Abstract
Second language (L2) acquisition is more susceptible to environmental and idiosyncratic factors than first language acquisition. Here, we used functional magnetic resonance imaging for L2 learners of different ages of first exposure (mean: 12.6 and 5.6 years) in a formal school environment, and compared the cortical activations involved in processing English sentences containing either syntactic or spelling errors, where the testing ages and task performances of both groups were matched. We found novel activation patterns in two regions of the left inferior frontal gyrus (IFG) that correlated differentially with the performances of the late and early learners. Specifically, activations of the dorsal and ventral triangular part (F3t) of the left IFG correlated positively with the accuracy of the syntactic task for the late learners, whereas activations of the left ventral F3t correlated negatively with the accuracy for the early learners. In contrast, other cortical regions exhibited differential correlation patterns with the reaction times (RTs) of the syntactic task. Namely, activations of the orbital part (F3O) of the left IFG, as well as those of the left angular gyrus, correlated positively with the RTs for the late learners, whereas those activations correlated negatively with the RTs for the early learners. Moreover, the task‐selective activation of the left F3O was maintained for both the late and early learners. These results explain individual differences in L2 acquisition, such that the acquisition of linguistic knowledge in L2 is subserved by at least two distinct inferior frontal regions of the left F3t and F3O. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: fMRI, frontal cortex, sentence processing, syntax, language acquisition
INTRODUCTION
The concept of a “critical” or “sensitive” period for first language (L1) acquisition is based on the loss of flexibility in cerebral reorganization due to acquired aphasia after puberty [Lenneberg, 1967], and it has been extended to second language (L2) acquisition, becoming the topic of debate [Birdsong and Molis, 2001; Flege et al., 1999; Friederici et al., 2002; Johnson and Newport, 1989]. It is possible that each language faculty is acquired through its own developmental process, and that the timing and duration of its sensitive period varies according to the type of acquisition (i.e., multiple sensitive periods hypothesis) [Flege, 1999; Seliger, 1978]. Because there is now evidence that some linguistic functions are separately executed in distinct cortical regions [Price, 2000; Sakai, 2005], it is all the more interesting to examine whether or not their acquisition processes differ, and if so, to what extent. Moreover, recent neuroimaging results have shown that both L1 and L2 are processed in the same cortical regions [Perani and Abutalebi, 2005; Sakai, 2005], indicating that the L2 acquisition mechanisms may be shared with L1 acquisition mechanisms.
In previous functional magnetic resonance imaging (fMRI) studies, we examined whether learning English past‐tense verbs as L2 knowledge would alter brain activation. For 2 months, 13‐year‐old students received training regarding English verbs during their regular English classroom education. When an English past‐tense task was contrasted with a verb‐matching task after the training period, activation was found primarily in the left inferior frontal gyrus (IFG), specifically the pars opercularis (F3op, Brodmann's area (BA) 44) and the pars triangularis (F3t, BA 45) of the left IFG [Sakai et al., 2004]. Moreover, the activation increase of the left dorsal F3op/F3t predicted the extent to which the individual participants improved their knowledge of the past tense. A recent fMRI study also reported greater activation of the left IFG in nonproficient English (L2) than in French (L1) during a sentence production task [Golestani et al., 2006]. To examine the effect of duration of exposure (DOE) to L2, we also tested 19‐year‐old participants who had studied English for 6 years. We found that activation of the left dorsal F3t (dF3t) was lower, corresponding to a higher proficiency level (PL) [Tatsuno and Sakai, 2005]. These results suggest that activation of the left dF3t increases with PL improvements at the initial stage of L2 acquisition, and that it becomes lower when a higher PL in L2 is attained [Indefrey, 2006; Sakai et al., 2005]. The acquisition of English past‐tense verbs as L2 knowledge is one typical example of morphosyntactic processes, and the left IFG may be required in the acquisition of syntax in general. To confirm the possibility that this region is actually involved in the acquisition of syntactic structures of sentences, more direct evidence regarding cortical activation for such syntactic knowledge is mandatory. The present study aimed to address this issue by examining the correlation between activations of each cortical region and individual performances, that is, each participant's mean accuracy or reaction times (RTs) that were specifically related to a task about syntactic structures of sentences. The second aim of the present study was to examine how participant's age of first exposure (AOE) to L2 in a formal school environment would affect the cortical activations during syntactic decision, further clarifying the nature of individual differences during language development. To dissociate the effects of AOE and DOE is not, however, within the scope of the present study; instead, we compared two groups of L2 learners with different AOEs (mean: 12.6 and 5.6 years) and DOE (2.4 and 8.7 years, respectively), whereas their developmental ages at the time of testing (15.1 and 14.3 years, respectively) were matched.
During the fMRI measurement, forced‐choice error‐detection tasks were performed: an English syntactic (Esyn) task and an English spelling (Espe) task. This paradigm is an extension of our previous experiment involving word‐order errors in English [Embick et al., 2000]. All sentence stimuli were visually presented, and the same set of sentences was used for both tasks. The Esyn task directly tested the correct use of English verbs in sentences [Levin, 1993], and the syntactic errors used in the Esyn task were basically related to argument structures of English verbs (Table I). For example, L2 learners tend to make a mistake of omitting an object, and this is because objects of transitive verbs can be omitted quite freely in many languages other than English [Cole, 1987; Park et al., 2004]. Similarly, the null‐subject (pro‐drop) is allowed in Japanese as well as in Spanish and Italian [Hyams, 1989], and Spanish speakers often accept English sentences without overt subjects [White, 1985]. Because the present paradigm explicitly requires judgment on the grammaticality of sentences, the acquisition of argument structures and their associated syntactic processes will be further elucidated. In the Espe task, on the other hand, typographical errors were included in half of the same set of sentences, to test the English orthography of words. The Espe task was used as a baseline control for the word recognition and the reading of English sentences as L2, as well as decision and response processes. Moreover, the Espe task served as the general control condition for each individual participant, and thus effects of overall developmental and educational differences (e.g., experiences and exposures in L2 that do not interact with sentence processing), if any, would be canceled out in the direct comparison of Esyn − Espe for each fMRI experiment. Here, the performances of both Esyn and Espe tasks were matched between the two participant groups, controlling the general level of intelligence and proficiency in L2. Furthermore, the cross‐linguistic effects of L1 and L2 combinations (e.g., translation) are also canceled out in Esyn − Espe, because they are common to both tasks using the same set of sentences. Cortical activations specific to sentence processing in L2 can thus be properly examined for individual participants.
Table I.
100 sets of syntactically normal and anomalous sentences
| Key sentence | Associated sentence | |
|---|---|---|
| Normal | Anomalous | |
| Can you put your bag on the table? | No, I can't put it there. | No, I can't put there. |
| Do you find a problem here? | No, I do not find one here. | No, I do not find here. |
| Do you like dogs very much? | Yes, I like them very much. | Yes, I like very much. |
| Do you play outside a lot? | No, I don't play outside a lot. | No, I play not outside a lot. |
| Do you smile often? | No, I do not smile often. | No, I smile not often. |
| Will you work today? | No, but I will work tomorrow. | |
| Do you often laugh? | Yes, I often laugh. | |
| Can you tell me the news now? | No, but I can tell it later. | No, but I can tell later. |
| Can you walk today? | No, I can't walk today. | No, I walk not today. |
| Do you enjoy tennis at school? | No, I don't enjoy it there. | No, I don't enjoy there. |
| Can you bring your bag here? | Yes, I can bring it there. | Yes, I can bring there. |
| Can you carry your bag now? | Yes, I can carry it now. | Yes, I can carry now. |
| Can you speak here? | No, I can't speak here. | No, I speak not here. |
| Will you fight here? | Yes, I will fight here. | |
| Do you often think about history? | Yes, I do. | Yes, I often think about. |
| Can you send this letter now? | No, but I can send it later. | No, I can't send. |
| Do you talk a lot? | No, I don't talk a lot. | No, I talk not a lot. |
| Do you really love Mary? | Yes, I really love her. | Yes, I really love. |
| Do you sleep a lot? | Yes, I sleep a lot. | |
| Do you often meet Mary? | No, I don't often meet her. | No, I don't often meet. |
| You have a test today. | You must begin the test at 9. | |
| You can bring John here. | John can come here. | You can come John. |
| There are some problems here. | Some problems still remain here. | You can remain some problems here. |
| A battle will happen here. | You can stop the battle. | |
| You have an old glass. | You can break the glass. | |
| There is a bag on the table. | You can lay your bag there. | You can lie your bag there. |
| There is a flower in the garden. | I can grow flowers here. | |
| There are some plants in the garden. | Plants can't exist without water. | You cannot exist plants without water. |
| You have a car. | I can move the car. | |
| There have been some accidents here. | Accidents often happen here. | You may happen accidents here. |
| John's house is here. | John lives here. | You live John. |
| There are many examinations at school. | We start examinations on Monday. | |
| You can kill many monsters in the game. | They will die in the game. | You can die them in the game. |
| This window is closed now. | You can open the window. | |
| You have a nice hairstyle. | You can change your hairstyle. | |
| I know the news. | The news will appear on TV. | You will appear the news on TV. |
| You can continue the game here. | The game lasts till 5. | You can last the game. |
| That door is open now. | You can close the door. | |
| You can sell your house. | You can increase its price. | |
| There is a strange sound in the room. | The sound will occur soon. | You can occur the sound. |
| John thinks in his room. | He thinks carefully. | |
| Many accidents happen on the road. | They often happen there. | They happen carefully. |
| A strange sound will occur in the room. | It sometimes occurs there. | It will occur hard there. |
| John speaks in the class. | He speaks carelessly. | |
| John smiles at Mary. | He smiles on purpose. | |
| There is a door over there. | He will open it carefully. | It will open carefully. |
| John laughs at Mary. | He laughs on purpose. | |
| John changes his hairstyle. | It changes a lot. | It changes carelessly |
| John plays in the park. | He plays there willingly. | |
| Some food still remains in the house. | It still remains there. | It remains there carelessly. |
| John works at the office. | He works hard. | |
| The news appears on TV today. | It appears quickly. | It appears on purpose. |
| John walks to school. | He walks cautiously. | |
| John begins the test. | It begins now. | It begins cautiously. |
| John talks in the room. | He talks carefully. | |
| These flowers grow in the garden. | They always grow quickly. | They grow carefully. |
| John sleeps on the floor. | He sleeps there willingly. | |
| It will rain today. | It will stop soon. | It will stop willingly. |
| John can move the car. | It moves slowly. | It moves carefully. |
| John is fighting in the war. | He fights cautiously. | |
| We have a baseball game today. | It will be a long game. | It will be a long laster. |
| John thinks in his room. | He is a famous thinker. | |
| This rose grows in the garden. | It is a nice flower. | It is a nice grower. |
| John often speaks in the class. | He is a good speaker. | |
| John often dances on the stage. | He is a famous dancer. | |
| John starts the train. | It is a slow train. | It is a slow starter. |
| John often swims in the sea. | He is a good swimmer. | |
| He will drop the book | It is a heavy book. | It is a heavy dropper. |
| John plays in the park. | He is a happy player. | |
| John might die soon. | It will be an early death. | He will be an early dier. |
| John works at the hospital. | He is a good worker. | |
| Many car crashes happen at the corner. | They are terrible accidents. | They are terrible happeners. |
| John is beginning the card game of poker. | It is a quiet game. | It is a quiet beginner. |
| John walks to school. | He is a fast walker. | |
| Some food still remains in the house. | It is a secret stock. | It is a secret remainer. |
| John often talks in the city hall. | He is a nice talker. | |
| John often sleeps on the street. | He is a street sleeper. | |
| John stops the battle. | It is a hard battle. | It is a hard stopper. |
| John is fighting in the war. | He is a strong fighter. | |
| John moves the car. | It is a quiet car. | It is a quiet mover. |
| John often comes to my house. | He will come to my house. | Soon will come. |
| I am famous in this hospital. | I am a famous doctor. | Am a famous doctor. |
| John might tell the news at school. | He will tell it at school. | He will tell at school. |
| John stopped the train at the station. | He will start it soon. | Soon will start it. |
| John begins the game here. | He begins it now. | He begins now. |
| John is kind in the class. | He is a kind teacher. | Is a kind teacher. |
| Spotty finds a cake in the kitchen. | He finds it quickly. | He finds quickly. |
| You are good at school. | You are a good student. | Are a good student. |
| John works in this hospital. | He works very much. | Very much works. |
| John thinks in his room. | He always thinks. | Always thinks. |
| Do you believe in Santa Claus? | Santa Claus really exists. | We can exist Santa Claus. |
| We have bad weather in this town. | We can't change the weather. | |
| John is starting the computer game. | He is a quiet starter. | |
| John is beginning the card game of poker. | He is a real beginner. | |
| John grows a rose. | He is a famous grower. | |
| I want to buy a violin. | We can play this violin like this. | This violin can play like this. |
| I want to buy a new car. | We must keep new cars inside. | New cars must keep inside. |
| John speaks English very well. | He can speak French, too. | Can speak French, too. |
| There once was an old man in the village. | There once lived an old man in the village. | |
| There was no solution to this problem. | There exists no solution to this problem even now. | |
MATERIALS AND METHODS
Participants
Thirty native Japanese speakers aged from 12 to 17 participated in two groups: late learners and early learners. All of the participants had studied English as L2 in Japanese schools, and the L2 knowledge referred to here was acquired in a formal school environment alone. The late learners were 18 students (10 females and eight males, ages at the time of retesting: 14.1–17.3 years, mean ± SD: 15.1 ± 1.2) from the Secondary Education School Attached to the Faculty of Education of the University of Tokyo, and their AOEs were 12.6 ± 0.2. The early learners were 12 students (eight females and four males, ages: 12.3–16.4 years, 14.3 ± 1.7) from Gyoshu Junior & Senior High School (Numazu‐shi, Japan) who had participated in an English immersion program, and their AOEs were 5.6 ± 0.9. Informed consent was obtained from all participants as well as from their parents/guardians. The study was approved by both the schools and by the institutional review board of the University of Tokyo, Komaba.
The late learners received 10 sessions of classroom training at school within 1–2 months, in which the correct usage of English verbs was tested with 10 examples for each session. Each example consisted of a key sentence followed by its associated two sentences, one of which contained a syntactic error in some cases (see Table I for 100 sets of a key sentence and its associated sentences with or without a syntactic anomaly). The students first tried to guess their grammaticality, and a teacher then provided them with the correct answers and a brief explanation. They further underwent two test sessions: Test 1 and Test 2, which refer to the fMRI measurement before and after the training, respectively. A new set of sentences with the same English verbs was used for Test 2 to ensure whether they had actually acquired the knowledge of syntactic structures, rather than merely memorizing the sentence examples used for Test 1 and the training. The early learners underwent a single fMRI measurement without any special training or instruction regarding English verbs.
Stimuli and Tasks
For the training and fMRI sessions, we selected 42 high‐frequency English verbs (20 transitive and 22 intransitive, including 12 unergatives and 10 unaccusatives [Yusa, 2003]) and made 200 sets of sentences using these verbs, 100 each for Test 1 and Test 2 (Table I). The same words including these verbs were used for the two Tests but with different word combinations. In each trial of the fMRI experiments, stimuli in yellow letters against a dark background were visually presented. For fixation, a red cross was always shown at the center of the screen. The stimulus presentation and behavioral data collection were controlled using LabVIEW software and interface (National Instruments, Austin, TX). The participants wore earplugs and an eyeglass‐like MRI‐compatible display (resolution: 800 × 600) (VisuaStim XGA, Resonance Technology, Northridge, CA).
At the initiation of every trial of 7 s, the word “Syntax” (Esyn) or “Spelling” (Espe) was presented as a cue for 400 ms, signaling the beginning of the task to be performed. Next, a set of two English sentences (Table I) were shown for 6,400 ms. The participants read the two sentences silently and indicated whether or not the sentences contained an error by pushing one of two buttons within 6,400 ms. The tasks were conducted in a block design, and five trials were tested in each of the Esyn and Espe blocks (35 s). Individual trials were regarded as independent events, and only trials with correct responses were used for analyses of RTs and activations. Japanese (L1) versions of both tasks, that is, Jsyn and Jspe, were also conducted in separate blocks of five trials each. The sequence of a single fMRI run was in the order of either Jsyn‐Espe‐Esyn‐Espe‐Jspe or Jspe‐Espe‐Esyn‐Espe‐Jsyn, and their order was counterbalanced across the participants. It might be possible that switching between two languages exerted a differential impact on the late and early learners, but it was advantageous to control general conditions within the same runs. Moreover, negative transfer effects of syntactic knowledge from L1 to L2, if any, can be controlled by Espe that separated Esyn from Jsyn and Jspe. For each participant, 10 fMRI runs were carried out in a single day.
fMRI Data Acquisition and Analyses
The fMRI scans were conducted using a 1.5 T MRI system (STRATIS II, Premium; Hitachi Medical Corporation, Tokyo, Japan). Using a gradient echo echo‐planar imaging sequence (repetition time = 7 s, acquisition time = 1850 ms, echo time = 50.5 ms, flip angle = 90°, field of view = 192 × 192 mm2, resolution = 3 × 3 mm2), we scanned 16 horizontal slices (each 6 mm thick and having a 1‐mm gap, covering z = −49 to 62 mm). We set the timing of the first slice acquisition to 4,700 ms after the onset of sentence stimuli, so that most of the task period was silent (i.e., without scanner noise). Group analyses were performed using SPM5 statistical parametric mapping software (Wellcome Department of Imaging Neuroscience, London, UK). We realigned the functional volume data in multiple runs, and removed runs that included data with a translation of >2 mm in one of the three directions and a rotation of >1.4°. Each individual brain was spatially normalized to the standard brain space as defined by the Montreal Neurological Institute (MNI), resampled every 3 mm using trilinear interpolation, and smoothed with an isotropic Gaussian kernel of 9 mm full width at half maximum. Low‐frequency noise and global changes in activity were further removed. The acquisition timing of each slice was corrected using the first slice as a reference for the functional imaging data. By using event‐related canonical hemodynamic response functions, activations during the presentation of sentence stimuli were analyzed. For random effects analyses, a contrast image was generated for each participant and used for intersubject comparisons.
We used a high stringent task contrast of Esyn − Espe or Jsyn − Jspe, and thus adopted a two stage procedure established in a previous study [Crinion et al., 2006], starting with a whole brain search using a liberal statistical threshold for the voxel level (uncorrected P < 0.005). An inclusive mask of Esyn/Jsyn (uncorrected P < 0.05) or Esyn − Espe (uncorrected P < 0.05 for correlation analyses and group comparisons) was applied to eliminate any deactivation under the reference condition. To exclude a false positive activation, we then focused on effects that replicated across our present and previous fMRI studies and used a small volume correction (SVC) at the level of corrected P < 0.05 (16 mm radius for the late learners and 10 mm radius for the early learners, depending on the number of participants). The center coordinates for the SVC are described in the Tables III, IV, and V. For independent replication of the fMRI data between the late and early learners, the combined probability of observing the same region in each group was less than uncorrected P = 2.5 × 10−5, corresponding to a Z score of 4.1. Statistical significance of correlation between the task performances and activations (parameter estimates) at a local maximum of activation was further confirmed using Spearman's rank correlation, which is resistant to outliers, that is, high leverage points.
Table III.
Activated regions for the late learners
| Brain region | BA | Side | x | y | z | Z | x | y | z |
|---|---|---|---|---|---|---|---|---|---|
| SVC | |||||||||
| Esyn − Espe: Test 1 | |||||||||
| LPMC | 6 | L | −33 | 6 | 39 | 4.0 | −39 | 6 | 36a |
| L | −48 | −9 | 39 | 3.8 | −39 | 3 | 42a | ||
| F3O | 47 | L | −42 | 12 | −12 | 3.1 | −45 | 24 | −9b |
| AG | 39 | L | −54 | −51 | 21 | 4.3 | −51 | −60 | 27c |
| 39 | L | −42 | −63 | 24 | 4.2 | −51 | −60 | 27c | |
| MTG | 21 | L | −66 | −39 | 3 | 3.9 | −54 | −42 | 3d |
| Esyn − Espe: Test 2 | |||||||||
| dF3t | 45 | L | −54 | 24 | 24 | 3.6 | −54 | 27 | 21a |
| F3O | 47 | L | −33 | 27 | −9 | 3.1 | −45 | 24 | −9b |
| AG | 39 | L | −39 | −72 | 33 | 3.1 | −45 | −75 | 36c |
| MTG | 21 | L | −54 | −45 | 6 | 3.7 | −54 | −42 | 3d |
| Esyn: Test 2 | Regressor: Acc (Esyn) | ||||||||
| dF3t | 45 | L | −57 | 24 | 15 | 3.1 | −54 | 27 | 21a |
| Esyn − Espe: Test 2 | Regressor: RTs (Esyn) | ||||||||
| F3O | 47 | L | −36 | 18 | −21 | 3.6 | −45 | 24 | −9b |
| AG | 39 | L | −39 | −75 | 33 | 4.5 | −45 | −75 | 36c |
| Jsyn − Jspe: Test 1 + Test 2 | |||||||||
| LPMC | 6 | L | −42 | 12 | 48 | 3.8 | −39 | 3 | 42a |
| F3t | 45 | L | −51 | 21 | 9 | 3.0 | −54 | 27 | 21a |
| MTG | 21 | L | −51 | −39 | 0 | 3.2 | −54 | −42 | 3d |
| 21 | R | 54 | −45 | 0 | 3.8 | 66 | −36 | 3b | |
| (Esyn − Espe) − (Jsyn − Jspe): Test 2 | |||||||||
| dF3t | 45 | L | −48 | 24 | 27 | 2.7 | −54 | 27 | 21a |
[Hashimoto and Sakai, 2002].
[Homae et al., 2003].
[Sakai et al., 2005].
[Suzuki and Sakai, 2003].
Stereotactic coordinates (x, y, z) in the MNI space are shown for each activation peak of Z values.
Acc, accuracy; AG, angular gyrus; BA, Brodmann's area; F3O, orbital part of the inferior frontal gyrus; F3t, triangular part of the inferior frontal gyrus (d, dorsal; v, ventral); L, left hemisphere; LPMC, lateral premotor cortex; MTG, middle temporal gyrus; RTs, reaction times.
Table IV.
Activated regions for the early learners
| Brain region | BA | Side | x | y | z | Z | x | y | z |
|---|---|---|---|---|---|---|---|---|---|
| SVC | |||||||||
| Esyn − Espe | |||||||||
| F3O | 47 | L | −45 | 36 | −9 | 3.0 | −45 | 30 | −15a |
| Jsyn − Jspe | |||||||||
| AG | 39 | L | −42 | −60 | 18 | 2.9 | −51 | −60 | 27a |
| MTG | 21 | L | −54 | −39 | 0 | 2.7 | −54 | −42 | 3b |
| Esyn − Espe | Regressor: Acc (−Esyn) | ||||||||
| vF3t | 45 | L | −42 | 27 | 0 | 2.9 | −51 | 24 | −3c |
| Esyn − Espe | Regressor: RTs (−Esyn) | ||||||||
| AG | 39 | L | −54 | −57 | 36 | 3.4 | −51 | −60 | 27a |
Table V.
Activated regions for the late (Test 2) and/or early learners
| Brain region | BA | Side | x | y | z | Z | x | y | z |
|---|---|---|---|---|---|---|---|---|---|
| SVC | |||||||||
| Esyn − Espe: Late − Early | |||||||||
| dF3t | 45 | L | −51 | 27 | 24 | 3.1 | −54 | 27 | 21a |
| Esyn − Espe: Late + Early | |||||||||
| LPMC | 6 | L | −45 | 9 | 45 | 3.1 | −39 | 3 | 42a |
| F3O | 47 | L | −42 | 27 | −9 | 4.0 | −45 | 30 | −15b |
| AG | 39 | L | −39 | −69 | 36 | 3.5 | −45 | −75 | 36b |
| MTG | 21 | L | −45 | −45 | 6 | 3.8 | −54 | −42 | 3c |
| Esyn − Espe: Late − Early | Regressor: Acc (Esyn) | ||||||||
| vF3t | 45 | L | −51 | 27 | 0 | 3.0 | −51 | 24 | −3d |
| Esyn − Espe: Late − Early | Regressor: RTs (Esyn) | ||||||||
| F3O | 47 | L | −36 | 33 | −15 | 2.9 | −45 | 30 | −15b |
| AG | 39 | L | −36 | −75 | 33 | 4.3 | −45 | −75 | 36b |
| 39 | L | −48 | −63 | 33 | 2.6 | −51 | −60 | 27b | |
RESULTS
Behavioral Data
Behavioral data for the Esyn and Espe tasks are shown in Figure 1A and Table II. The late learners consistently showed significant improvement from Test 1 to Test 2 in both tasks. According to a repeated‐measures analysis of variance (rANOVA) with task [Esyn, Espe] × test session [Test 1, Test 2], the accuracy data showed significant main effects of task (F(1, 17) = 69, P < 0.0001) and test session (F(1, 17) = 35, P < 0.0001) with no significant interaction (F(1, 17) = 0.1, P = 0.7). The RTs also showed significant main effects of task (F(1, 17) = 25, P = 0.0001) and test session (F(1, 17) = 25, P < 0.0001) with no significant interaction (F(1, 17) = 0.009, P = 0.9). For Test 2, however, the correlation between the accuracy and RTs of Esyn was not significant (r = 0.08, r s = 0.22, P = 0.4), and neither was the correlation between the accuracy and RTs of Espe (r = −0.36, r s = −0.33, P = 0.2). On the other hand, the accuracy of Esyn correlated significantly with that of Espe among the late learners for Test 2 (r = 0.77, Spearman rank correlation coefficient: r s = 0.71, P = 0.003; Fig. 1B). The correlation between the accuracy of Esyn and Espe for Test 1 was marginally significant (r = 0.50, r s = 0.45, P = 0.070), and the correlation between the accuracy of Esyn and Espe for the early learners was also marginally significant (r = 0.45, r s = 0.56, P = 0.065). These results suggest that the training for the late learners enabled them to effectively acquire the L2 knowledge of both syntax and spellings.
Figure 1.

Performance data in L2 for the late and early learners. A: Accuracy of the Esyn and Espe tasks. The performance data for Test 1 (before training) and Test 2 (after the training) are shown separately for the late learners; the early learners underwent no training for the present study. Error bars denote standard errors among the participants. Asterisks indicate the statistical significance (t‐test, P < 0.005). N.S., not significant (P > 0.2). B: Correlation of the performance data for the late learners. The accuracy of Esyn for Test 2 correlated significantly with that of Espe for Test 2 among the late learners.
Table II.
Behavioral data for each group and task condition
| Group | Accuracy (%) | RTs (ms) | ||
|---|---|---|---|---|
| Esyn | Espe | Esyn | Espe | |
| Late learners, Test 1 | 52.3 ± 8.0 | 69.3 ± 15.9 | 4,414 ± 588 | 4,137 ± 524 |
| Late learners, Test 2 | 63.5 ± 10.7 | 81.6 ± 13.3 | 3,753 ± 570 | 3,468 ± 476 |
| Early learners | 66.6 ± 12.1 | 86.4 ± 7.4 | 3,876 ± 478 | 3,525 ± 681 |
| Jsyn | Jspe | Jsyn | Jspe | |
| Early learners | 89.2 ± 7.4 | 83.7 ± 7.9 | 3,507 ± 619 | 3,485 ± 639 |
| Late learners, Test 1 | 89.8 ± 8.0 | 84.7 ± 9.8 | 3,707 ± 513 | 3,671 ± 409 |
| Late learners, Test 2 | 95.5 ± 4.3 | 88.6 ± 8.5 | 3,037 ± 563 | 3,105 ± 649 |
Data are shown as mean ± SD.
After the training, the accuracy of the Esyn and Espe tasks for the late learners became equally high with that for the early learners (Fig. 1A). According to an rANOVA with task × group [early learners, late learners for Test 2], the accuracy data showed a significant main effect of task (F(1, 28) = 114, P < 0.0001), but neither a significant main effect of group (F(1, 28) = 1.1, P = 0.3) nor a significant interaction (F(1, 28) = 0.2, P = 0.7). The RTs also showed a significant main effect of task (F(1, 28) = 27, P < 0.0001), but neither a significant main effect of group (F(1, 28) = 0.2, P = 0.7) nor a significant interaction (F(1, 28) = 0.3, P = 0.6). These results indicate that the task performances were matched between the two groups after the training. For the early learners, the correlation between the accuracy and RTs of Esyn was not significant (r = 0.02, r s = 0.12, P = 0.7), but the negative correlation between the accuracy and RTs of Espe was significant (r = −0.65, r s = −0.59, P = 0.05).
Behavioral data for the Jsyn and Jspe tasks are shown in Table II. The late learners showed improvement from Test 1 to Test 2 in both tasks, but this effect was simply due to repeating the same tasks. According to an rANOVA with task [Jsyn, Jspe] × test session [Test 1, Test 2], the accuracy data showed significant main effects of task (F(1, 17) = 9.3, P = 0.007) and test session (F(1, 17) = 16, P = 0.001) with no significant interaction (F(1, 17) = 0.7, P = 0.4). The RTs also showed a significant main effect of test session (F(1, 17) = 29, P < 0.0001), but neither a significant main effect of task (F(1, 17) = 0.05, P = 0.8) nor a significant interaction (F(1, 17) = 0.65, P = 0.4). At the time of the first testing, the performances of the Jsyn and Jspe tasks were comparable between the late and early learners. According to an rANOVA with task × group [early learners, late learners for Test 1], the accuracy data showed a significant main effect of task (F(1, 28) = 7.3, P = 0.01), but neither a significant main effect of group (F(1, 28) = 0.1, P = 0.7) nor a significant interaction (F(1, 28) = 0.01, P = 0.9). The RTs showed neither significant main effects of task (F(1, 28) = 0.2, P = 0.6) and group (F(1, 28) = 1.0, P = 0.3) nor a significant interaction (F(1, 28) = 0.01, P = 0.9). These results further confirm the matched general performances between the two groups.
Task‐Selective Activation Patterns for the Late Learners
Cortical activations selective to the Esyn task were identified by the contrast of Esyn − Espe, separately for Test 1 and Test 2 (Fig. 2A,B and Table III). For Test 1, the activation was scattered in both hemispheres, including the left lateral premotor cortex (LPMC, BA 6), the pars orbitalis (F3O, BA 47) of the left IFG, two separated regions in the left angular gyrus (AG, BA 39), and the left middle temporal gyrus (MTG, BA 21). The bilateral postcentral gyrus and parietal operculum (BA 43) also showed activation which was not directly related to linguistic functions. For Test 2, however, it is striking to observe that the activation clearly localized in the left hemisphere, and that the significant activation emerged in the left dF3t [(x, y, z) = (−54, 24, 21); a yellow circle in Fig. 2B], in addition to the language‐related regions of the left F3O, AG, and MTG. These results suggest that the left dF3t is a region most crucially involved in training to understand syntactic structures, consistent with our hypothesis that this region subserves syntactic processing.
Figure 2.
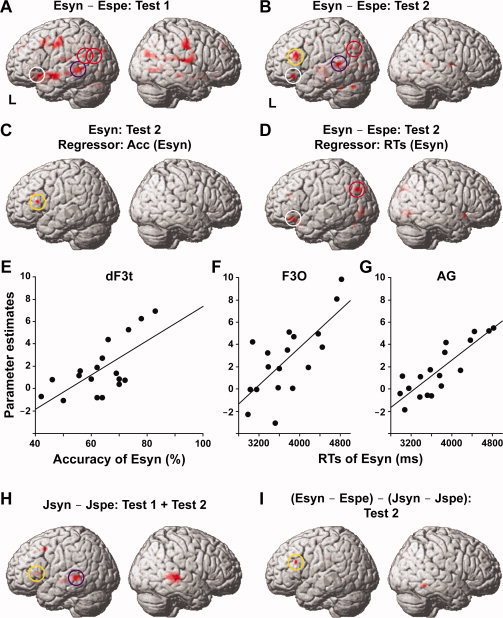
Task‐selective activation patterns for the late learners (Table III). A: Regions showing activation in Esyn − Espe for Test 1. The regions are projected onto a standard brain (L, left). B: Regions showing activation in Esyn − Espe for Test 2. Note the prominent activation of the left dF3t (yellow circle), which is absent in Fig. 2A. C: Regions showing activation in Esyn for Test 2, correlated with the accuracy (Acc) of Esyn for Test 2. Note the activation of the left dF3t (yellow circle). D: Regions showing activation in Esyn − Espe for Test 2, correlated with the RTs of Esyn for Test 2. Note the prominent activation of the left F3O (white circle) and AG (red circle). E: Significant correlation between activations in Esyn at the left dF3t and the accuracy of Esyn for Test 2. Each filled dot represents a late learner. F: Significant correlation between activations in Esyn − Espe at the left F3O and the RTs of Esyn for Test 2. G: Significant correlation between activations in Esyn − Espe at the left AG and the RTs of Esyn for Test 2. H: Regions showing activation in Jsyn − Jspe for both Test 1 and Test 2. Activation of the left MTG (purple circle) matches that in Fig. 2A, B. I: Regions showing activation in (Esyn − Espe) − (Jsyn − Jspe) for Test 2. Activation of the left dF3t (yellow circle) matches that in Fig. 2B,C.
Next, we tested whether or not the left dF3t activations predicted the extent to which each individual participant improved his/her knowledge of syntax in L2 after the training (i.e., Test 2). We found that the left dF3t activations in Esyn showed a significant correlation with the accuracy of Esyn (Fig. 2C, Table III). At the coordinates (−57, 24, 15) of the left dF3t, the activations in Esyn correlated significantly with the accuracy of Esyn (r = 0.68, r s = 0.54, P = 0.026; Fig. 2E). Regarding activations in Esyn − Espe, both the left dF3t [(−48, 27, 18), Z = 2.4, uncorrected P = 0.008] and ventral F3t (vF3t) [(−51, 27, −6), Z = 2.6, uncorrected P = 0.010] showed a positive correlation just below the threshold. The modulation of the left F3t activation cannot be explained merely by task difficulty or other domain‐general factors, because the easier the Esyn task became the more strongly the left F3t was activated.
In contrast, a correlation analysis with the RTs of Esyn (Test 2) revealed significant activation in the left F3O (a white circle in Fig. 2D) and AG (a red circle in Fig. 2D). The activations in Esyn − Espe correlated significantly with the RTs of Esyn at the coordinates (−39, 27, −12) of the left F3O (r = 0.72, r s = 0.68, P = 0.0049; Fig. 2F), as well as at (−39, −75, 33) of the left AG (r = 0.86, r s = 0.82, P = 0.0007; Fig. 2G). No significant activation showed negative correlation with the accuracy or RTs of Esyn. Here it is interesting to note that L1‐related activation in Jsyn − Jspe was observed in the left LPMC and F3t as well as in the bilateral MTG (Fig. 2H, Table III). For this analysis, data for both Test 1 and Test 2 were combined to enhance the consistent activation. Furthermore, the direct comparison between L2 and L1 (i.e., Esyn − Espe vs. Jsyn − Jspe for Test 2) revealed significant activation in the left dF3t (Fig. 2I). These results clearly indicate the critical roles of the left dF3t, F3O, and AG in L2.
Task‐Selective Activation Patterns for the Early Learners
In spite of the matched task performances of the late learners (Test 2) and the early learners (Fig. 1A), the early learners showed strikingly different modulation patterns of activations. In Esyn − Espe, we observed significant activation in the left F3O (a white circle in Fig. 3A, Table IV), but not in the left F3t. The left F3O activation which centered on the coordinates (−45, 36, −9), coincides with (−45, 30, −15) as reported in our recent study of L1 [Sakai et al., 2005]. In Jsyn − Jspe, the left AG and MTG were significantly activated for the early learners (Fig. 3B); the left MTG activation was consistent with that for the late learners (Fig. 2H). On the other hand, no significant activation showed positive correlation with the accuracy or RTs of Esyn. A correlation analysis in Esyn − Espe with the accuracy of Esyn as a negative regressor, however, revealed a significant activation in the left vF3t (a yellow circle in Fig. 3C, Table IV). The left vF3t activation, which centered on the coordinates (−42, 27, 0), also coincides with (−51, 24, −3) as reported previously [Momo et al., 2008]. At the coordinates (−42, 27, 0) of the left vF3t, activations in Esyn − Espe correlated negatively with the accuracy of Esyn (r = −0.76, r s = −0.73, P = 0.015; Fig. 3E), and so did the activations in Esyn (r = −0.62, r s = −0.61, P = 0.044). In accordance with our previous study [Tatsuno and Sakai, 2005], this proficiency‐dependent negative correlation suggests that the syntactic knowledge may have been consolidated in the left vF3t for the early learners.
Figure 3.

Task‐selective activation patterns for the early learners (Table IV). A: Regions showing activation in Esyn − Espe. Activation of the left F3O (white circle) matches that in Fig. 2A,B,D. B: Regions showing activation in Jsyn − Jspe. Note the activation of the left AG (red circle) and MTG (purple circle). C: Regions showing activation in Esyn − Espe, correlated negatively with the accuracy of Esyn. Note the prominent activation of the left vF3t (yellow circle). D: Regions showing activation in Esyn − Espe, correlated negatively with the RTs of Esyn. Note the activation of the left AG (red circle). E: Significant negative correlation between activations in Esyn − Espe at the left vF3t and the accuracy of Esyn. Each open dot represents an early learner. F: Significant negative correlation between activations in Esyn − Espe at the left AG and the RTs of Esyn.
In contrast, a correlation analysis in Esyn − Espe with the RTs of Esyn as a negative regressor revealed a significant activation in the left AG (a red circle in Fig. 3D). At the coordinates (−54, −57, 36) of the left AG, activations in Esyn − Espe correlated negatively with the RTs of Esyn (r = −0.84, r s = −0.81, P = 0.0071; Fig. 3F). The coordinates were anterolateral to those observed for the late learners with the RTs of Esyn as a positive regressor (Fig. 2D). Because the left AG was activated in Jsyn − Jspe for the early learners (Fig. 3B), more efficient processing in L2 with shorter RTs, which corresponded to more activations for the early learners, may be extrapolated to linguistic processing in L1.
Direct Comparisons Between the Late and Early Learners
Because some contrasting results were obtained between the late (Test 2) and early learners, we further performed direct group comparisons in Esyn − Espe. First, the late learners showed significantly more enhanced activation in the left dF3t (Fig. 4A, Table V) than the early learners, which strengthens the main results obtained for the late learners. Moreover, when both groups were combined, consistent activation in Esyn − Espe was observed in the left LPMC and F3O, in addition to the left AG and MTG (Fig. 4B). A differential correlation analysis in Esyn − Espe with the accuracy of Esyn as a positive regressor for the late learners and as a negative regressor for the early learners revealed a significant activation in the left vF3t alone (a yellow circle in Fig. 4C). At the coordinates (−51, 27, 0) of the left vF3t, activations in Esyn − Espe correlated differentially between the late and early learners with the accuracy of Esyn (late learners: r = 0.48, r s = 0.54, P = 0.025; early learners: r = −0.63, P = 0.03, r s = −0.48, P = 0.1; Fig. 4E). In contrast, a differential correlation analysis in Esyn − Espe with the RTs of Esyn resulted in a significant activation in the left F3O (a white circle in Fig. 4D) and the two separated regions in the left AG. At the coordinates (−33, 33, −15) of the left F3O, activations in Esyn − Espe also correlated differentially between the late and early learners with the RTs of Esyn (late learners: r = 0.64, r s = 0.49, P = 0.042; early learners: r = −0.46, P = 0.1, r s = −0.36, P = 0.2; Fig. 4F). These results from direct group comparisons further clarify the distinct functional roles of the left F3t and F3O in L2 acquisition.
Figure 4.

Task‐selective activation patterns for the late (Test 2) and/or early learners (Table V). A: Regions showing activation in Esyn − Espe for the group comparison of Late − Early. Note the prominent activation of the left dF3t (yellow circle), consistent with that in Fig. 2B,C,I. B: Regions showing activation in Esyn − Espe for combining both groups, that is, Late + Early. Note the prominent activation of the left F3O (white circle), consistent with that in Figs. 2A,B,D and 3A. C: Regions showing activation in Esyn − Espe for the group comparison of Late − Early, correlated with the accuracy of Esyn. Note the prominent activation of the left vF3t (yellow circle), consistent with that in Fig. 3C. D: Regions showing activation in Esyn − Espe for the group comparison of Late − Early, correlated with the RTs of Esyn. Note the activation of the left F3O (white circle) and AG (red circles), consistent with that in Figs. 2D and 3D. E: Significant correlation between activations in Esyn − Espe at the left vF3t and the accuracy of Esyn. Each filled dot represents a late learner, whereas each open dot represents an early learner. F: Significant correlation between activations in Esyn − Espe at the left F3O and the RTs of Esyn.
DISCUSSION
The results obtained for the two groups of L2 learners in a formal school environment are novel and striking in two respects. First, we found significant correlations between activations in multiple cortical regions and individual performances that were specifically related to syntactic structures of sentences. Second, regarding the second aim of the present study, the different AOE of the late and early learners actually affected activations during syntactic decision. More specifically, activations of the left dF3t (Fig. 2C,E) and vF3t (Fig. 4C,E) correlated positively with the accuracy of the Esyn task for the late learners, whereas activations of the left vF3t correlated negatively with that for the early learners (Figs. 3C,E and 4E). It should be noted that this group difference in brain activations was observed even when the testing ages and task performances of both groups were matched, with overall developmental and educational differences controlled by the Espe task. On the basis that cortical activation increases initially at the onset of L2 acquisition, followed by a reduction in activation during the consolidation of linguistic competence [Sakai, 2005], we speculate that the functional development of the left F3t was in an initial stage for the late learners and in a more advanced stage for the early learners. In contrast, other cortical regions exhibited differential correlation patterns with the RTs of the Esyn task. Namely, activations of the left F3O, as well as those of the left AG, correlated positively with the RTs for the late learners (Fig. 2D,F,G), whereas those activations correlated negatively with the RTs for the early learners (Figs. 3D,F and 4D,F). Moreover, the task‐selective activation of the left F3O was maintained for both the late and early learners (Figs. 2A,B, 3A, and 4B). These results explain individual differences in L2 acquisition, such that the acquisition of linguistic knowledge in L2 is subserved by at least two distinct inferior frontal regions of the left F3t and F3O.
Functional specialization in the left IFG has been extensively examined by recent human brain mapping studies. Accumulating evidence has indicated that the left F3op, dF3t, vF3t, and LPMC are all specialized in syntactic processing [Dapretto and Bookheimer, 1999; Embick et al., 2000; Friederici et al., 2000; Kinno et al., 2008; Momo et al., 2008; Musso et al., 2003; Stromswold et al., 1996; Suzuki and Sakai, 2003]. The left LPMC is located at the junction of the precentral sulcus and the inferior frontal sulcus, just dorsal to the left F3op/F3t, and this region was also activated in the present study (Figs. 2A,H and 3B). Recent fMRI studies have also tried to specify the function of this region during sentence processing including the operation of movement [Ben‐Shachar et al., 2004], syntactic linearization [Grewe et al., 2005], and processing of string patterns [Friederici et al., 2006]. We have previously demonstrated that these regions showed prominent activation for syntactic decision tasks even when they were directly compared with verbal short‐term memory tasks [Hashimoto and Sakai, 2002]. The activation of the left LPMC and F3op/F3t is thus related to the process of analyzing syntactic structures, which cannot be explained either by task difficulty or by verbal short‐term memory components. On the other hand, we have reported that the left F3t/F3O is specialized in the selection and integration of semantic information within sentences as revealed by a direct comparison of cortical activation during sentence comprehension tasks and lexical decision tasks under both auditory and visual conditions [Homae et al., 2002, 2003]. Moreover, we have recently clarified that the left F3t/F3O activation is indeed selective to sentence comprehension, irrespective of the modalities of sign and speech [Sakai et al., 2005]. These results indicate an essential and universal role of the left F3t/F3O, which subserves sentence comprehension [Sakai, 2005].
The detailed results obtained in the present study indicate more precise nature of the distinct functional roles of the frontal regions proposed above, namely, syntax (e.g., building syntactic structures) in the left F3t and sentence comprehension (e.g., reading off the meanings from the syntactic structures) in the left F3O. The syntactic knowledge tested here may have been consolidated for the early learners, thus leading to the negative correlation between the left vF3t activations and the accuracy of Esyn (Fig. 3C,E). Note, however, that some individuals in this group still showed the low accuracy of Esyn. In contrast, acquiring the ability of sentence comprehension usually takes longer and requires more elaborative processes, because sentence comprehension involves understanding not only the literal meanings of sentences but also their hidden meanings—“reading between the lines.” RTs for bilinguals might reflect differences in L2 proficiency, but the accuracy and RTs of Esyn showed no correlation at all for both the late and early learners (see Behavioral Data). The positive correlation between the left F3O activations and the RTs of Esyn among the late learners (Figs. 2D,F and 4D,F) suggests that the longer the sentences were read the more the left F3O was utilized. Among the early learners, in contrast, the relationship between the activations and the RTs of Esyn was just reversed, such that the shorter the sentences were read the more efficiently the left F3O and AG (Figs. 3D,F and 4D,F) were recruited. It is thus possible that the cortical activations reflect the processing load itself for sentence comprehension, which can be facilitated by the use of syntactic knowledge required by the Esyn task. Unlike the left F3t, however, the left F3O activations were independent of the accuracy of Esyn itself. These results support the parallel processing of syntax in the left F3t and sentence comprehension in the left F3O.
The activation of the left MTG both in L2 (Figs. 2A,B and 4B) and in L1 (Figs. 2H and 3B) matches with its supporting role in sentence processing reported previously for adult native speakers [Fiebach et al., 2005; Kinno et al., 2008; Momo et al., 2008; Suzuki and Sakai, 2003]. Moreover, the activation of the left AG basically paralleled that of the left MTG in L2 and L1, further suggesting common cortical mechanisms shared by L1 and L2. The activated regions in Jsyn − Jspe, which included not only the left LPMC, F3t, and MTG but also the right MTG (Fig. 2H), replicated those in Sv − Pv (Sv: a sentence task under the visual condition; Pv: a phrase task under the visual condition) reported previously in our fMRI study [Homae et al., 2003]. Furthermore, it is notable that the correlation of the left AG activation with RTs of Esyn also paralleled that of the F3O for both the late (Figs. 2D,G and 4D) and early (Figs. 3D,F and 4D) learners. The two separable regions in the left AG (Figs. 2D, 3D, and 4D) correspond to activation foci for sentence comprehension in visually presented Japanese Sign Language as well as in auditorily presented Japanese [Sakai et al., 2005]. The activation modulation of the left AG supports the proposal for its supporting role in lexico‐semantics [Sakai, 2005], which is essentially involved in sentence comprehension subserved by the left F3O.
In the present study, the DOE of the early learners (8.7 ± 1.7) was 6 years longer than that of the late learners (2.4 ± 1.3), and their AOEs were also different. We found that the left F3t activations on the Esyn task correlated positively with the accuracy for the late learners at the initial stage of L2 acquisition, whereas they correlated negatively for the more experienced early learners, even if their testing ages and task performances were matched. This result is consistent with our previous findings of the similar differential activation patterns in the left IFG [Sakai et al., 2004; Tatsuno and Sakai, 2005] (cf., the Introduction), in which the two participant groups differed in 6 years of DOE, whereas their AOEs were comparable. It is thus likely that the DOE effect, which specifically interacts with sentence processing, is more closely coupled with the left IFG activations than is the AOE effect. One previous fMRI study reported that the left IFG activation during grammatical judgments was greater for participants at a later AOE than for those at an earlier AOE, even when their high PLs were matched [Wartenburger et al., 2003]. However, our results indicate that the left IFG may show higher, lower, or comparable activation, depending on which stages of L2 acquisition are compared. It should also be noted that the groups' mean DOE to two languages in the study of Wartenburger et al. (2003) differed by 16 years, and thus the differences between those groups might have disappeared if DOE had been equated. Another fMRI study has suggested that DOE affects the left IFG activation even if AOEs are comparable [Perani et al., 2003], which is consistent with the importance of DOE noted above.
There are still remaining issues that were not addressed by the present study. First, further research is necessary to determine whether the left IFG activation depends on exposure to a language at a particular stage of the developing brain, especially during the first several years. This point is critical in order to clarify to what extent the L2 acquisition mechanisms are shared with L1 acquisition mechanisms. Second, it remains unknown whether the L2 knowledge and use learned in EFL (English as a foreign language) contexts examined here are identical to those acquired in ESL (English as a second language) contexts (e.g., in the United States). Notwithstanding these issues, the present study clearly demonstrates that it is promising to evaluate activations in a specific cortical region as a direct measure of L2 abilities in individual learners, thereby opening new horizons for the neuroscience of education.
Acknowledgements
We thank T. Tsumoto for his encouragement, Y. Ochi, K. Shiota, N. Saeki, and N. Komoro for their technical assistance, S. Matsukura and H. Matsuda for their administrative assistance.
REFERENCES
- Ben‐Shachar M,Palti D,Grodzinsky Y ( 2004): Neural correlates of syntactic movement: Converging evidence from two fMRI experiments. Neuroimage 21: 1320–1336. [DOI] [PubMed] [Google Scholar]
- Birdsong D,Molis M ( 2001): On the evidence for maturational constraints in second‐language acquisition. J Mem Lang 44: 235–249. [Google Scholar]
- Cole P ( 1987): Null objects in universal grammar. Ling Inq 18: 597–612. [Google Scholar]
- Crinion J,Turner R,Grogan A,Hanakawa T,Noppeney U,Devlin JT,Aso T,Urayama S,Fukuyama H,Stockton K,Usui K,Green DW,Price CJ ( 2006): Language control in the bilingual brain. Science 312: 1537–1540. [DOI] [PubMed] [Google Scholar]
- Dapretto M,Bookheimer SY ( 1999): Form and content: Dissociating syntax and semantics in sentence comprehension. Neuron 24: 427–432. [DOI] [PubMed] [Google Scholar]
- Embick D,Marantz A,Miyashita Y,O'Neil W,Sakai KL ( 2000): A syntactic specialization for Broca's area. Proc Natl Acad Sci USA 97: 6150–6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach CJ,Schlesewsky M,Lohmann G,von Cramon DY,Friederici AD ( 2005): Revisiting the role of Broca's area in sentence processing: Syntactic integration versus syntactic working memory. Hum Brain Mapp 24: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flege JE ( 1999): Age of learning and second language speech In: Birdsong D,editor. Second Language Acquisition and the Critical Period Hypothesis. Mahwah, NJ: Lawrence Erlbaum Associates; pp 101–131. [Google Scholar]
- Flege JE,Yeni‐Komshian GH,Liu S ( 1999): Age constraints on second‐language acquisition. J Mem Lang 41: 78–104. [Google Scholar]
- Friederici AD,Opitz B,von Cramon DY ( 2000): Segregating semantic and syntactic aspects of processing in the human brain: An fMRI investigation of different word types. Cereb Cortex 10: 698–705. [DOI] [PubMed] [Google Scholar]
- Friederici AD,Steinhauer K,Pfeifer E ( 2002): Brain signatures of artificial language processing: Evidence challenging the critical period hypothesis. Proc Natl Acad Sci USA 99: 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD,Bahlmann J,Heim S,Schubotz RI,Anwander A ( 2006): The brain differentiates human and non‐human grammars: Functional localization and structural connectivity. Proc Natl Acad Sci USA 103: 2458–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golestani N,Alario FX,Meriaux S,LeBihan D,Dehaene S,Pallier C ( 2006): Syntax production in bilinguals. Neuropsychologia 44: 1029–1040. [DOI] [PubMed] [Google Scholar]
- Grewe T,Bornkessel I,Zysset S,Wiese R,von Cramon DY,Schlesewsky M ( 2005): The emergence of the unmarked: A new perspective on the language‐specific function of Broca's area. Hum Brain Mapp 26: 178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R,Sakai KL ( 2002): Specialization in the left prefrontal cortex for sentence comprehension. Neuron 35: 589–597. [DOI] [PubMed] [Google Scholar]
- Homae F,Hashimoto R,Nakajima K,Miyashita Y,Sakai KL ( 2002): From perception to sentence comprehension: The convergence of auditory and visual information of language in the left inferior frontal cortex. Neuroimage 16: 883–900. [DOI] [PubMed] [Google Scholar]
- Homae F,Yahata N,Sakai KL ( 2003): Selective enhancement of functional connectivity in the left prefrontal cortex during sentence processing. Neuroimage 20: 578–586. [DOI] [PubMed] [Google Scholar]
- Hyams N ( 1989): The null subject parameter in language acquisition In: Jaeggli O,Safir KJ, editors. The Null Subject Parameter. Dordrecht: Kluwer Academic Publishers; pp 215–238. [Google Scholar]
- Indefrey P ( 2006): A meta‐analysis of hemodynamic studies on first and second language processing: Which suggested differences can we trust and what do they mean? Lang Learn 56: 279–304. [Google Scholar]
- Johnson JS,Newport EL ( 1989): Critical period effects in second language learning: The influence of maturational state on the acquisition of English as a second language. Cognit Psychol 21: 60–99. [DOI] [PubMed] [Google Scholar]
- Kinno R,Kawamura M,Shioda S,Sakai KL ( 2008): Neural correlates of noncanonical syntactic processing revealed by a picture‐sentence matching task. Hum Brain Mapp 29: 1015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenneberg EH ( 1967): Biological Foundations of Language. New York: Wiley. [Google Scholar]
- Levin B ( 1993): English Verb Classes and Alternations: A Preliminary Investigation. Chicago, IL: The University of Chicago Press. [Google Scholar]
- Momo K,Sakai H,Sakai KL ( 2008): Syntax in a native language still continues to develop in adults: Honorification judgment in Japanese. Brain Lang 107: 81–89. [DOI] [PubMed] [Google Scholar]
- Musso M,Moro A,Glauche V,Rijntjes M,Reichenbach J,Büchel C,Weiller C ( 2003): Broca's area and the language instinct. Nat Neurosci 6: 774–781. [DOI] [PubMed] [Google Scholar]
- Park DC,Polk TA,Park R,Minear M,Savage A,Smith MR ( 2004): Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci USA 101: 13091–13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D,Abutalebi J ( 2005): The neural basis of first and second language processing. Curr Opin Neurobiol 15: 202–206. [DOI] [PubMed] [Google Scholar]
- Perani D,Abutalebi J,Paulesu E,Brambati S,Scifo P,Cappa SF,Fazio F ( 2003): The role of age of acquisition and language usage in early, high‐proficient bilinguals: An fMRI study during verbal fluency. Hum Brain Mapp 19: 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ ( 2000): The anatomy of language: Contributions from functional neuroimaging. J Anat 197: 335–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai KL ( 2005): Language acquisition and brain development. Science 310: 815–819. [DOI] [PubMed] [Google Scholar]
- Sakai KL,Miura K,Narafu N,Muraishi M ( 2004): Correlated functional changes of the prefrontal cortex in twins induced by classroom education of second language. Cereb Cortex 14: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Sakai KL,Tatsuno Y,Suzuki K,Kimura H,Ichida Y ( 2005): Sign and speech: Amodal commonality in left hemisphere dominance for comprehension of sentences. Brain 128: 1407–1417. [DOI] [PubMed] [Google Scholar]
- Seliger HW ( 1978): Implications of a multiple critical periods hypothesis for second language learning In: Ritchie WC,editor. Second Language Acquisition Research: Issues and Implications. New York, NY: Academic Press; pp 11–19. [Google Scholar]
- Stromswold K,Caplan D,Alpert N,Rauch S ( 1996): Localization of syntactic comprehension by positron emission tomography. Brain Lang 52: 452–473. [DOI] [PubMed] [Google Scholar]
- Suzuki K,Sakai KL ( 2003): An event‐related fMRI study of explicit syntactic processing of normal/anomalous sentences in contrast to implicit syntactic processing. Cereb Cortex 13: 517–526. [DOI] [PubMed] [Google Scholar]
- Tatsuno Y,Sakai KL ( 2005): Language‐related activations in the left prefrontal regions are differentially modulated by age, proficiency, and task demands. J Neurosci 25: 1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartenburger I,Heekeren HR,Abutalebi J,Cappa SF,Villringer A,Perani D ( 2003): Early setting of grammatical processing in the bilingual brain. Neuron 37: 159–170. [DOI] [PubMed] [Google Scholar]
- White L ( 1985): The pro‐drop parameter in adult second language acquisition. Lang Learn 35: 47–62. [Google Scholar]
- Yusa N ( 2003): ‘Passive’ unaccusatives in L2 acquisition In: Clancy PM,editor.Japanese/Korean Linguistics, Vol. 11 Stanford, CA: Center for the Study of Language and Information Publications and Stanford Linguistic Association; pp 246–259. [Google Scholar]


