Abstract
Biological models of posttraumatic stress disorder (PTSD) suggest that patients will display heightened amygdala but decreased medial prefrontal activity during processing of fear stimuli. However, a rapid and automatic alerting mechanism for responding to nonconscious signals of fear suggests that PTSD may display heightened rather than decreased MPFC under nonconscious processing of fear stimuli. This study used functional magnetic resonance imaging to examine blood oxygenation level‐dependent signal changes during nonconscious presentation (16.7 ms, masked) of fearful and neutral faces in 15 participants with PTSD and 15 age and sex‐matched healthy control participants. Results indicate that PTSD participants display increased amygdala and MPFC activity during nonconscious processing of fearful faces. These data extend existing models by suggesting that the impaired MPFC activation in PTSD may be limited to conscious fear processing. Hum Brain Mapp, 2008. © 2007 Wiley‐Liss, Inc.
Keywords: fMRI, medial prefrontal cortex, amygdala, posttraumatic stress disorder, fear
INTRODUCTION
Posttraumatic stress disorder (PTSD) is an anxiety disorder that is characterized by excessive fear responses to potential threats. Biological models of PTSD propose that reduced MPFC activity leads to impaired inhibition over amygdala fear processing networks, resulting in amygdala hyperresponsitivity in PTSD [Bremner et al., 1999b; Rauch et al., 1998, 2000; Shin et al., 2006]. Consistent with biological models of PTSD, neuroimaging studies of PTSD have reported decreases in MPFC activity (particularly rostral anterior cingulate cortex) and increases in amygdala activity [e.g. Bremner et al., 1999a; Lanius et al., 2001, 2003; Shin et al., 1999, 2004, 2005; Williams et al., 2006b], though only a few of these studies reported concomitant decreases and increases [Shin et al., 2004, 2005; Williams et al., 2006]. Heightened amygdala activity in PTSD does not appear to be particularly robust during conscious processing of trauma‐relevant and fear stimuli [Shin et al., 2004; Williams et al., 2006b; see also Lanius et al., 2003 for discussion]; however, amygdala hyperreactivity in PTSD is particularly marked in response to rapidly presented stimuli [e.g. Armony et al., 2005; Rauch et al., 2000]. Although studies in healthy participants suggest that MPFC may display heightened activity during nonconscious processing of fear signals as part of a feedforward brainstem‐amygdala‐cortical “alarm” system [Liddell et al., 2005; Williams et al., 2004, 2006a], no studies have indexed the role of the MPFC during nonconscious fear processing in PTSD.
Both human and nonhuman animal studies indicate that the amygdala is central to the generation and maintenance of fear‐related emotional responses [Davis, 1992; Liberzon et al., 1999; Morgan and LeDoux, 1995]. Animal research in fear conditioning lend support to biological models of PTSD suggesting that exaggerated amygdala responses to fear reflect a lack of “top‐down” inhibition by the MPFC [Milad and Quirk, 2002; Morgan et al., 1993]. Neuroimaging studies in humans have also confirmed the role of the amygdala in the acquisition of conditioned fear in response to conscious fear conditioning paradigms and the ventromedial prefrontal cortex in inhibition [LaBar et al., 1998; Phelps et al., 2004]. Structural abnormalities have also been reported in PTSD, with reduced MPFC volumes found [Rauch et al., 2003; Woodward et al., 2006]. However, there is also evidence of amygdala influencing MPFC function [Garcia et al., 1999; Gilboa et al., 2004]. Indeed, we have recently reported that nonconscious processing of fearful facial expressions in healthy participants involves a feedforward, subcortical network that extends from the brainstem, through to the amygdala and into ventral anterior cingulate/MPFC and is characterized by a preponderance of positive connections between amygdala and MPFC (including ACC, BA9/10, BA24/32) [Das et al., 2005, 2007; Liddell et al., 2005; Williams et al., 2004, 2006a]. We proposed that this network (including amygdala and MPFC) provides a rapid and automatic alerting mechanism for responding to nonconscious signals of fear that is “vital for the automatic orienting of attention toward the stimulus and to highlight the stimulus for further cognitive evaluation” [Liddell et al., 2005]. Activation in the MPFC was observed in the inferior frontal gyrus and ventral anterior cingulate which are thought to be linked to novelty processing and assessment of emotional significance. Activation was also observed in the left anterior cingulate, which may be specifically involved in the redirection of attention because of nonconscious emotional signals. Therefore, it is possible that an enhanced amygdala and MPFC, as well as enhanced connections between these regions, will be observed in PTSD.
Four functional magnetic resonance imaging (fMRI) studies have examined PTSD responses to threat stimuli presented rapidly. One study that presented fearful faces at 33 ms in a backward masking paradigm found increased amygdala activity to masked fear faces in PTSD [Rauch et al., 2000]. Another study presented masked trauma‐relevant stimuli at 33 ms to civilian trauma survivors and in a whole‐brain analysis found reduced fronto‐parietal attentional activity and increased parahippocampal and left hippocampal activity [Sakamoto et al., 2005]. Although 33 ms presentation of stimuli is rapid, these are able to be consciously detected [Williams et al., 2004]. In one study that presented nonconscious masked faces for 16 ms, PTSD severity was associated with increased right amygdala for masked fear [Armony et al., 2005]. A further study examined masked combat stimuli presented at varying intervals from 20 to 80 ms, in combat victims and controls, and found more activation in visual cortex in PTSD [Hendler et al., 2003]. However, studies of nonconscious perception in PTSD that focus specifically on the MPFC as a region of interest (ROI) remain to be conducted.
The aim of this study therefore was to examine the impact of trauma on amygdala and MPFC responses to fearful faces presented entirely below the threshold for conscious detection (nonconscious). Consistent with the proposal of a feedforward brainstem‐amygdala‐cortical “alarm” system [Liddell et al., 2005; Williams et al., 2006a], we predicted that nonconscious processing of fear stimuli in PTSD will involve greater recruitment of amygdala and MPFC, that these regions would be positively connected in both patients and controls but that patients would display greater connectivity than controls, and that PTSD severity would be positively correlated with heightened MPFC activation thereby providing convergent evidence for the proposal that heightened MPFC is related to PTSD during nonconscious fear processing.
METHOD
Participants
Fifteen patients with PTSD (7 males, 8 females) of mean age 37.33 years (SD = 9.90) and 15 age and sex‐matched non‐traumatized controls (7 males, 8 females) of mean age 35.80 years (SD = 9.06) participated in the study. All participants were right‐handed. Diagnoses of PTSD were made by clinical consensus by two clinical psychologists (independent of the study), according to DSM‐IV criteria [American Psychiatric Association, 1994] using the Clinician Administered PTSD Scale (CAPS) [Blake et al., 1995]. PTSD participants included survivors of motor vehicle accidents (n = 7) and assault (n = 8); the mean time elapsed since trauma was 4.9 years (SD = 5.6) and the mean CAPS total score was 79.2 (SD = 22.2). Non‐trauma‐exposed and PTSD participants were excluded if they had a history of psychosis or substance abuse, brain injury, neurological disorder, or serious medical conditions related to the thyroid or heart. PTSD participants scored higher than control participants on the depression [PTSD: M = 11.60, SD = 5.56; Controls: M = 3.00, SD = 5.15; t (29) = 4.40, P < .001] and anxiety [PTSD: M = 15.27, SD = 5.09; Controls: M = 0.53, SD = 1.13; t (29) = 10.94, P < .001] scales of the Depression Anxiety Stress Scale [Lovibond and Lovibond, 1995]. All participants provided written informed consent to participate, in accordance with National Health and Medical Research Council guidelines.
Procedure
The methodological details and analysis procedures have been described previously [e.g. Das et al., 2005, 2007; Liddell et al., 2005; Williams et al., 2006a].
Stimulus presentation
Participants viewed grey‐scale face stimuli which had been selected from a standardized picture set [Gur et al., 2002] and consisted of four female and four male individuals depicting fear and neutral facial expressions. All faces were matched for overall luminosity and size, and were equally aligned on a black background template. Face stimuli were presented nonconsciously. Each sequence comprised 240 stimuli (120 fear and 120 neutral) in a pseudo‐random sequence of 30 blocks (comprising 8 fear or 8 neutral stimuli each). Each face (fear or neutral) stimulus was presented for 16.7 ms, followed by a 163.3 ms neutral mask. These durations were based on parameters established in a psychophysics experiment, undertaken using an equivalent block design task, that demonstrated that participants could not detect the content of nonconscious presentations. This timing was sufficient to saturate the retina, but precluded conscious awareness of the presence of the stimulus. Our protocol was based on signal detection [Macmillan, 1986], and provides an exhaustive criterion for ensuring unconscious processing of fear. The inter‐stimulus interval (ISI) was 1,088 ms. The ISI was jittered by ±200 ms to ensure that stimulus onset did not coincide with a constant slice position during image acquisition. Face stimuli were presented via a projector (Sanyo ProX, Multiverse Projector) and mirror system. Participants received standardized and synchronized visual and audio (through headphones) instructions and were asked to actively attend to the face stimuli, in preparation for a post‐scanning briefing about these stimuli. Participants were instructed to focus on the first face even though it may be difficult to see. To determine that the fearful and neutral faces were perceived with the appropriate emotional response, we subsequently asked participants to judge the facial emotion expression in a forced‐choice rating task during conscious (500 ms) presentation.
Imaging Acquisition and Analysis
Imaging was performed on a 1.5T Siemens Vision Plus scanner using an echoplanar protocol. A total of 90 functional T2*‐weighted volumes (3 per stimulus block) were acquired, comprising 15 noncontiguous slices parallel to the intercommissural (AC‐PC) line, with 6.6 mm thickness and TR = 3.3 s, TE = 40 ms, Flip angle = 90; with FOV 24 × 24 cm2, matrix size 128 × 128. Three initial “dummy” volumes were acquired to ensure blood oxygen level dependent (BOLD) saturation. Pre‐processing (realignment and unwarping, spatial normalization into standardized MNI space, smoothing using an 8 mm FWHM isotropic Gaussian kernel) and statistical analysis of fMRI data was conducted using statistical parametric mapping (SPM2, Wellcome Department of Neurology, London).
To test our a priori hypotheses, we conducted search ROI analyses for the bilateral amygdala and medial prefrontal cortex (including anterior cingulated) (MPFC), using the automated anatomical labeling (AAL) masks [Tzourio‐Mazoyer et al., 2002] and the WFU Pickatlas [Version 1.02, Maldjian et al., 2003]. We defined the MPFC as including the anterior cingulate (BA 24/32) and medial orbital to superior frontal structures (extending to BA9/10), consistent with previous PTSD neuroimaging studies [Rauch et al., 2000; Shin et al., 1999, 2004; Williams et al., 2004, 2006a, b; Zubieta et al., 1999]. All within‐ and between subject analyses employed random effects analyses using SPM2, in contrast to previous studies, which have employed fixed effects analyses [Bremner et al., 1999a; Lanius et al., 2002, 2003]. Random effect analysis takes into account individual subject variation and allows for results to be generalized beyond the selected group of participants.
Individual contrast images (fear versus neutral) were brought to the second level and examined using subtraction analyses (one and two‐sample t‐test designs), an alpha level of P < 0.05 (small volume corrected, svc) and an extent threshold of >3 voxels per cluster. Psychophysiological interaction (PPI) analyses were also conducted to determine the degree to which experimental manipulation changes the connectivity between amygdala (source region) and MPFC (target region). Briefly, the first eigenvariate time series of left and right amygdala for each individual was extracted from a 6 mm sphere centred on the central coordinate (left amygdala: x = −23.5, y = 1.95, z = −18.5; right amygdala: x = 27.1, y = −0.573, z = −18.8). Individual contrasts were then created, representing the interaction between the source region and the experimental condition, taken to the second level and examined using one and two‐sample t‐tests (alpha: P < 0.01 (svc), extent threshold: 10 voxels). Results from the two‐sample t‐tests were saved as binary masks and used in follow‐up ROI analyses to determine whether the locations that differed between‐group in the connectivity analyses overlapped those that differed between‐group in the subtraction (fear‐neutral) analyses. Finally, the association between PTSD severity (CAPS total scores) as well as depression (DASS scale) and BOLD signal changes in MPFC and amygdala networks was examined using correlational analysis to further index the relationship between the regions of interest and degree of PTSD and depression. A regression analysis was also conducted between PTSD severity and BOLD which removed the contribution of depression given the frequent comorbidity between PTSD severity and depression [Kessler et al., 1995]. Indeed, in the current PTSD sample, a significant correlation between depression and PTSD severity (r = 0.775, P = 0.002) was observed.
RESULTS
Behavioural Data
Both the PTSD and control participants were highly accurate in classifying fear (PTSD, 89%; Controls, 89%) and neutral (PTSD, 94%; Controls, 73%) faces. There was no difference between groups in facial identification, χ 2 (1, N = 30) = 2.70, P > 0.05).
Subtraction Analyses
Controls showed engagement of bilateral amygdala and ventral MPFC, whereas PTSD participants showed large activations of the bilateral amygdala and dorsal MPFC (including dorsal ACC). Relative to controls, PTSD participants showed excessively large amygdala and (predominantly dorsal) MPFC responses to nonconscious fear (Table I; Fig. 1).
Table I.
Significant activation within amygdale and MPFC ROIs for nonconscious fear in PTSD and control subjects
| Region | Sidea | MNI coordinatesb | Cluster sizec | T value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Control subjects | ||||||
| Amygdala | L | −16 | 2 | −16 | 11* | 2.71 |
| R | 18 | 4 | −16 | 8 | 2.64 | |
| Medial Prefrontal Cortex (ventral) | R | 8 | 58 | −12 | 12 | 2.27 |
| PTSD subjects | ||||||
| Amygdala | R | 24 | 2 | −12 | 135* | 3.30 |
| L | −26 | −2 | −12 | 133* | 3.10 | |
| Medial Prefrontal Cortex (dorsal) | L → R | −8 | 60 | 36 | 135 | 2.62 |
| PTSD > Controld | ||||||
| Amygdala | L | −28 | −2 | −26 | 121* | 3.85 |
| R | 26 | 4 | −28 | 151* | 3.30 | |
| Medial prefrontal/anterior cingulate cortex (dorsal) | L, R | 0 | 36 | 36 | 690* | 3.24 |
| Medial prefrontal cortex (ventral) | L, R | 0 | 50 | −12 | 98 | 2.48 |
An arrow (→) is used to indicate when a cluster of activation extends into the opposite hemisphere. The reported coordinate is associated with the greatest significance level.
MNI coordinates (x, y, z, in millimetres) refer to the voxel of maximum signal change in each region.
The cluster with the largest number of voxels in each region is reported.
Only findings for the PTSD > control between‐group contrast are displayed. No significant differences were observed for the Control > PTSD contrast.
Figure 1.
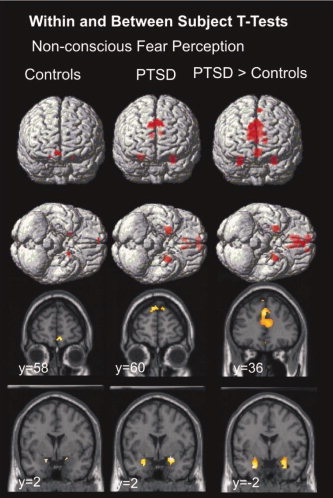
Significant activation within amygdala and MPFC ROIs for nonconscious fear in PTSD and control subjects.
Psychophysiological Interaction Analyses
Relative to controls, PTSD patients displayed increased connectivity between right amygdala (source region) and left dorsal MPFC (medial, superior frontal gyrus, MNI coordinates: −2 + 42 + 56, t = 3.03, P = 0.003, 12 voxels), but decreased connectivity between left amygdala (source region) and right rostral MPFC (rostral ACC, MNI coordinates: +16 + 46 + 20, t = 3.01, P = 0.003, 14 voxels) (Table I). Within‐subject findings indicated that these group differences were due to a positive covariation between right amygdala and MPFC in patients, but a negative covariation in controls. This pattern of covariation however was reversed for the left amygdala and MPFC; thus patients and controls displayed a negative and positive covariation, respectively. Follow‐up analyses revealed that while PTSD patients displayed excessively large MPFC responses relative to controls (788 voxels), connectivity differences were observed in only 7 of these voxels in which patients displayed negative connectivity and controls, positive connectivity.
Symptom Severity Correlations
In the PTSD group, PTSD symptom severity was negatively correlated with BOLD signal changes in dorsal MPFC (medial, superior frontal gyrus, MNI coordinates: +10 + 36 + 52, t = 3.70, P = 0.002, 201 voxels). Depression symptom severity (DASS: Depression total) was also negatively correlated with BOLD in MPFC (medial orbital, superior frontal gyrus, MNI coordinates, +0 + 50 −14, t = 4.12, P = 0.001, 219 voxels). Interestingly, PTSD symptom severity positively correlated with BOLD signal changes in dorsal MPFC (medial, superior frontal gyrus, MNI coordinates: −4 + 58 + 38, t = 7.33, P < 0.001, 1,423 voxels) when the contribution of depression was removed. Furthermore, the negative correlation between depression and BOLD remained after the contribution of PTSD severity was removed (medial, superior frontal gyrus, MNI coordinates: −4 + 58 + 38, t = 7.33, P < 0.001, 1,423 voxels). In addition, depression severity was positively correlated with BOLD signal changes in bilateral amygdala (MNI Coordinates: +24 −2 −22, t = 2.55, P = 0.012, 22 voxels; −20 + 2 −18, t = 2.06, P = 0.030, 12 voxels). PTSD symptom severity was not correlated with signal changes in the amygdala.
DISCUSSION
The current findings provide the first evidence of increased amygdala and MPFC activation in PTSD during nonconscious processing of fear and extend previous studies by demonstrating that although amygdala responses are significantly greater in PTSD participants, this is accompanied by an increase in MPFC activity in nonconscious processing. While these differences were particularly striking, we observed that differences in functional connectivity did not contribute to these findings. In addition, we found that PTSD severity was positively correlated with BOLD activity after accounting for the contribution of depression, thereby providing convergent evidence that heightened MPFC is related to PTSD during nonconscious fear processing.
Findings accord with evidence that PTSD individuals exhibit increased amygdala activation when viewing fearful faces presented at 33 ms and 16 ms [Armony et al., 2005; Rauch et al., 2000, respectively] and with models of hard‐wired amygdala‐based fear reactions [LeDoux, 1996]. This convergent evidence points to the conclusion that hyperresponsivity of the amygdala plays a key role in the pathophysiology of PTSD and that such activity related to fear perception may be best detected during nonconscious processing of fear [Morgan and LeDoux, 1995]. However, we report, for the first time, increased MPFC activity during nonconscious processing of fear signals in PTSD. This contrasts with a previous study that reported no activation in MPFC when participants viewed the same stimuli as those presented in the current study [Rauch et al., 2000]. Methodological issues may explain the differences between these two findings. While our study presented facial stimuli for 16.7 ms, the previous study presented stimuli for 33 ms. As noted in the introduction, stimuli may still be consciously detected when presented at 33 ms [Williams et al., 2004]. This distinction is particularly important given that “smidgens” of consciousness may interfere with the ability to examine the correlates of nonconscious perception [Bernat et al., 2001].
As predicted, functional connectivity findings indicated that PTSD patients displayed positive connectivity (relative to controls) with MPFC; this finding was specific to MPFC connections with the right amygdala only and did not contribute to the excessively large between group differences displayed in amygdala and MPFC. This finding accords with reports of an excitatory influence of amygdala on MPFC in PTSD individuals to consciously perceived trauma‐relevant stimuli [Gilboa et al., 2004] and with evidence of a feedforward activation in locus coerulus, amygdala, and MPFC in healthy controls during nonconscious processing of fear faces [Liddell et al., 2004]. These findings are also consistent with suggestions that the amygdala can modulate MPFC activity [Garcia et al., 1999]. In addition, PTSD patients also displayed negative connectivity between left amygdala and rostral MPFC indicating that enhanced connectivity in PTSD during nonconscious processing is lateralized to connections with right amygdala. In addition, this negative connectivity with right rostral MPFC was found to spatially overlap with regions in which PTSD patients also displayed excessively large MPFC responses relative to controls. While the reason for this negative connectivity is unclear, this overlap was only observed in 7 of the 788 voxels and is specific to connections with left amygdala. Interestingly, this negative connectivity in PTSD is consistent with findings reported in a previous PTSD study, although this was observed during conscious presentation of fearful versus happy facial expressions [Shin et al., 2005]. It is notable that small clusters of activation only, were displayed when connectivity differences between PTSD and control participants were examined. On the basis of previous studies that have reported an abundance of positive connectivity within the emotional circuitry during nonconscious processing of fear faces in healthy controls [Das et al., 2007; Williams et al., 2006a], we hypothesized that patients and healthy controls would display positive connectivity but that patients would display greater connectivity than controls. Findings of this study suggest therefore, that while differences in the connectivity of these regions are subtle, the activation observed within the regions themselves is excessive.
Severity of PTSD was negatively associated with MPFC activation during nonconscious processing of fear. Although this pattern is consistent with previous reports that PTSD is associated with impaired MPFC functioning during fear processing [Shin et al., 2005; Williams et al., 2006b], it was unexpected in the context of our finding that PTSD was characterized by increased MPFC relative to controls. Interestingly, PTSD symptom severity was positively correlated with BOLD signal changes in MPFC after the contribution of depression was removed, while the negative correlation between depression and BOLD remained after the contribution of PTSD severity was removed suggesting that the initial negative correlation between PTSD severity and BOLD was influenced by comorbid depression. It is possible therefore that the influence of comorbid depression in the more severe PTSD cases in our sample led to the observed associations between PTSD severity and decreased MPFC activation. Although imaging studies of depressed patients have generally not used fear processing paradigms, there is convergent evidence of reduced MPFC activation in depression [see Davidson et al., 2002, for review].
The conclusions from this study could be strengthened by methodological modifications in future studies. First, the absence of a trauma‐exposed no‐PTSD condition limits inferences about the influence of trauma exposure and PTSD on neural network responses. Second, whereas the use of fearful face stimuli indexes responses to generic fearful stimuli, it does not reflect response to trauma‐related cues. Future replications could usefully employ trauma‐specific cues to elicit fear responses. Third, the cross‐sectional nature of this study precludes inferences about the extent to which these responses are a function of PTSD or represent more trait‐like responses in these individuals. Future studies could measure responses before and after successful treatment of PTSD to address the relationship between these neural responses and symptomatology. Finally, this study did not index neural networks in association with autonomic arousal. A previous study suggests that neural networks respond differentially to trauma memories depending on the level of autonomic arousal present during the memory [Osuch et al., 2001].
CONCLUSIONS
This study provides the first demonstration that PTSD participants display greater activation within amygdala and MPFC during nonconscious fear processing. These data extend existing models by suggesting that the impaired MPFC activation in PTSD may be limited to conscious fear processing. The heightened capacity of the brain to respond to unconsciously perceived fear stimuli may explain why people with PTSD have difficulty controlling ongoing fear. These results point to a dysregulation of fear networks in PTSD individuals and suggest this fear dysregulation may be predominant at a nonconscious level. These data accord with evidence that people with PTSD display preconscious processing of fear stimuli [Harvey et al., 1996] and highlight the need for future research to carefully index biological responses to information that is processed at preconscious levels.
Acknowledgements
The authors are also grateful to Dr. Pritha Das for advice relating to SPM analysis.
REFERENCES
- American Psychiatric Association ( 1994): Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association. [Google Scholar]
- Armony A, Corbo V, Clément M‐H, Brunet A ( 2005): Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry 162: 1961–1963. [DOI] [PubMed] [Google Scholar]
- Bernat E, Shevrin H, Snodgrass M ( 2001): Nonconscious visual oddball stimuli evoke a P300 component. Clin Neurophysiol 112: 159–171. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM ( 1995): The development of a clinician administered PTSD scale. J Traum Stress 8: 75–90. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS ( 1999a): Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry 156: 1787–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS ( 1999b): Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biol Psychiatry 45: 806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Kemp AH, Liddell BJ, Brown KJ, Olivieri G, Peduto A, Gordon E, Williams LM ( 2005): Pathways for fear perception: modulation of amygdala activity by thalamo‐cortical systems. Neuroimage 26: 141–148. [DOI] [PubMed] [Google Scholar]
- Das P, Kemp AH, Flynn G, Harris AWF, Liddell BJ, Whitford TJ, Peduto A, Gordon E, Williams LM ( 2007): Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophrenia Research 90: 284–294. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K ( 2002): Depression: Perspectives from affective neuroscience. Ann Rev Psychol 53: 545–574. [DOI] [PubMed] [Google Scholar]
- Davis M ( 1992): The role of the amygdala in fear and anxiety. Annual Review of Neuroscience 15: 353–375. [DOI] [PubMed] [Google Scholar]
- Garcia R, Voulmba RM, Baudry M, Thompson RF ( 1999): The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature 402: 294–296. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, Bonne O ( 2004): Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biol Psychiatry 55: 263–272. [DOI] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE ( 2002): A method for obtaining 3‐dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods 115: 137–143. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Bryant RA, Rapee R ( 1996): Supraliminal and nonconscious processing of threat‐related material in post‐traumatic stress disorder. Cognit Ther Res 20: 613–623. [Google Scholar]
- Hendler T, Rotshtein P, Yeshurun Y, Weizmann T, Kahn I, Ben‐Bashat D, Malach R, Bleich A ( 2003): Sensing the invisible: Differential sensitivity of visual cortex and amygdala to traumatic content. Neuroimage 19: 587–600. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Hughes M, Nelson CB ( 1995): Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52: 1048–1060. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby C, Gore JC, LeDoux JE, Phelps EA ( 1998): Human amygdala activation during conditioned fear acquisition and extinction: A mixed‐trial fMRI study. Neuron 20: 937–946. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS ( 2001): Neural correlates of traumatic memories in posttraumatic stress disorder: A functional MRI investigation. Am J Psychiatry 158: 1920–1922. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, Gati JS, Menon RS ( 2002): Brain activation during script‐driven imagery induced dissociative responses in PTSD: A functional magnetic resonance imaging investigation. Biol Psychiatry 52: 305–311. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, Neufeld RWJ, Gati JS, Menon RS ( 2003): Recall of emotional states in posttraumatic stress disorder: An fMRI investigation. Biol Psychiatry 53: 204–210. [DOI] [PubMed] [Google Scholar]
- LeDoux J ( 1996): The emotional brain: The mysterious underpinnings of emotional life. New York: Touchstone Books. [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM ( 1999): Brain activation in PTSD in response to trauma‐related stimuli. Biol Psychiatry 45: 817–826. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, Gordon E, Williams LM ( 2005): A direct brainstem‐amygdala‐cortical 'alarm' system for nonconscious signals of fear. Neuroimage 24: 235–243. [DOI] [PubMed] [Google Scholar]
- Lovibond SH, Lovibond PF ( 1995): Manual for the Depression Anxiety Stress Scales, 2nd ed. Sydney: Psychology Foundation. [Google Scholar]
- Macmillan N ( 1986): The psychophysics of nonconscious perception. Behav Brain Sci 9: 38–39. [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA ( 2003): An automated method for neuroanatomic and cytoarchitectronic atlas‐based interrogation of fMRI data sets. NeuroImage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Milad M, Quirk GJ ( 2002): Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420: 70–74. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE ( 1995): Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci 109: 681–688. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE ( 1993): Extinction of emotional learning: Contribution of medial prefrontal cortex. Neurosci Lett 163: 109–113. [DOI] [PubMed] [Google Scholar]
- Osuch EA, Benson B, Geraci M, Podell D, Herscovitch P, McCann UD, Post RM ( 2001): Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biol Psychiatry 50: 246–253. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE ( 2004): Extinction learning in humans: Role of the amygdala and ventromedial prefrontal cortex. Neuron 43: 897–905. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Whalen PJ, Pitman RK ( 1998): Neuroimaging and the neuroanatomy of PTSD. CNS Spectrums 3: 30–41. [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK ( 2000): Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biol Psychiatry 47: 769–776. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Segal E, Pitman RK, Carson MA, McMullin K, Whalen PJ, Makris N ( 2003): Selectively reduced regional cortical volumes in posttraumatic stress disorder. Neuroreport 14: 913–916. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Fukuda R, Okuaki T, Rogers M, Kasai K, Machida T, Shirouzu I, Yamasue H, Akiyama T, Kato N ( 2005): Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder. Neuroimage 26: 813–821. [DOI] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, et al. ( 1999): Regional cerebral blood flow during script‐driven imagery in childhood sexual abuse‐related post‐traumatic stress disorder: A PET investigation. Am J Psychiatry 156: 575–584. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK ( 2004): Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with Posttraumatic stress disorder. Arch Gen Psychiatry 61: 168–176. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko M, Cavanagh SR, Krangek TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL ( 2005): A functional magnetic imaging resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 62: 273–281. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK ( 2006): Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci 1071: 67–79. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Williams LM, Liddell BJ, Rathjen J, Brown KJ, Gray J, Phillips M, Young A, Gordon E ( 2004): Mapping the time course of nonconscious and conscious perception of fear: An integration of central and peripheral measures. Hum Brain Mapp 21: 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, Gordon E ( 2006a): Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. J Neurosci 26: 9264–9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L, Kemp A, Felmingham K, Barton M, Olivieri G, Peduto AS, Gordon E, Bryant RA ( 2006b): Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage 29: 347–357. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, Eliez S ( 2006): Decreased anterior cingulate volume in combat‐related PTSD. Biol Psychiatry 59: 582–587. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Chinitz JA, Lombardi U, Fig LM, Cameron OG, Liberzon I ( 1999): Medial frontal cortex involvement in PTSD symptoms: A SPECT study. J Psychiatry Res 33: 259–264. [DOI] [PubMed] [Google Scholar]


