Abstract
Much of the rising health care costs in aging populations can be attributed to congenital disease and psychiatric and neurologic disorders. Early detection of changes related to these diseases can promote the development of new therapeutic strategies and effective treatments. Changes in tissue, such as damage resulting from continued functional abnormality, often exhibit a time‐delay before detection is possible. Methods for detecting functional alterations in endogenous brain fluctuations allow for an early diagnosis before tissue damage occurs, enabling early treatment and a more likely positive outcome. A literature review and comprehensive overview of the current state of knowledge about endogenous brain fluctuations is presented here. Recent findings of the association between various pathological conditions and endogenous fluctuations are discussed. A particular emphasis is placed on research showing the relationship between clinical measures and pathological findings to the dynamics of endogenous fluctuations of the brain. Recent discoveries of methods for detecting abnormal functional connectivity are discussed and future research directions explored. Hum Brain Mapp 2008. © 2008 Wiley‐Liss, Inc.
Keywords: low frequency fluctuations, BOLD fMRI, ICA, FFT, fractal dimension, Hurst exponent, functional connectivity
INTRODUCTION
Imaging Endogenous Brain Activity With Functional MRI
Functional magnetic resonance imaging (fMRI) reflects hemodynamic alterations related to brain function. Blood oxygen level dependent (BOLD) contrast used in fMRI is based on detecting changes in local deoxyhemoglobin concentration that correlates with local field potentials and multiunit activity in brain cortex [Logothetis et al., 2004; Ogawa et al., 1992]. Changes in paramagnetic deoxyhemoglobin reduces T2/T2* weighted image intensity by increasing local dephasing of spins. BOLD image signal change has been correlated to externally controllable neuronal activity in order to detect where the brain activates in response to functional alterations. Controlled activity is administered to the subject with external stimuli or the subject is monitored for specific measurable functions.
In diagnostic imaging fMRI has been used to differentiate eloquent cortex from removable tissue such as tumors and arterio‐venous malformations [Sunaert, 2006]. Continuous attention and motionless participation in response to the stimuli during an fMRI scan is challenging for many patients. Some diseases and medications alter the brain hemodynamics and make the detection of stimulus related responses with fMRI inaccurate [Lehericy et al., 2002; Kiviniemi et al., 2005].
Biswal et al. [ 1995] showed that low frequency BOLD signal oscillations in resting patients are synchronous in bilateral primary motor cortices. The authors suggested that the detected low frequency fluctuations (LFF) function much like information carrying AM/FM radio waves. They regarded this phenomenon as functional connectivity, the correlation between spatially remote neurophysiologic events [Friston et al., 1993]. These intrinsic, functionally connected fluctuations can map the functional cortices more extensively and concisely than single paradigm activation studies [Xiong et al., 1999]. Moreover the endogenous brain fluctuations are linearly superimposed with neuronal activity in motor cortex during finger tapping and modulate brain responses external stimuli such as pain [Boly et al., 2007; Fox et al., 2006].
The brain continuously uses 20–25% of blood flow volume and it is very active even during resting periods and sleep. The ongoing activity of the brain cortex fluctuates very prominently on low frequencies <0.1 Hz [Fox et al., 2005, 2007]. Recent advances have resulted in a differentiation of endogenous brain activity fluctuations from different physiological noise sources. This has enabled the detection of several resting state neuronal networks, including the default mode activity and primary sensory cortices [Beckmann et al., 2005; Fox et al., 2005; Fransson, 2005; Kiviniemi et al., 2003]. Data‐based analyses using independent components have shown the human brain detects more than 10 independent signal sources [Damoiseaux et al., 2007].
One of the most robustly detectable sources of fluctuation is located in the areas of the brain that have largest metabolic activity during rest. These areas tend to deactivate during increasing cognitive demand and activate in rest, hence the name default mode network [Fox et al., 2005]. An interesting feature of the default mode network is that the fluctuation in the default mode network is in opposite phase compared to signal detected in functional networks that respond to external stimuli, i.e., these networks are anticorrelated [Fox et al., 2005; Fransson, 2005].
For the detected alterations in endogenous oscillations of brain activity to be used in diagnostic purposes, clarification is needed: (i) the physiological origin of endogenous brain fluctuations needs to be determined, and (ii) robust markers of endogenous brain activity and fluctuations related to specific diseases decided. International consortiums, such as the Finnish‐Chinese NEURO‐project EDEN, are actively researching the physiologic factors modulating the spontaneous activity of BOLD signal, as well as developing novel methods for using spontaneous brain activity as a marker of brain disease.
Multimodal Findings of Endogenous Fluctuations
Several physiological metrics show significant low frequency activity that overlaps with the frequency of endogenous brain fluctuations. Most of these physiological measures correlate with the endogeneous fluctuations detected in the brain using BOLD contrast:
-
i
The DC‐baseline of electroencephalography (EEG) can detect infraslow oscillations (ISO, 0.02–1 Hz) in human brain. The ISO produce a widespread synchronization of neuronal excitability over the cortex in sleeping subjects. The ISO waves correlate with power oscillations in the faster alpha (8–12 Hz), beta (12–30 Hz), and delta (4–8 Hz) EEG rhythms [Vanhatalo et al., 2004]. Slow alpha and beta power oscillations on EEG correlate with BOLD signal fluctuations [Goldman et al., 2002; Laufs et al., 2003].
-
ii
Astrocytes and neurons present slow oscillations in vitro reflect metabolic activity of the cell [Geiger, 1963; Vern et al., 1988]. Blood oxygenation and tissue cytochrome oxidative metabolism also oscillate dominantly at low frequency (LF ∼0.1 Hz) and at (VLF ∼0.04 Hz) [Obrig et al., 2000]. An association exists between metabolic oscillations and both the EEG and BOLD signal oscillations [Moosmann et al., 2003].
-
iii
Vasomotor waves at very low frequencies have been shown to occur in brain tissue [Hudetz et al., 1998; Kleinfeld et al., 1998]. Vasomotor waves are autonomous precapillary resistance sphincter tone undulations affecting the circulatory variables like blood flow, volume, and oxygenation at low frequencies. The vasomotor wave amplitude increases and wave frequency decreases following perfusion pressure reduction. The opposite amplitude and frequency changes occur after perfusion increase [Hudetz et al., 1998; Kannurpatti et al. (2008)].
-
iv
Low frequency changes in respiratory rate, end tidal CO2 and cardiac rate correlate with to the detected BOLD oscillations [Birn et al., 2006; Schmueli et al., 2007; Wise et al., 2004]. These are regarded as physiological noise preventing the detection of true neuronal network fluctuations. Physiological LFF are correlated with BOLD signal of the brain cortex both negatively and positively with long time lags (from −10 to +27 s based on previous findings). The physiological origin of these shifted correlations may reflect the feedback mechanisms of vascular regulatory system; vasomotor waves induce blood flow resistance and volume alterations that result in cardiac and respiratory system counter‐action. The positive and negative correlation with BOLD signal may be the action and counteraction of this feedback regulation.
Origin of Endogenous Bold Fluctuations—A Theory Based on Recent Observations
BOLD signal is determined by the oxygen extraction fraction (OEF) of the brain tissue. OEF is formed by the balance of inflow of oxygenated blood into tissue versus tissue oxygen uptake by metabolism. Oxygen inflow and tissue metabolism both slowly fluctuate over time [Obrig et al., 2000]. The resulting OEF is affected by the fluctuations. Tissue layer specific neuronal spiking activity is a strong contributor to the correlation structure of the spontaneous BOLD signal fluctuations [Fox and Raichle, 2007; Pelled and Goelman, 2004]. In addition, the effects of simultaneously occurring metabolic and blood flow fluctuations (i–iv) coalesce and form the OEF alterations that regulate BOLD signal.
Likely due to neuronal activity, endogenous BOLD fluctuation can be used in identifying functional brain units and neuronal networks [Fox and Raichle, 2007]. As the BOLD signal correlates significantly with local field potentials, the baseline neuronal power oscillations affect the BOLD signal [Logothetis et al., 2004]. Characteristic neuronal activity fluctuations occurring in multiple frequency ranges in neuronal networks set their mark on top of the underlying blood flow oscillations [Fox et al., 2005, 2006; Mantini et al., 2007].
Endogenous fluctuations are strongly associated with several physiological variables, such as cardiac, vasomotor, and respiratory oscillations [Birn et al., 2006; Shmueli et al., 2007; Wise et al., 2004]. If physiological variables were the sole origin of BOLD signal fluctuations, the correlation maps of endogenous fluctuation signals and BOLD data should map vascular anatomy and CSF/brain interfaces of the brain. However, this is not the case. Thus, endogenous BOLD signal fluctuations represent a spatially and temporally independent interference pattern reflecting the simultaneous activity of several physiological fluctuations; metabolic, electrophysiological, and vasomotor in origin [Birn et al., 2006; Kannurpatti et al., 2007; Kiviniemi et al., 2003; Mantini et al., 2007; Shmueli et al., 2007]. The effects of these fluctuations affect the OEF by superimposing in a linear way. The linear interaction of neuronal activation and background fluctuation has been shown to occur during finger tapping [Fox et al., 2006]. Figure 1 shows a theoretical example of the linear superimposition of physiological fluctuations modulating deoxyhemoglobin concentration. The modelling is based on previous knowledge on fluctuation time lags [Obrig et al., 2000] and transit time delays [Biswal et al., 2003].
Figure 1.
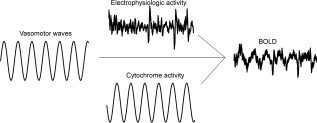
Theoretical sine wave model of 0.1 Hz fluctuation sources showing metabolic (cytochrome) activity, and vasomotor wave effects merging with more random electrophysiologic power oscillation effects on blood flow at the post‐capillary level. These three variables are major factors controlling OEF that determines the BOLD signal.
Factors Affecting Endogenous Fluctuations
Endogenous BOLD signal fluctuations can be increased or reduced. Hypercapnia and sevoflurane anesthesia are known to reduce temporal synchrony of the BOLD fluctuations [Biswal et al., 1997; Peltier et al., 2005]. Hypercapnia and isoflurane anesthesia increase regional blood flow, resulting in a reduction of the vasomotor fluctuations causing a lack of coherency in the BOLD fluctuations. In addition, hypercapnia may reduce the BOLD effect‐to‐noise by a ceiling effect thus also reducing the effects of neuronal fluctuations.
Intravenous anesthesia, hypocapnia, caffeine, and blood removal increase BOLD signal fluctuations. Midazolam sedation increases functional connectivity in the measured BOLD signal. All of the previous factors reduce of perfusion pressure, which increases vasomotor waves. Increased vasomotor waves cause the blood flow to be more synchronous, and therefore increases the correlation of measured BOLD signals [Hudetz et al., 1998; Kannurpatti et al., 2007; Kiviniemi et al., 2005].
Little is know about the effects of baseline neuronal activity intensity on endogenous physiological oscillations. Previous research has focused on changes in the mean BOLD signal intensity level following neuronal activity increase. BOLD signal fluctuations related to activation hyperemia may be strongly affected by the vasomotor wave or other physiological fluctuations. Obrig et al. [ 2000] noticed phase and amplitude differences in metabolism and oxy/deoxyhemoglobin concentration fluctuations between activated and resting brain cortex. The amount of brain activation needed to alter neuronal frequency‐band power oscillations and metabolic oscillations, as well as the way in which these fluctuation changes affect BOLD signal, is unknown. Multimodal recordings probing electrophysiological and metabolism can aid in clarifying this issue.
Functional connectivity is the measured correlation of voxel time courses of BOLD signals between different brain areas. The initial research by Biswal et al. [ 1995] was conducted in the absence of cited stimuli, noted as a relatively pure resting state. Effects like reduced vasomotor fluctuation frequency during activation hyperemia and post stimulus undershoot may alter endogenous oscillations. This would then affect functional connectivity correlations because of effects related more to the stimulus than inter‐neuronal connectivity. Waites et al. [ 2005] showed that functional connectivity increases when measured after stimulus. Functional connectivity measures during resting scans reflect more inter‐neuronal activity within brain networks than coherence of neuronal response to a given stimuli. The question then arises as to the meaning of endogenous fluctuations. A reasonable interpretation may define endogenous fluctuations as the response of a network of neurons to a single orchestrating neuron(s). Another possibility is defining endogenous fluctuations as cross talk of neuronal populations with certain time‐delay in small‐world networks.
Methods for Analyzing Fluctuations
Biswal et al. [ 1995] based functional connectivity measurements on well placed seed voxel time series correlations in fMRI. Exact knowledge of its features used in detecting functional connectivity is a distinctive strength of this method. Seed voxels can be measured with accuracy from probabilistic and histologic segmentations of normalized brain tissue. The measurement of negative and positive correlation of the detected seed voxel endogenous fluctuation yields a comprehensive overview of baseline activity differences in various brain activity between subjects [Zhou et al., 2007]. Seed voxel correlation is sensitive to errors in seed misplacement, which may occur after brain plasticity and other disease related shifting of brain functions. A recent approach by Zang et al. [ 2004] focuses on more regional connectivity such as regional homogeneity (named ReHo). ReHo estimates Kendall's coefficient of concordance between neighboring voxels and avoids the use of seed voxels. ReHo offers a fast mapping of the regional homogeneity of brain activity across the whole brain [Zang et al., 2004].
The endogenous brain oscillations of neuronal networks may be difficult to eradicate from complex BOLD signal because of unpredictable interference of vasomotor waves, electrophysiological, power oscillations, and other low frequency modulators of blood flow [Birn et al., 2006; Kannurpatti et al., 2007; Kiviniemi et al., 2003; Mantini et al., 2007; Shmueli et al., 2007]. Methods exists for effectively regressing physiologic noise, motion artifacts, and spin history distortions, and global image signal intensity alterations on the neuronal network signals [Birn et al., 2006; Maxim et al., 2005; Schmueli et al., 2007]. It is yet unclear as to what is the best way to preprocess resting state data. Should all of these preprocessing steps be applied or are we deleting some valuable information while regressing out physiological fluctuation related signals from the BOLD data with relatively long time delays?
Independent component analysis (ICA) can provide a data driven solution to the preprocessing and data analysis scheme. ICA can effectively separate signal sources reflecting endogenous brain activity fluctuations from noise sources [Calhoun et al., 2003; Greicius et al., 2004; Kiviniemi et al., 2003, 2004]. ICA separates motion, physiological pulsations, and technical artifacts into signal sources of their own. Time domain signals of these noise sources can then be regressed from the BOLD data if needed. The strength of ICA is in the data driven approach, which enables the detection of several relevant signal sources in the midst of disease related alterations in temporal or spatial features of BOLD signal [Beckman et al., 2005; Greicius et al., 2004, 2007; Kiviniemi et al., 2003].
ICA methods, such as FastICA, detect statistically independent signal sources by maximizing non‐Gaussianity of the joint‐density distributions of the data [Hyvärinen and Oja, 2000]. It has been shown to be beneficial to utilize the relatively large dimensionality of spatial domain instead of time domain in the separation of independent signal sources from the BOLD data. At present there are several free ICA software packages applicable for detecting spontaneous activity sources obtainable on‐line, such as GIFT (http://icatb.sourceforge.net/), MELODICA (http://www.fmrib.ox.ac.uk/fsl/), and RAICAR. These tools use several methods for estimating the number of components needed for each dataset. This has been a challenge in the implementation of ICA with imaging data. Another challenge with ICA is the lack of inherent ordering of detected signal sources. However, there are several automated ways to order the detected sources.
Smooth ongoing oscillations makes up only part of brain activity, particularly in the presence of pathological conditions. Pathological activity may present as a sudden or abnormally wide burst of excess activity occurring in addition to spontaneous fluctuations. A simple data‐based approach is to use two‐dimensional temporal cluster analysis (2dTCA). This approach produces a single BOLD signal reference waveform by calculating the number of voxels per a given time point higher (i.e., indicate activation) compared to previous time points. The use of the TCA reference waveform is similar to a typical activation study paradigm. The resulting maps can be used in detecting sudden bursts of activity [Morgan et al., 2007].
A method commonly used in the past for detecting periodic BOLD fluctuations was Fast Fourier Transformation (FFT) [Bandettini et al., 1993; Kiviniemi et al., 2000]. Kiviniemi et al. [ 2005] observed that BOLD signal power spectrum alters over a range of lowest frequencies after midazolam sedation. This alters the f −α power spectrum characteristics. Scale‐invariant f−α spectral behavior represents a system that has repeating elements over multiple frequency bands. Fractal dimension (D f, range 1–2) of a given signal can be estimated from the power spectral factor f−α: D f = (3 − α)/2. Another factor related to power spectral distribution is the Hurst exponent (H, range 0–1) which can be expressed as H = 2 − D f [Bullmore et al., 2001; Maxim et al., 2005]. A time series signal with maximal D f = 2 represents a process in which continuously varying signal deviations are sharply returned towards the stable mean signal. D f → 1 in a slowly fluctuating process that allows diffusion away from the mean (Sprott, 2003; Fig. 2). Figure 2 shows an example of a sedation‐related reduction in BOLD signal D f. Changes in the interaction (i.e., interference) of endogenous brain fluctuation sources may be reflected and quantified by measuring H and D f. However, these measures are not specific since different time vectors may represent the same value of D f. Projection of metrics free of scale may be used in reflecting disease‐related changes in multimodal approaches of brain activity measurement. EEG and BOLD data may add valuable information pertaining to brain pathology [Maxim et al., 2005].
Figure 2.
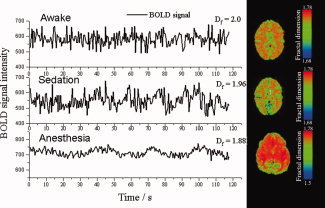
Three examples of BOLD signals from the visual cortex, with respective Df in different states of consciousness (left side). Examples color‐encoded Df on to the T2* weighted EPI image (TR 500, TE 40 ms) calculated in time domain (on right side). Subjects displayed on the top (awake) and middle sections (sedation) are the same subject (adult volunteer); the bottom section displays data for an anesthetized child subject. In sedated and anesthetized subjects the BOLD signal fluctuates in a less controlled way suggesting a reduced long term memory of the processes contributing to BOLD signal (Maxim et al., 2007).
Effects of Pathology on Endogenous Brain Activity Fluctuations
Functional connectivity of the endogenous brain oscillations has been successfully applied in detecting differences between diverse patient groups and controls. Li et al. [ 2002] were the original researchers to demonstrate changes in the mean inter‐voxel correlation coefficient of bilateral hippocampi in Alzheimers disease. These authors hypothesized the reduction of functional connectivity in hippocampi was due to the effects of neurofibrillary tangles and plaques. Lowe et al. [ 2002] detected reduced connectivity in multiple sclerosis. Low frequency BOLD synchrony reflected white matter tract integrity and was regarded as a marker of white matter disease. More recently, functional correlation coefficient alterations have been observed in several disease areas (e.g., depression) suggesting altered cortico–limbic connectivity [Anand et al., 2007].
Schizophrenia reduces functional connectivity of the default mode network fluctuations [Bluhm et al., 2007; Garrity et al., 2007]. Estimation of positive and negative associations of endogenous fluctuations in known anatomical segments resulted in detecting over and under‐connectivity in paranoid schizophrenia [Zhou et al., 2007]. Multidimensional scaling methods of brain activations show more variability in the interregional configurations in schizophrenic subjects. This suggests a disconnection in neuronal networks [Welchew et al., 2002]. The complexity and multitude of cognitive changes in schizophrenia simplifies with an analysis of abnormal resting state changes. Improper neuronal integration leads to excess introspection, abnormal ideas, and problems with coordination of movement and competition of information processing. These challenges can be addressed with further understanding of over‐ and under‐disintegration. [Grecius et al., 2004, 2007] published a significant set of findings based on the ICA method. These authors showed that the signal source of endogenous brain fluctuations is involved in a default‐mode network. Patients with Alzheimer disease (AD), as well as patients suffering from depression, showed detectable spatial signal sources differences in default mode activity. The ICA analysis was used to separate signal source components, from which the component best matching with a default mode template in normalized space was detected [Greicius et al., 2004]. The goodness‐of‐fit between the detected ICA signal source and the template could be used as a metric distinguishing AD subjects from normal age‐matching controls. Significant functional connectivity alterations were detected in key areas of episodic memory processes such as the hippocampi. The patient group suffering from depression showed abnormal functional connectivity of subgenual cingulate cortex to default mode network.
Abnormal regional homogeneity of the endogenous brain fluctuations has been detected in Alzheimers disease (AD) [He et al., 2007], attention deficit hyperactivity disorder (ADHD) [Zhu et al., 2006] and schizophrenia [Liu et al., 2006]. AD patients showed a reduced regional homogeneity in posterior cingulate gyrus and precuneus. The reduction was correlated with Mini‐Mental State Exam score. Regional brain atrophy and reduction in tissue metabolism in these brain areas were found to be associated with reducing local coherence. The same authors noticed a reduction in the low frequency amplitude of BOLD signal fluctuations in the same regions [Zang et al., 2007]. They concluded the reduction in endogenous brain oscillations leads to reduced regional homogeneity. Maxim et al. [ 2005] observed that BOLD signal LFF were more dominant in AD patients, and the Hurst exponent increased in multiple areas. This change was connected to alterations in D f of EEG in the same areas reflecting reductions in acetylcholinergic neuronal projections in the frontal brain [Maxim et al., 2005].
In an epilepsia model the BOLD signal increase was shown to precede electric activity increase [Mäkiranta et al., 2005]. The reversed relationship may have an impact on endogenous brain fluctuations and connectivity. Subjects having temporal epilepsia showed reduced functional connectivity of anterior and posterior cingulate gyrus, and, inferior and medial frontal gyrus. Remarkably the functional connectivity abnormality was detectable dispite normal activation of the same areas during language task [Waites et al., 2006]. The authors proposed four possible mechanisms behind the functional connectivity change: (i) interictal and ictal discharges, (ii) regional inhibition of (inter)ictal charges, (iii) medication, or (iv) plasticity changes in language networks. However these mechanisms do not cover the scope of temporal lobe epilepsy and the authors suggested default mode network under‐connectivity was present. More recent methods using temporal cluster analysis (2dTCA) have been able to detect interictal activity in the default mode network and other brain areas in epileptic subjects. BOLD with 2dTCA can depict epileptic activity over the whole brain, including deep subcortical structures inaccessible with common diagnostic tools such as an EEG [Morgan et al., 2007]. Combined EEG/fMRI studies have shown that inter‐ictal and generalized 3/s epileptic activity interferes with default mode system leading to impaired consciousness in the absence of detectable seizure or behavioral changes (Laufs, this issue). Reduction in precuneus connectivity in default mode is correlated to a decrease in consciousness (Greicius et al., in this issue, Laufs in this issue).
Monitoring Effects of Therapy
Midazolam sedation to Ramsey score three level (subject show little response to strong calls) increases the power of low frequency fluctuation and BOLD signal synchrony in primary functional regions and reduces default mode connectivity from precuneus [Kiviniemi et al., 2005; Greicius et al., this issue]. Peltier et al. [ 2005] observed that sevoflurane reduces functional connectivity. This difference is best explained by effects of pharmaceuticals on vasomotor waves; midazolam reduces and sevoflurane increases vasomotor waves. Drugs may have nontherapeutic side effects on vasomotor waves, metabolism or neuronal activity fluctuations. Cocaine, caffeine, and other chemical substances affecting vascular tone or neuronal activity affect endogenous fluctuations and need to be carefully monitored [Li et al., 2000]. Therefore conclusions drawn from studies of therapeutic drug effects based on low frequency functional connectivity measures should be carefully considered. Further research is needed to clarify the effects of a given drug.
Anand [ 2007] found that phase synchrony of resting state endogenous oscillations increases in limbic structures (amygdala, pallidostriatum, and medial thalamus) and anterior cingulate cortex depressed subjects after 6 weeks of antidepressant therapy. The authors emphasized well the difficulty in establishing the drug (sertraline) effect on hemodynamics, symptom change, or placebo. In another study subgenual cingulate showed excess connectivity to the default mode network in depression. Correlation coefficients within the area were determined to be a marker of refractoriness to depression treatment.
Future Directions
Research has established acceptable measures of endogenous oscillations that help to differentiate patient groups from controls and monitor treatment to therapy. Further work is needed to determine which of the sources of fluctuation are directly affected by a given disease. More precise knowledge is needed on BOLD signal fluctuations and their temporal formation, as well as ways in which a given disease alters tissue function. This knowledge will enable the development of new strategies for disease diagnostics and treatment. Multimodality approaches incorporating near‐infrared spectroscopy, noninvasive blood pressure measurements and EEG can be used to determine which of the underlying BOLD signal fluctuation sources are affected by a given disease.
Results of altered correlation of endogenous brain fluctuations in different areas reflect either localized coherence or longer range network connectivity alterations. Exploring disease related alterations in long and short distance communication in neuronal networks is an area of research with much potential. Correlation analysis does not show causality or directionality of the connections, which may be important in some diseases. Small world network analyses and Granger causality approaches do yield results of causality [Basset et al., 2006; Goebel et al., 2003]. In the clinical diagnostic setting, the detection of changes in spontaneous background brain activity needs to be identified using semiautomated computer interfaces. Disease‐specific modifications are advisable for methods such as ICA, 2dTCA, correlation based techniques and multiscale estimation tools. The aim is to make data‐based decisions using quantitative methods.
A method providing for a more direct measurement of neuronal activity or electric current than the BOLD signal would give a more precise estimate of neuronal tissue status. Methods like diffusion weighted adcfMRI may provide a more precise characterization of brain function [Le Bihan et al., 2007]. Mezer et al. [ 2007] used a BOLD signal spectrogram approach to functionally segment anatomical structures for both white and gray matter. The authors showed that BOLD signal frequency bands are tissue specific, and the relative frequency distributions in momentary spectrograms change continuously in time. Faster temporal sampling of brain signals is needed in order to avoid aliasing effects completely. Changing an MRI scanner into a magnetic resonance encephalographer via reducing spatial encoding is a method for obtaining a faster MRI signal [Henning et al., 2006]. Further research is needed to determine the usefulness of these methods in clinical science and diagnostics.
CONCLUSION
Endogenous brain fluctuations as a measure of functional abnormality enable the detection of disease in an early phase prior to structural damage or in the absence of detectable macroscopic abnormality. Detection and quantification of endogenous brain oscillations can be effectively used to estimate disease stage and effectiveness of treatment. Disease alters spatial configuration of endogenous fluctuation sources by reducing local activity because of tissue damage, by plasticity effects, or by producing abnormal coherent activity outside known networks. Scale‐free alterations of multiple fluctuation frequencies suggest a failure in neurovascular interaction. Tissue damage in gray matter layers and white matter tracts has led to alterations of temporal coherence of the fluctuations. Disease may be inhibiting or augmenting normal fluctuations in tissue metabolism, electrophysiological, or vascular levels. New algorithms evaluating neuronal network fluctuations offer tools for the development of disease specific treatment and monitoring schemes. However, care must be taken in differentiating changes due to medication and disease. Further research on the basic mechanisms of endogenous fluctuations is needed to better understand disease and medication‐related changes.
Acknowledgements
The author cordially thank Matthew J. Hayat, Ph.D., for editorial assistance.
REFERENCES
- Anand A,Li Y,Wang Y,Wu J,Gao S,Bukhari L,Mathews VP,Kalnin A,Lowe MJ ( 2007): Antidepressant effect on connectivity of the mood‐regulating circuit: An fMRI study. Neuropsychopharmacology 30: 1334–1344. [DOI] [PubMed] [Google Scholar]
- Bandettini PA,Jesmanowicz A,Wong EC,Hyde JS ( 1993): Processing strategies for time‐course data sets in functional MRI of the human brain. Magn Reson Med 30: 161–173. [DOI] [PubMed] [Google Scholar]
- Bassett DS,Meyer‐Lindenberg A,Achard S,Duke T,Bullmore E ( 2006): Adaptive reconfiguration of fractal small‐world human brain functional networks. Proc Natl Acad Sci USA 103: 19518–19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF,DeLuca M,Devlin JT,Smith SM ( 2005): Investigations into resting‐state connectivity using independent component analysis. Phil Trans Roy Soc Lond B Biol Sci 29: 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB,Yetkin FZ,Haughton VM,Hyde JS ( 1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34: 537–541. [DOI] [PubMed] [Google Scholar]
- Biswal B,Hudetz AG,Yetkin FZ,Haughton VM,Hyde JS ( 1997): Hypercapnia reversibly suppresses low‐frequency fluctuations in the human motor cortex during rest using echo‐planar MRI. J Cereb Blood Flow Metab 17: 301–308. [DOI] [PubMed] [Google Scholar]
- Biswal BB,Pathak AP,Ulmer JL,Hudetz AG ( 2003): Decoupling of the hemodynamic and activation‐induced delays in functional magnetic resonance imaging. J Comput Assist Tomo 27: 219–225. [DOI] [PubMed] [Google Scholar]
- Birn RM,Diamond JB,Smith MA,Bandettini PA ( 2006): Separating respiratory‐variation‐related fluctuations from neuronal‐activity‐related fluctuations in fMRI. Neuroimage 15: 1536–1548. [DOI] [PubMed] [Google Scholar]
- Bluhm RL,Miller J,Lanius RA,Osuch EA,Boksman K,Neufeld RW,Theberge J,Schaefer B,Williamson P ( 2007): Spontaneous low‐frequency fluctuations in the BOLD signal in schizophrenic patients: Anomalies in the default network. Schizophr Bull 33: 1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M,Balteau E,Schnakers C,Degueldre C,Moonen G,Luxen A,Phillips C,Peigneux P,Maquet P,Laureys S ( 2007): Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA 17: 12187–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET,Long ET,Suckling J,Fadili J,Calvert G,Zelaya F,Carpenter TA,Brammer M ( 2001): Colored noise and computational inference in neurophysiological (fMRI) time series analysis: Resampling methods in time and wavelet domains. Hum Brain Map 12: 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD,Adali T,Pekar JJ,Pearlson GD ( 2003): Latency (in)sensitive ICA. Group independent component analysis of fMRI data in the temporal frequency domain. Neuroimage 20: 1661–1669. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS,Rombouts SA,Barkhof F,Scheltens P,Stam CJ,Smith SM,Beckmann CF ( 2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 12: 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD,Raichle ME ( 2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700–711. [DOI] [PubMed] [Google Scholar]
- Fox MD,Snyder AZ,Vincent JL,Corbetta M,Van Essen DC,Raichle ME ( 2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD,Snyder AZ,Zacks JM,Raichle ME ( 2006): Coherent spontaneous activity accounts for trial‐to‐trial variability in human evoked brain responses. Nat Neurosci 9: 23–25. [DOI] [PubMed] [Google Scholar]
- Fransson P ( 2005): Spontaneous low‐frequency BOLD signal fluctuations: An fMRI investigation of the resting‐state default mode of brain function hypothesis. Hum Brain Mapp 26: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ,Frith CD,Liddle PF,Frackowiak RS ( 1993): Functional connectivity: The principal‐component analysis of large (PET) data sets. J Cereb Blood Flow Metab 13: 5–14. [DOI] [PubMed] [Google Scholar]
- Garrity AG,Pearlson GD,Mckiernan K,Lloyd D,Kiehl KA,Calhoun VD ( 2007): Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry 164: 450–457. [DOI] [PubMed] [Google Scholar]
- Geiger RS ( 1963): The behaviour of adult mammalian brain cell in culture. Int Rev Neurobiol 11: 1–52. [DOI] [PubMed] [Google Scholar]
- Greicius MD,Srivastava G,Reiss AL,Menon V ( 2004): Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci USA 101: 4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD,Flores BH,Menon V,Glover GH,Solvason HB,Kenna H,Reiss AL,Schatzberg AF ( 2007): Resting‐state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel R,Roebroeck A,Kim DS,Formisano E ( 2003) Investigating directed cortical interactions in time‐resolved fMRI data using vector autoregressive modeling and Granger causality mapping. Magn Reson Imaging 21: 1251–1261. [DOI] [PubMed] [Google Scholar]
- Goldman RI,Stern JM,Engel J Jr,Cohen MS ( 2002): Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport 13: 2487–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y,Wang L,Zang Y,Tian L,Zhang X,Li K,Jiang T ( 2007): Regional coherence changes in the early stages of Alzheimer's disease: A combined structural and resting‐state functional MRI study. Neuroimage 1: 488–500. [DOI] [PubMed] [Google Scholar]
- Henning J,Zhong K,Speck O ( 2007): MR‐Encephalography: Fast multi‐channel monitoring of brain physiology with magnetic resonance. Neuroimage 1: 212–219. [DOI] [PubMed] [Google Scholar]
- Hudetz AG,Biswal B,Shen H,Lauer KK,Kampine JP ( 1998): Spontaneous fluctuations in cerebral oxygen supply—An introduction In: Hudetx and Bruley, editors. Oxygen Transport to Tissue. New York: Plenum Press; pp 551–559. [DOI] [PubMed] [Google Scholar]
- Hyvärinen A,Oja E ( 2000): Independent component analysis: Algorithms and applications. Neural Netw 13: 411–430. [DOI] [PubMed] [Google Scholar]
- Kannurpatti SS,Biswal BB,Kim YR,Rosen BR ( 2008): Spatio temporal characteristics of low frequency BOLD signal fluctuations in isoflurane anesthetized rat brain. NeuroImage 40: 1738–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi V,Jauhiainen J,Pääkkö E,Vainionpää V,Oikarinen J,Rantala H,Tervonen O,Biswal BB ( 2000): Slow vasomotor fluctuation in the fMRI of the anesthetized child brain. Mag Reson Med 44: 378–383. [DOI] [PubMed] [Google Scholar]
- Kiviniemi V,Kantola J‐H,Jauhiainen J,Hyvärinen A,Tervonen O ( 2003): Independent component analysis of non‐deterministic fMRI signal sources. NeuroImage 19: 253–260. [DOI] [PubMed] [Google Scholar]
- Kiviniemi V,Kantola J‐H,Jauhiainen J,Tervonen O ( 2004): Comparison of methods for detecting nondeterministic fluctuation in fMRI. Magn Reson Imag 22: 197–203. [DOI] [PubMed] [Google Scholar]
- Kiviniemi V,Haanpää H,Kantola J‐H,Jauhiainen J,Vainionpää V,Alahuhta S,Tervonen O ( 2005): Midazolam sedation increases fluctuation and synchrony of the resting brain BOLD signal. Magn Reson Imag 23: 531–537. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D,Mitra PP,Helmchen F,Denk W ( 1998): Fluctuations and stimulus‐induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc Natl Acad Sci USA 95: 15741–15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H,Kleinschmidt A,Beyerle A,Eger E,Salek‐Haddadi A,Preibisch C,Krakow K ( 2003): Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci USA 100: 11053–11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D,Urayama S,Aso T,Hanakawa T,Fukuyama H ( 2006): Direct and fast detection of neuronal activation in the human brain with diffusion MRI. Proc Natl Acad Sci USA 103: 8263–8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehéricy S,Biondi A,Sourour N,Vlaicu M,du Montcel ST,Cohen L,Vivas E,Capelle L,Faillot T,Casasco A,Le Bihan D,Marsault C ( 2002): Arteriovenous brain malformations: Is functional MR imaging reliable for studying language reorganization in patients? Initial observations. Radiology 223: 672–682. [DOI] [PubMed] [Google Scholar]
- Liu H,Liu Z,Liang M,Hao Y,Tan L,Kuang F,Yi Y,Xu L,Jiang T ( 2006): Decreased regional homogeneity in schizophrenia: A resting state functional magnetic resonance imaging study. Neuroreport 17: 19–22. [DOI] [PubMed] [Google Scholar]
- Li SJ,Biswal B,Li Z,Risinger R,Rainey C,Cho JK,Salmeron BJ,Stein EA ( 2000): Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn Reson Med 43: 45–51. [DOI] [PubMed] [Google Scholar]
- Li S‐J,Li Z,Wu G,Zhang M‐J,Franczak M,Antuono PG ( 2002): Alzheimers disease: Evaluation2 of a functional MR imaging index as a marker. Radiology 225: 253–259. [DOI] [PubMed] [Google Scholar]
- Logothetis NK,Pauls J,Augath M,Trinath T,Oelterman A ( 2001): Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157. [DOI] [PubMed] [Google Scholar]
- Lowe MJ,Phillips MD,Lurito JT,Mattson D,Dzemidzic M,Mathews VP ( 2002): Multiple sclerosis: Low‐frequency temporal blood oxygen level‐dependent fluctuations indicate reduced functional connectivity—Initial results. Radiology 224: 184–192. [DOI] [PubMed] [Google Scholar]
- Maxim V,Sendur L,Fadili J,Suckling J,Gould R,Howard R,Bullmore E ( 2005): Fractional Gaussian noise functional MRI and Alzheimer's disease. NeuroImage 25: 141–158. [DOI] [PubMed] [Google Scholar]
- Mantini D,Perrucci MG,Del Gratta C,Romani GL,Corbetta M ( 2007): Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci USA 7;104: 13170–13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezer A,Assaf Y ( 2007): Resting state fMRI of the Thalamus. ISMRM, Abstract 31. [Google Scholar]
- Moosmann M,Ritter P,Krastel I,Brink A,Thees S,Blankenburg F,Taskin B,Obrig H,Villringer A ( 2003): Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. Neuroimage 20: 145–158. [DOI] [PubMed] [Google Scholar]
- Morgan VL,Gore JC,Abou‐Khalil B ( 2007): Cluster analysis detection of functional MRI activity in temporal lobe epilepsy. Epilepsy Res 76: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkiranta M,Ruohonen J,Suominen K,Niinimaki J,Sonkajärvi E,Kiviniemi V,Seppänen T,Alahuhta S,Jantti V,Tervonen O ( 2005): BOLD signal increase preceeds EEG spike activity—A dynamic penicillin induced focal epilepsy in deep anesthesia. Neuroimage 27: 715–724. [DOI] [PubMed] [Google Scholar]
- Obrig H,Neufang M,Rüdiger W,Kohl M,Steinbrink J,Einhäupl K,Villringer A ( 2000): Spontaneous low frequency oscillations of cerebral hemodynamics and metabolsim in human adults. NeuroImage 12: 623–639. [DOI] [PubMed] [Google Scholar]
- Ogawa S,Tank DW,Menon R,Ellermann JM,Kim S‐G,Merkle H,Ugurbil K ( 1992): Intrinsic signal changes accompanying sensory stimulation; Functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA 89: 5951–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelled G,Goelman G ( 2004): Different physiological MRI noise between cortical layers. Magn Res Med 52: 913–916 [DOI] [PubMed] [Google Scholar]
- Peltier SJ,Kerssens C,Hamann SB,Sebel PS,Byas‐Smith M,Hu X ( 2005): Functional connectivity changes with concentration of sevoflurane. Neuroreport 28: 16:285–288. [DOI] [PubMed] [Google Scholar]
- Shmueli K,van Gelderen P,de Zwart JA,Horovitz SG,Fukunaga M,Jansma JM,Duyn JH ( 2007): Low‐frequency fluctuations in the cardiac rate as a source of variance in the resting‐state fMRI BOLD signal. Neuroimage 38: 306–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L ( 1996): Circulation of the central nervous system In: Greger R,Windhorst U, editors. Comprehensive Human Physiology, Vol 1 Berlin: Springer‐Verlag. [Google Scholar]
- Sprott JC ( 2003): Chaos and Time‐Series Analysis. Oxford: Oxford University Press; p 226. [Google Scholar]
- Sunaert S ( 2006): Presurgical planning for tumor resectioning. J Magn Reson Imag 23: 887–905. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S,Tallgren P,Andersson S,Sainio K,Voipio J,Kaila K ( 2004): Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proc Natl Acad Sci USA 101: 5053–5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vern BA,Schuette WH,Leheta B,Juel VC,Radulovacki M ( 1988). Low‐frequency oscillations of cortical oxidative metabolism in waking and sleep. J Cereb Blood Flow Metab 8: 215–226. [DOI] [PubMed] [Google Scholar]
- Waites AB,Stanislavsky A,Abbot DF,Jackson GD ( 2005): Effect of prior cognitive state on resting state networks measured with functional connectivity. Human Brain Mapping 24: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites AB,Briellmann RS,Saling MM,Abbott DF,Jackson GD ( 2006): Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol 59: 335–343. [DOI] [PubMed] [Google Scholar]
- Welchew DE,Honey GD,Sharma T,Robbins TW,Bullmore ET ( 2002): Multidimensional scaling of integrated neurocognitive function and schizophrenia as a disconnexion disorder. Neuroimage 17: 1227–1239. [DOI] [PubMed] [Google Scholar]
- Wise RG,Ide K,Poulin MJ,Tracey I ( 2004): Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. NeuroImage 21: 1652–1664. [DOI] [PubMed] [Google Scholar]
- Xiong J,Parsons LM,Gao J‐H,Fox PT ( 1999): Interregional connectivity to primary motor cortex revealed using MRI resting state images. Hum Brain Map 8: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang YF,Jiang TZ,Lu YL,He Y,Tian LX ( 2004): Regional homogeneity approach to fMRI data analysis. NeuroImage. [DOI] [PubMed] [Google Scholar]
- Zang YF,He Y,Zhu CZ,Cao QJ,Sui MQ,Liang M,Tian LX,Jiang TZ,Wang YF ( 2007): Altered baseline brain activity in children with ADHD revealed by resting‐state functional MRI. Brain Dev 29: 83–91. [DOI] [PubMed] [Google Scholar]
- Zhou Y,Liang M,Tian L,Wang K,Hao Y,Liu H,Liu Z,Jiang T ( 2007): Functional disintegration in paranoid schizophrenia using resting‐state fMRI. Schizophrenia Research 97: 194–205. [DOI] [PubMed] [Google Scholar]
- Zhu CZ,Zang YF,Liang M,Tian LX,He Y,Li XB,Sui MQ,Wang YF,Jiang TZ ( 2005): Discriminative analysis of brain function at resting‐state for attention‐deficit/hyperactivity disorder. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv 8 (Pt 2): 468–475. [DOI] [PubMed] [Google Scholar]


