ABSTRACT
Nutrition is a crucial factor for growth and bone development in broiler chickens. Adjustments in dietary ingredients might affect bone development and consequently locomotion related problems. This study was designed to evaluate effects of dietary organic minerals (ORM), fish oil (FISH), and hydrolyzed collagen (COL) on growth performance and tibia characteristics of broiler chickens. A total of three hundred eighty four 1-day-old Ross 308 male broiler chickens were used in a complete randomized block design with 4 diet groups and 8 replicates per diet group. In the ORM diet, the inorganic macro and trace minerals were replaced by their organic varieties. In the FISH diet, palm oil and soybean oil were partly replaced by FISH. In the COL diet, soybean meal was partly replaced by COL. Results showed that the ORM and COL diet groups reached a higher body weight (BW) at 42 D of age than the FISH diet group, whereas the control group was in between. The feed conversion ratio between day 1 and 42 was lower in the ORM and COL diet groups than in both other diet groups. On day 28, 35, and 42, gait score (GS), Varus Valgus deformity, tibia length (TL), thickness, femoral and metatarsal head thickness (THT), mineral content (TMC), mineral density (TMD), breaking strength (TBS), stiffness (TSF), and energy to fracture (TEF) were measured (n = 3/replicate). The ORM diet group had higher TL at day 42, higher THT at day 28, higher TMC at day 42, higher TMD at day 28, 35, and 42, higher TBS at day 42, higher TSF at day 35 and 42, and higher TEF at day 42 compared to the FISH diet group, with the COL and control diet groups in between. It can be concluded that replacing dietary inorganic macro and trace minerals by their organic varieties seems to stimulate tibia dimensions, strength, and mineral content of broiler chickens. On the contrary, FISH appears to negatively affect tibia characteristics.
Keywords: Organic minerals, fish oil, collagen, broiler chicken, tibia characteristics
INTRODUCTION
Leg problem is one of the most important factors affecting health and welfare of broiler chickens nowadays (EFSA, 2010). Knowles et al. (2008) demonstrated that over 27.6% of the broiler chickens in the UK suffer from poor locomotion and 3.3% were unable to walk, especially in the last 2 wk of the growing period, and this resulted in pain and inability to reach feed and water (Bessei, 2006; Gocsik et al., 2017). De Jong and Guémené (2011) showed that approximately 50% of broiler chickens in Dutch flocks suffered from locomotion related problems at slaughter age. Locomotion-related problems, i.e., weak bones or joints may result in broken bones, punctured skins, and/or damaged muscles, especially when handling the chickens during depopulation or when processing in the slaughter plant. Next to welfare problems, carcass quality and slaughter revenues can also be negatively affected by leg problems (Yalçin et al., 1998; Kestin et al., 1999; Mench, 2004).
Most of the locomotion-related research in broiler chickens focused on improving tibia bone development and its strength (Ruff and Hughes, 1985; Kim et al., 2006), because the tibia is the most loaded and affected (by, e.g., bone pathologies) leg bone during the growth period, probably influencing the locomotion of the broiler chickens (Julian, 1998; Dibner et al., 2007).
Various factors to reduce locomotion-related problems in broiler chickens have been studied, and nutritional approaches seem to be very promising (Kidd, 2003; Calini and Sirri, 2007). Specific ingredients or nutrients in broiler chicken diets might positively influence bone development, thereby reducing locomotion related problems in later life (Yalçin et al., 1998; Oviedo-Rondon et al., 2006). Among others, organic macro and trace minerals, lipid composition, and collagen has been shown or suggested to affect leg bone development. Replacing inorganic by organic macro and trace minerals in broiler diets has shown to improve intestinal absorption of those minerals (Wedekind et al., 1991; Burrell et al., 2004; Wang and Xu, 2008), resulting in greater bio-availability and higher bone mineralization in broiler chickens.
Fish oil (FISH) is containing n-6 polyunsaturated fatty acids (PUFA), which differently affect osteoblast function and bone mineralization than the common fat sources of broiler diets containing n-3 PUFA, such as palm oil, maize oil, and soy bean oil (Watkins et al., 1996, 2003), but effects on broiler chickens bone development is largely unknown.
Finally, supplementation with hydrolyzed collagen (COL) in mice diets resulted in a higher bone mineral content and bone mineral density, and a higher concentration of type I collagen and proteoglycans in the bone matrix (Wu et al., 2004; Nomura et al., 2005; Guillerminet et al., 2010, 2012). It can be speculated that COL in broiler chicken diets might stimulate bone development, but evidence for that is very limited.
The main objectives of this study were to investigate effects of dietary organic macro and trace minerals, FISH, and COL on: 1) growth performance; 2) tibia characteristics; 3) locomotion; 4) leg disorders, and 5) bone development related blood parameters of broiler chickens.
MATERIALS AND METHODS
Experimental Design
A total of 4 dietary groups, including 1 control diet (CON) and 3 modified diets (FISH, COL, and organic minerals (ORM)) were compared. Each diet group was replicated 8 times. A total of 32 experimental pens within a complete randomized block design were used. Within each block of 4 pens, diet groups were randomly distributed. Pen was used as the experimental unit and each pen contained 12 male broiler chickens.
Animals and Experimental Procedures
The experiment was conducted at the Animal Sciences Department of Wageningen University and Research, Wageningen, The Netherlands. All procedures in this study were approved by the Central Commission Animal Experiments, The Hague, The Netherlands; approval number: 2016.D-0138.001.
A total of 384 one-day-old Ross 308 male broiler chickens from a 38-week-old breeder flock were obtained from a commercial hatchery (Lagerwey, Lunteren, The Netherlands). Chickens were vaccinated against infectious bronchitis (eye drop; MSD Animal Health, Boxmeer, The Netherlands) upon arrival at the research facility and against Newcastle disease (Nobilis ND Clone 30; eye drop; MSD Animal Health, Boxmeer, The Netherlands) at day 11 of age. Upon arrival at day 0, all chickens were individually weighed, wing-tagged, and randomly assigned to 32 pens in a climate-controlled room. Temperature was maintained at 32°C until day 3 and thereafter gradually reduced to 22°C at day 42. A continuous light program from arrival to day 3 and a 16L:8D light program from day 4 to 42 was applied. Chickens were raised from arrival to day 42 with ad libitum access to feed and water.
Experimental Diets
A 3-phase feeding program was applied; starter diets were provided from day 0 to 10, grower diets from day 11 to 28, and finisher diets from day 29 to 42. Dietary treatments were applied throughout all 3 phases. Four experimental diets were used in this experiment. They are as follows: 1) CON, 2) replacement of inorganic by organic macro (Ca, P) and trace minerals (I, Cu, Mn, Zn, Se; ORM group; ORM; full replacement was done, without changing the mineral level), 3) partly replacement of palm oil and soybean oil by FISH (fish oil group; FISH), and 4) partly replacement of soybean meal by COL (collagen group; COL). In the ORM diet, the inorganic macro minerals Ca and P, provided by limestone and monocalcium phosphate were partly replaced by Calfos (Darling Ingredients Inc., Eindhoven, The Netherlands), an organic Ca and P source originating from processed bones. This was done for 70.9% in the starter diet, for 59.6% in the grower diet, and for 44.7% in the finisher diet. The trace mineral premix with in ORM was completely replaced by a complete organic sourced trace mineral premix (Optimin, Trouw Nutrition, Tilburg, The Netherlands). In the FISH diet, palm oil and soybean oil were partly replaced by FISH (100% in starter diet, 86.5% in grower diet, 98.4% in finisher diet; Trouw Nutrition, Tilburg, The Netherlands). Fish oil content was 39.4 g/kg in the starter diet and 50 g/kg in the grower and finisher diet. In the COL diet, soybean meal was partly (10% in starter diet, 9.2% in grower diet, 11% in finisher diet) replaced by COL Hydro-P (Darling Ingredients Inc., Eindhoven, The Netherlands), originating from pigs. Collagen content was 25 g/kg in starter, grower, and finisher diet. All diets were produced and pelleted by Research Diet Services (Wijk bij Duurstede, The Netherlands) and analyzed for ash (ISO5984), dry matter (ISO6496), crude fibre (ISO6865), crude fat (ISO6492), crude protein (ISO5983), P (ISO6941), and Ca (ISO 6869). CON and ORM diets were analyzed for Fe, Cu, Mn, Zn, and Se. CON and FISH diet were analyzed for fatty acid composition, using the method described by Khan et al. (2009). Diet compositions and calculated and analyzed nutrient values are shown in Table 1.
Table 1.
Composition (%), calculated and analyzed nutrients of the experimental diets (g/kg, as-fed basis).
| Starter (0 to 10 D) | Grower (11 to 28 D) | Finisher (29 to 42 D) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ingredients | CON | FISH | COL | ORM | CON | FISH | COL | ORM | CON | FISH | COL | ORM |
| Corn | 47.00 | 47.00 | 47.00 | 47.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 |
| Soybean meal | 26.65 | 26.65 | 24.78 | 26.65 | 27.45 | 27.45 | 24.68 | 27.45 | 23.22 | 23.22 | 20.25 | 23.22 |
| Wheat | 14.92 | 14.92 | 14.92 | 14.92 | 15.51 | 15.51 | 15.51 | 15.51 | 21.27 | 21.27 | 21.27 | 21.27 |
| Potato protein | 2.50 | 2.50 | 2.50 | 2.50 | 2.20 | 2.20 | 2.20 | 2.20 | 2.23 | 2.23 | 2.23 | 2.23 |
| Soybean oil | 2.00 | – | 2.01 | 2.00 | 3.28 | 0.19 | 3.01 | 3.28 | 3.50 | 0.08 | 3.50 | 3.50 |
| Palm oil | 2.00 | – | 1.47 | 2.00 | 2.50 | 0.59 | 2.50 | 2.50 | 1.58 | – | 1.33 | 1.58 |
| Limestone | 1.61 | 1.61 | 1.61 | 0.69 | 1.33 | 1.33 | 1.34 | 0.74 | 1.05 | 1.05 | 1.06 | 0.63 |
| Monocalcium phosphate | 1.05 | 1.05 | 1.07 | – | 0.69 | 0.69 | 0.71 | – | 0.49 | 0.49 | 0.49 | – |
| Sodium bicarbonate | 0.42 | 0.42 | 0.34 | 0.42 | 0.34 | 0.34 | 0.28 | 0.34 | 0.34 | 0.34 | 0.34 | 0.34 |
| L-Threonine | 0.10 | 0.10 | 0.10 | 0.10 | 0.05 | 0.05 | 0.05 | 0.05 | 0.04 | 0.04 | 0.04 | 0.04 |
| L-Lysine HCL | 0.30 | 0.30 | 0.24 | 0.30 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| L-tryptophan | – | – | 0.01 | – | – | – | 0.01 | – | – | – | 0.01 | – |
| L-isoleucine | – | – | 0.01 | – | – | – | 0.02 | – | – | – | 0.03 | |
| DL-methionine | 0.30 | 0.30 | 0.30 | 0.30 | 0.26 | 0.26 | 0.27 | 0.26 | 0.23 | 0.23 | 0.23 | 0.23 |
| Phytase1 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Salt | 0.05 | 0.05 | 0.06 | 0.05 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 |
| Rovabio excel AP | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Diamol | – | – | – | 0.29 | – | – | 0.55 | 0.19 | – | – | 0.71 | 0.13 |
| Fish oil | – | 4.00 | – | – | – | 5.00 | – | – | – | 5.00 | – | – |
| Inorganic minerals2 | 0.50 | 0.50 | 0.50 | – | 0.50 | 0.50 | 0.50 | – | 0.50 | 0.50 | 0.50 | – |
| Organic minerals3 | – | – | – | 0.50 | – | – | – | 0.50 | – | – | – | 0.50 |
| Calfos4 | – | – | – | 1.68 | – | – | – | 1.09 | – | – | – | 0.78 |
| Hydrolyzed collagen5 | – | – | 2.50 | – | – | – | 2.50 | – | – | – | 2.50 | – |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Calculated nutrients | ||||||||||||
| AME (kcal/kg) | 2891.9 | 2894.3 | 2889.5 | 2891.8 | 2987.5 | 2987.5 | 2987.5 | 2987.5 | 3011.4 | 3009.1 | 3011.4 | 3011.4 |
| Dry matter | 882.19 | 882.17 | 883.34 | 881.94 | 883.25 | 883.25 | 885.53 | 883.09 | 881.50 | 881.47 | 884.03 | 881.38 |
| Ash | 57.78 | 57.86 | 56.61 | 54.51 | 51.39 | 51.42 | 55.28 | 49.28 | 44.57 | 44.61 | 50.05 | 43.06 |
| Crude protein | 205.70 | 205.75 | 218.96 | 205.75 | 204.59 | 204.57 | 214.18 | 204.57 | 190.52 | 190.52 | 199.22 | 190.52 |
| Crude fat | 64.87 | 64.3 | 59.40 | 64.86 | 82.04 | 81.98 | 78.95 | 82.05 | 74.98 | 74.92 | 72.06 | 74.98 |
| Crude fibre | 24.23 | 24.23 | 23.55 | 24.23 | 24.21 | 24.21 | 23.19 | 24.21 | 23.87 | 23.87 | 22.78 | 23.87 |
| Starch | 383.59 | 383.56 | 383.56 | 383.56 | 374.53 | 374.55 | 374.52 | 374.55 | 407.19 | 407.19 | 407.18 | 407.19 |
| Sugar | 38.94 | 38.94 | 36.93 | 38.94 | 36.69 | 36.69 | 36.71 | 36.69 | 36.53 | 36.53 | 33.34 | 36.53 |
| Ca | 9.20 | 9.21 | 9.20 | 9.21 | 7.59 | 7.59 | 7.60 | 7.60 | 5.88 | 5.88 | 5.91 | 5.91 |
| P | 5.89 | 5.88 | 5.83 | 5.84 | 5.07 | 5.07 | 4.96 | 5.03 | 4.51 | 4.51 | 4.41 | 4.48 |
| K | 8.31 | 8.31 | 7.96 | 8.31 | 8.44 | 8.45 | 7.89 | 8.44 | 7.69 | 7.69 | 7.09 | 7.69 |
| Na | 1.40 | 1.42 | 1.40 | 1.40 | 1.31 | 1.33 | 1.31 | 1.31 | 1.31 | 1.32 | 1.30 | 1.31 |
| Cl | 1.50 | 1.53 | 1.54 | 1.53 | 1.50 | 1.49 | 1.51 | 1.51 | 1.50 | 1.50 | 1.52 | 1.51 |
| LYS | 13.21 | 13.24 | 13.37 | 13.24 | 12.50 | 12.47 | 12.66 | 12.47 | 11.40 | 11.40 | 11.53 | 11.40 |
| dLYSp | 11.50 | 11.53 | 11.50 | 11.53 | 10.80 | 10.77 | 10.83 | 10.77 | 9.87 | 9.87 | 9.87 | 9.87 |
| dMETp | 5.84 | 5.86 | 5.93 | 5.84 | 5.44 | 5.44 | 5.57 | 5.44 | 5.00 | 5.00 | 5.11 | 5.00 |
| dM+Cp | 8.51 | 8.54 | 8.51 | 8.51 | 8.11 | 8.12 | 8.10 | 8.11 | 7.55 | 7.56 | 7.52 | 7.55 |
| dTHRp | 7.50 | 7.52 | 7.50 | 7.52 | 7.00 | 7.00 | 7.02 | 7.00 | 6.41 | 6.41 | 6.40 | 6.41 |
| dTRPp | 2.07 | 2.06 | 2.07 | 2.07 | 2.07 | 2.07 | 2.07 | 2.07 | 1.91 | 1.91 | 1.91 | 1.91 |
| dILEp | 7.60 | 7.60 | 7.60 | 7.60 | 7.60 | 7.60 | 7.60 | 7.60 | 7.00 | 7.00 | 7.00 | 7.00 |
| dARGp | 11.50 | 11.50 | 12.78 | 11.50 | 11.59 | 11.59 | 12.58 | 11.59 | 10.55 | 10.55 | 11.48 | 10.55 |
| dHISp | 4.65 | 4.65 | 4.65 | 4.65 | 4.66 | 4.66 | 4.56 | 4.66 | 4.32 | 4.32 | 4.20 | 4.32 |
| dVALp | 8.42 | 8.43 | 8.70 | 8.43 | 8.40 | 8.40 | 8.50 | 8.40 | 7.82 | 7.82 | 7.88 | 7.82 |
| dGLYp | 6.81 | 6.81 | 11.08 | 6.81 | 6.81 | 6.81 | 10.93 | 6.81 | 6.33 | 6.33 | 10.42 | 6.33 |
| dSERp | 8.72 | 8.72 | 9.09 | 8.72 | 8.73 | 8.73 | 8.91 | 8.73 | 8.12 | 8.12 | 8.26 | 8.12 |
| C18:2 | 23.65 | 12.01 | 23.02 | 23.65 | 30.40 | 13.43 | 28.84 | 30.40 | 30.61 | 12.28 | 30.16 | 30.61 |
| C18:3 | 2.04 | 3.49 | 2.02 | 2.04 | 3.03 | 4.41 | 2.79 | 3.03 | 3.16 | 4.30 | 3.12 | 3.16 |
| Retainable phosphorus | 4.40 | 4.40 | 4.40 | 4.40 | 3.71 | 3.71 | 3.69 | 3.70 | 2.80 | 2.80 | 2.80 | 2.80 |
| NSP | 141.24 | 141.24 | 137.88 | 141.24 | 141.13 | 141.13 | 136.02 | 141.13 | 138.53 | 138.53 | 133.03 | 138.53 |
| Electrolyte balance (mEq) | 231.05 | 231.35 | 221.05 | 230.25 | 230.76 | 231.69 | 216.45 | 230.57 | 211.12 | 211.91 | 195.41 | 210.83 |
| Analyzed nutrients | ||||||||||||
| Dry matter | 877.00 | 877.70 | 880.80 | 877.70 | 881.40 | 880.00 | 882.00 | 880.20 | 878.10 | 878.00 | 879.00 | 877.70 |
| Crude protein | 205.00 | 209.50 | 219.10 | 205.00 | 206.50 | 208.60 | 218.30 | 207.40 | 190.30 | 196.80 | 203.10 | 192.60 |
| Crude fat | 64.00 | 65.00 | 56.20 | 63.80 | 80.30 | 80.70 | 74.30 | 79.40 | 72.10 | 75.50 | 71.40 | 72.80 |
| Crude fiber | 28.40 | 28.00 | 29.00 | 29.30 | 28.60 | 26.60 | 25.90 | 26.90 | 26.20 | 23.40 | 25.80 | 25.10 |
| Ca | 8.60 | 8.40 | 8.50 | 8.50 | 7.20 | 7.10 | 7.20 | 6.90 | 5.60 | 5.80 | 5.80 | 5.50 |
| P | 5.80 | 5.60 | 5.80 | 5.80 | 5.10 | 5.00 | 4.90 | 5.00 | 4.40 | 4.30 | 4.40 | 4.50 |
| I | 0.35 | – | – | 0.38 | 0.32 | – | – | 0.22 | 0.28 | – | – | 0.24 |
| Cu | 0.15 | – | – | 0.13 | 0.13 | – | – | 0.14 | 0.12 | – | – | 0.15 |
| Mn | 0.10 | – | – | 0.11 | 0.09 | – | – | 0.11 | 0.11 | – | – | 0.09 |
| Zn | 0.09 | – | – | 0.10 | 0.09 | – | – | 0.10 | 0.09 | – | – | 0.09 |
| Se | 0.02 | – | – | 0.02 | 0.02 | – | – | 0.02 | 0.02 | – | 0.03 | |
Phytase provided up to 0.2% inclusion level: 245 g/kg rP and 103 g/kg Ca; between 0.2 and 0.6% inclusion level: 140 g/kg rP and 68 g/kg Ca.
Composition of inorganic premix provided per kg of diet: 12,000 IU vitamin A (source of vitamin A), 2,400 IU vitamin D3, 30 IU vitamin E (source of vitamin E), 1.5 mg vitamin K3, 2 mg vitamin B1, 7.5 mg vitamin B2, 10 mg d-pantothenic acid, 35 mg niacin amide, 200 μg biotin, 20 μg vitamin B12, 1 mg folic acid, 3.5 mg vitamin B6, 461 mg choline chloride, 80 mg Fe (as FeSO4⋅H2O), 12 mg Cu (as CuSO4⋅5H2O), 60 mg Zn (as ZnSO4⋅H2O), 85 mg Mn (as MnO), 0.4 mg Co (as CoSO4⋅7H2O), 0.8 mg I (as KI), 0.1 mg Se (as Na2SeO3⋅5H2O) and 50 mg anti-oxidant.
Composition of organic premix provided per kg of diet: 12,000 IU vitamin A (source of vitamin A), 2,400 IU vitamin D3, 30 IU vitamin E (source of vitamin E), 1.5 mg vitamin K3, 2 mg vitamin B1, 7.5 mg vitamin B2, 10 mg d-pantothenic acid, 35 mg niacin amide, 200 μg biotin, 20 μg vitamin B12, 1 mg folic acid, 3.5 mg vitamin B6, 461 mg choline chloride, 80 mg Fe (as Fe proteinate), 12 mg Cu (as Cu proteinate), 60 mg Zn (as Zn proteinate), 85 mg Mn (as Mn proteinate), 0.4 mg Co (as CoSO4⋅7H2O), 0.8 mg I (as KI), 0.1 mg Se (as Se selenite) and 50 mg anti-oxidant.
Composition of Calfos provided per kg of product: 100 g crude protein, 300 g calcium, 130 g phosphorus (113 g digestible phosphorus), 50 g moisture.
Composition of hydrolyzed collagen per kg of product: 920 g protein, 5 gr total fat, 50 gr moisture.
Data Collection, Sampling, and Measurements
All chickens were individually weighed on day 0, 10, 21, 28, 35, and 42. Feed intake (FI) was measured per pen for the starter, grower, and finisher period. Feed conversion ratios were calculated for all the three phases and over the whole growth period. Feed conversion ratio over the whole rearing period was also corrected to a body weight (BW) of 3,500 g corrected feed conversion ratio (CFCR) using following formula:
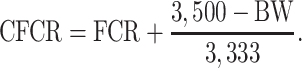 |
Mortality was determined per pen on daily basis. Gait score (GS) was evaluated in 2 randomly chosen chickens per pen, using the method of Kestin et al. (1992) on day 27, 34, and 41 and scored within a range of 0 (normal locomotion) to 5 (unable to stand). To investigate whether the used diets might affect home pen behavior, observations were performed on day 6, 13, 20, 27, 34, and 41 with morning and afternoon sessions using the scan sampling technique. During 6 to 8 min per day per pen, the number of chickens performing the following activities was scored: eating, drinking, walking, standing, resting, foraging, comfort behaviour, dust bathing, and perching.
On day 28, 35, and 42, three chickens per pen were randomly selected and sacrificed by cervical dislocation. At day 42, immediately after sacrificing, chickens were decapitated and a blood sample (10 mL) was obtained. Blood samples were centrifuged at 750 g for 10 min to obtain serum. Serum of the 3 samples per pen were pooled (1.5 mL) and frozen at −20°C until further analysis. Serum contents of Ca, P, and alkaline phosphatase (ALP; related to bone mineralization; Orimo, 2010) were analyzed photo-metrically, using a Cobas c701 analyser (Roche, Basel, Switzerland). Serum vitamin D3 (a major Ca and P regulator; Van Leeuwen et al., 2001) was analyzed using a 1,25-dihydroxyvitamin D3 ELISA Kit (Elabscience, Houston, Texas, United States), and serum parathyroid hormone (PTH a developmental regulator in cartilage and bone; Martin, 2016) was analyzed using a chicken PTH Kit (Elabscience, Houston, Texas, United States).
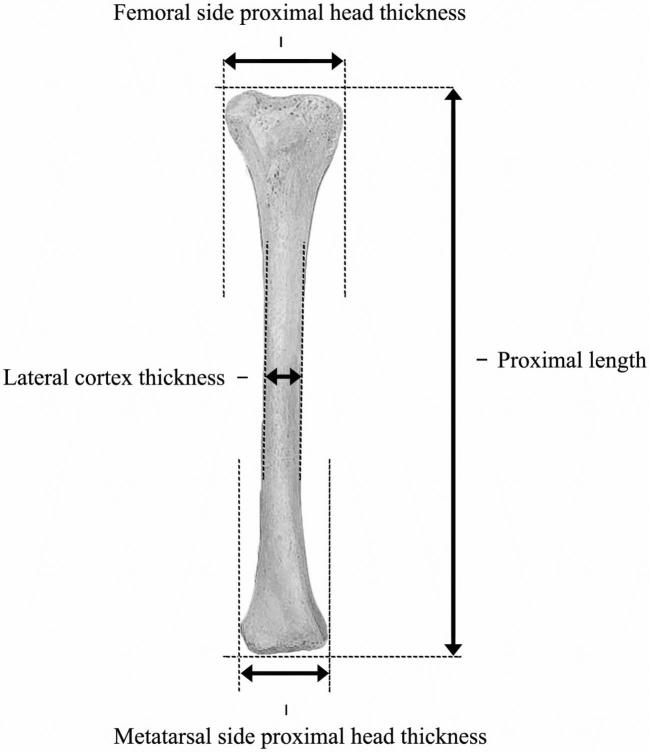
The 3 slaughtered chickens per pen were checked on Varus Valgus deformity by a veterinarian at each of three slaughtering days (day 28, 35, 42). Varus Valgus deformity was scored as present or not and each (small) deviation from normal was scored as present. Both tibia bones were collected, deboned, and frozen at −20°C. After thawing, proximal length (cm), lateral cortex thickness (cm), and proximal head thickness (cm) at both the femoral and metatarsal side were measured using a digital calliper (Figure 1). Tibia mineral content (TMC) (g) and tibia mineral density (TMD) (g/cm2) were analyzed using a dual-energy X-ray absorptiometry machine (Horizon DEXA System by Hologic, Tromp Medical, Castricum, The Netherlands) using pooled samples (2 pens or 6 tibias of the same group per scan).
Figure 1.
Morphological measurements of proximal length, lateral cortex thickness, femoral, and metatarsal side bone head thickness on tibia (cm).
Tibia bones were subjected to a three-point bending test (method described by Jungmann et al., 2007) using an Instron electromechanical universal testing machine (Instron, Norwood, Massachusetts, United States). Maximum load to break the tibia (N), tibia stiffness (TSF) (N/mm), and total energy to fracture (TEF) (N-mm) were calculated, where TSF (the slope of the linear part of the curve, N/mm) = dx/dy, where dx is the initial point (a) from x-axis (N), dy is the ultimate point (b) from y-axis (mm), and TEF (the area under the curve, N-mm) = 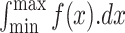 , where f (x) is the curve of y-axis, dx is linear integral equation (Figure 2).
, where f (x) is the curve of y-axis, dx is linear integral equation (Figure 2).
Figure 2.
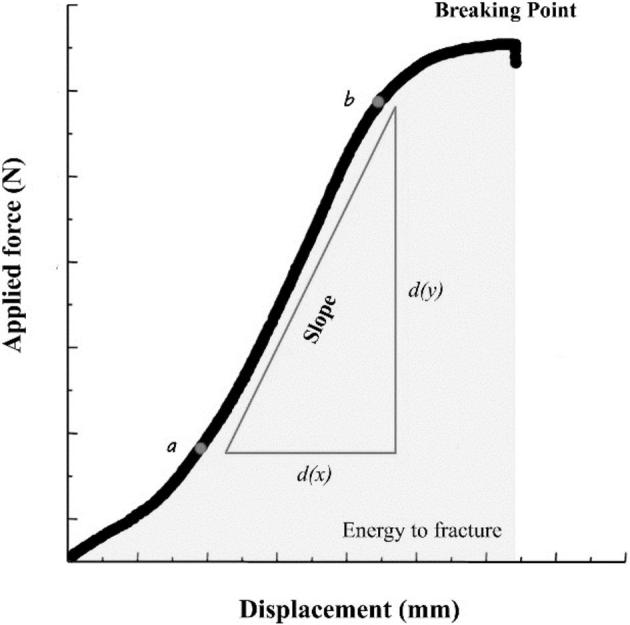
Demonstration of breaking strength (breaking point, N), stiffness (the slope of the linear part of the curve, N/mm), and energy to fracture (the area under the curve, N-mm) during applied force on tibia by Instron testing machine.
Statistical Analysis
All growth performance data (body weight, FI, feed conversion ratio, mortality), tibia characteristics (proximal length, lateral cortex thickness, proximal head thickness, TMC, TMD, breaking strength (TBS), TSF, and TEF), blood parameters (Ca, P, PTH, ALP, and 1.25-dihydroxy vitamin D3), and locomotion-related observations (home pen behavior and GS) were subjected to mixed model analysis using the PROC MIXED procedure. In one pen of the CON diet, 3 female chickens were present and this pen was removed from the experiment for all analyses. Pen was used as the experimental unit, except for TMC and TMD, where the combination of 2 pens was used as the experimental unit.
The overall statistical model used was
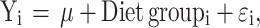 |
where Yi = the dependent variable, µ is overall mean, Diet group = Dietary group (i = CON, ORM diet, FISH diet, collagen diet), and Ɛ = the residual error term.
Varus Valgus deformity was subjected to generalized linear mixed model analysis, using the PROC GLIMMIX procedure in SAS, using same model (Version 9.4, July 2013, SAS Institute Inc., Cary, North Carolina, US). Block was used as a random effect. BW was added to the model as a covariable for tibia characteristics. Distribution of the means and residuals were examined to verify model assumptions. Results are presented as LSmeans ± SEM. When multiple comparisons were performed, the level of significance was corrected, using Bonferroni. Effects were considered to be significant when P ≤ 0.05.
RESULTS
Growth Performance
All growth performance parameters are presented in Table 2. At day 10, the FISH diet group had a lower BW compared to the other diet groups (Δ = 12 to 18 g, P = 0.001). At day 42, the COL and ORM diet groups had a higher BW than the FISH diet group (Δ = 107 to 138 g; P = 0.026), with the CON diet group in between and not different from the other diet groups. At the other measuring days, no difference in BW was observed between diet groups. Average daily feed intake was not influenced by diet group throughout the experiment. Chickens of the COL and ORM diet groups had a lower feed conversion ratio (FCR) during the finisher diet phase (day 29 to 42, Δ = 0.09 to 0.12; P = 0.041) and throughout the experiment (day 0 to 42, Δ = 0.06 to 0.09; P = 0.024) compared to the FISH and CON diet groups. No significant differences in FCR were found during the starter and grower phase. Mortality was low and not influenced by diet groups (1.30% on average).
Table 2.
Body weight, feed intake, feed conversion ratio, and mortality of broiler chickens fed a control, fish oil, collagen, and organic minerals diet.
| Measurement | Control | Fish Oil | Collagen | Organic Minerals | SEM | P value |
|---|---|---|---|---|---|---|
| n (pens) | 7 | 8 | 8 | 8 | ||
| Body weight (g) | ||||||
| 0 D | 48 | 48 | 48 | 48 | 1 | 0.967 |
| 10 D | 248a | 236b | 254a | 254a | 3 | 0.001 |
| 21 D | 983 | 957 | 975 | 988 | 15 | 0.445 |
| 28 D | 1,706 | 1,655 | 1,724 | 1,724 | 27 | 0.238 |
| 35 D | 2,618 | 2,593 | 2,647 | 2,683 | 30 | 0.208 |
| 42 D | 3,574a,b | 3,497b | 3,635a | 3,604a | 33 | 0.026 |
| Feed intake (g) | ||||||
| 0 to 10 D | 240 | 243 | 241 | 246 | 7 | 0.909 |
| 11 to 28 D | 1,957 | 1,953 | 1,956 | 1,934 | 24 | 0.853 |
| 29 to 42 D | 2,901 | 2,813 | 2,891 | 2,845 | 42 | 0.633 |
| 0 to 42 D | 5,098 | 5,009 | 5,087 | 5,025 | 76 | 0.586 |
| Feed conversion ratio (FI/BWG) | ||||||
| 0 to 10 D | 1.55 | 1.53 | 1.51 | 1.51 | 0.04 | 0.897 |
| 11 to 28 D | 1.61 | 1.62 | 1.60 | 1.57 | 0.03 | 0.683 |
| 29 to 42 D | 1.64b | 1.63b | 1.61b | 1.54a | 0.03 | 0.035 |
| 0 to 42 D | 1.58b | 1.59b | 1.56b | 1.51a | 0.02 | 0.047 |
| Corrected feed conversion ratio (FI/BWG) | ||||||
| 0 to 42 D | 1.56b | 1.55b | 1.54b | 1.48a | 0.01 | 0.024 |
| Mortality (%) | 1.04 | 1.04 | 2.08 | 1.04 | 0.01 | 0.887 |
Values within a row, lacking a common superscript differ (P ≤ 0.05).
Locomotion-Related Observations and Bone Development Related Blood Parameters
Home pen behavior parameters (eating, drinking, walking, standing, resting, foraging, comfort behavior, dust bathing, and perching) for each of the scanning days (day 6, 13, 20, 27, 34, and 41) are presented in Table A1 in the Appendix. Locomotion-related observation parameters (GS and TR) are presented in Table 3. No significant differences between diet groups were found for any of these parameters throughout the experiment. Age based differences were found in eating (P = 0.04), drinking (P = 0.003), walking (P = 0.003), standing (P = 0.004), resting (P < .0001), and foraging (P = 0.007) behaviors. Blood parameters (Ca, P, ALP, PTH, and 1,25-dihydroxyvitamin D3) are presented in Table 4. No significant differences between diet groups were found for any of these blood parameters.
Table 3.
Locomotion-related observations (gait score and varus Valgus deformity) of broiler chickens fed a control, fish oil, collagen, and organic mineral diet.
| Measurement | Control | Fish oil | Collagen | Organic minerals | SEM | P value |
|---|---|---|---|---|---|---|
| n (pens) | 7 | 8 | 8 | 8 | ||
| Gait score (score 1 to 5)1 | ||||||
| 27 D | 2.0 | 2.0 | 2.0 | 2.0 | -2 | -2 |
| 34 D | 2.47 | 2.33 | 2.34 | 2.22 | 0.02 | 0.254 |
| 41 D | 2.61 | 2.66 | 2.58 | 2.48 | 0.03 | 0.125 |
| Varus Valgus deformity (%3) | ||||||
| 27 D | 21 | 17 | 17 | 17 | 0.07 | 0.511 |
| 34 D | 58 | 66 | 58 | 45 | 0.09 | 0.083 |
| 41 D | 66 | 70 | 66 | 63 | 0.06 | 0.114 |
Method of Kestin et al. (1992), scored within a range of 0 (normal locomotion) to 5 (unable to stand).
Due to same scores in each diet group, SEM and P values were not calculated.
The percentage of chickens per diet group.
Table 4.
Blood parameters of broiler chickens at 42 D of age fed a control, fish oil, collagen, or organic mineral diet.
| Measurement | Control | Fish oil | Collagen | Organic minerals | SEM | P value |
|---|---|---|---|---|---|---|
| n (pens) | 7 | 8 | 8 | 8 | ||
| Calcium (mmol/L) | 2.60 | 2.51 | 2.58 | 2.64 | 0.05 | 0.506 |
| Phosphorus (mmol/L) | 1.83 | 1.91 | 1.86 | 1.82 | 0.03 | 0.242 |
| Parathyroid hormone (pg/mL) | 331.3 | 371.3 | 385.8 | 483.9 | 46.5 | 0.900 |
| Alkaline phosphatase (U/L) | 1134.9 | 1115.2 | 1137.2 | 1204.7 | 73.3 | 0.860 |
| 1,25-dihydroxyvitamin D3 (pg/mL) | 463.2 | 468.3 | 493.3 | 502.1 | 37.6 | 0.931 |
Tibia Characteristics
Tibia characteristics are presented in Table 5. At day 28, the femoral side of the proximal tibia head was thicker in ORM diet group compared to the FISH diet group (Δ = 0.11 cm; P = 0.047), with both other diet groups in between and not different from the ORM and FISH diet groups. Additionally, at day 28, TMD was higher in the ORM diet group than the other three diet groups (Δ = 0.09 to 0.17 g/cm2; P = 0.039).
Table 5.
Tibia characteristics of broiler chickens at 28, 35, and 42 D of age fed a control, fish oil, collagen, or organic mineral diet.
| Measurement | Control | Fish oil | Collagen | Organic minerals | SEM | P value |
|---|---|---|---|---|---|---|
| n (pens) | 7 | 8 | 8 | 8 | ||
| Tibia proximal length (cm) | ||||||
| 28D | 8.3 | 8.3 | 8.4 | 8.4 | 0.02 | 0.233 |
| 35 D | 10.5 | 10.5 | 10.6 | 10.6 | 0.04 | 0.263 |
| 42 D | 13.7ab | 13.4b | 13.9a | 14.0a | 0.09 | 0.012 |
| Tibia lateral cortex thickness (cm) | ||||||
| 28 D | 0.51 | 0.44 | 0.52 | 0.57 | 0.003 | 0.052 |
| 35 D | 0.84 | 0.83 | 0.84 | 0.85 | 0.002 | 0.996 |
| 42 D | 1.22 | 1.21 | 1.23 | 1.25 | 0.002 | 0.747 |
| Tibia proximal bone head thickness—femoral side (cm) | ||||||
| 28 D | 1.58ab | 1.54b | 1.59ab | 1.65a | 0.02 | 0.047 |
| 35 D | 2.54 | 2.52 | 2.54 | 2.55 | 0.03 | 0.884 |
| 42 D | 3.24 | 3.23 | 3.24 | 3.26 | 0.03 | 0.902 |
| Tibia proximal bone head thickness—metatarsal side (cm) | ||||||
| 28 D | 1.27 | 1.26 | 1.28 | 1.34 | 0.03 | 0.268 |
| 35 D | 2.22 | 2.22 | 2.24 | 2.25 | 0.02 | 0.887 |
| 42 D | 3.01 | 2.99 | 2.99 | 3.05 | 0.03 | 0.635 |
| Tibia mineral content1 (g) | ||||||
| 28 D | 7.58 | 7.57 | 7.70 | 7.89 | 0.17 | 0.469 |
| 35 D | 12.98 | 12.68 | 13.53 | 15.48 | 0.83 | 0.168 |
| 42 D | 17.32b | 17.00b | 15.77b | 20.79a | 0.84 | 0.013 |
| Tibia mineral density1 (g/cm2) | ||||||
| 28 D | 0.141b | 0.133b | 0.140b | 0.150a | 0.003 | 0.039 |
| 35 D | 0.262b | 0.250b | 0.251b | 0.277a | 0.006 | 0.018 |
| 42 D | 0.275b | 0.276b | 0.274b | 0.339a | 0.010 | 0.010 |
| Tibia breaking strength (N) | ||||||
| 28 D | 287 | 278 | 287 | 295 | 2.77 | 0.070 |
| 35 D | 302 | 297 | 302 | 307 | 3.74 | 0.651 |
| 42 D | 297b | 296b | 300b | 327a | 3.96 | 0.001 |
| Tibia stiffness (N/mm) | ||||||
| 28 D | 257 | 251 | 257 | 264 | 3.41 | 0.067 |
| 35 D | 270ab | 263b | 264b | 275a | 3.35 | 0.039 |
| 42 D | 263b | 261b | 267ab | 284a | 4.91 | 0.011 |
| Tibia energy to fracture (N-mm) | ||||||
| 28 D | 279 | 273 | 280 | 287 | 3.51 | 0.066 |
| 35 D | 293 | 283 | 289 | 295 | 3.84 | 0.158 |
| 42 D | 290b | 287b | 295ab | 307a | 4.54 | 0.013 |
Values within a row, lacking a common superscript differ (P ≤ 0.05).
Two pens were combined and used as the experimental unit for mineral content and mineral density parameters.
At day 35, TMD was higher in the ORM diet group than in the other 3 diet groups (Δ = 0.15 to 0.27 g/cm2; P = 0.018). Furthermore, at day 35, TSF was higher in the ORM diet group than in the FISH and COL diet groups (Δ = 11 N/mm; P = 0.039), with the CON diet group in between and not different from the other three diet groups.
At day 42, chickens of the COL and ORM diet groups had longer proximal tibia compared to the FISH diet group (Δ = 0.5 to 0.6 cm; P = 0.012), while the CON diet group was in between and not different from the other diet groups. Tibia mineral content (Δ = 3.47 to 5.02 g; P = 0.013), TMD (Δ = 0.063 to 0.065 g/cm2; P = 0.010), and TBS (Δ = 28 to 32 N; P = 0.001) were higher in the ORM diet group than in the 3 other diet groups. Tibia stiffness (Δ = 21 to 23 N/mm; P = 0.011) and TEF (Δ = 17 to 20 N-mm; P = 0.013) were higher in the ORM diet group than the CON and FISH diet groups, with the COL diet group in between and not different from the other diet groups.
DISCUSSION
Growth Performance
The results of this study showed that replacement of inorganic sourced macro and trace minerals by their organic varieties, and replacement of soybean meal by COL in broiler chicken diets might stimulate growth performance. At day 42, the chickens of the COL and ORM diet groups had a higher BW compared to the FISH diet group. The findings of the ORM diet group are in line with previous studies that showed that dietary organic sourced Ca and P (Tahir et al., 2012) and Cu, Fe, Mn, Zn (Bao et al., 2007; Abdallah et al., 2009; Ao et al., 2017) resulted in higher growth performance than their inorganic varieties, because of their functions in numerous biochemical reactions, increased bio-availability, and less antagonistic impact among each other. It can be speculated whether or not an increase of inorganic minerals compared to the current guidelines will result in comparable effects as when ORM are used.
Since hardly any research has been performed on the effects of dietary COL and FISH on growth performance of broiler chickens, it requires further investigation to understand the mechanisms involved. Possible explanations for a higher BW result of the COL diet group might be the protein quality and digestibility of collagen. In chickens, mice and humans, COL or gelatin has been demonstrated to improve protein digestion and absorption of peptides, rich in proline, hydroxyproline, and glycine (Oesser et al., 1999; Iwai et al., 2005; Ohara et al., 2007). In addition, the higher BW of the COL diet group can probably be explained by the higher protein content of the COL diets. The FISH diet group had a lower BW than the COL and ORM diet groups. These findings were opposite as compared to other studies, where the dietary FISH resulted in higher BW and lower FCR of broiler chickens (Lopez-Ferrer et al., 1999; Schreiner et al., 2005). The lower BW of the FISH diet group might be explained by undesirable lipid peroxidation in feed.
Despite a higher BW in the ORM and COL diet groups compared to the FISH diet group, FI was similar for all diet groups. As a result of this, lower FCR values for ORM and COL diet groups throughout the growing period, specifically during the finisher diet phase were found. These results on FCR are in accordance with previous studies, stating that broiler chickens fed organic Ca, P, Cu, Fe, Mn, Zn had lower FCR levels (Nollet et al., 2007; Abdallah et al., 2009; Tahir et al., 2012; Oliveira et al., 2015; Ao et al., 2017) than broiler chickens fed inorganic mineral varieties, probably again due to the higher bio-availability of the ORM varieties.
Locomotion-Related Observations and Bone Development Related Blood Parameters
In general, fast-growing broiler chickens suffering from leg problems demonstrated by low locomotion activities and gait abnormalities (Lewis and Hurnik, 1990; Corr et al., 2003; Bessei, 2006; Kittelsen et al., 2017). Whether this can be influenced by diet composition cannot be concluded from the current study, because no influence of the used diet compositions was found for home pen behavior observations, GS, and TR. Whether the lack of effects of the used diet composition is indeed true or due to, e.g., the non-commercial setup (small pens; low stocking density) and consequently the good litter quality in our study needs further investigations. However, looking more into detail to the results of the GS and rotated tibia, the ORM diet group showed, not significantly, the best GS and the lowest prevalence of TR at day 34 and 41. This might suggest that ORM not only affecting tibia characteristics, but also result in better leg quality and locomotion.
Looking to the age based behavioral changes, we expected less walking and more resting toward to slaughter age. However, this was not found in the current study, but more or less the opposite was found, which might be explained by the low stocking density in the pen, particularly after the removal of chickens at day 28 and 34 for slaughtering.
Regarding bone related blood parameters, literature demonstrates ambiguous results. Organic sourced Zn and Mn in broiler diets resulted in a lower serum ALP level than inorganic varieties of Zn and Mn (Yuan et al., 2011). Higher serum Cu and Fe levels were found in another broiler study applying organic sourced Zn in diet of broiler chickens compared to inorganic Zn (Yalçinkaya et al., 2012). Organic sourced Zn in broiler chickens diet resulted in higher Ca content in plasma compared to CON containing inorganic sourced Zn (Salim et al., 2008). Dobrzanski et al. (2008) concluded that organic sourced Cu in laying hen diets resulted in higher Cu levels in blood compared to inorganic sourced Cu in diet. To our knowledge, no studies have been conducted to determine the relationship between dietary COL or FISH on bone related blood parameters in chickens. In the current study, serum Ca, P, ALP, vitamin D3, and PTH levels were not influenced by diet groups. Due to limited comparative studies and their inconsistent results regarding the effects of dietary organic macro and trace minerals, COL and FISH on blood parameters in poultry or other species, further research is needed.
Tibia Characteristics
Tibia morphological measurements, such as tibia proximal length, and lateral cortex thickness have been used as indicators of bone quality in poultry (Leblanc et al., 1986; Krupski and Tatara, 2007; Charuta et al., 2013). The results obtained in this study indicated that chickens of the ORM diet group had significantly longer tibia at day 42 and thicker femoral and metatarsal side tibia heads at day 28 compared to the FISH diet group. These findings are in agreement with previous studies, indicating the stimulative effects of organic sourced minerals on tibia morphologic characteristics. Guinotte et al. (1991) reported that organic sourced Ca in broiler chicken diets resulted in higher tibia proximal length and lateral cortex thickness. Scott et al. (1982) reported that lower bio-availability of Zn and Mn led to shorter tibia proximal length, shorter lateral cortex thickness, and malformations on tibia. A low concentration of Zn in broiler diets also resulted in shorter tibia length (TL), due to its role in bone development by mechanisms influencing longitudinal bone growth at the growth plate (Starcher et al., 1980; Wang et al., 2002; Oviedo-Rondón et al., 2006). In the current study, morphological tibia characteristics of the ORM diet group were stimulated, which can be explained by higher mineral absorption and greater bio-availability. Dietary FISH had a negative effect on tibia morphological characteristics, which might be explained by undesirable lipid peroxidation in feed, as indicated for BW as well.
Tibia mechanical and biophysical measurements, such as bone mineral density (Watkins and Southern, 1992; Rath et al., 2000; Onyango et al., 2003; Kim et al., 2006; Shim et al., 2012), bone mineral content (Akpe et al., 1987; Onyango et al., 2003; Kim et al., 2006), and bone breaking strength (Merkley, 1981; Ruff and Hughes, 1985; Park et al., 2003; Kim et al., 2006) have been the most promising parameters to assess bone health in poultry, because of their relationships with locomotion and leg pathologies. A deficiency of Ca, P, and trace minerals in the diet result in lower bone mineral content and lower breaking strength (Bar et al., 2003; Sá et al., 2004; McDevitt et al., 2006). Ca and P are primarily essential for bone mineralization (Rath et al., 1999; Blake and Fogelman, 2002), and increasing Ca and P in broiler chicken diets might positively influence bone mineralization and bone Ca content, leading to a stronger bone (Driver et al., 2005a,b; Létourneau-Montminy et al., 2008). Current diets were optimized based on the CVB (CVB, 2012), but the advices for Ca and P in this table are not based on bone quality. It can be speculated that the current advised minimal levels are too limited for optimal bone development and bone strength. Instead of increasing dietary inorganic Ca and P content, changing the source of Ca and P and thereby increasing the bio-availability might work in the same way. In the current study, the highest TMC and the highest TMD at slaughter age were observed in the ORM diet group compared to all other diet groups. These findings are in accordance with El-Husseiny et al. (2012) who evaluated effects of replacement of organic Zn, Mn, and Cu on tibia mineralization.
Bone strength is generally assumed to be suboptimal in modern broiler chickens (Lilburn, 1994; Williams et al., 2004; Sherlock et al., 2010) and the strength is mostly related to the inorganic part of the bone, which is responsible for the hardness (Turner and Burr, 1993; Boivin and Meunier, 2002a,b; Bonser and Casinos, 2003). It has been shown that even if the mineral content of the diet can fulfill the requirements for growth performance, the mineral content level might be insufficient to meet the requirements for maximal bone strength (Bar et al., 2003; Sa et al., 2004). Hemme et al. (2005) reported that organic sourced P, because of its higher bio-availability, positively affected bone strength. Cu and Fe are vitally important for crosslinking of collagen, which gives the bone its breaking strength and elasticity (Dibner et al., 2007). Insufficient amount of available Cu and Fe resulted in lower bone strength, even if Ca and P levels were adequate (Medeiros et al., 1997). In the present study, TBS of the ORM diet group was highest at the slaughter age (day 42). This confirms previous studies that dietary organic Zn, Mn, Cu, Fe, Se and more available Ca and P resulted in higher bone breaking strength (McDevitt et al., 2006; Dibner et al., 2007; Ferket et al., 2009; El-Husseiny et al., 2012).
Tibia stiffness and TEF parameters are mostly related to the organic part of bone, which is responsible for flexibility (Velleman, 2000; Turner, 2006). In the current study, TSF and TEF reached the highest values in the ORM diet group. It is known that especially essential trace minerals play a role in the linkage between elastin and collagen, which supplies bone its stiffness and flexibility (Starcher et al., 1980; Dibner et al., 2007). These 2 parameters of COL diet group were also high at the slaughter age compared to CON and FISH diet groups, which might be explained by the increased collagen amount in bone and consequently more flexible bone structure.
In conclusion, the hypothesis of this study was that dietary ORM, FISH, and COL might positively affect tibia development in broiler chickens. The results of this study indeed showed that organic macro and trace minerals in the diet positively affect tibia characteristics, but these effects have not been found for the FISH and COL. Because of the best tibia characteristics for the ORM diet group and the lowest (not significant) values for GS and rotated tibia, it can be suggested that ORM in the diet of fast-growing broiler chickens might improve animal welfare and risk on injuries during depopulation, transport, and slaughtering.
SUPPLEMENTARY DATA
Table A1. Home pen behavior observation parameters of broiler chickens (%) fed FISH, COL, and organic minerals diets.
ACKNOWLEDGMENTS
This project was financed by the public-private partnership “Healthy Bones” (project number BO-47001-011). The financial support of the Ministry of Agriculture, Nature, and Food Quality, Aviagen, Darling Ingredients Inc., ForFarmers, Hubbard, Marel Stork Poultry Processing BV, Nepluvi, and Nutreco is gratefully acknowledged. The authors would like to thank Lagerwey hatchery (Lunteren, The Netherlands) for providing 1-day-old chicks; Martijn Nieuwenhout at Tromp Medical B.V. (Castricum, The Netherlands) for allowing us to use their X-ray machine. Stefan Veenstra, Henny Reimert, Ilona van der Anker-Hensen, Mehmet Can Güçlü and the animal care takers are acknowledged for their help during the experiment.
REFERENCES
- Abdallah A. G., El-Husseiny O. M., Abdel-Latif K. O.. 2009. Influence of some dietary organic mineral supplementations. Int. J. Poult. Sci. 8:291–298. [Google Scholar]
- Akpe M. P., Waibel P. E., Larntz K., Metz A. L., Noll S. L., Walser M. M.. 1987. Phosphorus availability bioassay using bone ash and bone densitometry as response criteria. Poult. Sci. 66:713–720. [DOI] [PubMed] [Google Scholar]
- Ao T., Macalintal L. M., Paul M. A., Pescatore A. J., Delles R. M., Cantor A. H., Ford M. J., Dawson K. A.. 2017. Effects of dietary supplementation of organic minerals on the performance of broiler chicks fed oxidised soybean oil. J. Appl. Anim. Nutr. 5:1–5. [Google Scholar]
- Bao Y. M., Choct M., Iji P. A., Bruerton K.. 2007. Effect of organically complexed copper, iron, manganese, and zinc on broiler performance, mineral excretion, and accumulation in tissues. J. Appl. Poult. Res. 16:448–455. [Google Scholar]
- Bar A., Shinder D., Yosefi S., Vax E., Plavnik I.. 2003. Metabolism and requirements for calcium and phosphorus in the fast-growing chicken as affected by age. Br. J. Nutr. 89:51–60. [DOI] [PubMed] [Google Scholar]
- Bessei W. 2006. Welfare of broilers: a review. World Poult. Sci. J. 62:455–466. [Google Scholar]
- Blake G. M., Fogelman I.. 2002. Methods and clinical issues in bone densitometry and quantitative ultrasonometry. Pages 1573–1585 in Principles of Bone Biology (Second Edition). Bilezikian J. P., Raisz L. G., Rodan G. A., ed. Academic Press, Massachusetts, US. [Google Scholar]
- Boivin G., Meunier P. J.. 2002a. The degree of mineralization of bone tissue measured by computerized quantitative contact microradiography. Calcif. Tissue Int. 70:503–511. [DOI] [PubMed] [Google Scholar]
- Boivin G., Meunier P. J.. 2002b. Effects of bisphosphonates on matrix mineralization. J. Musculoskelet. Neuronal. Interact.. 2:538–543. [PubMed] [Google Scholar]
- Bonser R. H. C., Casinos A.. 2003. Regional variation in cortical bone properties from broiler fowl-a first look. Br. Poult. Sci. 44:350–354. [DOI] [PubMed] [Google Scholar]
- Burrell A. L., Dozier W. A., Davis A. J., Compton M. M., Freeman M. E., Vendrell P. F., Ward T. L.. 2004. Responses of broilers to dietary zinc concentrations and sources in relation to environmental implications. Br. Poult. Sci. 45:225–263. [DOI] [PubMed] [Google Scholar]
- Calini F., Sirri F.. 2007. Breeder nutrition and offspring performance. Rev. Braz. J. Poult. Sci. 9:77–83. [Google Scholar]
- Charuta A., Dzierzęcka M., Pierzchała M., Cooper R. G., Poławska E., Horbańczuk J. O.. 2013. Sex-related differences of morphometric, densitometric, and geometric parameters of tibia and tarsometatarsal bone in 14-month-old ostriches (Struthio camelus). Poult. Sci. 92:2965–2976. [DOI] [PubMed] [Google Scholar]
- Corr S. A., Gentle M. J., McCorquodale C. C., Bennett D.. 2003. The effect of morphology on walking ability in the modern broiler: a gait analysis study. Anim. Welfare 12:159–171. [Google Scholar]
- CVB 2012. Veevoedertabel 2012 [Feeding table], Central Veevoeder Bureau. Lelystad. [Google Scholar]
- De Jong I. C., Guémené D.. 2011. Major welfare issues in broiler breeders. World Poult. Sci. J. 67:73–82. [Google Scholar]
- Dibner J. J., Richards J. D., Kitchell M. L., Quiroz M. A.. 2007. Metabolic challenges and early bone development. J. Appl. Poult. Res. 16:126–137. [Google Scholar]
- Dobrzañski Z., Korczyñski M., Chojnacka K., Górecki H., Opaliñski S.. 2008. Influence of organic forms of copper, manganese and iron on bioaccumulation of these metals and zinc in laying hens. J. Elementol. 13:309–319. [Google Scholar]
- Driver J. P., Pesti G. M., Bakalli R. I., Edwards H. M. Jr. 2005a. Calcium requirements of the modern broiler chicken as influenced by dietary protein and age. Poult. Sci. 84:1629–1639. [DOI] [PubMed] [Google Scholar]
- Driver J. P., Pesti G. M., Bakalli R. I., Edwards H. M. Jr. 2005b. Effects of calcium and nonphytate phosphorus concentrations on phytase efficacy in broiler chicks. Poult. Sci. 84:1406–1417. [DOI] [PubMed] [Google Scholar]
- El-Husseiny O. M., Hashish S. M., Ali R. A., Arafa S. A., El-Samee L. D. A., Olemy A. A.. 2012. Effects of feeding organic zinc, manganese and copper on broiler growth, carcass characteristics, bone quality and mineral content in bone, liver and excreta. Int. J. Poult. Sci. 11:368–377. [Google Scholar]
- European Food Safety Authority 2010. Standard sample description for food and feed. EFSA J. 8:1457. [Google Scholar]
- Ferket P. R., Oviedo-Rondón E. O., Mente P. L., Bohórquez D. V., Santos Jr A. A., Grimes J. L., Richards J. D., Dibner J. J., Felts V.. 2009. Organic trace minerals and 25-hydroxycholecalciferol affect performance characteristics, leg abnormalities, and biomechanical properties of leg bones of turkeys. Poult. Sci. 88:118–131. [DOI] [PubMed] [Google Scholar]
- Gocsik É., Silvera A. M., Hansson H., Saatkamp H. W., Blokhuis H. J.. 2017. Exploring the economic potential of reducing broiler lameness. Br. Poult. Sci. 58:337–347. [DOI] [PubMed] [Google Scholar]
- Guillerminet F., Beaupied H., Fabien-Soulé V., Tomé D., Benhamou C. L., Roux C., Blais A.. 2010. Hydrolyzed collagen improves bone metabolism and biomechanical parameters in ovariectomized mice: an in vitro and in vivo study. Bone 46:827–834. [DOI] [PubMed] [Google Scholar]
- Guillerminet F., Fabien-Soulé V., Even P., Tomé D., Benhamou C. L., Roux C., Blais A.. 2012. Hydrolyzed collagen improves bone status and prevents bone loss in ovariectomized C3H/HeN mice. Osteop. Int. 23:1909–1919. [DOI] [PubMed] [Google Scholar]
- Guinotte F., Nys Y., de Monredon F.. 1991. The effects of particle size and origin of calcium carbonate on performance and ossification characteristics in broiler chicks. Poult. Sci. 70:1908–1920. [DOI] [PubMed] [Google Scholar]
- Hemme A., Spark M., Wolf P., Paschertz H., Kamphues J.. 2005. Effects of different phosphorus sources in the diet on bone composition and stability (breaking strength) in broilers. J. Anim. Physiol. Anim. Nutr. 89:129–133. [DOI] [PubMed] [Google Scholar]
- Iwai K., Hasegawa T., Taguchi Y., Morimatsu F., Sato K., Nakamura Y., Higashi A., Kido Y., Nakabo, Ohtsuki K.. 2005. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J. Agric. Food Chem. 53:6531–6536. [DOI] [PubMed] [Google Scholar]
- Jungmann R., Schitter G., Fantner G. E., Lauer M. E., Hansma P. K., Thurner P. J.. 2007. Real-time microdamage and strain detection during micromechanical testing of single trabeculae. In Experimental and Applied Mechanics: SEM Annual Conference and Exposition, Springfield, Massachusetts, US, 3–6 June 2007. [Google Scholar]
- Julian R. J. 1998. Rapid growth problems: ascites and skeletal deformities in broilers. Poult. Sci. 77:1773–1780. [DOI] [PubMed] [Google Scholar]
- Kestin S. C., Su G., Sorensen P.. 1999. Different commercial broiler crosses have different susceptibilities to leg weakness. Poult. Sci. 78:1085–1090. [DOI] [PubMed] [Google Scholar]
- Kestin S. C., Knowles T. G., Tinch A. F., Gregory N. G.. 1992. The prevalence of leg weakness in broiler chickens and its relationship with genotype. Vet. Rec. 131:190–194. [DOI] [PubMed] [Google Scholar]
- Khan N. A., Cone J. W., Hendriks W. H.. 2009. Stability of fatty acids in grass and maize silages after exposure to air during the feed out period. Anim. Feed Sci. Tech. 154:183–192. [Google Scholar]
- Kidd M. T. 2003. A treatise on chicken dam nutrition that impacts on progeny. World Poult. Sci. J. 59:475–494. [Google Scholar]
- Kim W. K., Donalson L. M., Mitchell A. D., Kubena L. F., Nisbet D. J., Ricke S. C.. 2006. Effects of alfalfa and fructooligosaccharide on molting parameters and bone qualities using dual energy X-ray absorptiometry and conventional bone assays. Poult. Sci. 85:15–20. [DOI] [PubMed] [Google Scholar]
- Kittelsen K. E., David B., Moe R. O., Poulsen H. D., Young J. F., Granquist E. G.. 2017. Associations among gait score, production data, abattoir registrations, and postmortem tibia measurements in broiler chickens. Poult. Sci. 96:1033–1040. [DOI] [PubMed] [Google Scholar]
- Knowles T. G., Kestin S. C., Haslam S. M., Brown S. N., Green L. E., Butterworth A., Pope S. J., Pfeiffer D., Nicol C. J.. 2008. Leg disorders in broiler chickens: prevalence, risk factors and prevention. PLoS One 3:e1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupski W., Tatara M. R.. 2007. Interrelationships between densitometric, morphometric, and mechanical properties of the tibia in turkeys. Bull. Vet. Inst. Pulawy 51:621–626. [Google Scholar]
- Leblanc B., Wyers M., Cohn-Bendit F., Legall J. M., Thibault E., Florent J. M.. 1986. Histology and histomorphometry of the tibia growth in two turkey strains. Poult. Sci. 65:1787–1795. [DOI] [PubMed] [Google Scholar]
- Létourneau-Montminy M. P., Lescoat P., Narcy A.,Sauvant D.,Bernier J. F.,Magnin M., Pomar C., Nys Y., Jondreville C.. 2008. Effects of reduced dietary calcium and phytase supplementation on calcium and phosphorus utilisation in broilers with modified mineral status. Br. Poult. Sci. 49:705–715. [DOI] [PubMed] [Google Scholar]
- Lewis N. J., Hurnik J. F.. 1990. Locomotion of broiler chickens in floor pens. Poult. Sci. 69:1087–1093. [DOI] [PubMed] [Google Scholar]
- Lilburn M. S. 1994. Skeletal growth of commercial poultry species. Poult. Sci. 73:897–903. [DOI] [PubMed] [Google Scholar]
- Lopez-Ferrer S., Baucells M. D., Barroeta A. C., Grashorn M. A.. 1999. n-3 enrichment of chicken meat using fish oil: alternative substitution with rapeseed and linseed oils. Poult. Sci. 78:356–365. [DOI] [PubMed] [Google Scholar]
- Martin T. J. 2016. Parathyroid hormone-related protein, its regulation of cartilage and bone development, and role in treating bone diseases. Phys. Rev. 96:831–871. [DOI] [PubMed] [Google Scholar]
- McDevitt R. M., McEntee G. M., Rance K. A.. 2006. Bone breaking strength and apparent metabolisability of calcium and phosphorus in selected and unselected broiler chicken genotypes. Br. Poult. Sci. 47:613–621. [DOI] [PubMed] [Google Scholar]
- Medeiros D. M., Ilich J., Ireton J., Matkovic V., Shiry L., Wildman R.. 1997. Femurs from rats fed diets deficient in copper or iron have decreased mechanical strength and altered mineral composition. J. Trace Element Exp. Med. 10:197–203. [Google Scholar]
- Mench J. 2004. Lameness. Pages 3–17 in Measuring and Auditing Broiler Welfare. Weeks C. A., Butterworth A., ed. CABI, Wallingford, UK. [Google Scholar]
- Merkley J. W. 1981. The effect of sodium fluoride on egg production, egg quality, and bone strength of caged layers. Poult. Sci. 60:771–776. [DOI] [PubMed] [Google Scholar]
- Nollet L., Van der Klis J. D., Lensing M., Spring P.. 2007. The effect of replacing inorganic with organic trace minerals in broiler diets on productive performance and mineral excretion. J. Appl. Poult. Res. 16:592–597. [Google Scholar]
- Nomura Y., Oohashi K., Watanabe M., Kasugai S.. 2005. Increase in bone mineral density through oral administration of shark gelatin to ovariectomized rats. Nutrition 21:1120–1126. [DOI] [PubMed] [Google Scholar]
- Oesser S., Adam M., Babel W., Seifert J.. 1999. Oral administration of 14C labeled gelatin hydrolysate leads to an accumulation of radioactivity in cartilage of mice (C57/BL). J. Nutr. 129:1891–1895. [DOI] [PubMed] [Google Scholar]
- Ohara H., Matsumoto H., Ito K., Iwai K., Sato K.. 2007. Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. J. Agric. Food Chem. 55:1532–1535. [DOI] [PubMed] [Google Scholar]
- Oliveira T. F. B., Bertechini A. G., Bricka R. M., Kim E. J., Gerard P. D., Peebles E. D.. 2015. Effects of in ovo injection of organic zinc, manganese, and copper on the hatchability and bone parameters of broiler hatchlings. Poult. Sci. 94:2488–2494. [DOI] [PubMed] [Google Scholar]
- Onyango E. M., Hester P. Y., Stroshine R., Adeola O.. 2003. Bone densitometry as an indicator of percentage tibia ash in broiler chicks fed varying dietary calcium and phosphorus levels. Poult. Sci. 82:1787–1791. [DOI] [PubMed] [Google Scholar]
- Orimo H. 2010. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon Med. Sch. 77:4–12. [DOI] [PubMed] [Google Scholar]
- Oviedo-Rondón E. O., Ferket P. R., Havestein G. B.. 2006. Nutritional factors that affect leg problems in broilers and turkeys. Avian Poult. Bio. Rev. 17:89–103. [Google Scholar]
- Park S. Y., Birkhold S. G., Kubena L. F., Nisbet D. J., Ricke S. C.. 2003. Effect of storage condition on bone breaking strength and bone ash in laying hens at different stages in production cycles. Poult. Sci. 82:1688–1691. [DOI] [PubMed] [Google Scholar]
- Rath N. C., Balog J. M., Huff W. E., Huff G. R., Kulkarni G. B., Tierce J. F.. 1999. Comparative differences in the composition and biomechanical properties of tibiae of seven-and seventy-two-week-old male and female broiler breeder chickens. Poult. Sci. 78:1232–1239. [DOI] [PubMed] [Google Scholar]
- Rath N. C., Huff G. R., Huff W. E., Balog J. M.. 2000. Factors regulating bone maturity and strength in poultry. Poult. Sci. 79:1024–1032. [DOI] [PubMed] [Google Scholar]
- Ruff C. R., Hughes B. L.. 1985. Bone strength of height-restricted broilers as affected by levels of calcium, phosphorus, and manganese. Poult. Sci. 64:1628–1636. [Google Scholar]
- Sá L. M., Gomes P. C., Rostagno H. S., Albino L. F. T., Cecon P. R., D'Agostini P.. 2004. Calcium requirement for broiler chicks from 22 to 42 and 43 to 53 days old. Braz. J. Anim. Sci. 33:397–406. [Google Scholar]
- Salim H. M., Jo C., Lee B. D.. 2008. Zinc in broiler feeding and nutrition. Avian Bio. Res. 1:5–18. [Google Scholar]
- Schreiner M., Hulan H. W., Razzazi-Fazeli E., Böhm J., Moreira R. G.. 2005. Effect of different sources of dietary omega-3 fatty acids on general performance and fatty acid profiles of thigh, breast, liver and portal blood of broilers. J. Sci. Food Agric. 85:219–226. [Google Scholar]
- Scott M. L., Nesheim M. C., Yang R.. 1982. Essential inorganic elements. Pages 258–382 in Nutrition of the Chicken. 3rd ed M. L. Scott and Associates, New York, US. [Google Scholar]
- Sherlock L., Demmers T. G. M., Goodship A. E., McCarthy I. D., Wathes C. M.. 2010. The relationship between physical activity and leg health in the broiler chicken. Br. Poult. Sci. 51:22–30. [DOI] [PubMed] [Google Scholar]
- Shim M. Y, Karnuah A. B., Mitchell A. D., Anthony N. B., Pesti G. M., Aggrey S. A.. 2012. The effects of growth rate on leg morphology and tibia breaking strength, mineral density, mineral content, and bone ash in broilers. Poult. Sci. 91:1790–1795. [DOI] [PubMed] [Google Scholar]
- Starcher B. C., Hill C. H., Madaras J. G.. 1980. Effect of zinc deficiency on bone collagenase and collagen turnover. J. Nutr. 110:2095–2102. [DOI] [PubMed] [Google Scholar]
- Tahir M., Shim M. Y., Ward N. E., Smith C., Foster E., Guney A. C., Pesti G. M.. 2012. Phytate and other nutrient components of feed ingredients for poultry. Poult. Sci. 91:928–935. [DOI] [PubMed] [Google Scholar]
- Turner C. H. 2006. Bone strength: current concepts. Ann. New York Acad. Sci. 1068:429–446. [DOI] [PubMed] [Google Scholar]
- Turner C. H., Burr D. B.. 1993. Basic biomechanical measurements of bone: a tutorial. Bone 14:595–608. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen J. P. T. M., Van Driel M., Van Den Bemd G. J. C. M, Pols H. A. P.. 2001. Vitamin D control of osteoblast function and bone extracellular matrix mineralization. Crit. Rev. Eukaryot. Gene Expr. 11:1–3. [PubMed] [Google Scholar]
- Velleman S. G. 2000. The role of the extracellular matrix in skeletal development. Poult. Sci. 79:985–989. [DOI] [PubMed] [Google Scholar]
- Wang X., Fosmire G. J., Gay C. V., Leach R. M. Jr. 2002. Short-term zinc deficiency inhibits chondrocyte proliferation and induces cell apoptosis in the epiphyseal growth plate of young chickens. J. Nutr. 132:665–673. [DOI] [PubMed] [Google Scholar]
- Wang Y. B., Xu B. H.. 2008. Effect of different selenium source (sodium selenite and selenium yeast) on broiler chickens. Anim. Feed Sci. Technol. 144:306–314. [Google Scholar]
- Watkins B. A., Li Y., Lippman H. E., Feng S.. 2003. Modulatory effect of omega-3 polyunsaturated fatty acids on osteoblast function and bone metabolism. Prostaglandins, Leukot. Essent. Fatty Acids 68:387–398. [DOI] [PubMed] [Google Scholar]
- Watkins B. A., Shen C. L., Allen K. G., Seifert M. F.. 1996. Dietary (n-3) and (n-6) polyunsaturates and acetylsalicylic acid alter ex vivo PGE2 biosynthesis, tissue IGF-I levels, and bone morphometry in chicks. J. Bone Min. Res. 11:1321–1332. [DOI] [PubMed] [Google Scholar]
- Watkins K. L., Southern L. L.. 1992. Effect of dietary sodium zeolite A and graded levels of calcium and phosphorus on growth, plasma, and tibia characteristics of chicks. Poult. Sci. 71:1048–1058. [DOI] [PubMed] [Google Scholar]
- Wedekind K. J., Titgemeyer E. C., Twardock A. R., Baker D. H.. 1991. Phosphorus, but not calcium, affects manganese absorption and turnover in chicks. J. Nutr. 121:1776–1786. [DOI] [PubMed] [Google Scholar]
- Williams B., Waddington D., Murray D. H., Farquharson C.. 2004. Bone strength during growth: influence of growth rate on cortical porosity and mineralization. Calcif. Tissue Int. 74:236–245. [DOI] [PubMed] [Google Scholar]
- Wu J., Fujioka M., Sugimoto K., Mu G., Ishimi Y.. 2004. Assessment of effectiveness of oral administration of collagen peptide on bone metabolism in growing and mature rats. J. Bone Min. Met. 22:547–553. [DOI] [PubMed] [Google Scholar]
- Yalçin S., Settar P., Dicle O.. 1998. Influence of dietary protein and sex on walking ability and bone parameters of broilers. Br. Poult. Sci. 39:251–256. [DOI] [PubMed] [Google Scholar]
- Yalçinkaya I., Çinar M., Yildirim E., Erat S., Başalan M., Güngör T.. 2012. The effect of prebiotic and organic zinc alone and in combination in broiler diets on the performance and some blood parameters. Ital. J. Anim. Sci. 11:298–302. [Google Scholar]
- Yuan J., Xu Z., Huang C., Zhou S., Guo Y.. 2011. Effect of dietary Mintrex-Zn/Mn on performance, gene expression of Zn transfer proteins, activities of Zn/Mn related enzymes and fecal mineral excretion in broiler chickens. Anim. Feed Sci. Technol. 168:72–79. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.