Abstract
Being exposed to fear signals makes us feel threatened and prompts us to prepare an adaptive response. In our previous studies, we suggested that amygdala (AMG) and premotor cortex (PM) play a role in the preparation of the observers' motor response required by the situation. The present experiment aimed at assessing how interindividual differences in alexithymia—a personality trait associated with deficits in emotional reactivity and regulation—influence the neural network associated with the perception of fear. Using fMRI, we scanned 34 healthy subjects while they were passively observing fearful body expressions. Applying a dimensional approach, we performed correlation analyses between fear‐related brain areas and alexithymia scores among all participants. Using a categorical approach, we conducted a between‐group comparison (13 high vs. 12 low‐alexithymia subjects). Our results were threefold. First, the right AMG activity in response to fearful stimuli was negatively correlated with the level of difficulty to identify emotions. Second, PM activity was linked to reduced subjective emotional reactivity. Third, the between‐group comparison revealed greater activity in anterior cingulate cortex (ACC) for high than low‐alexithymia scorers. Moreover, the relationship between ACC and PM was in opposite direction in individuals with high (negative link) and low (positive link) alexithymia. Therefore, compared to our previous findings, we hereby further reveal how ACC interacts with PM to sustain self‐regulation of one's own emotional state in response to threatening social signals. Moreover, this neural mechanism could account for the description of the “cold‐blooded” personality of individuals with alexithymia. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: fear, body expressions, alexithymia, emotional regulation, fMRI
INTRODUCTION
Fearful expressions, and more specifically whole body expressions of fear, convey information not only about the other's state of mind but also about the actions undertaken by the frightened person. Such characteristic fearful behavior communicates a strong fear signal to observers. When facing such fearful signal, one has to rapidly understand it and initiate an adaptive reaction [Darwin, 1872; de Gelder et al., 2004; Frijda, 1986; LeDoux, 1995]. In our previous fMRI studies investigating the neural bases of perceiving whole body expressions of fear and anger [Grèzes et al., 2007; Pichon et al., 2008, 2009], we showed that the perception of threat signals is associated with increased activity in superior temporal sulcus (STS), amygdala (AMG), inferior frontal cortices, and premotor cortex (PM) and suggested that AMG and PM play a role in the preparation of the observers' motor response required by the situation. This adaptive response entails to identify the emotional expression one observes in the other and to self‐regulate one's own emotional state accordingly, two mechanisms that are supposed to be impaired in individuals with alexithymia [Berthoz et al., 2002; Lane et al., 1997; Luminet et al., 2003; Moriguchi et al., 2006, 2008].
Investigating the influence of interindividual differences in alexithymia level, in a population free of any psychiatric or neurological disorders, on the neural bases of the perception of fearful social cues is a promising model for relating patterns of brain activations with key components of social‐cognition processing. Indeed, alexithymia is a personality construct that encompasses a cluster of characteristics reflecting impaired emotion processing and regulation. All leading alexithymia researchers agree that this personality construct encompasses both affective and cognitive dimensions. Yet, the exact number of essential features of alexithymia has been debated. For Bermond and colleagues notably, reduced capacities for emotionalizing (the ease by which one experiences an emotional feeling or becomes emotionally aroused by emotion inducing events) is an essential element of the alexithymia phenomenon [Bermond et al., 1999]. Among the alexithymia dimensions, the affective ones reflect difficulties in differentiating and reporting emotional feelings and reduced subjective emotional excitability to emotion‐inducing events, whereas the cognitive ones concern preoccupations with the details of external events and limited imaginative capacity [Lumley et al., 2007; Sifneos, 1973]. Recent neuroimaging studies on socioaffective processing in alexithymia have stressed the implication of specific brain areas, including anterior cingulate cortex (ACC) [Kano et al., 2003, 2007; Moriguchi et al., 2007] (see Fig. 1), medio‐frontal gyrus [Berthoz et al., 2002; Mantani et al., 2005; Mériau et al., 2006; Moriguchi et al., 2006], parietal and premotor [Karlsson et al., 2008; Moriguchi et al., 2008; Reker et al., 2009 fusiform gyrus [Eichmann et al., 2008; Reker et al., 2009] as well as insula [Kano et al., 2007; Moriguchi et al., 2008; Reker et al., 2009; Silani et al., 2008], and AMG [Kugel et al., 2008; Li and Sinha, 2006; Reker et al., 2009]. Among those studies, few have investigated alexithymia related to threat stimuli: Kano et al. [ 2003] applied angry faces, Mériau et al. [ 2006] used fear and angry faces, and Moriguchi et al. [ 2007] showed pain pictures.
Figure 1.
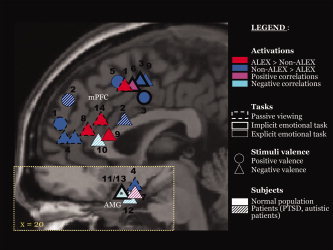
Alexithymia‐related activations during emotional tasks. The activations' peak of 14 studies revealed during either direct comparison between alexithymic and non‐alexithymic groups or correlation analyses with alexithymia scores (BVAQ‐B or TAS‐20) was superimposed on sagittal sections of the MNI brain, one for the medial frontal cortex (x = 0, anterior cingulate cortex and superior medial frontal gyrus) and one for the amygdala (x = 20); (1) [Berthoz et al., 2002a]; (2) [Huber et al., 2002]; (3) [Kano et al., 2003]; (4) [Leweke et al., 2004]; (5) [Mantani et al., 2005]; (6) [Mériau et al., 2006]; (7) [Li and Sinha, 2006]; (8) [Kano et al., 2007]; (9) [Moriguchi et al., 2007]; (10) [Frewen et al., 2008]; (11) [Kugel et al., 2008]; (12) [Silani et al., 2008]; (13) [Reker et al., 2009]; (14) this study; PTSD, posttraumatic stress disorder; *used ROI approach, and therefore coordinates were not available.
Although alexithymia includes dimensions related either to restricted emotional awareness or to a concrete thinking style [Bermond et al., 1999; Haviland, 1998; Sifneos, 1996], previous neuroimaging studies have tended to consider alexithymia as a single construct. Only Kugel et al. [ 2008] and recently Reker et al. [ 2009] have tested the influence of its various dimensions independently. Both studies revealed a specific association between AMG activity and the level of difficulty in identifying feelings in response to masked sad faces.
This study aimed at investigating the influence of interindividual differences in alexithymia on the neural network associated with the perception of fearful body expressions. Our goal was twofold: to identify brain activations specific to individuals displaying the characteristic combination of dimensions observed in alexithymia and to assess independently the influence of affective and cognitive dimensions of alexithymia on the fear network. In line with previous studies and the present meta‐analysis (see Fig. 1), we expected to replicate a group effect in medial prefrontal regions when perceiving threatening stimuli. Moreover, we expected to identify the relations between the alexithymia dimensions and brain activity in areas constituting the fear network, such as those previously revealed by Kugel et al. [ 2008] and Reker et al. [ 2009] between the difficulties in identifying feelings and the level of AMG activity. For this purpose, we selected participants in a nonclinical population with a questionnaire investigating five of the core facets of alexithymia (Bermond–Vorst Alexithymia Questionnaire). We put together a sample representative of the alexithymia scores' distribution in the population at large, with the objective of adopting both a dimensional approach to estimate the link between each of the five alexithymia dimensions and fear‐related brain areas and a categorical approach to test the effect of the high versus low levels of global alexithymia.
MATERIALS AND METHODS
Participants
We screened 201 young men for alexithymia using the Bermond–Vorst Alexithymia Questionnaire [BVAQ‐B, Zech and Luminet, 1999]. This procedure enabled to select 34 right‐handed men free of any history of neurological or psychiatric disorder and free of fMRI‐related exclusion criteria, who accepted to participate in the fMRI study. Following the routinely legal procedure for fMRI studies, all subjects met a medical doctor before the scan. This procedure allowed to verify inclusion criteria, such as right‐handedness as assessed by the Edinburgh Handedness Scale [Oldfield, 1971] and good or corrected (with contact lens) visual acuity. In‐line with our objectives, we first built two groups with maximally divergent BVAQ‐B scores: the high‐alexithymia (13 HA) and low‐alexithymia (12 LA) groups had BVAQ‐B scores that were respectively above the 75th (>51) and below the 25th (<46) percentiles of the BVAQ‐B scores' distribution of the initial 201 men sample. Finally, only nine men with an intermediate score could be recruited to built the third group (46 < BVAQ‐B < 51). All the participants gave their informed written consent. The study was approved by the local Ethics Committee and was conducted in accordance with the Declaration of Helsinki.
Self‐Report Questionnaires
In‐line with the consensus that alexithymia includes at least four core facets [Lumley et al., 2007; Sifneos, 1996], we used the BVAQ unlike most of previous neuroimaging studies, which used the TAS‐20 (see below). Our choice was based on the fact that in addition to measure the lack of imaginative/fantasmatic capacity, this questionnaire includes a supplementary dimension: the absence of subjective reactions when facing an emotional event. Moreover, this instrument is more independent of current dysphoric affects than the TAS‐20 [e.g. Berthoz et al., 2000; Kooiman et al., 2002; Lumley et al., 2007].
The participants completed French versions of the following self‐report questionnaires:
The Bermond‐Vorst Alexithymia Questionnaire version B [BVAQ‐B, Zech and Luminet, 1999] includes 20 items that measure five dimensions; three dimensions that Bermond and colleagues labeled affective: difficulties verbalizing emotional experience (B1), poor insight into one's emotional experiences (B3), low‐emotional reactivity to emotion‐inducing events (B4), and two dimensions that Bermond and colleagues labeled cognitive: poor daydreaming, lack of fantasies (B2) and difficulties analyzing one's own emotional states and reactions (B5). For French samples, the upper and lower BVAQ‐B cutoff scores are respectively [Deborde et al., 2008]: BVAQ‐B ≥ 53 and BVAQ‐B ≤ 43. Among the 34 study participants, the BVAQ‐B total scale demonstrated satisfying internal consistency (Cronbach's α = 0.71). Whereas this was the case for the BVAQ‐B B1 (Cronbach's α = 0.75), B2 (Cronbach's α = 0.68) and B5 subscales (Cronbach's α = 0.61), BVAQ‐B B3 (Cronbach's α = 0.38), and B4 subscales (Cronbach's α = 0.43) showed lower internal consistencies.
The 20‐item Toronto Alexithymia Scale [TAS‐20, Loas et al., 1995] captures two affective and one cognitive alexithymic features, respectively: difficulties identifying feelings (T1, equivalent to B3), difficulties describing feelings (T2, equivalent to B1), and a concrete thinking style (T3, equivalent to B5). For French samples, the upper and lower TAS‐20 cutoff scores are, respectively, [Loas et al., 1996]: TAS‐20 ≥ 56 and TAS‐20 ≤ 44.
The 13‐item Beck Depression Inventory [BDI‐13, Collet and Cotraux, 1986] measures the level of current depression.
The Spielberger State‐Trait Anxiety Inventory Form‐Y [Bruchon‐Schweitzer and Paulhan, 1993]: the first 20 items ask subjects to report the extent of their anxiety at present (STAI‐S); the last 20 items ask respondents to indicate the intensity of their anxiety in general (STAI‐T).
Self‐Report Questionnaires' Statistical Analyses
To adopt a dimensional approach, we performed correlation analyses between the self‐report scores among all the participants (n = 34). As the scores were found not normally distributed using a Shapiro–Wilk test, we used nonparametric Spearmann's coefficients of correlation. To adopt a categorical approach, we performed a Mann–Whitney test to estimate the effect of alexithymia category (HA vs. LA) on these scores.
fMRI Stimuli and Experimental Design
Subjects were first scanned and then performed a behavioral experiment on the same materials.
Materials
Recording of stimuli involved 12 professional actors (six females, six males), performing the neutral action of opening a door in front of them, react to a specified encounter and close the door again. The fear version of the scenario required actors to react to something or someone that frightened them. Importantly, faces were blurred such that only information from the body was available. Static materials of fearful and neutral actions were obtained by selecting the frame at the perceived apex of each video‐clip. Details can be found elsewhere [see Grèzes et al., 2007; Pichon et al., 2008].
fMRI experimental design
Here, the analyses focus on the comparison between fearful and neutral body expressions. The full experiment was however a 2 × 2 factorial design consisting in neutral and fearful body actions presented in either still or dynamic format. Each experiment included two scanning sessions. During each session, a total of 136 stimuli were presented: 24 stimuli of each category (dynamic fear, static fear, dynamic neutral, and static neutral), 10 oddball stimuli (Odd) (upside‐down video‐clips), and 30 null events (black screen). A stimulus lasted 3 s and was followed by a black screen of 600 ms. Order of stimuli was fully randomized. Subjects performed an oddball task: they were asked to press the button each time an upside‐down video‐clip appeared, so that trials of interest were uncontaminated by motor response.
fMRI data acquisition
Images were acquired using a 3‐T whole‐body imager equipped with a circular polarized head coil. For each participant, we first acquired a high‐resolution structural T1‐weighted anatomical image (inversion‐recovery sequence, 1 × 0.75 × 1.22 mm) parallel to the AC–PC plane, covering the whole brain. For functional imaging, we used a T2*‐weighted echo‐planar sequence at 36 interleaved 3.5‐mm‐thick axial slices with 1‐mm gap (TR = 2995 ms, TE = 35 ms, flip angle = 80°, FOV = 19.2 × 19.2 cm, 64 × 64 matrix of 3 × 3 mm voxels). For each session, 173 volumes were acquired.
fMRI Statistical Analyses
Image analysis was performed with SPM2 (http://www.fil.ion.ucl.ac.uk/spm). The first four volumes of each functional session were discarded to allow for equilibration effects. The remaining 169 functional images were reoriented, slice‐time corrected to the middle slice, and spatially realigned to the first volume. These images were normalized to the standard MNI template and subsampled at an isotropic voxel size of 2 mm. The normalized images were smoothed with an isotropic 6‐mm FWHM Gaussian kernel.
Statistical analysis was also carried out using SPM2. At the first level, the five following conditions were modeled for each session and subject: two where subjects saw static or dynamic fearful body expressions (Fs and Fd, respectively), two where subjects saw dynamic or static neutral body expressions (Ns and Nd, respectively), and one with the oddball stimuli. Null events were implicitly modeled. The BOLD response to the stimulus onset for each event‐type was convolved with a canonical hemodynamic response function of 3 s. Also included for each subject session were six covariates capturing residual movement‐related artefacts (three rigid‐body translations and three rotations determined from initial image coregistration), and a single covariate representing the mean (constant) BOLD signal over scans. The data were high‐pass filtered with a frequency cut‐off at 128 s.
We first performed a random effect whole‐brain analysis (ANOVA implemented in SPM2). For each subject at the first‐level, we created images of parameter estimates consisting in means of linear contrasts for each condition (Fd, Fs, Nd, and Ns). These four contrast images were then entered into a second‐level random effect analysis consisting in an ANOVA within‐subjects (n = 34). A nonsphericity correction was applied for variance differences between subjects.
The main effect Fear versus Neutral (independently of whether the stimuli where static or dynamic) was calculated [(Fs + Fd) − (Ns + Nd)]) to reveal the previously identified fear network [Grèzes et al., 2007; Pichon et al., 2009].
Then, the effect of alexithymia was investigated.
First, using a region of interest (ROI) approach, the links between the levels of activity in 12 brain areas of the fear network and self‐report scores was examined. The choice of these 12 regions was based on previous studies showing consistent activation of these brain areas in response to the perception of threatening bodily expressions [Grèzes et al., 2007; Pichon et al., 2008, 2009]. These ROIs (10‐mm radius spheres), centered on peak coordinates revealed in the main effect of fear (Fear vs. Neutral), were extracted for each individual mean contrast values (MarsBaR software). Correlation coefficients between individual mean values and BVAQ‐B scores were calculated. When a correlation was significant, equivalent correlation analyses were performed with TAS‐20 scores.
Second, using a categorical approach, to compare emotional processing in high‐alexithymia (13 HA) and low‐alexithymia (12 LA) subjects, we created images of parameter estimates for the contrast Fear versus Neutral actions [(Fs + Fd) − (Ns + Nd)] for each subject at the first level. Then, these contrast images were entered into an ANOVA with two groups comprising a factor. A nonsphericity correction was applied for variance differences between groups. We finally calculated the contrasts between groups to reveal brain areas that were significantly more activated in one group compared to the other.
All statistical parametric maps were thresholded at k = 10 voxels, P ≤ 0.001 (uncorrected). These maps were overlaid on the MNI template and labeled using the atlas of Duvernoy [ 1999] and the anatomy toolbox (http://www.fzjuelich.de/ime/spm_anatomy toolbox; [Eickhoff et al., 2005]).
Behavioral Experiment
We performed a recognition task after the scan to check that, in subclinical subjects, there was no difference in performances [recognition rates and reaction times (RT)] between high‐ and low‐alexithymia subjects. Participants were presented the same stimuli again and instructed to determine whether the emotion portrayed by the body expressions was fear or neutral. The percentage of correct emotion recognition (ER) and their corresponding RT were calculated.
Statistical analyses of behavioral data
First, we performed Spearmann correlations between ER and RT and alexithymia total scores (BVAQ‐B, TAS‐20). Second, repeated measures ANOVA [Group (HA vs. LA) × Emotion (Fear vs. Neutral)] were calculated for both ER and RT. Greenhouse–Geisser adjustment of degree of freedom was performed, when appropriate, and adjusted P values were reported.
RESULTS
Self‐Report Scores (n = 34, Table I)
Table I.
Participant self‐report questionnaires' scores and after‐scan task performances (n = 34)
| Mean (SD) | Mann–Whitney testa (n = 25) | ||||
|---|---|---|---|---|---|
| All (n = 34) | LA (n = 12) | HA (n = 13) | Z | P | |
| Age | 21.26 (2.39) | 20.08 (1.08) | 21.93 (2.89) | −2.010 | 0.060 |
| P sychometric data | |||||
| BVAQ‐B | 48.06 (10.85) | 36.17 (3.81) | 59.15 (5.78) | −4.248 | ≤0.001 |
| B1 | 12.06 (3.66) | 9.17 (3.16) | 14.31 (2.32) | −3.419 | ≤0.001 |
| B2 | 8.18 (3.49) | 6.25 (2.14) | 10.38 (4.01) | −2.502 | 0.011 |
| B3 | 9.32 (2.54) | 7.75 (2.99) | 10.31 (1.44) | −2.199 | 0.030 |
| B4 | 10.06 (3.29) | 7.92 (2.81) | 12.23 (2.35) | −3.285 | ≤0.001 |
| B5 | 8.44 (3.81) | 5.08 (0.67) | 11.92 (3.17) | −4.304 | ≤0.001 |
| TAS‐20 | 47.23 (12.53) | 38.00 (12.32) | 54.46 (9.45) | −2.997 | 0.002 |
| T1 | 16.09 (5.71) | 14.50 (7.01) | 17.15 (4.49) | −1.718 | 0.087 |
| T2 | 14.09 (4.94) | 11.25 (5.41) | 16.23 (4.19) | −2.263 | 0.022 |
| T3 | 17.06 (5.21) | 12.25 (3.22) | 21.97 (3.75) | −3.897 | ≤0.001 |
| BDI‐13 | 1.76 (2.16) | 0.83 (1.53) | 2.15 (2.76) | −1.475 | 0.205 |
| STAI | |||||
| State (STAI‐S) | 31.68 (9.32) | 27.42 (8.21) | 33.08 (9.07) | −1.662 | 0.098 |
| Trait (STAI‐T) | 37.59 (10.47) | 36.58 (12.26) | 35.77 (7.57) | −0.436 | 0.689 |
| Behavioral data | |||||
| Recognition (%) | 85.96 (3.91) | 86.20 (5.14) | 85.79 (3.57) | −0.545 | 0.611 |
| Fear | 77.30 (7.25) | 79.17 (8.43) | 74.94 (6.46) | −1.582 | 0.123 |
| Neutral | 94.61 (6.57) | 93.23 (8.57) | 96.64 (4.28) | −1.116 | 0.264 |
| Reaction time (ms) | 565.42 (116.41) | 582.83 (86.06) | 555.04 (158.51) | −0.653 | 0.538 |
| Fear | 561.95 (110.76) | 572.25 (91.85) | 558.12 (148.63) | −0.272 | 0.810 |
| Neutral | 568.90 (131.87) | 593.41 (93.66) | 551.96 (178.58) | −0.870 | 0.406 |
Note: SD, standard deviation; HA, group with high alexithymia; LA, group with low alexithymia; B1 = T3, difficulty in verbalizing emotions; B2, difficulty in fantasizing; B3 = T1, difficulty in identifying emotions; B4, low‐emotional reactivity; B5=T3, concrete thinking style.
Nonparametric test for the comparison between HA and LA groups.
We found a positive correlation between the BVAQ‐B and TAS‐20 total scores (r = 0.542, P = 0.001) and their corresponding factors (B1/T2: r = 0.702, B3/T1: r = 0.717, B5/T3: r = 0.717; all P < 0.001). Regarding the associations between alexithymia and dysphoric affects, we found a positive correlation between TAS‐20 total and BDI‐13 scores (r = 0.455, P = 0.007), TAS‐20 total, and STAI scores (TAS‐20/STAI‐S: r = 0.458, P = 0.006; TAS‐20/STAI‐T: r = 0.410, P = 0.016). This was not the case for BVAQ‐B scores (BVAQ‐B/BDI‐13: r = 0.227, P = 0.196; BVAQ‐B/STAI‐S: r = 0.232, P = 0.187; BVAQ‐B/STAI‐T: r = −0.055, P = 0.757). Regarding the alexithymia subscores, only those measuring the difficulties in identifying feelings were correlated with dysphoric affects' scores (B3/BDI‐13: r = 0.419, P = 0.014; B3/STAI‐S: r = 0.552, P = 0.001; B3/STAI‐T: r = 0.480, P = 0.004; T1/BDI‐13: r = 0.513, P = 0.002; T1/STAI‐S: r = 0.446, P = 0.008; T1/STAI‐T: r = 0.606, P < 0.001).
Moreover, we found no significant difference between the HA and LA groups for the BDI‐13 and STAI scores.
Behavioral Measures (Table I)
There was no main effect of Group [F ER(1,23) = 0.053, P = 0.820; F RT(1,23) = 0.289, P = 0.596], and no Group x Emotion interaction [F ER(1.88,43.18) = 1.731, P = 0.191; F RT(3,69) = 0.795, P = 0.501]. The analyses (Mann–Whitney tests) of each emotion separately (Fear and Neutral) revealed no group differences in performances (Table I). No significant correlations were found between the BVAQ‐B total score and recognition rates or reaction times for fearful [r ER = −0.307, P = 0.078; r RT = −0.227, P = 0.197) or neutral stimuli (r ER = 0.236, P = 0.180; r RT = −.253, P = 0.149). Similarly, no significant correlations were found between TAS‐20 scores and behavioral measures.
fMRI Data
ANOVA within group: Main effect of Fear (n = 34, Table II)
Table II.
Brain regions showing amplitude effects when subjects (n = 34) perceived Fearful versus Neutral bodily expressions and fear‐related areas differently activated between groups with high and low alexithymia
| Brain regions | MNI coordinates | Z score | Nb of voxels | ||
|---|---|---|---|---|---|
| x | y | z | |||
| All | |||||
| L Inferior frontal gyrus, pars orbitalis (BA47) | −34 | 30 | 2 | 4.58 | 45 |
| R Inferior frontal gyrus, pars triangularis (BA45) | 52 | 28 | 0 | 6.18 | 453 |
| L Inferior frontal gyrus, pars triangularis (BA45) | −48 | 24 | −6 | 4.58 | 45 |
| R Inferior frontal gyrus, pars opercularis (BA44) | 54 | 16 | 20 | 3.61 | 21 |
| L Inferior frontal gyrus, pars opercularis (BA44) | −48 | 14 | 18 | 4.63 | 388 |
| L Supplementary motor area | −6 | −20 | 54 | 5.17 | 217 |
| R Supplementary motor area | 6 | −10 | 70 | 3.74 | 17 |
| L Premotor cortex | −48 | −12 | 54 | 4.76 | 55 |
| R Premotor cortex | 58 | −2 | 44 | 4.11 | 86 |
| R Middle temporal gyrus | 62 | 42 | −2 | 6.11 | 2253 |
| R Superior temporal sulcus | 52 | −28 | −10 | 5.92 | |
| R Temporoparietal junction | 58 | −42 | 16 | 5.63 | |
| R Temporal pole | 48 | 0 | −34 | 4.49 | |
| L Superior temporal sulcus | −60 | −52 | 12 | 5.35 | 1461 |
| L Temporoparietal junction | −56 | −50 | 26 | 4.24 | |
| L Linual gyrus | −20 | −98 | −14 | 5.71 | 214 |
| Brain stem | 6 | −20 | −10 | 5.43 | 155 |
| L caudate nucleus | −6 | 6 | 6 | 3.80 | 20 |
| R Amygdala/hippocampus | 18 | −6 | −24 | 3.59 | 11 |
| L Amygdala | −28 | 0 | −26 | 3.57 | 13 |
| HA > LA | |||||
| R Anterior cingulate cortex (BA32) | 6 | 34 | 24 | 4.05 | 27 |
| R Cuneus | 16 | −64 | 32 | 3.95 | 17 |
| LA > HA | |||||
| L Inferior Parietal Lobule (BA40) | −56 | −44 | 46 | 4.24 | 25 |
| L Precuneus (BA7) | −12 | −68 | 64 | 4.21 | 54 |
| L Superior Parietal Lobule | −28 | −58 | 52 | 4.16 | 18 |
| R Premotor cortex (BA6) | 18 | 0 | 70 | 3.57 | 11 |
| R Middle temporal gyrus | 48 | −38 | −6 | 3.46 | 22 |
Height and extent threshold: P < 0.001 uncorrected and k = 10 voxels.
Whole body expressions of fear induced greater activation in bilateral inferior frontal gyrus (IFG), STS, supplementary motor area (SMA), PM, AMG, temporoparietal junction (TPJ), and right temporal pole (TP).
Correlation analyses (n = 34, Table III, Fig. 2)
Table III.
Correlations between brain regions showing amplitude effects in response to Fearful versus Neutral stimuli (fear‐related network) and BVAQ‐B scores (n = 34)
| Brain regions | BVAQ | B1 | B2 | B3 | B4 | B5 |
|---|---|---|---|---|---|---|
| R IFG (BA45) | 0.166 | 0.032 | 0.039 | 0.079 | 0.085 | 0.079 |
| L IFG (BA45) | −0.123 | −0.196 | 0.218 | 0.055 | −0.025 | −0.248 |
| R STS | −0.123 | −0.291 | 0.162 | 0.076 | −0.118 | −0.062 |
| L STS | 0.235 | 0.099 | 0.175 | 0.318 | −0.005 | 0.169 |
| R PM | −0.309a | −0.180 | −0.189 | −0.136 | −0.348* | −0.210 |
| L PM | −0.037 | −0.077 | −0.054 | 0.134 | −0.099 | 0.153 |
| R IFG (BA44) | −0.112 | −0.157 | −0.254 | 0.122 | −0.149 | −0.009 |
| L IFG (BA44) | −0.020 | 0.061 | −0.032 | 0.095 | 0.071 | −0.121 |
| R Temporal pole | −0.297 | −0.169 | −0.016 | −0.298 | −0.227 | −0.239 |
| R AMG | −0.219 | −0.074 | −0.093 | −0.420* | 0.090 | −0.185 |
| L AMG | −0.155 | −0.090 | −0.001 | −0.329b | −0.100 | 0.061 |
| R ACC | 0.377* | 0.291 | 0.066 | 0.170 | 0.367* | 0.304 |
*P < 0.05; a P = 0.075; b P = 0.057.
Value: Spearmann coefficients of correlation.
In yellow: correlations that did not survey when controlling for BDI or STAI scores.
Note: B1, difficulty in verbalizing emotions; B2, difficulty in fantasizing; B3, difficulty in identifying emotions; B4, low‐emotional reactivity; B5, concrete thinking style.
Figure 2.
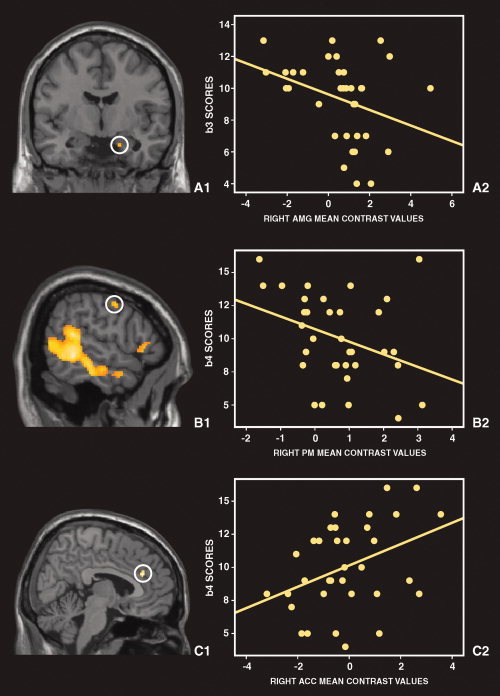
Correlations between activity in key regions of the fear network and alexithymia scores. A: The right amygdala (AMG): A1—Group average activation of amygdala superimposed on a coronal section of the MNI brain. A2—Negative correlation between AMG activity and BVAQ‐B B3 scores, which measure subjects' difficulties in identifying emotions. B: The right premotor cortex (PM): B1—Group average activation of the PM superimposed on a sagittal section of the MNI brain. B2—Negative correlation between PM activity and BVAQ‐B B4 scores that measure low emotional reactivity. C: The right anterior cingulate cortex (ACC): C1—Group average activation of ACC revealed by the comparison between HA and LA groups, superimposed on a sagittal section of the MNI brain. C2—Positive correlation between ACC activity and BVAQ‐B B4 scores that measure low emotional reactivity. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
We observed significant negative correlations between the BVAQ‐B B3 score (difficulties in identifying emotions) and right AMG activity (and a trend with left AMG, P = 0.057), between TAS‐20 total and T1 (similar to B3) scores and right AMG activity (TAS‐20/rAMG: r = −0.436, P = 0.010; T1/rAMG: −0.469, P = 0.005), between the BVAQ‐B B4 score (low‐emotional reactivity) and right PM activity, and a trend between BVAQ‐B total score and PM activity (r = −0.309, P = 0.075).
As B3 and T1 factors were linked to BDI‐13 scores on the one hand, and AMG activity was linked to BDI‐13 scores (r = −0.421, P = 0.013), on the other hand, we performed partial correlations (controlling for BDI‐13 scores) and found that the link between B3 scores and AMG activity disappeared (r = −0.211, P = 0.238, ddl = 31). The link between TAS‐20 scores and AMG activity was reduced and remained as a trend only after controlling for depressivity (r = −0.326, P = 0.064, ddl = 31), whereas the link between T1 scores and AMG activity remained (r = −0.355, P = 0.043, ddl = 31).
As multiple correlation analyses were computed between psychological measurements and hemodynamic response in 12 different ROIs, the possibility of false positives was increased. However, a more conservative corrected threshold would raise the risk of false negatives. Importantly, although the present correlational results provide useful information on the features of hemodynamic response in each ROI in an exploratory manner, they are only suggestive values and need to be replicated in future studies.
ANOVA between groups (13 HA vs. 12 LA; Table II)
The between‐group comparison for the main effect of Fear revealed greater activations in HA than LA in right ACC (BA24/BA32) and cuneus. Conversely, the LA activated significantly more than the HA the right PM, as well as the left precuneus, inferior parietal lobule, superior parietal lobule, and postcentral gyrus.
From the literature and the present meta‐analysis (see Fig. 1), we had a priori hypothesis on the link between alexithymia and ACC only, and therefore further investigated this region exclusively. First, in the two groups separately, we examined the ACC level of activity in our two conditions of interest (Fear and Neutral) relative to baseline (indexed by the beta values). We observed that beta values were negative when compared with the baseline. In HA, activity was higher for Fear than Neutral, whereas in LA, activity was lower for Fear than Neutral. Second, correlation analyses with the five BVAQ‐B dimensions scores in the whole sample (n = 34) showed that ACC activity was positively correlated with BVAQ‐B total scores, which was driven by a significant correlation with B4 scores (low‐emotional reactivity) only (Table III). One limit of these results is the use of self‐report questionnaires. Indeed, providing information about ones' own difficulties in socioaffective behaviors raises the problem of the capacity of subjects to report such difficulties without exaggerating or underreporting them. This can result in distorted responses. Studies in which both self‐ and observer‐evaluations are used will be more illuminating.
Brain areas intercorrelations (see Fig. 3)
Figure 3.
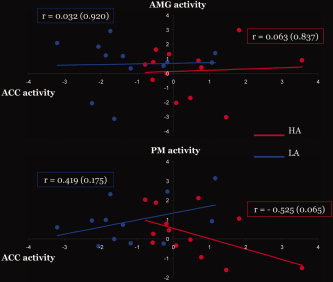
Correlations between three key brain areas (ACC, PM, and AMG) in high and low‐alexithymia scorers. Correlation analyses between AMG (top) and PM (bottom) activities and ACC activity in subjects with high alexithymia (in red) and low alexithymia (in blue). The dots correspond to subjects' mean contrast values in a 10‐mm ROI (AMG and PM) for the contrast Fear versus Neutral relative to the mean contrast values in the ACC ROI extracted from the same contrast. Note that the slopes between ACC and PM are significantly different between the two groups and in opposite direction (HA: r = −0.525; LA: r = 0.419), whereas the slopes between ACC and AMG did not differ between the two groups.
We observed correlations between alexithymia scores and activity in right AMG and PM as well as stronger activation in HA compared to LA in the ACC. As both AMG and PM have strong connections with ACC [see Amodio and Frith, 2006 for review], the link between ACC and AMG activities, and between ACC and PM, were explored here. Correlation analyses on all participants revealed no significant association. We then performed correlation analyses within HA and LA groups separately. We found no link between AMG and ACC activity in response to fearful stimuli in either groups (HA: r = 0.196, P = 0.503; LA: r = −0.224, P = 0.484). However, we found a trend for a negative correlation between ACC and right PM activity in HA (r = −0.525, P = 0.065) but not in LA (r = 0.419, P = 0.175). More importantly, the slopes were significantly different between the two groups (an univariate ANOVA with group as a random factor, ACC activity as the covariate and PM activity as the dependent variable revealed a significant Group × ACC activity interaction: F(1,21) = 6.205, P = 0.021).
DISCUSSION
The goal of this study was to assess the relations between individual differences in alexithymia and the neural activations triggered by observing threatening emotional actions. We used the BVAQ‐B multidimensional questionnaire to select the study participants. Moreover, alexithymia was also assessed with the TAS‐20, and the BDI‐13 and the STAI were used to measure dysphoric affects. Perceiving fearful body actions was associated with activations in bilateral STS, AMG, IFG, SMA, PM, TPJ, and TP, a fear‐related network similar to that reported in our previous fMRI studies [de Gelder et al., 2004; Grèzes et al., 2007].
Our critical findings are threefold. First, the level of difficulty in identifying emotions was negatively linked to AMG activity, a structure known to play a critical role in a brain network mediating automatic fear responses [Amaral, 2003; Blanchard and Blanchard, 1972; LeDoux, 2000]. Second, both alexithymia total scores and low emotional reactivity scores were negatively related to PM activity, a structure implicated in motor simulation [Rizzolatti et al., 2001] and in motor preparation [Hoshi and Tanji, 2004; Passingham, 1993], whereas only low emotional reactivity scores were negatively related to ACC activity. Finally, using a categorical approach, we showed that high‐BVAQ‐B scorers, when compared with low‐BVAQ‐B scorers, had similar levels of dysphoric affects and similar behavioral performances, but they had stronger ACC activity. Different patterns of correlation between ACC and PM activities were found among these two groups. We suggest that these results may reflect an altered control of adaptive behavioral response to negative emotional signals in high‐alexithymia subjects.
We first show that the more subjects report difficulties in identifying emotions, the less activity is observed in AMG during the perception of fearful behaviors (see Fig. 2). An extensive body of research has established that lesions of AMG or temporal pole in nonhuman primates [Amaral, 2003; Blanchard and Blanchard, 1972; LeDoux, 2000] and in humans [Adolphs and Tranel, 1999; Adolphs et al., 1994, 1995, 2001; Calder et al., 1996] cause severe deficits in recognizing aversive emotions, especially fear. In line with this, our result suggests that subjects who experience difficulties in emotional insight have an impaired sensitiveness to socially threatening stimuli. This is consistent with works that investigated how participant's subjective evaluation of emotional signals influenced brain responses to these cues [Canli et al., 2000; Garavan et al., 2001; Phan et al., 2003]. Notably, AMG was found less activated when subjects rated as low their emotional reactions to specific pictures [Canli et al., 2000]. This is also in line with what has been recently suggested by Bylsma et al.'s review [Bylsma et al., 2008]: they proposed that the blunted response of AMG in depressive adults to fearful stimuli could be seen as the neural basis of a reduced emotional reactivity. In this study, we show that the link between the level of AMG activation and the difficulties in identifying feelings was partially explained by the depression levels of the participants. Further studies, also using eye movements and autonomic responses recordings, could help to clarify whether the negative relation between subjective difficulties in identifying one's own emotion and AMG activity reveal reduced appraisal or reactivity to emotional stimuli.
The second main finding is that the more subjects show global alexithymia, the less there is activity in PM during fear perception (see Fig. 2). More specifically, lower PM activity was linked to reduced subjective emotional reactivity. It has been shown in macaque monkeys and in humans that PM is involved during the execution and the observation of an action [diPellegrino et al., 1992; Fogassi et al., 1998; Grafton et al., 1996; Grèzes et al., 2003; Keysers and Gazzola, 2007; Rizzolatti et al., 1996]. The automatic activation of motor representations or simulation during action observation is considered crucial for the understanding of others' motor behaviors [Gallese et al., 2004; Grèzes and Decety, 2001; Sommerville and Decety, 2006]. It has been recently suggested that by itself this simulation process is also an essential component for understanding other's emotions [Carr et al., 2003; Gallese et al., 2004; Preston and e Waal, 2002]. One possible explanation for the diminished PM activity is related to a reduced resonance to emotional actions. But two studies [Karlsson et al., 2008; Moriguchi et al., 2008], using video‐clips of human actions and emotions (positive and negative), have shown the reverse pattern, that is, greater activity in the premotor and sensorimotor cortex in high‐alexithymia scorers (than in low‐alexithymia scorers), which has been hypothesized to reflect a tendency, in high‐alexithymia scorers, to experience physical sensations in emotional situations [Karlsson et al., 2008], or an overtendency to simulate other's behaviors [Moriguchi et al., 2008].
Nevertheless, besides their communicative function, emotions are also adaptive by preparing the organism for behavioral response to the current environment [Darwin, 1872; Frijda, 1986; LeDoux, 1995]. Our study indeed confirmed that both PM and AMG are activated by socially threatening body expressions [de Gelder et al., 2004; Grèzes et al., 2007; Pichon et al., 2008]. The PM is known to be implicated in externally driven actions [Hoshi and Tanji, 2004; Passingham, 1993], and its present activation could also reflect the preparation of an adapted motor action [Hoshi and Tanji, 2004] in response to the perception of fear signals. Here, the observed coordinates (z MNI: 44) are located at the border between ventral and dorsal PM [Tomassini et al., 2007], whereas the ones found by Morigushi et al. ([2008]; z MNI: 72) and Karlsson et al. ([ 2008]; z MNI: 65) are located exclusively in dorsal PM. The difference in localization may be due to the fact that Morigushi et al. [2008] have used nonemotional videos (goal‐directed movements) and Karlsson et al. [ 2008] pooled emotions together, irrespectively of their valence. In the current study, the PM coordinates are similar to those reported in previous research during observation of threatening expressions [Whalen et al., 2001, z MNI = 46; Grèzes et al., 2007, z = 40; Pichon et al., 2008; z = 52; Pichon et al., 2009; z = 52]. Moreover, in monkey, stimulation of this part of the PM (the polysensory zone PZ in the dorsal part of F4) elicits protective movements [Graziano and Cooke, 2006]. Because the AMG plays a critical role in initiating adaptive behavioral response to the perception of social signals via its connections with subcortical areas and PM cortex [Amaral and Price, 1984; Avendano et al., 1983], another possible explanation for the observed diminished PM activity is that of an attenuated tendency to react appropriately to social situations. Yet, both of the above‐mentioned mechanisms could account for the lack of expressiveness that has been described in alexithymia [Taylor et al., 2008].
Third, high‐alexithymia subjects had greater ACC activity than low‐alexithymia subjects. Lane et al. [ 1997] have conceptualized alexithymia as a poor capacity to consciously experience emotional feelings and coined the term “blindfeel” (i.e., the emotional equivalent of blindsight; [Lane et al, 1997]). Furthermore, they speculated it would be associated with an abnormal participation of the ACC during emotional arousal on the basis that the level of activity in dorsal ACC to emotion‐inducing stimuli was related to high‐emotional awareness [Lane et al., 1998; see also McRae et al., 2008].
Yet, only low‐negative associations were reported between alexithymia and level of emotional awareness scores [e.g., Berthoz et al., 2000; Bydlowski et al., 2005; Lane et al., 2000]. Using group comparisons or correlation analyses, all the previous studies revealed associations between ACC activation and alexithymia (see Fig. 1). Nonetheless, it should be noted that 50% of them reported a negative link, 40% a positive link, and 10% showed both type of associations (i.e., high‐alexithymia individuals activated more the rostral ACC, whereas low‐alexithymia ones activated more its dorsal part, [Moriguchi et al., 2007]). In line with the results of Moriguchi et al. ([ 2007]; processing of pictures depicting painful actions), we observed greater rostral ACC activity in the high‐alexithymia group. So far, in previous studies that have used emotional facial expressions, alexithymia was associated either with greater dorsal ACC (12 12 48) ([Mériau et al., 2006]; implicit processing of the emotional faces) or with lower dorsal ACC (14 6 48) activity ([Kano et al., 2003]; explicit recognition of angry faces). Hence, the extent to which these discrepancies are related to the experimental variables, including tasks and stimuli, and the gender of the study participants should be addressed in future studies.
In the current study, ACC hyperactivity is located at the boundary between anterior part of rostral ACC (arACC), which is particularly activated by emotional tasks, and posterior part of rostral ACC (prACC), which is mostly activated by cognitive tasks [Amodio and Frith, 2006; Bush et al., 2000]. This subregion of ACC, for which we found a main effect of alexithymia trait, is considered crucial for efficient interactions between affective and cognitive processes [Amodio and Frith, 2006]. It was previously revealed during voluntary suppression of unpleasant affects elicited by aversive pictures [Phan et al., 2005] and during explicit control of subjective emotional arousal induced by threatening stimuli [Ochsner et al., 2004; Rubino et al., 2007]. Here, the more activity in this region, the less participants reported being emotionally aroused (BVAQ B4 score). In line with those findings, we suggest that higher ACC activity in high‐alexithymia individuals may reflect a mechanism restricting the harmful, unpleasant impact of a negative event. Alexithymia is indeed known to be associated with maladaptive coping strategies, notably emotional inhibition and immature defensive styles [Helmes et al., 2008]. Consistently, our results further revealed that the relationship between ACC and PM is in opposite direction in high‐alexithymia subjects (r = −0.525) and in low‐alexithymia subjects (r = 0.419) (see Fig. 3). This is not the case for the link between ACC and AMG [but see Mériau et al., 2006]. Both ACC and PM were found to be related to poor emotional reactivity (B4), whereas the AMG was found to be related to poor emotion identification (B3 and T1). As the part of medial prefrontal cortex, for which we found a group effect, has robust connections with premotor areas in monkeys [Barbas et al., 1999], we propose the present ACC/PM link in high‐alexithymia individuals could sustain inhibitory control of adaptive behavioral response to threatening emotional signals.
Finally, our results further demonstrate that alexithymia is a valid model for investigating the neural correlates of individual differences in inner emotional experiences and behavioral socioaffective skills. Alexithymia has been reported in about 15% of the normal population and is considered a vulnerability factor for various medical and psychiatric disorders [Taylor and Bagby, 2004]. Our study showed that, in nonclinical population, individuals who display prototypic alexithymia seem to overregulate their emotional state, in absence of any instruction, when confronted to emotional stimuli. We believe it would be informative to examine whether this strategy of regulation is similar in healthy and clinical individuals with alexithymia. In fact, regarding our results and the different models of alexithymia that have been proposed, it is plausible that high‐alexithymia subjects in nonclinical population use an inhibitory strategy when faced to emotional events, whereas high‐alexithymia patients in clinical populations are no more able of self‐regulation.
CONCLUSION
Being exposed to fear signals makes us feel threatened and prompts us to prepare an adaptive response. Our previous studies [de Gelder et al., 2004; Grèzes et al., 2007; Pichon et al., 2008] suggested that AMG and PM play a role in the preparation of the observers' motor response required by the situation. Using interindividual differences in alexithymia, a personality trait associated with deficits in emotional reactivity and emotion regulation, this study further reveals how anterior cingulate cortex interacts with PM to sustain self‐regulation of one's own emotional state in response to threatening social signals. On the behavioral level, this neural mechanism could account for the description of the “cold‐blooded” personality and “stiff wooden posture” of prototypic individuals with alexithymia [Taylor et al., 1997].
Acknowledgements
We are grateful to J.L. Anton, M. Roth, B. Nazarian, and B. Wicker for their help in fMRI scanning, to F. Maloumian for technical help, and to M. Wessa and C. Chevallier for their comments on previous versions of this manuscript.
REFERENCES
- Adolphs R, Tranel D ( 1999): Preferences for visual stimuli following amygdala damage. J Cogn Neurosci 11: 610–616. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR ( 1994): Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372: 669–672. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR ( 1995): Fear and the human amygdala. J Neurosci 15: 5879–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H ( 2001): Emotion recognition from faces and prosody following temporal lobectomy. Neuropsychology 15: 396–404. [DOI] [PubMed] [Google Scholar]
- Amaral DG ( 2003): The amygdala, social behavior, and danger detection. Ann NY Acad Sci 1000: 337–347. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL ( 1984): Amygdalo‐cortical projections in the monkey (Macaca fascicularis). J Comp Neurol 230: 496. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD ( 2006): Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci 7: 268–277. [DOI] [PubMed] [Google Scholar]
- Avendano C, Price JL, Amaral DG ( 1983): Evidence for an amygdaloid projection to premotor cortex but not to motor cortex in the monkey. Brain Res 264: 111–117. [DOI] [PubMed] [Google Scholar]
- Barbas H, Ghashghaei H, Dombrowski SM, Rempel‐Clower NL ( 1999): Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory‐related areas in the rhesus monkey. J Comp Neurol 410: 343–367. [DOI] [PubMed] [Google Scholar]
- Bermond B, Vorst HC, Vingerhoets AJ, Gerritsen W ( 1999): The Amsterdam Alexithymia Scale: Its psychometric values and correlations with other personality traits. Psychother Psychosom 68: 241–251. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Ouhayoun B, Parage N, Kirzenbaum M, Bourgey M, Allilaire JF ( 2000): Etude préliminaire de validation française de l'échelle de niveaux de conscience émotionnelle chez des patients déprimés et des contrôles. [Preliminary study for french validation of the levels of emotional awareness scale on depressed patients and controls]. Ann Med‐Psychol 158: 665–672. [Google Scholar]
- Berthoz S, Artiges E, Van de Moortele P‐F, Poline JB, Rouquette S, Consoli SM, Martino JL ( 2002): Effect of impaired recognition and expression of emotions on frontocingulate cortices: An fMRI study of men with alexithymia. Am J Psychiatry 159: 961–967. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ ( 1972): Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol 81: 281–290. [DOI] [PubMed] [Google Scholar]
- Bruchon‐Schweitzer M, Paulhan I ( 1993): Manuel Français de l'Echelle d'Anxiété‐Trait et d'Anxiété‐Etat de. Paris: Spielberger. [Google Scholar]
- Bush G, Luu P, Posner MI ( 2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 222. [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J ( 2008): A meta‐analysis of emotional reactivity in major depressive disorder. Clin Psychol Rev 28: 676–691. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Young AW, Rowland D, Perrett DI, Hodges JR, Etcoff NL ( 1996): Facial emotion recognition after bilateral amygdala damage: Differentially severe impairment of fear. Cogn Neuropsychol 13: 699–745. [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JDE, Cahill L ( 2000): Event‐related activation in the human amygdala associates with later memory for individual emotional experience. J Neurosci 20: 99RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL ( 2003): Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci USA 100: 5497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet L, Cotraux J ( 1986): Inventaire abrégé de la dépression de Beck (13 items). Etude de validité concurrente avec les échelles de Hamilton et de ralentissement de Widlöcher. Encephale 12: 77–79. [PubMed] [Google Scholar]
- Darwin C ( 1872): The Expression of the Emotions in Man and Animals. London: John Murray. [Google Scholar]
- de Gelder B, Snyder J, Greve D, Gerard G, Hadjikhani N ( 2004): Fear fosters flight: A mechanism for fear contagion when perceiving emotion expressed by a whole body. Proc Natl Acad Sci USA 101: 16701–16706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deborde A‐S, Berthoz S, Wallier JM, Fermanian J, Falissard B, Jeammet P, Corcos M ( 2008): The Bermond‐Vorst Alexithymia Questionnaire Cutoff Scores: A study in eating‐disorder and control subjects. Psychopathology 41: 43–49. [DOI] [PubMed] [Google Scholar]
- diPellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G ( 1992): Understanding motor events: A neurophysiological study. Exp Brain Res 91: 176–180. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM ( 1999): The Human Brain. Surface, Three‐Dimensional Sectional Anatomy with MRI, and Blood Supply. Vienna: Springer‐Verlag. [Google Scholar]
- Eichmann M, Kugel H, Suslow T ( 2008): Difficulty identifying feelings and automatic activation in the fusiform gyrus in response to facial emotion. Percept Motor Skills 107: 915–922. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K ( 2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Fadiga L, Rizzolatti G ( 1998): Neurons responding to the sight of goal directed hand/arm actions in the parietal area PF (7b) of the macaque monkey. Soc Neurosci Abstr 24: 257. [Google Scholar]
- Frewen PA, Lanius RA, Dozois DJ, Neufeld RWJ, Pain C, Hopper JW,Densmore M, Stevens TK ( 2008): Clinical and neural correlates of alexithymia in posttraumatic stress disorder. Ann NY Acad Sci 1071: 397–400. [DOI] [PubMed] [Google Scholar]
- Frijda NH ( 1986): The Emotions. Cambridge: Cambridge University Press. [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G ( 2004): A unifying view of the basis of social cognition. Trends Cogn Sci 8: 396–403. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pendergrass CA, Cara J, Ross TJ, Stein EA, Risinger RC ( 2001): Amygdala response to both positively and negatively valenced stimuli. Neuroreport 12: 2779–2783. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Arbib MA, Fadiga L, Rizzolatti G ( 1996): Localization of grasp representations in humans by positron emission tomography. II. Observation compared with imagination. Exp Brain Res 112: 103–111. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Cooke DF ( 2006): Parieto‐Frontal Interactions, Personal Space, and Defensive Behavior. Neuropsychologia 44: 845–859. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Decety J ( 2001): Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta‐analysis. Human Brain Mapp 12: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes J, Armony JL, Rowe J, Passingham RE ( 2003): Activations related to “mirror” and “canonical” neurones in the human brain: An fMRI study. Neuroimage 18: 928–937. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Pichon S, de Gelder B ( 2007): Perceiving fear in dynamic body expressions. NeuroImage 35: 959–967. [DOI] [PubMed] [Google Scholar]
- Haviland MG ( 1998): The validity of the California Q‐set alexithymia prototype. Psychosomatics 39: 536–539. [DOI] [PubMed] [Google Scholar]
- Helmes E, McNeill PD, Holden RR, Jackson C ( 2008): The construct of alexithymia: Associations with defense mechanisms. J Clin Psychol 64: 318–331. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J ( 2004): Functional specialization in dorsal and ventral premotor areas. Prog Brain Res 143: 507–511. [DOI] [PubMed] [Google Scholar]
- Huber M, Herholz K, Habedank B, Thiel A, Muller‐Kuppers M, Ebel H, Subic‐Wrana C, Kohle K, Heiss WD ( 2002): Different patterns of regional brain activation during emotional stimulation in alexithymics in comparison with normal controls. Psychother Psychosom Med Psychol 52: 469–478. [DOI] [PubMed] [Google Scholar]
- Kano M, Fukudo S, Gyoba J, Kamachi M, Tagawa M, Mochizuki H, Itoh M, Hongo M, Yanai K ( 2003): Specific brain processing of facial expressions in people with alexithymia: An H215O‐PET study. Brain 126: 1474–1484. [DOI] [PubMed] [Google Scholar]
- Kano M, Hamaguchi T, Itoh M, Yanai K, Fukudo S ( 2007): Correlation between alexithymia and hypersensitivity to visceral stimulation in human. Pain 132: 252–263. [DOI] [PubMed] [Google Scholar]
- Karlsson H, Naatanen P, Stenman H ( 2008): Cortical activation in alexithymia as a response to emotional stimuli. Br J Psychiatry 192: 32–38. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V ( 2007): Integrating simulation and theory of mind: From self to social cognition. Trends Cogn Sci 11: 194–196. [DOI] [PubMed] [Google Scholar]
- Kooiman CG, Spinhoven P, Trijsburg RW ( 2002): The assessment of alexithymia: A critical review of the literature and a psychometric study of the Toronto Alexithymia Scale‐20. J Psychosom Res 53: 1083–1090. [DOI] [PubMed] [Google Scholar]
- Kugel H, Eichmann M, Dannlowski U, Ohrmann P, Bauer J, Arolt V, Heindel W, Suslow T ( 2008): Alexithymic features and automatic amygdala reactivity to facial emotion. Neurosci Lett 435: 40–44. [DOI] [PubMed] [Google Scholar]
- Lane RD, Ahern GL, Schwartz GE, Kaszniak AW ( 1997): Is alexithymia the emotional equivalent of blindsight? Biol Psychiatry 42: 834–844. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE ( 1998): Neural correlates of levels of emotional awareness: Evidence of an interaction between emotion and attention in the anterior cingulate cortex. J Cogn Neurosci 10: 525. [DOI] [PubMed] [Google Scholar]
- LeDoux JE ( 1995): Emotion: Clues from the brain. Annu Rev Psychol 46: 209–235. [DOI] [PubMed] [Google Scholar]
- LeDoux JE ( 2000): Emotion circuits in the brain. Annu Rev Neurosci 23: 155–84. [DOI] [PubMed] [Google Scholar]
- Leweke F, Stark R, Milch W, Kurth R, Schienle A, Kirsch P, Stingl M, Reimer C, Vaitl D ( 2004): Patterns of neuronal activity related to emotional stimulation in alexithymia. Psychother Psychosom Med 54: 437–444. [DOI] [PubMed] [Google Scholar]
- Li CS, Sinha R ( 2006): Alexithymia and stress‐induced brain activation in cocaine‐dependent men and women. J Psychiatry Neurosci 31: 115–121. [PMC free article] [PubMed] [Google Scholar]
- Loas G, Fremaux D, Marchand MP ( 1995): Factorial structure and internal consistency of the French version of the twenty‐item Toronto Alexithymia Scale in a group of 183 healthy proband. Encephale 21: 117–122. [PubMed] [Google Scholar]
- Loas G, Otmani O, Fremaux D, Lecercle C, Duflot M, Delahousse J ( 1996): External validity, reliability and basic score determination of the Toronto Alexithymia Scales (TAS and TAS‐20) in a group of alcoholic patients. Encephale 22: 35–40. [PubMed] [Google Scholar]
- Luminet O, Taylor GJ, Bagby RM ( 2003): La mesure de l'alexithymie In: Corcos M, Speranza M, editors. Psychopathologie de l'Alexithymie. Paris: Dunod. [Google Scholar]
- Lumley MA, Neely LC, Burger AJ ( 2007): The assessment of alexithymia in medical settings: Implications for understanding and treating health problems. J Person Assess 89: 230–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantani T, Okamoto Y, Shirao N, Okada G, Yamawaki S ( 2005): Reduced activation of posterior cingulate cortex during imagery in subjects with high degrees of alexithymia: A functional magnetic resonance imaging study. Biol Psychiatry 57: 982–990. [DOI] [PubMed] [Google Scholar]
- McRae K, Reiman EM, Fort CL, Chen K, Lane RD ( 2008): Association between trait emotional awareness and dorsal anterior cingulate activity during emotion is arousal‐dependent. NeuroImage 41: 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mériau K, Wartenburger I, Kazzer P, Prehn K, Lammers CH, van der Meer E, Villringer A, Heekeren HR ( 2006): A neural network reflecting individual differences in cognitive processing of emotions during perceptual decision making. NeuroImage 33: 1016–1027. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Lane RD, Maeda M, Mori T, Nemoto K, Matsuda H, Komaki G ( 2006): Impaired self‐awareness and theory of mind: An fMRI study of mentalizing in alexithymia. NeuroImage 32: 1472–1482. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Decety J, Ohnishi T, Maeda M, Mori T, Nemoto K, Matsuda H, Komaki G ( 2007): Empathy and judging other's pain: An fMRI study of alexithymia. Cereb Cortex 17: 2223–2234. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Decety J, Hirakata M, Maeda M, Matsuda H, Komaki G ( 2008): The human mirror neuron system in a population with deficient self‐awareness: An fMRI study in alexithymia. Human Brain Mapp 9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ ( 2004): For better or for worse: Neural systems supporting the cognitive down‐ and up‐regulation of negative emotion. Neuroimage 23: 483–499. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Passingham RE ( 1993): The Frontal Lobes and Voluntary Action. Oxford: Oxford University Press. [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Decker LR, Noll DC, Nichols TE, Britton JC, Liberzon I ( 2003): Activation of the medial prefrontal cortex and extended amygdala by individual ratings of emotional arousal: A fMRI study. Biol Psychiatry 53: 211–215. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME ( 2005): Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry 57: 210–219. [DOI] [PubMed] [Google Scholar]
- Pichon S, de Gelder B, Grèzes J ( 2008): Emotional modulation of visual and motor areas by dynamic body expressions of anger. Soc Neurosci 3: 199–212. [DOI] [PubMed] [Google Scholar]
- Pichon S, de Gelder B, Grèzes J ( 2009): Two different faces of threat. Comparing the neural systems for recognizing fear and anger in dynamic body expressions. NeuroImage 47: 1873–1883. [DOI] [PubMed] [Google Scholar]
- Preston SD, e Waal FB ( 2002): Empathy: Its ultimate and proximate bases. Behav Brain Sci 25: 1–20. [DOI] [PubMed] [Google Scholar]
- Reker M, Ohrmann P, Rauch AV, Kugel H, Bauer J, Dannlowski U, Arolt V, Heindel W, Suslow T ( 2009): Individual differences in alexithymia and brain response to masked emotion faces. Cortex In Press, Corrected Proof. [DOI] [PubMed]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L ( 1996): Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res 3: 131–141. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V ( 2001): Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci 2: 661–670. [DOI] [PubMed] [Google Scholar]
- Rubino V, Blasi G, Latorre V, Fazio L, d'Errico I, Mazzola V, Caforio G,Nardini M,Popolizio T,Hariri A,Arciero G,Bertolino A ( 2007): Activity in medial prefrontal cortex during cognitive evaluation of threatening stimuli as a function of personality style. Brain Res Bull 74: 250–257. [DOI] [PubMed] [Google Scholar]
- Sifneos PE ( 1973): The prevalence of alexithymic characteristics in psychosomatic patients. Psychother Pschosom 22: 255–262. [DOI] [PubMed] [Google Scholar]
- Sifneos PE ( 1996): Alexithymia: Past and present. Am J Psychiatry 153: 137–142. [DOI] [PubMed] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U ( 2008): Levels of emotional awareness and autism: An fMRI study. Soc Neurosci 3: 97–112. [DOI] [PubMed] [Google Scholar]
- Sommerville JA, Decety J ( 2006): Weaving the fabric of social interaction: Articulating developmental psychology and cognitive neuroscience in the domain of motor cognition. Psychonom Bull Rev 13: 179–200. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Bagby RM ( 2004): New trends in alexithymia research. Psychother Psychosom 73: 68–77. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Bagby RM, Parker JDA ( 1997): Disorders of affect regulation alexithymia in medical and psychiatric illness. Cambridge: Cambridge University Press. [Google Scholar]
- Taylor GJ, Bagby RM, Parker JDA ( 2008): Disorders of affect regulation alexithymia in medical and psychiatric illness. Cambridge: Cambridge University Press. [Google Scholar]
- Tomassini V, Jbabdi S, Klein JC, Behrens TEJ, Pozzilli C, Matthews PM, Rushworth MFS, Johansen‐Berg H ( 2007): Diffusion‐weighted imaging tractography‐based parcellation of the human lateral premotor cortex identifies dorsal and ventral subregions with anatomical and functional specializations. J Neurosci 27: 10259–10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zech E, Luminet O ( 1999): Alexithymia and its measurement: Confirmatory factor analyses of the 20‐item Toronto Alexithymia Scale and the Bermond‐Vorst Alexithymia Questionnaire. Eur J Person 13: 511–532. [Google Scholar]


