Abstract
Neuroimaging research examining correlates of adolescent behavioral maturation has focused largely on issues related to higher cognitive development. Currently few studies have explored neural correlates of emotional reactivity in adolescent groups. In this study, we sought to examine the nature of posterior cingulate activation during situations of moral dilemma in normal adolescents. We focused on this region because of emerging evidence that suggests its role in emotionally self‐relevant mental processing. Ten healthy teenagers, aged from 14 to 16 years, underwent three fMRI sequences designed to examine (i) brain responses during moral dilemma; (ii) brain responses during passive viewing of the moral dilemma outcome; and (iii); “deactivation” during a simple cognitive task compared with resting‐state activity. Our main finding was that during moral dilemma, all subjects showed significant activation of the posterior cingulate cortex, and more variable activation of the medial frontal cortex and angular gyrus. Interestingly, these findings were replicated in each subject using the passive viewing task, suggesting that the previous pattern was not specific to moral reasoning or decision making. Finally, six of the ten subjects showed deactivation of the same posterior cingulate region during the cognitive task, indicating some commonality of function between posterior cingulate activity during moral dilemmas and rest. We propose that these posterior cingulate changes may relate to basic neuronal activities associated with processing self‐relevant emotional stimuli. Given the high single‐subject reproducibility of posterior cingulate activations, our findings may contribute to further characterize adolescent emotional reactivity in developmental neuroimaging studies. Hum Brain Mapp, 2008. © 2007 Wiley‐Liss, Inc.
Keywords: fMRI, emotion, brain development, maturation, limbic system, cingulate cortex
INTRODUCTION
Adolescence represents a period of significant behavioral maturation in humans, where individuals' typically heightened emotional arousability during puberty is gradually met with a stronger capacity for cognitive, social, and moral regulation [Steinberg, 2005]. However, for many, adolescence is also a time of increased risk to a range of emotional disturbances that may disrupt normal developmental trends or increase vulnerability to certain psychopathologies [Silk, et al. 2003]. As such, the development of objective assessments of emotionally relevant brain states in adolescent groups may serve as a potential tool for investigating, or even predicting, maladaptive behaviors en route.
Over the past decade, there has been a particular interest in characterizing the neural basis of cognitive maturation in adolescence using functional magnetic resonance imaging (fMRI) [Casey et al., 2005; Paus, 2005; Steinberg, 2005]. However, as discussed recently by Paus [2005], very few studies have examined aspects of social and emotional maturation in adolescent subjects; an equally important feature of behavioral development. In the current study, we sought to examine specific brain responses to situations of moral dilemma in adolescents using fMRI procedures adapted for the clinical environment [e.g., Pujol et al., 2000]. We chose the moral dilemma task for two primary reasons; first, because moral dilemmas are inherently emotionally provocative [Greene et al., 2001], and because emotional self‐regulation has a critical influence on the socialization and moral development of adolescents [Levesque et al., 2004]. Second, with respect to similar studies in adults, the moral dilemma paradigm has been associated with a consistent pattern of activations of the anterior medial prefrontal cortex, angular gyrus, and ventral posterior cingulate cortex [Greene and Haidt, 2002; Greene et al., 2001; Harenski and Hamann, 2006; Moll, et al., 2002a, b, 2005]. Interestingly, these brain regions frequently show task‐induced deactivation in fMRI studies (i.e., activity specific to resting‐state periods) [Johnson et al., 2002; Raichle et al., 2001].
In this study, we were particularly interested in the nature of posterior cingulate activity in adolescent subjects given this region's apparent sensitivity to different forms of emotional stimulation [Bromm, 2001; Maddock, 1999; Nielsen et al., 2005; Phan et al., 2002; Vogt and Laureys, 2005]. This sensitivity appears to generalize to situations of explicit emotional engagement, for example, during tasks of emotional word processing and face‐perception [Fredrikson et al., 1995; Maddock et al., 2001, 2003; Phillips et al., 1998; Sprengelmeyer et al., 1998], as well as implicit engagement during self‐directed attention or evaluation, and autobiographical memory [Johnson et al., 2002; Kircher et al., 2001, 2002; Maddock et al., 2001; Sugiura et al. 2005]. In a study of emotional word processing by Maddock et al. [2003], activation of the posterior cingulate region was related to the emotional salience of word stimuli, as opposed to their emotional valence. In a study by Piefke et al., [2003], prominent activation of the posterior cingulate was linked to the implicit emotional content of subjects' autobiographical memories, again irrespective of emotional valence. One proposal based on such studies, is that the posterior cingulate region may respond to the general emotional content of events, particularly when the nature of processing is self‐relevant [Vogt et al., 2006].
Because the associations between posterior cingulate function and emotional, self‐relevant processing have been only demonstrated in adult populations, it is unknown whether they may be similarly represented in adolescent subjects given the developmental issues outlined above. While the activity of other regions involved in emotional self‐regulatory processes, such as the medial frontal cortex, has been associated with less emotional control in adolescents versus adults [Williams et al., 2006], basic data is lacking on the nature of the posterior cingulate activity in adolescents. Focusing on this region, we predicted that healthy adolescents would show robust activation of the ventral (i.e. most genuinely limbic) portion of the posterior cingulate cortex, consistent with the heightened emotional arousability of this age group. To our knowledge, there have been no fMRI studies at present that have specifically focused on the posterior cingulate region in adolescent subjects.
The current study therefore sought to address three primary questions; first, consistent with previous studies of adult subjects, do adolescents also demonstrate a similar responsiveness of the posterior cingulate region during situations of moral dilemma? To examine this, we designed a series of moral dilemma scenarios that were specific to the subjects' social environment. The moral dilemmas were then incorporated into a traditional block‐design fMRI paradigm, appropriate for examining sustained brain responses.
Second, if posterior cingulate activation is present in adolescents during moral dilemma, to what extent is it specific to the act of moral judgment, as opposed to a more general response associated with the task context? To test this, we included a second dilemma condition, where subjects passively contemplated the consequences of their previous moral judgments, which in all cases depicted negative outcomes. By retaining the specific context of the dilemma, but removing the need for moral judgment, we were able to test whether observed activations depend on the presence of doubt preceding a moral judgment.
Finally, with use of a resting‐state condition, we also investigated the anatomical overlap of moral dilemma activations with relative task‐induced deactivations also frequently observed within the posterior cingulate region. We anticipated that the region of activity would be similar for both situations, supporting some functional commonality between resting‐state activity and the regional activation associated with the dilemma tasks.
SUBJECTS AND METHODS
Subjects
We recruited ten adolescents (four females) with a mean age of 15 years (ranging 14–16 years) from the general community. All subjects were of similar socio‐demographic background and had no history of psychiatric or neurological disorder. All subjects were right‐handed and presented with normal or corrected‐to‐normal visual acuity. Written informed consent was obtained from all subjects and their parents prior to participation. The study was approved by local institutional research and ethics committees.
Experimental Design
This study involved three separate fMRI assessments; (1) brain activations during moral dilemma; (2) brain activations during passive viewing of the moral dilemma outcome; and (3) brain activity during rest compared with a simple control task (“simple choice deactivation”). For each assessment, we used an identical 3AB block‐design consisting of six 30‐s blocks, totaling 3 min in duration.
Moral dilemma
Two developmental psychologists and a pediatric neurologist selected 18 scenarios that were considered suitable for constructing moral dilemmas in this adolescent cohort. The scenario of each moral dilemma was conveyed to the subjects in the form of artist‐sketched cartoons (see Fig. 1 and Table I). Within the week prior to each fMRI session, subjects' were familiarized with the 18 dilemma scenarios to ensure comprehension and that each scenario would be recalled when cued on the actual scanning day. During the training session, the scenarios were presented as a nonpersonal event involving an imaginary person and in no instance here was the subject required to give his or her own moral judgment.
Figure 1.
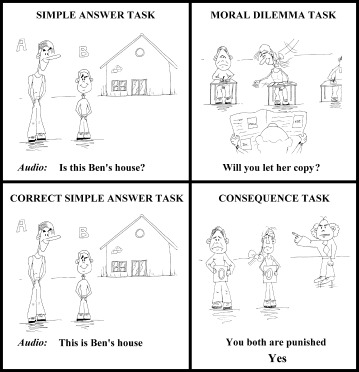
Example of the cartoon sketches used in this study. The top‐left picture gives an example of a typical control task situation (i.e. simple answer condition). In this example, subjects were familiarized before scanning with the following story structure (translated from Catalan); “Ben and Alex are two friends. Today they are going to play videogames at this house, which is Ben's house. They don't often go to Alex's house in the city.” During scanning, subjects' response to the picture was prompted with; “Is this Ben's house?” The top‐right picture gives an example of a typical moral dilemma scenario. In this example, subjects were familiarized before scanning with the following story structure; “You are doing a difficult exam and your girlfriend wants your answers. If you show her, the teacher will catch you, and you will be punished. If not, she will break‐up with you.” During scanning, subjects' response to the picture was prompted with; “Will you let her copy?” The bottom picture gives an example of the stimuli used in the consequence of judgment condition. The left bottom picture depicts a neutral outcome from the control task described above. The right bottom picture depicts one of two possible outcomes from the moral dilemma described above.
Table I.
The basic topics that were used to construct eighteen moral dilemmas for adolescents
| 1 | To tell on your friend or allow the teacher punish your whole class. |
| 2 | To lie your parents about your school grades or own up and be punished. |
| 3 | To tell the teacher that you are being bullied or to say nothing. |
| 4 | To tell your best friend that his/her boyfriend/girlfriend was kissing another boy/girl. |
| 5 | To lie to the teacher about your home work. |
| 6 | To be punished by your parents or stay out late at a party with your friends. |
| 7 | To steal something small from a shop to be popular with your friends. |
| 8 | To leave your little brother alone at home and meet your girlfriend/boyfriend for a while. |
| 9 | To smoke a cigarette with your friends and risk being caught by your parents. |
| 10 | To stick up for your friend in a fight and risk being hurt yourself. |
| 11 | To tell your mother that you think your father is having an affair. |
| 12 | To let your girlfriend/boyfriend copy your exam answers and risk being punished. |
| 13 | To keep a wallet and money that you found or return it to the person. |
| 14 | To impress your friends and keep playing a game or leave early and help your mother as you promised. |
| 15 | To break‐up with your girlfriend/boyfriend because your parents don't like them. |
| 16 | To drive your parents car without a license to impress your boyfriend/girlfriend. |
| 17 | To miss a great party with your friends or cause a big argument between your parents. |
| 18 | To spend your money on something you want instead of buying your father a good birthday present. |
To characterize brain responses to moral dilemma, we developed an appropriate control condition that was matched to the dilemma scenarios in terms of memory‐load as well as the visual and descriptive complexity of each dilemma cartoon. In this condition, a further set of 18 sketches were developed depicting ordinary situations/events that would latter cue a simple yes/no answer during fMRI (Fig. 1). In contrast to the dilemma condition, the situations or events selected for this control condition were specifically non self‐referential, and did not involve any emotional information or moral conflict. This condition was intended to control for low‐level sensory, memory and motor processes associated with the moral dilemma condition. Similar to the dilemma situations, subjects were also familiarized with this “simple answer” condition on the same day, within 1‐week prior to actual scanning.
Each dilemma and control cartoon was visually presented via a laptop computer running presentation software (http://www.neurobehavioralsystems.com). Stimuli were back‐projected onto a viewing screen at the subject's feet and were viewed with prism glasses attached to the head‐coil. During the presentation of each dilemma, subjects were voice‐prompted for their moral judgments, now acting as the protagonists of each dilemma. Following a 4‐s presentation, subjects were given 1 s to respond (yes or no) by raising their index (yes) or index and middle fingers (no), which were recorded by an experimenter. Similarly, during the simple answer condition, subjects were prompted for appropriate yes/no responses as cued from the picture scenes (Fig. 1). For both conditions, the presentation and response to each picture was made within 5 s, and each block contained six picture presentations.
Passive viewing of the moral dilemma outcome (consequence of judgment task)
For each of the 18 dilemma scenarios, two pictures depicting the alternative consequences of each dilemma were developed (i.e. 36 pictures). Consistent with the theme of each dilemma, each consequence was presented with a negative outcome (e.g. Fig. 1). The presentation of these consequences were tailored for each subject based on his/her recorded responses and was accompanied by a short verbal explanation of the consequence of each judgment. Subjects were not required to make a response to this condition. As a control condition for this task, subjects were again visually presented with simple answer conditions, but in this instance were told the correct answer for each situation and were not required to make a response. We refer to this condition hereon as the correct simple answer condition. The presentation of all picture scenarios was again made within a 5‐s window.
Resting‐state (simple choice deactivation)
Following the above two assessments, subjects underwent a final resting‐state sequence, where 30‐s blocks of rest (dot visual fixation) were alternated in the same 3AB design with a repeated presentation of the simple answer condition described above (1). The intention of this assessment was to examine functional deactivation during low‐demanding cognitive task compared with the rest. Presentation of the simple answer condition followed the same procedures detailed above.
Data Acquisition
The imaging protocol was adapted for the clinical environment, which involved image acquisitions of reduced slice number, covering the primary regions of interest. A 1.5 T Signa system (General Electric, Milwaukee, WI, USA) equipped with echo‐speed gradients and single‐shot echoplanar imaging (EPI) software was used. Functional sequences consisted of gradient recalled acquisition in the steady state (time of repetition [TR], 3,000 ms; time of echo [TE], 50 ms; pulse angle, 90°) within a field of view of 24 cm, with a 96 × 64‐pixel matrix (interpolated to 128 × 128 pixels when reconstructed), a slice thickness of 6 mm, and inter‐slice gap of 3 mm (acquisition voxel size, 2.5 × 3.8 × 6 mm3). Eight interleaved slices, parallel to the anterior–posterior commissure (AC‐PC) line, were acquired to cover the whole‐brain and ensure inclusion of our posterior cingulate region of interest within at least three continuous slices. In each case, the second last slice was placed on the AC‐PC line. The functional time series consisted of 60 consecutive images obtained during each of the 3‐min assessments. Foam padding was used to limit head movements within the coil.
Anatomical MRI examinations were also acquired for each individual including an eight‐slice two‐dimensional spoiled gradient (SPGR) sequence (TR, 100 ms; TE, 3.3 ms, flip angle 45°) matching with the functional acquisition, and a three‐dimensional (3D) SPGR sequence with 60–70 contiguous slices (TR, 40 ms; TE, 3 ms; flip angle, 30°; field of view, 26 cm; acquisition matrix, 256 × 192 pixels; slice thickness, 2.5 mm).
fMRI Analysis
Image preprocessing and analysis was performed on an independent workstation (Ultra 60; Sun Microsystems, Mountain View, CA), using commercially available software (FuncTool, Advantage Windows, version 3.1; GE Medical Systems). Motion correction was applied by removing gradual movement‐related signal variations (linear drifts) using linear regression analysis. Spatial smoothing of the data was performed in‐plane by applying a 4 × 4‐voxel (4 × 4 × 1) square kernel to the interpolated 128 × 128‐pixel images. The analysis procedures adopted for obtaining statistical parametric maps using FuncTool have been previously described [Pujol et al., 1998, 1999, 2000] and involve the voxel‐by‐voxel calculation of the Student t value obtained when comparing differences in signal intensity associated with the different study conditions. Statistical parametric t maps are displayed with a pseudocolor activation scheme and are superimposed on corresponding anatomical images.
In this study, we were primarily interested in examining the nature of posterior cingulate activations during the three functional assessments among each individual subject. The three contrasts of interest were; (i) moral dilemma minus simple answer condition; (ii) consequence of judgment minus correct simple answer condition, (iii) resting‐state minus simple answer condition. For each individual, activations were considered significant if corresponding to a P value of <0.0001 and spatial extent of greater than four contiguous voxels. This threshold corresponds to a corrected P value of P < 0.05, based on our previously described empirical‐correction for multiple comparisons [Pujol et al., 2000]. Native‐space functional anatomical coordinates were transformed into standard space [Talairach and Tournoux, 1988] using the 3D anatomical MRI exams acquired for each individual.
The posterior cingulate region considered in this study corresponded to the anatomical posterior cingulate gyrus involving the retrosplenial and posterior cingulate cortices. The anterior limit of the posterior cingulate gyrus was operatively defined by a vertical line at the midpoint between anterior and posterior commissures [Pujol et al., 2002], the inferior limit was the corpus callosum, the superior limit the cingulate sulcus, and the posterior limit the precuneus. Based on relevant anatomical and functional distinctions within the posterior cingulate gyrus [Vogt et al., 2006], activations were described in relation to the dorsal (rostral) and ventral (caudal) posterior cingulate divisions proposed by Vogt et al. [2006], with their border centered at −5.4 cm from the vertical plane at the anterior commissure and 1.97 cm from the bi‐commissural line.
RESULTS
Subjects' behavioral responses to the moral dilemma conditions were made with a 100% response rate (i.e. no missed responses). For the simple answer condition, subjects also gave correct responses with the desired ceiling level of accuracy at 100%.
The primary finding of this study was that when comparing the conditions of moral dilemma with the simple answer condition, there was significant and focal activation of the ventral posterior cingulate cortex in all adolescents studied (Brodmann areas BA 23/31 in each case). Figure 2 shows the typical pattern of brain activation associated with moral dilemma in a representative adolescent while Figure 3A shows the pattern of corresponding posterior cingulate activations for all subjects (see also Table II). Moral dilemma was also associated with suprathreshold activation of other regions, including the anterior medial frontal cortex (six of ten subjects, BA 9/10/32), inferior parietal cortex (eight of ten subjects, BA 39), and left premotor/prefrontal cortex (two of ten subjects, BA 6/9 and 44/45). For those individuals who showed activation of the inferior parietal lobe during moral dilemma (i.e. angular gyrus), this was mainly bilateral, but with a tendency for a greater magnitude of activation of the left‐hemisphere (Fig. 3A).
Figure 2.
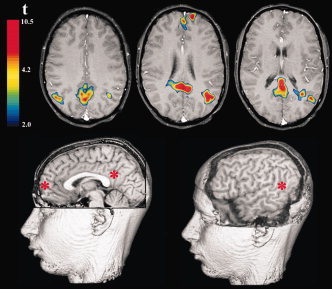
Typical pattern of brain activation associated with moral dilemma in a representative adolescent (Subject 1). Transverse‐slices presented in the top‐row display the spatial extent of activations in the posterior cingulate cortex; anterior medial prefrontal cortex and bilateral angular gyrus. The rendered 3D brains provide a sagittal representation of the three regional clusters on this same individual. Displayed asterisks mark the peak center of maximal activation of the three clusters. Activations correspond to t values greater than 4.2, P < 0.0001 (uncorrected). Images are displayed in radiological format. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 3.
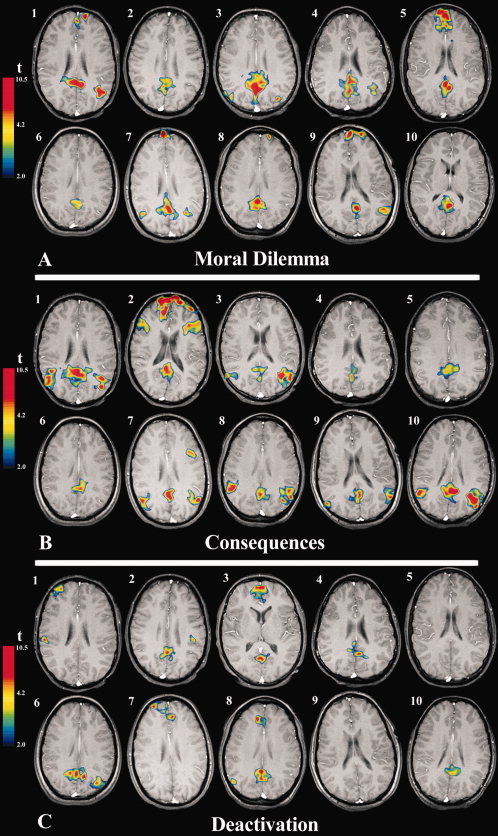
Individual subject activations of the posterior cingulate cortex. (A) (top); transverse images showing the magnitude and extent of peak activations in all 10 subjects during the moral dilemma condition. (B) (middle); transverse images showing the magnitude and extent of peak activations in all 10 subjects during the consequence of judgment condition. (C) (bottom); transverse images showing the magnitude and extent of peak deactivations in six of the ten subjects with the contrast “visual‐fixation” minus “simple answer condition.” In all images, functional activations correspond to t values greater than 4.2, P < 0.0001 (uncorrected). Images are displayed in radiological format. Subjects 2, 6, 7, and 8 = female subjects. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table II.
Posterior cingulate activation for the three study contrasts
| Moral dilemma | Consequence of judgment task | Simple choice deactivation | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Talairach coord.a | t value | Cluster size (mm2) | Talairach coord.a | t value | Cluster size (mm2) | Talairach coord.a | t value | Cluster size (mm2) | ||||||
| X | Y | Z | X | Y | Z | X | Y | Z | |||||||
| 1 | 2 | −46 | 24 | 7.4 | 633 | 3 | −51 | 24 | 7.3 | 451 | – | – | – | – | – |
| 2 | 5 | −58 | 26 | 4.8 | 40 | 4 | −46 | 17 | 6.2 | 524 | 1 | −56 | 25 | 5.3 | 60 |
| 3 | 4 | −56 | 32 | 10.5 | 679 | 4 | −61 | 22 | 4.7 | 24 | −1 | −56 | 14 | 5.0 | 28 |
| 4 | −5 | −49 | 24 | 5.5 | 316 | 1 | −62 | 22 | 4.8 | 18 | −2 | −57 | 23 | 5.6 | 137 |
| 5 | 1 | −53 | 19 | 6.9 | 376 | 6 | −58 | 27 | 4.7 | 35 | – | – | – | – | – |
| 6 | 2 | −52 | 23 | 4.5 | 36 | −3 | −46 | 23 | 6.5 | 77 | 4 | −56 | 22 | 5.6 | 190 |
| 7 | −2 | −56 | 25 | 6.1 | 295 | 4 | −51 | 25 | 6.5 | 355 | – | – | – | – | – |
| 8 | 1 | −42 | 26 | 7.1 | 292 | 1 | −51 | 26 | 5.3 | 78 | 3 | −42 | 26 | 6.4 | 344 |
| 9 | −2 | −53 | 15 | 6.6 | 81 | −2 | −57 | 15 | 5.2 | 137 | – | – | – | – | – |
| 10 | −1 | −54 | 11 | 6.5 | 113 | −10 | −53 | 20 | 7.9 | 478 | −10 | −52 | 20 | 5.5 | 39 |
Coordinates of the local points of maximal activation included in the cluster.
In the second contrast, which compared the consequence of judgment condition with the correct simple answer condition, we again found significant activations of the posterior cingulate region in all adolescents (BA 23/31). Figure 3B shows this pattern of activation in each subject when passively viewing the consequences of their moral judgments. As can be seen, there were more variable suprathreshold activations of the anterior medial frontal and inferior parietal cortices, both within and between‐subjects. Figure 4 illustrates, for each individual, the relative spatial proximity of posterior cingulate activations observed during both moral dilemma and consequence of judgment conditions.
Figure 4.
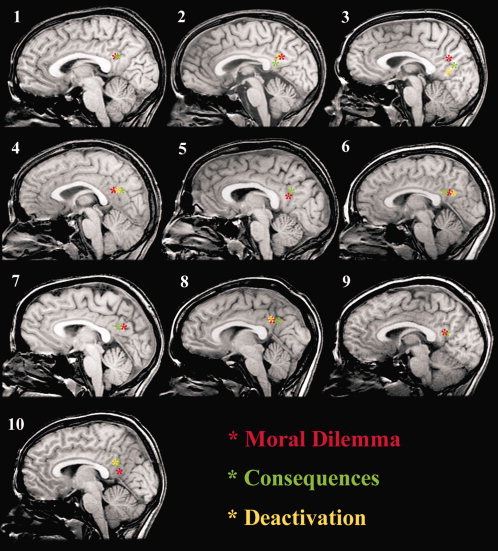
High‐resolution mid‐line sagittal images of all subjects illustrating the relative anatomical overlap of posterior cingulate activations characterized during (i) moral dilemma (red asterisk); (ii) consequence of judgment (green asterisk); and (iii); functional deactivations using resting‐state data (yellow asterisk). Subjects 2, 6, 7, and 8 = female subjects. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In a further post‐hoc group comparison of the moral dilemma and consequence of judgment conditions using paired‐samples Wilcoxon Signed Ranks tests, we found no significant differences in the spatial location (x, y, z dimensions), activation magnitude (t value) or extent (cluster‐size) between the two conditions.
As a final analysis, we also investigated the anatomical resemblance, in terms of spatial location, of the above posterior cingulate activations for each subject and simple choice deactivation in our third assessment that involved the contrast visual‐fixation (resting‐state) vs. simple answer condition. For six of the ten subjects, there was significant deactivation of the posterior cingulate cortex (BA 23/31) during the simple answer condition, indicating, conversely, greater relative activation of this region during rest (Fig. 3C). As illustrated in Figures 4 and 5, the peak posterior cingulate deactivation, when present, occurred in the same approximate region activated during moral dilemma. In addition, we found that three of the four subjects showing no significant deactivation at the established threshold, showed a cluster greater than four voxels with P < 0.01 within the region activated during moral dilemmas. This analysis indicates that deactivation was generally present across subjects, although it occurred at a low threshold in some cases.
Figure 5.
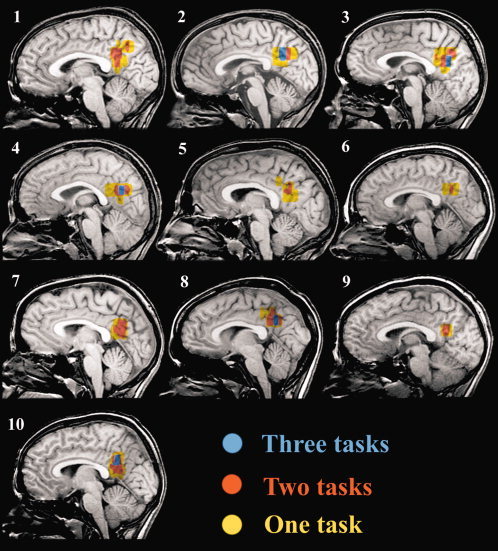
Mid‐line sagittal images for all subjects illustrating, irrespective of task type, the relative functional overlap of posterior cingulate activations between the three study contrasts (i.e., yellow indicates activation in one of the three study tasks, red any combination of two tasks, and blue activation during the three tasks). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Because of a study limitation in the number of acquired slices (total eight slices to cover the brain), a voxelwise analysis comparing the rest and moral dilemma conditions was not feasible (mainly due to head displacement along the z axis as these conditions were acquired in different sequences). Nevertheless, at the group level, posterior cingulate activation magnitude (peak t value) in “moral dilemma vs simple answer condition” was significantly greater than deactivation in “rest vs. simple answer condition” (Wilcoxon Z = −2.0; P = 0.047) in a paired comparison including all 10 subjects. Results in Table II and Figure 3 suggest that the posterior cingulate region was more strongly activated during moral dilemma (vs. simple answer condition) than during rest (vs. simple answer condition) in five of the ten adolescents (subjects 1, 5, 7, and 9 with significant activation in moral dilemma only and subject 3 showing the largest study activation).
DISCUSSION
We assessed brain responses to situations of moral dilemma in adolescents, with particular interest in characterizing the nature of posterior cingulate activations among individual subjects. We used a block‐design fMRI paradigm to evaluate sustained patterns of brain activity during the experience of moral dilemma and its judgment. Our primary finding was that when comparing the dilemma scenarios with an appropriate control condition, all adolescents showed significant, focal activations of the posterior cingulate cortex.
The result of the main study contrast (moral dilemma vs. simple answer condition) suggests that the dilemma paradigm does allow for the reliable assessment of posterior cingulate functional activation in adolescent subjects (Fig. 3A). Our broader, ongoing interest here involves the development of reliable, but nonsophisticated means to assess, at the level of brain function, general aspects of emotional reactivity and maturity across the adolescent period. As a first approach, the dilemma task appears to be effective in engaging our posterior cingulate region‐of‐interest—a region that has become more frequently associated with aspects of emotional responsiveness in adult fMRI studies (see below).
A second aim of this study was to further test the specificity of posterior cingulate activation during the dilemma paradigm, that is, whether or not the experience of moral dilemma itself would be primarily responsible for the observed patterns of activation. To explore this, we included a second condition where subjects contemplated negative representations of the consequences of their moral judgments, thus reducing the psychological element of doubt associated with the moral dilemmas. The pattern of activations obtained with this contrast (consequence of judgment vs. correct simple answer condition) largely replicated that observed during moral dilemmas (Fig. 3B), corresponding again with focal activation of the posterior cingulate region among all subjects. Figures 4 and 5 highlight the relative spatial overlap of activations characterized during both the moral dilemma and consequence of judgment conditions within each subject, where activations cluster around the ventral (caudal) extent of the posterior cingulate cortex [Vogt et al., 2006].
The resemblance of findings observed during both moral dilemma tasks may help to constrain the meaning of posterior cingulate activation in our study. The primary moral dilemma condition is a complex self‐referential task that involves processing emotional information, conflict, moral reasoning and decision‐making. Although we cannot totally rule out that moral reasoning and decision‐making activities are also relevant during the consequence condition (i.e., this task might remind the subject of the decision process to some extent), posterior cingulate activation during this condition is probably more closely related to self‐referential emotional processing (a feature common to both tasks).
Indeed, one interpretation of the above findings is that posterior cingulate activity during both tasks reflects self‐directed attention and implicit emotional engagement with moral challenges. This proposal draws upon recent, parallel evidence from fMRI studies in adult populations implicating the posterior cingulate cortex in processes related to self‐appraisal and self‐monitoring [Johnson et al., 2002; Kircher et al., 2001; Sugiura et al., 2005], as well as various explicit forms of emotion perception [Fredrikson et al., 1995; Maddock et al., 2001, 2003; Phillips et al., 1998; Sprengelmeyer et al., 1998]; see meta‐analyses by [Bromm, 2001; Maddock, 1999; Nielsen et al., 2005; Phan et al., 2002]. The moral dilemma paradigm combines elements of both of the above, because it is primarily a self‐referential experimental challenge and is inherently emotionally engaging [Greene et al., 2001]. In anatomical terms, the ventral posterior cingulate receives strong afferent input from other areas with established links to emotional and social behaviors, including, notably, the subgenual anterior cingulate cortex and medial orbital frontal cortex [Carmichael and Price, 1995; Morris et al., 1999; Vogt and Pandya, 1987; Vogt et al., 2001]. The four‐component cingulum model proposed by Vogt couches a particular role for the ventral posterior cingulate region in self‐relevant behavioral‐monitoring, suggesting that it may respond to the emotional (and nonemotional) content of events, assessing for their self‐relevance via interactions with subgenual anterior cingulate cortex [Vogt et al., 2006]. Interestingly, the emerging link between posterior cingulate activity and self‐directed mental activity has been informed to a large extent by analyses of resting‐state brain activity, typically characterized as fMRI deactivations in task‐oriented studies [Raichle et al., 2001]. This activity is, similarly, thought to represent self‐directed cognition during the resting‐state or introspective‐thinking in the absence of external stimuli and/or directed attention. These data have been corroborated by evidence that such deactivations are methodologically reduced by self‐referential aspects of a task, particularly when involving emotion or rumination [Gusnard et al., 2001; Maddock, 1999; McGuire et al., 1996]. To examine the anatomical resemblance of such deactivations with the nature of activations described above, we compared resting‐state blocks (i.e. visual‐fixation) with the simple answer condition. Consistent with the above, six of the ten adolescents showed significant posterior cingulate deactivations, which, as illustrated in Figures 3C and 4, occurred in the same approximate region characterized during both of the activation tasks. Despite some greater variability in incidence of these deactivations, this finding suggests that there is some phenomenological overlap between brain activity that is intrinsic to the resting‐state, but also engaged by the dilemma challenges.
While previous studies have commented on the apparent similarity between posterior cingulate activations during emotionally‐evocative tasks and deactivation induced by cognitive paradigms, no study has directly examined both phenomena in the same assessment, or across individuals. By including this analysis here, and subsequently confirming a high degree of overlap among the three study contrasts, we further emphasize the notion that resting fMRI is not an emotionally neutral state. To this end, one way to conceptualize the effects of the dilemma tasks on posterior cingulate activity is that, instead of activating this region above baseline, they fail to deactivate an already high posterior cingulate activity at rest. Although not confirmed by direct contrast, our results suggest that the dilemma tasks appear to sustain, with a tendency towards increasing, this resting activity, compared with the typical decreases seen with cognitive tasks (e.g. Fig. 3C). Our assumption, which will require further study, is that posterior cingulate activation during moral dilemma tasks is more closely related to the enhancement of self‐referential processes, as in the default “resting state,” than to the specific components of these tasks (e.g. moral reasoning).
The characterization of posterior cingulate activations and deactivations described above, overall, formed part of a broader pattern of activations that included other regions also implicated in fMRI studies of moral dilemma and self‐relevant mental processing, such as the anterior medial frontal cortex and inferior parietal regions at the level of the angular and supramarginal gyri [Greene and Haidt, 2002; Greene, et al. 2001; Harenski and Hamann, 2006; Moll, et al. 2002a, b, 2005]. However, relative to the posterior cingulate foci, these activations were considerably more variable among subjects, both in terms of their activation thresholds and extents. It could be reasoned, with respect to equivalent studies in adults, that these regions are modulated in a developmental capacity beyond the current age studied, particularly because medial frontal activations are typically also evident in adult studies. Williams et al. [2006] provided some evidence to suggest significant functional maturation of brain regions involved in emotional processing between the adolescent to early adulthood years and beyond. In an early study, we reported increases in the size of the corpus callosum occurring throughout the late adolescent years into early adulthood [Pujol et al., 1993]. This large white matter bundle provides primary interhemispheric connections to distributed cortical regions, including the posterior cingulate, medial frontal and angular gyrus network implicated here. Other data also suggests that there may be a differential rate of gray‐matter maturation across the ages of 4–21 years, whereby the frontal pole region matures earliest, the angular gyrus around puberty, and medial wall regions towards the end of the late adolescent period [Gogtay et al., 2004].
There are certain limitations to our study that should be considered. We acquired functional data using a clinical protocol that allowed for a limited number of images per sequence and whole‐brain coverage using eight slices. Although slice‐selection was made to capture those regions implicated by other studies of moral dilemma in adults, it is likely that an improved sequence would also improve the functional results reported here. Nevertheless, it is important to reiterate that our ongoing intention is to develop functional probes of adolescent emotionality that could be reproduced in the clinical setting. While this study represents the first phase of such efforts, the strong within and between‐subject characterization of posterior cingulate activation among each adolescent is encouraging. Additionally, an important next step forward will be to supplement functional imaging data with a detailed characterization of individual differences in state and trait behavioral features, including experimental measures of emotional engagement (i.e. psychophysiological parameters) as well as standardized mood‐state and personality/temperament ratings. Ultimately, this approach may begin to establish more sensitive functional markers of emotional disturbance in adolescent groups with relevance to the study of emerging or high‐risk psychopathological states. We note that although pathologically heightened activity of the posterior cingulate cortex has been reported in disorders, such as major depression [Ho et al., 1996], schizophrenia [Andreasen et al., 1997; Haznedar et al., 1997] and obsessive‐compulsive disorder [McGuire et al., 1994; Perani et al., 1995], which each involve significant emotional disturbances, its relevance to these symptoms and their typical first‐onset during the adolescent period remains largely unexplored.
Acknowledgements
The authors thank the members of the Fundació Carme Vidal of Girona and Hector Ortiz, MSc, for their contributions.
REFERENCES
- Andreasen NC, O'Leary DS, Flaum M, Nopoulos P, Watkins GL, Boles Ponto LL, Hichwa RD ( 1997): Hypofrontality in schizophrenia: Distributed dysfunctional circuits in neuroleptic‐naive patients. Lancet 349: 1730–1734. [DOI] [PubMed] [Google Scholar]
- Bromm B ( 2001): Brain images of pain. News Physiol Sci 16: 244–249. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL ( 1995): Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 363: 615–641. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S ( 2005): Imaging the developing brain: What have we learned about cognitive development? Trends Cogn Sci 9: 104–110. [DOI] [PubMed] [Google Scholar]
- Fredrikson M, Wik G, Annas P, Ericson K, Stone‐Elander S ( 1995): Functional neuroanatomy of visually elicited simple phobic fear: Additional data and theoretical analysis. Psychophysiology 32: 43–48. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF III, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM ( 2004): Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101: 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J, Haidt J ( 2002): How (and where) does moral judgment work? Trends Cogn Sci 6: 517–523. [DOI] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD ( 2001): An fMRI investigation of emotional engagement in moral judgment. Science 293: 2105–2108. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME ( 2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenski CL, Hamann S ( 2006): Neural correlates of regulating negative emotions related to moral violations. Neuroimage 30: 313–324. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Luu C, Hazlett EA, Siegel BV Jr, Lohr J, Wu J, Haier RJ, Bunney WE Jr ( 1997): Decreased anterior cingulate gyrus metabolic rate in schizophrenia. Am J Psychiatry 154: 682–684. [DOI] [PubMed] [Google Scholar]
- Ho AP, Gillin JC, Buchsbaum MS, Wu JC, Abel L, Bunney WE Jr ( 1996): Brain glucose metabolism during non‐rapid eye movement sleep in major depression. A positron emission tomography study. Arch Gen Psychiatry 53: 645–652. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP ( 2002): Neural correlates of self‐reflection. Brain 125: 1808–1814. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Senior C, Phillips ML, Rabe‐Hesketh S, Benson PJ, Bullmore ET, Brammer M, Simmons A, Bartels M, David AS ( 2001): Recognizing one's own face. Cognition 78: B1–B15. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Brammer M, Bullmore E, Simmons A, Bartels M, David AS ( 2002): The neural correlates of intentional and incidental self processing. Neuropsychologia 40: 683–692. [DOI] [PubMed] [Google Scholar]
- Levesque J, Joanette Y, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M ( 2004): Neural basis of emotional self‐regulation in childhood. Neuroscience 129: 361–369. [DOI] [PubMed] [Google Scholar]
- Maddock RJ ( 1999): The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends Neurosci 22: 310–316. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH ( 2001): Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104: 667–676. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH ( 2003): Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp 18: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire PK, Bench CJ, Frith CD, Marks IM, Frackowiak RS, Dolan RJ ( 1994): Functional anatomy of obsessive‐compulsive phenomena. Br J Psychiatry 164: 459–468. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Paulesu E, Frackowiak RS, Frith CD ( 1996): Brain activity during stimulus independent thought. Neuroreport 7: 2095–2099. [PubMed] [Google Scholar]
- Moll J, de Oliveira‐Souza R, Bramati IE, Grafman J ( 2002a): Functional networks in emotional moral and nonmoral social judgments. Neuroimage 16: 696–703. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira‐Souza R, Eslinger PJ, Bramati IE, Mourao‐Miranda J, Andreiuolo PA, Pessoa L ( 2002b): The neural correlates of moral sensitivity: A functional magnetic resonance imaging investigation of basic and moral emotions. J Neurosci 22: 2730–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira‐Souza R, Krueger F, Grafman J ( 2005): Opinion: The neural basis of human moral cognition. Nat Rev Neurosci 6: 799–809. [DOI] [PubMed] [Google Scholar]
- Morris R, Petrides M, Pandya DN ( 1999): Architecture and connections of retrosplenial area 30 in the rhesus monkey (Macaca mulatta). Eur J Neurosci 11: 2506–2518. [DOI] [PubMed] [Google Scholar]
- Nielsen FA, Balslev D, Hansen LK ( 2005): Mining the posterior cingulate: Segregation between memory and pain components. Neuroimage 27: 520–532. [DOI] [PubMed] [Google Scholar]
- Paus T ( 2005): Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci 9: 60–68. [DOI] [PubMed] [Google Scholar]
- Perani D, Colombo C, Bressi S, Bonfanti A, Grassi F, Scarone S, Bellodi L, Smeraldi E, Fazio F ( 1995): [18F]FDG PET study in obsessive‐compulsive disorder. A clinical/metabolic correlation study after treatment. Br J Psychiatry 166: 244–250. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I ( 2002): Functional neuroanatomy of emotion: A meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Bullmore ET, Howard R, Woodruff PW, Wright IC, Williams SC, Simmons A, Andrew C, Brammer M, David AS ( 1998): Investigation of facial recognition memory and happy and sad facial expression perception: An fMRI study. Psychiatry Res 83: 127–138. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR ( 2003): Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain 126: 650–668. [DOI] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Junque C, Marti‐Vilalta JL, Capdevila A ( 1993): When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann Neurol 34: 71–75. [DOI] [PubMed] [Google Scholar]
- Pujol J, Conesa G, Deus J, Lopez‐Obarrio L, Isamat F, Capdevila A ( 1998): Clinical application of functional magnetic resonance imaging in presurgical identification of the central sulcus. J Neurosurg 88: 863–869. [DOI] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A ( 1999): Cerebral lateralization of language in normal left‐handed people studied by functional MRI. Neurology 52: 1038–1043. [DOI] [PubMed] [Google Scholar]
- Pujol J, Roset‐Llobet J, Rosines‐Cubells D, Deus J, Narberhaus B, Valls‐Sole J, Capdevila A, Pascual‐Leone A ( 2000): Brain cortical activation during guitar‐induced hand dystonia studied by functional MRI. Neuroimage 12: 257–267. [DOI] [PubMed] [Google Scholar]
- Pujol J, Lopez A, Deus J, Cardoner N, Vallejo J, Capdevila A, Paus T ( 2002): Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. Neuroimage 15: 847–855. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS ( 2003): Adolescents' emotion regulation in daily life: Links to depressive symptoms and problem behavior. Child Dev 74: 1869–1880. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H ( 1998): Neural structures associated with recognition of facial expressions of basic emotions. Proc Biol Sci 265: 1927–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L ( 2005): Cognitive and affective development in adolescence. Trends Cogn Sci 9: 69–74. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Watanabe J, Maeda Y, Matsue Y, Fukuda H, Kawashima R ( 2005): Cortical mechanisms of visual self‐recognition. Neuroimage 24: 143–149. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988). Co‐Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical. [Google Scholar]
- Vogt BA, Laureys S ( 2005): Posterior cingulate, precuneal and retrosplenial cortices: Cytology and components of the neural network correlates of consciousness. Prog Brain Res 150: 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN ( 1987): Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol 262: 271–289. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt LJ, Perl DP, Hof PR ( 2001): Cytology of human caudomedial cingulate, retrosplenial, and caudal parahippocampal cortices. J Comp Neurol 438: 353–376. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S ( 2006): Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage 29: 452–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, Peduto A, Gordon E ( 2006): The mellow years? Neural basis of improving emotional stability over age. J Neurosci 26: 6422–6430. [DOI] [PMC free article] [PubMed] [Google Scholar]


