotin, 22,000 mcg; menadione, 330 mg; thiamine, 400 mg; riboflavin, 860 mg; pantothenic acid, 2,000 mg; pyridoxine, 430 mg; niacin, 6,500 mg; folic acid, 220 mg; choline, 60,000 mg; iron, 6,000 mg; and copper, 1,000 mg.
On day 14, all birds were necropsied for intestinal lesion scores (duodenum, jejunum, ileum, ceca, and colon). Scores were assessed blindly using a scale of 0 (none) to 4 (high) (Johnson and Reid, 1970). Liver, pancreas, bursa of fabricius, spleen, and small intestine from 2 birds with average BW close to the cage average were isolated and weighed. The small intestine was emptied of its content prior to weighing. Additional 1 bird per treatment was randomly selected and jejunum immediately located and excised at duodenal loop and 2 cm anterior to Markel diverticulum. Two segments (∼3 cm) of mid-jejunum were excised and placed in buffered formalin for histomorphology analysis. The other segment was used to collect mucosa scrapings for IgA analysis (Leung et al., 2019a).
Sample Processing and Analyses
Individual egg yolks were used for measuring IgY using the commercial kit (Pierce Chicken IgY Purification Kit, 44,918) with minor modification. Briefly separated and weighed yolk was homogenized, and 1 mL samples were taken and mixed with 5 mL of de-lipidation reagent. Samples were incubated at 4˚C for 24 h before centrifugation for 15 min at 10,000 × g in 4˚C. The supernatant was pipetted out and 0.5 mL of supernatant was mixed for 2 min with IgY precipitation reagent and incubated for 24 h at 4˚C. Samples were centrifuged for 15 min at 10,000 × g in 4˚C. The supernatant was discarded, and the formed pellet was retained by adding 0.5 mL PBS (28,372, Thermo Fisher, Waltham MA) followed by reading at 280 nm. The rest of supernatant was used for measuring IgA. Jejunal mucosal samples were diluted in a 4-fold volume of PBS (wt/wt) and analyzed for total protein content using a kit (Pierce BCA Protein Assay Kit, Thermo Fisher Scientific, Waltham, MA). Then, the concentration of IgA in the egg yolk and jejunal mucosal samples was measured using Chicken IgA ELISA Kit according to the manufacturer's instruction (RK00583, ABclonal, Woburn, MA).
Fixed jejunal tissues were cut into a longitudinal cross section and embedded in paraffin wax. The tissues were then sectioned (5 μm) and stained with hematoxylin and eosin for morphological measurements. Villus height (VH) and crypt depth (CD) were measured (μm) with a calibrated micrometer for each tissue using a Leica DMR microscope (Leica Microsystems, Wetzlay, Germany). The ratio of villi height to crypt depth (VCR) was calculated by dividing VH by CD.
Statistical Analysis
The IgA and IgY in the egg yolk were analyzed using the Student's t-test with egg as experimental unit. All the other data were statistically analyzed using GLM procedures of SAS (9.4, SAS Inst. Inc., Cary, NC) with cage as experimental unit. The pre-challenge growth performance data and the lesion scores (just for challenged birds) were analyzed using a two-way ANOVA in a 2 (sex ♀ and ♂) × 4 (feed; control then control, YB then control, control then YB, and YB then YB). Other parameters were analyzed using a 3-way ANOVA in a 2 (sex) × 2 (Eimeria challenge: no and yes) × 4 (feed). An α level of P < 0.05 was used as the criterion for statistical significance.
RESULTS
IgY and IgA in the Egg Yolk
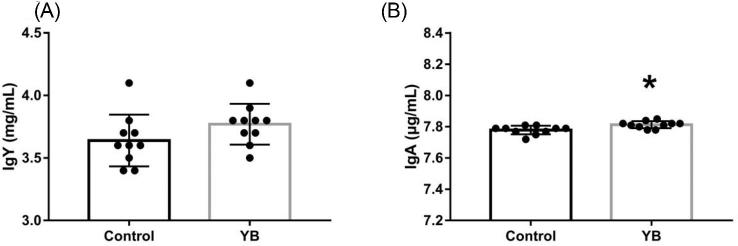
As shown in Figure 2, there was no difference in the level of IgY in the egg yolk from BB fed control and YB diets (P > 0.05, Figure 2A). However, feeding YB increased the level of IgA in the egg yolk (P < 0.05, Figure 2B).
Figure 2.
The effects of feeding YB to broiler breeders on the levels of Ig Y and Ig A in the yolk of their eggs. Asterisk indicates a significant difference, P < 0.05. YB, yeast bioactives; Ig, immunoglobulin.
Pre-Challenge Growth Performance (Day 0 to 9)
As shown in Table 3, there was no (P > 0.05) interaction between feed and sex on ADFI, ADG, FCR, and BW. Neither feed nor sex affected (P > 0.05) ADFI. Male broilers had lower FCR and a higher ADG and BW than the female broilers (P < 0.05). The YB did not affect the ADG, FCR and BW (P > 0.05) in the first 9 D of life.
Table 3.
The effects of feeding yeast bioactives (YB) to broiler breeders and/or their offspring on growth performance of broiler chicks before challenge with Eimeria (day 0 to 9).1
| ADFI, | ADG, | FCR, | BW, | ||
|---|---|---|---|---|---|
| Item | g/bird/day | g/bird/day | g/g | g/bird | |
| Main effect, sex | |||||
| Male | 24.49 | 23.62a | 1.05b | 235.9a | |
| Female | 24.70 | 19.61b | 1.27a | 200.2b | |
| SEM | 1.126 | 0.786 | 0.040 | 7.102 | |
| Main effect, feed | |||||
| Broiler breeder | Broiler chicks | ||||
| Control | Control | 24.73 | 22.71 | 1.13 | 228.1 |
| YB | Control | 24.81 | 20.64 | 1.22 | 209.4 |
| Control | YB | 24.18 | 21.65 | 1.06 | 218.0 |
| YB | YB | 24.65 | 21.45 | 1.22 | 216.7 |
| SEM | 1.592 | 1.112 | 0.056 | 10.04 | |
| Probabilities (P-value) | |||||
| Sex | 0.894 | <0.001 | <0.001 | <0.001 | |
| Feed | 0.992 | 0.627 | 0.433 | 0.627 | |
| Sex × Feed | 0.777 | 0.994 | 0.487 | 0.993 | |
Means assigned different letter superscripts within a response criterion differ, P < 0.05.
BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; FCR, feed conversion ratio.
post-challenge growth performance (day 9 to 14)
As shown in Table 4, there was no (P > 0.05) interaction among the 3 factors including the feed, Eimeria, and sex on the ADFI, ADG, FCR, and BW. All the 3 factors did not affect (P > 0.05) the ADFI of the broilers. Eimeria challenge increased the FCR and decreased ADG and BW of the broilers (P < 0.05). Male broilers had a higher BW than the female broilers (P < 0.05). The feed and sex have no effects on ADG and FCR of the broilers (P > 0.05).
Table 4.
The effects of feeding yeast bioactives (YB) to broiler breeders and/or their offspring on growth performance of broiler chicks upon challenge with Eimeria (day 9 to 14).1
| Item | ADFI, g/bird/day | ADG, g/bird/day | FCR, g/g | BW, g/bird | |
|---|---|---|---|---|---|
| Main effect, sex | |||||
| Male | 46.0 | 31.4 | 1.46 | 393.0a | |
| Female | 44.3 | 31.4 | 1.41 | 357.0b | |
| SEM | 1.908 | 0.506 | 0.076 | 7.977 | |
| No | 47.7 | 37.0a | 1.29b | 407.5a | |
| Yes | 42.6 | 25.8b | 1.65a | 342.6b | |
| SEM | 1.908 | 0.506 | 0.076 | 7.977 | |
| Main effect, feed | |||||
| Broiler breeder | Broiler chicks | ||||
| Control | Control | 47.0 | 32.5 | 1.45 | 390.5 |
| YB | Control | 44.3 | 31.7 | 1.40 | 376.3 |
| Control | YB | 44.2 | 30.0 | 1.47 | 359.5 |
| YB | YB | 45.0 | 31.4 | 1.43 | 373.7 |
| SEM | 2.699 | 0.716 | 0.107 | 11.282 | |
| Probabilities (P-value) | |||||
| Sex | 0.521 | 0.938 | 0.872 | 0.003 | |
| Eimeria challenge | 0.062 | <0.001 | <0.001 | <0.001 | |
| Feed | 0.872 | 0.121 | 0.326 | 0.296 | |
| Sex × Eimeria challenge | 0.859 | 0.146 | 0.141 | 0.418 | |
| Sex × feed | 0.926 | 0.351 | 0.145 | 0.891 | |
| Eimeria challenge × feed | 0.663 | 0.849 | 0.573 | 0.310 | |
| Sex × Eimeria challenge × feed | 0.224 | 0.280 | 0.421 | 0.575 | |
Means assigned different letter superscripts within a response criterion differ, P < 0.05.
BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; FCR, feed conversion ratio.
Visceral Organs
As shown in Table 5, there was no (P > 0.05) interaction among the 3 factors including the feed, Eimeria, and sex on pancreas and small intestine weight. There was an interaction (P < 0.05) effect between Eimeria and sex on liver weight such that, in female broilers, unchallenged group had lighter (P < 0.05) liver than that of Eimeria challenged group whereas liver weight of male broilers was not affected by Eimeria challenge (P > 0.05). Eimeria challenge increased liver and small intestine weight and decreased pancreas weight (P < 0.05). In addition, male broilers had higher liver weight than the female broilers (P < 0.05).
Table 5.
The effects of feeding yeast bioactives (YB) to broiler breeders and/or their offspring on organ weight in broiler chickens challenged with Eimeria.1
| Item | Liver,mg/g BW | Pancreas, mg/g BW | Small intestine, mg/g BW | |
|---|---|---|---|---|
| Sex × Eimeria challenge | ||||
| Sex | Eimeria challenge | |||
| Male | No | 35.4a | 4.02 | 47.6 |
| Male | Yes | 36.1a | 3.63 | 62.7 |
| Female | No | 32.7b | 4.48 | 54.3 |
| Female | Yes | 36.1a | 3.51 | 66.7 |
| SEM | 0.598 | 0.182 | 1.919 | |
| Main effects, sex | ||||
| Male | 35.8a | 3.82 | 55.1 | |
| Female | 34.4b | 3.99 | 60.5 | |
| SEM | 0.402 | 0.129 | 1.357 | |
| Main effects, Eimeria challenge | ||||
| No | 34.1b | 4.25a | 50.9b | |
| Yes | 36.1a | 3.57b | 64.7a | |
| SEM | 0.402 | 0.129 | 1.357 | |
| Main effect, feed | ||||
| Broiler breeder | Broiler chicks | |||
| Control | Control | 34.3 | 3.91 | 57.1 |
| YB | Control | 35.7 | 3.98 | 59.6 |
| Control | YB | 35.7 | 3.88 | 56.0 |
| YB | YB | 34.7 | 3.88 | 58.5 |
| SEM | 0.568 | 0.182 | 1.919 | |
| Probabilities (P-value) | ||||
| Sex | 0.020 | 0.352 | 0.006 | |
| Eimeria challenge | <0.001 | <0.001 | <0.001 | |
| Feed | 0.201 | 0.981 | 0.572 | |
| Sex × Eimeria challenge | 0.019 | 0.115 | 0.490 | |
| Sex × feed | 0.342 | 0.385 | 0.051 | |
| Eimeria challenge × feed | 0.356 | 0.952 | 0.568 | |
| Sex × Eimeria challenge × feed | 0.804 | 0.669 | 0.099 | |
Means assigned different letter superscripts within a response criterion differ, P < 0.05.
BW, body weight.
Immune Organs and IgA in Jejunal Mucosa
As shown in Table 6, there was no (P > 0.05) interaction among the 3 factors including the feed, Eimeria, and sex on the bursa of fabricius weight and the concentration of IgA in jejunal mucosa. There was an interaction (P < 0.05) effect between Eimeria and sex on spleen weight such that the spleen of Eimeria challenged female broilers was heavier (P < 0.05) than that of unchallenged ♀, but Eimeria had no effect on male broilers spleen weight (P > 0.05). Eimeria challenge increased spleen weight and the concentration of IgA in jejunal mucosa (P < 0.05). In addition, male broilers had heavier spleen than the female broilers (P < 0.05). The 3 factors (feed, Eimeria, and sex) did not affect the relative weight of bursa of fabricius (P > 0.05).
Table 6.
The effects of feeding yeast bioactives (YB) to broiler breeders and/or their offspring on the weight of immune organs and the concentration of immunoglobulin A (IgA) in jejunum mucosa of broiler chickens challenged with Eimeria.1
| Items | Bursa, mg/g BW | Spleen, mg/g BW | IgA, μg/mg protein | |
|---|---|---|---|---|
| Sex × Eimeria challenge | ||||
| Sex | Eimeria challenge | |||
| Male | No | 1.978 | 0.937b | 0.432 |
| Male | Yes | 1.816 | 0.983b | 0.696 |
| Female | No | 1.786 | 0.913b | 0.443 |
| Female | Yes | 1.868 | 1.187a | 0.738 |
| SEM | 0.081 | 0.044 | 0.088 | |
| Main effect, sex | ||||
| Sex | ||||
| Male | 1.897 | 0.960b | 0.585 | |
| Female | 1.827 | 1.050a | 0.570 | |
| SEM | 0.057 | 0.031 | 0.062 | |
| Main effect, Eimeria challenge | ||||
| No | 1.882 | 0.925b | 0.438b | |
| Yes | 1.842 | 1.085a | 0.717a | |
| SEM | 0.057 | 0.031 | 0.062 | |
| Main effect, feed | ||||
| Broiler breeder | Broiler chicks | |||
| Control | Control | 1.865 | 1.051 | 0.554 |
| YB | Control | 1.845 | 0.954 | 0.567 |
| Control | YB | 1.947 | 1.013 | 0.595 |
| YB | YB | 1.792 | 1.000 | 0.593 |
| SEM | 0.081 | 0.044 | 0.088 | |
| Probabilities (P-value) | ||||
| Sex | 0.384 | 0.045 | 0.865 | |
| Eimeria challenge | 0.618 | <0.001 | 0.003 | |
| Feed | 0.588 | 0.491 | 0.985 | |
| Sex × Eimeria challenge | 0.133 | 0.011 | 0.992 | |
| Sex × feed | 0.067 | 0.418 | 0.769 | |
| Eimeria challenge × feed | 0.138 | 0.358 | 0.934 | |
| Sex × Eimeria challenge × feed | 0.662 | 0.600 | 0.996 | |
Means assigned different letter superscripts within a response criterion differ, P < 0.05.
BW, body weight.
Intestinal Lesion Scores and Jejunal Histomorphology
No lesion scores were detected in the intestinal tract of unchallenged broilers. As shown in Table 7, feed and sex did not affect duodenum, jejunum, ileum, cecum, and colon lesion score (P > 0.05). There was no (P > 0.05) interaction among the 3 factors including the feed, Eimeria, and sex on the VH, CD, and VCR (Table 8). Eimeria challenge increased jejunal CD and decreased the VH and VCR (P < 0.05). The VH of broilers in the control + YB and YB + YB treatments was higher than that in the control + control and YB + control treatments (P < 0.05).
Table 7.
The effects of feeding yeast bioactives (YB) to broiler breeders and/or their offspring on intestinal lesion scores of broiler chicks challenged with Eimeria.1
| Item | Duodenum | Jejunum | Ileum | Ceca | Colon | |
|---|---|---|---|---|---|---|
| Main effect, sex | ||||||
| Male | 3.295 | 2.604 | 0.104 | 0.000 | 0.000 | |
| Female | 3.438 | 2.656 | 0.125 | 0.000 | 0.000 | |
| SEM | 0.100 | 0.086 | 0.039 | 0.000 | 0.000 | |
| Main effect, feed | ||||||
| Broiler breeder | Broiler chicks | |||||
| Control | Control | 3.208 | 2.542 | 0.042 | 0.000 | 0.000 |
| YB | Control | 3.208 | 2.563 | 0.125 | 0.000 | 0.000 |
| Control | YB | 3.490 | 2.542 | 0.125 | 0.000 | 0.000 |
| YB | YB | 3.558 | 2.875 | 0.167 | 0.000 | 0.000 |
| SEM | 0.130 | 0.111 | 0.051 | 0.000 | 0.000 | |
| Probabilities (P-value) | ||||||
| Sex | 0.275 | 0.642 | 0.681 | – | – | |
| Feed | 0.119 | 0.106 | 0.361 | – | – | |
| Sex × Feed | 0.456 | 0.154 | 0.067 | – | – | |
Scale: 0 (none) to 4 (high) (Johnson and Reid, 1970).
All the lesion scores of intestinal tract in unchallenged broiler chicks were zeros.
Table 8.
The effects of feeding YB to broiler breeders and/or their offspring on the jejunal histomorphology of broiler chicks challenged with Eimeria.1
| Item | VH, μm | CD, μm | VCR | |
|---|---|---|---|---|
| Main effects, sex | ||||
| Sex | ||||
| Male | 759.3 | 217.2 | 3.91 | |
| Female | 749.2 | 218.5 | 3.85 | |
| SEM | 3.944 | 3.146 | 0.078 | |
| Main effects, Eimeria challenge | ||||
| No | 932.2a | 168.2b | 5.60a | |
| Yes | 576.3b | 267.5a | 2.16b | |
| SEM | 3.944 | 3.146 | 0.078 | |
| Main effects, feed | ||||
| Broiler breeder | Broiler chicks | |||
| Control | Control | 740.1b | 211.7 | 3.81 |
| YB | Control | 736.4b | 219.7 | 3.77 |
| Control | YB | 767.5a | 219.6 | 3.95 |
| YB | YB | 773.0a | 220.3 | 4.00 |
| SEM | 5.578 | 4.449 | 0.110 | |
| Probabilities (P-value) | ||||
| Sex | 0.075 | 0.762 | 0.580 | |
| Eimeria challenge | <0.001 | <0.001 | <0.001 | |
| Feed | <0.001 | 0.478 | 0.425 | |
| Sex × Eimeria challenge | 0.647 | 0.821 | 0.909 | |
| Sex × Feed | 0.380 | 0.953 | 0.957 | |
| Eimeria challenge × Feed | 0.739 | 0.088 | 0.309 | |
| Sex × Eimeria challenge × Feed | 0.601 | 0.993 | 0.989 | |
Means assigned different letter superscripts within a response criterion differ, P < 0.05.
VH, villus height; CD, crypt depth; VCR, VH/CD.
DISCUSSION
Concerns on antibiotic resistance have led to decreased antibiotic use for growth promotion and increased focus on alternatives (Niewold, 2007; Huyghebaert et al., 2011; Kiarie et al., 2013; Kiarie and Mills, 2019). Dietary immunomodulation is a key component that can enhance the productivity and integrity of the immune system in farm animals under limited use of AGP (Kogut and Klasing, 2009; Yitbarek et al., 2013; Kogut, 2017). There are numerous studies that reported that injection of hens with various pathogens increased transfer of pathogen-specific antibodies to offspring via egg (Rahman et al., 2002; Tini et al., 2002; Hamal et al., 2006). Other investigations focused on injecting hens with non-microbial antigens to increase egg antibodies. For example, progeny from chicken and turkey hens injected with jack bean urease showed improved growth performance linked to maternally transferred urease antibodies that reduced toxic ammonia production in the GIT (Pimentel and Cook, 1988; Pimentel et al., 1991). Feeding broiler chicken hyperimmune egg yolk antibodies against various neuropeptides (cholecystokinin, neuropeptide Y) and eicosanoids improved growth and feed efficiency (Cook, 2004).
The mucosal of the chick small intestine constitutes a highly dynamic interface with the external environment through the delivery, processing, and absorption of nutrients (Wijtten et al., 2012). Enterocytes undergo rapid development from non-polar cellular structure to fully functioning, elongated, polar, enterocyte structure in the first 2 wk of life (Wijtten et al., 2012). During this process, the immature intestinal cells may exhibit low resistance to luminal stress (Geyra et al., 2001). Specifically, the newly hatched chicks are susceptible to enteric pathogens because their immune system is not fully developed (Bar-Shira and Friedman, 2005; Friedman et al., 2012). During the first week of the post-hatching period, the immune system is not mature enough to produce its own B lymphocytes and as such chick's humoral immunity depends on maternal antibodies received from the egg yolk. It has been reported that the amount of IgY and IgA deposited in the egg and the levels transferred to the offspring were directly related to the circulating levels of these Ig in the dam (Hamal et al., 2006). The IgY is predominantly present in the egg yolk and IgA in the egg white as a result of mucosal secretion in the oviduct (Rose et al., 1974). However, IgA can also be found in the egg yolk. For example, in a comparative study of hatching eggs from two commercial BB lines, the concentration of IgA ranged from 15.5 to 22 µg/mL and 7.0 to 14 µg/mL in the egg yolk and white, respectively (Hamal et al., 2006).
In the present study we fed BB YB known for their intestinal health benefits and immunomodulatory properties (Jensen et al., 2008; Alizadeh et al., 2016b; Leung et al., 2019a,b). The concentration of IgY and IgA was analyzed in yolk in the present study. The observed concentration of yolk IgY was less than a range of 8 to 11 mg/mL (Ulmer-Franco et al., 2012) but more than a range of 1.2 to 2.3 mg/mL (Hamal et al., 2006) in hatching eggs; the discrepancy might due to different age and breed of the BB. Yeast bioactives did not affect the level of IgY in the egg yolk but increased the level of IgA. Although not measured in the present study, IgA was detected in the amniotic fluid of embryonating eggs and in the digestive tract of 19-D embryos (Rose et al., 1974). Indeed, previous study showed that the higher IgA level in the eggs may result in the higher IgA level in the chicks during their early life (Hamal et al., 2006). Thus, although we did not examine intestinal mucosal concentration of IgA in the chicks upon hatching, IgA levels in day old chicks were expected to have increased in concurrence with observed levels in the yolk.
Eimeria replicates within the intestinal wall inducing inflammation and necrosis of the mucosa barrier and underlying tissue (Chapman, 2014). Lesion formation in the intestinal epithelium results in nutrient malabsorption, diarrhea and growth impairment (Williams, 2005; Chapman, 2009). In the present study, Eimeria challenge significantly increased FCR and decreased growth in agreement with our previous studies using the same challenge protocol (Kim et al., 2017; Leung et al., 2019a,b). Eimeria challenge significantly increased liver weight and decreased pancreas weight. The pancreas plays a vital role in secreting various digestive enzymes, and previous study demonstrated that Eimeria challenge may suppress the pancreas development (Russell and Ruff, 1978), which may be linked to the negative consequences on digestive capacity (Kim et al., 2017). Moreover, Eimeria challenge significantly increased small intestine weight in line with the findings of Kim et al. (2017). It has been reported that locations of lesions vary depending on the species, and the species used in the current study mainly targeted the duodenum and jejunum (Chapman, 2014). The observed lesion scores corroborate our previous studies employing similar challenge protocol in cage and floor housing (Kim et al., 2017; Leung et al., 2019a,b). Eimeria challenge significantly increased the CD and decreased the VH and VCR of jejunum, which agreed with the study of Leung et al. (2019b). Moreover, the Eimeria challenge significantly increased spleen weight and the concentration of IgA in jejunal mucosa suggesting that the Eimeria challenge stimulated immune response in line with the findings of previous studies (Gao et al., 2009; Leung et al., 2019a).
Previous studies have reported that supplementation of broiler starter diets with YB improved growth performance linked to immunomodulation (Munyaka et al., 2012; Yitbarek et al., 2013; Leung et al., 2019a). It has been suggested that YB could complement maternal Ig (Gao et al., 2009; Munyaka et al., 2012; Yitbarek et al., 2013; Alizadeh et al., 2016a). We hypothesized that broiler chicks hatched from eggs of BB fed YB and subsequently reared on diets with and without YB will show improved growth performance and resilience to Eimeria challenge linked to immunomodulation (Friedman et al., 2003; Bar-Shira and Friedman, 2005). However, we did not observe effects of feeding YB to BB and/or chicks on jejunal mucosal IgA or immune organ weights in presence or absence of Eimeria. The pathway, amount, and kinetics of total IgA transferred to the egg and subsequently to the chicks have not been studied in detail. However, several studies have shown that maternal IgA does not appear to be taken up into the circulation of the offspring and has its major function in the newly hatched chick as a protective Ig in the GIT (Hamal et al., 2006; Gadde et al., 2017). Plasma IgA levels in the chicks were lowest on day 3 post-hatch and approached adult levels by day 21 post-hatch (Hamal et al., 2006). This suggested that the mucosal IgA observed in the present study was mainly due endogenous production initiated by antigens encountered at mucosal surfaces.
Feeding YB to BB and/or their offspring did not affect growth performance before or after Eimeria challenge or organ weights. Leung et al. (2019a) fed broiler chickens nucleotides rich yeast extract and challenged them with Eimeria on day 10 post-hatch. The concentration of plasma and mucosal IgA and immune organ weights (bursa, spleen and thymus) were determined at day 5 and 25 post-challenge. There were no diet effects on day 5 post-challenge measurements; however, birds fed yeast nucleotides showed heavier bursa weight on day 25 post-challenge. Although feeding YB to BB or/and their offspring did not affect lesion scores there was an independent effect of YB on jejunal histomorphology. The VH of broilers in the control + YB and YB + YB treatments were significantly higher than that in the control + control and YB + control treatments suggesting that feeding YB to BB and/or chicks can improve intestinal digestive and absorptive function. It is interesting that there were 2 significant interaction effects between the Eimeria challenge and gender on liver and spleen weight which may be mainly due to BW discrepancy between male and female chicks.
In poultry, developmental events important for immuno-competence are initiated during the embryonic period and continue in the early weeks following hatching (Rudrappa and Humphrey, 2007). In this context, the protective role of maternal antibodies is of interest due to the precocial nature of chicken (Friedman et al., 2012). These maternal antibodies are provided during the process of egg formation and continue to function in the hatchling until its own immune response can take over (Friedman et al., 2012). We demonstrated that feeding YB to BB increased the level of IgA in the egg yolk indicating that manipulation of breeder diets could be used to enrich hatching eggs with antibodies. Eimeria challenge significantly decreased pancreas weight, damaged the intestinal tract, deteriorated jejunal histomorphology, stimulated immune response, and resulted in decreased growth performance of chicks. Feeding YB to BB and to the chicks improved their jejunal histomorphology independent of Eimeria challenge. It is well known the quality of hatching eggs and therefore chick quality is dependent on BB age (Gous et al., 2010). Therefore, the present study was limited to a proof of concept as it only captured a short duration of BB hatching egg production cycle and one dose of YB. Future investigations should focus on the impact of different YB doses during hatching eggs production cycle and subsequent impact on progeny growth performance, health, livability, and meat yield. Such data would facilitate recommendations for commercial application.
ACKNOWLEDGMENTS
Supported by Ontario Agri-Food Innovation Alliance, Natural Sciences and Engineering Research Council of Canada, and Canadian Bio-Systems Inc. Assistance of Mohsen Mohammadigheisar, Conor Voth, Tanka Khanal, Sara Robinson, Juan Sanchez, and Emily Kim appreciated. Our appreciation is extended to Dr John Barta and his lab for the Eimeria culture and lesion scoring. Z. Lu was a recipient of a full scholarship from Nanjing Agricultural University Graduate International Academic Exchange Fund.
REFERENCES
- Akbari Moghaddam Kakhki R., Lu Z., Thanabalan A., Leung H., Mohammadigheisar M., Kiarie E.. 2019. Eimeria challenge adversely affected long bone attributes linked to increased resorption in 14-day-old broiler chickens. Poult. Sci. 98:1615–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh M., Munyaka P., Yitbarek A., Echeverry H., Rodriguez-Lecompte J. C.. 2016a. Maternal antibody decay and antibody-mediated immune responses in chicken pullets fed prebiotics and synbiotics. Poult. Sci. 96:58–64. [DOI] [PubMed] [Google Scholar]
- Alizadeh M., C. J., Rodriguez-Lecompte J. C., Yitbarek A., Sharif S., Crow G., Slominski B. A.. 2016b. Effect of yeast-derived products on systemic innate immune response of broiler chickens following a lipopolysaccharide challenge. Poult. Sci. 95:2266–2273. [DOI] [PubMed] [Google Scholar]
- Aviagen 2014. Ross 708 broiler: nutrients specification. p 10 Aviagen, Huntsville, AL. [Google Scholar]
- Aviagen 2018. Ross 708 broiler: parent stock management guidelines. p 188 Aviagen, Huntsville, AL. [Google Scholar]
- Bar-Shira E., Friedman A.. 2005. Expression of innate immune functions in developing broiler gut associated lymphoid tissue in the immediate pre and posthatch period. Poul. Sci. 84:27–27. [Google Scholar]
- Calder P. C., Krauss-Etschmann S., de Jong E. C., Dupont C., Frick J. S., Frokiaer H., Heinrich J., Garn H., Koletzko S., Lack G., Mattelio G., Renz H., Sangild P. T., Schrezenmeir J., Stulnig T. M., Thymann T., Wold A. E., Koletzko B.. 2006. Early nutrition and immunity - progress and perspectives. Br. J. Nutr. 96:774–790. [PubMed] [Google Scholar]
- CCAC 2009. The care and use of farm animals in research, teaching and testing. C. C. o. A. Care, O. Ottawa, Canada. [Google Scholar]
- Cervantes H. M. 2015. Antibiotic-free poultry production: is it sustainable. J. Appl. Poult. Res. 24:91–97. [Google Scholar]
- Chapman H. D. 2009. A landmark contribution to poultry science–prophylactic control of coccidiosis in poultry. Poult. Sci. 88:813–815. [DOI] [PubMed] [Google Scholar]
- Chapman H. D. 2014. Milestones in avian coccidiosis research: a review. Poult. Sci. 93:501–511. [DOI] [PubMed] [Google Scholar]
- Cook M. E. 2004. Antibodies: alternatives to antibiotics in improving growth and feed efficiency. J. Appl. Poult. Res. 13:106–119. [Google Scholar]
- Ferket P. R. 2012. Embryo epigenomic response to breeder management and abstract from a conference nutrition XXIV World's Poultry Congress. p 1–11, Salvador, Bahia, Brazil. [Google Scholar]
- Friedman A., Bar-Shira E., Sklan D.. 2003. Ontogeny of gut associated immune competence in the chick. World Poult. Sci. J. 59:209–219. [Google Scholar]
- Friedman A., Elad O., Cohen I., Bar Shira E.. 2012. The gut associated lymphoid system in the post-hatch chick: dynamics of maternal IgA. Isr. J. Vet. Med. 67:75–81. [Google Scholar]
- Gadde U., Kim W. H., Oh S. T., Lillehoj H. S.. 2017. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health Res. Rev. 18:26–45. [DOI] [PubMed] [Google Scholar]
- Gao J., Zhang H. J., Wu S. G., Yu S. H., Yoon I., Moore D., Gao Y., Yan H. J., Qi G. H.. 2009. Effect of Saccharomyces cerevisiae fermentation product on immune functions of broilers challenged with Eimeriatenella. Poult. Sci. 88:2141–2151. [DOI] [PubMed] [Google Scholar]
- Geyra A., Uni Z., Sklan D.. 2001. Enterocyte dynamics and mucosal development in the post-hatch chick. Poult. Sci. 80:776–782. [DOI] [PubMed] [Google Scholar]
- Gous R. M. 2010. Nutritional limitations on growth and development in poultry. Livest. Sci. 130:25–32 [Google Scholar]
- Hamal K. R., Burgess S. C., Pevzner I. Y., Erf G. F.. 2006. Maternal antibody transfer from dams to their egg yolks, egg whites, and chicks in meat lines of chickens. Poult. Sci. 85:1364–1372. [DOI] [PubMed] [Google Scholar]
- Huyghebaert G., Ducatelle R., Van Immerseel F.. 2011. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 187:182–188. [DOI] [PubMed] [Google Scholar]
- Jensen G. S., Patterson K. M., Yoon I.. 2008. Yeast culture has anti-inflammatory effects and specifically activates NK cells. Comp. Immunol. Microbiol. Infect. Dis. 31:487–500. [DOI] [PubMed] [Google Scholar]
- Johnson J., Reid W. M.. 1970. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 28:30–36. [DOI] [PubMed] [Google Scholar]
- Kiarie E., Romero L. F., Nyachoti C. M.. 2013. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr. Res. Rev. 26:71–88. [DOI] [PubMed] [Google Scholar]
- Kiarie E. G., Mills A.. 2019. Role of feed processing on gut health and function in pigs and poultry: conundrum of optimal particle size and hydrothermal regimens. Frontiers in Vet. Sci. 6:19. doi: 10.3389/fvets.2019.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Leung H., Akhtar N., Li J., Barta J. R., Wang Y., Yang C., Kiarie E.. 2017. Growth performance and gastrointestinal responses of broiler chickens fed corn-soybean meal diet without or with exogenous epidermal growth factor upon challenge with Eimeria. Poult. Sci. 96:3676–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasing K. C. 2007. Nutrition and the immune system. Br. Poult. Sci. 48:525–537. [DOI] [PubMed] [Google Scholar]
- Kogut M. H. 2017. Issues and consequences of using nutrition to modulate the avian immune response. J. Appl. Poult. Res. 26:605–612. [Google Scholar]
- Kogut M. H., Klasing K.. 2009. An immunologist's perspective on nutrition, immunity, and infectious diseases: introduction and overview. J. Appl. Poult. Res. 18:103–110. [Google Scholar]
- Leung H., patterson R., Barta J. R., Kiarie E.. 2019a. Performance, nutrient utilization and relative immune organ weights in broiler chickens fed corn-soybean meal diets without or with yeast nucleotides upon challenge with Eimeria. Poult. Sci. 10.3382/ps/pez213. [DOI] [Google Scholar]
- Leung H., A Yitbarek, Snyder R., Patterson R, Barta J. R., Karrow N., Kiarie E.. 2019b. Responses of broiler chickens to Eimeria challenge when fed a nucleotide-rich yeast extract. Poultry Sci. 98:1622–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja C. 1983. A comparative-study of postnatal-growth and organ development in some species of birds. Growth 47:317–339. [PubMed] [Google Scholar]
- Munyaka P. M., Echeverry H., A. Yitbarek, Camelo-Jaimes G., Sharif S., Guenter W., House J. D. J. D., Rodriguez-Lecompte J. C.. 2012. Local and systemic innate immunity in broiler chickens supplemented with yeast-derived carbohydrates. Poult. Sci. 91:2164–2172. [DOI] [PubMed] [Google Scholar]
- Murdoch B. M., Murdoch G. K., Greenwood S., McKay S.. 2016. Nutritional influence on epigenetic marks and effect on livestock production. Frontiers in Genet. 7:182. doi: 10.3389/fgene.2016.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewold T. A. 2007. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poult. Sci. 86:605–609. [DOI] [PubMed] [Google Scholar]
- Pimentel J. L., Cook M. E.. 1988. Improved growth in the progeny of hens immunized with jackbean urease1. Poult. Sci. 67:434–439. [DOI] [PubMed] [Google Scholar]
- Pimentel J. L., Cook M. E., Jonsson J. M.. 1991. Research note: increased growth of chicks and poults obtained from hens injected with jackbean urease1. Poult. Sci. 70:1842–1844. [DOI] [PubMed] [Google Scholar]
- Rahman M. M., Bari A. S. M., Giasuddin M., Islam M. R., Alam J., Sil G. C., Rahman M. M.. 2002. Evaluation of maternal and humoral immunity against Newcastle disease virus in chicken. Int. J. Poult. Sci. 1:161–163. [Google Scholar]
- Rose M. E., Orlans E., Buttress N.. 1974. Immunoglobulin classes in the hen's egg: their segregation in yolk and white. Euro. J. Immunol. 4:521–523. [DOI] [PubMed] [Google Scholar]
- Rudrappa S. G., Humphrey B. D.. 2007. Energy metabolism in developing chicken lymphocytes is altered during the embryonic to post-hatch transition. J. Nutr. 137:427–432. [DOI] [PubMed] [Google Scholar]
- Russell J. Jr., Ruff M. D.. 1978. Eimeria spp.: influence of coccidia on digestion (amylolytic activity) in broiler chickens. Exp. Parasitol. 45:234–240. [DOI] [PubMed] [Google Scholar]
- Stanley D., Geier M. S., Hughes R. J., Denman S. E., Moore R. J.. 2013. Highly variable microbiota development in the chicken gastrointestinal tract. Plos One 8:e84290 10.1371/journal.pone.0084290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tini M., Jewell U. R., Camenisch G., Chilov D., Gassmann M.. 2002. Generation and application of chicken egg-yolk antibodies. Comp. Biochem. Physiol. Part A: Mol. Integrat. Physiol. 131:569–574. [DOI] [PubMed] [Google Scholar]
- Ulmer-Franco A. M., Cherian G., Quezada N., Fasenko G. M., McMullen L. M.. 2012. Hatching egg and newly hatched chick yolk sac total IgY content at 3 broiler breeder flock ages. Poult. Sci. 91:758–764. [DOI] [PubMed] [Google Scholar]
- Uni Z., Ferket P. R., Tako E., Kedar O.. 2005. In ovo feeding improves energy status of late-term chicken embryos. Poult. Sci. 84:764–770. [DOI] [PubMed] [Google Scholar]
- Uni Z., Ferket R. P.. 2004. Methods for early nutrition and their potential. World Poult. Sci. J. 60:101–111. [Google Scholar]
- Uni Z., Noy Y., Sklan D.. 1999. Posthatch development of small intestinal function in the poult. Poult. Sci. 78:215–222. [DOI] [PubMed] [Google Scholar]
- Wijtten P. J. A., Langhout D. J., Verstegen M. W. A.. 2012. Small intestine development in chicks after hatch and in pigs around the time of weaning and its relation with nutrition: a review. Acta Agric. Scandinavica, Section A - Anim. Sci. 62:1–12. [Google Scholar]
- Williams R. B. 2005. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 34:159–180. [DOI] [PubMed] [Google Scholar]
- Yitbarek A., Rodriguez-Lecompte J. C., Echeverry H. M., Munyaka P. M., Barjesteh N., Sharif S., Camelo-Jaimes G.. 2013. Performance, histomorphology, and toll-like receptor, chemokine, and cytokine profile locally and systemically in broiler chickens fed diets supplemented with yeast-derived macromolecules. Poult. Sci. 92:2299–2310. [DOI] [PubMed] [Google Scholar]