Abstract
It has been suggested that in humans the mirror neuron system provides a neural substrate for imitation behaviour, but the relative contributions of different brain regions to the imitation of manual actions is still a matter of debate. To investigate the role of the mirror neuron system in imitation we used fMRI to examine patterns of neural activity under four different conditions: passive observation of a pantomimed action (e.g., hammering a nail); (2) imitation of an observed action; (3) execution of an action in response to a word cue; and (4) self‐selected execution of an action. A network of cortical areas, including the left supramarginal gyrus, left superior parietal lobule, left dorsal premotor area and bilateral superior temporal sulcus (STS), was significantly active across all four conditions. Crucially, within this network the STS bilaterally was the only region in which activity was significantly greater for action imitation than for the passive observation and execution conditions. We suggest that the role of the STS in imitation is not merely to passively register observed biological motion, but rather to actively represent visuomotor correspondences between one's own actions and the actions of others. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: fMRI, imitation, mirror neuron system
INTRODUCTION
Motor imitation involves observing the action of another individual and matching one's own movements to those actions being observed [Decety et al.,2002]. Imitation has been suggested to rely on the mirror neuron system [Brass and Heyes,2005; Buccino et al.,2004; Heyes,2001; Iacoboni,2005; Iacoboni and Dapretto,2006; Rizzolatti,2005; Rizzolatti et al.,2001; Rizzolatti and Craighero,2004]. Mirror neurons are visuomotor neurons that fire both when an action is performed and when a similar or identical action is passively observed [Rizzolatti and Craighero,2004]. A mirror system or network in the human brain should therefore be defined by its common activation during passive observation of action and during execution of actions without visual stimulation [Chong et al.,2008]. If such a mirror network is the basis for imitation, then imitation should rely on the same areas that are commonly recruited for action observation and execution. Previous brain imaging studies that have investigated imitation, however, have not examined the overlap or conjunction between action observation, execution, and imitation. Therefore, the relative roles of different brain regions within an action observation/execution network during imitation remain unknown. The aim of the present study was to independently identify the brain regions that respond to both observation and execution of movements (both word‐cued and self‐selected) and to investigate the differential role of these regions during imitation.
Mirror neurons were first observed using microelectrode recordings of single neurons in area F5 of the monkey premotor cortex [di Pellegrino et al.,1992; Gallese et al.,1996; Rizzolatti et al.,1996a] and later also in the PF/PFG complex within the inferior parietal cortex [Fogassi and Luppino,2005; Fogassi et al.,2005; Gallese et al.,2002]. Neurons in the superior temporal sulcus (STS) also respond to the passive observation of actions [Jellema et al.,2000; Perrett et al.,1989], but they have not generally been considered to form part of the mirror‐neuron network because they do not appear to be endowed with motor properties [Rizzolatti and Craighero,2004]. Early brain imaging studies that compared brain activity during perceived and executed actions [Decety et al.,1997; Iacoboni et al.,2001; Rizzolatti et al.,1996b] suggested that an analogous frontoparietal mirror neuron system exists in humans. Since then, many studies using TMS, EEG, MEG, PET and fMRI have confirmed these early results. It is widely believed that Brodmann area 44, in the posterior inferior frontal gyrus (IFG), is the human mirror‐equivalent of monkey area F5 and that the rostral inferior parietal lobule (IPL) is the human mirror‐equivalent of area PF/PFG (for reviews, see Fabbri‐Destro and Rizzolatti [2008], Iacoboni and Dapretto [2006], Rizzolatti [2005], Rizzolatti et al. [2001], and Rizzolatti and Craighero [2004]). However, it has been suggested that the evidence for a circuit in humans with “mirror” properties analogous to those observed in monkeys is relatively weak [Decety and Grèzes,1999; Dinstein et al.,2008; Grèzes and Decety,2001; Turella et al.,2009]. One issue is that, in humans, there appear to be many more areas that exhibit “mirror” properties based on their common activation during observation and execution of action—including the ventral and dorsal premotor cortex, the supplementary and presupplementary motor areas, the superior parietal lobule, and the intraparietal region—than would be expected based on the original monkey studies [Gazzola and Keysers,2009; Turella et al.,2009]. If mirror areas in humans are truly widespread throughout the cortex, the explanatory power of a “mirror system” with unique functional properties is diminished. A further issue is that most brain imaging studies have examined neural activity for just a single condition at a time—passive observation, execution, or imitation—rather than testing for regions of overlap between all conditions, which a strict definition of the mirror system requires [Fabbri‐Destro and Rizzolatti,2008]. A recent study by Gazzola and Keysers [2009] directly tested for common areas of activation for observed and executed actions in humans. They found reliable overlap in two regions in which mirror neurons have been found in monkeys: (1) the ventral premotor area (BA 6/44) and (2) the inferior parietal cortex (area PF). In addition, however, they observed reliable overlap in the dorsal premotor cortex, supplementary motor area, middle cingulate, middle temporal gyrus, somatosensory cortex, and superior parietal lobule.
Assuming a similar mirror neuron network exists in humans and monkeys, the question remains whether this network supports imitation. The fact that monkeys appear to be relatively poor imitators [Visalberghi and Fragaszy,2001; Whiten and Ham,1992] suggests that imitation is not the primary function of the nonhuman mirror neuron system. Iacoboni and Dapretto [2006] have suggested a “core circuit” for imitation that includes three regions. Two of these regions form part of the mirror neuron network: the posterior inferior frontal gyrus (IFG) plus adjacent ventral premotor cortex (PMC), and the rostral part of the inferior parietal lobule (IPL). The third region, the posterior part of the superior temporal sulcus (STS), is outside of the mirror neuron system. Iacoboni and coworkers [Iacoboni,2005; Iacoboni et al.,2001; Iacoboni and Dapretto,2006] have suggested that the main function of the STS is to provide visual input to the mirror neuron system and to allow matching between sensory predictions of imitative motor plans and a visual description of observed actions.
Apart from these three regions, other studies examining imitation have reported involvement of the superior parietal lobule [Chaminade et al.,2002; Iacoboni et al.,1999; Jackson et al.,2006; Koski et al.,2003; Vogt et al.,2007; Williams et al.,2007] and dorsal premotor area [Aziz‐Zadeh et al.,2006; Buccino et al.,2004; Grèzes et al.,2003; Koski et al.,2002,2003; Vogt et al.,2007; Williams et al.,2007]. The study of Gazzola and Keysers [2009] also identified these regions as overlapping for observation and execution of movement; however, the function of these areas for imitation and their relationship with the mirror network are still not clear.
In the current study, we aimed to examine the roles of mirror network areas in imitation. As noted earlier, most of the studies that have investigated the mirror system in humans have not included the necessary conditions to test for mirror properties as defined by the original single‐cell work in monkeys [Dinstein et al.,2008; Gazzola and Keysers,2009; Turella et al.,2009]. Instead of simply comparing levels of activity between the different conditions, as in typical fMRI studies, we specifically used a conjunction analysis to identify areas commonly active during all conditions of passive observation, execution, and imitation of action. Another problem with previous investigations is that they have typically involved visual stimulation in both the observation and execution conditions, thus potentially yielding vision‐related activity in both. A true mirror area should be active during execution conditions without visual stimulation [Dinstein et al.,2007]. In our fMRI study, participants were required to observe, imitate, and execute actions—in the latter condition in the absence of any visual stimuli. Two separate execution conditions were examined: participants either selected an appropriate action on the basis of a word‐cue, or they self‐selected an action pseudorandomly from a learned set. During word‐cued execution, visual and language areas of the brain should be active, whereas during self‐selected execution working memory and response selection areas should be involved [Cunnington et al.,2002]. Crucially, the conjunction between these two execution conditions should involve just those areas associated with the common processes of planning, initiation, and control of movement.
Several previous studies have examined observation or imitation of actions directed toward objects [Aziz‐Zadeh et al.,2006; Buccino et al.,2004; Chaminade et al.,2002; Decety et al.,2002; Gazzola and Keysers,2009; Grèzes et al.,2003], actions involving the pantomimed use of objects [Chong et al.,2008; Decety et al.,1997; Montgomery et al.,2007], or either communicative or meaningless finger gestures [Chaminade et al.,2005; Cunnington et al.,2006; Decety et al.,1997; Dinstein et al.,2007; Iacoboni et al.,1999; Montgomery et al.,2007]. Studies in monkeys suggest that mirror neurons respond only to actions directed toward objects, although they still respond when objects are not visible but inferred to be present [Kohler et al.,2002; Umiltà et al.,2001]. In humans, both goal‐directed actions with the object present and goal‐directed pantomimed actions [Buccino et al.,2001; Montgomery et al.,2007] appear to involve the mirror system, as do communicative actions [Montgomery et al.,2007]. Similarly, our previous study identified movement‐selective mirror neurons during observation and execution of actions involving the pantomimed use of objects [Chong et al.,2008]. In the current study, we employed the same pantomimed actions as in our previous work [Chong et al.,2008] to avoid brain activity associated with object processing that has been found in studies of object‐directed actions [Buccino et al.,2001].
In this study, we aimed to examine the roles of mirror network areas in imitation. We first identified the observation/execution network by determining regions that were commonly active across all conditions of execution, observation, and imitation. We then tested for any differences in activation between conditions within these commonly active regions, to investigate their relative roles in passive observation, execution, and imitation of action.
MATERIALS AND METHODS
Participants
Twenty neurologically healthy volunteers participated in the experiment (mean age = 22.6; standard deviation 2.72 years; ten males). All participants were right‐handed as assessed by the Edinburgh Handedness Inventory [Oldfield, 1971] and had normal or corrected‐to‐normal visual acuity. Volunteers were informed about the potential health risks and gave informed consent prior to entering the study. The study was approved by the Medical Research Ethics Committee of The University of Queensland.
Experimental Design
Stimuli
Stimuli (Fig. 1A) were movie clips of six pantomimed, goal‐directed hand actions (clicking a pen; hammering a nail; scratching a surface; shooing a fly; shooting a gun; stroking a cat), taken from the study of Chong et al. [2008]. The object toward which each action was directed (e.g., pen, hammer) was not present in any of the clips, but the goal of each action was self‐evident to participants, and this was verified behaviorally prior to the imaging session. Each action was carefully chosen to be as distinct from the others as possible. The movie clips were 1 s in duration and showed images of the action being performed by the right hand of an actor viewed from an allocentric (i.e., participant's own) perspective against a black background. Only the torso and right hand of the actor were visible. In the observation condition, participants were required to watch the video clips passively. In the imitation condition, participants watched the identical clips and were required to imitate the actions they observed. For example, when participants saw the video of a hand hammering a nail, they were required to perform an identical hammering action themselves. Participants were asked to begin movements as soon as they saw the video clips, but were not specifically required to synchronize the timing of their own actions with the video clips. An infrared camera installed in the scanning room allowed the experimenter to monitor participants' hand actions and record errors. During the word‐cued execution condition, participants were shown one of six nouns associated with each of the six actions: PEN, NAIL, SURFACE, FLY, GUN, or CAT. The use of verbs as word cues was specifically avoided because action words have previously been shown to activate mirror areas in the left prefrontal cortex [Rüschemeyer et al.,2007]. During the self‐selected execution condition, participants viewed a green cross at fixation and were required to select their own action to perform from the set of six learned actions. Prior to scanning, participants were trained to perform each action accurately with their right hand, matching the actions as closely as possible with those depicted in the video clips.
Figure 1.
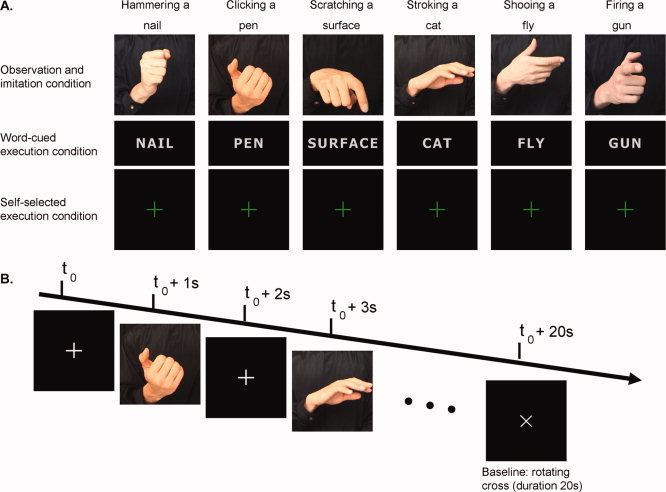
Stimulus displays and experimental protocol. (A) Overview of the stimuli used in the four conditions. (B) Sequence of events within a typical block of the observation and imitation conditions. Pictures are from the videos presented in the observation and imitation trials. Note that the hand actions were always depicted as dynamic displays for 1 s.
Scanning session
The four action conditions were run in separate blocks. The six different hand actions were presented and repeated in a random order during the observation and imitation trials (Fig. 1B), and the six different words were presented and repeated in a random order during the word‐cued execution trials. Each condition lasted for 20 s and was followed by a 20‐s baseline period in which the fixation cross rotated through 90° every 2 s. Each block lasted for 4 min. Brain activity during the observation condition, the two execution conditions, and the imitation condition was compared with the resting baseline. Each of the four conditions was pseudorandomized so that each condition appeared in each position (first, second, third, or fourth) and no condition was repeated immediately after itself. Participants were evenly allocated to one of the four condition orders. The condition order was repeated so that each participant completed each condition twice for a total of eight blocks. A cardboard cuff was placed over the participant's arm throughout the scanning session so that they could not see their own hand while they were performing the actions. This was done to ensure that all visual stimulation involved the video displays only and not the added visual stimulus of seeing one's own hand move during the execution and imitation conditions.
fMRI methods
A 4‐Tesla Bruker‐Siemens MRI scanner was used to acquire the images. Functional MRI scans used an echo‐planar imaging (EPI) sequence with repetition time (TR) = 2,000 ms, echo time (TE) = 30 ms, flip angle = 90°, 64 × 64 volume element (voxel) matrix, 32 axial slices, 3.59 × 3.59 mm in‐plane resolution, 3.5 mm slice thickness with slice spacing = 3.85 mm. The first four T R periods from each functional run were removed to allow for steady‐state tissue magnetization. A total of 122 EPI volumes were collected for each run, and eight runs were performed by each participant. Standard protocols were then followed to acquire high‐resolution, T1‐weighted structural images from each participant.
Data analysis
Data were processed and analyzed using SPM5 (Wellcome Department of Imaging Neuroscience, Institute of Neurology, London; http://www.fil.ion.ucl.ac.uk/spm), implemented in Matlab (Mathworks, USA). All experiments were preprocessed in the same way. Following correction for differences in timing of slice acquisition within a volume, EPI volumes were realigned to the middle image for movement correction using a least‐squares approach and six‐parameter rigid body spatial transformations [Friston et al.,1995]. A mean EPI volume was obtained during realignment, and the structural MRI was coregistered with that mean volume. The structural scan was normalized to the Montreal Neurological Institute T1 template [Friston et al.,1995] using nonlinear basis functions. The same deformation parameters were applied to the EPI volumes. The EPI volumes were spatially smoothed using a 7 mm FWHM filter. The smoothed, normalized single‐subject EPI data were analyzed using observation, imitation, word‐execution, and self‐selection blocks as effects of interest. Standard (no overall grand mean scaling) random‐effects analyses were then performed, in which each participant's response was considered as a variable drawn from the population [Holmes and Friston,1998]. For each participant, statistical parametric maps were generated from linear contrasts for each condition minus baseline. These contrasts of parameter estimates were then included in a one‐way repeated‐measures ANOVA in a second‐level analysis. We performed a conjunction analysis (using conjunction‐null hypothesis) across all four conditions, as described by Nichols et al. [2005], using a threshold of P < 0.001 uncorrected, with a minimum cluster size >5 voxels. The conjunction‐null hypothesis, unlike the global‐null hypothesis (see Friston et al. [2005] and Nichols et al. [2005]), ensures that significant voxels are above threshold in every one of the four conditions.
To compare levels of activation between the four conditions, we then extracted the parameter estimates from the peak voxels in each of the clusters that were significant in the conjunction analysis and performed pairwise comparisons between conditions using repeated‐measures t‐tests (P ≤ 0.01). Anatomical localization was carried out by visual comparison of the MRI‐projected sections with corresponding slices from the Duvernoy [1999] brain atlas.
RESULTS
Behavioral Data
Participants were observed by infrared video monitoring during the scans, and error rates were recorded to ensure that the task was performed correctly. None of the participants displayed overt movement during any of the acquisitions in the passive observation condition. Errors in the execution and imitation conditions were very rare, accounting for <2% of all trials. A one‐way repeated‐measures ANOVA found no difference between the number of correctly executed trials in the Word‐cued Execution (M = 118.15, 98.46% correct trials), Self‐Selected Execution (M = 118.75, 98.96% correct trials), and Imitation conditions (M = 118.1, 98.42% correct trials), F(2,38) = 0.797, P = 0.458.
Neuroimaging Data
Common activation across conditions: Conjunction analysis
We first identified regions that were active across all four conditions of observation, execution (×2), and imitation. The conjunction analysis across the four conditions revealed six regions (left superior temporal sulcus, right superior temporal sulcus, left dorsal premotor cortex, right dorsal premotor cortex, left superior parietal lobule, and left supramarginal gyrus) that were significantly active (Table I, Fig. 2).
Table I.
Six clusters above threshold (P < 0.001, voxel threshold > 5) in the conjunction analysis of all four conditions
| Locus | Cluster size | MNI | Z value | Self‐selected minus observation | Word‐cued minus observation | Imitation minus self‐selected | Imitation minus word‐cued | Imitation minus observation | ||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||||
| Left STS | 13 | −51 | −63 | 6 | 3.55 | ns | ns | Z = 5.16; P < 0.001 | Z = 4.46; P < 0.001 | Z = 4.13; P < 0.001 |
| Right STS | 48 | 51 | −51 | 6 | 3.93 | ns | ns | Z = 3.66; P < 0.001 | Z = 3.77; P < 0.001 | Z = 4.32; P < 0.001 |
| Left SPL | 50 | −33 | −45 | 54 | 4.22 | Z = 4.54; P < 0.001 | Z = 4.67; P < 0.001 | ns | ns | Z = 5.47; P < 0.001 |
| Left SMG | 6 | −48 | −36 | 30 | 3.45 | Z = 3.57; P < 0.001 | Z = 3.48; P < 0.001 | ns | ns | Z = 4.62; P < 0.001 |
| Left dPM | 14 | −51 | −3 | 48 | 3.74 | Z = 3.34; P < 0.001 | Z = 2.77; P = 0.003 | ns | ns | Z = 4.13; P < 0.001 |
| Right dPM | 13 | 48 | 3 | 48 | 3.94 | Z = 2.70; P = 0.003 | Z = 2.34; P = 0.01 | ns | ns | Z = 4.12; P < 0.001 |
STS, superior temporal sulcus; SPL, superior parietal lobule; SMG, supramarginal gyrus; dPM, dorsal premotor cortex; ns, no suprathreshold voxel.
For each of the six clusters we extracted the parameter estimates from the peak voxels in each of the clusters that were significant in the conjunction analysis and performed pairwise comparisons between conditions using repeated‐measures t‐tests.
All the significant (≤0.01) results are presented.
Figure 2.
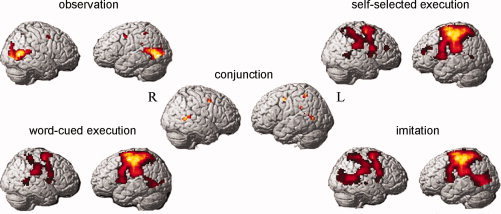
Results from the fMRI analyses. Display of the significant clusters (P < 0.001 uncorr., voxel threshold > 5) in the conjunction analysis and each of the four conditions minus baseline (P < 0.001 uncorr.) displayed on a rendered brain.
Comparisons between conditions: Pairwise contrasts
We performed pairwise comparisons between each of the four conditions in each of the six regions identified in the conjunction analysis. Significant results (P ≤ 0.01) are shown in Table I. Activity was significantly greater in the imitation condition compared with all other conditions in the left and right superior temporal sulcus (see Fig. 3). Within these bilateral STS regions, there was no significant difference in activity elicited by the two execution conditions (word‐cued and self‐selected) and the observation condition (Table I, Fig. 3). This effect of imitation within the STS was not evident in any of the other three regions of interest. By contrast, activity in the left dorsal premotor cortex, right dorsal premotor cortex, left superior parietal lobule, and left supramarginal gyrus was significantly greater in each of the three conditions involving execution—word‐cued execution, self‐selected execution, and imitation—than in the observation‐alone condition (Table I, Fig. 3).
Figure 3.
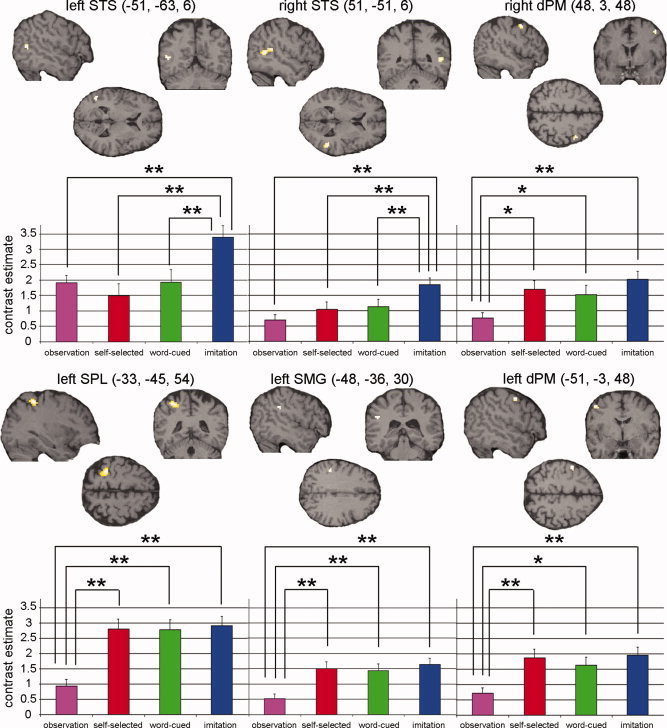
Comparisons between conditions in each of the regions identified in the conjunction analysis. Coronal, axial, and sagittal sections displayed at an uncorrected threshold of P < 0.001 + contrast estimate and S.E. of the most significant voxel in that cluster. Significant differences (*P ≤ 0.01, **P ≤ 0.001, see Table I) between the conditions are shown. STS, superior temporal sulcus; SPL, superior parietal lobule; SMG, supramarginal gyrus; dPM, dorsal premotor cortex.
DISCUSSION
The aim of the current study was to examine the roles of mirror network areas in manual imitation. To achieve this aim, we first performed a conjunction analysis to identify regions that were significantly active in all four conditions of observation, execution, and imitation. This analysis revealed a bilateral network that included the bilateral dorsal premotor area, left supramarginal gyrus, left superior parietal lobule, and bilateral STS (see Fig. 2). These cortical sites extend beyond the classical mirror neuron regions of the IFG and IPL. Our findings are consistent with a meta‐analysis of fMRI and PET studies [Grèzes and Decety,2001] that examined neural activity associated with action execution, action simulation, and action observation. That study found a significant overlap between activation patterns for execution and observation of actions in the dorsal premotor cortex, the supramarginal gyrus, and the superior parietal lobe. The STS was also found to be involved during action observation in several studies.
We found two distinct patterns of neural response across conditions (see Fig. 3). The responses in the parietal and premotor areas were more motor‐related (see Fig. 3). This pattern involved the IPL, SPL, and dorsal premotor cortex and showed equivalent activity for all conditions involving movement execution (imitation, plus self‐selected and word‐cued execution), but less activity for passive observation. The other pattern of activity involved the STS bilaterally and appeared more imitation‐related (see Fig. 3). These superior temporal regions showed more activation during the imitation condition than all of the other conditions.
The inferior frontal gyrus was not significantly active across all the conditions in our study. In the observation‐only condition (see Fig. 2), we did not find activation in the IFG (similar to Jackson et al. [2006] and Jonas et al. [2007]). Iacoboni et al. (1999) did find increased activation in Broca's area during imitation compared with execution and observation, but Williams et al. (2006) showed no evidence of activation in this region during imitation compared with execution in an identical task, even when they lowered the threshold to P = 0.05 uncorrected. Because a small set of actions was used in our study, it could be argued that the ventral premotor cortex and IFG were not particularly active during passive observation because the actions were over‐learned and that these regions are more involved in learning of novel hand movements [Buccino et al.,2004] than of familiar hand movements [Goldenberg and Karnath,2006; Tanaka and Inui,2002]. This seems unlikely, however, because an fMRI study by Calvo‐ Merino et al. [2005] on action observation in dancers showed that the ventral premotor cortex was more active during observation of learned actions compared with nonlearned actions. Our results are in line with previous studies that suggest that areas 44 and 45 are not crucial for imitation [Makuuchi,2005; Molenberghs et al.,2009].
The dorsal premotor area, on the other hand, seems to be a region that is more consistently activated during imitation [Molenberghs et al.,2009] and was found to be active in the conjunction of all conditions in our study. This region is more active during imitation of goal‐directed actions compared with non‐goal‐directed actions [Koski et al.,2002]; during specular imitation compared with motor control tasks [Koski et al.,2003]; during imitation versus performance of nonmatching actions [Williams et al.,2007]; and during imitation versus passive action observation [Molnar‐Szakacs et al.,2005] and shows common activation for imitation and observation of an object being grasped [Grèzes et al.,2003; Vogt et al.,2007]. This suggests that in the frontal lobe the dorsal premotor area rather than the inferior frontal gyrus is crucial for imitation.
In our study, the posterior STS showed significant bilateral activation in all the conditions. The largest activity was found during the imitation condition. In an fMRI study specifically designed to examine activity in the STS, Iacoboni et al. [2001] found significantly greater STS activation during imitation compared with an execution control and observation condition. Our study is different in several ways. First, Iacoboni et al. [2001] did not have a self‐selected execution condition, and so it is not clear if the STS was also active during motor responses without a visual cue. Second, they tested for increases in STS activation between conditions, but not for the critical conjunction across conditions, and so it is not clear whether the STS was significantly active in all conditions. In our study, the left and right STS were significantly active in all four conditions compared with baseline. Third, Iacoboni et al. [2001] selected the STS as a region of interest based on activation from an earlier study, making it difficult to rule out involvement of cortical areas beyond their temporal ROI. To circumvent this limitation, we first identified the observation/execution network in a conjunction analysis across the whole brain. We found that the STS alone showed a selective imitation‐related response. Finally, instead of the simple finger movements used by Iacoboni et al. [2001], we used pantomimed, goal‐directed hand movements that more closely match the goal‐directed hand movements used to activate mirror neurons in monkeys [Gallese et al.,1996].
Interestingly, and in contrast to the other regions identified, the STS responded equally to executed actions and to actions passively observed in our study. Given previous suggestions that the STS is crucial for the perception of biological motion [Allison et al.,2000; Castelli et al.,2000; Grèzes and Decety,2001; Grossman et al.,2000; Jellema et al.,2000; Perrett et al.,1989; Perrett and Emery,1994], it is puzzling that we found equivalent activity within the STS for passive observation of action as for the execution of movement in the absence of any visual stimuli. It is unlikely that the human STS has motor properties, because single cell recordings in an homologous region in monkeys suggests that STS neurons do not respond during executed actions [Rizzolatti and Craighero,2004]. Others [Iacoboni,2005; Iacoboni et al.,2001; Iacoboni and Dapretto,2006] have suggested that the STS receives an efference copy of motor plans from the frontoparietal mirror system, which is then used for matching with higher‐order visual descriptions of the observed action within the STS. The STS activity we observed during movement execution is consistent with a role for the STS in receiving an efference copy of the planned action [Iacoboni and Dapretto,2006]. It is also possible that activity within the STS during movement execution arises because participants also imagine the movement to be performed. The STS is active during mental imagery of biological motion involving point‐light display animations [Grossman and Blake,2001], but previous studies that have specifically examined motor imagery have not revealed activation of the STS [Grèzes and Decety,2001]. It is therefore unclear whether the STS activity we observed in the movement execution condition should be attributed to visual or motor imagery of pantomimed hand actions, or to some other factor. This question will need to be addressed in future studies.
Our results do clearly point to a role of the STS in imitation, since bilateral regions of the posterior STS showed significantly greater activation during imitation than all other conditions. One could argue that the increased activity in the STS during the imitation condition compared with the observation condition is due to an increase of attention to biological motion. We think that this explanation is unlikely, because in our study, we observed equal activation in the execution conditions without biological motion compared with the observation condition. This suggests that the STS must do more than merely encoding biological motion.
Evidence from studies of stroke patients suggests an important role for the STS in imitation. Merians et al. [1997] reported a patient with apraxia due to a temporal lobe lesion who had greater problems imitating gestures than performing the same gestures to verbal command. More recently, in a study of 32 stroke patients, Tessari et al. [2007] reported that of the individuals with selective deficits imitating meaningless hand actions, all had overlapping lesions involving the STS and ventral angular gyrus. Tessari et al. [2007] suggested that the STS forms part of a direct visuomotor pathway, as opposed to an indirect semantic pathway, whereby visual representations of observed actions can access the motor system for imitation. This view is broadly consistent with Iacoboni's [2005] theory that the STS provides a higher‐order visual description of observed actions to the mirror neuron system that is necessary for imitation. By contrast, Goldenberg and Karnath [2006] found no evidence for any influence of STS lesions on imitation in their lesion‐mapping study of 44 left‐hemisphere stroke patients. Their findings implicate the left IFG and left IPL as regions critical for imitation of meaningless finger and hand actions, respectively.
The STS is also suggested to play a key role in identifying the intentions of others via analysis of “social” biological motion [Allison et al.,2000; Saxe et al.,2004]. The use of pantomimed actions in our study, which may be considered to have a more communicative, social function in everyday life, could have recruited the STS to a greater extent than actions performed toward real objects (as studied by Gazzola and Keysers [2009]). Nonetheless, previous studies of action observation in which the object was actually present have also shown involvement of the STS during imitation, consistent with our results [Chaminade et al.,2002; Decety et al.,2002]. In a study examining reciprocal imitation, Decety et al. [2002] showed that the left STS was more involved when participants imitated the actions of an experimenter compared with when they observed the experimenter imitating their own self‐selected actions. Decety et al. [2002] therefore suggested that the STS is crucial for making self–other distinctions during imitation, when observed and executed actions match.
Crucially, the STS appears to register the congruence between observed actions and intended goals, both for hand actions [Pelphrey et al.,2004a] and for shifts of gaze [Pelphrey et al.,2003]. In a social context, Pelphrey et al. [2004b] found that activity within the STS is enhanced for so‐called “mutual gaze,” in which the observer's eye‐gaze matches that of the observed person. Behaviorally, Miall et al. [2006] have shown that performing actions enhances the visual perception of congruent actions, suggesting that motor processes associated with action planning and execution somehow feed back to influence the visual processing and perception of others' actions when those actions match. Our results, showing enhanced activity of the STS during imitation, may therefore reflect a role of the STS in registering the congruence between one's own actions and the actions of others, and perhaps enhancing the perceptual interpretation of others' actions when they match the observer's own motor plans [e.g., Miall et al.,2006]. In this way, the STS may provide an interface between one's own actions and the actions he or she observes, providing a neural substrate for various aspects of social cognition more generally.
Acknowledgements
The authors wish to thank Trevor Chong and Darren Tan for their contributions to the study.
REFERENCES
- Allison T, Puce A, McCarthy G ( 2000): Social perception from visual cues: Role of the STS region. Trends Cogn Sci 4: 267–278. [DOI] [PubMed] [Google Scholar]
- Aziz‐Zadeh L, Koski L, Zaidel E, Mazziotta J, Iacoboni M ( 2006): Lateralization of the human mirror neuron system. J Neurosci 26: 2964–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Heyes C ( 2005): Imitation: Is cognitive neuroscience solving the correspondence problem? Trends Cogn Sci 9: 489–495. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ ( 2001): Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. Eur J Neurosci 13: 400–404. [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund HJ, Rizzolatti G ( 2004): Neural circuits underlying imitation learning of hand actions: An event‐related fMRI study. Neuron 42: 323–334. [DOI] [PubMed] [Google Scholar]
- Calvo‐Merino B, Glaser DE, Grèzes J, Passingham RE, Haggard P ( 2005): Action observation and acquired motor skills: An FMRI study with expert dancers. Cereb Cortex 15: 1243–1249. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happė F, Frith U, Frith C ( 2000): Movement and mind: A functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage 12: 314–325. [DOI] [PubMed] [Google Scholar]
- Chaminade T, Meltzoff AN, Decety J ( 2002): Does the end justify the means? A PET exploration of the mechanisms involved in human imitation. Neuroimage 15: 318–328. [DOI] [PubMed] [Google Scholar]
- Chaminade T, Meltzoff AN, Decety J ( 2005): An fMRI study of imitation: Action representation and body schema. Neuropsychologia 43: 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong TT‐J, Cunnington R, Williams MA, Kanwisher N, Mattingley JB ( 2008): fMRI adaptation reveals mirror neurons in human inferior parietal cortex. Curr Biol 18: 1576–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E ( 2002): The preparation and execution of self‐initiated and externally‐triggered movement: A study of event‐related fMRI. Neuroimage 15: 373–385. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Robinson S, Moser E ( 2006): The selection of intended actions and the observation of others' actions: A time‐resolved fMRI study. Neuroimage 29: 1294–1302. [DOI] [PubMed] [Google Scholar]
- Decety J, Grèzes J ( 1999): Neural mechanisms subserving the perception of human actions. Trends Cogn Sci 3: 172–178. [DOI] [PubMed] [Google Scholar]
- Decety J, Grèzes J, Coster N, Perani D, Jeannerod M, Procyk E, Grassi F, Fazio F ( 1997): Brain activity during observation of actions. Influence of action content and subject's strategy. Brain 120: 1763–1777. [DOI] [PubMed] [Google Scholar]
- Decety J, Chaminade T, Grèzes J, Meltzoff AN ( 2002): A PET exploration of the neural mechanisms involved in reciprocal imitation. Neuroimage 15: 265–272. [DOI] [PubMed] [Google Scholar]
- Dinstein I, Hasson U, Rubin N, Heeger DJ ( 2007): Brain areas selective for both observed and executed movements. J Neurophysiol 98: 1415–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Thomas C, Behrmann M, Heeger DJ ( 2008): A mirror up to nature. Curr Biol 18: R13–R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G ( 1992): Understanding motor events: A neurophysiological study. Exp Brain Res 91: 176–180. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM ( 1999): The Human Brain: Surface, Three Dimensional Sectional Anatomy with MRI, and Blood Supply, 2nd ed New York: Springer. [Google Scholar]
- Fabbri‐Destro M, Rizzolatti G ( 2008): Mirror neurons and mirror systems in monkeys and humans. Physiology 23: 171–179. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Luppino G ( 2005): Motor functions of the parietal lobe. Curr Opin Neurobiol 15: 626–631. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G ( 2005): Parietal lobe: From action organization to intention understanding. Science 308: 662–667. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J, Heather JD, Frackowiak RSJ ( 1995): Spatial realignment and normalization of images. Hum Brain Mapp 2: 165–189. [Google Scholar]
- Friston KJ, Penny WD, Glaser DE ( 2005): Conjunction revisited. Neuroimage 25: 661–667. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G ( 1996): Action recognition in the premotor cortex. Brain 119: 593–609. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fogassi L, Fadiga L, Rizzolatti G ( 2002): Action representation and the inferior parietal lobule In: Prinz W, Hommel B, editors. Common Mechanisms in Perception and Action: Attention and Performance, Vol. XIX New York: Oxford University Press; pp 247–266. [Google Scholar]
- Gazzola V, Keysers C ( 2009): The observation and execution of actions share motor and somatosensory voxels in all tested subjects: Single‐subject analyses of unsmoothed fMRI Data. Cereb Cortex 9: 1239–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G, Karnath HO ( 2006): The neural basis of imitation is body part specific. J Neurosci 26: 6282–6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes J, Decety J ( 2001): Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta‐analysis. Hum Brain Mapp 12: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes J, Armony JL, Rowe J, Passingham RE ( 2003): Activations related to “mirror” and “canonical” neurones in the human brain: An fMRI study. Neuroimage 18: 928–937. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Blake R ( 2001): Brain activity evoked by inverted and imagined biological motion. Vision Res 41: 1475–1482. [DOI] [PubMed] [Google Scholar]
- Grossman E, Donnelly M, Price R, Pickens D, Morgan V, Neighbor G, Blake R ( 2000): Brain areas involved in perception of biological motion. J Cogn Neurosci 12: 711–720. [DOI] [PubMed] [Google Scholar]
- Heyes C ( 2001): Causes and consequences of imitation. Trends Cogn Sci 5: 253–261. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ ( 1998): Generalisability, random effects and population inference. Neuroimage 7: S754. [Google Scholar]
- Iacoboni M ( 2005): Neural mechanisms of imitation. Curr Opin Neurobiol 15: 632–637. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M ( 2006): The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci 7: 942–951. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G ( 1999): Cortical mechanisms of human imitation. Science 286: 2526–2528. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Koski LM, Brass M, Bekkering H, Woods RP, Dubeau MC, Mazziotta JC, Rizzolatti G ( 2001): Reafferent copies of imitated actions in the right superior temporal cortex. Proc Natl Acad Sci USA 98: 13995–13999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J ( 2006): Neural circuits involved in imitation and perspective‐taking. Neuroimage 31: 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellema T, Baker CI, Wicker B, Perrett DI ( 2000): Neural representation for the perception of the intentionality of actions. Brain Cogn 442: 280–302. [DOI] [PubMed] [Google Scholar]
- Jonas M, Siebner HR, Biermann‐Ruben K, Kessler K, Bäumer T, Büchel C, Schnitzler A, Münchaub A ( 2007): Do simple intransitive finger movements consistently activate frontoparietal mirror neuron areas in humans? Neuroimage 36: T44–T53. [DOI] [PubMed] [Google Scholar]
- Kohler E, Keysers C, Umilta MA, Fogassi L, Gallese V, Rizzolatti G ( 2002): Hearing sounds, understanding actions: Action representation in mirror neurons. Science 297: 846–848. [DOI] [PubMed] [Google Scholar]
- Koski L, Wohlschläger A, Bekkering H, Woods RP, Dubeau M, Mazziotta JC, Iacoboni M ( 2002): Modulation of motor and premotor activity during imitation of target‐directed actions. Cereb Cortex 12: 847–855. [DOI] [PubMed] [Google Scholar]
- Koski L, Iacoboni M, Dubeau MC, Woods RP, Mazziotta JC ( 2003): Modulation of cortical activity during different imitative behaviors. J Neurophysiol 89: 460–471. [DOI] [PubMed] [Google Scholar]
- Makuuchi M ( 2005): Is Broca's area crucial for imitation? Cereb Cortex 15: 563–570. [DOI] [PubMed] [Google Scholar]
- Merians AS, Clark M, Poizner H, Macauley B, Gonzalez‐Rothi LJ, Heilman K ( 1997): Visual‐imitative dissociation apraxia. Neuropsychologia 35: 1483–1490. [DOI] [PubMed] [Google Scholar]
- Miall RC, Stanley J, Todhunter S, Levick C, Lindo S, Miall JD ( 2006): Performing hand actions assists the visual discrimination of similar hand postures. Neuropsychologia 44: 966–976. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB ( 2009): Is the mirror neuron system involved in imitation? A short review and meta‐analysis. Neurosci Biobehav Rev 33: 975–980. doi:10.1016/j.neubiorev.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Molnar‐Szakacs I, Iacoboni M, Koski L, Mazziotta JC ( 2005): Functional segregation within pars opercularis of the inferior frontal gyrus: Evidence from fMRI studies of imitation and action observation. Cereb Cortex 15: 986–994. [DOI] [PubMed] [Google Scholar]
- Montgomery KJ, Isenberg N, Haxby JV ( 2007): Communicative hand gestures and object‐directed hand movements activated the mirror neuron system. Soc Cogn Affect Neurosci 2: 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J ( 2005): Valid conjunction inference with the minimum statistic. Neuroimage 25: 653–660. [DOI] [PubMed] [Google Scholar]
- Oldfield R ( 1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Singerman JD, Allison T, McCarthy G ( 2003): Brain activation evoked by perception of gaze shifts: The influence of context. Neuropsychologia 41: 156–170. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G ( 2004a): Grasping the intentions of others: The perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. J Cogn Neurosci 16: 1706–1716. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Viola RJ, McCarthy G ( 2004b): When strangers pass: Processing of mutual and averted social gaze in the superior temporal sulcus. Psychol Sci 15: 598–603. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Emery NJ ( 1994): Understanding the intentions of others from visual signals: Neurophysiological evidence. Curr Psychol Cogn 13: 683–694. [Google Scholar]
- Perrett DI, Harries MH, Bevan R, Thomas S, Benson PJ, Mistlin AJ, Chitty AJ, Hietanen JK, Ortega JE ( 1989): Frameworks of analysis for the neural representation of animate objects and actions. J Exp Biol 146: 87–113. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G ( 2005): The mirror neuron system and its function in humans. Anat Embryol 210: 419–421. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L ( 2004): The mirror‐neuron system. Annu Rev Neurosci 27: 169–192. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L ( 1996a): Premotor cortex and the recognition of motor actions. Cogn Brain Res 3: 131–141. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Paulesu E, Perani D, Fazio F ( 1996b): Localization of grasp representations in humans by PET: 1. Observation versus execution. Exp Brain Res 111: 246–252. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V ( 2001): Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci 2: 661–670. [DOI] [PubMed] [Google Scholar]
- Rüschemeyer S, Brass M, Friederici AD ( 2007): Comprehending prehending: Neural correlates of processing verbs with motor stems. J Cogn Neurosci 19: 855–865. [DOI] [PubMed] [Google Scholar]
- Saxe R, Xiao DK, Kovacs G, Perrett DI, Kanwisher N ( 2004): A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia 42: 1435–1446. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Inui T ( 2002): Cortical involvement for action imitation of hand/arm postures versus finger configurations: An fMRI study. Neuroreport 13: 1599–1602. [DOI] [PubMed] [Google Scholar]
- Tessari A, Canessa N, Ukmar M, Rumiati RI ( 2007): Neuropsychological evidence for a strategic control of multiple routes in imitation. Brain 130: 1111–1126. [DOI] [PubMed] [Google Scholar]
- Turella L, Pierno AC, Tubaldi F, Casteillo U ( 2009): Mirror neurons in humans: Consisting or confounding evidence? Brain Lang 108: 10–21. [DOI] [PubMed] [Google Scholar]
- Umiltà MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C, Rizzolatti G ( 2001): I know what you are doing: A neurophysiological study. Neuron 31: 155–165. [DOI] [PubMed] [Google Scholar]
- Visalberghi E, Fragaszy D ( 2001): Do monkeys ape? Ten years after In: Dautenhahn K, Nehaniv C, editors. Imitation in Animals and Artifacts. Boston, MA: The MIT Press; pp 471–499. [Google Scholar]
- Vogt S, Buccino G, Wohlschläger AM, Canessa N, Shah NJ, Zilles K, Eickhoff SB, Freund H‐J, Rizzolatti G, Fink GR ( 2007): Prefrontal involvement in imitation learning of hand actions: Effects of practice and expertise. Neuroimage 37: 1371–1383. [DOI] [PubMed] [Google Scholar]
- Whiten A, Ham R ( 1992): On the nature and evolution of imitation in the animal kingdom: Reappraisal of a century of research. Adv Study Behav 21: 239–283. [Google Scholar]
- Williams JHG, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A ( 2006): Neural mechanisms of imitation and “mirror neuron” functioning in autistic spectrum disorder. Neuropsychologia 44: 610–621. [DOI] [PubMed] [Google Scholar]
- Williams JHG, Whiten A, Waiter GD, Pechey S, Perrett DI ( 2007): Cortical and subcortical mechanisms at the core of imitation. Soc Neurosci 2: 66–78. [DOI] [PubMed] [Google Scholar]


